- Department of Gastrointestinal Surgery, Chongqing Medical University, Yongchuan Hospital, Chongqing, China
Purpose: The purpose of the current meta-analysis was to analyze whether intraoperative blood loss (IBL) influenced the complications and prognosis of gastric cancer patients after gastrectomy.
Methods: We systematically searched the PubMed, Embase and Cochrane library databases on November 29, 2021. The Newcastle-Ottawa scale was used to evaluate the quality of included studies. This meta-analysis uses RevMan 5.3 for data analysis.
Results: A total of nine retrospective studies were included in this meta-analysis, involving 4653 patients. In terms of short-term outcomes, the Larger IBL group has a higher complication rate (OR = 1.94, 95% CI, 1.44 to 2.61, P < 0.0001) and a longer operation time (OR = 77.60, 95% CI, 41.95 to 113.25, P < 0.0001) compared with the smaller IBL group, but the Larger IBL group had higher total retrieved lymph nodes (OR = 3.68, 95% CI, 1.13 to 6.24, P = 0.005). After pooling up all the HRs, the Larger IBL group has worse overall survival (OS) (HR = 1.80, 95% CI, 1.27 to 2.56, P = 0.001) and disease-free survival (DFS) (HR = 1.48, 95% CI, 1.28 to 1.72, P < 0.00001).
Conclusion: Larger IBL increased operation time and postoperative complications, and decreased OS and DFS of gastric cancer patients. Therefore, surgeons should be cautious about IBL during operation.
Introduction
Gastric cancer (GC) is currently the fourth largest malignant tumor worldwide and the second largest cause of cancer-related deaths in the world (1, 2). There are various treatments for GC, among which radical gastrectomy is the essential method (3–7). The surgical methods include total gastrectomy and partial gastrectomy, accompanied by lymph node dissection (8).
The influence of intraoperative blood loss (IBL) on the short-term outcomes and long-term prognosis after surgery is concern for surgeons (9). Studies reported that the amount of IBL was associated with colorectal cancer and pancreatic cancer after surgery (9–11).
There has been controversy about the influence of IBL on the short-term outcomes and long-term prognosis of GC patients after gastrectomy (12–19). Some studies reported that IBL did not affect the prognosis (12), while some studies reported that larger IBL had an adverse effect on the outcomes of GC patients (13–19). Therefore, the purpose of this meta-analysis was to explore whether IBL influenced the complications and prognosis of GC patients after gastrectomy.
Methods
This meta-analysis was conducted on the basis of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (20).
Search Strategy
Two researchers independently searched the English literature in PubMed, Embase and Cochrane Library databases, and the search date was November 29, 2021. The search items were as follows: (“blood loss” OR “intraoperative blood loss”) AND (“gastric cancer” OR “gastric carcinoma” OR “gastric neoplasms” OR “stomach cancer” OR “stomach carcinoma” OR “stomach neoplasms”). In addition, we also searched the references of all included articles to avoid omissions.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1. Original study; 2. The patients were pathologically diagnosed with GC and underwent surgical treatment; 3. There was a comparison between the smaller intraoperative blood loss (SIBL) group and the larger intraoperative blood loss (LIBL) group in the study; 4. The results of the study included at least short-term outcomes or long-term prognosis. The exclusion criteria included: 1. Non-original studies such as reviews, meeting, comments, case report, etc.; 2. Short-term outcomes and long-term prognostic were insufficient.
Data Extraction
The data of the included studies were extracted as follows: 1. Study information included first author, country, publication year, study design, IBL definition, sample size; 2. Baseline information included sex, age, BMI, adjuvant chemotherapy, tumor size, tumor staging and tumor differentiation; 3. Surgical information included operation time, the scope of resection and the number of lymph node dissection; 4. Prognosis included long-term prognosis such as overall survival (OS) and disease-free survival (DFS). The data were independently extracted and cross-checked by two reviewers, and if there was a disagreement, the group discussion resolved it.
Quality Assessment
Two reviewers used the Newcastle-Ottawa Scale (NOS) to assess the quality of the included studies independently (21). The NOS evaluates the quality of studies based on three categories (selection, comparability, and results). The scores were 0–9, of which 9 were high-quality studies, 7–8 were medium-quality studies, and other scores were low-quality studies. If there were differences in the evaluation process, the group discussion resolved it.
Statistical Analysis
Categorical variables were analyzed with 95% confidence interval (CI) odds ratio (OR); continuous variables were analyzed with 95% CI mean difference (MD); and survival variables were analyzed with 95% CI hazard ratio (HR) for analysis. Among them, HR was extracted from multivariate analysis and/or univariate analysis or estimated from the Kaplan-Meier survival curve (22, 23). The heterogeneity of each study was evaluated by I2 and Chi-square test: I2 > 50% indicated high heterogeneity, using random effects model, P < 0.1 was considered statistically significant; I2 ≤ 50% was using fixed effects model, P < 0.05 was considered statistically significant (24, 25). This meta-analysis used RevMan 5.3 (Cochrane Collaboration, London, United Kingdom) for data analysis.
Results
Study Selection
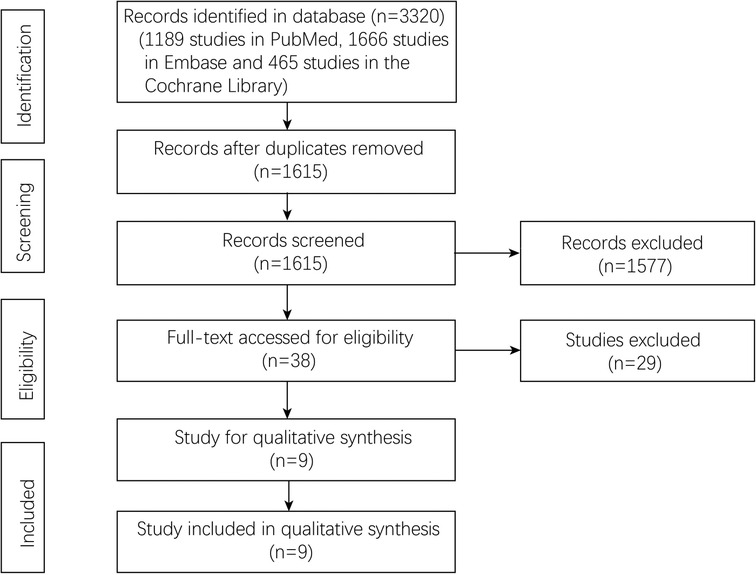
A total of 3320 studies were included through the initial literature search. After deleting 1705 duplicate studies, 1615 studies remained. And 1577 studies were excluded through the screening of titles and abstracts. After evaluating the full texts of the remaining 38 studies, according to the inclusion and exclusion criteria, nine studies (12–19, 26) published from 2000 to 2021 were finally included. The screening process was shown in Figure 1.
Study Characteristics
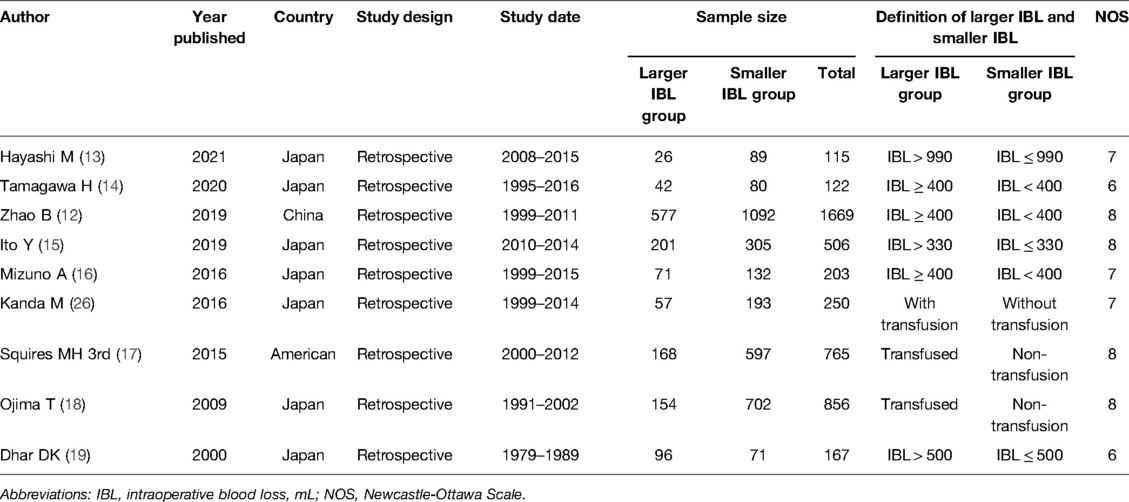
This meta-analysis included nine relevant studies with a total of 4653 patients including 1392 patients in the LIBL group and 3261 patients in the SIBL group. Nine studies were retrospective studies, of which seven were from Japan and the remaining two were from China and American. The publishing year was from 2000 to 2021 and the study date was from 1979 to 2016. The cut-off value was according to the transfusion, 330, 400, 500 and 990 mL. The specific information of the included studies and the NOS score were summarized in Table 1.
Baseline Information
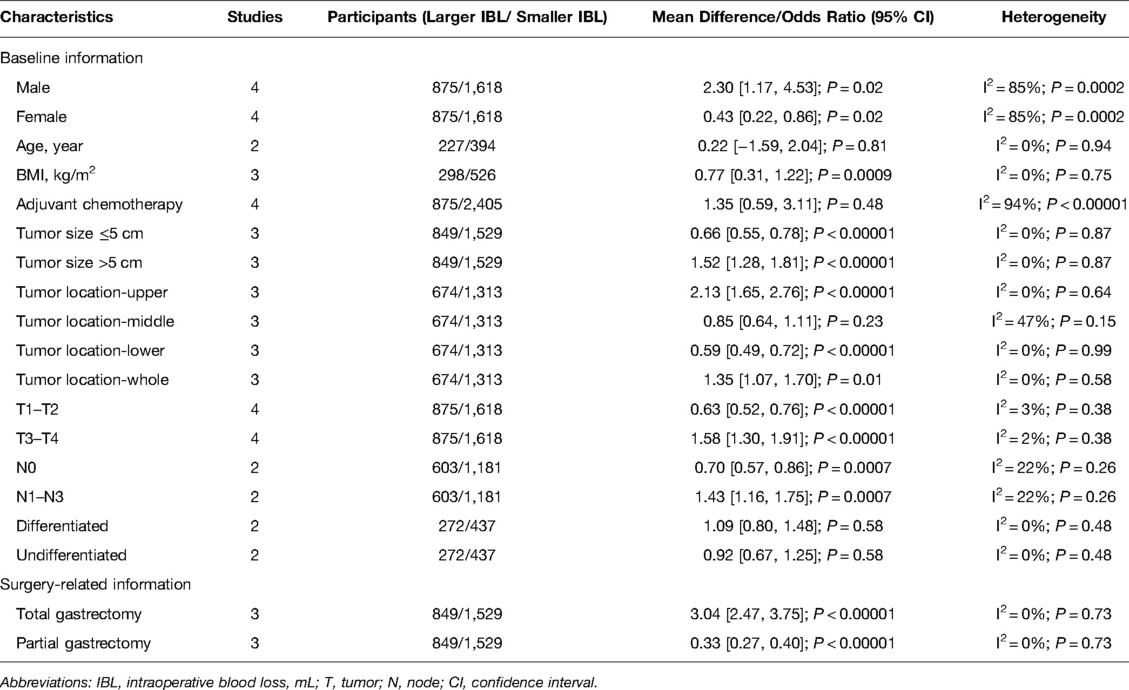
The baseline information included sex, age, body mass index (BMI), adjuvant chemotherapy, tumor size, tumor T stage, tumor N stage and tumor differentiation. Through analysis, we found that the LIBL group had more males (OR = 2.30, 95% CI, 1.17 to 4.53, P = 0.02), lower BMI (OR = 0.77, 95% CI, 0.31 to 1.22, P = 0.0009), more tumors located in the upper stomach (OR = 2.13, 95% CI, 1.65 to 2.76, P < 0.00001) and larger tumors (Tumor size ≤5 cm: OR = 0.66, 95% CI, 0.55 to 0.78, P < 0.00001; Tumor size >5 cm: OR = 1.52, 95% CI, 1.28 to 1.81, P < 0.00001), more T3-4 (OR = 1.58, 95% CI, 1.30 to 1.91, P < 0.00001) and more N1-3 (OR = 1.43, 95% CI, 1.16 to 1.75, P = 0.007). In addition, there was no significant difference between the two groups in terms of age (OR = 0.22, 95% CI, −1.59 to 2.04, P = 0.81), adjuvant chemotherapy (OR = 1.35, 95% CI, 0.59 to 3.11, P = 0.48), tumor location in the middle stomach (OR = 0.85, 95% CI, 0.64 to 1.11, P = 0.23) or tumor differentiation (OR = 1.09, 95% CI, 0.80 to 1.48, P = 0.58). (Table 2).
Surgical Information and Short-Term Outcomes
The surgical information included the operation time, the extent of resection, the number of lymph node dissections and postoperative complications (including infection, enteroparalysis, venous thrombosis and anastomotic leakage). Through analysis, it could be found that the SIBL group has shorter operation time (OR = 77.60, 95% CI, 41.95 to 113.25, P < 0.0001) (Figure 2B), less portion of total gastrectomy (OR = 3.04, 95% CI, 2.47 to 3.05, P < 0.00001) (Table 2), and lower postoperative complications (OR = 1.94, 95% CI, 1.44 to 2.61, P < 0.0001) (Figure 2A), but the Larger IBL group had higher total retrieved lymph nodes (OR = 3.68, 95% CI, 1.13 to 6.24, P = 0.005). (Figure 2C).
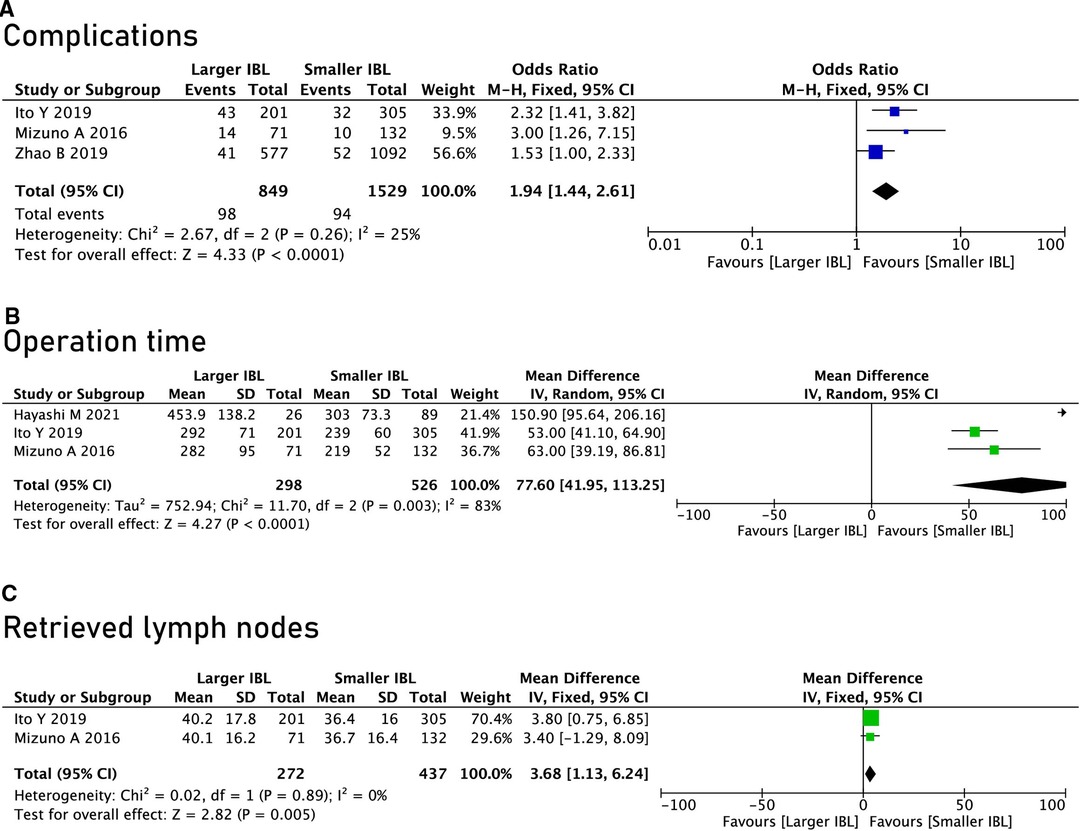
Figure 2. Surgical information and postoperative complications. (A) Complications; (B), Operation time; (C), Retrieved lymph nodes.
Overall Survival and Disease-Free Survival
A total of 9 studies were included in this meta-analysis, of which 6 studies reported OS and 7 studies reported DFS. Through summary analysis, we found that the OS (HR = 1.80, 95% CI, 1.27 to 2.56, P = 0.001) and DFS (HR = 1.48, 95% CI, 1.28 to 1.72, P < 0.00001) of the SIBL group were better than the LIBL group. (Figure 3).

Figure 3. Overall survival and disease free survival between the Larger IBL group and the Smaller IBL group. Note: IBL: intraoperative blood loss, mL.
Sensitivity Analysis and Funnel Plot
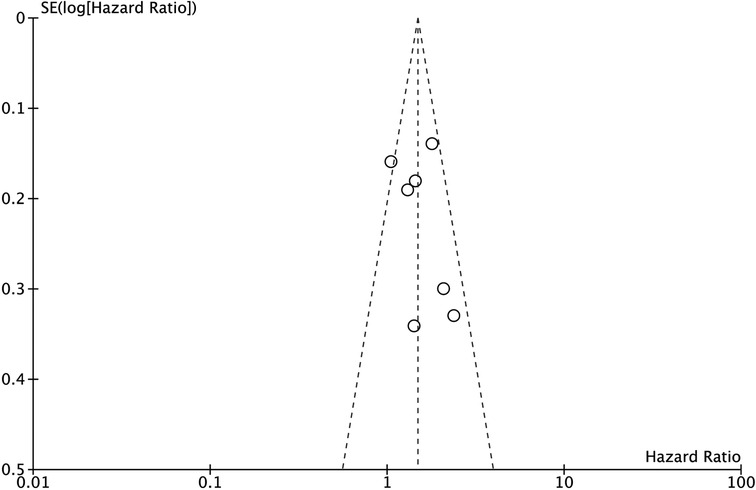
Sensitivity analysis was performed by excluding each study one by one, and the results of the excluded study remain unchanged. Moreover, the funnel plot of DFS was analyzed, and the funnel plot was visually symmetrical. (Figure 4).
Discussion
This meta-analysis included nine studies, including 4653 patients. The SIBL group had shorter operation time, a greater proportion of partial gastrectomy, and a lower incidence of postoperative complications and smaller number of lymph node dissections. Furthermore, the SIBL group had better OS and DFS than the LIBL group.
IBL was often an indicator of the difficulty of gastrointestinal surgery. It has been reported in esophageal cancer and colorectal cancer: Larger IBL were related to increased postoperative complications, and IBL also affected prognosis. In gastric cancer, there were similar reports, but there was some controversy. Zhao et al. (12) reported that IBL did not independently affect the survival of patients after gastrectomy; Hayashi et al. (13) reported that IBL was an independent prognostic factor after gastrectomy; Dhar et al. (19) reported that controlling IBL might improve the survival rate of patients after gastrectomy.
Factors affecting the gastrectomy complications included age, BMI, tumor stage, etc. (27, 28) In this study, large IBL was found to increase complications. The possible reason was that large IBL reduced the body’s immunity, thereby increasing the incidence of complications (29). Therefore, surgeons should operate carefully during surgery to minimize IBL.
In this study, we found that large IBL reduced OS, and the possible reasons were as follows: 1. Large IBL might promote tumor extravasation and hematogenous spread, leading to tumor recurrence and metastasis, especially peritoneal metastasis, thereby affecting the survival. The mechanism might be that the accumulated blood in the peritoneal cavity may provide a favorable microenvironment for the growth of tumor cells. In addition, a large number of angiogenic factors that activate platelets and leukocytes, such as vascular endothelial growth factor (VEGF), could lead to cell proliferation and tumor progression (30). 2. The patient’s immune nutritional status played an important role in tumor immunity (31) and large IBL might hinder anti-tumor immunity, thereby affecting survival (29). 3. Large IBL tended to increase the odds of allogeneic transfusion, which resulted in altered cellular immunity, suppression of natural killer cell activity, and induction of regulatory T cells. These might lead to relative immunosuppression and may allow disseminated or residual microscopic tumor cells to avoid immunodetection, which often led to early recurrence after gastrectomy (32–35).
There was still some important information that could not be included in the meta-analysis due to the limited amount of data. For example, Hayashi et al. (13) reported that the operation time might have an impact on the complications of gastrectomy; Ito et al. (15) reported that the operation method will affect the complications of gastrectomy. Therefore, more relevant information should be reported in future studies.
There were some limitations to this meta-analysis. Firstly, this study included only nine retrospective studies with a total of 4653 patients, with relatively small sample size; secondly, the included studies had different cut-off values for IBL groupings, which might result in heterogeneity; thirdly, most of the included studies were studies on Asian populations and the conclusions may have certain limitations. Finally, some survival data were directly extracted from the Kaplan-Meier survival curve, which may lead to inaccuracies. Therefore, in the future, further high-quality, large-sample, multi-center, and long-term follow-up randomized controlled trials were needed to accurately assess the impact of IBL on short-term outcomes and long-term prognosis after GC surgery.
In conclusion, Larger IBL increased operation time and postoperative complications, and decreased OS and DFS of gastric cancer patients. Therefore, surgeons should be cautious about IBL during operation.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
Data extraction, Z-LW, D-CX and XZ; quality assessments, XZ; data analysis, XZ and Z-LW; writing-origin draft, Z-LW and D-CX; writing-review and editing, XZ, Z-LW and D-CX. All authors contributed to the article and approved the submitted version.
Acknowledgments
We acknowledge all of the authors whose publications are referred to in our article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Varon C, Azzi-Martin L, Khalid S, Seeneevassen L, Ménard A, Spuul P. Helicobacters and cancer, not only gastric cancer? Semin Cancer Biol. (2021) S1044-579X(21)00219-4. doi: 10.1016/j.semcancer.2021.08.007. [Epub ahead of print]34425210
2. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. (2017) 6(1):79. doi: 10.1186/s13643-017-0473-z
3. Tao W, Cheng YX, Liu XY, Zhang B, Yuan C, Peng D, et al. A simple predictive index of the abdominal shape for postoperative complications after laparoscopy-assisted distal gastrectomy for gastric cancer. Front Surg. (2021) 8:768434. doi: 10.3389/fsurg.2021.768434
4. Capelli G, Tonello AS, Chiminazzo V, Lorenzoni G, Bao QR, Marchet A, et al. Validation of a nomogram to predict long term outcomes after curative surgery for gastric cancer in an Italian cohort of patients. J Visc Surg. (2021) S1878-7886(21)00137-5. doi: 10.1016/j.jviscsurg.2021.09.001. [Epub ahead of print]34794901
5. Peng D, Cheng YX, Tao W, Zou YY, Qian K, Zhang W. Onco-metabolic surgery: a combined approach to gastric cancer and hypertension. Cancer Manag Res. (2020) 12:7867–73. doi: 10.2147/CMAR.S260147
6. Tao W, Liu XY, Cheng YX, Kang B, Zhang H, Yuan C, et al. Does extended intraoperative peritoneal lavage really bring benefit on patients with gastric cancer? A meta-analysis of published clinical trials. Front Oncol. (2021) 11:715040. doi: 10.3389/fonc.2021.715040
7. Navashenaq JG, Shabgah AG, Banach M, Jamialahmadi T, Penson PE, Johnston TP, et al. The interaction of helicobacter pylori with cancer immunomodulatory stromal cells: new insight into gastric cancer pathogenesis. Semin Cancer Biol. (2021) S1044-579X (21)00248-0. doi: 10.1016/j.semcancer.2021.09.014. [Epub ahead of print]34600095
8. Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. (2005) 241(1):27–39. doi: 10.1097/01.sla.0000149300.28588.23
9. Kang B, Liu XY, Li ZW, Yuan C, Zhang B, Wei ZQ, et al. The effect of the intraoperative blood loss and intraoperative blood transfusion on the short-term outcomes and prognosis of colorectal cancer: a propensity score matching analysis. Front Surg. (2022) 9:837545. doi: 10.3389/fsurg.2022.837545
10. Mörner ME, Gunnarsson U, Jestin P, Svanfeldt M. The importance of blood loss during colon cancer surgery for long-term survival: an epidemiological study based on a population based register. Ann Surg. (2012) 255(6):1126–8. doi: 10.1097/SLA.0b013e3182512df0
11. Nagai S, Fujii T, Kodera Y, Kanda M, Sahin TT, Kanzaki A, et al. Impact of operative blood loss on survival in invasive ductal adenocarcinoma of the pancreas. Pancreas. (2011) 40(1):3–9. doi: 10.1097/MPA.0b013e3181f7147a
12. Zhao B, Huang X, Lu H, Zhang J, Luo R, Xu H, et al. Intraoperative blood loss does not independently affect the survival outcome of gastric cancer patients who underwent curative resection. Clin Transl Oncol. (2019) 21(9):1197–206. doi: 10.1007/s12094-019-02046-6
13. Hayashi M, Yoshikawa T, Yura M, Otsuki S, Yamagata Y, Morita S, et al. Intraoperative blood loss as an independent prognostic factor for curative resection after neoadjuvant chemotherapy for gastric cancer: a single-center retrospective cohort study. Surg Today. (2021) 51(2):293–302. doi: 10.1007/s00595-020-02114-3
14. Tamagawa H, Aoyama T, Kano K, Numata M, Atsumi Y, Hara K, et al. The impact of intraoperative blood loss on the long-term prognosis after curative resection for borrmann type IV gastric cancer: a retrospective multicenter study. Anticancer Res. (2020) 40(1):405–12. doi: 10.21873/anticanres.13967
15. Ito Y, Kanda M, Ito S, Mochizuki Y, Teramoto H, Ishigure K, et al. Intraoperative blood loss is associated with shortened postoperative survival of patients with stage II/III gastric cancer: analysis of a multi-institutional dataset. World J Surg. (2019) 43(3):870–7. doi: 10.1007/s00268-018-4834-0
16. Mizuno A, Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, et al. Adverse effects of intraoperative blood loss on long-term outcomes after curative gastrectomy of patients with stage II/III gastric cancer. Dig Surg. (2016) 33(2):121–8. doi: 10.1159/000443219
17. Squires MH 3rd, Kooby DA, Poultsides GA, Weber SM, Bloomston M, Fields RC, et al. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: a 7-Institution analysis of 765 patients from the US gastric cancer collaborative. J Am Coll Surg. (2015) 221(3):767–77. doi: 10.1016/j.jamcollsurg.2015.06.012
18. Ojima T, Iwahashi M, Nakamori M, Nakamura M, Naka T, Katsuda M, et al. Association of allogeneic blood transfusions and long-term survival of patients with gastric cancer after curative gastrectomy. J Gastrointest Surg. (2009) 13(10):1821–30. doi: 10.1007/s11605-009-0973-9
19. Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Watanabe R, et al. Long-term survival of transmural advanced gastric carcinoma following curative resection: multivariate analysis of prognostic factors. World J Surg. (2000) 24(5):588–93; discussion 593–4. doi: 10.1007/s002689910099
20. Moher D, Liberati A, Tetzlaff J. Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
21. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
22. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
24. Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. (2008) 14(5):951–7. doi: 10.1111/j.1365-2753.2008.00986
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
26. Kanda M, Kobayashi D, Tanaka C, Iwata N, Yamada S, Fujii T, et al. Adverse prognostic impact of perioperative allogeneic transfusion on patients with stage II/III gastric cancer. Gastric Cancer. (2016) 19(1):255–63. doi: 10.1007/s10120-014-0456-x
27. Adachi W, Kobayashi M, Koike S, Rafique M, Nimura Y, Kuroda T, et al. The influence of excess body weight on the surgical treatment of patients with gastric cancer. Surg Today. (1995) 25(11):939–45. doi: 10.1007/BF00312377
28. Sun ZW, Du H, Li JR, Qin HY. Constructing a risk prediction model for anastomotic leakage after esophageal cancer resection. J Int Med Res. (2020) 48(4):300060519896726. doi: 10.1177/0300060519896726
29. Bruns CJ, Schäfer H, Wolfgarten B, Engert A. Effect of intraoperative blood loss on the function of natural killer cells in tumors of the upper gastrointestinal tract. Langenbecks Arch Chir Suppl Kongressbd. (1996) 113:146–9.9101816
30. Kamei T, Kitayama J, Yamashita H, Nagawa H. Intraoperative blood loss is a critical risk factor for peritoneal recurrence after curative resection of advanced gastric cancer. World J Surg. (2009) 33(6):1240–6. doi: 10.1007/s00268-009-9979-4
31. Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. (2011) 98(2):268–74. doi: 10.1002/bjs.7305
32. Chen G, Zhang FJ, Gong M, Yan M. Effect of perioperative autologous versus allogeneic blood transfusion on the immune system in gastric cancer patients. J Zhejiang Univ Sci B. (2007) 8(8):560–5. doi: 10.1631/jzus.2007.B0560
33. Baumgartner JM, Silliman CC, Moore EE, Banerjee A, McCarter MD. Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg. (2009) 208(1):110–9. doi: 10.1016/j.jamcollsurg.2008.08.012
34. Heiss MM, Fraunberger P, Delanoff C, Stets R, Allgayer H, Ströhlein MA, et al. Modulation of immune response by blood transfusion: evidence for a differential effect of allogeneic and autologous blood in colorectal cancer surgery. Shock. (1997) 8(6):402–8. doi: 10.1097/00024382-199712000-00002
Keywords: intraoperative blood loss, gastric cancer, surgery, prognosis, outcomes
Citation: Wen Z, Xiao D and Zhou X (2022) Does Intraoperative Blood Loss Affect the Short-Term Outcomes and Prognosis of Gastric Cancer Patients After Gastrectomy? A Meta-Analysis. Front. Surg. 9:924444. doi: 10.3389/fsurg.2022.924444
Received: 20 April 2022; Accepted: 26 May 2022;
Published: 14 June 2022.
Edited by:
Francesco Frattini, ASST Sette Laghi, ItalyReviewed by:
Andrea Balla, Hospital “San Paolo”, ItalyMarta Goglia, Sapienza University of Rome, Italy
Copyright © 2022 Wen, Xiao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiong Zhou eGlvbmd6aG91MDAxMUAxNjMuY29t
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Ze-Lin Wen
Ze-Lin Wen Xiong Zhou
Xiong Zhou