- 1Department of Animal Science, Faculty of Agriculture, Ege University, Izmir, Türkiye
- 2Department of Animal Science, Faculty of Agriculture, Ataturk University, Erzurum, Türkiye
- 3Department of Nano-Science and Nano-Engineering, Institute of Science and Technology, Ataturk University, Erzurum, Türkiye
- 4Department of Animal Science, College of Agriculture and Natural Resources, University of Tehran, Alborz, Karaj, Iran
- 5Department of Life Sciences, Western Caspian University, Baku, Azerbaijan
- 6Department of Industrial Engineering, University of Applied Sciences Technikum Wien, Vienna, Austria
Introduction: The purpose of this study was to investigate how in vitro gas production (GP) and ruminal fermentation characteristics were affected by increasing concentrations of green algae plant (C. reinhardtii) extracts in combination with nanoparticles MgO and MgS.
Methods: A solution containing 0.1 M MgCl2 was prepared in 300 mL for the green production of MgCl nanoparticles. The mixture was refluxed for two hours at 85°C using a reflux condenser after 10 mL of pomegranate plant extract was added. The green algal plant (C. reinhardtii), which has many non-toxic antioxidants, was used as a carbon source to produce carbon quantum dots (CQD). Chemical analysis was conducted in accordance with AOAC (2005) recommendations. Rumen fluid from recently slaughtered calves is used to produce in vitro gas immediately following slaughter. Analysis of variance (ANOVA) was performed on the obtained data from the in vitro study in a completely randomized design using the mixed model of SAS (version 9.4; Inc., Cary NC, USA).
Results and Discussion: The variance analysis results and the average values of the chemical compositions were significantly influenced by the extracts (all p < 0.0001). In this line, the values of net gas, pH, OMD, ME, NEl, and ME were found to be the highest for Algae + 50 MgO and the lowest for Algae + 50 MgS, respectively (all p < 0.0001). These promising results imply that extracts from C. Reinhardtii may be able to mitigate the adverse consequences of rumen fermentation. To precisely ascertain the impact particular Rhodophyta on greenhouse gas emissions, additional investigation is needed.
Introduction
In response to the increasing population and the need to provide animal protein, along with the lack of animal feed resources, humans and animals have competed for agricultural resources (1, 2). Thus, Sustainability of livestock production is currently a research priority due to the increasing demand for food by the growing world population. It has been predicted that green algae (Chlamydomonas reinhardtii) can provide biomass and animal feed in the future (3). Green algae are substantially more productive in terms of biomass than other photosynthetic organisms, and more crucially, growing microalgae does not compete with food crops on arable ground (4). It is possible to use the algae as a non-traditional alternative feed source owing to their efficacy in converting solar energy, independence from external environmental conditions, and high production rate compared to conventional crops (4).
Besides contributing to greenhouse gas emissions, methane loss is one of the greatest negative factors in ruminant production (5–7). Although causing energy loss in the rumen, CH4 production reduces rumen acidity and keeps the rumen environment below normal via using H+ ions by methanogenic bacteria (8). The concentration of dihydrogen in the rumen depends on factors such as methanogen growth and the rate of feed fermentation. Methane generation and volatile fatty acid production are determined by the equilibrium between pathways that create and combine metabolic hydrogen (9). A variety of methane inhibitors can prevent methane-related energy losses in ruminants and provide economic and ecological benefits (10).
Numerous resources have focused on the reduction of CH4 generation, especially energy loss from methane production. In addition, studies on the transformation of fermentation products into chemicals useful for animals have been accompanied in the recent years. Accordingly, to reduce enteric methane production, unsaturated fatty acids (11, 12), lysozyme (13), organic acid salts (14), S. cerevisiae (15), enzymes (15), and ethyl acetate (16) are added to ruminant diets. Unlike specific CH4 inhibitors, these compounds generally affect and suppress microorganism growth (17). Consequently, the feed value is reduced due to adverse effects on rumen fermentation. Many researchers suggest that, instead of adding additives that are thought to affect the rumen microbiome, the use of carbon quantum dots (CQD), magnesium sulfide (MgS) and magnesium oxide (MgO) nanoparticles, which are known as hydrogen receptors, is an appropriate alternative (18–21). However, there is a lack of information about the evaluating anti-methanogenic capabilities of nanoparticles of C. reinhardtii in in vitro system. Thus, this study assessed, using an in vitro gas and methane generation approach, the green algal (C. reinhardtii) anti-methanogenic capabilities with and without nanoparticles.
Materials and methods
Green synthesis and structural characterization of CQD, MgS and MgO NPs
Preparation of algae extract
For the green synthesis of MgCl NPs, 300 mL of a solution containing 0.1 M MgCl2 was prepared. 10 mL of pomegranate algae extract was added to the solution and refluxed for 2 h at 85°C under a reflux condenser. It was then placed in a reactor via Teflon tube. Hydrothermal reactions were performed at 180–195°C for 4 h to reduce nano-particle (NP) size. The precipitated MgO NPs were washed first via pure water and ethyl alcohol. They were preserved in an atmosphere free of moisture after being dried for 48 h at 60°C in an oven. MgS NPs were synthesized using the same procedure. 1 mol of Na2S was added to the synthesis medium and the same process was repeated to synthesize MgS NPs. In the synthesis of CQD, the green algae (C. reinhardtii), known for its high non-toxic antioxidant content, was used as a carbon source. For this purpose, the algae extract was placed in a reactor containing sodium citrate as a reducing agent. CQD was synthesized by incubating at 180–195°C for 8 h.
Characterization of CQD, MgS and MgO NPs
Green-synthesised CQD, MgS and MgO NPs were characterized at the High Technology Application and Research Center of Eastern Anatolia (DAYTAM) at Atatürk University. X-ray microscopy (XRD) and FTIR analyses were performed for the characterization of CQD, MgS, and MgO NPs. The synthesized CQD, MgS, and MgO nanoparticles were characterized, including their size and morphology.
Chemical analyses
AOAC (71) guidelines were followed for chemical analyses. Kjeldahl was used to determine N content (AOAC, 71, Method 984.13). For the determination of Acid Detergent Fiber (ADF) and Neutral Detergent Fiber (NDF), Van Soest et al. (22) were used.
In vitro gas production
In vitro gas production is performed by taking rumen fluid from newly slaughtered cattle (as soon as they are slaughtered), as mentioned by Palangi et al. (10). Using a method validated by Menke and Steingass (23), it was found that 0.2 g of treated (CQD, MgS, and MgO nanoparticles at levels of 0.50, 100 ppm) and ground (1 mm) green algae (C. reinhardtii) samples were incubated in rumen fluid via 100 mL standardized glass syringes to measure in vitro gas production. Methane and gas volumes of feed samples were measured 24 h after incubation.
Statistical analysis
The mixed model of SAS version 9.4 (SAS Institute, Inc., Cary, NC, United States) was used in a completely randomized design to examine the data gathered from the in vitro study. The following model was used to statistically analyze the experiment:
where μ is the overall mean for each parameter, Ti is the effect of treatment, and Eij is residual error. Differences among sample means with p < 0.05 were accepted as statistically significant.
Results
Characterization of CQD, MgS, and MgO nanoparticles
XRD analysis
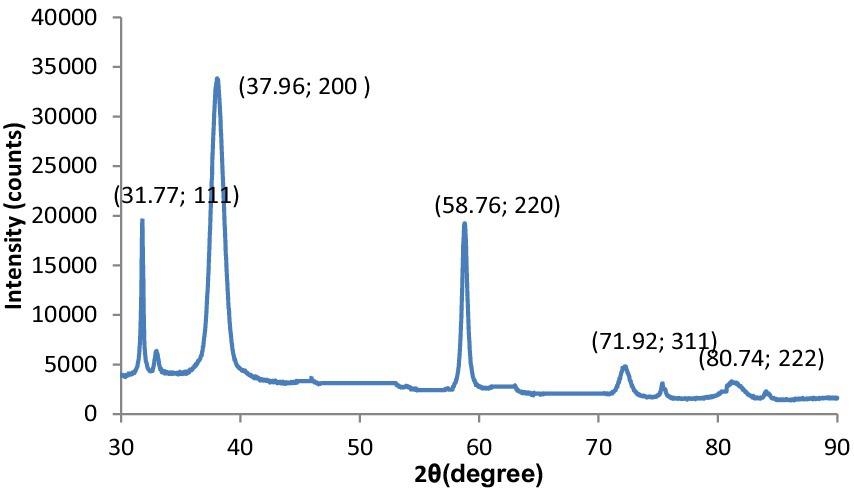
The fundamental method for examining crystal size, phase purity, and crystal structure is X-ray diffraction (XRD) examination. As shown in Figure 1, the XRD pattern of the synthesized MgO exhibits various peaks corresponding to the (111), (200), (220), (311), and (222) reflection planes.
The particle size of the synthesized MgO NPs was determined from the Debye–Scherrer equation: D = K/cos (θ).
The MgO NPs’ median dimension and d-spacing values have been determined to be 20 nm and 0.25 nm, correspondingly.
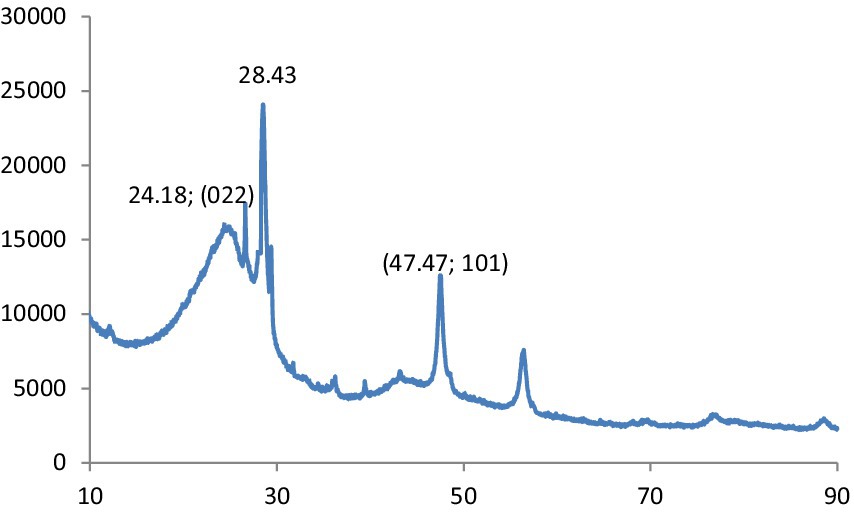
Figure 2 shows the XRD pattern of CNPs. The XRD pattern exhibited an intense peak at 2θ = 22.90° and a weak peak at 2θ = 41.60°, corresponding to the (022) and (101) diffraction patterns of graphite carbon, respectively.
FTIR analysis
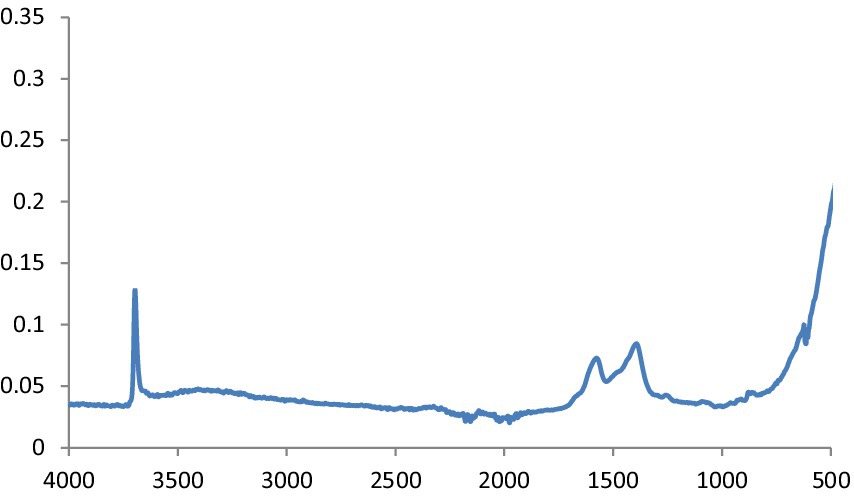
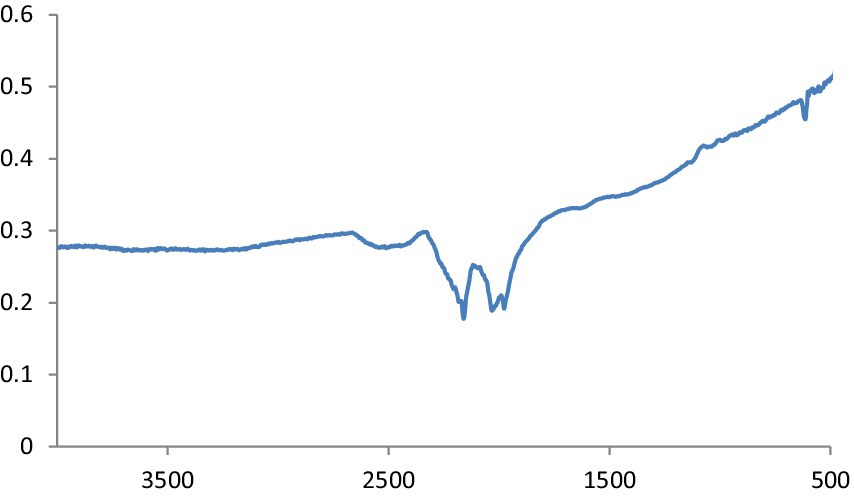
MgO NPs are characterized using the FTIR spectrum (Figure 3). In ambient settings, the spectra were captured at wavelengths ranging from 400 to 4,000 cm−1. The peak at 651.94 cm−1 indicates the stretching peak vibration of Mg-O bond, confirming that the obtained product is magnesium oxide. Moreover, H2O adsorption on the metal surface is indicated by the peaks at 1552.0 cm−1 and 3520.0 cm−1.
The potential biomolecules in charge of the reduction of MgS NPs by green synthesis were found using Fourier transform infrared spectroscopy (FTIR) analysis. The FTIR spectra of MgS NPs made using Na2S and pomegranate algae extract are displayed in Figure 4. In the spectrum, bands were observed at 3603.5, 1,725, 1,550, 1,232, 972 and 613 cm−1. Particularly, the sharp band at 1,725 cm−1 represents the C=O vibrations specific to the structure of flavonoids that can be found in pomegranate extract.
TEM analysis
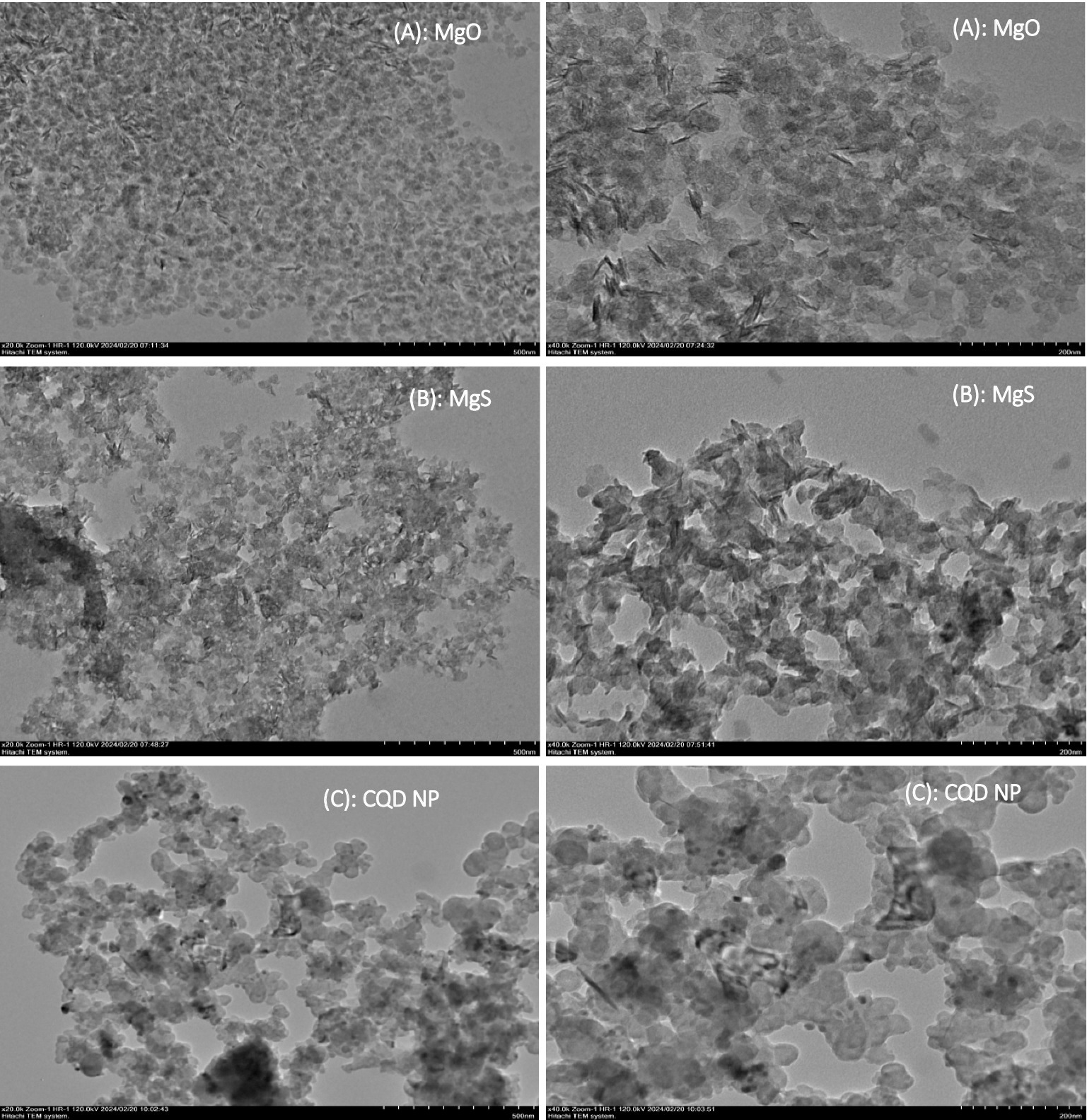
The characterisation of MgO NP production using pomegranate extract is depicted in Figure 5A, which is an image captured using a transmission electron microscope (TEM). Here, the scale bars are 500 and 200 nm. The images of TEM analysis of MgS NPs were taken and show the structures of MgS NPs (Figure 5B). The shape of these NPs was a small layer formation with a nearly spherical arrangement on a smooth surface. They had diameters ranging from 20–60 ± 1.6 nm and an average diameter of 55 ± 3.8 nm.
Chemical composition
The nutrient composition and relative feed value of algal at various concentrations are indicated in Table 1. The extracts impacted significantly the chemical compositions (all p < 0.0001). Regarding fiber fractions such as ADF and NDF, the highest values were recorded for Algae +100 MgS. In contrast, in related to the CP and EE fractions, Algae +50 Mgs had the highest values (p < 0.0001).
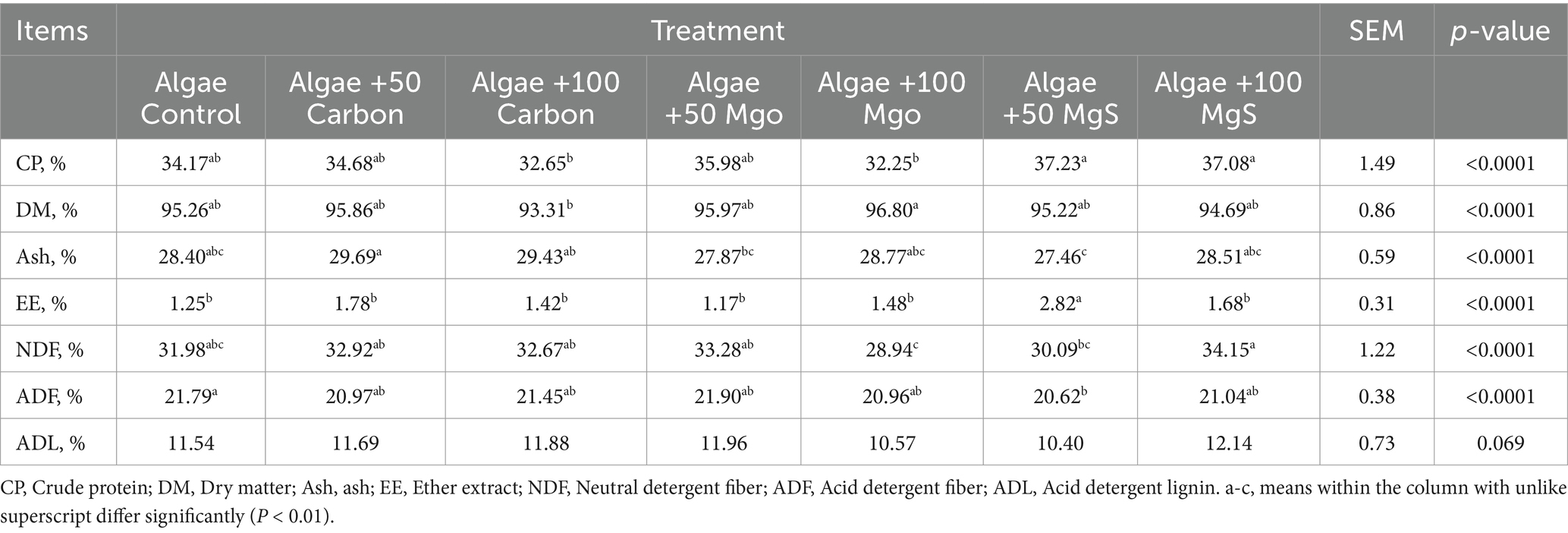
Table 1. Chemical nutrient composition and relative feed value of increasing doses of Algae at different levels of nanoparticles.
In vitro fermentation and gas production
The effects of algae extracts on in vitro rumen fermentation profiles are shown in Table 2. The parameters of gas production, pH, and OMD have influenced significantly by the different extracts (all p < 0.0001); the highest and lowest values regarding net gas, pH, OMD, ME, and NEl were observed for Algae +50 MgO and Algae +50 MgS, respectively. The converse mentioned trend was observed for Algae +50 MgO and Algae +50 MgS in related to CH4 production (p < 0.0001). Totally, not only measured total gas volume but also most of the measured parameters from the Algae-based rumen fluid were significantly influenced by the different nanoparticles (p < 0.0001).
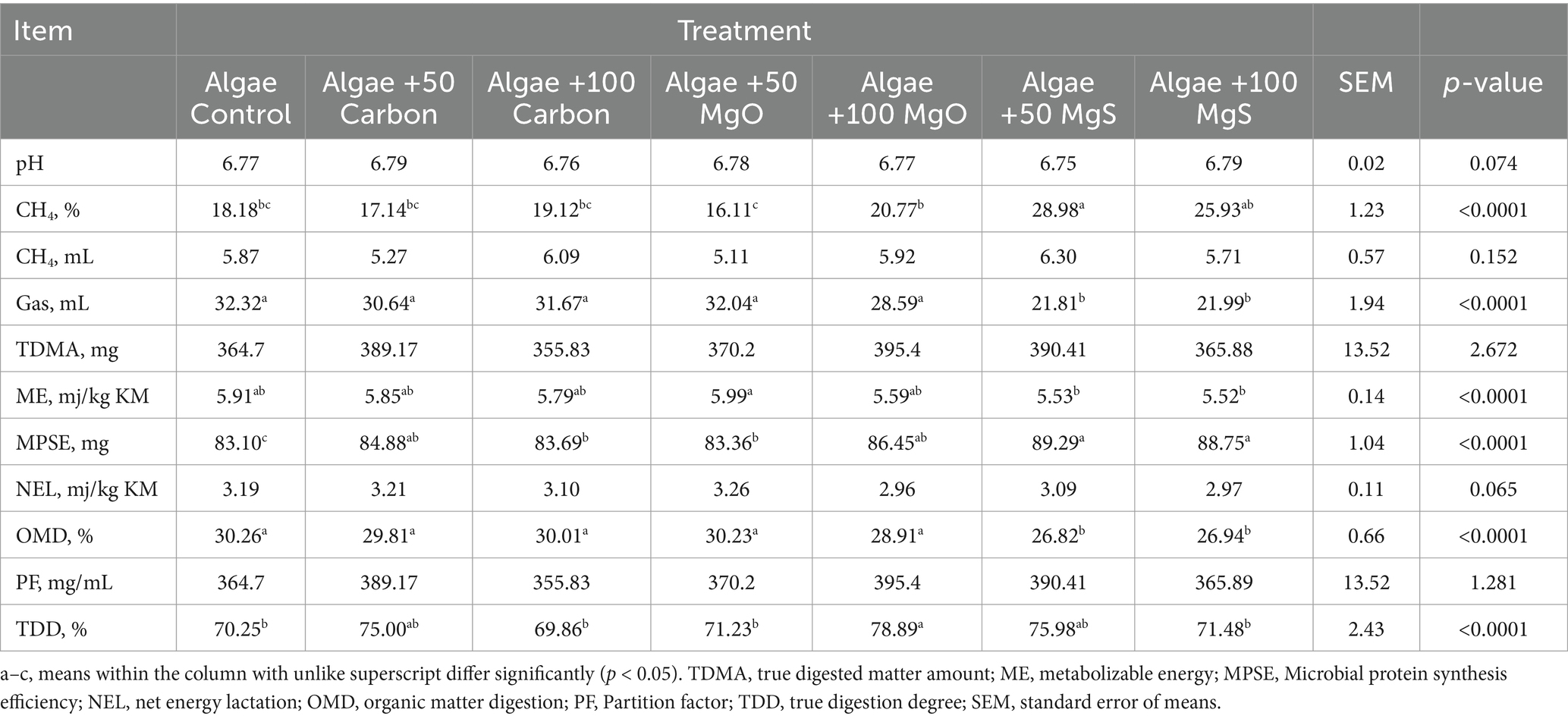
Table 2. Effects of nanoparticles on in vitro gas, methane production quantities, and rumen fermentation variables of algae.
VFA parameters
Table 2 shows the volatile fatty acid (VFA) composition of rumen fluid. The effects of extracts on total VFA (TVFA) were substantial (p < 0.001), with the highest value found in the “Algae +100 MgS” group (163.12 mM) and the lowest in the CON group (139.59 mM). For the individual VFA, extracts have resulted in fluctuated amounts between treatments, in which the treatments influenced the individual VFA significantly (p < 0.001).
Discussion
Characterization of CQD, MgS, and MgO nanoparticles
XRD analysis
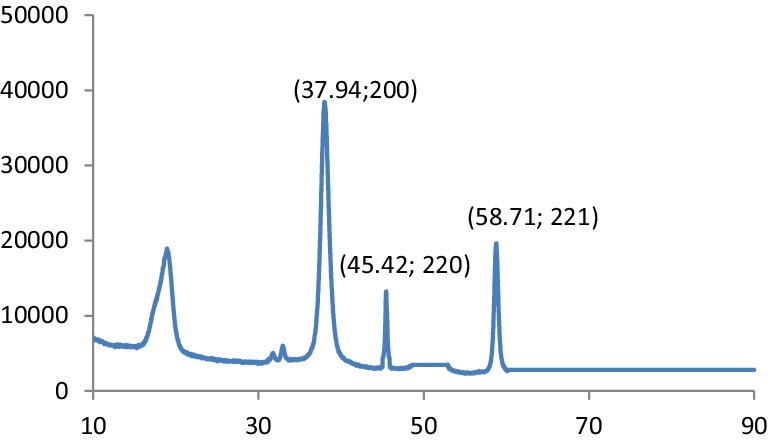
The observed peaks demonstrate the cubic structure of MgO and assign it to the pure phase of periclase MgO. In the spectra of other phases, no additional peaks could be seen. It confirmed that the prepared MgO was crystallized and was free of impurities. In addition, the presented peaks exhibit higher intensity and narrower spectral widths, indicating the product is in good condition. The XRD graph obtained for the crystallographic analysis of synthesized MgS nanomaterials is given in Figure 6. The 2θ values for MgS NPs peak at 37.94° (200), 45.42 (220) and 58.71° (221) at 200, 210 and 222. The characteristic peaks of the XRD spectrum at 2θ = 45.45° can be indexed at (220). Literature-based findings are consistent with the results obtained (24).
FTIR analysis
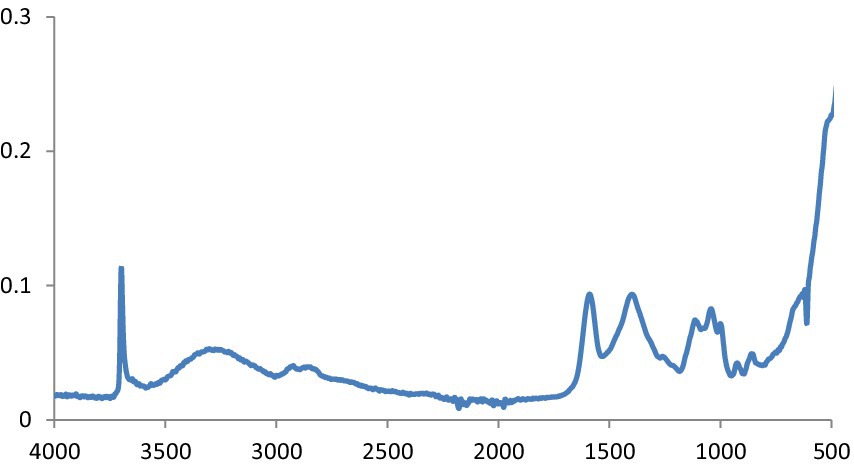
This is defined as OH stretching and bending, respectively. The metal-oxygen frequencies for the respective metal oxides published in the literature and observed frequencies coincide reasonably well. Using this method, MgO NPs can be analyzed for their chemical composition and surface properties (25). The -C-H bending vibrations in the aromatic amine groups of the flavonoid structure are linked to the absorption band at 550 cm−1. Additionally, the peak at 972 cm−1 shows the existence of MgS NPs as well as the distinctive C-S bond structure peaks. Under the aliphatic chain structure, the observed 613.4 cm-1 peak is part of the –CH2 group. The pomegranate algae extract’s bioactive components were verified using FTIR spectrum (26). Using this analysis, it is possible to determine the biomolecules involved in the synthesis of MgS NP. Figure 7 displays the carbon quantum dots of NP according to FTIR spectra. The band at around 3,242 cm−1 is indicative of OH stretching vibration, which may arise from either the hydroxyl groups found in C black NP or water absorption. The peak recorded at 1,652 cm−1 was exclusively found in pure CB and was ascribed to the material’s C=C stretching vibration. Peaks at 2,040, 2,166, and 2,015 cm−1 are ascribed to the nanocarbon structure’s carbonyl group and C–O stretching (27).
TEM analysis
TEM analysis is a very critical methodology to describe the particle size distribution, average particle size and shape of NPs. The produced MgO nanoparticles are less than 10 nm in size and spherical, as confirmed by TEM examination, despite being aggregated (28). The current green synthesis method approach has enabled the use of a simple and low-cost reducing agent for single-phase MgS NPs. This approach offers an effective method to synthesize MgS NPs in a non-toxic manner (24). Transmission electron microscopy (TEM) evaluated the morphology of pure carbon NP samples. These NPs are the samples showing the highest level of modification and are shown in Figure 5C. The TEM image shows semi-spherical primary particles with an average size ranging from 15 to 65 nm. These primary particles were formed and held together by agglomeration, resulting in agglomerates. The results corroborate other published studies in the literature, and the conclusions are consistent with current literature (29).
Chemical composition
The present results on the chemical composition of macroalgae were in line with previous reports (30, 31). In disagreement with our findings, a recent meta-analysis of 47 published papers containing a broad variety of macroalgae was conducted and demonstrated that the average content of CP, NDF, ADF, and organic matter (OM) was 734.2, 189.2, 321.3, and 208.5 g/kg DM, respectively (32). Additionally, to confirm our findings Min et al. (33) published the different levels of CP (7.8 to 38.1% DM), NDF (16.6 to 43.1% DM), ADF (6.6 to 13.1% DM), and EE (0.3 to 3.9% DM) across eight macroalgae species. It’s important to note that the chemical composition and bioactive content of macroalgae are impacted by their taxonomic classification (brown, green, or red), and vary across genera and species. Seasonal fluctuations may also impact their composition during the growing and harvesting periods (31, 34). All algae and nanoparticles tested in our study had acceptable chemical compositions, particularly as a protein source; however, they should be included in a TMR ration to determine their potential advantages.
The current study’s findings about NDF and ADF were congruent with those of Mahmood Ameen (35). Feeds typically comprise 100–120 g/kg DM of ash. The crude ash levels of the feeds were comparable to those reported by Kamalak et al. (36) and Karabulut et al. (37). Differences in nutrient composition of feeds between studies may be qualified to numerous elements, such as climate, fertilization, species and type, harvesting time, feed storage conditions, and vegetative phase (36, 38). Also, it has been stated that the in vitro gas production level is affected by the nutrient composition of feedstuff, the presence of compounds inhibiting (such as tannins) gas production, the microflora and microfauna content of the rumen fluid (donor animal’s diet), and the quality of fermentation provided (36, 38).
Microalgae have mass balances ranging from 630 to 1,170 g kg−1, although proximate analysis seldom provides 100% (39). Our investigation’s findings regarding the mass balance deficit suggest that other soluble components such as B vitamins, nonprotein nitrogen, chlorophyll, and soluble carbohydrates may be responsible. Microalgae fiber is low in hemicellulose and lacks lignin, even though it has a high fiber content (50–55% of total carbohydrate) (40). This enhances the probability that the protein will be readily available due to its lack of lignin complexation. In addition, the cell wall fraction in microalgae is highly digestible (41). Drewery et al. (42) found that supplementing post-extraction algal residue (CP = 179 g kg−1 DM) increased OM digestibility in steers fed oat straw (CP; 45 g kg−1 DM). Similarly, Tetracystis sp., N. bacillaris, and C. vulgaris have a higher lipid content, which improves the calorie density of the diet. It has been widely shown that lipids frequently diminish enteric CH4 emissions from ruminants (43, 44).
In vitro fermentation and gas production
The post-fermentation pH ranged from 6.75 to 7.79 among algal-extract treatments, demonstrating that algae supplementation promotes a more alkaline environment during microbial fermentation. Carbohydrates are the primary source of substrate for the creation of acetate and butyrate during ruminal fermentation, and as byproducts, CO2 and hydrogen (H2), are used by methanogenic archaea to produce CH4 (9). Furthermore, according to Kholif et al. (45), microalgae promote carbohydrate fermentation by rumen microbes, which is consistent with what was observed with the addition of microalgae and was attributed to the microalgae’s fulvic acids, which can provide carbon to ruminal microorganisms (46) and thus favor microbial growth and increase DMD. In turn, the increased degradability resulted in higher production of SCFA and ME, ascribed to enhanced carbohydrate degradation (45).
Although not investigated in the current study, the increase in SFCA and ME with microalgae might be due to increased activity of the fibrolytic bacteria (47) and increased propionate production. In contrast, decrease of SFCA and ME are attributed to a reduction in other SCFAs, such as acetate (48). In the meantime, the effects on DMD and SCFA associated with the content and degradability of feed carbohydrates may be reflected in the computed variations in CH4 per unit of SCFA, ME, and OM (49).
Biogas production (BG) is intimately related to feed degradability and, as a result, the availability of highly-fermented nutrients for rumen microbial activity and growth (15). Although their production is predominantly reliant on the fermentation of carbohydrates to SCFA and proteins, and BG is mostly made up of CO2 and CH4, their contribution to BG is negligible in comparison to that of carbohydrates (50). Furthermore, the production of acetate and butyrate during rumen fermentation produces more gas than the formation of propionate, accounting for the majority of the BG (51).
Natural compounds of microalgae have been proposed as potential methods for controlling rumen fermentation, contributing to CH4 generation (52, 53). A previous in vitro investigation (54) demonstrated that Schizochytrium spp. inhibited CH4. Furthermore, several research (55, 56) found an increase in CH4-producing bacteria and protozoa, demonstrating that not all microalgae have CH4-reducing properties.
The anti-methanogenic effect observed in this study has been reported in studies involving other microalgae (Spirulina platensis, Chlorella vulgaris, and Schizochytrium spp.). The studies attribute this effect to the presence of docosahexaenoic acid (C22:6 n − 3) and eicosapentaenoic acid (C20:5 n − 3), polyunsaturated acids that decrease the concentration of acetate and increase propionate, which results in reduction the abundance of methanogenic archaea, the primary microorganisms producing CH4 (15, 51, 52). Likewise, Sheng et al. (57) found that humic compounds, including fulvic and humic acids, can lower CH4 production in ruminants. They ascribed this to a decrease of the molar proportion of protozoa and acetate populations (58), which minimizes the amount of H2 available for CH4 production (59).
The addition of the microalgae reduced BG production in the current study, which is in line with Elghandour et al. (15), who observed that the BG decreased with the addition of the microalgae Schizochytrium spp. and associated it with the antimicrobial and cytotoxic effects of the compounds of the microalgae (60), as well as the long-chain fatty acid profile (48). Also, it is likely that the microalgae have modified the structure of the microbial community during fermentation which is caused variations in the final fermentation products, including the SCFA profile (61).
Among the treatments, the highest amount of gas produced was observed for the MgS nanoparticles group. Additionally, the MgO treatments demonstrated a notable decrease in the production of methane, which indicate the ability of MgO nanoparticles to meet the needs of rumen bacteria during the incubation period (62). The two main sources of in vitro gas generation are carbon dioxide and methane, which are derived directly from microbial fermentation, and carbon dioxide released from a bicarbonate buffer, obtained indirectly by buffering short-chain fatty acids. Menke and Steingass (23) affirm that the only variables influencing gas generation are the feed’s physical and chemical composition. The fermentation rate, however, could be impacted by modifications in ruminal microbial activity.
VFA parameters
Volatile fatty acids (VFAs) have been considered one of the most significant factors in achieving anaerobic fermentation. According to Makkar (63), fluctuations in gas production might alter the amounts or ratios of VFA produced. VFAs’ hydrophobic qualities enable them to penetrate the bilayer structure of the bacterial cell’s plasma membrane (64). Therefore, by changing the membrane structure and increasing its flowability and permeability, they can lower the rate of bacterial growth (65).
Previous research has shown that adding red algae (Asparagopsis taxiformis) and lipid-extracted microalgae to forage diets dramatically boosted propionate and butyrate levels in the rumen (66). This is deemed advantageous since previous research demonstrated that the energy from propionate was used more efficiently than energy from acetate (67, 68). Lodge-Ivey et al. (68) found that adding lipid-extracted algae (Chlorella or Nannochloropsis) to the diet increased total rumen VFA content, which is in consistent with our findings. In contrast to our findings, it has been proposed that the high lipid content of Chlorella may suppress cellulolytic bacteria in the rumen and finally reduction of total VFA (53). Furthermore, adding algae to a corn silage-based diet raised ruminal pH and reduced total VFA by up to 18% after 19 days (69). Other in vitro investigations (53–55, 70) found that supplementation with DHA-rich microalgae or marine algae increased ruminal propionate while decreasing overall VFA and CH4 synthesis. The differences could be attributed to variances in supplementation levels and oil extraction.
Conclusion
The results of our study indicate that the use of Algae+50 Mgo nano-particles, viable feed additive, highest in CP and EE, can reduce methane emission and gas production. Furthermore, all the treatments containing Algae decreased in vitro gas production. Also, addition of the Algae+50 Mgo nano-particles improved fermentation kinetics, VFAs, and nutrients’ degradability compared to the other experimental treatments. These results are promising and suggest that the applied extracts could mitigate undesirable outcomes of rumen fermentation. Although more research is necessary to clarify the exact effects of the extracts on the aforementioned indices.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
VP: Conceptualization, Data curation, Project administration, Software, Writing – original draft, Writing – review & editing. AdK: Methodology, Data curation, Writing – original draft. MM: Supervision, Data curation, Writing – original draft. HN: Methodology, Data curation, Writing – original draft. HÜ: Methodology, Data curation, Writing – original draft. AlK: Methodology, Data curation, Writing – original draft. AF: Methodology, Data curation, Writing – original draft. AM: Methodology, Data curation, Writing – original draft. ML: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Scientific and Technological Research Council of Türkiye (TUBITAK) under Grant Number 123R066.
Acknowledgments
The authors thank TUBITAK for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Byerly, T. Competition between animals and man for agricultural resources. In: New protein foods: animal protein supplies, Altschul AM. Academy Press, Inc. A Subsidiary of Harcourt Brace Jovanovich, vol. 3 (2013). 72.
2. Weber, GM, and Windisch, W. Producing sufficient animal-source protein for the growing world population In: Springer, Cham: Sustainable Nutrition in a Changing World (2017). 321–34.
3. Vanthoor-Koopmans, M, Wijffels, RH, Barbosa, MJ, and Eppink, MH. Biorefinery of microalgae for food and fuel. Bioresour Technol. (2013) 135:142–9. doi: 10.1016/j.biortech.2012.10.135
4. Moheimani, NR, Vadiveloo, A, Ayre, JM, and Pluske, JR. Nutritional profile and in vitro digestibility of microalgae grown in anaerobically digested piggery effluent. Algal Res. (2018) 35:362–9. doi: 10.1016/j.algal.2018.09.007
5. Bowen, JM, Cormican, P, Lister, SJ, McCabe, MS, Duthie, C-A, Roehe, R, et al. Links between the rumen microbiota, methane emissions and feed efficiency of finishing steers offered dietary lipid and nitrate supplementation. PLoS One. (2020) 15:e0231759. doi: 10.1371/journal.pone.0231759
6. Pryce, JE, and Haile-Mariam, M. Symposium review: genomic selection for reducing environmental impact and adapting to climate change. J Dairy Sci. (2020) 103:5366–75. doi: 10.3168/jds.2019-17732
7. Huhtanen, P, Bayat, A, Lund, P, Hellwing, ALF, and Weisbjerg, MR. Variation in feed efficiency hampers use of carbon dioxide as a tracer gas in measuring methane emissions in on-farm conditions. J Dairy Sci. (2020) 103:9090–5. doi: 10.3168/jds.2020-18559
8. Kumar, S, Puniya, AK, Puniya, M, Dagar, SS, Sirohi, SK, Singh, K, et al. Factors affecting rumen methanogens and methane mitigation strategies. World J Microbiol Biotechnol. (2009) 25:1557–66. doi: 10.1007/s11274-009-0041-3
9. Ungerfeld, EM. Metabolic hydrogen flows in rumen fermentation: principles and possibilities of interventions. Front Microbiol. (2020) 11:589. doi: 10.3389/fmicb.2020.00589
10. Palangi, V, Macit, M, and Bayat, A. Mathematical models describing disappearance of Lucerne hay in the rumen using the nylon bag technique. South Afr J Anim Sci. (2020) 50:7. doi: 10.4314/sajas.v50i5.9
11. Garcia, F, Brunetti, MA, Lucini, EI, Turcato, S, Moreno, MV, Frossasco, GP, et al. Essential oils from Argentinean native species reduce in vitro methane production. Rev Investig Agropecu. (2018) 44:76–83.
12. Martin, C, Ferlay, A, Mosoni, P, Rochette, Y, Chilliard, Y, and Doreau, M. Increasing linseed supply in dairy cow diets based on hay or corn silage: effect on enteric methane emission, rumen microbial fermentation, and digestion. J Dairy Sci. (2016) 99:3445–56. doi: 10.3168/jds.2015-10110
13. Biswas, AA, Lee, SS, Mamuad, LL, Kim, S-H, Choi, Y-J, Bae, G-S, et al. Use of lysozyme as a feed additive on in vitro rumen fermentation and methane emission. Asian Australas J Anim Sci. (2016) 29:1601–7. doi: 10.5713/ajas.16.0575
14. Elghandour, M, Kholif, A, Salem, A, De Oca, RM, Barbabosa, A, Mariezcurrena, M, et al. Addressing sustainable ruminal methane and carbon dioxide emissions of soybean hulls by organic acid salts. J Clean Prod. (2016) 135:194–200. doi: 10.1016/j.jclepro.2016.06.081
15. Elghandour, M, Vázquez, J, Salem, A, Kholif, A, Cipriano, M, Camacho, L, et al. In vitro gas and methane production of two mixed rations influenced by three different cultures of Saccharomyces cerevisiae. J Appl Anim Res. (2017) 45:389–95. doi: 10.1080/09712119.2016.1204304
16. Boussaada, A, Arhab, R, Calabrò, S, Grazioli, R, Ferrara, M, Musco, N, et al. Effect of Eucalyptus globulus leaves extracts on in vitro rumen fermentation, methanogenesis, degradability and protozoa population. Ann Anim Sci. (2018) 18:753–67. doi: 10.2478/aoas-2018-0006
17. McGinn, S, Beauchemin, K, Coates, T, and Colombatto, D. Methane emissions from beef cattle: effects of monensin, sunflower oil, enzymes, yeast, and fumaric acid. J Anim Sci. (2004) 82:3346–56. doi: 10.2527/2004.82113346x
18. Wang, T, Zhang, D, Dai, L, Chen, Y, and Dai, X. Effects of metal nanoparticles on methane production from waste-activated sludge and microorganism community shift in anaerobic granular sludge. Sci Rep. (2016) 6:25857. doi: 10.1038/srep25857
19. Ünşar, EK, and Perendeci, NA. What kind of effects do Fe2O3 and Al2O3 nanoparticles have on anaerobic digestion, inhibition or enhancement? Chemosphere. (2018) 211:726–35. doi: 10.1016/j.chemosphere.2018.08.014
20. Fujinawa, K, Nagoya, M, Kouzuma, A, and Watanabe, K. Conductive carbon nanoparticles inhibit methanogens and stabilize hydrogen production in microbial electrolysis cells. Appl Microbiol Biotechnol. (2019) 103:6385–92. doi: 10.1007/s00253-019-09946-1
21. Wang, R, Si, HB, Wang, M, Lin, B, Deng, JP, Tan, LW, et al. Effects of elemental magnesium and magnesium oxide on hydrogen, methane and volatile fatty acids production in in vitro rumen batch cultures. Anim Feed Sci Technol. (2019) 252:74–82. doi: 10.1016/j.anifeedsci.2019.04.009
22. Pv, VS, Robertson, JB, and Lewis, BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. (1991) 74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2
23. Menke, HH, and Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev. (1988) 28:7–55. doi: 10.1007/s11240-018-1512-8
24. Nalci, OB, Nadaroglu, H, Pour, AH, Gungor, AA, and Haliloglu, K. Effects of ZnO, CuO and γ-Fe 3 O 4 nanoparticles on mature embryo culture of wheat (Triticum aestivum L.). Plant Cell Tissue Organ Cult. (2019) 136:269–77.
25. Mahadevaiah, R, Lalithamba, HS, Shekarappa, S, and Hanumanaika, R. Synthesis of Nα-protected formamides from amino acids using MgO nano catalyst: study of molecular docking and antibacterial activity. Sci Iran. (2017) 0:3002–13. doi: 10.24200/sci.2017.4491
26. Taleatu, B, Omotoso, E, Arbab, E, Lasisi, R, Makinde, W, and Mola, G. Microstructural and optical properties of nanocrystalline MgS thin film as wide band gap barrier material. Appl Phys A. (2015) 118:539–45. doi: 10.1007/s00339-014-8753-0
27. Vijaya, S, and Deepa, C. Facile green synthesis of carbon nanoparticles using medicinally Murraya koenigii shoots. J Environ Nanotechnol. (2017) 6:01–4. doi: 10.13074/jent.2017.03.171232
28. Munjal, S, Singh, A, and Kumar, V. Synthesis and characterization of MgO nanoparticles by orange fruit waste through green method. Int J Adv Res Comput Sci. (2017) 4:36–42.
29. Andrade-Guel, M, Ávila-Orta, CA, Cadenas-Pliego, G, Cabello-Alvarado, CJ, Pérez-Alvarez, M, Reyes-Rodríguez, P, et al. Synthesis of nylon 6/modified carbon black nanocomposites for application in uric acid adsorption. Materials. (2020) 13:5173. doi: 10.3390/ma13225173
30. Guinguina, A, Hayes, M, Gröndahl, F, and Krizsan, SJ. Potential of the red macroalga Bonnemaisonia hamifera in reducing methane emissions from ruminants. Animals. (2023) 13:2925. doi: 10.3390/ani13182925
31. Lee-Rangel, HA, Roque-Jiménez, JA, Cifuentes-López, RO, Álvarez-Fuentes, G, Cruz-Gómez, ADL, Martínez-García, JA, et al. Evaluation of three marine algae on degradability, in vitro gas production, and CH4 and CO2 emissions by ruminants. Fermentation. (2022) 8:17. doi: 10.3390/fermentation8100511
32. Sofyan, A, Irawan, A, Herdian, H, Harahap, MA, Sakti, AA, Suryani, AE, et al. Effects of various macroalgae species on methane production, rumen fermentation, and ruminant production: a meta-analysis from in vitro and in vivo experiments. Anim Feed Sci Technol. (2022) 294:115503. doi: 10.1016/j.anifeedsci.2022.115503
33. Min, BR, Parker, D, Brauer, D, Waldrip, H, Lockard, C, Hales, K, et al. The role of seaweed as a potential dietary supplementation for enteric methane mitigation in ruminants: challenges and opportunities. Anim Nutr. (2021) 7:1371–87. doi: 10.1016/j.aninu.2021.10.003
34. Pandey, D, Mansouryar, M, Novoa-Garrido, M, Næss, G, Kiron, V, Hansen, H, et al. Nutritional and anti-methanogenic potentials of macroalgae for ruminants. (2021). doi: 10.19103/AS.2021.0091.14
35. Mamhood Ameen, S. Potential nutritive value and methane production of some ruminant feedstuffs from north of Iraq estimated using an in vitro gas technique: Fen Bilimleri Enstitüsü. (2005).
36. Kamalak, A, Canbolat, Ö, Gürbüz, Y, and Ozay, O. Prediction of dry matter intake and dry matter digestibilities of some forages using the gas production technique in sheep. Turk J Vet Anim Sci. (2005) 29:517–23.
37. Karabulut, A, Canbolat, O, Kalkan, H, Gurbuzol, F, Sucu, E, and Filya, I. Comparison of in vitro gas production, metabolizable energy, organic matter digestibility and microbial protein production of some legume hays. Asian Australas J Anim Sci. (2007) 20:517–22. doi: 10.5713/ajas.2007.517
38. Canbolat, Ö, Kara, H, and Fİlya, İ. Comparison of in vitro gas production, metabolizable energy, organic matter digestibility and microbial protein production of some legume hays (2013).
40. Tibbetts, SM, Whitney, CG, MacPherson, MJ, Bhatti, S, Banskota, AH, Stefanova, R, et al. Biochemical characterization of microalgal biomass from freshwater species isolated in Alberta, Canada for animal feed applications. Algal Res. (2015) 11:435–47. doi: 10.1016/j.algal.2014.11.011
41. Moore, KJ, and Jung, H-JG. Lignin and fiber digestion. J Range Manag. (2001) 54:420–30. doi: 10.2458/azu_jrm_v54i4_moore
42. Drewery, M, Sawyer, J, Pinchak, W, and Wickersham, T. Effect of increasing amounts of postextraction algal residue on straw utilization in steers. J Anim Sci. (2014) 92:4642–9. doi: 10.2527/jas.2014-7795
43. Boadi, D, Benchaar, C, Chiquette, J, and Massé, D. Mitigation strategies to reduce enteric methane emissions from dairy cows: update review. Can J Anim Sci. (2004) 84:319–35. doi: 10.4141/A03-109
44. Beauchemin, KA, McGinn, SM, and Petit, HV. Methane abatement strategies for cattle: lipid supplementation of diets. Can J Anim Sci. (2007) 87:431–40. doi: 10.4141/CJAS07011
45. Kholif, A, Gouda, G, Olafadehan, O, and Abdo, M. Effects of replacement of Moringa oleifera for berseem clover in the diets of Nubian goats on feed utilisation, and milk yield, composition and fatty acid profile. Animal. (2018) 12:964–72. doi: 10.1017/S1751731117002336
46. Ghasimi, DS, Aboudi, K, de Kreuk, M, Zandvoort, MH, and van Lier, JB. Impact of lignocellulosic-waste intermediates on hydrolysis and methanogenesis under thermophilic and mesophilic conditions. Chem Eng J. (2016) 295:181–91. doi: 10.1016/j.cej.2016.03.045
47. Beauchemin, KA, Ungerfeld, EM, Eckard, RJ, and Wang, M. Fifty years of research on rumen methanogenesis: lessons learned and future challenges for mitigation. Animal. (2020) 14:s2–s16. doi: 10.1017/S1751731119003100
48. Burnett, V, Jacobs, J, Norng, S, and Ponnampalam, E. Feed intake, liveweight gain and carcass traits of lambs offered pelleted annual pasture hay supplemented with flaxseed (Linum usitatissimum) flakes or algae (Schizochytrium sp.). Anim Prod Sci. (2016) 57:877–83. doi: 10.1071/AN15230
49. Astudillo-Neira, R, Muñoz-Nuñez, E, Quiroz-Carreno, S, Avila-Stagno, J, and Alarcon-Enos, J. Bioconversion in ryegrass-fescue hay by pleurotus ostreatus to increase their nutritional value for ruminant. Agriculture. (2022) 12:534. doi: 10.3390/agriculture12040534
50. Getachew, G, Blümmel, M, Makkar, H, and Becker, K. In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Anim Feed Sci Technol. (1998) 72:261–81. doi: 10.1016/S0377-8401(97)00189-2
51. Sucu, E. In vitro studies on rumen fermentation and methanogenesis of different microalgae and their effects on acidosis in dairy cows. Fermentation. (2023) 9:229. doi: 10.3390/fermentation9030229
52. Mavrommatis, A, Skliros, D, Sotirakoglou, K, Flemetakis, E, and Tsiplakou, E. The effect of forage-to-concentrate ratio on schizochytrium spp.-supplemented goats: modifying rumen microbiota. Animals. (2021) 11:52. doi: 10.3390/ani11092746
53. Fievez, V, Boeckaert, C, Vlaeminck, B, Mestdagh, J, and Demeyer, D. In vitro examination of DHA-edible micro-algae: 2. Effect on rumen methane production and apparent degradability of hay. Anim Feed Sci Technol. (2007) 136:80–95. doi: 10.1016/j.anifeedsci.2006.08.016
54. Durmic, Z, Moate, PJ, Eckard, R, Revell, DK, Williams, R, and Vercoe, PE. In vitro screening of selected feed additives, plant essential oils and plant extracts for rumen methane mitigation. J Sci Food Agric. (2014) 94:1191–6. doi: 10.1002/jsfa.6396
55. Tsiplakou, E, Abdullah, M, Skliros, D, Chatzikonstantinou, M, Flemetakis, E, Labrou, N, et al. The effect of dietary Chlorella vulgaris supplementation on micro-organism community, enzyme activities and fatty acid profile in the rumen liquid of goats. J Anim Physiol Anim Nutr. (2017) 101:275–83. doi: 10.1111/jpn.12521
56. Mickdam, E, Khiaosa-Ard, R, Metzler-Zebeli, BU, Klevenhusen, F, Chizzola, R, and Zebeli, Q. Rumen microbial abundance and fermentation profile during severe subacute ruminal acidosis and its modulation by plant derived alkaloids in vitro. Anaerobe. (2016) 39:4–13. doi: 10.1016/j.anaerobe.2016.02.002
57. Sheng, P, Ribeiro, GO, Wang, Y, and McAllister, TA. Humic substances reduce ruminal methane production and increase the efficiency of microbial protein synthesis in vitro. J Sci Food Agric. (2019) 99:2152–7. doi: 10.1002/jsfa.9407
58. Hassan, A, Salem, A, Elghandour, M, Hafsa, SA, Reddy, P, Atia, S, et al. Humic substances isolated from clay soil may improve the ruminal fermentation, milk yield, and fatty acid profile: a novel approach in dairy cows. Anim Feed Sci Technol. (2020) 268:114601. doi: 10.1016/j.anifeedsci.2020.114601
59. Ranilla, M, Jouany, JP, and Morgavi, D. Methane production and substrate degradation by rumen microbial communities containing single protozoal species in vitro. Lett Appl Microbiol. (2007) 45:675–80. doi: 10.1111/j.1472-765X.2007.02251.x
60. Scholz, B, and Liebezeit, G. Screening for biological activities and toxicological effects of 63 phytoplankton species isolated from freshwater, marine and brackish water habitats. Harmful Algae. (2012) 20:58–70. doi: 10.1016/j.hal.2012.07.007
61. Meehan, DJ, Cabrita, AR, Silva, JL, Fonseca, AJ, and Maia, MR. Effects of Chlorella vulgaris, Nannochloropsis oceanica and Tetraselmis sp. supplementation levels on in vitro rumen fermentation. Algal Res. (2021) 56:102284. doi: 10.1016/j.algal.2021.102284
62. Chanzanagh, EG, Seifdavati, J, FMA, G, Benamar, HA, and Sharifi, RS. Effect of ZnO nanoparticles on in vitro gas production of some animal and plant protein sources. Kafkas Üniv Vet Fak Derg. (2018) 24
63. Makkar, HP. In vitro screening of feed resources for efficiency of microbial protein synthesis In: In vitro screening of plant resources for extra-nutritional attributes in ruminants: nuclear and related methodologies. ed. Philip E. Vercoe school of animal biology the university of Western Australia crawley WA, Australia: Springer (2010). 107–44.
64. Burt, S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. (2004) 94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022
65. Newbold, C, McIntosh, F, Williams, P, Losa, R, and Wallace, R. Effects of a specific blend of essential oil compounds on rumen fermentation. Anim Feed Sci Technol. (2004) 114:105–12. doi: 10.1016/j.anifeedsci.2003.12.006
66. Kinley, RD, de Nys, R, Vucko, MJ, Machado, L, and Tomkins, NW. The red macroalgae Asparagopsis taxiformis is a potent natural antimethanogenic that reduces methane production during in vitro fermentation with rumen fluid. Anim Prod Sci. (2016) 56:282–9. doi: 10.1071/AN15576
67. Chalupa, W. Manipulating rumen fermentation. J Anim Sci. (1977) 45:585–99. doi: 10.2527/jas1977.453585x
68. Lodge-Ivey, S, Tracey, L, and Salazar, A. Ruminant nutrition symposium: the utility of lipid extracted algae as a protein source in forage or starch-based ruminant diets. J Anim Sci. (2014) 92:1331–42. doi: 10.2527/jas.2013-7027
69. Boeckaert, C, Vlaeminck, B, Dijkstra, J, Issa-Zacharia, A, Van Nespen, T, Van Straalen, W, et al. Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. J Dairy Sci. (2008) 91:4714–27. doi: 10.3168/jds.2008-1178
70. Machado, L, Magnusson, M, Paul, NA, de Nys, R, and Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS One. (2014) 9:e85289. doi: 10.1371/journal.pone.0085289
Keywords: gas production, nanoparticles, methane emission, in vitro, green algae
Citation: Palangi V, Kaya A, Macit M, Nadaroglu H, Ünlü HB, Kaya A, Fekri A, Mammadov A and Lackner M (2025) Comparative anti-methanogenic ability of green algae (C. reinhardtii) with/without nanoparticles: in vitro gas and methane production. Front. Vet. Sci. 12:1492230. doi: 10.3389/fvets.2025.1492230
Edited by:
Sandeep K. Malyan, Dyal Singh Evening College, IndiaReviewed by:
Pankaj Kumar, Gurukul Kangri University, IndiaVineet Kumar, Central University of Rajasthan, India
Copyright © 2025 Palangi, Kaya, Macit, Nadaroglu, Ünlü, Kaya, Fekri, Mammadov and Lackner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valiollah Palangi, dmFsaW9sbGFoLnBhbGFuZ2lAZWdlLmVkdS50cg==; Maximilian Lackner, bWF4aW1pbGlhbi5sYWNrbmVyQHRlY2huaWt1bS13aWVuLmF0
 Valiollah Palangi
Valiollah Palangi Adem Kaya
Adem Kaya Muhlis Macit2
Muhlis Macit2 Hayrunnisa Nadaroglu
Hayrunnisa Nadaroglu Ashkan Fekri
Ashkan Fekri Ayaz Mammadov
Ayaz Mammadov Maximilian Lackner
Maximilian Lackner