
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Vet. Sci. , 06 February 2023
Sec. Veterinary Dentistry and Oromaxillofacial Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fvets.2023.1022838
 So Shirahata1,2‡
So Shirahata1,2‡ Naoki Iwashita1,3‡
Naoki Iwashita1,3‡ Rie Sasaki1
Rie Sasaki1 Ryota Nomura4†
Ryota Nomura4† Masaru Murakami5
Masaru Murakami5 Junya Yasuda6
Junya Yasuda6 Hidemi Yasuda7
Hidemi Yasuda7 Kuniyasu Nakajima8
Kuniyasu Nakajima8 Hiroaki Inaba9
Hiroaki Inaba9 Michiyo Matsumoto-Nakano9
Michiyo Matsumoto-Nakano9 Kazuhiko Nakano4
Kazuhiko Nakano4 Jumpei Uchiyama10
Jumpei Uchiyama10 Tomoki Fukuyama1*
Tomoki Fukuyama1*Previous research has demonstrated that Porphyromonas gulae (P. gulae) significantly contributes to the development of periodontal disease in dogs. Porphyromonas gulae is divided into three subtypes according to the 41-kDa filamentous appendage (fimA), defined as types A, B, and C. This study aimed to elucidate the association between fimA type of P. gulae with the number of permanent teeth, reflecting the severity of periodontal disease. Two hundred twenty-five dogs were categorized by P. gulae fimA type as negative, type A dominant, type B dominant, and type C dominant. The stage of periodontal disease in P. gulae-positive dogs increased with age, particularly in type C dominant dogs. Correspondingly, the number of permanent teeth in P. gulae fimA type C-dominant dogs was significantly lower than that of P. gulae-negative dogs, suggesting there is a significant association between fimA type of P. gulae and the number of permanent teeth resulting from the development of periodontal disease.
Periodontal disease is extremely common in dogs and is associated with serious systemic diseases (1–6). Studies have reported that 84% of Beagles over the age of 3 and 100% of Poodles over the age of 4 develop periodontal disease (5–9). Current living environment, type and frequency of dental care, and infectious diseases such as viral and bacterial diseases are involved in the progression of periodontal disease. However, the mechanisms underlying the development of periodontal disease in dogs require further investigation.
Recently, Porphyromonas gulae (P. gulae), a gram-negative black pigmented anaerobic bacterium, was identified as one of the possible pathogens that causes periodontal disease in dogs, and it causes the onset and exacerbation of periodontitis (10). Porphyromonas gulae can be subtyped into three groups according to the genotype of Fimbrillin (fimA; a pathogenic protein that forms fimbria on the surface of the bacterium): types A, B, and C (11, 12). FimA is expressed on the cell surface of P. gulae and plays a pivotal role in adherence to the teeth and may induce the pathogenic features of periodontitis (11, 12). Studies in a mouse abscess model demonstrated that type B causes significantly greater systemic inflammation than type A. Moreover, type C induces even higher systemic inflammation than the other genotypes (11, 12). Indeed, type C is considered to be the most virulent of the genotypes, as it is predominant in the oral cavities of dogs with severe periodontitis (12, 13). However, previous studies only demonstrated fimA Type C may be found more frequently in severe disease samples and have the ability to cause more inflammation than other types in a mouse model, this does not prove causation of periodontitis in dogs. Therefore, the association between fimA type and clinical features of periodontal disease is not yet fully understood. The present study aimed to elucidate the possible association between fimA types of P. gulae and the number of permanent teeth resulting from the severity of periodontal disease in dogs. We hypothesized that clinical features of canine periodontitis and the number of permanent teeth were linked with fimA types of P. gulae, as seen in the murine study described above, and that type B and C were more virulent than type A.
This study was performed cross-sectionally, and all protocols of this study were conducted in accordance with the Animal Care and Use Program of Azabu University (Approval No. 200318-1). The study was explained to all owners, who provided written informed consent for approval of their pets participation. Severity of periodontal disease and number of permanent teeth were compared between genotypes of fimA in each age group. Oral swab specimens were collected from the gingival margin of the maxillary right or left canine and fourth premolar using a microbrush (Microapplicator fine, FEED Corporation, Yokohama, Japan) as described previously (10). Detection of P. gulae and genotype of fimA were determined using previously described PCR-based methods (11, 12). Periodontal disease stage was grossly evaluated according to previously described criteria (American Veterinary Dental College. https://avdc.org/avdc-nomenclature/) under general anesthesia and categorized as follows: normal (clinically normal), Stage 1 (gingivitis only, without attachment loss), Stage 2 (early periodontitis), Stage 3 (moderate periodontitis), and Stage 4 (advanced periodontitis). Disease stage was determined based on the most severely affected tooth (typically the maxillary premolar). Clinicians participating in this study had longer than 10 years clinical experience and were trained and certified by the special course of Small animal dental society of Japan. For the periodontal disease stages and number of teeth, data are expressed as the mean ± standard error of mean. After the normality of dependent variables and homogeneity of variances have been confirmed by Shapiro-Wilk test and Brown-Forsythe test, respectively. The relation of periodontal disease stages and presence/absence of P. gulae were analyzed as a two-way analyses of variance (ANOVA) design (Type III sum-of-squares), followed by Šídák's multiple comparisons test. Secondary, the relation of periodontal disease stages and various genotype groups were analyzed as a two-way ANOVA design (Type III sum-of-squares), followed by Dunnett's multiple comparison tests (comparison with negative). For number of residual teeth, as the complete lack of variation in the <50 months P. gulae-negative group, to dichotomize the dependent variable as full dentition vs. <42 teeth and run the analysis as a logistic regression in only 50–100 months and >100 months age groups. Statistical significance was estimated at 5 and 1% levels of probability (Prism 9, GraphPad Software, San Diego, CA). Power analysis has also been performed to confirm the sample size of this study was appropriate. The proportion of various genotype groups in each age group were analyzed with a Chi square test. There was no significant interaction between factors in every ANOVAs.
The study was performed among 225 dogs (age 6–211 months, dog breed: toy poodle, chihuahua, dachshund, maltese, pomeranian, yorkshire terrier, miniature schnauzer, papillon, and mix) with mild-to-severe periodontal disease, at five Japanese animal clinics (Primo Animal Hospital Sagamiharachuo, Sagamiono Primo Animal Hospital, Atsugi Primo Animal Hospital, Yasuda Veterinary Clinic, and Nakajima Animal Hospital). Dogs with a recent (last 6 months) history of antibiotics usage were excluded from the study. Dogs were divided into three groups based on age: <50 months (~4 years; n = 50), 50–100 months (~4–8 years; n = 60), and >100 months (~8 years; n = 115). The proportion of P. gulae-positive dogs increased with age (Figure 1A). In particular, the proportion of P. gulae fimA type C-dominant dogs significantly increased with age compared to other genotypes (20% in <50 months group, >40% in 50–100 and >100 months groups). The proportion of P. gulae fimA type C-dominant dogs is higher in the 50–100 and >100 month groups vs. the <50 month group. When we compared the severity of periodontal disease between presence and absence of P. gulae, a significant increase was observed in dog with P. gulae compared to P gulae-negative dogs in all age groups. When we compared the severity of periodontal disease between genotypes (Figure 1B), a significant (compared to the negative group) increase was observed in dogs with dominant fimA type A and fimA type C compared to P. gulae-negative dogs in the <50-months age group (the PD stage of type C was twice as many as that of the negative group). In the 50–100-months age group, significant increases (~50% of the PD stage was increased compared to the negative group) were observed in fimA type C. In the >100-months group, the presence of all genotypes of P. gulae significantly increased the stage of periodontal disease compared to that in P. gulae-negative dogs. Strikingly, P. gulae type C infections significantly associated with periodontal disease stages at all ages directly or indirectly (there is a possibility other bacterium in the community could be being influenced by P. gulae). Finally, the association between the genotype of P. gulae and the number of permanent teeth was examined (Figure 1C). According to the results of a logistic regression analysis between full dentition and <42 teeth in 50–100 and >100 months age groups, the number of dogs with full dentition in P. gulae-positive groups decreased with age, however, a significant decrease was observed only in dogs with dominant fimA type B and C compared to P. gulae-negative dogs in the >100-months group (Figure 2 shows representative image of dogs in the 50–100 months). These findings indicate that there is a significant association between the presence of P. gulae and the number of permanent teeth.
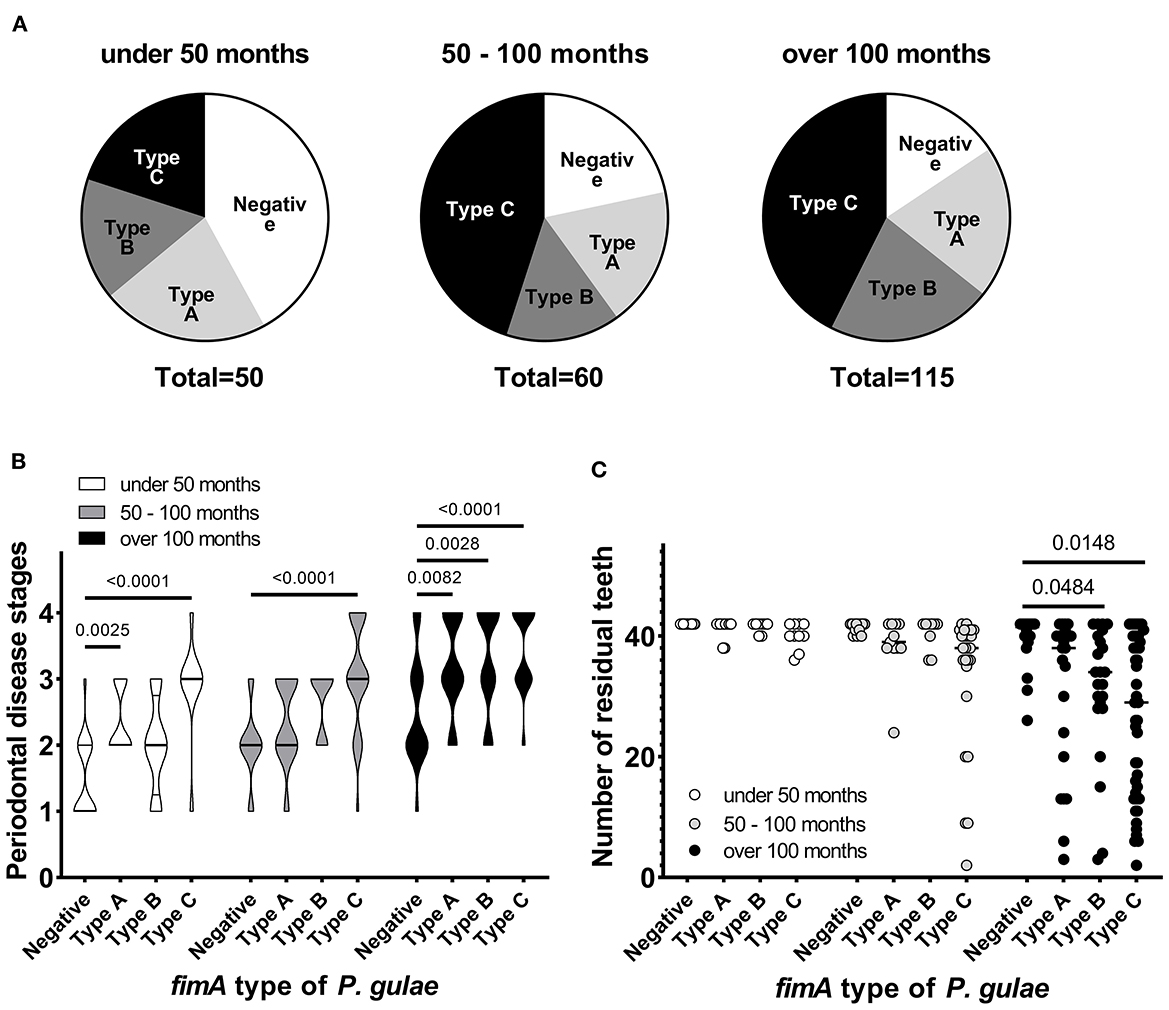
Figure 1. Contribution of fimA type of Porphyromonas gulae to periodontal disease. (A) Porphyromonas gulae carriage and associated genotype. (B) Association between periodontal disease stage and fimA type of P. gulae in each age group (Dunnett's multiple comparison test vs. the P. gulae-negative group). (C) Association between fimA type of P. gulae and the number of permanent teeth in each age group (a logistic regression analysis between full dentition and <42 teeth in 50–100 and >100 months age groups).
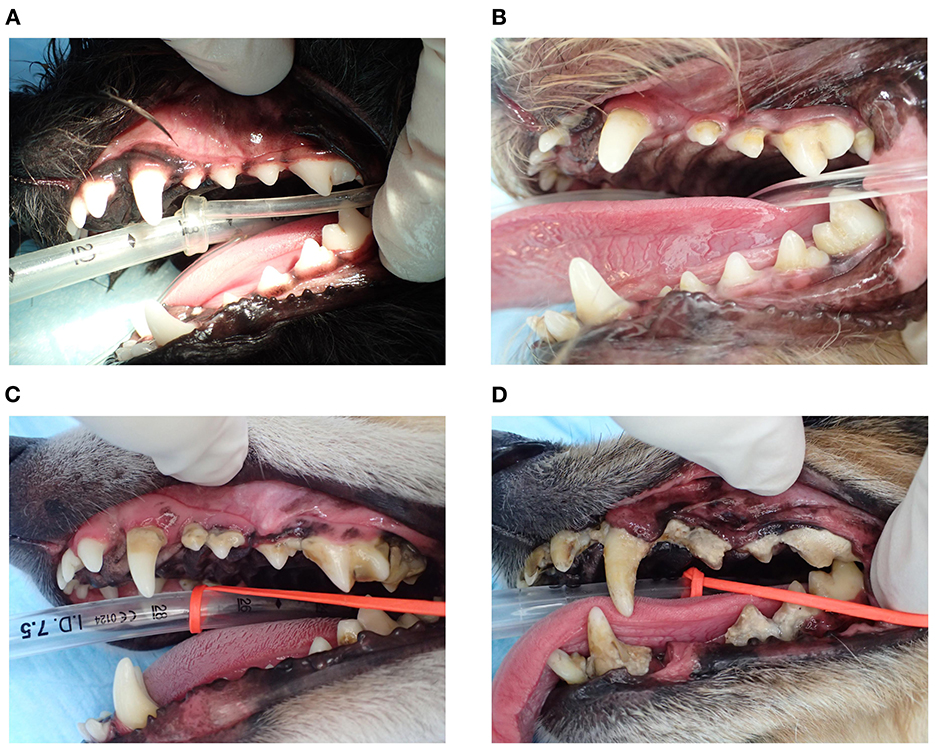
Figure 2. Representative image of gingival and teeth of dogs in the 50–100-months age group. (A) A subject negative for Porphyromonas gulae, (B) fimA -type A P. gulae, (C) fimA type B P. gulae, and (D) fimA type C P. gulae.
To the best of our knowledge, this is the first epidemiological study to demonstrate a link between the genotype of P. gulae and the number of permanent teeth as a clinical feature of periodontal disease. In the early life stage (under 50 months), there are significant impacts of fimA type C infection on periodontal disease stages compared to the negative group. Adverse impact of fimA type C was also demonstrated according to the significant increase of periodontal disease stage and the significant decrease in permanent teeth in older stages (50–100 months and over 100 months) within this genotype. On the other hand, it is still uncertain why fimA type C is increasing with advancing age since there is no longitudinal knowledge to know if fimA Type changes within an individual over time. The proportion of other types of fimA (A and B) is not influenced by age, therefore, it is unlikely fimA type A and B are changed to type C during life. Possible transition of fimA type within an individual over time is under investigation as a next step of this project in our group.
As an initial step, P. gulae fimA genotype should be diagnosed as early as possible, as this information could alert pet owners and veterinarians to take all necessary steps to control the development of the disease. Our findings clearly indicate that P. gulae genotype is associated with periodontal disease severity. Thus, the genotyping of P. gulae fimA type during basic check-ups might be a future option better to understand the oral environment. In all advanced cases of periodontal disease, appropriate dental care should be undertaken to avoid further disease development. It has been reported that P. gulae is susceptible to clindamycin, which has been approved for the treatment of periodontal disease in dogs (14, 15). Recently, our group showed that combination therapy using clindamycin and IFN-α treatment can improve periodontal condition and reduce P. gulae in dogs (13).
Taken together, our findings demonstrate that there is significant association between the presence of P. gulae fimA type C and the reduced number of permanent teeth resulting from the development of periodontal disease. However, it should be noted that there are several limitations in the current report including reliance on the score for the most severe tooth in the mouth to reflect periodontal disease stage, and no longitudinal knowledge to know if fimA Type changes within an individual over time.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ supplementary material.
The animal study was reviewed and approved by Animal Care and Use Program of Azabu University. Written informed consent was obtained from the owners for the participation of their animals in this study.
SS, NI, RN, MM, JY, HY, HI, MM-N, KN, JU, and TF contributed to the study conception and design. SS, NI, RS, RN, MM, JY, HY, HI, KN, MM-N, KN, JU, and TF performed material preparation, data collection, and analysis. TF wrote the first draft of the manuscript. All authors commented on the earlier versions of the manuscript, read, and approved the final manuscript.
We thank Fumitoshi Asai and Ayaka Kotemori for his valuable suggestions to this study. We also would like to thank Editage (www.editage.com) for English language editing.
NI was employed by Bioalchemis. JY was employed by SPECTRUM LAB. JAPAN Co., LTD. HY was employed by AlphaVets Co., LTD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Shirai M, Nomura R, Kato Y, Murakami M, Kondo C, Takahashi S, et al. Short communication: distribution of Porphyromonas gulae fimA genotypes in oral specimens from dogs with mitral regurgitation. Res Vet Sci. (2015) 102:49–52. doi: 10.1016/j.rvsc.2015.07.009
2. Kawabata Y, Ekuni D, Miyai H, Kataoka K, Yamane M, Mizutani S, et al. Relationship between prehypertension/hypertension and Periodontal disease: a prospective cohort study. Am J Hypertens. (2016) 29:388–96. doi: 10.1093/ajh/hpv117
3. Liccardo D, Cannavo A, Spagnuolo G, Ferrara N, Cittadini A, Rengo C, et al. Periodontal disease: a risk factor for diabetes and cardiovascular disease. Int J Mol Sci. (2019) 20:1414. doi: 10.3390/ijms20061414
4. Borsa L, Dubois M, Sacco G, Lupi L. Analysis the link between Periodontal diseases and Alzheimer's disease: a systematic review. Int J Environ Res Public Health. (2021) 18:9312. doi: 10.3390/ijerph18179312
5. Wallis C, Holcombe LJ. A review of the frequency and impact of periodontal disease in dogs. J Small Anim Pract. (2020) 61:529–40. doi: 10.1111/jsap.13218
6. Kouki MI, Papadimitriou SA, Kazakos GM, Savas I, Bitchava D. Periodontal disease as a potential factor for systemic inflammatory response in the dog. J Vet Dent. (2013) 30:26–9. doi: 10.1177/089875641303000103
7. Marshall MD, Wallis CV, Milella L, Colyer A, Tweedie AD, Harris S, et al. A longitudinal assessment of periodontal disease in 52 Miniature Schnauzers. BMC Vet Res. (2014) 10:166. doi: 10.1186/1746-6148-10-166
8. Hoffmann T, Gaengler P. Epidemiology of periodontal disease in poodles. J Small Anim Pract. (1996) 37:309–16. doi: 10.1111/j.1748-5827.1996.tb02396.x
9. Kortegaard HE, Eriksen T, Baelum V. Periodontal disease in research beagle dogs–an epidemiological study. J Small Anim Pract. (2008) 49:610–6. doi: 10.1111/j.1748-5827.2008.00609.x
10. Kato Y, Shirai M, Murakami M, Mizusawa T, Hagimoto A, Wada K, et al. Molecular detection of human periodontal pathogens in oral swab specimens from dogs in Japan. J Vet Dent. (2011) 28:84–9. doi: 10.1177/089875641102800204
11. Nomura R, Shirai M, Kato Y, Murakami M, Nakano K, Hirai N, et al. Diversity of fimbrillin among Porphyromonas gulae clinical isolates from Japanese dogs. J Vet Med Sci. (2012) 74, 885–91. doi: 10.1292/jvms.11-0564
12. Yamasaki Y, Nomura R, Nakano K, Inaba H, Kuboniwa M, Hirai N, et al. Distribution and molecular characterization of Porphyromonas gulae carrying a new fimA genotype. Vet Microbiol. (2012) 161:196–205. doi: 10.1016/j.vetmic.2012.07.026
13. Nomura R, Inaba H, Yasuda H, Shirai M, Kato Y, Murakami M, et al. Inhibition of Porphyromonas gulae and periodontal disease in dogs by a combination of clindamycin and interferon alpha. Sci Rep. (2020) 10:3113. doi: 10.1038/s41598-020-63861-4
14. Senhorinho GN, Nakano V, Liu C, Song Y, Finegold SM, Avila-Campos MJ, et al. Occurrence and antimicrobial susceptibility of Porphyromonas spp. and Fusobacterium spp in dogs with and without periodontitis. Anaerobe. (2012) 18:381–5. doi: 10.1016/j.anaerobe.2012.04.008
Keywords: dog, periodontal disease, Porphyromonas gulae, fimA type, number of permanent teeth
Citation: Shirahata S, Iwashita N, Sasaki R, Nomura R, Murakami M, Yasuda J, Yasuda H, Nakajima K, Inaba H, Matsumoto-Nakano M, Nakano K, Uchiyama J and Fukuyama T (2023) Possible association of fimA genotype of Porphyromonas gulae with the severity of periodontal disease and the number of permanent teeth in dogs. Front. Vet. Sci. 10:1022838. doi: 10.3389/fvets.2023.1022838
Received: 19 August 2022; Accepted: 12 January 2023;
Published: 06 February 2023.
Edited by:
Ana Nemec, University of Ljubljana, SloveniaReviewed by:
Rok Gašperšič, University of Ljubljana, SloveniaCopyright © 2023 Shirahata, Iwashita, Sasaki, Nomura, Murakami, Yasuda, Yasuda, Nakajima, Inaba, Matsumoto-Nakano, Nakano, Uchiyama and Fukuyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomoki Fukuyama,  dC1mdWt1eWFtYUBhemFidS11LmFjLmpw
dC1mdWt1eWFtYUBhemFidS11LmFjLmpw
†Present address: Ryota Nomura, Department of Pediatric Dentistry, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
‡These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.