
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Surg. , 07 June 2023
Sec. Visceral Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fsurg.2023.1184322
This article is part of the Research Topic Advances in Proctology and Colorectal Surgery View all 45 articles
 Giovanni Viel1*†
Giovanni Viel1*† Francesco A. Ciarleglio1*†
Francesco A. Ciarleglio1*† Marco Frisini1,‡
Marco Frisini1,‡ Stefano Marcucci1,‡
Stefano Marcucci1,‡ Stefano Valcanover1,‡
Stefano Valcanover1,‡ Emma Bragantini2,‡
Emma Bragantini2,‡ Mattia Barbareschi2,‡
Mattia Barbareschi2,‡ Liliana Mereu3,‡
Liliana Mereu3,‡ Saverio Tateo3,‡
Saverio Tateo3,‡ Elettra Merola4
Elettra Merola4 Franco Armelao4,‡
Franco Armelao4,‡ Giovanni De Pretis4,‡
Giovanni De Pretis4,‡ Marco Brolese5,‡
Marco Brolese5,‡ Nicola L. Decarli2,‡
Nicola L. Decarli2,‡ Alberto Brolese1,§
Alberto Brolese1,§
Appendiceal tumors are incidentally detected in 0.5% cases of appendectomy for acute appendicitis and occur in approximately 1% of all appendectomies. Here, we report two cases of appendiceal collision tumors in two asymptomatic women. In both cases, imaging revealed right-lower-quadrant abdominal masses, which were laparoscopically resected. In both cases, histological examinations revealed an appendiceal collision tumor comprising a low-grade appendiceal mucinous neoplasm and well-differentiated neuroendocrine neoplasm (NEN). For complete oncological control, right hemicolectomy was performed in one patient for the aggressive behavior of NEN; however, histology revealed no metastasis. The other patient only underwent appendectomy. No further treatment was recommended. According to the latest guidelines, exact pathology needs to be defined. Proper management indicated by a multidisciplinary team is fundamental.
Primary appendiceal tumors are rare entities in heterogeneous group of tumors, with an incidence of approximately 1.2 case per 100,000 people annually in the United States (1). They are most commonly found incidentally in a surgical specimen after appendectomy for acute appendicitis. However, their pathology and classification remain controversial. Hence, a new classification of these neoplasms was published in the World Health Organization (WHO) Classification of tumors, 5th edition, 2019 (2). Mucinous neoplasm and neuroendocrine neoplasm (NEN) are the most frequent benignant and malignant lesions (3).
When tumor components are composed by two adjacent, different but separate neoplasms from 2 different cellular lines, they are called collision tumor (3, 4). Appendiceal collision tumors are rare entities. Only 13 cases have been reported in the international literature to date. Here, we present two new cases comprising a low-grade appendix mucinous neoplasm (LAMN) and a well-differentiated NEN, which were managed differently.
A 49-year-old Caucasian woman with no significant medical history visited an ambulatory gynecology clinic for a routine check-up. Transvaginal ultrasonography revealed an oval mass with a mixed content measuring 74 mm × 44 mm in diameter, suggesting a dermoid cyst or an ovarian fibroma. Abdominal magnetic resonance imaging (MRI) also described a tumor close to the right ovarian gland (69 mm × 40 mm × 46 mm), with contrast enhancement in the arterial phase and clear margins afterward and a small nodulation inside (Figures 1A,B). Metastases or peritoneal deposits were not noted. Remarkably, Ca-125 and Ca 19-9 values were 10.7 and 42 U/ml (normal values: <35 and <37 U/ml), respectively. Thus, gynecologists performed laparoscopic surgery and found an appendiceal neoplasm intraoperatively. The surgery was completed with an appendectomy and a peritoneal biopsy performed by a general surgeon consultant. The specimen was removed through the umbilical port in an extraction bag, with no cystic lesion rupture. Intraoperative frozen sections indicated a LAMN. Macroscopically, the resected specimen showed an 8.5 cm-long appendix with a cystic neoformation measuring 8 cm × 5.5 cm × 5 cm, with mucinous content. At 1 cm proximal to the appendiceal cecal margin, another yellow node measuring 2.1 cm in diameter was detected. On histological examination, the bigger mass was described as a LAMN with acellular mucus confined to the wall (TNM Classification 8th edition 2016: pTis), whereas the smaller nodule was described as a NEN G1, characterized by mesenteric fat and visceral serous membrane involvement measuring 0.9 and 0.5 mm, respectively (Figure 1C). Perineural tumoral invasion without angiolymphatic invasion was observed. Immunohistochemical analysis revealed positivity for cromogranin A (Cg A) (Figure 1D) and synaptophysin, with a Ki-67 proliferation index of 0.4% (Figure 1E) (TNM Classification 8th edition 2016: pT2G1). Moreover, peritoneal biopsy was negative for tumor seeding. No complications occurred, and the patient was discharged on postoperative day (POD) 4. A multidisciplinary team analyzed the case and decided to perform segmental colectomy with lymph node dissection Finally, robot-assisted right hemicolectomy was performed. On POD 5, the patient was discharged after a regular postoperative course. Histologically, the specimen had no residual tumor and no nodal involvement (19 nodes). No adjuvant therapy was recommended. At 6 and 12 months follow-up, total body CT scan and assessment of serological markers showed no evidence of recurrence.
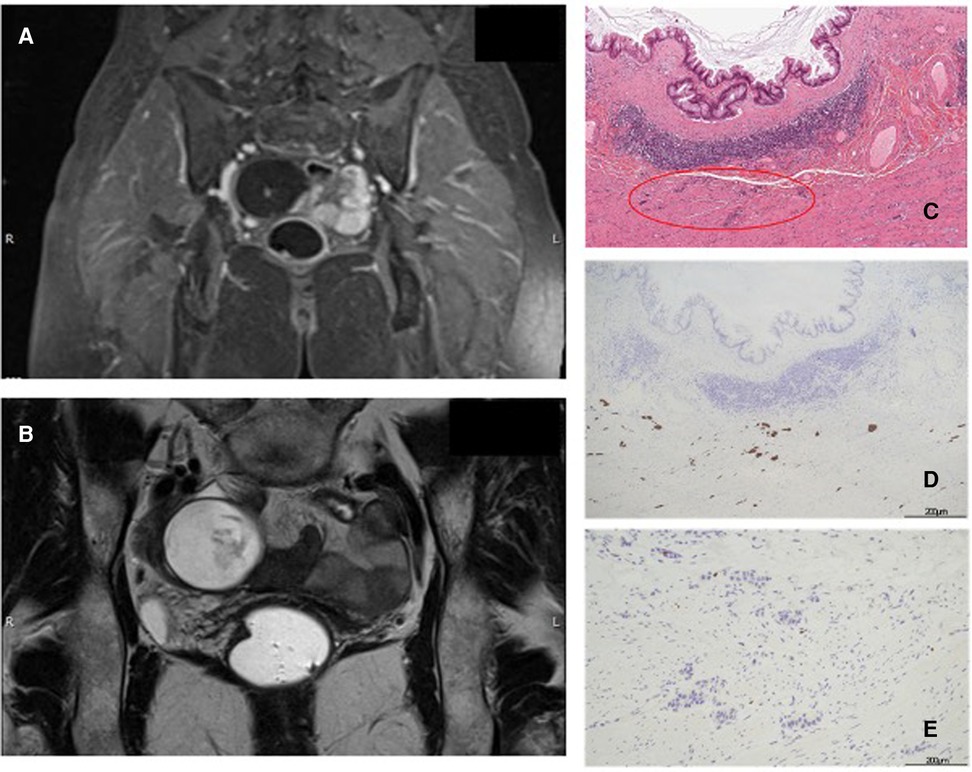
Figure 1. (A,B): Abdominal MR T1 and T2-weighted images. (C): LAMN and NEN (red circle), hematoxylin and eosin (H & E) staining 10×. (D): Immunohistochemistry positive for Cg A. (E): Immunohistochemistry for Ki-67: proliferation index of 0.4%.
A 59-year-old Caucasian asymptomatic woman underwent an abdominal ultrasound which revealed a right pelvic mass. Her Ca-125 value was 3.2 U/ml (normal value: < 35 U/ml). Abdominal MRI revealed a cystic oval mass [diameters 3.7 cm × 4.5 cm × 6.4 cm; hyperintense in T2-weighted images (Figure 2A) and hypointense in T1-weighted images Figure 2B)] in the right uterus space. Final radiological diagnosis was hydrosalpinx. Hence, the patient underwent laparoscopic surgical treatment. A general consultant surgeon performed appendectomy and appendiceal tumor (diameter 5 cm) with a smooth surface and stretched elastic consistency was found. No pelvic organ was involved. Subsequently, the patient demonstrated no complications, and on POD 2, she was discharged.
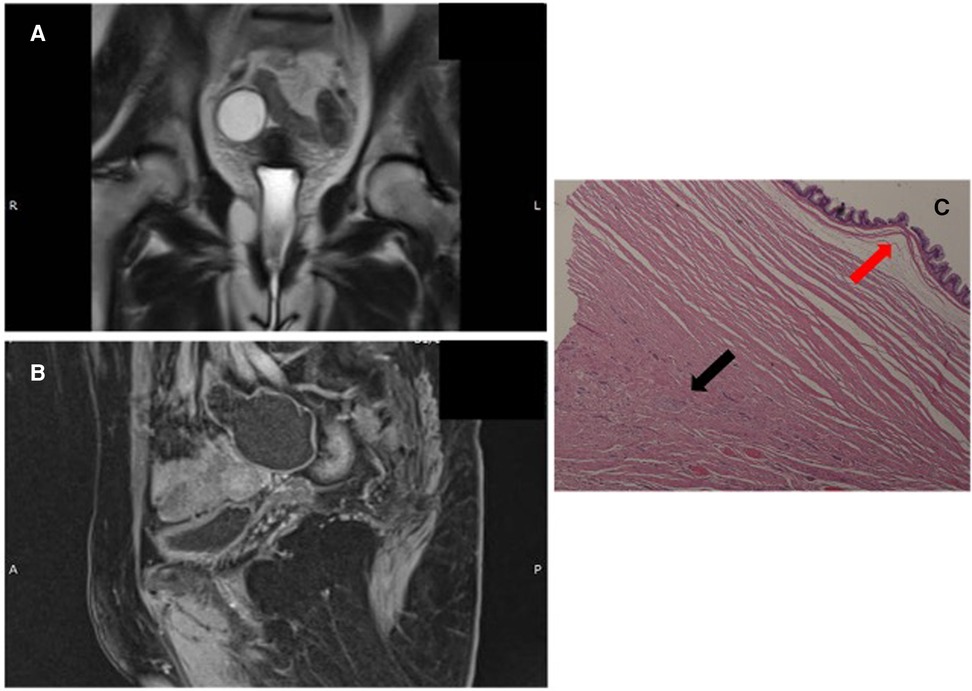
Figure 2. (A,B): abdominal MR T2- and T1-weighted images. (C): LAMN (red arrow) and NEN G1 (black arrow), (hematoxylin and eosin (H & E) staining).
Gross morphology of the resected specimen showed a 9 cm-long cyst-like dilated appendix measuring 6 cm in diameter. The appendix was filled with thick mucus. Histologically (Figure 2C), the specimen appeared to be a LAMN with a fully thick mucus on the appendicular wall, but no peri-appendicular adipose tissue was involved (TNM Classification 8th edition 2016: pTis). A NEN G1 (9 mm × 7 mm) limited to the muscularis layer was identified in the proximal section of the appendix, with no serous and perivisceral fat invasion and no vascular or perineural neoplastic invasion. However, on immunohistochemical evaluation, Cg A and synaptophysin were positive. The Ki-67 proliferation index was 1% (TNM Classification 8th edition 2016: pT1G1). Additionally, the specimen had negative surgical margins. The multidisciplinary team did not recommend any adjuvant therapy. At 6 and 12 months follow-up, total body CT scan, abdominal ultrasound, and serological markers' assessment showed no evidence of recurrence.
Appendiceal tumors are extremely rare entities, usually detected incidentally following an emergent appendectomy for acute appendicitis in approximately 1% (1) of cases and occurring in approximately 1%–2% of all appendectomies (5).
Incidental diagnosis of asymptomatic patients in the course of another examination is relatively common, as noted in the two cases described.
According to the 5th edition of the WHO classification (2), appendiceal tumors are classified into several histological types, such as serrated lesions and polyps, mucinous neoplasms, adenocarcinomas, goblet cell adenocarcinoma, and NEN.
The mucinous tumors of the appendix are categorized into serrated polyps, hyperplastic polyps, LAMNs, high-grade appendiceal mucinous neoplasms (HAMNs), and mucinous adenocarcinomas (2, 6). Mucinous neoplasms are characterized by a dilated appendix containing luminal mucin. High secretion by these tumors can cause appendiceal rupture and tumoral cell dissemination in the peritoneal cavity. LAMNs are among the most common borderline neoplasms of the appendix, with an incidence of 0.3% in a recent series of appendectomy specimens (5). Histological examination show high-grade atypical glands with an infiltrative pattern extended through the muscularis mucosae. LAMNs comprise well-differentiated glands inside the muscularis mucosae, with dissecting mucin or epithelium and they do not exhibit infiltrative epithelial invasion of the appendiceal wall (2, 7–10).
Moreover, among the most common types of primary malignant lesion of the appendix are appendiceal NENs, with an incidence of approximately 0.15 per 100,000 people annually (11). The Ki-67 index determines the tumor grading according to the WHO and European Neuroendocrine Tumor Society classifications (2, 12). Generally, neuroendocrine tumors (NET) of the appendix are either G1 (more than 80%) (13) or G2 (14). These neoplasms appear as yellowish, well-demarcated nodules arising in any part of the appendix. Microscopically, they have uniform polygonal tumor cells frequently arranged in large nests (2).
Collision tumors results from the proliferation cellular lines. They are two distinct but adiacent neoplasms, retaining a transition between the two. Otherwise, a multidirectional differentiation of cells from a single tumor results in a combined neoplasm (3, 4).
The association between mucinous and neuroendocrine appendiceal tumors is an uncommon event with only few cases described (15). We found only 13 cases in 10 papers on PubMed research (Tables 1A,B). Our cases are appendiceal collision tumors, because both showed histologically distinct type of neoplastic cells with epithelial and neuroendocrine origin occurring in the same region the components, although intimately juxtaposed, are not intermixed and do not show transition, consistent with Singh NG et al.'s definition (16). The first case was of a LAMN containing acellular mucus confined to the wall; it was associated with a smaller NEN G1 nodule with mesenteric fat and visceral serous membrane involvement measuring 0.9 and 0.5 mm, respectively (Figure 1C). Perineural tumoral invasion without angiolymphatic invasion was also evident. In the second case, histological examination (Figure 2C) showed a LAMN with fully thick mucus on the appendicular wall; however, we did not observe the involvement of periappendicular adipose tissue associated with NEN G1, which was limited to the muscularis layer without serous, perivisceral, and vascular invasion. The mean age at diagnosis of patients with appendiceal collision tumors is 43 ± 12 years (23–60 years), with prevalence in women (8/5).
Clinical presentation is not specific and is characterized by a wide spectrum of findings and symptoms. Patients may have specific symptoms of clinical acute appendicitis or colorectal carcinoma syndrome or even nonspecific symptoms. The diagnosis is usually made incidentally in the course of another examination. Our patients did not report any symptoms, including NEN-related symptoms (weight loss, diarrhoea, or cutaneous flushing).
The role of tumor markers is still insufficiently defined. An elevated serum carcinoembryonic antigen (CEA) level was reported in 3 cases of the literature (15, 16, 34). In our study, only Case 1 had slightly elevated CA 19-9 levels.
Preoperative diagnosis of appendiceal collision tumor is often incidental because this entity has no special radiological or clinical features (17). An eventual preoperative biopsy generally detects only one histological component, and it may only identify a mixed histology in only one-third of cases (18). Incidental radiological findings of a pelvic mass could be the first evidence of the disease in asymptomatic patients. CT scan is the gold standard preoperative diagnostic imaging test; it shows a cystic mass of liquid density adjacent to the caecum and at a retrocecal location in most cases (19). Unfortunately, mass dimensions and radiological characteristics on CT scan and MRI in some cases cannot identify the origin of tumors, particularly if the origin is ovarian or appendicular (20). In both our cases, radiological findings were compatible with both origins, and the final evidence of an appendiceal disease was determined only during surgery.
Gold standard treatment is surgery for selected case. Laparoscopic approach appears to be a safe and feasible option for not advanced cases (15). Appendectomy alone is the treatment of choice when benign lesions, such as adenoma or LAMN with negative margins and NEN of <1 cm, are present (21–23). In adenocarcinoma or NEN of >2 cm with the involvement of the appendiceal base, segmental colectomy with lymph node dissection for tumor staging is indicated (5, 21, 25). Right hemicolectomy should also be considered in NEN of 1 cm–2 cm with serosal involvement, Ki-67 proliferative index of >2%, location at the base of the appendix, and angioinvasion or neuroinvasion (5, 12, 21–25).
Initially, we performed a laparoscopic appendectomy with peritoneum biopsy in one case. Through the laparoscopic exploration, a pseudomyxoma peritonei was excluded. Postoperative morbidity was not observed. The effect of two different histological components increases the complexity of therapeutic approach because it is not yet clear whether biological behavior depends on a larger or more aggressive component (17).
Histologic findings are relevant to the prognosis and treatment of patients, and the management of collision tumors is guided by component neoplasms (25). Generally, the more aggressive histological pattern determines the clinical evolution of the disease (26). Duffy et al. (27) suggested that the treatment should be more aggressive in a collision tumor with major neuroendocrine components and high grading. Therefore, in relation to the pathology, we performed a simple appendectomy in one case and a minimally invasive right hemicolectomy for the more aggressive behaviour of NEN in the other case.
The most important factor for improving the outcome is early and accurate diagnosis with adequate histopathological examination to confirm the presence of two components within the same neoplasm (28). Immunohistochemical tests are the cornerstone in identifying a large number of these tumors, from adenomas or adenocarcinomas with several neuroendocrine cells to classical neuroendocrine tumors with focal exocrine/epithelial elements (17).
Moreover, adjuvant chemotherapy for collision tumor has not been evaluated in prospective randomized trials. Adjuvant chemotherapy is not recommended for low-grade, well-differentiated mucinous tumors and should only be considered in cancers with invasive features such as lymphovascular or lymph node involvement (29). Prevention or delayed neuroendocrine syndrome is not supported by randomized evidence from the perioperative setting of pure G2 or G3 NENs (30). However, advanced appendiceal NEN treatment with somatostatin analogs (SSAs) as the first-line approach is associated with more prolonged progression-free survival; however, in patients with a progressive disease despite receiving treatment with SSAs, further therapeutic modalities may include temozolomide-based chemotherapy (30, 31).
In previous studies, recurrent disease was only found in 3 patients with metastasis at the first operation (16, 26, 32, 33).
Long-term surveillance and follow-up are necessary for both tumor types according to final pathological reports. However, there are no suggested guidelines for an optimal postoperative follow-up (15).
Appendiceal collision tumors are rare diseases; therefore, they continue to be challenging for physicians. Unfortunately, the small sample size of this study does not allow for definitive conclusions to be made. Considering the controversy relating to its definition, the limited diagnostic ability of biopsies, and the lack of awareness of this diagnosis within the scientific community, the disease remains underestimated. Currently, no shared guidelines are available. Moreover, the definitive diagnosis can be achieved only after surgery because NEN could be overlooked during diagnosis because of its small dimension. Therefore, each patient must be managed case by case, and a multidisciplinary team, including gynecologists, surgeons, radiologist, oncologist and pathologists with expertise in NENs, is important for appropriate management of patients. This approach involves various health professionals from different organizations to provide utmost care and advanced treatment to patients based on latest available insights into the disease.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by APSS Santa Chiara Hospital Trento, Italy. The patients/participants provided their written informed consent to participate in this study.
Conceptualization, GV; Data curation, EM, FA and GDP; Formal analysis, MF and NDL; Investigation, SM and MB; Validation, SV, EB, MB, LM and ST; Writing—review & editing, FC and AB. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Van de Moortele M, De Hertogh G, Sagaert X, Van Cutsem E. Appendiceal cancer: a review of the literature. Acta Gastroenterol Belg. (2020) 83(3):441–8.33094592
2. Ahadi M, Sokolova A, Brown I, Chou A, Gill AJ. The 2019 world health organization classification of appendiceal, colorectal and anal canal tumours: an update and critical assessment. Pathology. (2021) S0031–3025(20):31020–5. doi: 10.1016/j.pathol.2020.10.010
3. Villa M, Sforza D, Siragusa L, Guida AM, Ciancio Manuelli M, Pirozzi BM, et al. A low-grade appendiceal mucinous neoplasia and neuroendocrine appendiceal collision tumor: a case report and review of the literature. Am J Case Rep. (2021) 22:e927876. doi: 10.12659/AJCR.927876
4. Pecorella I, Memeo L, Ciardi A, Rotterdam H. An unusual case of colonic mixed adenoendocrine carcinoma: collision versus composite tumor. A case report and review of the literature. Ann Diagn Pathol. (2007) 11(4):285–90. doi: 10.1016/j.anndiagpath.2006.03.011
5. Inoue A, Murata K, Komori T, Takeda T, Fujii M, Yamaguchi T, et al. Open versus laparoscopic surgery for primary appendiceal tumors: a large multicenter retrospective propensity score-matched cohort study in Japan. Surg Endosc. (2021) 35(10):5515–23. doi: 10.1007/s00464-020-08046-w
6. Carr NJ, Bibeau F, Bradley RF, Dartigues P, Feakins RM, Geisinger KR, et al. The histopathological classification, diagnosis and differential diagnosis of mucinous appendiceal neoplasms, appendiceal adenocarcinomas and pseudomyxoma peritonei. Histopathology. (2017) 71(6):847–58. doi: 10.1111/his.13324
7. Fish R, Renehan AG, Punnett G, Aziz O, Fulford P, Selvasekar C, et al. Referral and treatment pathways for pseudomyxoma peritonei of appendiceal origin within a national treatment programme. Colorectal Dis. (2018) 20(10):888–96. doi: 10.1111/codi.14310
8. Hajjar R, Dubé P, Mitchell A, Sidéris L. Combined mucinous and neuroendocrine tumours of the appendix managed with surgical cytoreduction and oxaliplatin-based hyperthermic intraperitoneal chemotherapy. Cureus. (2019) 11(1):e3894. doi: 10.7759/cureus.3894
9. Overmann MJ, Asare EA, Compton CC. Appendix carcinoma. In: Amin MB, Edge SB, Greene F, Compton CC, Gershenwald JE, Brookland RK, et al. editors. AJCC Cancer staging manual. 8th ed. Chicago: Springer; (2017). p. 237–50.
10. Rymer B, Forsythe RO, Husada G. Mucocoele and mucinous tumours of the appendix: a review of the literature. Int J Surg. (2015) 18:132–5. doi: 10.1016/j.ijsu.2015.04.052
11. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One hundred years after “carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. (2008) 26(18):3063–72. doi: 10.1200/JCO.2007.15.4377
12. Pape UF, Niederle B, Costa F, Gross D, Kelestimur F, Kianmanesh R, et al. ENETS Consensus guidelines for neuroendocrine neoplasms of the appendix (excluding goblet cell carcinomas). Neuroendocrinology. (2016) 103(2):144–52. doi: 10.1159/000443165
13. Volante M, Grillo F, Massa F, Maletta F, Mastracci L, Campora M, et al. Neuroendocrine neoplasms of the appendix, colon and rectum. Pathologica. (2021) 113(1):19–27. doi: 10.32074/1591-951X-230
14. Shen C, Yin Y, Chen H, Tang S, Yin X, Zhou Z, et al. Neuroendocrine tumors of colon and rectum: validation of clinical and prognostic values of the world health organization 2010 grading classifications and European neuroendocrine tumor society staging systems. Oncotarget. (2017) 8(13):22123–34. doi: 10.18632/oncotarget.13641
15. Tan HL, Tan GH, Teo M. Two rare cases of appendiceal collision tumours involving an appendiceal mucinous neoplasm and carcinoid. BMJ Case Rep. (2016) 2016:bcr2015213938. doi: 10.1136/bcr-2015-213938
16. Singh NG, Mannan AA, Kahvic M, Nur AM. Mixed adenocarcinoma-carcinoid (collision tumor) of the appendix. Med Princ Pract. (2011) 20(4):384–6. doi: 10.1159/000324870
17. Schizas D, Katsaros I, Michalinos A, Damaskos C, Garmpis N, Ntomi V, et al. Collision tumors of the gastrointestinal tract: a systematic review of the literature. Anticancer Res. (2018) 38(11):6047–57. doi: 10.21873/anticanres.12955
18. Karkouche R, Bachet JB, Sandrini J, Mitry E, Penna C, Côté JF, et al. Colorectal neuroendocrine carcinomas and adenocarcinomas share oncogenic pathways. A clinico-pathologic study of 12 cases. Eur J Gastroenterol Hepatol. (2012) 24(12):1430–7. doi: 10.1097/MEG.0b013e3283583c87
19. Govaerts K, Lurvink RJ, De Hingh IHJT, Van der Speeten K, Villeneuve L, Kusamura S, et al. Appendiceal tumours and pseudomyxoma peritonei: literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur J Surg Oncol. (2021) 47(1):11–35. doi: 10.1016/j.ejso.2020.02.012
20. Dellaportas D, Vlahos N, Polymeneas G, Gkiokas G, Dastamani C, Carvounis E, et al. Collision tumor of the appendix: mucinous cystadenoma and carcinoid. A Case Report. Chirurgia (Bucur). (2014) 109(6):843–5.25560511
21. Matias-García B, Mendoza-Moreno F, Blasco-Martínez A, Busteros-Moraza JI, Diez-Alonso M, Garcia-Moreno Nisa F. A retrospective analysis and literature review of neoplastic appendiceal mucinous lesions. BMC Surg. (2021) 21(1):79. doi: 10.1186/s12893-021-01091-9
22. Guaitoli E, Gallo G, Cardone E, Conti L, Famularo S, Formisano G, et al. Consensus statement of the Italian polispecialistic society of young surgeons (SPIGC): diagnosis and treatment of acute appendicitis. J Invest Surg. (2021) 34(10):1089–103. doi: 10.1080/08941939.2020.1740360
23. Ussia A, Vaccari S, Gallo G, Grossi U, Ussia R, Sartarelli L, et al. Laparoscopic appendectomy as an index procedure for surgical trainees: clinical outcomes and learning curve. Updates Surg. (2021) 73(1):187–95. doi: 10.1007/s13304-020-00950-z
24. Marano L, D'Ignazio A, Cammillini F, Angotti R, Messina M, Marrelli D, et al. Comparison between 7th and 8th edition of AJCC TNM staging system for gastric cancer: old problems and new perspectives. Transl Gastroenterol Hepatol. (2019) 4:22. doi: 10.21037/tgh.2019.03.09
25. Sholi AN, Gray KD, Pomp A. Management and outcome of an appendiceal collision tumour composed of neuroendocrine and mucinous neoplasms. BMJ Case Rep. (2019) 12(7):e229414. doi: 10.1136/bcr-2019-229414
26. Baena-del-Valle J, Palau-Lázaro M, Mejía-Arango M, Otero J, Londoño-Schimmer E, Cortes N, et al. Well differentiated neuroendocrine tumor of the appendix and low-grade appendiceal mucinous neoplasm presenting as a collision tumor. Rev Esp Enferm Dig. (2015) 107(6):396–8.26031879
27. Duffy A, Shia J, Klimstra D, Temple L, O'Reilly EM. Collision tumor of the large bowel in the context of advanced pregnancy and ulcerative colitis. Clin Colorectal Cancer. (2008) 7(6):402–5. doi: 10.3816/CCC.2008.n.054
28. Kanthan R, Tharmaradinam S, Asif T, Ahmed S, Kanthan SC. Mixed epithelial endocrine neoplasms of the colon and rectum—an evolution over time: a systematic review. World J Gastroenterol. (2020) 26(34):5181–206. doi: 10.3748/wjg.v26.i34.5181
29. Shaib WL, Assi R, Shamseddine A, Alese OB, Staley C 3rd, Memis B, et al. Appendiceal mucinous neoplasms: diagnosis and management. Oncologist. (2017) 22(9):1107–16. doi: 10.1634/theoncologist.2017-0081 Erratum in: Oncologist.28663356
30. Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, et al. ENETS Consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. (2016) 103(2):186–94. doi: 10.1159/000443172
31. Alexandraki KI, Kaltsas GA, Grozinsky-Glasberg S, Chatzellis E, Grossman AB. Appendiceal neuroendocrine neoplasms: diagnosis and management. Endocr Relat Cancer. (2016) 23(1):R27–41. doi: 10.1530/ERC-15-0310
32. Sugarbaker PH, Ben-Yaacov A, Hazzan D, Nissan A. Synchronous primary neuroendocrine and mucinous epithelial tumors present in the same appendix. Case report of 2 patients. Int J Surg Case Rep. (2020) 67:76–9. doi: 10.1016/j.ijscr.2020.01.022
33. Rossi G, Bertolini F, Sartori G, Bigiani N, Cavazza A, Foroni M, et al. Primary mixed adenocarcinoma and small cell carcinoma of the appendix: a clinicopathologic, immunohistochemical, and molecular study of a hitherto unreported tumor. Am J Surg Pathol. (2004) 28(9):1233–9. doi: 10.1097/01.pas.0000128666.89191.48
34. Ekinci N, Gün E, Avcı A, Er A. Coexistence of low-grade mucinous neoplasm and carcinoid (collision tumor) within multiple appendiceal diverticula: a case report. Turk J Surg. (2018) 31:1–3. doi: 10.5152/turkjsurg.2018.3877
35. Cafaro MA, Yaryura Montero JG, Bianco A, Petersen ML, Cárdenas Villa RD, Cardozo Dutra B, et al. Tumor de colisión apendicular: neoplasia epitelial mucinosa y tumor neuroendocrino [appendiceal collision tumor: mucinous epithelial neoplasm and neuroendocrine tumor]. Rev Fac Cien Med Univ Nac Cordoba. (2020) 77(2):113–6. Spanish. doi: 10.31053/1853.0605.v77.n2.25071
Keywords: appendiceal tumors, collision tumor, low-grade appendiceal mucinous neoplasm, neuroendocrine neoplasm NEN, appendectomy
Citation: Viel G, Ciarleglio FA, Frisini M, Marcucci S, Valcanover S, Bragantini E, Barbareschi M, Mereu L, Tateo S, Merola E, Armelao F, De Pretis G, Brolese M, Decarli NL and Brolese A (2023) Appendiceal collision tumors: case reports, management and literature review. Front. Surg. 10:1184322. doi: 10.3389/fsurg.2023.1184322
Received: 11 March 2023; Accepted: 10 May 2023;
Published: 7 June 2023.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
Yogesh Vashist, Asklepios Tumorzentrum Hamburg, Asklepios Klinik Altona, Germany© 2023 Viel, Ciarleglio, Frisini, Marcucci, Valcanover, Bragantini, Barbareschi, Mereu, Tateo, Merola, Armelao, De Pretis, Brolese, Decarli and Brolese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco A. Ciarleglio ZnJhbmNlc2NvLmNpYXJsZWdsaW9AYXBzcy50bi5pdA== Giovanni Viel Z2lvdmFubmkudmllbEBhcHNzLnRuLml0
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
§Last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.