
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Vet. Sci., 30 January 2023
Sec. Comparative and Clinical Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1116854
A correction has been applied to this article in:
Corrigendum: Non-invasive methods to assess muscle function in dogs: a scoping review
Skeletal muscle function can be affected by multiple disorders in dogs of which cranial cruciate ligament rupture or disease (CCLD) is one of the most common. Despite the significance of this condition only sparse research exists regarding assessment of muscle function in dogs. This scoping review aimed to identify the non-invasive methods for canine muscle function assessments that have been reported in the literature in the past 10 years. A systematic literature search was conducted 1st March 2022 across six databases. After screening, 139 studies were considered eligible for inclusion. Among the included studies, 18 different muscle function assessment categories were identified, and the most frequently reported disease state was CCLD. We included an attempt to elucidate the clinical applicability of the 18 reported methods, as experts were asked to subjectively assess the methods for their clinical relevance as well as their practical applicability in dogs with CCLD.
Impaired skeletal muscle function is frequently a consequence of orthopaedic disorders. A positive outcome for orthopaedic disorders is dependent on optimal return to normal muscle function. One of the most common orthopaedic disorders causing lameness in dogs is cranial cruciate ligament disease (CCLD) (1–5). In CCLD, the function of the skeletal muscles is particularly challenged, as the normal stabilising synergism between the large muscles around the canine stifle joint and the cruciate ligaments is disrupted (1). It has been found that the extension and flexion angles of surgically treated CCLD stifles are inferior to healthy joints, and that the active range of motion is impaired in the surgically treated limb at long-term assessment (6). Further, it has been found that 6–18% of dogs seen by a veterinarian for reasons related to CCLD are subsequently euthanized due to this diagnosis (3, 7). Reasons for euthanasia include treatment costs and the risk of persistent lameness (3, 6, 7). Due to the incidence and long-term consequences of CCLD, it is critical for surgeons and physiotherapists to have tools to evaluate the functional capacity of patients. Muscle function evaluations could help target therapy and thereby improve the outcome of surgical interventions.
Skeletal muscle function can be measured and evaluated in different ways. Muscle activity can be evaluated by e.g., electromyography and acoustic myography (AMG), the latter also called mechanomyography (8). While muscle mass and tone may be estimated by simple palpation, objective information about mass and muscular health can be obtained from multi-frequency bio-impedance or ultrasound (9, 10). Magnetic resonance imaging (MRI) and computed tomography (CT) are other imaging methods available to evaluate muscles (11, 12). However, a major disadvantage of these two methods is the necessity for the animals to be anaesthetized or at least heavily sedated to ensure that they do not move (13, 14). Muscle function can also be evaluated biomechanically e.g., gait analysis where estimations of kinetic–and kinematic parameters quantify the patients' ambulation (15, 16).
The role of skeletal muscles in orthopaedic diseases such as CCLD emphasises the clinical relevance to muscle function assessment in dogs and an overview of methods for this purpose would be helpful. A scoping review is an approach to systematically identify and map the literature on a specific topic in a replicable way (17, 18). No previous scoping reviews of muscle function assessment in dogs exist. Thus, it is appropriate to use this approach to identify and categorise the available literature on this topic.
The objective of this scoping review was to identify and map the primary research literature on non-invasive methods for assessing muscle function in dogs using formal scoping review methodology. In addition, we also aimed to evaluate the relevance and applicability of the methods in clinical settings, in relation both to their value as muscle function assessors and to the complexity and costs of these procedures.
The scoping review followed the Guidance for conducting systematic scoping reviews from Joanna Briggs Institute (19) and was reported according to the PRISMA Extension for Scoping Reviews (PRISMA-ScR) with subheadings corresponding to the recommendations (17).
Our protocol for the current scoping review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis: Extension for Scoping Review Guidelines (PRISMA-P) 2015 statement (20). The final version of the protocol was uploaded and registered prospectively in the Open Science Framework 1st March 2022 (https://osf.io/r7ja2).
For studies to be included in the scoping review, the study population had to be live dogs or other animals in the canid family (foxes, wolves etc.). Studies had to address a muscle function assessment method. The context element of this scoping review was limited to non-invasive clinically relevant settings. Thus, studies were excluded if the muscle function assessment involved an invasive step (e.g., needle electrodes, blood samples, sedation, or anaesthesia). Studies comprising both invasive and non-invasive settings were included if these were adequately described to make it clear which parts of the study were eligible.
Language was restricted to English, and publication date to the last 10 years (2012-) to ensure current applicability in clinical settings. Book chapters and reviews were excluded to avoid subjective opinions and the risk of double reporting. When we identified reviews, we checked the reference list for eligible studies and included them if they were missed by our search.
To identify potentially relevant studies, a systematic literature search was conducted 1 March 2022 across the following bibliographic databases: Web of Science (RRID:SCR_022706), CAB Abstracts (CABI) (RRID:SCR_016467), Ovid MEDLINE® ALL (RRID:SCR_002185), AGRICOLA (RRID:SCR_008158), Scopus (RRID:SCR_022559) and Embase (RRID:SCR_001650). The databases were searched from 2012 to 1st March 2022. The search strategy was discussed and refined through team discussions and in collaboration with experienced librarians from Copenhagen University Library. The search results for all six databases were exported to the reference manager software EndNote (EndNote™ version 20, Clarivate, Philadelphia, USA, RRID:SCR_014001), and duplicates were removed. When online access to studies was not possible, full texts were requested by contacting the author(s) via either ResearchGate [www.researchgate.net, an academic social network, (RRID:SCR_006505)] or by personal inquiry via e-mail.
The literature search was prepared using preliminary searches of Web of Science and Ovid MEDLINE® ALL to identify studies on the topic. The relevant records were then analysed for specific words contained in the title or the abstract, and these words were used to develop the full search strategy. The final literature search performed (by KHD) 1st March 2022 is shown in Table 1 and examples of the full electronic search strategy (for Embase and Web of Science) can be found online in Appendix I (https://osf.io/sdnxj).
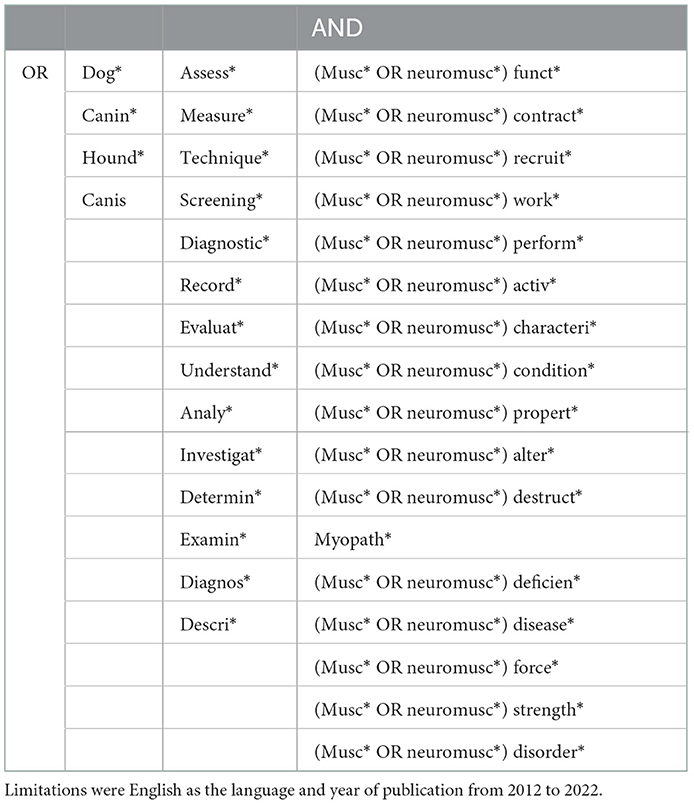
Table 1. Search strategy for the databases: Web of Science, CAB Abstracts, Ovid MEDLINE® ALL, AGRICOLA, Scopus and Embase.
All records retrieved were exported from EndNote and imported into Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia (available at www.covidence.org, RRID:SCR_016484). Additional duplicates were automatically removed by Covidence before starting the screening process.
Initially, the reviewers aligned their understanding of the inclusion and exclusion criteria to increase consistency of the review process. Two independent reviewers (KHD and TA) reviewed the titles and abstracts of all the records for eligibility. A third independent reviewer (MKZ) evaluated discrepancies. Subsequently, the three independent reviewers (KHD, TA and MKZ) went through the potentially eligible records, and decided the final included records based on a full-text analysis. Each of the reviewers screened 2/3 of the total number and shared half of their screening with each of the two other reviewers. The whole screening process in Covidence was blinded, also for the reviewers themselves when discrepancies had to be solved. Reasons for exclusion were determined for each study: Invasiveness due to anaesthesia/sedation, invasiveness due to other conditions than anaesthesia (e.g., blood samples or electrodes), wrong outcome or intervention, inadequate description of intervention (e.g., not mentioned whether the electromyographic method was invasive or surface, or whether MRI or CT required anaesthesia), wrong population (in silico studies or a study population other than canids), wrong source of literature (not considered to be original quantitative research e.g., book chapters or reviews), intervention on cadavers, language other than English, wrong year of publication and study duplicate (i.e., the search both included a proceeding and an article of the same study). If full text versions of studies were not available online, and there was no response from direct contact to the authors after two attempts over 1 month, or if it was impossible to find contact information for authors via Google Scholar (www.scholar.google.com, RRID:SCR_008878), records were excluded as “not available.”
A data extraction table was jointly developed by all three reviewers to determine which variables to extract. Categories of muscle function assessment methods were continuously added to the table as they were identified from the eligible studies. Independently, two reviewers continuously charted data from each eligible study. At the end of data extraction, the data-charting forms were compared, and disagreements were resolved by consensus or, if consensus could not be reached, with input from the third reviewer. Authors were not contacted for clarification or to obtain additional information on incomplete studies.
Information collected from each study included author(s), publication title, year of publication, journal, one or more muscle function assessment methods, study population details, country, context (e.g., disease), aim/objective, intervention, and outcome/key findings. The final version of the data extraction form of all included studies is available online in Appendix II (https://osf.io/7j4yk).
The results were presented descriptively in tables and supplemented with appropriate graphical presentations. When the number of observations within each outcome measure was higher than 18, the most frequent results were presented. Studies were grouped by the type of muscle function assessment method(s) they included, allowing identification and mapping of the range of muscle function assessment methods currently applied in dogs. Further, the studies were grouped by the journals they were published in, by the countries and by the context of the studies (specific diseases or basic research). Information on the clinical relevance and clinical applicability of the identified methods was obtained by two clinical experts (JM and AV) who were asked to grade the level of clinical relevance of the methods: Grading 1–4 (poor, fair, good, excellent) for the potential quality of information provided to the clinician on muscle function in dogs with CCLD. Correspondingly, two experts in biomechanics (TA and MKZ) were asked to grade the level of applicability (method compliance) of the methods, including cost, training of staff, space requirements, time requirements etc. Method applicability was also graded from 1 to 4 (poor, fair, good, excellent). The clinical value gradings were then plotted against the method applicability gradings to identify methods with high value/high applicability, high clinical value/low applicability etc. During the method grading, the experts did not know the frequency of the identified methods applied in the included studies. The documents that the experts were given before grading can be seen online in Appendix III (https://osf.io/gf64p).
In total, 3,105 citations were identified from searches of the electronic databases and 139 studies were considered eligible for inclusion in this scoping review (Figure 1).
The characteristics and relevant data charted from each of the included sources is provided in Appendix II, available online (https://osf.io/7j4yk). Eighteen different muscle function assessment categories were identified among the included studies and the percentage distribution of all observations (n = 248) is shown (Figure 2): subjective evaluation (consisting of a synthesis of multiple clinical assessments e.g., observation of gait or identification of muscle atrophy without any grading) (37/248, 14.9%), limb circumference (17/248, 6.9%), muscle condition score (MCS) (18/248, 7.3%), goniometry (12/248, 4.8%), scoring systems (lameness/pain scores etc.) (24/248, 9.7%), 6-min walk test (2/248, 0.8%), surface electromyography (sEMG) (16/248, 6.5%), AMG (3/248, 1.2%), electrical impedance myography (EIM) (2/248, 0.8%), force plate/force transducer/instrumented carpet/pressure walkway (35/248, 14.1%), treadmill with force plates (9/248, 3.6%), video analysis with markers (25/248, 10.1%), video analysis without markers (11/248, 4.4%), fluoroscopy with markers (1/248, 0.4%), accelerometry and pedometry (12/248, 4.8%), pressure algometry (1/248, 0.4%), infrared thermography (5/248, 2.0%), or ultrasound (18/248, 7.3%).
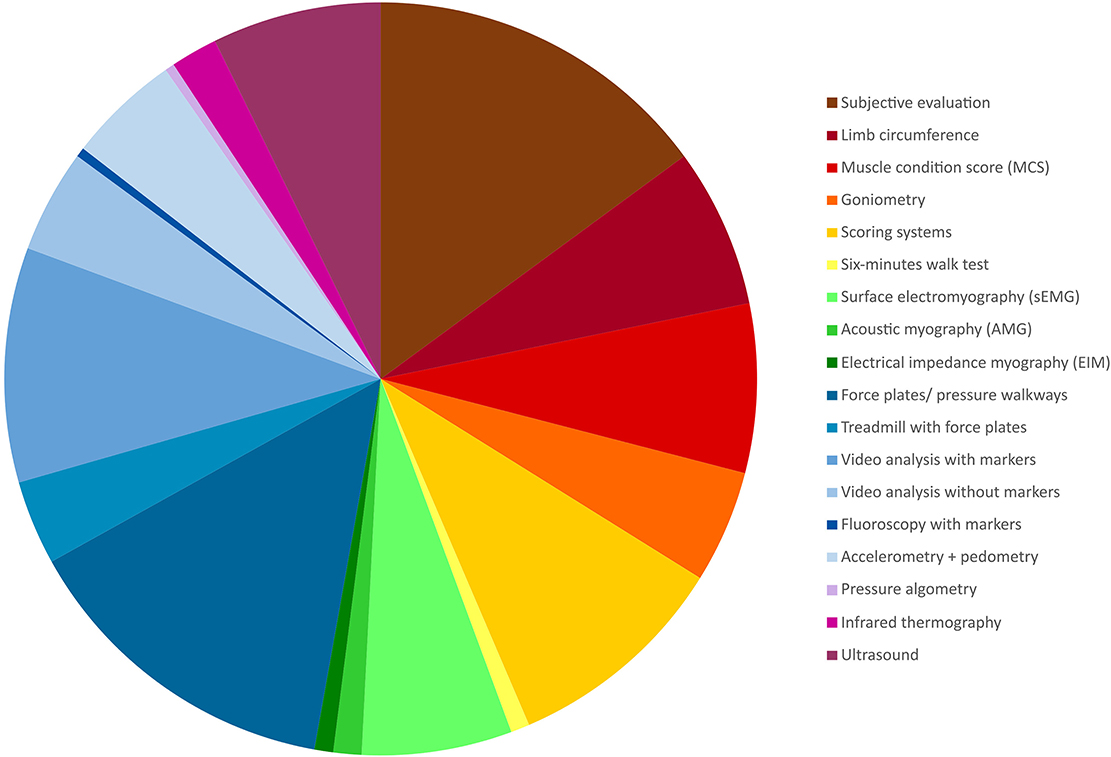
Figure 2. Pie chart visualising the distribution of the identified method categories based on all observations (n = 248).
The method category called “scoring systems” comprised several validated and non-validated scoring systems primarily focusing on lameness or pain. This category (scoring systems), representing 24 of the included studies (three of them applying two scoring systems), was divided into 14 subcategories (Table 2).
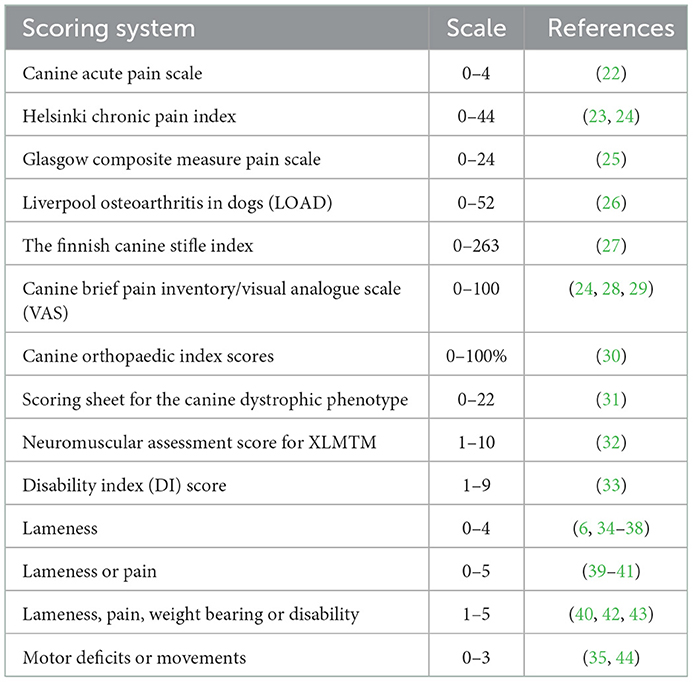
Table 2. Unfolding the method category “scoring systems” including 24 studies (XLMTM: X-linked myotubular myopathy).
The number of studies represented within each muscle function assessment category varied from one to 37, and the number of muscle function assessment methods per study varied from one to five. The mean (SD) size of the study population in the included studies was 35.1 (76.3), the lower quartile was 6, the median 11.5, and the upper quartile of the study population was 25.75.
The most frequent context area among the included studies was basic research on healthy dog(s) (48 studies) followed by CCLD (16 studies) (Table 3).
The percentage distribution of the identified 18 muscle function assessment methods among all the included studies (n = 139) and studies focusing on CCLD [n = 16 (1, 6, 14, 34, 35, 39, 40, 45–53)] are shown in Figure 3. Ten out of the 18 muscle function assessment methods were applied in studies on CCLD. A greater representation of limb circumference, goniometry, scoring systems and force plates/pressure walkways was observed for CCLD studies when compared with all the included studies. Video analysis without markers, accelerometry/pedometry, and ultrasound were some of the methods that were not applied among the CCLD studies. Scoring systems were applied in five of the CCLD studies and covered simple lameness scores but with different scales (6, 34, 35, 39, 40).
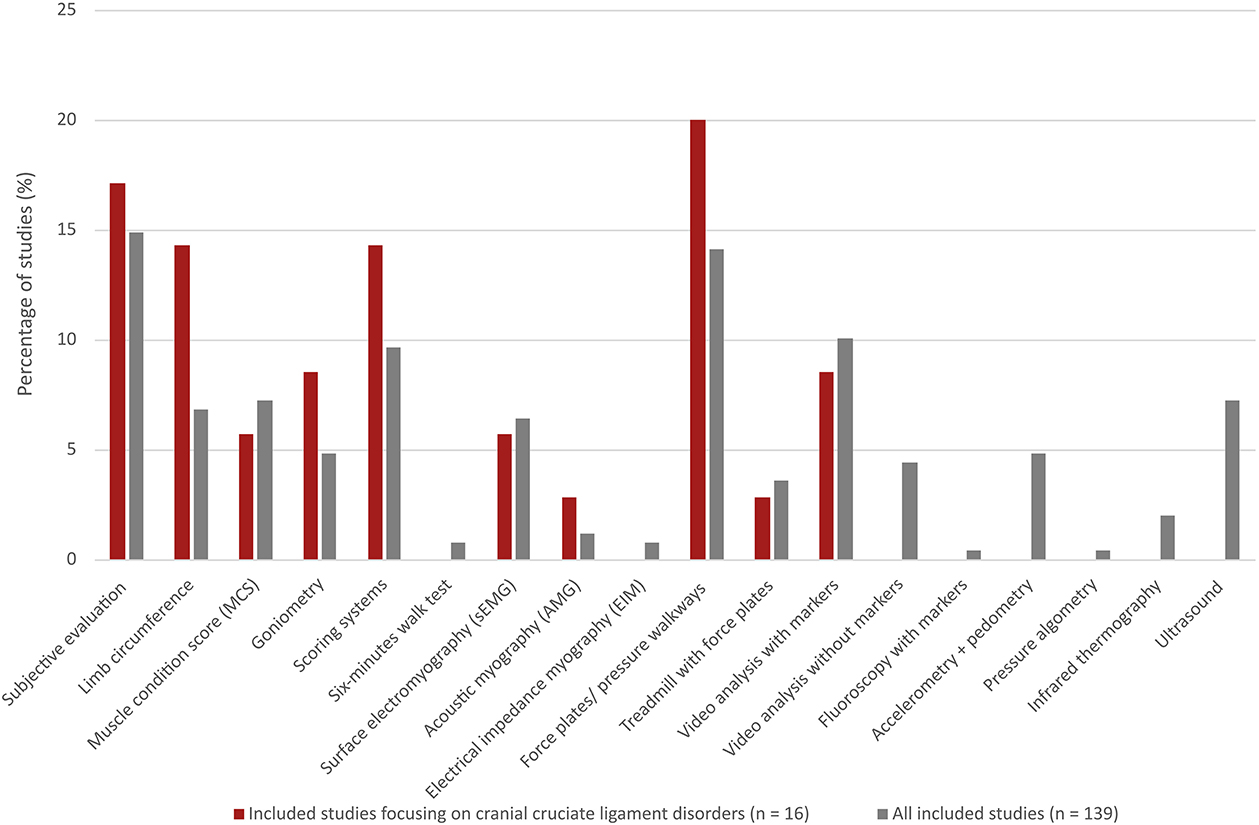
Figure 3. Percentage distribution of 18 different muscle function assessment methods among all the included studies (n = 139, grey bars) compared with the distribution in only a small part of these studies focusing on cranial cruciate ligament disease (n = 16, red bars).
The method grading made by clinical experts and experts in biomechanics are shown in Figure 4. Subjective evaluation, scoring systems, limb circumference, MCS and goniometry were graded as methods with high applicability. AMG, sEMG and EIM were graded as methods with the most clinically relevant outcome for dogs with CCLD.
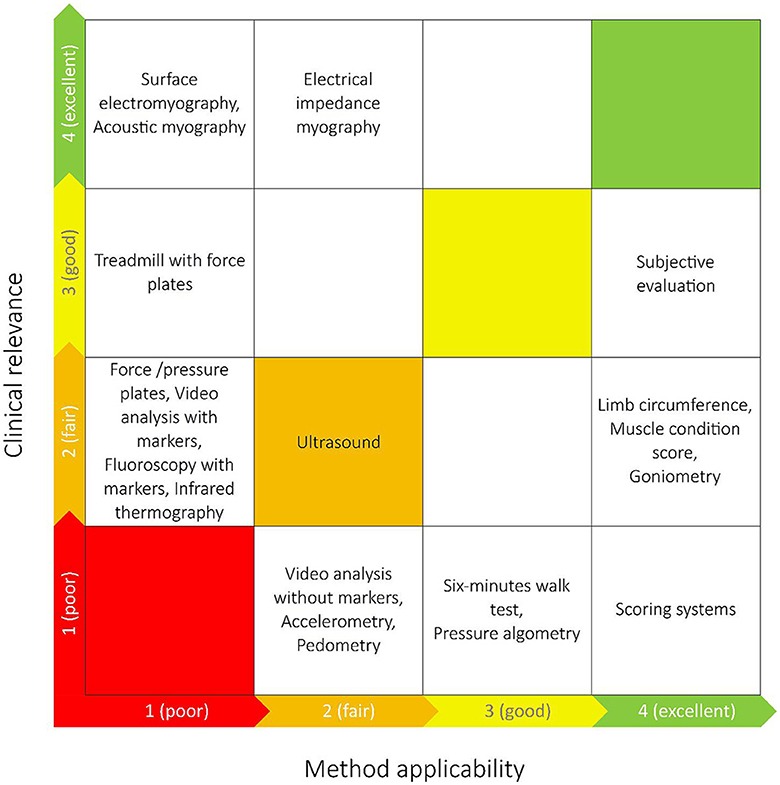
Figure 4. The grading of the identified muscle function assessment methods made by experts in biomechanics (x-axis) and clinical experts (y-axis) in relation to dogs with cranial cruciate ligament disease. The most optimal techniques are placed closer to the upper right corner, and the least optimal closest to the lower left corner.
Figure 5 shows the geographical distribution of the included studies; 23 countries were represented. The most frequent countries were the United States (50 studies), Brazil (17 studies), Germany (12 studies), Italy (10 studies) and The United Kingdom (10 studies).
In general, the distribution of the methods applied in studies within the same country were as diverse as between countries. As an example, 17 studies included from Brazil covered 12 of the identified 18 method categories without any obvious links between the studies. However, there were exceptions for some of the countries: two out of three of the included studies from Austria (54, 55) were from the same group applying the same methods, and the two studies from Denmark used AMG (56, 57). Further, five out of six studies from Finland were from the same research group but applied different methods in the studies (6, 27, 35, 47, 58). Accordingly, the included 12 studies from Germany were conducted by four research groups (59–70). The German studies had a relatively high fraction of the objective, quantitative methods focusing on kinematics and kinetics e.g., six out of the nine studies applying a treadmill with force plates were German.
The 16 included studies focusing on CCLD were conducted in 9 different countries from five continents, and with the United States as the most represented country (n = 6).
There were 65 scientific journals represented among the included studies; the most frequent journals were PLOS ONE (12 studies), Veterinary Surgery (9 studies), Veterinary and Comparative Orthopaedics and Traumatology (8 studies), and Acta Veterinaria Scandinavica (8 studies) (Table 4).
The aim was to identify and map current non-invasive muscle function assessment methods in dogs and to evaluate their applicability and relevance in clinical settings. We included 139 studies published in a variety of international peer-reviewed journals. Most of the identified studies represented basic research in healthy dogs. However, CCLD was the most frequently studied disease. The muscle function assessment methods in the included studies were divided into 18 categories and ranged from subjective examinations and simple measurements to complex and advanced methods. Muscle atrophy evaluation was one of the simple assessments. Muscle atrophy was identified by palpation without any grading (the method “subjective evaluation”), by muscle condition scoring the patient, and by measuring the limb circumference. Accordingly, lameness (i.e., altered muscle function in a limb) and pain were identified by clinical examinations and by scoring systems, the latter making the outcome quantitative. Pain scoring is not by definition considered directly related to muscle function. Nevertheless, if a dog perceives pain, this will often be coincident with reduced or altered muscle function. Further, pain assessment is defined as the fourth vital sign alongside temperature, pulse and respiration examinations, making pain assessment a way to capture more patients with altered muscle function (71). Unfolding the “scoring systems” category revealed that there was not a single standardised pain or lameness scoring system for dogs reported in the included studies. Further, there were no specific scoring systems for dogs with CCLD. As such, different scales of lameness grading were used (6, 34, 35, 39, 40), making it difficult to compare results between studies. Seven percent of the identified methods assessed muscle function by sEMG and AMG. Using these methods, the activity of specific muscles was investigated, and in some studies asymmetries between right- and left-sided muscles were quantified (46, 51). An integral part of most studies was gait analysis, since this term covers the entire range from visual observation of gait to quantified outcome measures as, for example, kinetic studies or kinematic studies using reflective markers attached to the dog (16). Subjective evaluation was the most frequently identified method category among all the included studies, while objective assessment using kinetic outcome measures was the second most frequent category in the included studies, and the most frequent category among studies focusing on CCLD.
The clinical relevance of evaluating surgical techniques and surgical outcomes for CCLD patients can explain why lameness, pain scoring, and biomechanical outcomes were represented among the included CCLD studies.
The clinical relevance of evaluating surgical techniques and surgical outcomes for CCLD patients may explain why lameness, pain scoring, and biomechanical outcomes were represented among the included CCLD studies, whereas the 6-min walk test and accelerometry/pedometry were not. This may be because the last-mentioned methods are primarily used to evaluate diseases that affect gait velocity, such as heart and respiratory diseases (72, 73). Dogs can establish compensatory movement patterns due to lameness, and thereby maintain their velocity. Thus, the 6-min walk test and accelerometry/pedometry are probably poor methods to evaluate surgical CCLD outcomes and therefore not represented among the included CCLD studies.
For most of the included studies, muscle function assessment was not the central purpose. Rather, the identified muscle function assessment methods were supplemental outcome measures. In general, the choice of muscle function assessment method in a given study depends on factors such as availability of equipment and the research question to be answered. It is possible that the percentage distribution of the identified methods in the present study would have been different if muscle function assessment were the main purpose of all the included studies.
The experts' grading of the muscle function assessment methods aimed at a clinical evaluation of the identified methods for dogs with CCLD. Given that CCLD was the most frequently represented disease state among the included studies, we found it appropriate to centre the expert evaluation on dogs with CCLD. The experts in biomechanics evaluated the subjective evaluation, scoring systems, limb circumference, MCS and goniometry to be the most applicable methods in clinical settings. The clinical experts evaluated sEMG, AMG, and EIM to be the methods with the most relevant information for dogs with CLLD. Subjective evaluation (consisting of a synthesis of multiple clinical assessments e.g., observation of gait or identification of muscle atrophy without any grading) was the muscle function assessment method placed closest to the top right corner in Figure 5 and thereby had the best combined grading made by the experts. The expert evaluation reflects the potential value of each method in the context of managing CCLD in practise, but the specific purpose of every study is key in the choice of muscle function assessment method.
The geographical distribution of the included studies was almost the same for the total included studies as for those focusing on CCLD. None of the included German studies focused on CCLD, but the same biomechanical method, e.g., the treadmill, were applied by other investigators on dogs with CCLD (49).
All the methods captured in this scoping review were indirect to varying degrees in the way they evaluated muscle function. However, the wide range of presented methods revealed good options to assess different aspects of muscle function in either research or clinical practises in the future. Given the high method applicability and clinical relevance of subjective evaluation, development of formalised, structured clinical assessment tools may be of value to future research.
Our scoping review has some limitations. Firstly, despite using broad search terms in the search strategy, it is possible that eligible studies were missed by our search. Further, this scoping review did not specifically search for biomechanical studies as the objective was to identify studies assessing the muscles per se, and the search strategy was designed specifically to capture such studies. However, since many biomechanical studies were captured by our search, a wider search strategy including biomechanical terms could potentially have found more eligible studies and increased the overall frequency of methods including force plates and motion capture/movement analysis. Additionally, several studies were excluded due to non-invasiveness as an inclusion criterion. Our intention was to exclude invasive experimental procedures not transferable to clinical settings. Since sedation is commonly performed in clinical practise, it could be argued that it would have been acceptable and relevant to include studies with sedated or anaesthetized dogs, provided that the muscle assessment method itself was non-invasive. However, most of the studies which were excluded due to anaesthesia used CT or MRI, which is typically accessible only in larger clinics. The expert evaluations of the included muscle function assessment methods are subjective but reflect the potential value of each method in the context of managing CCLD in practice.
The width and depth of the literature on muscle function assessment methods in dogs was identified by this scoping review. The literature included simple case studies conducted in clinical settings as well as highly advanced basic research on experimental dogs. We observed that simple subjective evaluation was the most frequently used method for muscle function in dogs, and it was also the method with the best combined rating made by the experts in this scoping review. Kinetic methods were the most frequently reported in studies on dogs with CCLD. In total, 18 muscle function assessment categories were identified, with limited standardisation of muscle function assessment. This highlights the need for more and ideally high-quality research to establish consensus within muscle function assessment methods both in general and for specific patient groups, such as dogs with CCLD.
KD was the primary person developing the review protocol and the search strategy, conducted the literature search, and the first draft of the manuscript. KD, TA, and MZ were involved in the eligibility screening, discussed the findings, extracted the relevant data, and discussed ideas on how to present data in the best way. JM and AV acted as expert clinicians in grading the muscle assessment methods. TA and MZ acted as experts in biomechanics. All authors discussed the results and contributed to the revision of the manuscript, read, and approved the submitted version.
KD was supported by a stipendium for the Faculty of Health and Medical Sciences.
We would like to thank the librarians Annemette Møller Hansen and Anne Cathrine Trumpy from Copenhagen University Library, and Lene Kaad from University College Copenhagen for the assistance with our search strategy. Further, we would like to thank Adrian Harrison for reading the manuscript.
Adrian Harrison is a co-supervisor for KD's PhD project. Adrian Harrison established the company MyoDynamik Aps in 2011 that offers AMG equipment.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1116854/full#supplementary-material
Appendix I. Search strategy (https://osf.io/sdnxj).
Appendix II. Data extraction form (https://osf.io/7j4yk).
Appendix III. Documents for the clinical experts and experts in biomechanics (https://osf.io/gf64p).
1. Adrian CP, Haussler KK, Kawcak CE, Palmer RH, McIlwraith CW, Reiser RF, et al. Gait and electromyographic alterations due to early onset of injury and eventual rupture of the cranial cruciate ligament in dogs: a pilot study. Vet Surg. (2019) 48:388–400. doi: 10.1111/vsu.13178
2. Vilensky JA, Oconnor BL, Brandt KD, Dunn EA, Rogers PI, Delong CA. Serial kinematic analysis of the unstable knee after transection of the anterior cruciate ligament - temporal and angular changes in a canine model of osteoarthritis. J Orthop Res. (1994) 12:229–37. doi: 10.1002/jor.1100120212
3. Engdahl K, Emanuelson U, Hoglund O, Bergstrom A, Hanson J. The epidemiology of cruciate ligament rupture in an insured swedish dog population. Sci Rep. (2021) 11:9546. doi: 10.1038/s41598-021-88876-3
4. Spinella G, Arcamone G, Valentini S. Cranial cruciate ligament rupture in dogs: review on biomechanics, etiopathogenetic factors and rehabilitation. Vet Sci. (2021) 8:186. doi: 10.3390/vetsci8090186
5. Johnson JA, Austin C, Breur GJ. Incidence of canine appendicular musculoskeletal disorders in 16 veterinary teaching hospitals from 1980 through 1989. Vet Comp Orthop Traumatol. (1994) 7:56–69. doi: 10.1055/s-0038-1633097
6. Molsa SH, Hyytiainen HK, Hielm-Bjorkman AK, Laitinen-Vapaavuori OM. Long-term functional outcome after surgical repair of cranial cruciate ligament disease in dogs. BMC Vet Res. (2014) 10:266. doi: 10.1186/s12917-014-0266-8
7. Boge GS, Engdahl K, Bergstrom A, Emanuelson U, Hanson J, Hoglund O, et al. Disease-related and overall survival in dogs with cranial cruciate ligament disease, a historical cohort study. Prev Vet Med. (2020) 181:7. doi: 10.1016/j.prevetmed.2020.105057
8. Cooper MA, Herda TJ, Vardiman JP, Gallagher PM, Fry AC. Relationships between Skinfold Thickness and Electromyographic and Mechanomyographic Amplitude Recorded During Voluntary and Non-Voluntary Muscle Actions. J Electromyogr Kinesiol. (2014) 24:207–13. doi: 10.1016/j.jelekin.2013.12.005
9. Bartels EM, Andersen EL, Olsen JK, Kristensen LE, Bliddal H, Danneskiold-Samsoe B, et al. Muscle assessment using multi-frequency bioimpedance in a healthy danish population aged 20-69 years: a powerful non-invasive tool in sports and in the clinic. Physiol Rep. (2019) 7:e14109. doi: 10.14814/phy2.14109
10. Kara M, Ata AM, Kaymak B, Ozcakar L. Ultrasound imaging and rehabilitation of muscle disorders part 2: nontraumatic conditions. Am J Phys Med Rehabil. (2020) 99:636–44. doi: 10.1097/PHM.0000000000001352
11. Kaiser S, Harms O, Konar M, Staudacher A, Langer A, Thiel C, et al. Clinical, radiographic, and magnetic resonance imaging findings of gastrocnemius musculo - tendinopathy in various dog breeds. Vet Comp Orthop Traumatol. (2016) 29:515–21. doi: 10.3415/VCOT-16-01-0015
12. Bullen LE, Evola MG, Griffith EH, Seiler GS, Saker KE. Validation of ultrasonographic muscle thickness measurements as compared to the gold standard of computed tomography in dogs. PeerJ. (2017). 5:e2926. doi: 10.7717/peerj.2926
13. Mostafa AA, Griffon DJ, Thomas MW, Constable PD. Morphometric characteristics of the pelvic limb musculature of labrador retrievers with and without cranial cruciate ligament deficiency. Vet Surg. (2010) 39:380–9. doi: 10.1111/j.1532-950X.2010.00657.x
14. Frank I, Duerr F, Zanghi B, Middleton R, Lang L. Diagnostic ultrasound detection of changes in femoral muscle mass recovery after tibial plateau levelling osteotomy in dogs. Vet Comp Orthop Traumatol. (2019) 32:394–400. doi: 10.1055/s-0039-1688985
15. Shin JH, Greer B, Hakim CH, Zhou Z, Chung YC, Duan Y, et al. Quantitative phenotyping of duchenne muscular dystrophy dogs by comprehensive gait analysis and overnight activity monitoring. PLoS ONE. (2013) 8:e59875. doi: 10.1371/journal.pone.0059875
16. Carr BJ, Canapp SO, Zink MC. Quantitative comparison of the walk and trot of border collies and labrador retrievers, breeds with different performance requirements. PLoS ONE. (2015) 10:e0145396. doi: 10.1371/journal.pone.0145396
17. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. Prisma extension for scoping reviews (Prisma-Scr): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
18. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
19. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. (2015) 13:141–6. doi: 10.1097/XEB.0000000000000050
20. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (Prisma-P) 2015: elaboration and explanation. Br Med J. (2015) 350:g7647. doi: 10.1136/bmj.g7647
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.02.003
22. Altinkaya N, Cagatay S, Necati E. Neurological physiotherapy in labrador retriever dog with paraparesis: a case report. Int J Physiotherapy. (2020) 7:192–5. doi: 10.15621/ijphy/2020/v7i5/778
23. Hulea CI, Cristina RT. Monitoring and therapeutics of joint pain in dogs. Medicamentul Veterinar / Vet Drug. (2018) 12:47–59.
24. Silva N, Luna SPL, Joaquim JGF, Coutinho HD, Possebon FS. Effect of acupuncture on pain and quality of life in canine neurological and musculoskeletal diseases. Can Vet J-Revue Veter Canadienne. (2017) 58:941–51.
25. Nutt AE, Knowles TG, Nutt NG, Murrell JC, Carwardine D, Meakin LB, et al. Influence of muscle-sparing lateral thoracotomy on postoperative pain and lameness: a randomized clinical trial. Vet Surg. (2021) 50:1227–36. doi: 10.1111/vsu.13599
26. von Pfeil DJF, Steinberg EJ, Dycus D. Arthroscopic tenotomy for treatment of biceps tendon luxation in two apprehension police dogs. J Am Vet Med Assoc. (2020) 257:1157–64. doi: 10.2460/javma.2020.257.11.1157
27. Hyytiainen HK, Morelius M, Lappalainen AK, Bostrom AF, Lind KA, Junnila JJT, et al. The finnish canine stifle index: responsiveness to change and intertester reliability. Vet Rec. (2020) 186:604. doi: 10.1136/vr.105030
28. Barthelemy NP, Griffon DJ, Ragetly GR, Carrera I, Schaeffer DJ. Short- and long-term outcomes after arthroscopic treatment of young large breed dogs with medial compartment disease of the elbow. Vet Surg. (2014) 43:935–43. doi: 10.1111/j.1532-950X.2014.12255.x
29. de Oliveira Reusing MS, do Amaral CH, Zanettin KA, Weber SH, Villanova JA. Effects of hydrotherapy and low-level laser therapy in canine hip dysplasia: a randomized, prospective, blinded clinical study. Revue Veterinaire Clinique. (2021) 56:177–84. doi: 10.1016/j.anicom.2021.08.001
30. Schulz KS, Ash KJ, Cook JL. Clinical outcomes after common calcanean tendon rupture repair in dogs with a loop-suture tenorrhaphy technique and autogenous leukoreduced platelet-rich plasma. Vet Surg. (2019) 48:1262–70. doi: 10.1111/vsu.13208
31. Barthelemy I, Uriarte A, Drougard C, Unterfinger Y, Thibaud JL, Blot S. Effects of an immunosuppressive treatment in the grmd dog model of duchenne muscular dystrophy. PLoS ONE. (2012) 7:e48478. doi: 10.1371/journal.pone.0048478
32. Snyder JM, Meisner A, Mack D, Goddard M, Coulter IT, Grange R, et al. Validity of a neurological scoring system for canine x-linked myotubular myopathy. Hum Gene Ther Clin Dev. (2015) 26:131–7. doi: 10.1089/humc.2015.049
33. Gordon-Evans WJ, Johnson AL, Knap KE, Griffon DJ. The effect of body condition on postoperative recovery of dachshunds with intervertebral disc disease treated with postoperative physical rehabilitation. Vet Surg. (2019) 48:159–63. doi: 10.1111/vsu.13142
34. de Barros LP, Ribeiro LRR, Pereira CDC, Ferreira FLM, da Conceicao M, Dias L. Prospective clinical assessment of tibial tuberosity advancement for the treatment of cranial cruciate ligament rupture in dogs. Acta Cir Bras. (2018) 33:684–9. doi: 10.1590/s0102-865020180080000004
35. Hyytiainen HK, Molsa SH, Junnila JT, Laitinen-Vapaavuori OM, Hielm-Bjorkman AK. Ranking of physiotherapeutic evaluation methods as outcome measures of stifle functionality in dogs. Acta Vet Scand. (2013) 55:29. doi: 10.1186/1751-0147-55-29
36. Lee S, Lee JM, Park H, Won S, Cheong J. Rehabilitative effect of intramuscular electrostimulation after reconstruction of medial patellar luxation in small sized dog. J Vet Clin. (2015) 32:16–21. doi: 10.17555/jvc.2014.02.32.1.16
37. Smith MJ, Cook JL, Kuroki K, Jayabalan PS, Cook CR, Pfeiffer FM, et al. Comparison of a novel bone-tendon allograft with a human dermis-derived patch for repair of chronic large rotator cuff tears using a canine model. Arthroscopy. (2012) 28:169–77. doi: 10.1016/j.arthro.2011.08.296
38. Spinella G, Davoli B, Musella V, Dragone L. Observational study on lameness recovery in 10 dogs affected by iliopsoas injury and submitted to a physiotherapeutic approach. Animals. (2021) 11:419. doi: 10.3390/ani11020419
39. Hoon QJ, Mouatt J, Corfield G, Moses PA. Surgical management of bilateral semitendinosus fibrotic myopathy and cranial cruciate ligament disease in a German shepherd dog. Australian Vet Pract. (2019) 49:5–10.
40. Hussain S, Jayaprakash R, Shafiuzama M, Nissar S, Sridhar R, George RS. Effects of early postoperative rehabilitation with physiotherapy in the cranial cruciate ligament ruptured dogs stabilized with extra capsular technique. Ind J Ani Res. (2019) 53:1104–8. doi: 10.18805/ijar.B-3624
41. Riley LM, Satchell L, Stilwell LM, Lenton NS. Effect of massage therapy on pain and quality of life in dogs: a cross sectional study. Vet Rec. (2021) 189:e586. doi: 10.1002/vetr.586
42. Gibson MA, Brown SG, Brown NO. Semitendinosus myopathy and treatment with adipose-derived stem cells in working German shepherd police dogs. Can Vet J-Revue Vet Can. (2017) 58:241–6.
43. Mocchi M, Bari E, Dotti S, Villa R, Berni P, Conti V, et al. Canine mesenchymal cell lyosecretome production and safety evaluation after allogenic intraarticular injection in osteoarthritic dogs. Animals. (2021) 11:3271. doi: 10.3390/ani11113271
44. Giza EG, Plonek M, Nicpon JM, Wrzosek MA. Electrodiagnostic studies in presumptive primary hypothyroidism and polyneuropathy in dogs with reevaluation during hormone replacement therapy. Acta Vet Scand. (2016) 58:32. doi: 10.1186/s13028-016-0212-9
45. Brown NP, Bertocci GE, States GJR, Levine GJ, Levine JM, Howland DR. Development of a canine rigid body musculoskeletal computer model to evaluate gait. Front Bioeng Biotechnol. (2020) 8:150. doi: 10.3389/fbioe.2020.00150
46. Hayes GM, Granger N, Langley-Hobbs SJ, Jeffery ND. Abnormal reflex activation of hamstring muscles in dogs with cranial cruciate ligament rupture. Vet J. (2013) 196:345–50. doi: 10.1016/j.tvjl.2012.10.028
47. Hyytiainen HK, Molsa SH, Junnila JJT, Laitinen-Vapaavuori OM, Hieim-Bjorkman AK. Developing a testing battery for measuring dogs' stifle functionality: the finnish canine stifle Index (Fcsi). Vet Rec. (2018) 183:324. doi: 10.1136/vr.104588
48. Petazzoni M, Buiatti M. Simultaneous fluoroscopic-guided tibial plateau leveling and fracture reduction for the concurrent treatment of chronic cranial cruciate ligament tear and comminuted diaphyseal fracture of the tibia and fibula in a dog. J Am Vet Med Assoc. (2019) 254:613–8. doi: 10.2460/javma.254.5.613
49. Ragetly CA, Griffon DJ, Hsu MKI, Klump LM, Hsiao-Wecksler ET. Kinetic and kinematic analysis of the right hind limb during trotting on a treadmill in labrador retrievers presumed predisposed or not predisposed to cranial cruciate ligament disease. Am J Vet Res. (2012) 73:1171–7. doi: 10.2460/ajvr.73.8.1171
50. Santarossa A, Gibson TWG, Kerr C, Monteith GJ, Durzi T, Gowland S, et al. Body composition of medium to giant breed dogs with or without cranial cruciate ligament disease. Vet Surg. (2020) 49:1144–53. doi: 10.1111/vsu.13434
51. Varcoe GM, Manfredi JM, Jackson A, Tomlinson JE. Effect of tibial plateau levelling osteotomy and rehabilitation on muscle function in cruciate-deficient dogs evaluated with acoustic myography. Comp Exerc Physiol. (2021) 17:435–45. doi: 10.3920/CEP200085
52. White DA, Harkin KR, Roush JK, Renberg WC, Biller D. Fortetropin inhibits disuse muscle atrophy in dogs after tibial plateau leveling osteotomy. PLoS ONE. (2020) 15:e0231306. doi: 10.1371/journal.pone.0231306
53. Yoon HY, Kim KH, Jeong SW. Clinical evaluation of tightrope cranial cruciate ligament technique for treatment of cranial cruciate ligament deficiency in dogs. J Vet Clin. (2012) 29:455–9.
54. Bockstahler B, Krautler C, Holler P, Kotschwar A, Vobornik A, Peham C. Pelvic limb kinematics and surface electromyography of the vastus lateralis, biceps femoris, and gluteus medius muscle in dogs with hip osteoarthritis. Vet Surg. (2012) 41:54–62. doi: 10.1111/j.1532-950X.2011.00932.x
55. Breitfuss K, Franz M, Peham C, Bockstahler B. Surface electromyography of the vastus lateralis, biceps femoris, and gluteus medius muscle in sound dogs during walking and specific physiotherapeutic exercises. Vet Surg. (2015) 44:588–95. doi: 10.1111/j.1532-950X.2014.12302.x
56. Fuglsang-Damgaard LH, Harrison AP, Vitger AD. Altered muscle activation in agility dogs performing warm-up exercises: an acoustic myography study. Comp Exerc Physiol. (2021) 17:251–62. doi: 10.3920/CEP190076
57. Vitger AD, Bruhn-Rasmussen T, Pedersen EO, Fuglsang-Damgaard LH, Harrison AP. The impact of water depth and speed on muscle fiber activation of healthy dogs walking in a water treadmill. Acta Vet Scand. (2021) 63:46. doi: 10.1186/s13028-021-00612-z
58. Hyytiainen HK, Blomvall L, Hautala M, Lappalainen AK. Reliability of a new bite force measure and biomechanics of modified long attack in police dogs. Animals. (2021) 11:874. doi: 10.3390/ani11030874
59. Andrada E, Reinhardt L, Lucas K, Fischer MS. Three-dimensional inverse dynamics of the forelimb of beagles at a walk and trot. Am J Vet Res. (2017) 78:804–17. doi: 10.2460/ajvr.78.7.804
60. Abdelhadi J, Wefstaedt P, Nolte I, Schilling N. Fore-aft ground force adaptations to induced forelimb lameness in walking and trotting dogs. PLoS ONE. (2012) 7:e52202. doi: 10.1371/journal.pone.0052202
61. Fischer S, Anders A, Nolte I, Schilling N. Compensatory load redistribution in walking and trotting dogs with hind limb lameness. Vet J. (2013) 197:746–52. doi: 10.1016/j.tvjl.2013.04.009
62. Fischer S, Nolte I, Schilling N. Adaptations in muscle activity to induced, short-term hindlimb lameness in trotting dogs. PLoS ONE. (2013) 8:e80987. doi: 10.1371/journal.pone.0080987
63. Fuchs A, Anders A, Nolte I, Schilling N. Limb and back muscle activity adaptations to tripedal locomotion in dogs. J Exp Zool A Ecol Genet Physiol. (2015) 323:506–15. doi: 10.1002/jez.1936
64. Goldner B, Fuchs A, Nolte I, Schilling N. Kinematic adaptations to tripedal locomotion in dogs. Vet J. (2015) 204:192–200. doi: 10.1016/j.tvjl.2015.03.003
65. Kutschenko A, Manig A, Mönnich A, Bryl B, Alexander CS, Deutschland M, et al. Intramuscular tetanus neurotoxin reverses muscle atrophy: a randomized controlled trial in dogs with spinal cord injury. J Cachexia Sarcopenia Muscle. (2022) 13:443–53. doi: 10.1002/jcsm.12836
66. Lehmann SV, Andrada E, Taszus R, Koch D, Fischer MS. Three-dimensional motion of the patella in French bulldogs with and without medial patellar luxation. BMC Vet Res. (2021) 17:76. doi: 10.1186/s12917-021-02787-z
67. Lorke M, Willen M, Lucas K, Beyerbach M, Wefstaedt P, Escobar HM, et al. Comparative kinematic gait analysis in young and old beagle dogs. J Vet Sci. (2017) 18:521–30. doi: 10.4142/jvs.2017.18.4.521
68. Schafer W, Hankel J. Energy consumption of young military working dogs in pre-training in Germany. Animals. (2020) 10:1753. doi: 10.3390/ani10101753
69. Söhnel K, Rode C, De Lussanet MHE, Wagner H, Fischer MS, Andrada E. Limb dynamics in agility jumps of beginner and advanced dogs. J Exp Biol. (2020) 223:jeb202119. doi: 10.1242/jeb.202119
70. Wachs K, Fischer MS, Schilling N. Three-dimensional movements of the pelvis and the lumbar intervertebral joints in walking and trotting dogs. Vet J. (2016) 210:46–55. doi: 10.1016/j.tvjl.2015.12.009
71. Monteiro BP, Lascelles BDX, Murrell J, Robertson S, Steagall PVM, Wright B. 2022 WSAVA guidelines for the recognition, assessment and treatment of pain. J Small Anim Pract. (2022). doi: 10.1111/jsap.13566. [Epub ahead of print].
72. Galindo-Zamora V, von Babo V, Eberle N, Betz D, Nolte I, Wefstaedt P. Kinetic, kinematic, magnetic resonance and owner evaluation of dogs before and after the amputation of a hind limb. BMC Vet Res. (2016) 12:20. doi: 10.1186/s12917-016-0644-5
Keywords: dogs, muscle function, methods, non-invasive, cranial cruciate ligament rupture
Citation: Dahl KH, Zebis MK, Vitger AD, Miles JE and Alkjær T (2023) Non-invasive methods to assess muscle function in dogs: A scoping review. Front. Vet. Sci. 10:1116854. doi: 10.3389/fvets.2023.1116854
Received: 15 December 2022; Accepted: 12 January 2023;
Published: 30 January 2023.
Edited by:
Isaac Karimi, Razi University, IranReviewed by:
Antonello Bufalari, Dipartimento di Medicina Veterinaria di Perugia, ItalyCopyright © 2023 Dahl, Zebis, Vitger, Miles and Alkjær. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathrine Højte Dahl,  a2F0aHJpbmVoZEBzdW5kLmt1LmRr
a2F0aHJpbmVoZEBzdW5kLmt1LmRr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.