
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Surg. , 15 September 2022
Sec. Neurosurgery
Volume 9 - 2022 | https://doi.org/10.3389/fsurg.2022.968906
 Chenghua Yuan1,2,3,4,5
Chenghua Yuan1,2,3,4,5 Jian Guan1,2,3,4,5
Jian Guan1,2,3,4,5 Yueqi Du1,2,3,4,5
Yueqi Du1,2,3,4,5 Zeyu Fang1,2,3,4,5
Zeyu Fang1,2,3,4,5 Xinyu Wang1,2,3,4,5
Xinyu Wang1,2,3,4,5 Qingyu Yao1,2,3,4,5
Qingyu Yao1,2,3,4,5 Can Zhang1,2,3,4,5
Can Zhang1,2,3,4,5 Zhenlei Liu1,2,3,4,5
Zhenlei Liu1,2,3,4,5 Kai Wang1,2,3,4,5
Kai Wang1,2,3,4,5 Wanru Duan1,2,3,4,5
Wanru Duan1,2,3,4,5 Xingwen Wang1,2,3,4,5
Xingwen Wang1,2,3,4,5 Zuowei Wang1,2,3,4,5
Zuowei Wang1,2,3,4,5 Hao Wu1,2,3,4,5
Hao Wu1,2,3,4,5 Fengzeng Jian1,2,3,4,5*
Fengzeng Jian1,2,3,4,5*
Background: Patients with syringomyelia who present with new neurological symptoms after posterior fossa decompression (PFD) are not uncommon. However, systematic reports on different pathologies are few in the literature.
Objective: The purpose of this study was to summarize our experience for failed PFD.
Methods: Between January 2015 and December 2019, 85 consecutive failed PFD patients were identified. The neurological courses were summarized with Klekamp J (KJ) or mJOA score system for all patients. Long-term results were summarized with Kaplan-Meier method.
Results: Twenty-eight consecutive patients underwent FMDD (Foramen magnum and foramen of Magendie dredging) (Group I), extradural PFD and manipulation of tonsil was significantly associated with lower failure rates. Twenty patients underwent craniocervical fixation (Group II), nine underwent local spinal segment decompression (Group III), six underwent CSF diversion procedures, and one were treated for persistent pain by radiofrequency. Neuropathic pain was most significantly improved in Group I while swallowing improved in Group II within 1 year after the surgery. In the long term, late postoperative deterioration-free possibility in Group II was better than in Group I. All patients in Group III improved (P = 0.0088). Six cases of CSF diversion procedures were relieved in a short time. Pain in one patient persisted after PFD, and trial of radiofrequency failed.
Conclusion: Not only does the recurrent cerebrospinal fluid flow obstruct the foramen magnum, but also spinal pathologies and craniocervical instabilities may occur. This study provides the largest summarized clinical experience that may assist surgeons with different therapeutic decisions for failed PFD.
Syrinx are most commonly associated with craniocervical anomalies, other causes include trauma, infections, degeneration, and tumors (1, 2). Syringomyelia may have multiple causes that require different treatment strategies. Posterior fossa decompression (PFD) remains the most commonly used treatment for patients with syringomyelia-Chiari complex; although important surgical details for such patients remain debatable (3). Persistent, recurrent, or worse syringomyelia after PFD occurs in 10%–50% of cases (4, 5). Repeat PFD has been the first-line procedure for failed PFD. Atlantoaxial fusion and other possible spinal pathology treatments or shunting procedures are other treatment options (6–9).
To date, few reports have been published on the indications and treatment choices for persistent or recurrent syrinx, or worsening neurologic symptoms after PFD (5, 7, 8). Moreover, existing articles do not systematically summarize the treatment processes for syringomyelia based on the different underlying pathologies.
The aim of this study was to review our experience for clinically treating patients with syringomyelia for whom PFD had previously failed.
The study was reviewed and approved by the local ethics committee with waiver of informed consent from patients given its retrospective nature.
Between January 2015 and December 2019, 85 consecutive patients with failed PFD for syringomyelia were treated at our institution. For most patients, the first surgery had been conducted at other institutions. The indications for first surgery included: progressive neurological symptoms or syringomyelia.
The indications for secondary surgery included: (1) new, recurrent, or progressive neurological symptoms (independent of syrinx size), (2) large or swollen syringomyelia with obvious spinal cord compression (10), (3) progression of syringomyelia over time despite no or few symptoms, or other obvious pathology. Patients were excluded if they had a history of shunt placement, infection, multiple decompression, or incomplete follow-up data. The patients were categorized according to the flow chart shown in Figure 1.
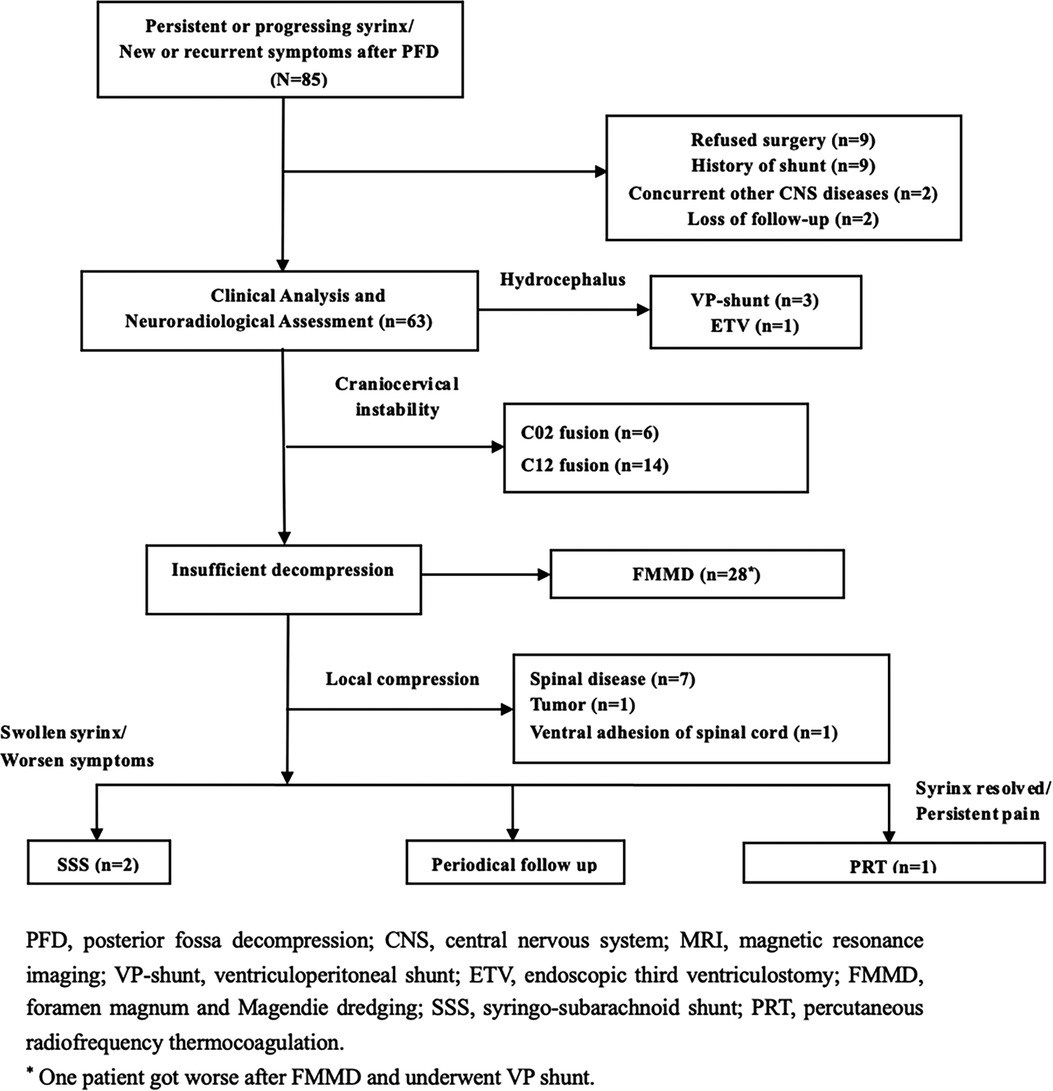
Figure 1. The treatment algorithm categorization and surgical strategy of 85 consecutive failed PFD patients between 2015 and 2019.
KJ scores (11) and mJOA were used to evaluate the clinical manifestations of Group I, II and III before and after surgery. Long-term results were summarized with Kaplan-Meier statistics.
Patients were divided into three groups according to their neurological symptoms, radiological findings; and postoperative outcomes of the first PFD.
Patients were categorized as Group I if insufficient decompression (Figure 2) was achieved. Clinical symptoms were attributed to the brainstem, cerebellum, and lower cranial nerves, or if the upper segment of the syrinx was at the foramen magnum level (10, 12). “Good decompression” was defined as effective spinal cord decompression where the original obstruction to CSF flow was caused by the ectopic cerebellar tonsil, as observed on MR images, and reappearance of the subarachnoid cisterns (Figure 2) of the posterior fossa between the brainstem and cerebellar tonsil.
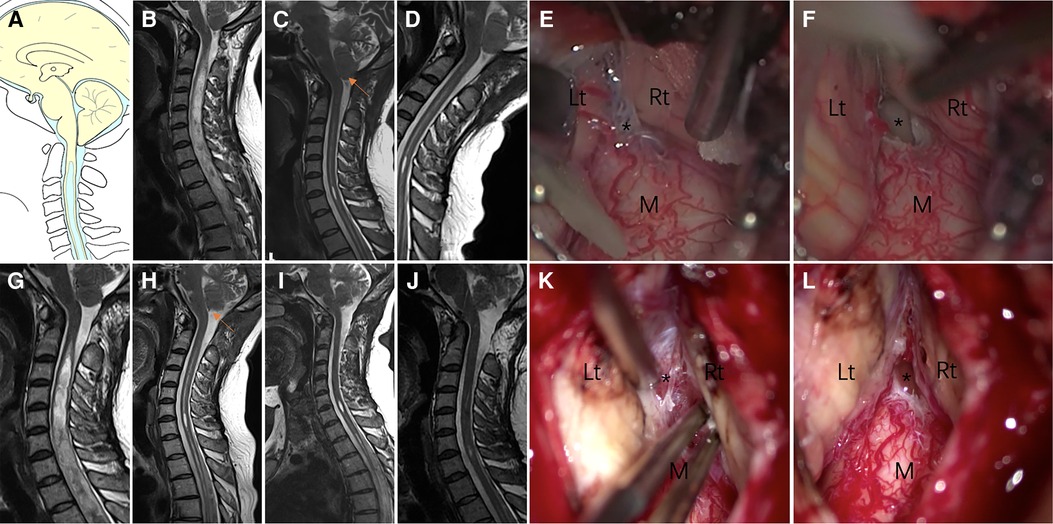
Figure 2. Case presentation of Group I, a 31-year-old woman presented with upper extremity paresthesia. (A) Schematic drawings of the foramen magnum region, sagittal view. Midsagittal T2-weighted MRI scans of the cranio-cervical before the initial craniocervical decompression surgery (B), 3 (C) months after the initial surgery, and 6 (D) months after the initial surgery are shown. (E,F) An obvious veil obstructing the foramen of Magendie (asterisk). (G) 1 year after the initial surgery. Postoperative MRI after 3 months (H), 6 months (I) and 1 year (J) of revision surgery showing good decompression of the posterior fossa. (K,L) Veil obstructing the foramen of Magendie was removed and tonsil was coagulated. Lt, left tonsil; Rt, right tonsil; M, medulla oblongata.
Patients were placed in this group if their dynamic radiographic and reconstructive CT images indicated cranio-cervical instability, and if their clinical symptoms were related to the brainstem, cerebellum, and lower cranial nerves.
If preoperative MR images demonstrated obvious local spinal pathology, the patient was categorized in Group III. For these patients, direct local segment decompression, removal of the lesion, and arachnoid lysis were the surgical options considered.
Our detailed surgical techniques for FMMD in patients with failed PFD have been published previously (8, 13). In brief, all patients in Group I underwent secondary suboccipital decompression combined with careful intradural exploration (8). Different techniques were adopted based on herniation status of the cerebellar tonsils, i.e., low-power bipolar electrocoagulation for mild herniation or medialized hypertrophy and subpial resection for obvious herniation. Electrocoagulation was used to seal the pial opening to ensure hemostasis. Maintaining the integrity of the pia mater is also essential for preventing potential adhesion, scarring, or recurrence. For other pathologies like inter-tonsillar arachnoid adhesions, tonsil to medulla arachnoid adhesions, tonsil to dura mater arachnoid adhesions, and arachnoid veil, micro scissors are used to separate the adhesions (8).
Patients in Group II underwent direct posterior reduction and fixation. The main challenge of this revision surgery was the presence of an occipital defect; the initial operation had destroyed the posterior nuchal attachment and the neck muscles, which caused subsidence at the cranial-cervical junction. In the second surgery, we distracted the facet joints, restored the lost perpendicular height, and then reduced the horizontal translation. The following techniques for posterior fixation were used:
(a) C1 lateral mass screw and C2 pedicle screw fixation (14).
(b) Occiput to C2 fixation with C2 pedicle screws (15).
The techniques for cervical spondylosis compression include anterior or posterior decompression (16). For lumbar compression fracture, some authors have suggested an anatomy-based comprehensive classification of spinal osteotomies (17). Most astrocytoma was biopsied; however, tumor resection using an inside-out technique can be adopted (18). As for patient who develop ventral cyst of the spinal cord after PFDD, we tried cyst incision and drainage.
The relationship between CM and hydrocephalus is interesting. There is limited evidence for surgical management of CM associated with hydrocephalus (19). SSS (syringo-subarachnoid shunt) should be the last procedure to be considered because of its higher failure rate (20).
Persistent neurogenic pain after decompression is not uncommon. SCS (Spinal cord stimulation) is an effective method for the treatment of pain (21, 22). Pulsed radio frequency (PRF), which is a type of undifferentiated pain treatment method, uses radio frequency current to produce high voltage pulse in a short time; this blocks nociceptive afferent pathways (23). However, PRF was failed.
Follow-up information was obtained during outpatient visits or by telephone interviews. Treatment success was defined as sustained improvement of preoperative symptoms or stabilization of previously progressing symptoms. Treatment failure was defined as postoperative neurological deterioration. Patients were assessed at postoperative, 3 months and 12 months postoperatively for neurological recovery using the KJ or mJOA scores (24). Long-term results were summarized with Kaplan-Meier statistics in three groups. Patients also underwent postoperative radiography, reconstructive CT, and MRI to determine the volume of syrinx, decompression, bone graft status, and reduction state.
The baseline data of the Group I and II of patients were summarized using descriptive statistics: Student's t test, the Mann–Whitney U test, the chi-square test or Fisher's exact test (SPSS 25.0, Chicago, IL). The KM method were employed to compare the deterioration-free survival between the three groups (R version 4.0.2).
Group I had 28 patients (8 men and 20 women with a mean age of 40.5 ± 12.5). Group II had 20 patients who presented typically with swallowing problems, and compared to Group I, had a longer interval between the first and second surgeries (39 ± 33 vs. 60 ± 24 months). Hypesthesia, weakness of the limb(s), and Neuropathic pain were the top three main complaints. The clinical presentations and underlying etiologies are summarized in Table 1.
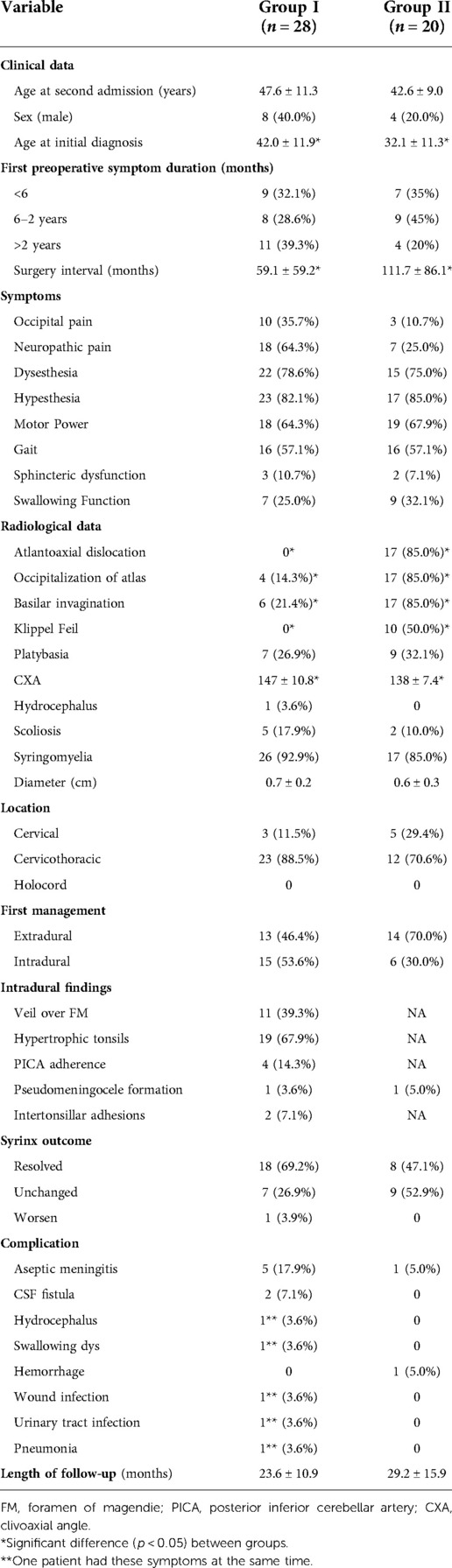
Table 1. Clinical or radiological data and management strategies of Group I and II patients (n = 48).
All 28 patients in Group I with insufficient decompression were treated with FMDD. Intraoperatively, management of intradural factors included addressing adhesion between the cerebellar tonsils and dura mater arachnoid, adhesion of the PICA or its branches, descent of the cerebellar tonsil and medulla (Figure 2). Most (78.6%) of patients either clinically improved or remained stable. Five patients were diagnosed with aseptic meningitis, which was treated successfully by intermittent lumbar puncture. Two patients developed a CSF fistula after the operation. In one patient, dysphagia was not relieved and fever persisted after decompression. The symptoms were controlled after VP shunt. However, the patient died 3 years later.
The twenty patients in Group II with craniocervical instability were treated with either posterior C1–C2 fixation (n = 14) (Figure 3) or occipitocervical fixation (n = 6). All patients achieved complete decompression after posterior reduction and fixation. Clinical improvement was achieved in nearly all patients. Only two patients experienced any intra- hemorrhage or postoperative fever complications.
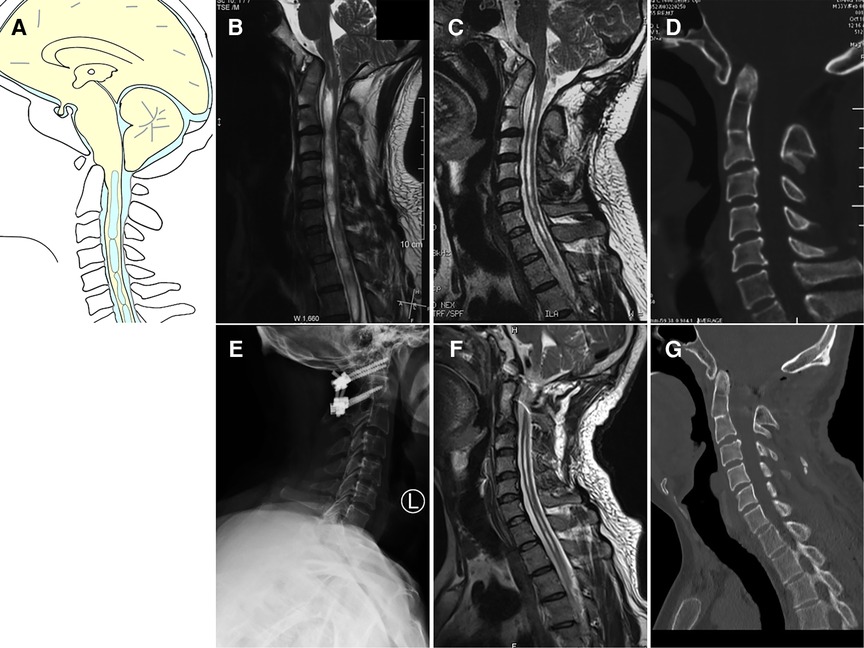
Figure 3. Case presentation of Group II, a 33-year-old man with dizzineess who received initial suboccipital decompression without fusion 6 months ago. The symptoms had worsened gradually. (A) Schematic drawings of the foramen magnum, sagittal view. (B) Preoperative MRI (sagittal view) showed ventral compression of the cervicomedullary junction, Chiari malformation and syringomyelia. (C) Preoperative MRI scan showed syringomyelia was reduced. (D) Preoperative CT scan showed basilar invagination, atlantoaxial dislocation and occipital bone defect. (E) Postoperative X showed C1 lateral mass screw and C2 pedicle screw fixation. (F) Postoperative MRI showed good decompression of the cervicomedullary junction. (G) Postoperative CT indicated complete reduction of both basilar invagination and atlantoaxial dislocation.
Regarding symptom improvement after surgery (Table 2), for patients in Groups I and II, substantial symptom improvements were noted in neuropathic pain, dysphagia, whereas the remainder of symptoms only improved marginally. Radiological improvements were noted in 69.2% or 47.1%, individually. The overall complication rate for Group I and II was 28.6% and 10.0%, respectively.
As for the six degenerative spinal disease patients in Group III (Table 3), clinical improvement or stable was achieved in all patients. One patient with cervical disc herniation was treated with anterior cervical decompression and fusion at the C4/5 level (Figure 4). Postoperative MR images revealed favorable decompression. Another patient with lumbar compression fracture was treated with L2 vertebral column resection and cage implantation (25). The third patient had tumor, which was misdiagnosed as CM and syringomyelia, and was treated by PFD. This patient was transferred to our hospital, where a total tumor resection was performed under electrophysiological monitoring (Figure 5). The pathological findings of the biopsy specimens indicated astrocytoma. The last patient had ventral cyst of spinal cord after PFDD. This patient was transferred to our hospital, where a cyst incision and drainage was performed under electrophysiological monitoring (Figure 6). In terms of long-term prognosis, the possibility of deterioration-free possibility in Group II was better than that in Group I, and all patients got improved in Group III [P = 0.0088, (Figure 7)].
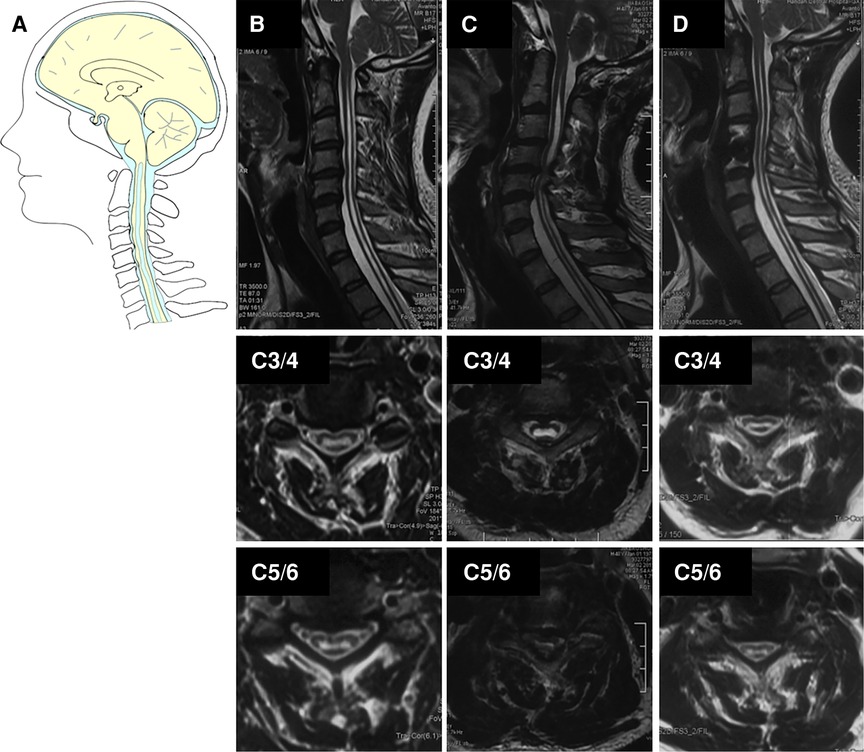
Figure 4. Case presentation of cervical disc herniation in Group III, (A) schematic drawings of the foramen magnum, sagittal view. Sagittal (upper), axial (middle, C3/4), and axial (down, C5/6). (B) Preoperative MRI showed the Chiari I malformation and syrinx. (C) Postoperative MRI after 4 years showed obvious C4/5-disc herniation and enlarged cervical syrinx above C4/5 level (red arrow). (D) A postoperative MRI after ACDF showed the cervical spinal canal was completely decompressed and the syrinx above C4/5 was partially resolved and lower extremity inflexibility improved.
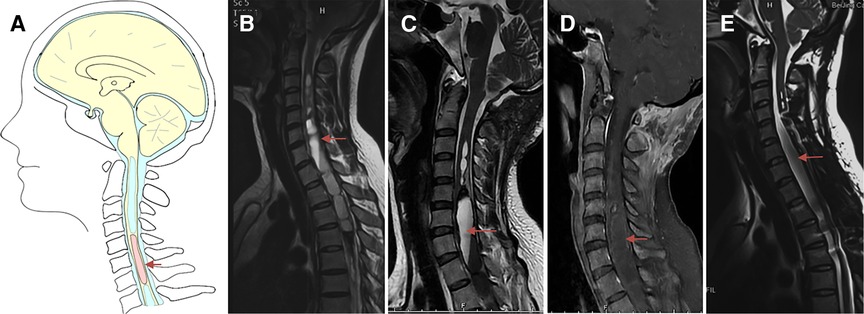
Figure 5. Case presentation of spinal cord tumor in Group III, a 28-year-old woman with progressive weakness and numbness of both lower limbs who received failed suboccipital decompression in local hospital 1.5 months ago. (A) Schematic drawings of the cervical spine, sagittal view. (B) Preoperative sagittal T2-weighted MRI showed large syringomyelia. (C,D) Postoperative enhanced MRI showed partial bone defects of posterior inferior border of occipital bone, spinal cord thickening with abnormal signal at the level of C5-T6, central canal dilation at C3-5 and T7-9, previous intramedullary hemorrhage at T10-11 level. (E) Postoperative MRI after 6 months showed obvious reduction of syringomyelia. Under electrophysiological monitoring, the tumor was completely removed, and the postoperative pathological results were astrocytoma, WHO grade I. Arrow: spinal cord tumor.
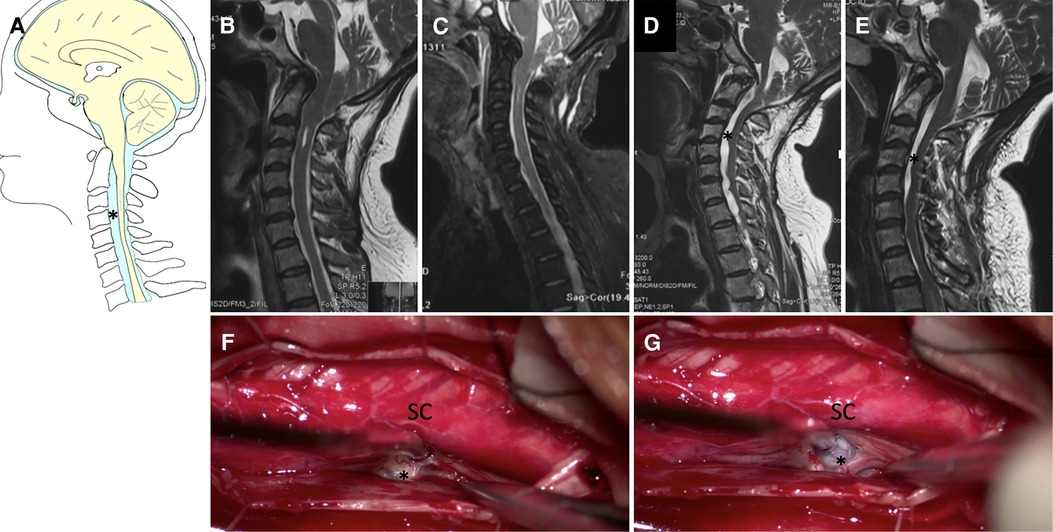
Figure 6. Case presentation of ventral cyst of spinal cord in Group III, (A) schematic drawings of the cervical spine, sagittal view. (B) Preoperative sagittal T2-weighted MRI showed small syringomyelia. (C) 2 weeks, (D) 3 months later after the first surgery MRI showed partial bone defects of posterior inferior border of occipital bone, ventral cyst of spinal cord. (E) Postoperative MRI after second surgery showed obvious reduction of cyst. Under electrophysiological monitoring, the cyst was incised and drained (F,G). SC, spinal cord. Asterisk: Ventral cyst.
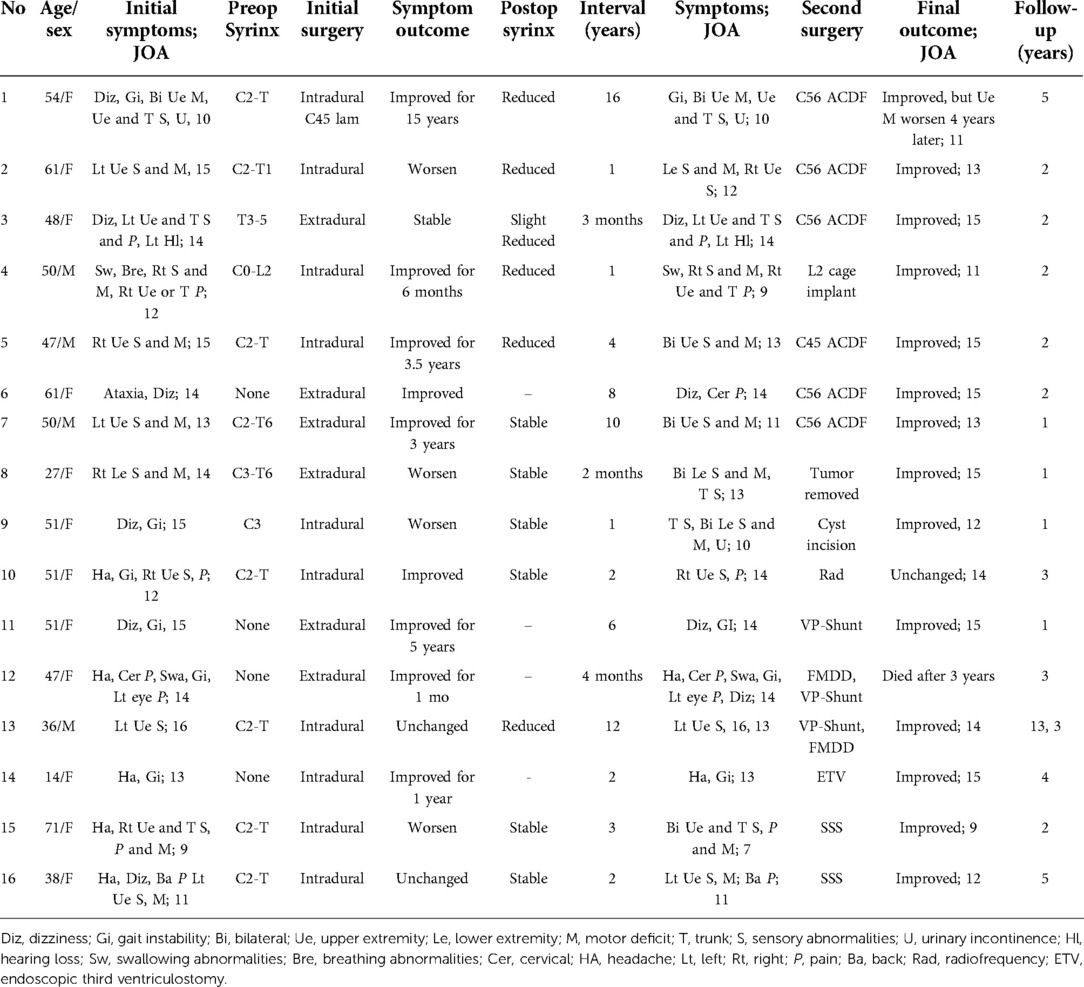
Table 3. Clinical or radiological data and management strategies of other patients except Group I and II (n = 16).
For six patients with CSF shunt, two patients with syrinx-subarachnoid shunt were relieved or stable, while for the other one patient with ETV was relieved. The other three patients with ventriculoperitoneal shunt were all relieved in a short period. One patient died 3 years later, and one patient deteriorated 10 years later and underwent decompression again.
For the patient with persistent pain after decompression, the patient's pain lasted until two years after operation. Trial of cervical nerve root radiofrequency treatment (twice) resulted, in relief of postoperative pain for a short time; the pain recurred after about two months.
Conditions such as CMI, trauma, arachnoiditis, spinal degenerative diseases, and epidural and extramedullary tumors often lead to occlusion of the subarachnoid space followed by disturbances in CSF flow resulting in syringomyelia (25–27). However, for intramedullary tumors or vascular lesions, transudation and tumor cell secretion are responsible for syrinx progression (28). The causes of treatment failure in patients with syringomyelia have been investigated previously (5, 7, 8, 29). However, there is a lack of systematic summary of large number of cases.
For each case of symptomatic syringomyelia, irrespective of previous surgery status, it is important to exclude hydrocephalus and brain tumor as underlying causes. This is followed by detailed evaluations to determine the symptomatology both before and after the initial decompression surgery, as well as symptom changes over time. In this revision study, we noted that the incidence of occipital headache was low, which was related with the effect of first bone decompression. Clinical symptoms associated with the brainstem, cerebellum, or lower cranial nerves, including swallowing dysfunctions and ataxia, usually improved in patients in Groups II. However, for non-functional symptoms, such as neuropathic pain, the clinical improvements were obvious in these Groups I patients. Indeed, hypesthesia was a common complaint of patients included in this study. This type of hypesthesia often remained after decompression. Therefore, it is necessary to tell a patient before the operation that their hypesthesia may not improve even after successful decompression except slight paresthesia. The medical history of some patients revealed an interval of clinical stability after the first PFD, with later re-emergence of swallowing dysfunctions. These symptoms suggest that the deterioration is related to the foramen magnum (8).
In our cohort, the foramen magnum area was first evaluated by comparing the preoperative and postoperative MRI findings for the first failed PFD, including the size of the cisterna magna and extent of decompression of cerebellar ptosis, pseudo meningocele formation, anterior compression of the odontoid, and/or craniocervical instability. Several researchers (8, 10, 30) have suggested that the upper segment of the syrinx near the foramen of magendie indicates underlying pathological changes in the foramen magnum area, which can be removed at the craniocervical level to restore free CSF flow.
Another important factor in the assessment of patients in whom PFD initially failed is the course of a syrinx after surgery. If a syrinx has returned, the reason for this needs to be investigated and the syrinx may need to be treated at the original area. Persistent syrinx may also be due to insufficient decompression or other underlying pathology. In our cohort, operative records from other institutions rarely contained video of the first decompression. If syrinx enlargement was above the compression level, it was likely that the new symptoms were related to local compression. If all of these factors were excluded based on MR images, whole-spine or enhanced MRI was performed to evaluate the patient for other pathologies. The main objective when performing a secondary decompression in patients for whom the initial PFD failed is clinical stabilization of the progressive clinical course. For the majority of the patients, periodical follow-up was recommended because their clinical symptoms were stable or only changed slightly, and because their syrinx had reduced in size (26).
While it is difficult to anticipate intraoperative findings until the dura is opened, accessing information related to the previous decompression surgery, such as the extent of bony resection or patency of the foramen of Magendie, is important when performing a secondary surgery, as it may guide the surgeon's decisions regarding whether to explore the subarachnoid space and whether to resect or coagulate the tonsils.
The secondary decompression may assist in identifying the cause of the first failed decompression. Intradural manipulation may cause underlying adhesions, but extradural decompression is often not sufficient to reestablish free CSF flow. The importance of opening the foramen of Magendie during PFD has been reported (8, 29, 31–33).
Indeed, preoperative instability can be easily overlooked. If dynamic radiographs or CT images show excessive activity at the craniocervical junction or upper cervical spine during flexion and extension, it is necessary to consider decompression combined with appropriate fusion. Some patients with craniocervical instability may not be identified because of unstable compensation before the operation, but this instability can become aggravated after the operation due to separation of the cervical muscles from the occipital region.
Surgical revision of craniocervical instability is difficult, especially for those patients who have previously undergone PFD. The large occipital defect and the loss of anatomical structure on the scar plane causes this dilemma.
In Group II, patients whose first surgery involved PFD without fusion had aggravated postoperative symptoms. Instability and disruption of the posterior tension band may have led to the failure of the first operation. In this case, the strategy we used during the secondary surgery was first to distract the facet joints, and then to restore the lost perpendicular height and reduce the level shift. In Group II, some patients were instrumented with C1 lateral mass screw and C2 pedicle screw fixation, and the other patients received occiput to C2 fixation with C2 pedicle screws (14, 15, 34). Detailed surgical strategies based on anatomy and preoperative planning are important to avoid hypoglossal nerve root and vertebral artery horizontal segment.
Extramedullary lesions that produce chronic spinal cord compression are a factor in the formation of syrinx. Compressive pathogenic factors include cervical disc herniation, basal impression, post-traumatic compression fracture, and arachnoid cyst (28, 35). Although cervical spondylosis is a common condition, it is rarely associated with syringomyelia (16, 36, 37). Syringomyelia is rarely related to spinal cord injuries (25, 38). The detailed medical history of a patient in Group III with this presentation has been summarized and published previously (25).
The most common tumor types associated with syrinx are ependymomas and hemangioblastomas, but astrocytoma are rare (28, 39, 40). Spinal arachnoiditis cyst is a rare complication of subarachnoid hemorrhage (41).
Some authors suggest shunting of the syrinx as an alternative choice to failed surgery (6). However, the surgeons at our institute avoid shunting unless the underlying pathology related to the syrinx cannot be found by existing methods. There is no high-level evidence for surgical management of syringomyelia associated with hydrocephalus (42). Studies analyzing the possible reasons for developing hydrocephalus after PFD are also limited.
It is not uncommon that syringomyelia related neuropathic pain does not relieve continuously after decompression. Common methods of pain relief include analgesics, radiofrequency and spinal cord stimulation; (21–23) in our patient use of the PRF were unsuccessful.
Although this is a small cases series, the descriptions of the revision surgeries for failed PFD-related syringomyelia, including the various methods and techniques used, may help other surgeons plan similar revision surgeries. It is also important for surgeons to understand the key underlying pathology that led to failure of the initial surgery. Etiologies for the failed initial operation are multifactorial.
This study had some limitations, particularly its retrospective design, which may have introduced bias related to the way surgeons operate at our center. Furthermore, most of the patients included in this study were referred to us after undergoing the first PFD at other institutions. Therefore, the factual incidence of recurrent symptoms or syringomyelia in the group is difficult to determine.
The management of patients with PFD failure depends on their underlying pathology. The different surgical techniques for patients with syringomyelia should be carefully reevaluated to reduce failure rates.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
CY: Writing – Original, Draft Data Curation; JG: Writing – Reviewing and Editing, Visualization; YD: Software, Draft Data Curation; ZF: Software, Draft Data Curation; XW: Software, Draft Data Curation; QY: Software, Draft Data Curation; CZ: Software; KW: Resources; ZL: Methodology, Resources; WD: Resources; XW: Resources, Visualization; ZW: Resources, Visualization; HW: Resources; FJ: Writing – Reviewing and Editing, Project administration. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Holly LT, Batzdorf U. Chiari malformation and syringomyelia. J Neurosurg Spine. (2019) 31(5):619–28. doi: 10.3171/2019.7.SPINE181139
2. Milhorat TH, Johnson WD, Miller JI, Bergland RM, Hollenberg-Sher J. Surgical treatment of syringomyelia based on magnetic resonance imaging criteria. Neurosurgery. (1992) 31(2):231–44; discussion 244–235. doi: 10.1227/00006123-199208000-00008
3. Chenghua Y, Min W, Wei L, Xinyu W, Fengzeng J. Comparison of foramen magnum decompression with and without duraplasty in the treatment of adult Chiari malformation type I: a meta-analysis and systematic review. Turk Neurosurg. (2022). doi: 10.5137/1019-5149.JTN.35727-21.5. [Epub ahead of print]35652180
4. Alfieri A, Pinna G. Long-term results after posterior fossa decompression in syringomyelia with adult Chiari type I malformation. J Neurosurg Spine. (2012) 17(5):381–7. doi: 10.3171/2012.7.SPINE12272
5. Soleman J, Bartoli A, Korn A, Constantini S, Roth J. Treatment failure of syringomyelia associated with Chiari I malformation following foramen magnum decompression: how should we proceed? Neurosurg Rev. (2018) 42(3):705–14. doi: 10.1007/s10143-018-01066-0
6. Soleman J, Roth J, Bartoli A, Rosenthal D, Korn A, Constantini S. Syringo-subarachnoid shunt for the treatment of persistent syringomyelia following decompression for Chiari type I malformation: surgical results. World Neurosurg. (2017) 108:836–43. doi: 10.1016/j.wneu.2017.08.002
7. Klekamp J. Neurological deterioration after foramen magnum decompression for Chiari malformation type I: old or new pathology? J Neurosurg Pediatr. (2012) 10(6):538–47. doi: 10.3171/2012.9.PEDS12110
8. Yuan C, Guan J, Du Y, Zhang C, Ma L, Yao Q, et al. Repeat craniocervical decompression in patients with a persistent or worsening syrinx: a preliminary report and early results for 8 cases. World Neurosurg. (2020) 138:e95–e105. doi: 10.1016/j.wneu.2020.02.015
9. Heiss JD, Suffredini G, Smith R, DeVroom HL, Patronas NJ, Butman JA, et al. Pathophysiology of persistent syringomyelia after decompressive craniocervical surgery. Clinical article. J Neurosurg Spine. (2010) 13(6):729–42. doi: 10.3171/2010.6.SPINE10200
10. Guan J, Yuan C, Zhang C, Ma L, Yao Q, Cheng L, et al. A novel classification and its clinical significance in Chiari I malformation with syringomyelia based on high-resolution MRI. Eur Spine J. (2021) 30(6):1623–34. doi: 10.1007/s00586-021-06746-y
11. Klekamp J. Surgical treatment of Chiari I malformation–analysis of intraoperative findings, complications, and outcome for 371 foramen magnum decompressions. Neurosurgery. (2012) 71(2):365–80; discussion 380. doi: 10.1227/NEU.0b013e31825c3426
12. Batzdorf U, McArthur DL, Bentson JR. Surgical treatment of Chiari malformation with and without syringomyelia: experience with 177 adult patients. J Neurosurg. (2013) 118(2):232–42. doi: 10.3171/2012.10.JNS12305
13. Guan J, Yuan C, Zhang C, Ma L, Yao Q, Cheng L, et al. Intradural pathology causing cerebrospinal fluid obstruction in syringomyelia and effectiveness of foramen Magnum and foramen of magendie dredging treatment. World Neurosurg. (2020) 44:e178–e88. doi: 10.1016/j.wneu.2020.08.068
14. Harms J, Melcher RP. Posterior C1–C2 fusion with polyaxial screw and rod fixation. Spine. (2001) 26(22):2467–71. doi: 10.1097/00007632-200111150-00014
15. Jian FZ, Chen Z, Wrede KH, Samii M, Ling F. Direct posterior reduction and fixation for the treatment of basilar invagination with atlantoaxial dislocation. Neurosurgery. (2010) 66(4):678–87; discussion 687. doi: 10.1227/01.NEU.0000367632.45384.5A
16. Vicenty-Padilla JC, De Jesus O. Interval recovery of syringomyelia in Chiari I malformation patient with acute cervical trauma after anterior decompression: case report and review of literature. World Neurosurg. (2018) 118:240–2. doi: 10.1016/j.wneu.2018.06.229
17. Schwab F, Blondel B, Chay E, Demakakos J, Lenke L, Tropiano P, et al. The comprehensive anatomical spinal osteotomy classification. Neurosurgery. (2014) 74(1):112–20; discussion 120. doi: 10.1227/NEU.0000000000000182o
18. Rashad S, Elwany A, Farhoud A. Surgery for spinal intramedullary tumors: technique, outcome and factors affecting resectability. Neurosurg Rev. (2018) 41(2):503–11. doi: 10.1007/s10143-017-0879-z
19. Massimi L, Pennisi G, Frassanito P, Tamburrini G, Di Rocco C, Caldarelli M. Chiari type I and hydrocephalus. Childs Nerv Syst. (2019) 35(10):1701–9. doi: 10.1007/s00381-019-04245-6
20. Iwasaki Y, Hida K, Koyanagi I, Abe H. Reevaluation of syringo-subarachnoid shunt for syringomyelia with Chiari malformation. Neurosurgery. (2000) 46(2):407–12; discussion 412–403. doi: 10.1097/00006123-200002000-00026
21. Lu Y, Mao P, Wang G, Tao W, Xiong D, Ma K, et al. Spinal cord stimulation for chronic intractable trunk or limb pain: study protocol for a Chinese multicenter randomized withdrawal trial (CITRIP study). Trials. (2020) 21(1):834. doi: 10.1186/s13063-020-04768-3
22. Campos WK, Almeida de Oliveira YS, Ciampi de Andrade D, Teixeira MJ, Fonoff ET. Spinal cord stimulation for the treatment of neuropathic pain related to syringomyelia. Pain Med. (2013) 14(5):767–8. doi: 10.1111/pme.12064
23. Bonaldi G, Baruzzi F, Facchinetti A, Fachinetti P, Lunghi S. Plasma radio-frequency-based diskectomy for treatment of cervical herniated nucleus pulposus: feasibility, safety, and preliminary clinical results. AJNR Am J Neuroradiol. (2006) 27(10):2104–11.17110676
24. Klekamp J. Chiari I malformation with and without basilar invagination: a comparative study. Neurosurg Focus. (2015) 38(4):E12. doi: 10.3171/2015.1.FOCUS14783
25. Yuan C, Guan J, Jian F. Rapid progression of acute cervical syringomyelia: a case report of delayed complications following spinal cord injury. J Spinal Cord Med. (2022) 45(1):155–9. doi: 10.1080/10790268.2020.1733336
26. Yuan C, Yao Q, Zhang C, Jian F. Spontaneous resolution of syringomyelia with a 16-year serial magnetic resonance imaging follow-up: a case report and literature review. World Neurosurg. (2019) 130:432–38. doi: 10.1016/j.wneu.2019.07.138
27. Yuan C, Guan J, Du Y, Fang Z, Wang X, Yao Q, et al. Spinal obstruction-related vs. Craniocervical junction-related syringomyelia: a comparative study. Front Neurol. (2022) 13:900441. doi: 10.3389/fneur.2022.900441
28. Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery. (1994) 35(5):865–73; discussion 873. doi: 10.1227/00006123-199411000-00010
29. Tubbs RS, Webb DB, Oakes WJ. Persistent syringomyelia following pediatric Chiari I decompression: radiological and surgical findings. J Neurosurg. (2004) 100(5 Suppl Pediatrics):460–4. doi: 10.3171/ped.2004.100.5.0460
30. Talacchi A, Meneghelli P, Borghesi I, Locatelli F. Surgical management of syringomyelia unrelated to Chiari malformation or spinal cord injury. Eur Spine J. (2016) 25(6):1836–46. doi: 10.1007/s00586-015-4262-x
31. Sacco D, Scott RM. Reoperation for Chiari malformations. Pediatr Neurosurg. (2003) 39(4):171–8. doi: 10.1159/000072467
32. Dlouhy BJ, Dawson JD, Menezes AH. Intradural pathology and pathophysiology associated with Chiari I malformation in children and adults with and without syringomyelia. J Neurosurg Pediatr. (2017) 20(6):526–41. doi: 10.3171/2017.7.PEDS17224
33. Menezes AH, Greenlee JDW, Dlouhy BJ. Syringobulbia in pediatric patients with Chiari malformation type I. J Neurosurg Pediatr. (2018) 22(1):52–60. doi: 10.3171/2018.1.PEDS17472
34. Goel A, Desai KI, Muzumdar DP. Atlantoaxial fixation using plate and screw method: a report of 160 treated patients. Neurosurgery. (2002) 51(6):1351–6; discussion 1356–7. doi: 10.1097/00006123-200212000-00004
35. Lee JH, Chung CF, Kim HJ. Decompression of the spinal subarachnoid space as a solution for syringomyelia without Chiari malformation. Spinal Cord. (2002) 40(10):501–6. doi: 10.1038/sj.sc.3101322
36. Ball JR, Little NS. Chiari malformation, cervical disc prolapse and syringomyelia–always think twice. J Clin Neurosci. (2008) 15(4):474–6. doi: 10.1016/j.jocn.2006.10.026
37. Kato N, Tanaka T, Nagashima H, Arai T, Hasegawa Y, Tani S, et al. Syrinx disappearance following laminoplasty in cervical canal stenosis associated with Chiari malformation–case report. Neurol Med Chir (Tokyo). (2010) 50(2):172–4. doi: 10.2176/nmc.50.172
38. Schurch B, Wichmann W, Rossier AB. Post-traumatic syringomyelia (cystic myelopathy): a prospective study of 449 patients with spinal cord injury. J Neurol Neurosurg Psychiatry. (1996) 60(1):61–7. doi: 10.1136/jnnp.60.1.61
39. Minehan KJ, Brown PD, Scheithauer BW, Krauss WE, Wright MP. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. (2009) 73(3):727–33. doi: 10.1016/j.ijrobp.2008.04.060
40. Yuan C, Yao Q, Cheng L, Zhang C, Ma L, Guan J, et al. Prognostic factors and nomogram prediction of survival probability in primary spinal cord astrocytoma patients. J Neurosurg Spine. (2021):1–12. doi: 10.3171/2021.1.SPINE202017
41. Atallah E, Dang S, Rahm S, Feghali J, Nohra C, Tjoumakaris S, et al. Rare case of diffuse spinal arachnoiditis following a complicated vertebral artery dissection. J Clin Neurosci. (2018) 52:132–4. doi: 10.1016/j.jocn.2018.03.005
Keywords: syringomyelia, posterior fossa decompression, revision, pathology, c12 dislocation
Citation: Yuan C, Guan J, Du Y, Fang Z, Wang X, Yao Q, Zhang C, Liu Z, Wang K, Duan W, Wang X, Wang Z, Wu H and Jian F (2022) Neurological deterioration after posterior fossa decompression for adult syringomyelia: Proposal for a summarized treatment algorithm. Front. Surg. 9:968906. doi: 10.3389/fsurg.2022.968906
Received: 14 June 2022; Accepted: 29 August 2022;
Published: 15 September 2022.
Edited by:
Jason A. Ellis, Lenox Hill Hospital, United StatesReviewed by:
Jianfeng Liu, Hebei Medical University, China© 2022 Yuan, Guan, Du, Fang, Wang, Yao, Zhang, Liu, Wang, Duan, Wang, Wang, Wu and Jian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengzeng Jian amlhbmZlbmd6ZW5nQHh3aC5jY211LmVkdS5jbg==
Specialty Section: This article was submitted to Neurosurgery, a section of the journal Frontiers in Surgery
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.