
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Surg. , 07 September 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.971068
Background: Although the incidence of a single meningioma or a single aneurysm is common, cases of multiple meningiomas combined with multiple aneurysms are rarely reported, and surgical treatment of the coexisting situation is also relatively uncommon.
Case presentation: A 38-year-old male patient presented to the neurosurgery department of our center with a headache. Examination revealed only symptoms of headache. Laboratory tests showed only decreased total protein and albumin. Magnetic resonance imaging showed preoccupation with the frontal lobe and the right temple bone. Magnetic resonance angiography and digital subtraction angiography showed two aneurysms in the anterior communicating artery and right anterior cerebral artery. Based on a combination of the patient’s history and imaging, we hypothesized that the patient was simultaneously suffering from meningioma and an aneurysm, and both of them are multiple. The patient underwent tumor resection and clipping procedure based on this hypothesis in one surgery. Intraoperative biopsy proved to be a meningioma. The patient was discharged on the 10th postoperative day, and a postoperative follow-up suggested no complications.
Conclusion: Multiple meningiomas combined with multiple aneurysms are rare to be reported in the same patient. For those unruptured intracranial aneurysms (UIAs) located in the visual field of craniotomy prepared for brain tumorlike meningioma, it is possible to do the clipping as well. When the meningiomas are multiple, fitted with the surgical indication, and located in a position that cannot be treated in one surgery, this may lead to a two-stage operation, no matter where the UIAs are located.
Although intracranial tumor seems to have totally different pathogenesis from cerebral vascular diseases like aneurysm, there are still scattered reports about the coexistence of meningiomas and aneurysms (1–10). The incidence of meningioma is about 0.5% (8). The rate of occurrence of multiple meningiomas is 8%. The existence of multiple meningiomas and multiple aneurysms is even rarer to be reported.
Here, we treat a patient with three meningiomas and three aneurysms, and we discuss the etiology based on the clinical and histological findings.
A literature review was performed. The PubMed database was screened for meningioma and aneurysm cases from 1997 to 2022 according to the PRISMA using the following key terms: “meningioma and aneurysm,” “meningioma co-exist with aneurysm,” and “multiple meningiomas and multiple aneurysms (Table 1).”
A 38-year-old man was admitted to the neurosurgery department of our center for treatment of headaches, complaining of a 1-week history of headaches. Prior to admission, a magnetic resonance imaging (MRI) scan had been performed at the previously visited hospital, and no invasive methods or medications were administered to the patient. On admission, we performed routine laboratory tests such as blood count, liver and kidney function, and coagulation. The patient had normal muscle strength, normal sensation, and good function of intracranial nerves on physical examination. Total protein was 63.9 g/L, and albumin was 38.5 g/L.
On the first day after admission, an MRI scan was performed on the patient.
The size of skull base cerebral occupancy was 41 × 31 mm, and the falx occupancy was 10 × 10 mm. The signal of two occupancies showed isointensity in both T1 and T2. The enhanced MRI report showed an unbalanced enhancement of the occupancy; the center of the occupancy was not enhanced (Figures 1–3). We also found a 7 × 6 mm enhancing node under the right temple bone.
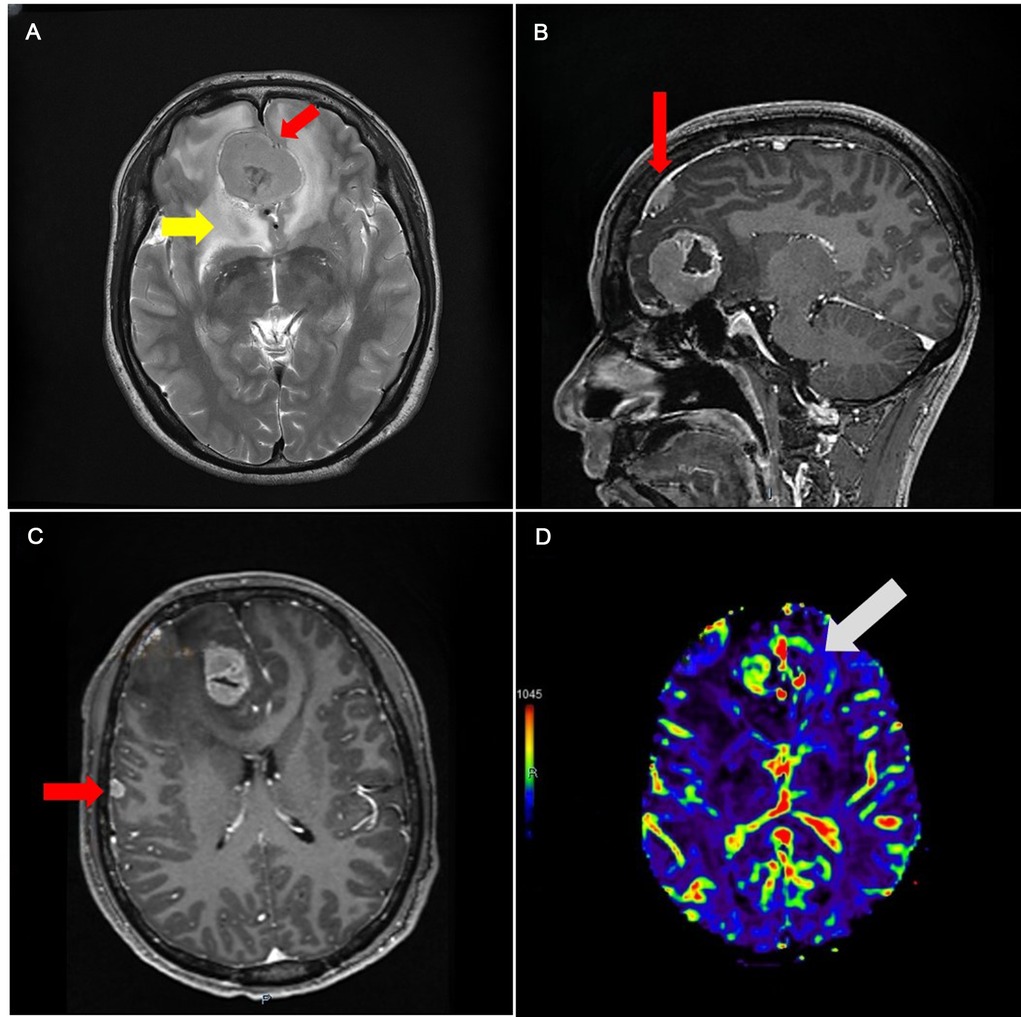
Figure 1. (A) Magnetic resonance image (MRI) image showing isointensity occupancy in T2 series with a clear border compared to the brain tissue (red arrowhead), surrounded by edema (yellow arrowhead). (B) Enhanced series showing the falx (red arrowhead). (C) Lesion with fair enhancement under the right temple bone. (D) CBV showing that the skull base area has more but not fair blood volume.
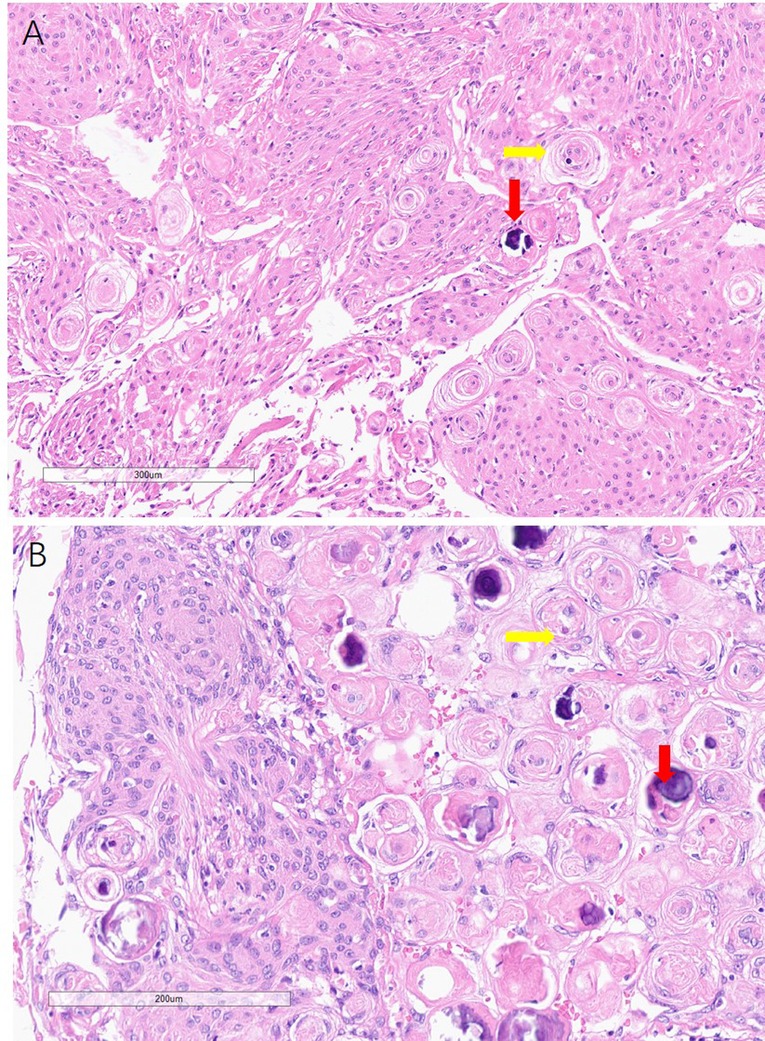
Figure 3. Histology of the meningiomas, showing psammoma bodies (yellow arrowhead) and calcification (read arrowhead) in both falx and skull base meningiomas.
Magnetic resonance spectroscopy shows that, according to the normal brain tissue, the Cr peak was slightly increased but the N-acetyl aspartate (NAA) peak was not remarkable. Perfusion weighted imaging shows that the occupancy has the feature of increased cerebral blood flow and cerebral blood volume (CBV) and delated mean transit time and time to peak (Figure 1). Magnetic resonance angiography (MRA) shows a cystic vascular change in both anterior communicating artery (ACOA) and right anterior cerebral artery (R-ACA) (A2), while the A1 portion of R-ACA does not appear to have this change.
The patient underwent a digital subtraction angiography (DSA) on the third day after admission, as the local hospital report mentioned an intracranial aneurysm. The result shows that three aneurysms are located in the ACOA and R-ACA (A2 + A3) (Figure 2).
Based on the patient's history, signs, symptoms, and supplement findings, we made a primary diagnosis of three occupancies, inferring them as meningiomas, but a definitive diagnosis would require biopsy results. The aneurysm was sure. According to the patient’s condition, a surgery plan was made. We decided to perform resection, clipping, and indocyanine green choroidal (ICG) angiography together.
On the sixth day after admission, we decided to perform tumor resection and aneurysm.
We exposed the brain tissue through an interhemispheric fissure approach, and a tumor of about 1 cm was seen besides the falx, with normal blood supply; we covered it with cotton pads, and then the frontal lobe near the midline was lifted to reveal the tumor at the anterior skull base; the tumor was attached to the anterior skull base and falx cerebral. The blood supply of the tumor surface was normal, the tumor texture was slightly benevolent, and the color was grayish white. Tumor surface had a capsule, the boundary with the surrounding normal brain tissue was clear, and the surrounding brain tissue was obviously edema. We freed the tumor along the tumor capsule and resected the skull base tumor.
After the resection of the skull base, we lifted the frontal lobe to expose the right frontal base, the A1 segment of the anterior cerebral artery, and the bilateral A2 segment; the anterior communicating artery was exposed along the direction of the interhemispheric fissure. The sizes of the aneurysms were 4 mm * 6 mm, 2 mm * 2 mm, and 4 mm * 2 mm. After the temporary aneurysm clip was used to block A1, the back of the neck of the aneurysm was fully dissociated, and three aneurysm clips were successfully applied to the anterior communicating aneurysm. The A2 and A3 segments of the aneurysm were successfully clipped with an aneurysm clip. Then, ICG fluoroscopy showed that the aneurysm was not developed, and the A1 and bilateral A2 arteries were developed smoothly.
Then, the right para-falx meningioma was resected and completely removed. The surgery was successful.
On the third postoperative day, an MRI review showed that the skull base and the falx occupancies had been resected, and computed tomography (CT) angiography showed that all three aneurysms had been successfully clipped.
The histologies of the skull base and falx occupancy were both reported to be endothelial meningiomas (Figure 3). The immunohistochemical technique shows EMA(+), GFAP(−), Ki-67(2%), PR(+), SSTR2(+), VIMENTIN(+).
The patient had minor epilepsy once on the third day after the surgery; sodium valproate was given, and epilepsy never happened again.
CT reviews of the brain and lungs were also performed three times on the first, third, and sixth postoperative days to check the bleeding and lesions. After the recovery time, the patient stated that the headache had disappeared.
Follow-up was planned for 3 months after discharge. The patient reported no discomfort, such as headache or epilepsy, during the follow-up time.
Meningioma has an incidence rate of 16%–23%, accounting for 30% of intracranial tumors, making it the most common type of intracranial tumor after glioma. The incidence increases with age and is often seen in people aged 60–70 years. The female-to-male ratio is 2:1, which may be associated with progesterone and PR-positive meningioma. The absence of NF2 is a risk factor, and ionizing radiation (IR) exposure also plays a role as well (11). It has been reported that only 1.1% of patients with meningioma have a coexisting aneurysm (8).
The initial diagnosis of an intracranial occupancy or aneurysm is not difficult to make nowadays with supplement examination methods. Modern imaging techniques such as MRI, CT, and DSA can easily detect occupancies such as glioma and meningioma and ventricular abnormalities like aneurysms. Meningioma presents isointensity on T1-weighted sequences and hyperintensity on T2-weighted and fluid-attenuated inversion recovery sequences. In addition, the “dural-tail sign” can also suggest the presence of a meningioma, which highly matches with the imaging of our patient (12). MRA has high sensitivity for intracranial aneurysms, but it is still difficult to investigate the presence of small or occultly located aneurysms. In our case, the A2 aneurysm can only be found when the DSA is performed.
In our case, all the aneurysms of the patient were located in the edema area originating from the meningioma in the frontal lobe, which creates the possibility to perform resection and clipping in one surgery. In 1994, Stevenson et al. presented a case report of a patient with multiple aneurysms, multiple meningiomas, and multiple subcutaneous angiolipomas. They performed two surgeries on this patient due to the anatomical position of the meningiomas and aneurysms. The gap between surgeries was 5 months. The similarity is that we both resected the meningioma in the frontal lobe and clipped the aneurysm at the end of the therapy. The difference between these two cases is that in preoperative time, MRI was performed on the patient. According to the imaging result, the location and the border of the tumor were clearer than those in the CT scan. What needs to be mentioned here is that multiple surgical targets may make intraoperative neuronavigation inaccurate; real-time navigation like intraoperative ultrasound will be reliable for multiple existing brain tumors or the brain shift (13). We did not perform the surgery on the occupancy placed in the right temple bone; instead, we chose to observe and follow up. In their case, during craniotomy, an en plaque meningioma was also found and they resected it (5).
We retained the abnormal occupancy under the temple bone because it is smaller than 1.5 cm, so we chose to follow up, considering both the size of the occupancy and the patient’s family income. In the 3-month follow-up, the occupancy under the temple bone seems asymptomatic, which makes our plan reasonable. In their research, no symptom has been reported during the 5 months of follow-up. Still, the second stage of surgery was performed on the patient.
The course of the seizure after the surgery was unknown. According to the situation of the patient’s MRI scan, the range of edema around the meningioma was large, and 3 days after the resection, the CT scan still showed a low-density area around the original position of the occupancy. These together could lead to the disturbance of neuroelectrophysiology, which could explain the single seizure after the surgery (14). The tumor volume seems to have no significant effect on causing the postoperative seizure (15). After giving sodium valproate regularly, the seizure never happened before the discharge and in the 3-month follow-up.
No research has proved the mechanistic correlation between a meningioma and an aneurysm. In previous research, patients with meningiomas have a higher chance of suffering from intracranial vascular abnormalities (including intracranial aneurysm) due to hypertension and tumor volume (2). According to Fischer et al. in their research, the incidence of meningiomas and aneurysms occurring together was 1.1%, which is lower than the average incidence of meningiomas and aneurysms alone. In their study, they hypothesized that higher-grade meningiomas alter the hemodynamics of the blood-supplying arteries and, therefore, lead to aneurysm formation; there is no relationship between a meningioma and a cerebral aneurysm, and the coexistence of brain tumor and vascular malformation is just an accident (1).
Further investigation is needed to determine the relationship between tumor volume and aneurysm formation. In our case, we can assume that the increased blood supply in the tumor changes the hemodynamics of the anterior circulation, thus causing aberrant flow, leading to mechanical overload and a shift in tensile forces and forming an aneurysm (4, 16). We can also assume that edema caused by the obstruction of venous reflux due to the skull base meningioma has no role in the formation of aneurysms, but it surely leads to a seizure postoperation. It has been reported that the appearance of a single meningioma and a single aneurysm seems to be on the same side or nearby, making this hypothesis more convincing (17, 18). In one case reported by Tanaka et al., after the resection of the frontal lobe meningioma, the aneurysm in the internal carotid artery (ICA) immediately ruptured (19).
However, this explanation cannot cover all cases, as there are still meningiomas located in the area where aneurysms are located on the other side (6). In our case, the distant occupancy in the right temple bone appears asymptomatic in a 3-month follow-up. So, further investigation and follow-up are needed.
There is still room for improvement in terms of diagnosis and treatment. According to the 2021 European Association of Neuro-Oncology (EANO) guidance on the diagnosis and management of meningioma, although positron emission tomography (PET) is not included in the standard treatment procedure, it could be an option to distinguish the tumor from healthy tissue (12). Considering the patient’s income, we finally decided not to perform PET on the patient. Instead, MR sequences and CT images are sufficient to show the basic imaging features of the tumor and to decide on the initial plan of surgery. MRA has its advantage in showing aneurysms, but in our case, the A3 segment aneurysm is only reported in the DSA scan, which informs that DSA still places itself as the “gold standard” for the intracranial aneurysm. From another perspective, DSA seems not to be necessary for this patient; the size of the aneurysm placed in the A2 segment is limited. Considering that the patient does not have hypertension or other risk factor, it will be just fine not to check it.
Stereotactic radiosurgery (SRS) like Gamma knife is also a possible way to treat meningiomas under the right temple bone and the possible residual recurrence of the falx and skull base meningiomas. First, for those meningiomas that have infiltrated the vessels, the skull base, or the cranial nerves, SRS reduces the residual (20, 21). Second, after the surgery, the patient did not complain of the headache. SRS is also effective for nonsymptomatic meningiomas (21). The same field of vision of excision makes the clipping of the three aneurysms seem like an “accident”, but this can lead to the treatment presented here—if the aneurysm is located in close proximity to the optic nerve after the removal of the skull base meningioma, then clipping is reasonable, even if the aneurysm is in the range of the edema area caused by the tumor (6). For the meningioma, it is also good to observe whether the tumor size is smaller than 10 mm or it does not disturb the neurofunction (11).
It is rare for multiple meningiomas and multiple aneurysms to be present in a single patient. Considering the possible association between tumor and ventricular abnormality, this case could provide a good indication and reference for subsequent clinical and scientific researchers in terms of histology, diagnosis, treatment, and prognosis. Further studies need to focus on the mechanism of the association between intracranial meningiomas and aneurysms. We hope our research can play the role of a supplement to guide the treatment of coexisting meningiomas and aneurysms.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
R-jW wrote the paper. X-lW and J-cC provided the case. X-lW provided all imaging pictures. J-cC provided the histology image. FX and J-cC reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fischer BR, Palkovic S, Holling M, Niederstadt T, Jeibmann A, Wassmann H. Coexistence of cerebral aneurysm and meningioma—pure accident? Clin Neurol Neurosurg. (2009) 111(8):647–54. doi: 10.1016/j.clineuro.2009.05.016
2. Kim Y-H, Lee YJ, Han JH, Ahn S, Lee J, Kim JH, et al. Association of intracranial aneurysms and meningiomas: a case-control study. J Neurosurg. (2015) 123(2):357–61. doi: 10.3171/2014.10.JNS14710
3. Kanamori M, Tomita T, Sasaki T, Murakami K, Takahashi N, Kakehata S, et al. Subarachnoid hemorrhage in a patient with a meningioma and an unruptured aneurysm. Neurol Med Chir (Tokyo). (2013) 53(5):343–6. doi: 10.2176/nmc.53.343
4. Pia HW, Obrador S, Martin JG. Association of brain tumours and arterial intracranial aneurysms. Acta Neurochir (Wien). (1972) 27(3):189–204. doi: 10.1007/BF01401881
5. Tachikawa T, Adachi J-I, Nishikawa R, Matsutani M. An anterior ethmoidal artery aneurysm associated with an olfactory groove meningioma. Case illustration. J Neurosurg. (2002) 97(6):1479. doi: 10.3171/jns.2002.97.6.1479
6. Takeda N, Nishihara M, Yamanishi S, Kidoguchi K, Hashimoto K. Strategy for patients with co-existence of meningioma and intracerebral aneurysm, especially unruptured aneurysm (—seven cases and review of the literature—). J Clin Neurosci. (2017) 45:236–42. doi: 10.1016/j.jocn.2017.07.032
7. Babbitz JD, Harsh GR. Concomitant ectatic posterior communicating artery and tentorial meningioma as a source of oculomotor palsy: case report. Neurosurgery. (2005) 57(6):E1316. doi: 10.1227/01.NEU.0000187448.96386.03
8. Javalkar V, Guthikonda B, Vannemreddy P, Nanda A. Association of meningioma and intracranial aneurysm: report of five cases and review of literature. Neurol India. (2009) 57(6):772–6. doi: 10.4103/0028-3886.59475
9. Stevenson JC, Choksey MS, McMahon J, Crawford PJ. Multiple cerebral aneurysms, multiple meningiomas and multiple subcutaneous angiolipomas: a case report. J Br J Neurosurg. (1994) 8(4):477–81. doi: 10.3109/02688699408995118
10. Sluis WM, Rinkel GJE, Velthuis BK, Ruigrok YM. The association of intracranial aneurysms and meningiomas: a hospital-based case-control study. Eur J Neurol. (2018) 25(1):e5. doi: 10.1111/ene.13479
11. Ogasawara C, Philbrick BD, Adamson DC. Meningioma: a review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines. (2021) 9(3):4. doi: 10.3390/biomedicines9030319
12. Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, et al. EANO Guideline on the diagnosis and management of meningiomas. Neuro Oncol. (2021) 23(11):1821–34. doi: 10.1093/neuonc/noab150
13. Boukobza M, Cebula H, Pop R, Kouakou F, Sadoun A, Coca HA, et al. Cystic meningioma: radiological, histological, and surgical particularities in 43 patients. Acta Neurochir (Wien). (2016) 158(10):1955–64. doi: 10.1007/s00701-016-2898-x
14. Yoshioka H, Hama S, Taniguchi E, Sugiyama K, Arita K, Kurisu K. Peritumoral brain edema associated with meningioma: influence of vascular endothelial growth factor expression and vascular blood supply. Cancer. (1999) 85(4):936–44. doi: 10.1002/(SICI)1097-0142(19990215)85:4<936::AID-CNCR23>3.0.CO;2-J
15. Hess K, Spille DC, Adeli A, Sporns PB, Brokinkel C, Grauer O, et al. Brain invasion and the risk of seizures in patients with meningioma. J Neurosurg. (2018) 130(3):789–96. doi: 10.3171/2017.11.JNS172265
16. Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. (2016) 12(12):699–713. doi: 10.1038/nrneurol.2016.150
17. Dolenc VV, Pregelj R, Slokan S, Skrbec M. Anterior communicating artery aneurysm associated with tuberculum sellae meningioma—case report. Neurol Med Chir (Tokyo). (1998) 38(8):485–8. doi: 10.2176/nmc.38.485
18. Ogino M, Nakatsukasa M, Nakagawa T, Murase I. Ruptured anterior communicating artery aneurysm encased in a tuberculum sellae meningioma. Case report. J Neurosurg. (1999) 91(5):871–4. doi: 10.3171/jns.1999.91.5.0871
19. Tanaka S, Kobayashi M, Ichinose T, Oikawa N, Kinoshita M, Yoshikawa A, et al. Intraoperative rupture of intracerebral aneurysm immediately after meningioma resection: a case report. BMC Neurol. (2022) 22(1):135. doi: 10.1186/s12883-022-02664-8
20. Ganau M, Foroni RI, Gerosa M, Zivelonghi E, Longhi M, Nicolato A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part I: therapeutic strategies. Tumori. (2014) 100(4):459–65. doi: 10.1177/1636.17912
Keywords: falx brain tumor, multiple aneurysms, case report, imaging, multiple meningiomas
Citation: Wei R, Wu X, Xia F and Chen J (2022) Case report and literature review: Treatment of multiple meningiomas combined with multiple unruptured aneurysms in a single operation. Front. Surg. 9:971068. doi: 10.3389/fsurg.2022.971068
Received: 16 June 2022; Accepted: 27 July 2022;
Published: 7 September 2022.
Edited by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomReviewed by:
Salvatore Chibbaro, Strasbourg University Hospital, France© 2022 Wei, Wu, Xia and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-cao Chen Y2hlbmppbmNhb0Bob3RtYWlsLmNvbQ==
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Abbreviations MRI, magnetic resonance imaging; CT, computed tomography; TP, total protein; ALB, albumin; CBV, cerebral blood volume; ACA, anterior cerebral artery; DSA, digital subtraction angiography; NAA, N-acetyl aspartate; IR, ionizing radiation; ICA, internal carotid artery.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.