
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Vet. Sci. , 13 March 2024
Sec. Veterinary Infectious Diseases
Volume 11 - 2024 | https://doi.org/10.3389/fvets.2024.1367328
This article is part of the Research Topic Antimicrobials Alternatives for the Prevention and Treatment of Veterinary Infectious Diseases: Volume II View all 9 articles
 Sehyeong Ham1†
Sehyeong Ham1† Jeongmin Suh1†
Jeongmin Suh1† Jieun Kim2
Jieun Kim2 Min Jeong Gu2
Min Jeong Gu2 Min Ah Park2
Min Ah Park2 Eunseon Oh2
Eunseon Oh2 Jun-Ok Moon2
Jun-Ok Moon2 Chanhee Chae1*
Chanhee Chae1*Background: The in vitro and in vivo anti-inflammatory and anti-oxidative effects of an amino acid (AA) blend (tryptophan, threonine, and methionine) in pigs.
Objective: This study aimed to evaluate the in vitro anti-inflammatory and anti-oxidative effects of an AA blend on intestinal porcine epithelial cells (IPEC-J2) and the in vivo anti-inflammatory and anti-oxidative effects in pigs experimentally challenged with Salmonella Typhimurium.
Methods: IPEC-J2 were pretreated with an AA blend for 25 h and then treated with lipopolysaccharide (LPS), deoxynivalenol (DON), or H2O2 for in vitro evaluation. A controlled standard diet supplemented with 0.3% of the AA blend was orally fed to the treated group pigs for 14 days, beginning at 21 days of age. At the end of the feeding period, pigs were orally inoculated with Salmonella Typhimurium.
Results: Pre-treatment with the AA blend reduced LPS/DON-induced interleukin (IL)-8 mRNA as a measurement of the anti-inflammatory effect and H2O2-induced reactive oxygen species (ROS) as a measurement of the anti-oxidative effect on IPEC-J2. Feeding with an AA blend resulted in a reduction of proinflammatory (tumor necrosis factor-α, IL-6, and IL-8) cytokine levels, while treated pigs experienced an increase in anti-inflammatory IL-10 cytokine in their sera. The addition of an AA blend-supplemented pig feed resulted in significantly lower Salmonella-induced cecal lesion scores compared to untreated pigs.
Discussion: Supplementation of feed with an AA blend reduced intestinal inflammation and pathology in pigs and may be applied for the control of Salmonella Typhimurium infection, as demonstrated in this study.
The most widely spread of all salmonellae is Salmonella enterica serovar Typhimurium (S. typhimurium), which is associated with enterocolitis and is the second most frequently isolated serotype from pigs (1). Necrosis of cryptic and surface enterocytes (whether local or diffuse) is revealed during histopathological examination for signs of this infection. During the acute stages of the disease, the lamina propria and submucosa contain neutrophils. Salmonella Typhimurium promotes its own colonization through the exploitation of inflammation (2). This S. typhimurium-induced intestinal inflammation overcomes colonization resistance with a profound dysbiosis of the colonic microbial community structure (3, 4).
Pigs experimentally infected with Salmonella Typhimurium displayed an acute inflammatory response (5). Salmonella infection induces the expression of various inflammatory cytokines (6). TNF-α is expressed during Salmonella infection and is associated with the host’s inflammatory responses in the intestinal tract (6). Systemic and mucosal antibody response development occurs through the production of interleukin (IL)-6 by TNF-α production (7). Salmonella Typhimurium produces IL-8, the main function of which is to act as a neutrophil chemo-attractant and activating factor while stimulating the production of proinflammatory cytokines (8, 9). On the other hand, the expression of IL-10 is also increased in the intestinal tract during Salmonella infection, where it downregulates inflammatory responses (6). In addition to inflammatory cytokines, reactive oxygen species (ROS) participate in the progression of an inflammatory reaction. An enhanced ROS generated by neutrophils at the site of inflammation is involved in damage to vascular endothelial cells and tissue injury (10).
Controlling inflammation may therefore be the key to preventing S. typhimurium infection in pigs. Amino acids (AAs) and their use in the intestinal tract are gaining attention as inflammatory regulators. Three of the key essential AAs for pigs are methionine (Met), threonine (Thr), and tryptophan (Trp) (11–13), and each plays an important role in inflammation regulation in growing pigs (12, 14). Although supplementation of piglet diets with tryptophan reduced the negative impact of Escherichia coli K88 at weaning, supplementation with threonine was not sufficient to reduce the negative impact of S. typhimurium (15, 16). The present study evaluated the anti-inflammatory effect of three AAs combined into a feed additive blend (Trp + Thr + Met) provided in pigs experimentally challenged with S. typhimurium.
All of the methods were previously approved by the Seoul National University Institutional Animal Care and Use Committee (Approval No. SNU-220621-3).
The AA evaluated in this study were blended to contain L-methionine (L-MET eco, 95% purity, CJ Cheiljedang Co., Ltd.), L-threonine (THR Pro, 80% purity, CJ Cheiljedang Co., Ltd.), and L-tryptophan (TRP Pro, 60% purity, CJ Cheiljedang Co., Ltd.). Tryptophan and threonine were granule-type feed-grade AA that were produced in Corynebacterium glutamicum. The AA blend was created at a weight ratio of 55 (Met):43 (Thr):2 (Trp).
Intestinal porcine epithelial cells (IPEC-J2, DSMZ No. ACC701, BWE, Germany) were seeded in T75 cell culture flasks (70,075, SPL Lifesciences, Gyeonggi-do, Korea) and cultured with Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12, Thermo Fisher Scientific, Waltham, United States) containing 10% fetal bovine serum (FBS, SV30207.02, HyClone, Cytiva, Australia), 1% insulin–transferrin–selenium–ethanolamine (ITS-X, 51500-056, Thermo Fisher Scientific, Waltham, United States), and 1% penicillin–streptomycin (15140-122, Thermo Fisher Scientific, Waltham, United States) at 37°C in a humidified atmosphere with 5% CO2. The cells were sub-cultured once every 3 to 4 days (twice a week) until the confluence reached 90%. For sub-culture, the cells were detached from the flask by trypsin/EDTA (GIBCO, NY, United States), centrifuged at 300 × g for 3 min, re-suspended, and re-seeded at a concentration of 5.0 × 105 cells in 25 mL of complete cell culture medium per T75 flask (17).
IPEC-J2s were seeded at 1.0 × 105 cells/well (1 mL per well) in 24-well plates and cultured overnight. Cells were pretreated with or without AA blend for 24 h and challenged with 1 μg/mL of deoxynivalenol (DON, D0156, Sigma Aldrich, St. Louis, United States) and 1 μg/mL of lipopolysaccharide (LPS, L2630, Sigma Aldrich) for 3 h.
After these treatments, the cells were washed with cold PBS and harvested for total RNA extraction and the quantification of IL-8 mRNA. The percent reduction of IL-8 was calculated following the equation below:
where RGE is relative gene expression.
Total RNA was extracted from IPEC-J2 using the easy-spin RNA extraction kit (17,221, iNtRON, Korea). The RNA purity and concentration were measured using spectrophotometry (QIAxpert System, Qiagen, Hilden, Germany).
A quantitative reverse transcription PCR (RT-qPCR) was performed on the Rotor-Gene Q 2plex platform (Qiagen) in a final volume of 20 μL using the AccuPower GreenStar RT-qPCR PreMix (K-6403, Bioneer, Korea). The PCR reaction mixture contained 10 μL of 2X master mix, 2 μL of each primer (10 pmol/μL), 2 μL of template RNA, and 4 μL of DEPC-DW. The mixture was added into a PCR tube, and the PCR consisted of cDNA synthesis at 50° C for 15 min and pre-denaturation at 95° C for 5 min, followed by 40 cycles at 95\u00B0C for 15 s, 55° C for 30 s, and 72° C for 30 s. The primers were designed with Primer-Blast1 based on the published cDNA sequence in the Gene Bank. The relative abundance of targeted genes was calculated according to the 2−ΔΔCt method and normalized to the mean expression of GAPDH, which serves as the internal reference gene. Information on the detected genes and primers is shown in Table 1.
IPEC-J2s were seeded at 1.5 × 104 cells/well (100 μL per well) in a 96-well plate. After overnight incubation at 37°C, cells were pretreated with or without the AA blend for 18 h and then washed with Hank’s Balanced Salt Solution (HBSS, 14175095, Thermo Fisher Scientific, Waltham, United States). To determine the amount of ROS, cells were incubated with 10 μM DCF-DA (2′, 7′-dichlorofluorescin-diacetate, D6883, Sigma Aldrich, St. Louis, United States) for 30 min and then treated with 0.5 mM H2O2 for 30 min. The fluorescence was read at 485 nm for excitation and 530 nm for emission with a fluorescence microplate reader (BioTek Synergy H1). The percent reduction of ROS was calculated following the equation below:
where RFU is the relative fluorescence unit.
The strain of S. enterica used in this study belongs to serovar Typhimurium, derived from the pig-virulent strain KVCC-BA1300432. This strain was kindly supplied by the Korean Veterinary Culture Collection (Gimcheon-si, Gyeongsangbuk-do, Republic of Korea).
Twenty-four 21-day-old pigs were purchased from a Salmonella-free herd. A commercial ELISA test (Swine Salmonella Ab Test, IDEXX Laboratories Inc., Westbrook, ME, United States) was used to test pig serology for Salmonella upon arrival at the Seoul National University facility. Twenty-four, 21-day-old pigs were randomly distributed into three groups (8 pigs per group). The diet was formulated according to the nutritional recommendations (18, 19).
At −14 days post-challenge (dpc, 21 days of age), pigs in the AA+ST group were started on a controlled standard diet feed supplemented with 0.3% of an AA blend. At 0 days post-challenge (dpc, 35 days of age), pigs in the AA+ST and ST groups were orally inoculated twice within 4 h using 1 mL of a growth medium containing 3.3 × 109 CFU/mL of S. enterica serovar Typhimurium. Pigs in the control group were orally inoculated twice within 4 h with 1 mL of sterile saline solution. At 14 dpc (49 days of age), pigs were sedated by an intravenous injection of sodium pentobarbital and then euthanized by electrocution as previously described (20). Tissues were collected from each pig at necropsy.
Blood samples were collected from all pigs at −14, 0, 3, 7, and 14 dpc.
Rectal temperatures were recorded at 0, 1, 2, 3, 4, 5, 6, 7, and 14 dpc at the same time by the same personnel. A fecal scoring system was defined according to the following scale: 0 (normal), 1 (semisolid feces without blood), 2 (watery feces without blood), and 3 (blood-tinged feces) (4).
Pig weight was measured at −14 (21 days of age), 0 (35 days of age), and 14 (49 days of age) dpc throughout the study. An average daily weight gain (ADWG = grams/pig/day) was calculated over three time points: (i) between −14 and 0 dpc, (ii) between 0 and 14 dpc, and (iii) between −14 and 14 dpc at the study conclusion. The difference between the initial final weights was divided at each of these three time points by the number of days in the corresponding period to calculate ADWG. All data were obtained in a blinded manner.
Serum samples were collected for the quantification of tumor necrosis factor-α (TNF-α), IL-6, IL-8, and IL-10, as assayed using a commercial ELISA kit (Porcine TNF-alpha Quantikine ELISA Kit, Porcine IL-6 Quantikine ELISA Kit, Porcine IL-8/CXCL8 Quantikine ELISA Kit, and Porcine IL-10 Quantikine ELISA Kit, R&D Systems, Inc., Minneapolis, MN, United States). The results were expressed as pg./mL.
Serum superoxide dismutase (SOD) activity was measured using commercial kits (OxiSelect™ Superoxide Dismutase Activity Assay Kit, Cell Biolabs, Inc., San Diego, CA, United States).
A commercial protein carbonyl fluorometric assay was used for the measurement of protein carbonyl content in serum (Cell Biolabs, Inc., San Diego, CA, United States). A commercial thiobarbituric acid reactive substances assay kit was used for the direct quantitative measurement of malondialdehyde in serum samples.
A scoring system for microscopic cecal lesions was defined according to the following scale: 0 (normal), 1 (mild neutrophilic infiltrate without submucosal infiltrates), 2 (moderate neutrophilic infiltrate with or without submucosal infiltrate), and 3 (marked neutrophilic infiltrate with or without submucosal infiltrate) (21).
For the in vitro experiment, differences among the experimental data were assessed using a one-way ANOVA, followed by Tukey’s post-hoc test and F-protected test. For the in vivo experiment, a normal distribution was determined with the Shapiro–Wilk on these data. Whether or not the groups had statistically significant differences between them at various time points was then determined by performing a one-way ANOVA. For further evaluation, a post-hoc test for a pairwise comparison with Tukey’s adjustment was conducted with a statistical significance result from the one-way ANOVA test. A Kruskal–Wallis test was additionally performed only in cases where the normality assumption was not met. The results that showed statistical significance from the Kruskal–Wallis test were further evaluated with the Mann–Whitney test to compare the differences among the groups. The results were reported in p-values, and p-values of <0.05 were considered significant.
AA blend treatment significantly reduced (p < 0.05) LPS/DON-induced IL-8 expression to 49.7% ± 2.3% (2,000 ppm of AA blend), 19.1% ± 0.3% (500 ppm of AA blend), and 22.5% ± 12.1% (125 ppm of AA blend) compared to that of control (Figure 1A).
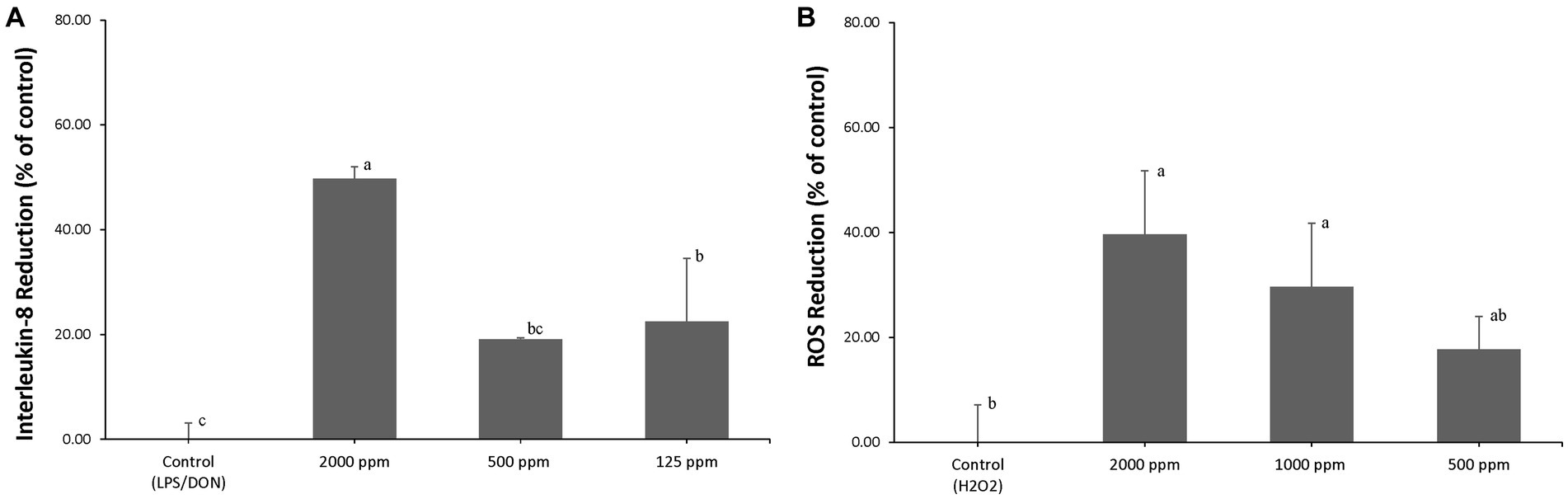
Figure 1. (A) IPEC-J2 were pretreated with an AA blend (0, 125, 500, and 2,000 ppm) for 24 h, and inflammation was induced by exposure to 1 μg/mL LPS and 1 μg/mL DON. Percent (%) of IL-8 reduction was calculated by (RGE of challenged cells – RGE of pretreated and challenged cells)/RGE of challenged cells × 100, where RGE is relative gene expression. Different letters mean statistically significant differences (p < 0.05). (B) IPEC-J2s were pretreated with an AA blend (0, 500, 1,000, and 2,000 ppm) for 24 h, and oxidative stress was induced by exposure to 0.5 mM H2O2. Percent (%) of ROS reduction was calculated by (RFU of challenged cells – RFU of pretreated and challenged cells)/RFU of challenged cells × 100, where RFU is relative fluorescence units (p < 0.05).
AA blend treatment significantly reduced (p < 0.05) H2O2-induced ROS to 39.7% ± 12.1% (2,000 ppm of AA blend), 29.6% ± 12.1% (1,000 ppm of AA blend), and 17.7% ± 6.3% (500 ppm of AA blend) compared to that of control (Figure 1B).
The mean rectal temperature was significantly higher (p < 0.05) in pigs (40.98 ± 0.66) from the ST group at 1 dpc compared to those of the control group pigs (40.20 ± 0.28) (Figure 2A). Pigs in the AA+ST and control groups had significantly (p < 0.05) lower fecal scores than those in the ST group at 1 to 7 dpc. Pigs in the control group had significantly (p < 0.05) lower fecal scores than those in the ST group at 14 dpc (Figure 2B).
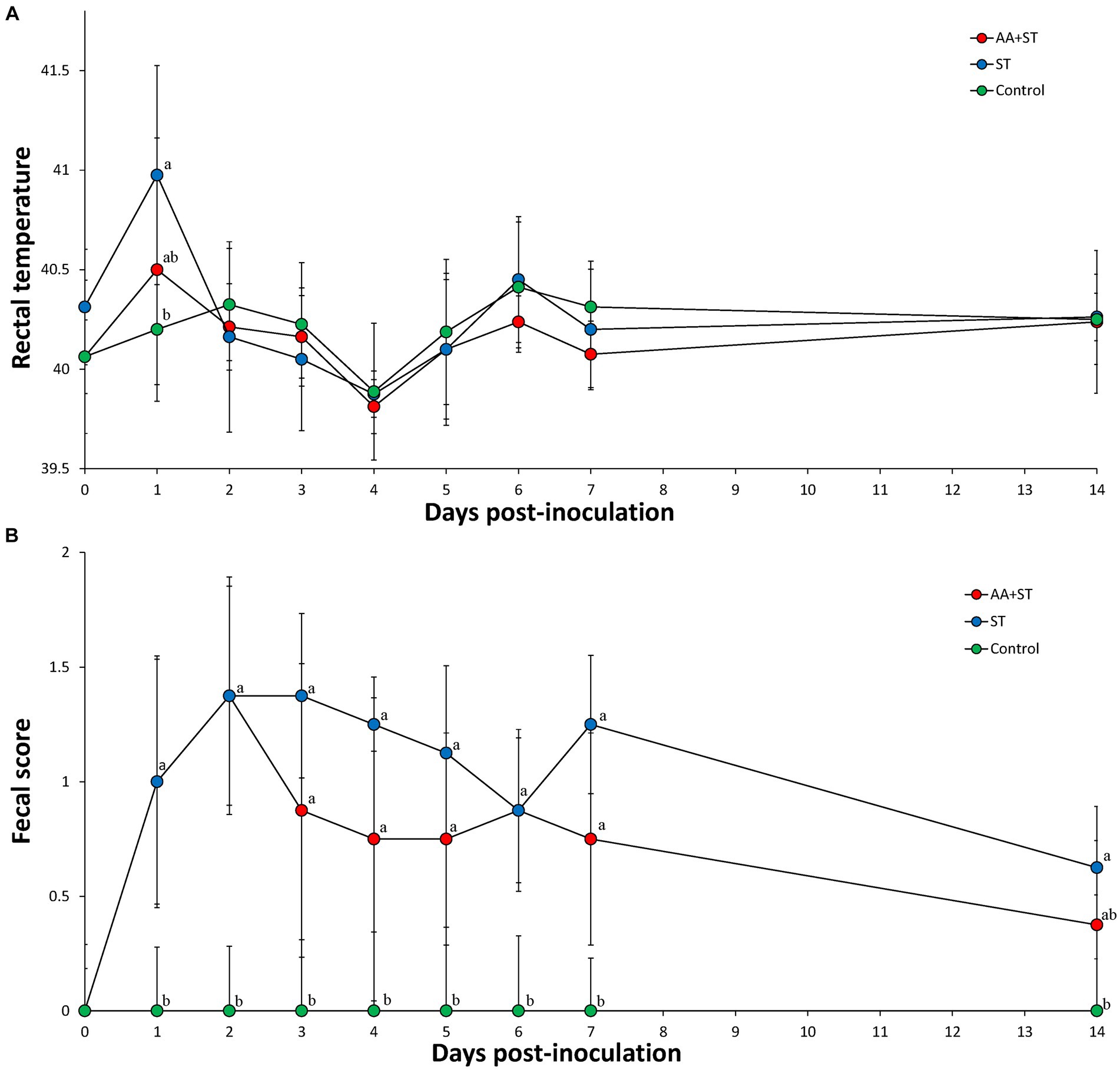
Figure 2. (A) Body temperature. (B) Fecal score. Variation is expressed as the standard deviation. Pigs were fed a control standard diet supplemented with an amino acid blend, followed by oral inoculation with Salmonella enterica serovar Typhimurium (AA+ST group), oral inoculation with Salmonella enterica serovar Typhimurium without supplementation with an amino acid blend (ST group), and control pigs. Different letters within a sampling point mean statistically significant differences (p < 0.05).
The body weight and ADWG of the pigs were measured at −14, 0, and 14 dpc, where significant differences among the three groups were not found.
Serum TNF-α levels were significantly (p < 0.05) lower in the AA+ST and control groups at 7 and 14 dpc than those from the ST groups (Figure 3A). Serum IL-6 levels were significantly (p < 0.05) higher in the ST group at 3, 7, and 14 dpc than those from the control group. Serum IL-6 levels were significantly (p < 0.05) lower in the AA+ST and control groups at 14 dpc than those from the ST group (Figure 3B). Serum IL-8 levels were significantly (p < 0.05) lower in the AA+ST and control groups at 3, 7, and 14 dpc than those from the ST group (Figure 3C). Serum IL-10 levels were significantly (p < 0.05) lower in the AA+ST and control groups at 3 dpc than those from the ST group (Figure 3D).
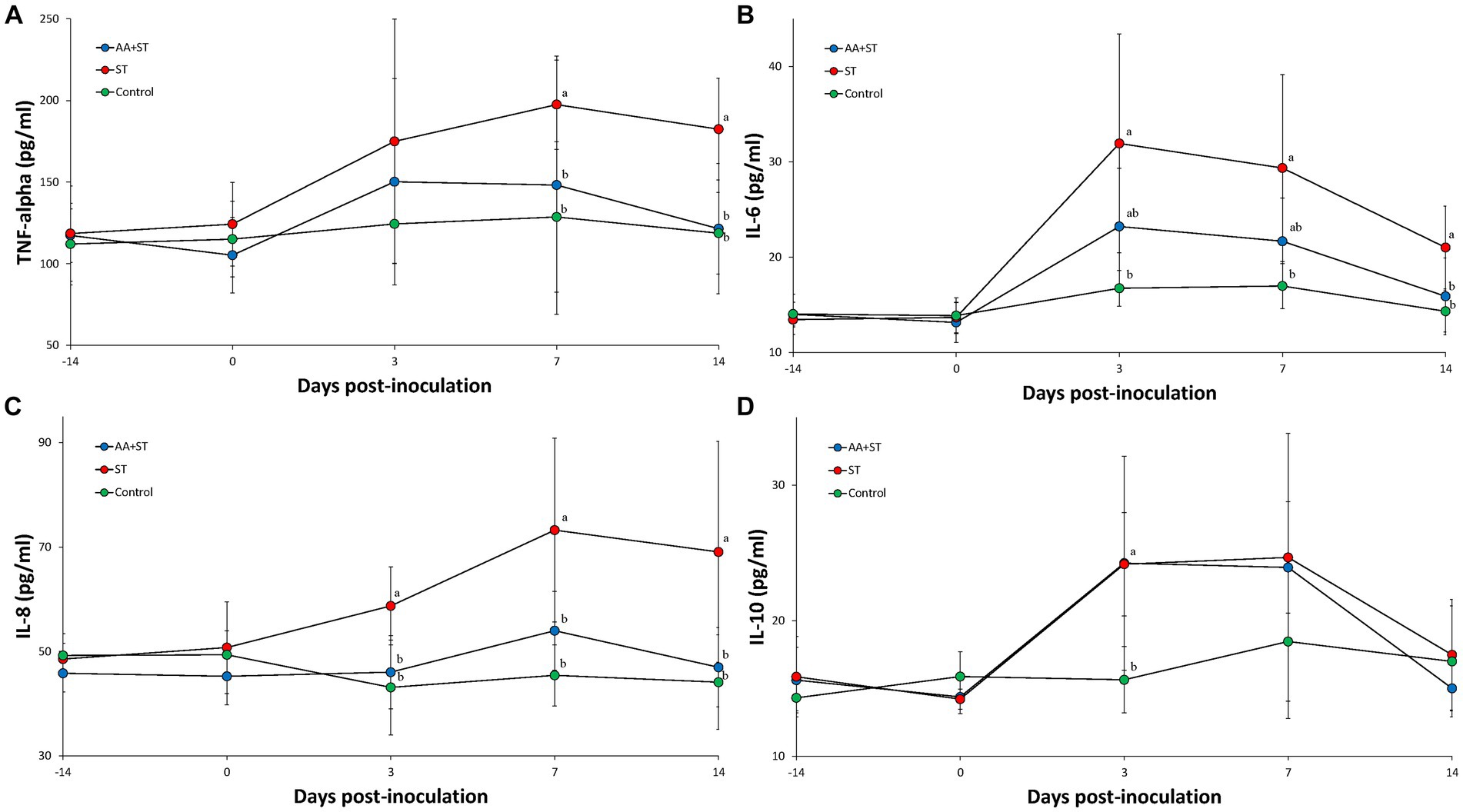
Figure 3. Levels of cytokines in serum. Variation is expressed as the standard deviation. (A) tumor necrosis factor-α. (B) interleukin-6. (C) interleukin-8. (D) interelukin-10. Pigs were fed a control standard diet supplemented with an amino acid blend, followed by oral inoculation with Salmonella enterica serovar Typhimurium (AA+ST group), oral inoculation with Salmonella enterica serovar Typhimurium without supplementation with an amino acid blend (ST group), and control pigs. Different letters within a sampling point mean statistically significant differences (p < 0.05).
Superoxide dismutase activity.
Serum SOD activities were significantly (p < 0.05) higher in the ST group at 3 and 7 dpc than those from the control groups and tend to increase in the AA+ST and control groups at 3 and 7 dpc than those from the ST group (Figure 4A).

Figure 4. (A) Superoxide dismutase activity. (B) Levels of protein carbonyl in serum. (C) Levels of malondialdehyde in serum. Pigs were fed a control standard diet supplemented with an amino acid blend, followed by oral inoculation with Salmonella enterica serovar Typhimurium (AA+ST group), Pigs were oral inoculation with Salmonella enterica serovar Typhimurium without supplementation with an amino acid blend (ST group), and control pigs. Different letters within a sampling point mean statistically significant differences (p < 0.05).
Serum protein carbonyl (Figure 4B) and malondialdehyde (Figure 4C) levels were significantly (p < 0.05) higher in the ST group at 3 and 7 dpc than those from the control groups and tend to increase in the AA+ST and control groups at 3 and 7 dpc than those from the ST group.
Pigs in the AA+ST (lesion score 1.28 ± 0.44, Figure 5A) and control lesion score 0 ± 0, Figure 5B) groups had significantly (p < 0.05) lower Salmonella lesion scores than that in the ST ((lesion score 2.05 ± 0.67, Figure 5C) group at 14 dpc. Pigs in the AA+ST group had significantly higher Salmonella lesion scores than those in the control group at 14 dpc.

Figure 5. Histopathology in Salmonella-induced cecal lesions. (A) Mild cecal lesions with mild neutrophilic infiltrate in pigs were fed a control standard diet supplemented with an amino acid blend, followed by oral inoculation with Salmonella enterica serovar Typhimurium (AA+ST group). (B) Normal cecum in control pigs. (C) Severe cecal lesions with moderate neutrophilic infiltrate and ulceration (arrows) in pigs were oral inoculation with Salmonella enterica serovar Typhimurium without supplementation with an amino acid blend (ST group).
Supplementation with an AA blend (Met + Thr + Trp) has been used successfully to improve gut health and immune function, thus potentially alleviating the negative effects of Salmonella Typhimurium infection on pigs. This experimental challenge study evaluated the growth performance of pigs through the measurement of ADWG. Due to the small number of pigs in each group and the shorter period of observation post-S. typhimurium challenge, a significant difference in ADWG between treated (AA + ST) and untreated (ST) groups was not observed.
Gastrointestinal disorders are identified through the biomarker measurement of inflammatory cytokines (22). Pigs that were experimentally infected with S. typhimurium exhibited a positive correlation of histological lesions with serum levels of TNF-α that were sensitive to tissue injury (23). The addition of an AA blend (Met + Thr + Trp) supplemented into pig feed increased the presence of an anti-inflammatory cytokine, IL-10, while reducing the amount of proinflammatory cytokines such as TNF-α, IL-6, and IL-8, as measured through serum samples from pigs experimentally challenged with S. typhimurium. The change in the amount of pro- and anti-inflammatory cytokines induced through feed supplementation with an AA blend reduced the severity of Salmonella-induced cecal lesions. Ample scientific evidence has been collected demonstrating that an AA blend used as an additive in pig feed is effective as an anti-inflammatory. Supplementation of methionine numerically increased the amount of anti-inflammatory IL-10 cytokine content in the jejunum of piglets experiencing intrauterine growth retardation (24). Of the three AAs used in supplementation, tryptophan blocks TNF-α-induced IL-8 secretion, which specifically provides strong anti-inflammatory effects on intestinal epithelial cells (25). The use of threonine supplementation in a high-fiber diet did not sufficiently maintain pig growth performance when challenged with S. typhimurium. (15). Supplementation of feed with an AA blend provided additional benefits, rendering it a great anti-inflammatory substance. It also reduced the severity of Salmonella-induced cecal lesions in pigs experimentally challenged with S. typhimurium. These results indicate that the supplementation of feed with an AA blend reduces intestinal inflammation and pathology in the pig.
Salmonella infection has been associated with the induction of oxidative stress, a condition characterized by an imbalance between the production of ROS and the cellular antioxidant defense mechanisms. This is manifested by an increase in oxidative stress markers such as MDA, indicating lipid peroxidation, and changes in antioxidant defenses, such as alterations in SOD activity. These molecular changes reflect the complex dynamics of the host–pathogen interaction and the impact of oxidative stress on cellular components. (2, 5).
To evaluate the anti-inflammatory activity of the AA blend, an in vitro examination was first performed using porcine intestinal epithelial cells. A cell-based in vitro evaluation system is a useful model to screen feed additive candidates for their functional effect and efficacy prior to an in vivo trial. In this study, the condition of intestinal inflammation was established by treatment with the combination of bacterial endotoxin (LPS) and mycotoxin (DON), or H2O2 to IPEC-J2s. In vitro studies demonstrated that pretreatment with the AA blend exerted a reduction of LPS/DON-induced inflammatory responses by downregulating IL-8 mRNA expression and ROS reduction under H2O2-induced oxidative stress. Furthermore, in vivo AA blend supplementation showed similar anti-inflammatory and anti-oxidative effects, especially reducing inflammatory cytokines, as expected in an in vitro study.
Salmonellosis is not currently well controlled by an effective strategy. Due to the large antigenic diversity of Salmonella, along with the fact that piglets are exposed extremely early in life (even in primary breeding herds), vaccination has produced mixed successes as a prevention and control strategy (26). With the emergence of antibiotic-resistant bacteria, antibiotics provide little efficacy against Salmonella. Treatment of salmonellosis is further hindered due to plasmids prevalent within S. enterica that encode for antimicrobial resistance (27). Various antibiotics have been used in therapeutic trials to treat severe Salmonella infections in pigs, but offer little merit (28). The Korean government began a restriction on antibiotic use as feed additives in 2005 due to overuse and misuse by producers. As a result, Salmonella control and management rely on alternative methodologies to traditional vaccination and antibiotic use. The results of this study demonstrate that the supplementation of feed with an AA blend was one of these successful alternative methods that could be used in the application of S. typhimurium control.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The animal study was approved by Seoul National University Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
SH: Data curation, Formal analysis, Investigation. JS: Data curation, Formal analysis, Investigation. JK: Data curation, Formal analysis, Investigation. MG: Data curation, Formal analysis, Investigation. MP: Data curation, Formal analysis, Investigation. EO: Data curation, Formal analysis, Investigation. J-OM: Data curation, Formal analysis, Investigation. CC: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was supported by contract research funds (Grant no. 550-20220079) of the Research Institute for Veterinary Science (RIVS) from the College of Veterinary Medicine.
JK, MG, MP, EO, and J-OM were employed by Application Center, CJ Blossom Park, Suwon, Republic of Korea.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Griffith, RW, Schwartz, KJ, and Meyerholz, DK. Salmonella In: BE Straw, JJ Zimmerman, S D’Allaire, and DJ Taylor, editors. Diseases of swine. 9th ed. Ames, Iowa: Wiley-Blackwell (2006)
2. Balaji, R, Wright, KJ, Hill, CM, Dritz, SS, Knoppel, EL, and Minton, JE. Acute phase responses of pigs challenged orally with Salmonella typhimurium. J Anim Sci. (2000) 78:1885–91. doi: 10.2527/2000.7871885x
3. Chirullo, B, Pesciaroli, M, Drumo, R, Ruggeri, J, Razzuoli, E, Pistoia, C, et al. Salmonella Typhimurium exploits inflammation to its own advantage in piglets. Front Microbiol. (2015) 6:985. doi: 10.3389/fmicb.2015.00985
4. Lupp, C, Robertson, ML, Wickham, ME, Sekirov, I, Champion, OL, Gaynor, EC, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. (2007) 2:119–29. doi: 10.1016/j.chom.2007.06.010
5. Stecher, B, Robbiani, R, Walker, AW, Westendorf, AM, Barthel, M, Kremer, M, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. (2007) 5:2177–89. doi: 10.1371/journal.pbio.0050244
6. Eckmann, L, and Kagnoff, MF. Cytokines in host defense against Salmonella. Microbes Infect. (2001) 3:1191–00. doi: 10.1016/S1286-4579(01)01479-4
7. Ramsay, AJ, Husband, AJ, Ramshaw, IA, Bao, S, Matthaei, KI, Koehler, G, et al. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. (1994) 264:561–3. doi: 10.1126/science.8160012
8. Oppenheim, JJ, Zachariae, CO, Mukaida, N, and Matsushima, K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. (1991) 9:611–48. doi: 10.1146/annurev.iy.09.040191.003153
9. Vitiello, M, D'Isanto, M, Galdiero, M, Raieta, K, Tortora, A, Rotondo, P, et al. Interleukin-8 production by THP-1 cells stimulated by Salmonella enterica serovar typhimurium porins is mediated by AP-1, NF-κB and MAPK pathways. Cytokine. (2004) 27:15–24. doi: 10.1016/j.cyto.2004.03.010
10. Mittal, M, Siddiqui, MR, Tran, K, Reddy, SP, and Malik, AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. (2014) 20:1126–67. doi: 10.1089/ars.2012.5149
11. Htoo, JK, and Morales, J. Bioavailability of L-methionine relative to DL-methionine as a methionine source for weaned pigs. J Anim Sci. (2016) 94:249–52. doi: 10.2527/jas.2015-9796
12. Bravo, R, Matito, S, Cubero, J, Paredes, SD, Franco, L, Rivero, M, et al. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age. (2013) 35:1277–85. doi: 10.1007/s11357-012-9419-5
13. Floc’h, NL, and Sève, B. Catabolism through the threonine dehydrogenase pathway does not account for the high first-pass extraction rate of dietary threonine by the portal drained viscera in pigs. Br J Nutr. (2005) 93:447–56. doi: 10.1079/BJN20051375
14. Nichols, NL, and Bertolo, RF. Luminal threonine concentration acutely affects intestinal mucosal protein and mucin synthesis in piglets. J Nutr. (2008) 138:1298–03. doi: 10.1093/jn/138.7.1298
15. Wellington, MO, Agyekum, AK, Hamonic, K, Htoo, JK, Van Kessel, AG, and Columbus, DA. Effect of supplemental threonine above requirement on growth performance of Salmonella typhimurium challenged pigs fed high-fiber diets. J Anim Sci. (2019) 97:3636–47. doi: 10.1093/jas/skz225
16. Liu, G, Gu, K, Wang, F, Jia, G, Zhao, H, Chen, X, et al. Tryptophan ameliorates barrier integrity and alleviates the inflammatory response to enterotoxigenic Escherichia coli K88 through the CaSR/Rac1/PLC-γ1 signaling pathway in porcine intestinal epithelial cells. Fron Immunol. (2021) 12:748497. doi: 10.3389/fimmu.2021.748497
17. Vergauwen, H. The IPEC-J2 cell line In: K Verhoeckx, P Cotter, I López-Expósito, C Kleiveland, T Lea, and A Mackie, editors. The impact of food bioactives on health: in vitro and ex vivo models. Cham, CH: Springer (2015)
18. Rostagno, HS, Teixeira Albino, LF, Lopez Donzele, J, Gomes, PC, de Oliveira, RF, Lopes, DC, et al. Brazilian tables for poultry and swine: Composition of feedstuffs and nutritional requirments. Viçosa Brazil: Universidade Federal de Viçosa (2011).
19. National Research Council (NRC). Nutrient requirements of swine. 11th ed. Washington DC: National Academies Press (2012).
20. Beaver, BV, Reed, W, Leary, S, McKiernan, B, Bain, F, Schultz, R, et al. 2000 report of the AVMA panel on euthanasia. J Am Vet Med Assoc. (2001) 218:669–96. doi: 10.2460/javma.2001.218.669
21. Opriessnig, T, Madson, DM, Roof, M, Layton, SM, Ramamoorthy, S, Meng, XJ, et al. Experimental reproduction of porcine circovirus type 2 (PCV2)-associated enteritis in pigs infected with PCV2 alone or concurrently with Lawsonia intracellularis or Salmonella typhimurium. J Comp Pathol. (2011) 145:261–70. doi: 10.1016/j.jcpa.2010.12.016
22. Piñeiro, C, Piñeiro, M, Morales, J, Andrés, M, Lorenzo, E, del Pozo, M, et al. Pig-MAP and haptoglobin concentration reference values in swine from commercial farms. Vet J. (2009) 179:78–84. doi: 10.1016/j.tvjl.2007.08.010
23. López-Colom, P, Yu, K, Barba-Vidal, E, Saco, Y, Martín-Orúe, SM, Castillejos, L, et al. I-FABP, pig-MAP and TNF-α as biomarkers for monitoring gut-wall integrity in front of Salmonella typhimurium and ETEC K88 infection in a weaned piglet model. Res Vet Sci. (2019) 124:426–32. doi: 10.1016/j.rvsc.2019.05.004
24. Zhang, H, Li, Y, Chen, Y, Ying, Z, Su, W, Zhang, T, et al. Effects of dietary methionine supplementation on growth performance, intestinal morphology, antioxidant capacity and immune function in intra-uterine growth-retarded suckling piglets. J Anim Physiol Anim Nutr. (2019) 103:868–81. doi: 10.1111/jpn.13084
25. Mine, Y, and Zhang, H. Calcium-sensing receptor (CaSR)-mediated anti-inflammatory effects of L-amino acids in intestinal epithelial cells. J Agric Food Chem. (2015) 63:9987–95. doi: 10.1021/acs.jafc.5b03749
26. Wales, AD, and Davies, RH. Salmonella Vaccination in Pigs: A Review. Zoonoses Public Health. (2017) 64:1–13. doi: 10.1111/zph.12256
27. Emond-Rheault, JG, Hamel, J, Jeukens, J, Freschi, L, Kukavica-Ibrulj, I, Boyle, B, et al. The Salmonella enterica plasmidome as a reservoir of antibiotic resistance. Microorganisms. (2020) 8:1016. doi: 10.3390/microorganisms8071016
Keywords: amino acid blend, cytokine, inflammation, Salmonella Typhimurium, porcine
Citation: Ham S, Suh J, Kim J, Gu MJ, Park MA, Oh E, Moon J-O and Chae C (2024) The in vitro and in vivo anti-inflammatory and anti-oxidative effects of an amino acid blend supplemented feed on pigs experimentally challenged with Salmonella Typhimurium. Front. Vet. Sci. 11:1367328. doi: 10.3389/fvets.2024.1367328
Received: 08 January 2024; Accepted: 14 February 2024;
Published: 13 March 2024.
Edited by:
Ambreen Ashar, North Carolina State University, United StatesReviewed by:
Muhammad Shoaib, Chinese Academy of Agricultural Sciences, ChinaCopyright © 2024 Ham, Suh, Kim, Gu, Park, Oh, Moon and Chae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chanhee Chae, c3dpbmVAc251LmFjLmty
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.