- 1Information Systems Department, University of Haifa, Haifa, Israel
- 2Department of Mathematics, Ariel University, Ariel, Israel
- 3Department of Small Animal Medicine and Surgery, University of Veterinary Medicine Hannover, Hannover, Germany
- 4Cats Protection, National Cat Centre, Sussex, United Kingdom
- 5School of Veterinary Medicine and Animal Science, São Paulo State University (Unesp), São Paulo, Brazil
- 6Joseph Bank Laboratories, School of Life Sciences, University of Lincoln, Lincoln, United Kingdom
Facial landmarks, widely studied in human affective computing, are beginning to gain interest in the animal domain. Specifically, landmark-based geometric morphometric methods have been used to objectively assess facial expressions in cats, focusing on pain recognition and the impact of breed-specific morphology on facial signaling. These methods employed a 48-landmark scheme grounded in cat facial anatomy. Manually annotating these landmarks, however, is a labor-intensive process, deeming it impractical for generating sufficiently large amounts of data for machine learning purposes and for use in applied real-time contexts with cats. Our previous work introduced an AI pipeline for automated landmark detection, which showed good performance in standard machine learning metrics. Nonetheless, the effectiveness of fully automated, end-to-end landmark-based systems for practical cat facial analysis tasks remained underexplored. In this paper we develop AI pipelines for three benchmark tasks using two previously collected datasets of cat faces. The tasks include automated cat breed recognition, cephalic type recognition and pain recognition. Our fully automated end-to-end pipelines reached accuracy of 75% and 66% in cephalic type and pain recognition respectively, suggesting that landmark-based approaches hold promise for automated pain assessment and morphological explorations.
1 Introduction
Facial expressions are universally acknowledged as key indicators of emotional states in mammals (1, 2). The link between facial expressions and emotions in humans has received considerable attention in research (3, 4). All mammals are known to display facial expressions (5). Analogously to humans, they are believed to communicate emotional states. This leads to the increase in interest in the study of facial expressions in the context of animal emotion and welfare studies (6–9).
Facial expressions and behavior also form an important part of animals' non-verbal communication. As such, it's essential that they are easily noticeable and interpretable by the receiver, implying that these signals must possess a degree of universality in their display. Yet, morphological characteristics in domesticated, and specifically in companion animals, may greatly vary due to selective breeding by humans (10–12), potentially affecting the distinctness of their facial expressions (13). However, the impact of this morphological diversity on the visual clarity of expressions and other forms of social signaling, as well as on the behavior and communication of companion animals, remains largely unstudied.
Although our understanding of facial visual signals, such as those caused by facial expressions and their relationship to animals' internal states, is quite limited, there has been some advancement mainly focusing on pain expressions. One of the most prevalent methods for pain assessment involves scoring by trained human experts. Species-specific pain assessment tools, known as grimace scales, focus on changes in an animal's facial features, and together with behavioral pain scales, they have been developed and validated for nearly all commonly domesticated species (with the notable exception of the dog, given their exceptional facial morphological diversity). Originally developed for rodents, these grimace scales have since been adapted for a range of mammalian species, including rats (14), rabbits (15), horses (16), pigs (17), ferrets (18), sheep (19, 20), and cats (21, 22).
In the context of studying facial appearance and visual signals, domestic cats are a particularly interesting exemplar in several aspects. First of all, cats display a wide variety of breed types and morphological features (although not as extreme as dogs), including a wide range of head shapes, from brachycephalic (e.g., the Persian) to dolichocephalic (e.g., the Siamese) (23). Additionally, the color of the coat, markings, and length of the fur are other elements that could influence the evaluation of facial expression in animals (24, 25), which potentially influence the ability to detect changes in facial expression in cats. Thus, they are a good initial exemplar for tackling the challenge of facial morphological diversity, which will need to be addressed for the more widespread use of AI in facial processing in animals. At the species level, domestic cats also show a diverse array of facial expressions, the majority of which have been systematically captured by the Cat Facial Action Coding System (CatFACS), linked to facial muscles (26). Changes in cats' facial shape have been linked to effective states such as fear, frustration, relaxed engagement, and pain (21, 22, 27). The available methods for cat pain assessment include three validated scales: the UNESP-Botucatu multidimensional composite pain scale (MCPS) (28), the Glasgow composite measure pain scale (CMPS) (29) and the Feline Grimace Scale (FGS) (22). However, all of these methods rely on the subjective judgments of humans, which may influence their reliability and validity. This leads to the need for the development of more objective methods for scoring and assessing pain, which are less susceptible to human bias.
Geometric morphometric analysis is a powerful tool that has been explored in the context of quantifying cat facial shape changes (13, 30). It uses points (facial landmarks) positioned on objects as proxies for shape. The landmark coordinates reflect their reciprocal locations, with differences in such locations across objects measuring the amount of shape variation. For instance, Finka et al. (30) applied this approach to quantify cat facial shape changes associated with pain. Images of 29 domestic short-haired female cats undergoing ovariohysterectomy were manually annotated using 48 landmarks specifically chosen for their relationship with underlying facial musculature and their relevance to cat-specific facial action units. A significant relationship was found between pain-linked Principal Components related to facial shape variation and the UNESP-Botucatu MCPS tool (28). In a similar manner, this approach was extended by Finka et al. (13) to explore the impact of cat breed and cephalic type variation on the relative positioning of facial landmarks. Major variations in baseline facial landmark configurations were identified within a population of common domestic cat breeds and diverse cephalic shapes. Variations in relative landmark positions were evident at both the cephalic and breed levels and were identified across all facial regions, including the ears, eyes, cheeks, mouth, and nose. Furthermore, while facial landmarks were able to differentiate between “pain” and “no pain” facial features in images of domestic short-haired cats, the painful cats of this breed were not reliably different from the neutral faces of other breeds.
These findings, which demonstrate that the geometric cat face model contains important visual information relevant to pain but is also potentially susceptible to “noise” caused by breed and cephalic variation in baseline features, formed a starting point for using machine learning (ML) techniques for automated recognition of cat pain in our previous work (31, 32). For example, in (31), the 48 facial landmarks suggested in Finka et al. (13, 30) were used in an ML model for cat pain recognition reaching above 72% on the dataset of Finka et al. (30). As this dataset was limited to young, adult female cats of a single breed and submitted to only one type of postoperative pain condition, this approach was subsequently extended to a more morphologically diverse dataset in Feighelstein et al. (32), reaching an even higher accuracy of detection of 77%, using the cat face model landmarks.
These results further indicated that the scheme of the 48 landmarks from Finka et al. (30) can contain useful visual signals sufficient for accurate recognition of internal states such as pain (30, 32), or for comparative studies across cat breeds and cephalic types (13). Feighelstein et al. (32) presented another interesting application of the geometric cat face model for investigating the explainability of pain recognition models by looking at average heat per landmark to understand more informative areas of the cat face for the ML model in pain recognition.
However, the landmark method heavily relied on the time-consuming and labor-intensive manual annotation of landmarks: in the case of Martvel et al. (33), it took skilled and trained annotators over 5.5 min to annotate one facial image, and our wider experience indicates this is typical for this type of activity. Martvel et al. (33, 34), developed an automated detector for cat facial landmarks, having introduced the first available annotated dataset with 48 landmarks: the Cat Facial Landmarks in the Wild (CatFLW) dataset (34). The model for automated landmark localization is based on a convolutional neural networks model and uses a magnifying ensemble method. Its performance in terms of normalized mean error (NME) was comparable and, in some cases, outperformed other models of this type with respect to human facial landmark localization. However, the question of whether the developed detector is useful for practical applications of cat facial analysis and whether automated landmark detection is sensitive enough for such tasks has remained open.
This study systematically investigates automated landmark-based approaches using three benchmark challenges related to cat facial analysis, which have been previously explored in the literature (30–32): breed, cephalic type, and pain recognition. We utilized the two datasets from these earlier studies to assess the performance of various landmark-based automated pipelines on these tasks. Additionally, we examined how their performance was impacted when the precise but labor-intensive manual landmarking process was substituted with a fully automated detection system.
2 Methods
2.1 Datasets
The dataset relating to cat pain was collected previously under the ethical approvals of the Institutional Animal Research Ethical Committee of the FMVZ-UNESP-Botucatu (protocol number of 20/2008) and the University of Lincoln, (UID: CoSREC252) as per Finka et al. (30). The dataset relating to cat breeds was collected previously under the ethical approvals of the Institutional Animal Research Ethical Committee of the FMVZ-UNESP-Botucatu (protocol number 20/2008). The use of this dataset and the generation of the data were approved by the delegated authority of Nottingham Trent University, Research Ethics Committee, as per Finka et al. (13). The current protocol using these datasets was reviewed by the Ethical Committee of the University of Haifa, and no further approval was required. All experiments were performed in accordance with relevant guidelines and regulations.
The images in both datasets were manually annotated with 48 facial landmarks. Examples of images with the annotation structure are shown in Figure 1. Specific details of landmark placements and their relevance to facial musculature and CatFACS action units are provided in Finka et al. (13, 30). While images themselves were not augmented, the ground truth and detected landmarks underwent normalization and centering to enhance the robustness of subsequent analyses. Normalization scales the landmark coordinates so that they fall between 0 and 1, and centering translates the landmarks so that their centroid (mean position of all landmarks) coincides with the origin of the coordinate system. Additional augmentation techniques on data include shifting random landmarks, complete landmark shifting, and rotations.

Figure 1. Cat images with 48 facial landmarks, breed, and cephalic type. Images are taken from the public Oxford IIIT Cats dataset (https://www.kaggle.com/datasets/imbikramsaha/cat-breeds).
2.1.1 The Cat Breed Dataset
This dataset included 1,662 images of cat facial images annotated across n = 18 common breeds. The images were sourced from Oxford Pet Dataset (35) and Google images. The breeds were further divided by Finka et al. (13) into three categorical cephalic types: dolichocephalic, mesocephalic, and brachycephalic. Dolichocephalic, or “long-headed” cats, have elongated faces with lengths greater than the width. Mesocephalic, or “middle-headed” cats have square-like faces with approximately equal width and length of the face. Brachycephalic, or “short-headed” cats, have flat faces, and the muzzle area is located closer to the eyes. Figure 2 presents average landmark annotations for the three cephalic types. The distribution of images over the types and breeds is provided in Table 1.
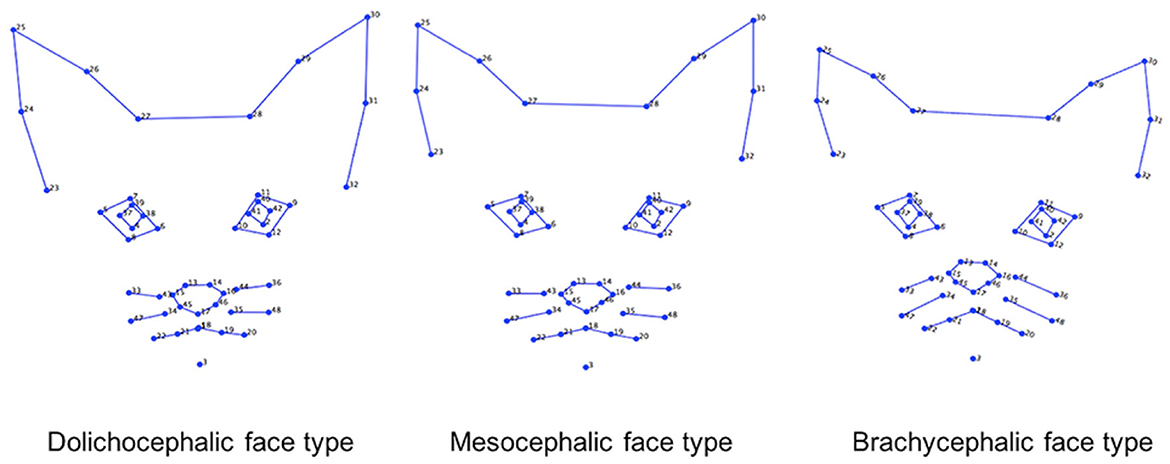
Figure 2. Average facial landmarks (annotated manually) for the three different cephalic types (13).
2.1.2 The Cat Pain Dataset
We used the dataset generated as part of a previous study by Finka et al. (30). The raw data comprised of footage from 29 healthy domestic short-haired female cats undergoing ovariohysterectomy as described in Brondani et al. (28). Cats were recorded at different time points corresponding to varying intensities of pain: pre-surgery (between 18–24 h during the preoperative period), 1-h post-surgery (between 30 min and 1 h after the end of the surgery, and prior to administration of additional analgesics), and post-rescue analgesia (approximately 4 h after postoperative analgesia). The final dataset contains images from 26 cat individuals with 232 images of “No Pain” (pre-surgery stage and post-rescue analgesia stage), and 232 images of “Pain” (1-h post surgery), overall 464 images.
2.2 The cat facial landmarks automated detector
The cat facial landmark detector used here is presented in Martvel et al. (33). This AI pipeline uses a magnifying method to localize landmarks, taking an image as input and producing 48 cats' facial landmarks. First, it localizes the face (so no preprocessing of the image is required), then determines five regions of interest (ears, eyes, and the whiskers area), and then localizes landmarks in each of these regions. The model was trained on the Cat Facial Landmarks in the Wild (CatFLW) dataset (34), which contains 2091 facial images of cats annotated with the 48 landmark scheme from Finka et al. (30).
2.3 Machine learning models
We have formulated the following benchmark tasks to be addressed:
1. Cat breed recognition: given a facial image of a cat, detect the cat's breed out of 18 classes (the full list of the 18 breeds is presented in Table 1).
2. Cephalic type recognition: given a facial image of a cat, detect its cephalic type out of 3 classes (dolichocephalic, mesocephalic or brachycephalic, see Table 1).
3. Pain recognition: given a facial image of a cat, detect whether it is in pain (binary “Pain”/“No pain” classification).
Figure 3 presents a high-level overview of the AI pipelines studied.
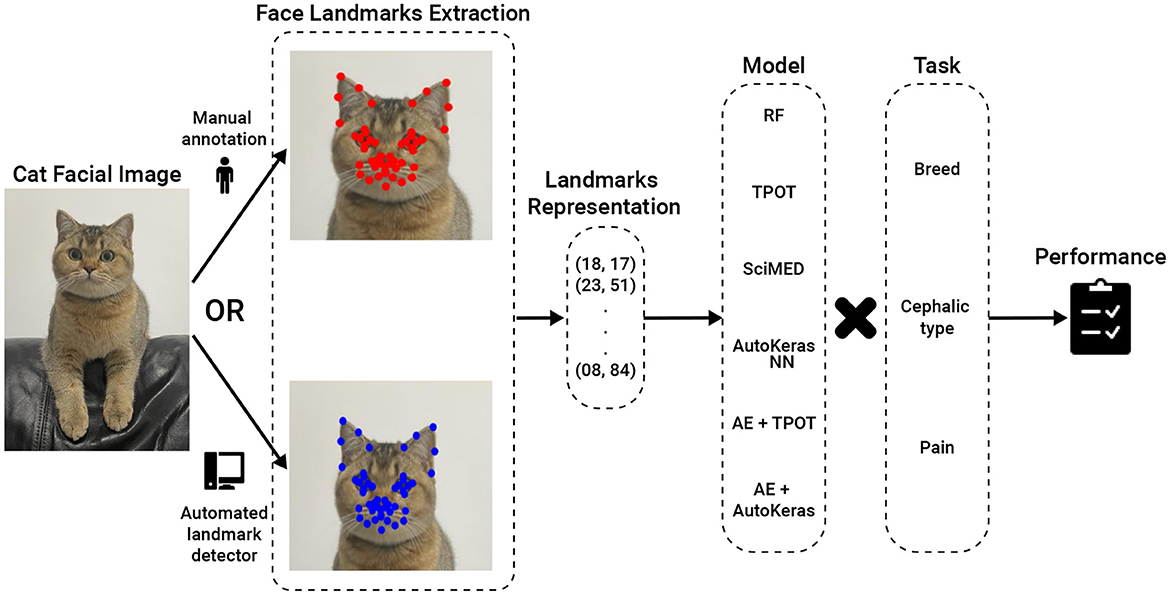
Figure 3. AI Pipeline Overview. The input is a cat facial image, which can be annotated either manually or using an automated landmark detector. The intermediate stage is 48 landmark coordinates, which are then fed to a machine learning model for classification. RF, Random Forest; TPOT, Tree-Based Pipeline Optimization Tool; SciMed, Scientist-Machine Equation Detector; NN, neural network; AE, AutoEncoder.
For the first two tasks, the models were trained using the Cat Breed Dataset. We tested a sequence of ML models with increasing levels of complexity. Each model was used for both the breed and cephalic type recognition classification tasks as the input features are identical, and only the target features are altered. Initially, we tested the Random Forest (RF) model (36) with a grid-search hyperparameter tuning (picked manually) (37) and bidirectional elimination for the feature selection (38). Specifically, we used a mixture of cost-complexity and SAT-based post-pruning on the trees in the RF model to obtain better generalization (39, 40). Afterward, we tested the Tree-Based Pipeline Optimization Tool (TPOT), a genetic algorithm-based automatic ML library (41). TPOT produces a full ML pipeline, including feature selection engineering, model selection, model ensemble, and hyperparameter tuning. In an orthogonal testing direction, we used the Scientist-Machine Equation Detector (SciMED) symbolic regression model, which searches for an analytical function between the input features and the target feature (42). Next, we moved to the deep-learning realm as it is known to be able to handle high-dimensional and complex tasks (43–45), as the ones we deal with. To this end, we initially developed our own neural network (NN) model using a manual trial-and-error process. Namely, we obtain a feed-forward (i.e., fully connected) NN with three layers (96, 64, 32, and 18), separated by dropout layers with p = 0.1 drop rate. The Adam optimizer with a learning rate of 10−4 is used for the training procedure with a batch size of 8. The cross-entropy loss was utilized given the categorical nature of the problem, and the primary metric for model evaluation during training was accuracy. To test a larger-scale NN architecture scale, we tested AutoKeras (46), an automatic deep-learning library that automatically searches for NN architectures and training hyper-parameters. Following this promising direction, we came to the conclusion that the input feature space is not informative as the data represents locations in an image (in a Cartesian coordinate system). As such, we decided to divide the classification and input data representation tasks. We repeated the TPOT and AutoKeras tests such that both were obtained as an input a 16-dimensional input generated from a fully connected AutoEncoder NN (47) (AE) with three layers for the encoder and decoder parts (96, 64, 32, 16, 32, 64, and 96).
The third task was previously explored on the Cat Pain Dataset in Feighelstein et al. (31), where manually annotated facial landmarks were used. We aimed to study an end-to-end automated pipeline, focusing specifically on how the pain recognition model performance is affected when we replace manually annotated landmarks with automatically detected ones. For a fair comparison, we followed the same preprocessing scheme as Feighelstein et al. (31). Namely, a preprocessing pipeline took as input images annotated with 48 landmarks, produced a set of multi-region vectors, and then introduced them into the classification model. During the preprocessing phase, the landmarks were centered as part of face alignment and vectorized based on the four facial regions. We used the same structured, fully connected NN for the classification as in the breed/type classification. The model is trained during ten epochs, optimizing a cross-entropy loss function using an Adam optimizer with a 0.1 learning rate and a batch size of 32. On each epoch, the training set is normalized using standard scaling and augmented. We again chose the model's hyperparameters that achieved the best (minimal) validation loss.
As a validation method for the first and second tasks (carried out on the Cat Breed Dataset), we used 5-fold cross-validation. For the third task, which was carried out in a different (balanced) Cat Pain Dataset, we used the stricter leave-one-subject-out cross-validation with no subject overlap (48). Due to the relatively low numbers of cats (n = 27 after dataset balancing) in the dataset, following this method is more appropriate (8, 49). By separating the subjects used for training, validation, and testing, respectively, we enforce generalization to unseen subjects and ensure that no specific features of an individual are used for classification. For evaluation of the facial landmark detector performance, the normalized mean error (NME) (50) and normalized root mean squared error (NRMSE) metrics using the inter-ocular distance (the distance between the outer corners of the eyes) were used to measure average detector errors. Additionally, to inspect how well breeds and cephalic types are separated when using manual vs. automatically detected landmarks, we used the t-distributed stochastic neighbor embedding (t-SNE) (51) transformation to visualize the high-dimensional data in two dimensions. This transformation provides a visual and more intuitive representation of the model's learned feature space. Finally, each of the models was tested with manually annotated landmarks and with automatically detected landmarks using the detection pipeline from (33) described above. The model's performance was measured using accuracy and F1-score.
3 Results
3.1 Landmark detection
Table 2 presents the average normalized mean error (NME) and normalized root mean squared error (NRMSE) measurements on the automatic detection of facial landmarks as compared to manual annotations. The largest error is observed for the dolichocephalic breeds and the smallest—for the mesocephalic ones. In terms of breeds, the “easiest” breed (with minimal error) for landmark detection is the (brachycephalic) British Shorthair, and the “hardest” breed (with maximal error) is the (dolichocephalic) Oriental Shorthair.
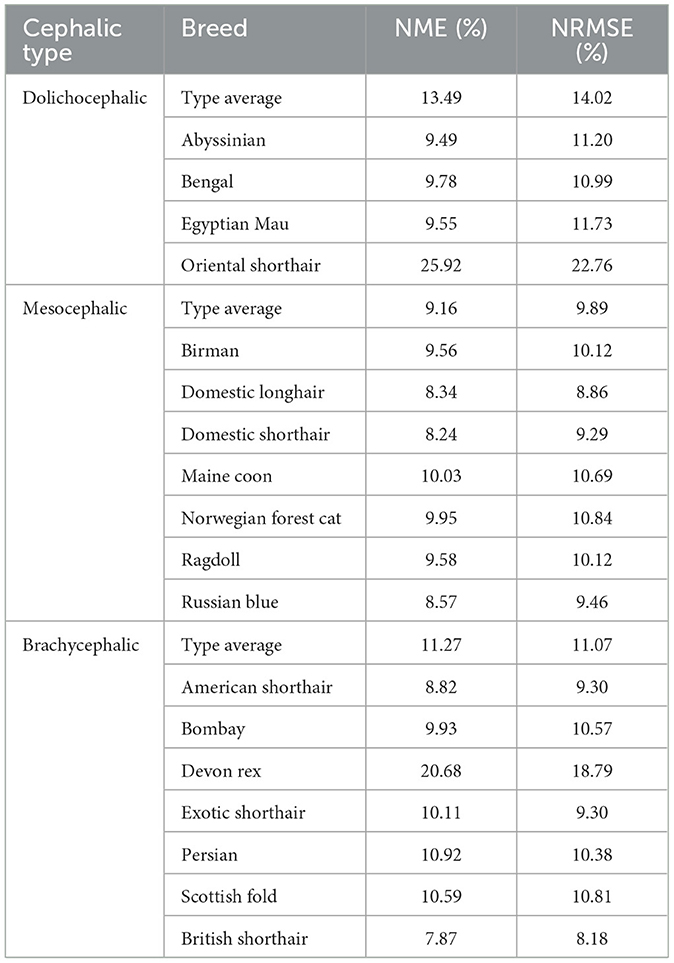
Table 2. Average normalized mean error (NME) and normalized root mean squared error (NRMSE) for different cephalic types and breeds (in %).
3.2 Breed and cephalic type recognition
Figures 4, 5 present the t-SNE visualization of the 18 breeds and 3 cephalic types, respectively, using both manual and automatically detected landmarks. The visualization shows the possibility for a better potential separation between breeds and types when using the former; it also shows a better separation between cephalic types than between breeds when using automatically detected landmarks.

Figure 4. T-distributed stochastic neighbor embedding (t-SNE) distributions for breed recognition: manual landmarks (left) and automatically detected landmarks (right).
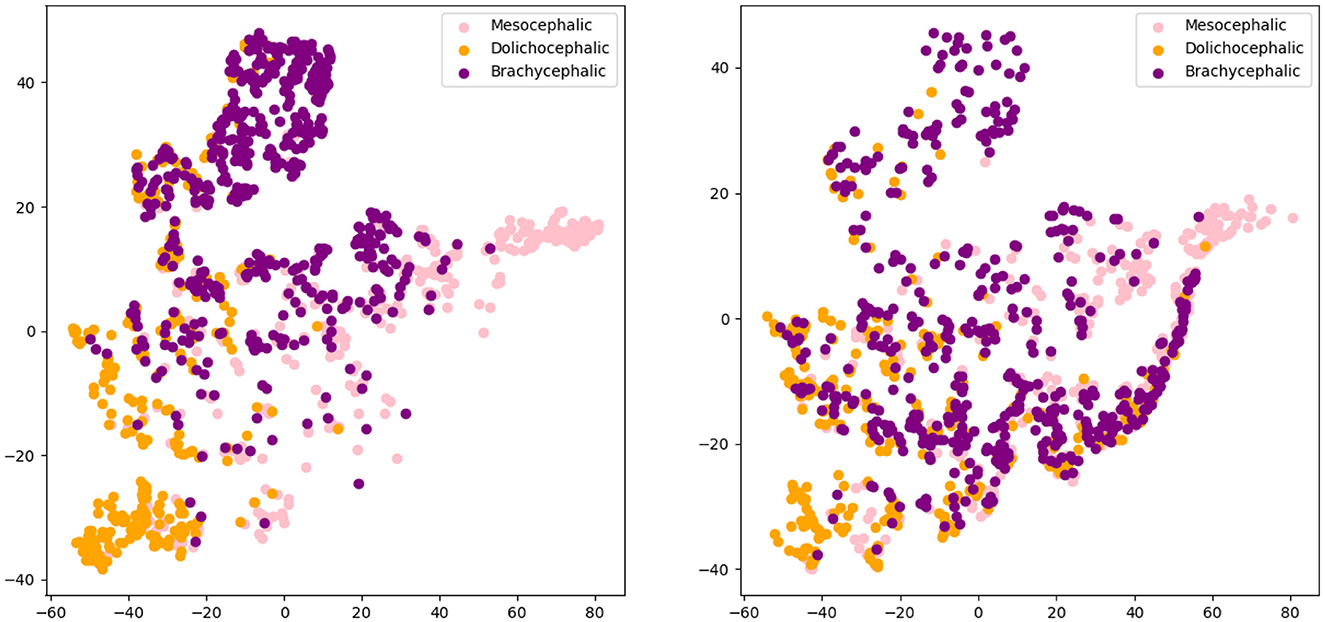
Figure 5. T-distributed stochastic neighbor embedding (t-SNE) distributions for cephalic type recognition: manual landmarks (left) and automatically detected landmarks (right).
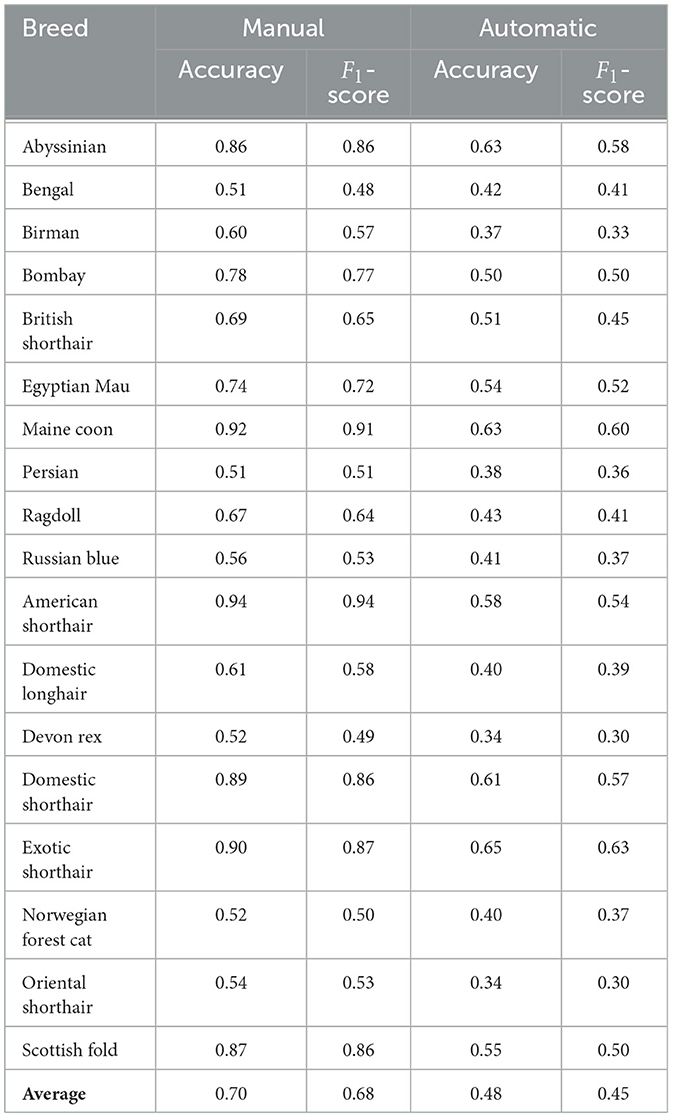
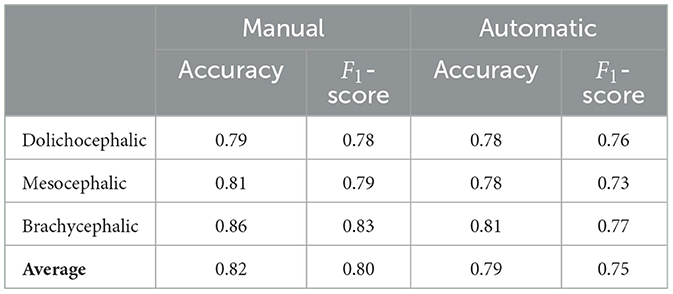
Table 3 presents a comparison of the performance of pipelines in the first two tasks, using different architectures, with the best being AE+AutoKeras in both. Tables 4, 5 present the metrics in a break-down to breeds and types using AE+AutoKeras model.
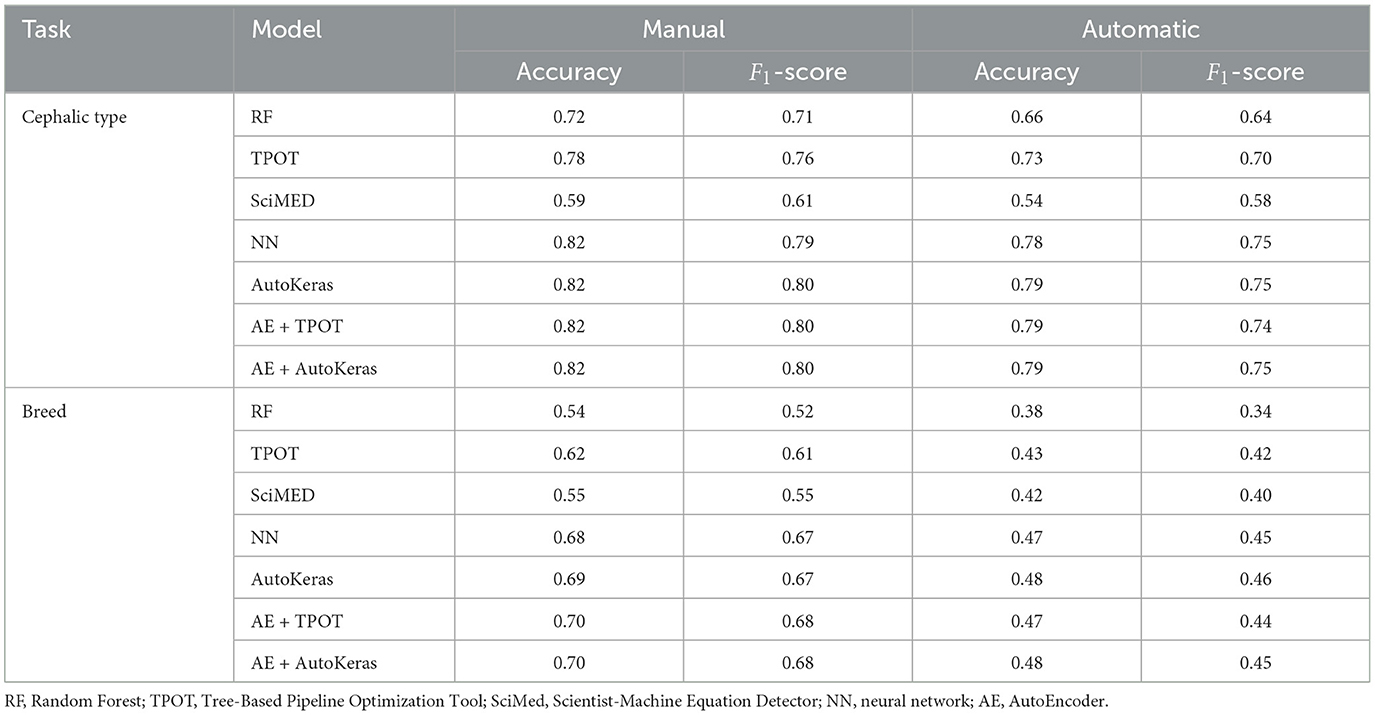
Table 3. Comparison of model performance for manual and automatic landmarks for the tasks of cephalic type and breed recognition.
3.3 Pain recognition
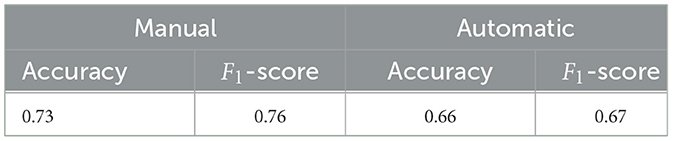
Table 6 presents the performance of the pain recognition pipeline for manual and automatically detected landmarks. Performance for the latter is lower, reaching only 66% accuracy, with the former performing with 73%.
4 Discussion
The facial landmark scheme of 48 landmarks, systematically developed based on cat facial anatomy in Finka et al. (30), has demonstrated its utility in several applications. That includes geometric morphometric methods for accurately quantifying changes in cat facial features (13, 30), landmark-based AI models for pain recognition (31, 32) and enhancing the explainability of deep learning models related to facial analysis of cats (32).
The annotation process is, however, extremely laborious and time-consuming. According to Martvel et al. (33), it takes skilled and trained annotators over 5.5 min to annotate each image. For the Cat Breed Dataset alone, this translates to more than 161 hours of manual annotation work for a relatively small dataset, underscoring the pressing need for automation of this process.
Automated localization of facial landmarks, also known as fiducial points (52), is a cornerstone of the field of automated human facial analysis. It has numerous applications for face alignment, feature extraction, facial expression recognition, head pose estimation, eye gaze tracking, facial unit recognition, and many more tasks (53–57); which has been addressed by a growing body of work. In the human domain, affective computing is a well-developed discipline, integrating aspects of facial expression analysis and gesture recognition with advanced, real-time emotion recognition platforms such as Noldus Face Reader, Microsoft Azure Cognitive Services, Affectiva AFFDEX, and Emotient FACET. Moreover, AI automatic pain estimation applications for assessing human pain from facial expressions have already been integrated into clinical settings for non-verbal patients. An example is PainChek (58), which uses facial landmarking techniques and has already been applied for patients with dementia and infants (59, 60).
The question arises: how close are we to developing mobile applications like “CatPainChek” or “Cat Google Translate” that can accurately interpret cats' affective states? To address this, it's crucial to scrutinize the methodologies used in creating AI models for recognizing human affective states. Human face and gesture analysis has existed as a research area since the 1970s, and a vast number of datasets and manual annotations of facial expressions and emotional states were available to boost the development of AI algorithms and models. These datasets contained annotations of millions of frames and, in many cases, were created using actors. For instance, the Actor Study Database, published in Seuss et al. (61), contains 68 minutes of high-quality videos of facial expressions performed by 21 actors, whose tasks ranged from displaying specific Action Units and their combinations at different intensities to enactment of a variety of emotion scenarios. Clearly, compiling datasets of cat body language and facial expressions tied to their emotional and welfare states is significantly more complex than it is for humans since cats obviously cannot serve as “actors” nor can we be totally confident about their emotional state, although scientific frameworks for inferring different emotional states in non-human animals are being developed (62). These include whole subject and contextual evaluation in addition to facial analysis. Indeed, pain is perhaps comparatively the easier affective state that we can both ethically and practically operationalize with certainty compared to other, more complex feline emotional states.
The development of automated facial analysis techniques for animals is just beginning to emerge. Broomé et al. (49) review state-of-the-art studies in the field. Critical to this is the need for valid benchmarks, which are commonly available in human domains. In machine learning research, benchmarks typically consist of well-defined datasets, evaluation metrics, and specific tasks or challenges. Benchmarks are crucial in research as they facilitate the objective comparison of different approaches, promoting transparency and reproducibility in scientific findings. For instance, in the human domain, there are numerous benchmark datasets [such as the Cohn-Kanade dataset (63), the Toronto face database (64), the Actor Study Database (61) and many more]. This lack of similar datasets for animals is a significant hindrance to progress in the field but is not insurmountable with appropriate investment. Species-specific benchmarking resources can promote comparison between approaches and systematize the field. Another issue discussed in Broome et al. is considerations of ethics and privacy, especially when producing datasets with animal participants where emotional states and especially pain are induced. This often makes it difficult to make datasets publicly accessible.
The contributions of the current study address this gap in several dimensions. First of all, we evaluated the usefulness of the automated detector of cat facial landmarks on three non-trivial machine learning tasks: breed, cephalic type, and pain recognition. As anticipated, substituting manually identified landmarks with their automatically detected equivalents led to a decrease in performance across all tasks. The key question is to what extent this reduction in accuracy is an acceptable trade-off for achieving complete automation. The pain recognition task presented a 7% drop in accuracy and 9% drop in F1-score. The cephalic type task presented only 5% drop in F1-score for the best model. The breed recognition task turned out to be the most sensitive in this context, showing a drop in 23% in F1-score for the best model. It should be noted that since the type and breed recognition tasks used unbalanced datasets, F1-score is the more informative metric for performance in this case.
Going deeper into the break-down to breeds, Table 4 shows that the largest drop in performance (moving from manual to automatic) is observed in Americal Short Hair (40%), Scottish Fold (36%) and Maine Coon (31%). The smallest drop occurs in Bengal (7%); however, the performance of the model is very low for both. The breed recognition task turns out to be extremely difficult for landmark-based models even when using manual landmarks: the best model reaches 68% in F1-score. As illustrated in Figure 6, this difficulty arises from the subtle differences in facial landmarks within certain breeds like the British Shorthair and Ragdoll. Despite belonging to distinct cephalic types, these breeds exhibit very similar geometric structures [this could be due to the two-dimensional geometric information not sufficiently factoring in muzzle length; it could also be introduced by labeling “errors” due to the breed and type labeling methodology used in Finka et al. (13)]. Other breeds, however, such as the Scottish Fold and Maine Coon, have distinct visual characteristics that set them apart, contributing to the relatively higher accuracy observed in their classification. Landmark-based approaches seem not to be the optimal choice for breed classification. Moving to black-box models has the price of losing explainability; however, such approaches seem more promising for this type of task. For instance, Ráduly et al. (65) reach very high accuracy in dog breed classification using deep learning techniques (although their dataset is much larger than the one used in this study).
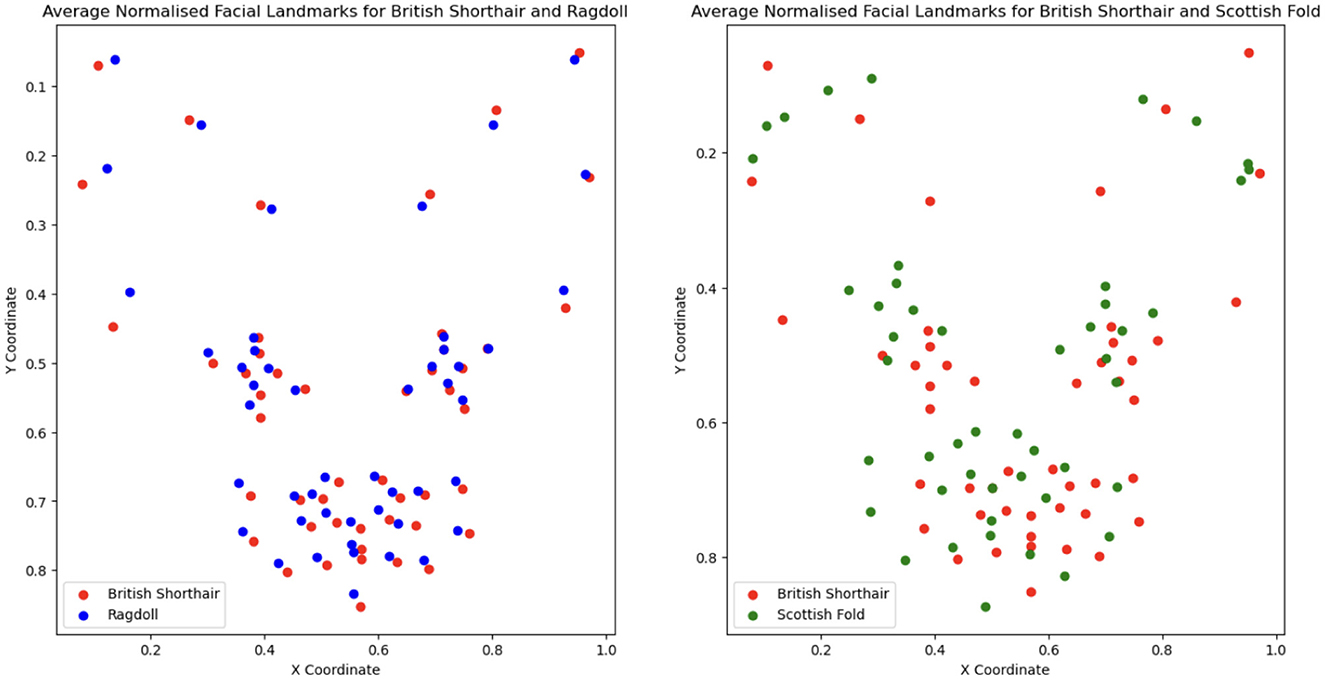
Figure 6. Comparison of breed-averaged normalized manual facial landmarks for British Shorthair, Ragdoll, and Scottish Fold.
Our models are much more successful with the cephalic type classification task, on the other hand, which presents small differences between manual and automated landmarks and has a high performance of 80% in F1-score. Scrutinizing the breakdown to the types on Table 5, the best performance is for the brachycephalic type, which also exhibits the largest drop in accuracy when moving to automated landmarks. This could be explained by the illustration in Figure 2, which shows the brachycephalic average landmarks have the most distinctive structure out of the three types.
The considerable differences in performance both in landmark detection and classification among various breeds (even within one cephalic type) and cephalic types can potentially be partly attributed to the unbalanced representation of each category in our existing datasets. Based on the results of Sexton et al. (24), we can also assume that the color of the coat and markings are other elements that influence the evaluation of landmark detection. It's plausible that training our automated detector on specific cephalic types or breeds could enhance its accuracy for those particular groups. However, the cat pain datasets currently available lack a diverse range of breeds and cephalic types. A valuable avenue for future research would be to enlarge these datasets and investigate the potential of models tailored to specific breeds and types within “pain” and “no pain” classifications.
This study has created landmark-based benchmark challenges for cat facial analysis by providing datasets, annotations, and benchmark models as a point of reference. Our aim is for these benchmarks to act as a foundational resource, enabling researchers to improve these results and develop new methods for analyzing cat facial expressions. In particular, it is important to highlight the difficulty in landmark detection of dolichocephalic breeds such as the Oriental short hair. The morphological traits of these breeds are quite extreme, and future datasets should aim to include more samples from these breeds.
In this context, another recent study by Steagall et al. (66) should be mentioned, which provided a deep learning pipeline for cat pain recognition based on facial landmarks. The study used an alternative reduced scheme of 37 landmarks, developing also an automated detector for them. Their pipeline is significantly outperformed by the current pipeline in terms of landmarks detection accuracy (their pipeline reaching only 16.76% NRMSE at best on aligned faces and worse on non-aligned, compared to the results in Table 2 on non-aligned images), while using fewer landmarks. However, it further highlights the utility of automated cat facial landmark detection and the importance of systematizing this emerging field. It is important to recognize, furthermore, that the creation of multiple landmark schemes that are difficult to compare poses a significant challenge to the systematic progression of the field. Future advancements in landmarking techniques should aim to build upon existing frameworks, leveraging the progress already made in this area.
5 Conclusions
In this paper we systematically explored the usefulness of the automated cat facial landmark detector introduced in Martvel et al. (33) for three tasks related to facial analysis: breed, cephalic type and pain recognition. The breed recognition pipeline performed below chance level, indicating that deep learning approaches are a better fit for this task. Our fully automated end-to-end pipelines reached accuracy of 75% and 66% in cephalic type and pain recognition respectively, suggesting that landmark-based approaches hold promise for automated pain assessment and morphological explorations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by University of Lincoln and Hanover University of Veterinary Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
GM: Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. TL: Conceptualization, Formal analysis, Software, Supervision, Writing – original draft, Writing – review & editing. MF: Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. SM: Data curation, Methodology, Writing – original draft, Writing – review & editing. IS: Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. LF: Data curation, Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. DM: Writing – original draft, Writing – review & editing. HV: Data curation, Writing – original draft, Writing – review & editing. AZ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was partially supported by the Data Science Research Center at the University of Haifa.
Acknowledgments
We thank Yaron Yossef and Nareed Farhat for their technical support, Ephantus Kanyugi for data management, and Mary the cat for providing the inspiration for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Descovich KA, Wathan J, Leach MC, Buchanan-Smith HM, Flecknell P, Framingham D, et al. Facial expression: an under-utilised tool for the assessment of welfare in mammals. In: ALTEX. (2017). doi: 10.14573/altex.1607161
2. Mota-Rojas D, Marcet-Rius M, Ogi A, Hernández-Ávalos I, Mariti C, Martínez-Burnes J, et al. Current advances in assessment of dog's emotions, facial expressions, and their use for clinical recognition of pain. Animals. (2021) 11:3334. doi: 10.3390/ani11113334
4. Russell JA, Bachorowski JA, Fernández-Dols JM. Facial and vocal expressions of emotion. Ann Rev Psychol. (2003) 54:329–49. doi: 10.1146/annurev.psych.54.101601.145102
5. Diogo R, Abdala V, Lonergan N, Wood B. From fish to modern humans-comparative anatomy, homologies and evolution of the head and neck musculature. J Anat. (2008) 213:391–424. doi: 10.1111/j.1469-7580.2008.00953.x
6. Boneh-Shitrit T, Feighelstein M, Bremhorst A, Amir S, Distelfeld T, Dassa Y, et al. Explainable automated recognition of emotional states from canine facial expressions: the case of positive anticipation and frustration. Sci Rep. (2022) 12:22611. doi: 10.1038/s41598-022-27079-w
7. Merkies K, Ready C, Farkas L, Hodder A. Eye blink rates and eyelid twitches as a non-invasive measure of stress in the domestic horse. Animals (Basel). (2019) 9:562. doi: 10.3390/ani9080562
8. Andresen N, Wöllhaf M, Hohlbaum K, Lewejohann L, Hellwich O, Thöne-Reineke C, et al. Towards a fully automated surveillance of well-being status in laboratory mice using deep learning: Starting with facial expression analysis. PLoS ONE. (2020) 15:e0228059. doi: 10.1371/journal.pone.0228059
9. Gleerup KB, Forkman B, Lindegaard C, Andersen PH. An equine pain face. Vet Anaesth Analg. (2015) 42:103–14. doi: 10.1111/vaa.12212
10. Helms J, Brugmann S. The origins of species-specific facial morphology: the proof is in the pigeon. Integr Comp Biol. (2007) 47:338–42. doi: 10.1093/icb/icm051
11. Wilkinson S, Lu ZH, Megens HJ, Archibald AL, Haley C, Jackson IJ, et al. Signatures of diversifying selection in European pig breeds. PLoS Genet. (2013) 9:e1003453. doi: 10.1371/journal.pgen.1003453
12. Himmler SM, Modlinska K, Stryjek R, Himmler BT, Pisula W, Pellis SM. Domestication and diversification: a comparative analysis of the play fighting of the Brown Norway, Sprague-Dawley, and Wistar laboratory strains of (Rattus norvegicus). J Comp Psychol. (2014) 128:318. doi: 10.1037/a0036104
13. Finka LR, Luna SP, Mills DS, Farnworth MJ. The application of geometric morphometrics to explore potential impacts of anthropocentric selection on animals' ability to communicate via the face: the domestic cat as a case study. Front Veter Sci. (2020) 7:606848. doi: 10.3389/fvets.2020.606848
14. Sotocina SG, Sorge RE, Zaloum A, Tuttle AH, Martin LJ, Wieskopf JS, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol Pain. (2011) 7:1744–8069. doi: 10.1186/1744-8069-7-55
15. Keating SCJ, Thomas AA, Flecknell PA, Leach MC. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS One. (2012) 7:e44437. doi: 10.1371/journal.pone.0044437
16. Dalla Costa E, Minero M, Lebelt D, Stucke D, Canali E, Leach MC. Development of the Horse Grimace Scale (HGS) as a pain assessment tool in horses undergoing routine castration. PLoS ONE. (2014) 9:e92281. doi: 10.1371/journal.pone.0092281
17. Di Giminiani P, Brierley VL, Scollo A, Gottardo F, Malcolm EM, Edwards SA, et al. The assessment of facial expressions in piglets undergoing tail docking and castration: toward the development of the piglet grimace scale. Front Veter Sci. (2016) 3:100. doi: 10.3389/fvets.2016.00100
18. Reijgwart ML, Schoemaker NJ, Pascuzzo R, Leach MC, Stodel M, de Nies L, et al. The composition and initial evaluation of a grimace scale in ferrets after surgical implantation of a telemetry probe. PLoS ONE. (2017) 12:e0187986. doi: 10.1371/journal.pone.0187986
19. McLennan KM, Rebelo CJ, Corke MJ, Holmes MA, Leach MC, Constantino-Casas F. Development of a facial expression scale using footrot and mastitis as models of pain in sheep. Appl Anim Behav Sci. (2016) 176:19–26. doi: 10.1016/j.applanim.2016.01.007
20. Häger C, Biernot S, Buettner M, Glage S, Keubler L, Held N, et al. The Sheep Grimace Scale as an indicator of post-operative distress and pain in laboratory sheep. PLoS ONE. (2017) 12:e0175839. doi: 10.1371/journal.pone.0175839
21. Holden E, Calvo G, Collins M, Bell A, Reid J, Scott E, et al. Evaluation of facial expression in acute pain in cats. J Small Animal Pract. (2014) 55:615–21. doi: 10.1111/jsap.12283
22. Evangelista MC, Watanabe R, Leung VS, Monteiro BP, O'Toole E, Pang DS, et al. Facial expressions of pain in cats: the development and validation of a Feline Grimace Scale. Sci Rep. (2019) 9:1–11. doi: 10.1038/s41598-019-55693-8
23. Künzel W, Breit S, Oppel M. Morphometric investigations of breed-specific features in feline skulls and considerations on their functional implications. Anat Histologia, Embryol. (2003) 32:218–223. doi: 10.1046/j.1439-0264.2003.00448.x
24. Sexton CL, Buckley C, Lieberfarb J, Subiaul F, Hecht EE, Bradley BJ. What is written on a dog's face? Evaluating the impact of facial phenotypes on communication between humans and canines. Animals. (2023) 13:2385. doi: 10.3390/ani13142385
25. Bloom T, Trevathan-Minnis M, Atlas N, MacDonald DA, Friedman HL. Identifying facial expressions in dogs: a replication and extension study. Behav Processes. (2021) 186:104371. doi: 10.1016/j.beproc.2021.104371
26. Caeiro CC, Burrows AM, Waller BM. Development and application of CatFACS: are human cat adopters influenced by cat facial expressions? Appl Anim Behav Sci. (2017) 189:66–78. doi: 10.1016/j.applanim.2017.01.005
27. Bennett V, Gourkow N, Mills DS. Facial correlates of emotional behaviour in the domestic cat (Felis catus). Behav Processes. (2017) 141:342–50. doi: 10.1016/j.beproc.2017.03.011
28. Brondani JT, Mama KR, Luna SP, Wright BD, Niyom S, Ambrosio J, et al. Validation of the English version of the UNESP-Botucatu multidimensional composite pain scale for assessing postoperative pain in cats. BMC Vet Res. (2013) 9:1–15. doi: 10.1186/1746-6148-9-143
29. Reid J, Scott E, Calvo G, Nolan A. Definitive Glasgow acute pain scale for cats: validation and intervention level. Veter Rec. (2017) 108:104208. doi: 10.1136/vr.104208
30. Finka LR, Luna SP, Brondani JT, Tzimiropoulos Y, McDonagh J, Farnworth MJ, et al. Geometric morphometrics for the study of facial expressions in non-human animals, using the domestic cat as an exemplar. Sci Rep. (2019) 9:1–12. doi: 10.1038/s41598-019-46330-5
31. Feighelstein M, Shimshoni I, Finka LR, Luna SP, Mills DS, Zamansky A. Automated recognition of pain in cats. Sci Rep. (2022) 12:9575. doi: 10.1038/s41598-022-13348-1
32. Feighelstein M, Henze L, Meller S, Shimshoni I, Hermoni B, Berko M, et al. Explainable automated pain recognition in cats. Sci Rep. (2023) 13:8973. doi: 10.1038/s41598-023-35846-6
33. Martvel G, Shimshoni I, Zamansky A. Automated detection of cat facial landmarks. Int J Comput Vision. (2023) 132:3103–3118. doi: 10.1007/s11263-024-02006-w
34. Martvel G, Farhat N, Shimshoni I, Zamansky A. CatFLW: cat facial landmarks in the wild dataset. arXiv [Preprint]. arXiv:230504232. (2023).
35. Parkhi OM, Vedaldi A, Zisserman A, Jawahar CV. Cats and dogs. In: 2012 IEEE Conference on Computer Vision and Pattern Recognition. (2012). p. 3498–3505. doi: 10.1109/CVPR.2012.6248092
36. Rokach L. Decision forest: twenty years of research. Inform Fusion. (2016) 27:111–25. doi: 10.1016/j.inffus.2015.06.005
37. Liu R, Liu E, Yang J, Li M, Wang F. Optimizing the hyper-parameters for SVM by combining evolution strategies with a grid search. In: Intelligent Control and Automation: International Conference on Intelligent Computing, ICIC 2006 Kunming, China, August 16–19, 2006. Springer Berlin Heidelberg (2006). p. 712–721. doi: 10.1007/11816492_87
38. Liu H, Yu L. Toward integrating feature selection algorithms for classification and clustering. IEEE Trans Knowl Data Eng. (2005) 17:491–502. doi: 10.1109/TKDE.2005.66
39. Andreas LP, Salvatore JS. Cost complexity-based pruning of ensemble classifiers. Knowl Inf Syst. (2001) 3:449–69. doi: 10.1007/PL00011678
40. Lazebnik T, Bunimovich-Mendrazitsky S. Decision tree post-pruning without loss of accuracy using the SAT-PP algorithm with an empirical evaluation on clinical data. Data Knowl Eng. (2023) 145:102173. doi: 10.1016/j.datak.2023.102173
41. Olson RS, Moore JH. TPOT: A tree-based pipeline optimization tool for automating machine learning. In: Workshop on Automatic Machine Learning. PMLR (2016). p. 66–74.
42. Keren LS, Liberzon A, Lazebnik T. A computational framework for physics-informed symbolic regression with straightforward integration of domain knowledge. Sci Rep. (2023) 13:1249. doi: 10.1038/s41598-023-28328-2
43. Tokarev KE, Zotov VM, Khavronina VN, Rodionova OV. Convolutional neural network of deep learning in computer vision and image classification problems. IOP Confer Series. (2021) 786:012040. doi: 10.1088/1755-1315/786/1/012040
44. Wu S, Roberts K, Datta S, Du J, Ji Z, Si Y, et al. Deep learning in clinical natural language processing: a methodical review. J Am Med Inform Assoc. (2020) 27:457–70. doi: 10.1093/jamia/ocz200
45. Lazebnik T, Rosenfeld A. A computational model for individual scholars' writing style dynamics. arXiv [Preprint]. arXiv:2305.04900 (2023).
46. Jin H, Song Q, Hu X. Auto-keras: an efficient neural architecture search system. In: Proceedings of the 25th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining. Association for Computing Machinery (2019). p. 1946–1956. doi: 10.1145/3292500.3330648
47. Bank D, Koenigstein N, Giryes R. Autoencoders. In: Rokach L, Maimon O, Shmueli E., , editors. Machine Learning for Data Science Handbook. Cham: Springer (2006).
48. Refaeilzadeh P, Tang L, Liu H. Cross-validation. In: Liu L, Özsu MT., , editors. Encyclopedia of Database Systems. Boston, MA: Springer (2009). doi: 10.1007/978-0-387-39940-9_565
49. Broomé S, Gleerup KB, Andersen PH, Kjellstrom H. Dynamics are important for the recognition of equine pain in video. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition. (2019). p. 12667–12676. doi: 10.1109/CVPR.2019.01295
50. Lai S, Chai Z, Li S, Meng H, Yang M, Wei X. Enhanced normalized mean error loss for robust facial landmark detection. In: BMVC. (2019). p. 111.
51. Cai TT, Ma R. Theoretical foundations of t-SNE for visualizing high-dimensional clustered data. J Mach Learn Res. (2022) 23:1–54.
52. Wu Y, Ji Q. Facial landmark detection: a literature survey. Int J Comput Vis. (2019) 127:115–42. doi: 10.1007/s11263-018-1097-z
53. Akinyelu AA, Blignaut P. Convolutional neural network-based technique for gaze estimation on mobile devices. Front Artif Intell. (2022) 4:796825. doi: 10.3389/frai.2021.796825
54. Al-Eidan RM, Al-Khalifa HS, Al-Salman AS. Deep-learning-based models for pain recognition: a systematic review. Appl Sci. (2020) 10:5984. doi: 10.3390/app10175984
55. Malek S, Rossi S. Head pose estimation using facial-landmarks classification for children rehabilitation games. Pattern Recognit Lett. (2021) 152:406–12. doi: 10.1016/j.patrec.2021.11.002
56. Yang J, Zhang F, Chen B, Khan SU. Facial expression recognition based on facial action unit. In: 2019 Tenth International Green and Sustainable Computing Conference (IGSC). IEEE (2019). p. 1–6. doi: 10.1109/IGSC48788.2019.8957163
57. Ma J, Li X, Ren Y, Yang R, Zhao Q. Landmark-based facial feature construction and action unit intensity prediction. Mathem Problems Eng. (2021) 2021:1–12. doi: 10.1155/2021/6623239
58. Atee M, Hoti K, Hughes JD. A technical note on the PainChek system: a web portal and mobile medical device for assessing pain in people with dementia. Front Aging Neurosci. (2018) 10:117. doi: 10.3389/fnagi.2018.00117
59. Babicova I, Cross A, Forman D, Hughes J, Hoti K. Evaluation of the Psychometric Properties of PainChek registered in UK Aged Care Residents with advanced dementia. BMC Geriatr. (2021) 21:1–8. doi: 10.1186/s12877-021-02280-0
60. Hoti K, Chivers PT, Hughes JD. Assessing procedural pain in infants: a feasibility study evaluating a point-of-care mobile solution based on automated facial analysis. Lancet Digital Health. (2021) 3:e623–34. doi: 10.1016/S2589-7500(21)00129-1
61. Seuss D, Dieckmann A, Hassan T, Garbas JU, Ellgring JH, Mortillaro M, et al. Emotion expression from different angles: a video database for facial expressions of actors shot by a camera array. In: 2019 8th International Conference on Affective Computing and Intelligent Interaction (ACII). IEEE (2019). p. 35–41. doi: 10.1109/ACII.2019.8925458
62. Mills DS. Perspectives on assessing the emotional behavior of animals with behavior problems. Curr Opin Behav Sci. (2017) 16:66–72. doi: 10.1016/j.cobeha.2017.04.002
63. Lucey P, Cohn JF, Kanade T, Saragih J, Ambadar Z, Matthews I. The extended Cohn-Kanade dataset (CK+): a complete dataset for action unit and emotion-specified expression. In: 2010 IEEE Computer Society Conference on Computer Vision and Pattern Recognition Workshops. IEEE (2010). p. 94–101. doi: 10.1109/CVPRW.2010.5543262
64. Susskind JM, Hinton GE, Movellan JR, Anderson AK. Generating facial expressions with deep belief nets. Affect Comput Emot Model Synt Recogn. (2008) 2008:421–440.
65. Ráduly Z, Sulyok C, Vadászi Z, Zölde A. Dog breed identification using deep learning. In: 2018 IEEE 16th International Symposium on Intelligent Systems and Informatics (SISY). IEEE (2018). p. 000271–000276. doi: 10.1109/SISY.2018.8524715
Keywords: artificial intelligence, facial landmark, cat behavior, cat morphology, pain assessment
Citation: Martvel G, Lazebnik T, Feighelstein M, Meller S, Shimshoni I, Finka L, Luna SPL, Mills DS, Volk HA and Zamansky A (2024) Automated landmark-based cat facial analysis and its applications. Front. Vet. Sci. 11:1442634. doi: 10.3389/fvets.2024.1442634
Received: 02 June 2024; Accepted: 11 November 2024;
Published: 09 December 2024.
Edited by:
Daniel Mota-Rojas, Metropolitan Autonomous University, MexicoReviewed by:
Greta Veronica Berteselli, University of Milan, ItalyAdriana Domínguez-Oliva, Metropolitan Autonomous University, Mexico
Copyright © 2024 Martvel, Lazebnik, Feighelstein, Meller, Shimshoni, Finka, Luna, Mills, Volk and Zamansky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Zamansky, YW5uYXphbUBpcy5oYWlmYS5hYy5pbA==
 George Martvel1
George Martvel1 Teddy Lazebnik
Teddy Lazebnik Sebastian Meller
Sebastian Meller Stelio P. L. Luna
Stelio P. L. Luna Daniel S. Mills
Daniel S. Mills Holger A. Volk
Holger A. Volk Anna Zamansky
Anna Zamansky