- 1Clinical Orthopedics, Department of Clinical and Molecular Science, School of Medicine, Università Politecnica delle Marche, Ancona, Italy
- 2Clinic of Adult and Paediatric Orthopedic, Azienda Ospedaliero-Universitaria, Ospedali Riuniti di Ancona, Ancona, Italy
Heterotopic ossification (HO) is a pathologic condition in which aberrant lamellar bone deposits in soft tissues, outside of the normal skeleton. Pathogenesis is still unclear, but different risk factors are known. Here we report a case of a 14 year-old girl presenting with pain in the medial calcaneal region and evidence of a rapidly growing, firm and solid neoformation. The lesion was diagnosed 6 years earlier, but it was consistently smaller and asymptomatic so that the patient did not undergo any follow up. The patient had no previous trauma or surgery, no other risk factors for HO and did not show any clinically evident HO in other districts. Xray and CT showed a heterogeneous bony lesion in the context of soft tissues, isolated from the calcaneus. After complete excision, histological analysis confirmed the diagnosis of HO. In conclusion, lone non congenital HO can occur regardless of known risk factors. Small HO lesion may also enter a proliferative phase without evidence of triggering events. More studies are required to better understand etiopathogenesis of HO in these clinical settings.
Introduction
Heterotopic ossification (HO) is a pathologic condition in which aberrant lamellar bone deposits in soft tissues, outside of the normal skeleton. Apart from rare cases of genetic forms, the majority of HO cases are acquired and they can be either spontaneous or post-traumatic.
The early identification of patients with HO can be difficult. Clinical signs are variable and nonspecific, ranging from inflammatory signs such as pain, erythema, swelling and warmth to increased joint stiffness and limited range of motion (ROM) of the involved joint. Asymptomatic masses are not uncommon presentations, as well (1).
Here we report a case of a skeletally immature girl without any known risk factor, with a diagnosis of lone HO lesion of the calcaneal region of the right foot.
Case Description
A 14-year-old girl presented to our hospital with pain and discomfort in the medial region of the right hindfoot and evidence of a solid and firm mass reported to be rapidly growing during the previous weeks.
She was evaluated six years earlier for a painless, slowly enlarging mass in the same region. Anamnestic evaluation at the time revealed no history of acute trauma or repetitive minor trauma, as well as a negative familiarity. Complete clinical examination confirmed the absence of other similar lesions.
Plain X-ray demonstrated a neoformation in the context of soft tissue with calcifications inside and undefined limits (Figure 1A). Ultrasound (US) had shown a poorly defined mass of mixed echogenicity measuring approximately 6 mm × 8 mm, with hyperechoic areas similar to bone signal. No cystic component or abnormal Doppler flow was found.
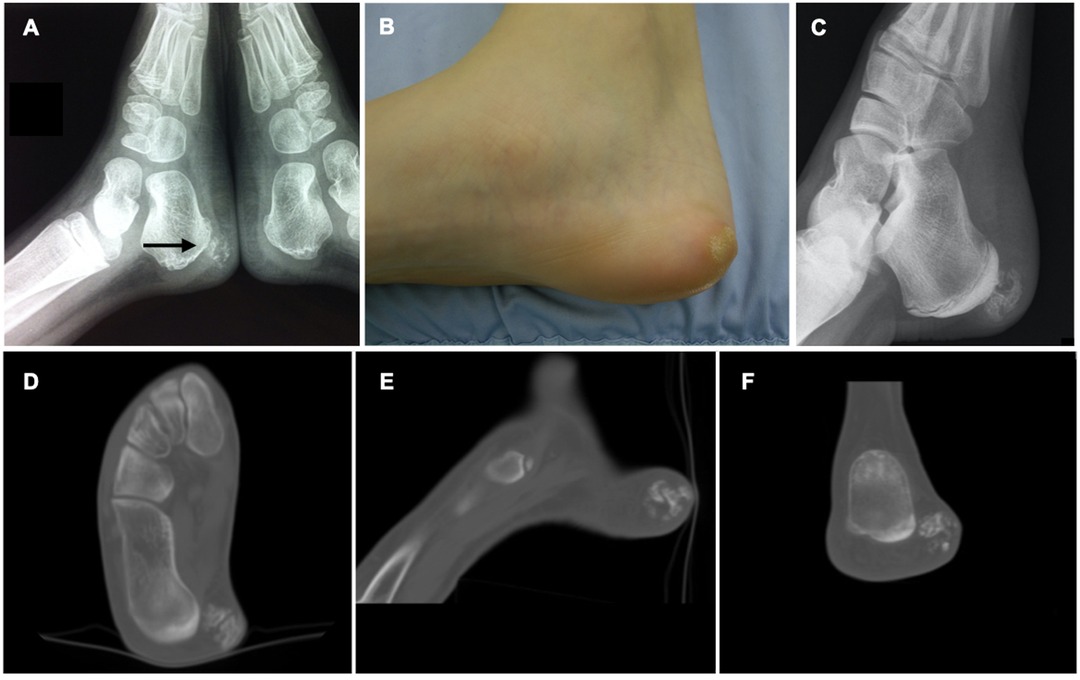
Figure 1. (A) Old X-ray showing the clinically silent lesion at the age of 6 years. (B) Clinical picture showing the mass covered with areas of hyperkeratosis. (C) New X-ray at time of presentation showed increased size of the lesion. (D–F) Preoperative CT section of the lesion.
For approximately six years she had been completely asymptomatic and had not been doing any clinical or radiological follow up.
At the time of presentation local examination revealed moderate oedema and hyperkeratosis in the posterior-medial side of the right hindfoot with evidence of a hard, firm mass measuring about 20 mm in diameter (Figure 1B). No sign of local inflammation was evident and neurovascular examination was normal.
A new X-ray examination demonstrated that the lesion was enlarged and contained more areas of ossification (Figure 1C).
CT scan (Figures 1D–F) showed the mass to be made of mixed bone and non-ossified areas. The lesion was clearly separated from the calcaneal cortical bone and measured approximately 14 × 18 mm in its maximum diameter.
A complete excision of the mass was performed through a small lesion-centered incision (Figures 2A,B). The mass was clearly separated from calcaneal periosteum and macroscopic radical excision was achieved. Histopathological examination confirmed the lesion to be made of “multiple foci of stromal osseous metaplasia.”, thus supporting the diagnosis of HO (Figure 2C).
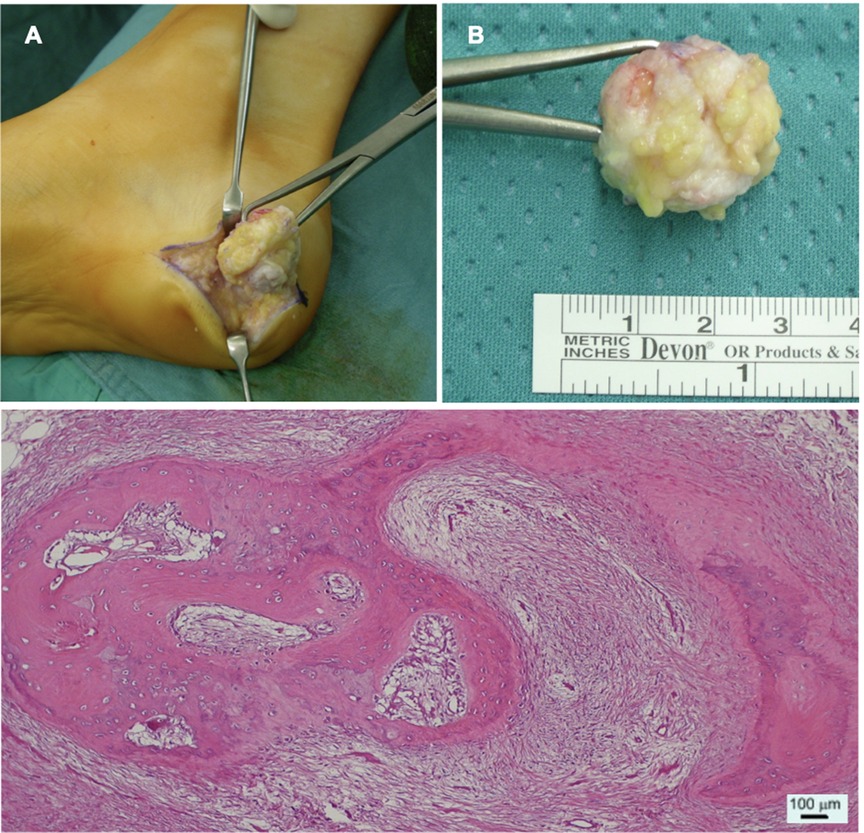
Figure 2. (A) Intraoperative image of the excision of the mass. (B) Picture of the sample measuring approximately 2.5 × 1.5 × 2 cm. (C) Section of the lesion as seen with optic microscopy and Hematoxylin & Eosin staining. The specimen shows “mixed fibrous-adipose dermo-epidermal tissue with foci of stromal osseous metaplasia”, as confirmed also by pathology report.
Radical excision of the mass was demonstrated with Xray and confirmed during follow up (Figure 3).

Figure 3. Xray 6 months after surgery confirming complete excision of the lesion without residual pathology.
No perioperative and postoperative, confirmed or suspected adverse events were recorded.
The patient was discharged without other treatments but with a strict clinical and radiological follow up, to detect early eventual local relapses or secondary lesions. During the programmed follow up the patient did not complain about local al distant symptoms in any way potentially related with relapses or secondary lesions. Thus, given the absence of secondary lesions and family history, the patient was still excluded from whole exome sequencing protocol.
Moreover, after 6 years of follow up, the patient is still asymptomatic and has experienced no signs or symptoms suggestive of local relapses or distant secondary lesions. (Figure 4 - Timetable).
Discussion
Heterotopic ossification (HO) is a pathological condition in which qualitatively normal bone tissue aberrantly forms in extra-skeletal tissues. The ultimate pathogenetic cause is the alteration of the usual process that regulates the timing and location of bone formation. This aberrant growth takes place not within the muscle fibers, but between muscle planes. Furthermore, these lesions are isolated from the skeletal bone, and even if in contact they do not interfere with the periosteal anatomy.
The most common clinical presentation is an acquired singular neoformation in a young adult. In approximately 75% of the cases a local trauma can be identified as the triggering event and repetitive microtrauma are highly suspected in the remaining (2). The other predisposing conditions are: surgical exposures in orthopedic surgery (3), fractures or dislocations (4), spinal cord injury and other neurological and metabolic disorders (2, 5).
Rare hereditary forms include Fibrodysplasia Ossificans Progressiva (FOP) and Progressive Osseous Heteroplasia (POH), with different genetical backgrounds and pathological appearances.
African-American ethnicity was clearly recognized as an independent risk factor for development of HO after total hip arthroplasty (THA). Male sex was also suggested as an independent risk factor but in this case evidence is conflicting (6, 7).
Beyond these few known risk factors and epidemiological notes, the exact pathogenesis of heterotopic ossification is still unclear. Chalmers et al. (8) described three conditions necessary to HO formation: the recruitment of osteogenic precursor cells (9), an event that triggers the differentiation of mesenchymal cells into bone-forming cells and a suitable tissue microenvironment that supports bone formation.
Different studies have demonstrated that an insufficient inhibitory response that causes overexpression of BMP signaling (7, 8) may play a role in eliciting the differentiation of osteogenic cells. Many other cytokines have also been claimed to play a role, even though no definitive mechanisms have been identified, yet (12).
A predisposing local environment may be induced by inflammation (13), tissue hypoxia (14), alteration in peripheral nerve system (PNS) activity and neuro-inflammation (15), prolonged immobilization (16), PTH or calcitonin alterations and mismatch in Ca/P ratio (17).
Histologically, early HO lesions are characterized by a consistent number of proliferating cells that may mimic a sarcoma. Mature HO, on the other side, is clearly identified by the specific peripheral ossification pattern. These lesions are well contained in a dense fibrous pseudocapsule (18). Radiological appearance reflects histological stadium. Early non-ossified lesions are barely visible, while mature HO is clearly recognizable by the peripherical pattern of ossification, also known as “eggshell ossification”. CT is even more specific in identifying the zonal pattern (19). In contrast, MRI may sometimes be misleading due to the heterogeneous appearance, the presence of peripheral oedema and the possibility of core contrast enhancement that may pose diagnostic questions (20). Clinical signs are often consequence of local inflammation, nerve compression or stiffness. Moreover, only with a clinical examination, it is sometimes difficult to differentiate the early phase of HO from deep venous thrombosis (DVT), cellulitis, osteomyelitis or from bone-forming tumors.
Conservative treatment includes physical therapy, bisphosphonates or NSAIDs. Clinical evidence supporting the use of bisphosphonates is limited. Prophylactic radiation and indomethacin have proven to reduce the incidence of postoperative HO, especially in the setting of hip arthroplasty (1, 21).
Surgical excision is often necessary for the treatment of severe HO limiting mobility or causing pain or neurologic symptoms. The optimal timing for surgery is still controversial, but there is a general attitude towards giving importance to the clinical setting regardless the stage of the lesion. Indeed, early excision of HO is not associated with higher rates of recurrence (22).
In this report we presented a case of a lone, non-congenital and non-familiar HO lesion in the medial calcaneal region of a 14-year-old girl. After 6 years without symptoms, the patient experienced sudden onset of pain and rapid growth of the mass. The patient neither had risk factors nor evident triggering causes and was otherwise healthy and without any signs of similar lesions in other districts. She denied local trauma either of major or minor entity, local previous surgery or prolonged immobilization. Family history was completely negative for similar lesions and the patient did not report any signs or symptoms potentially related to secondary lesions. The complete excision of the mass led to complete resolution of the local symptoms. Follow up was performed as routine, and no signs of local relapses or secondary lesions were reported. After 6 years of follow up the patient is completely asymptomatic.
The major limitation of this report is the absence of a genetic evaluation. Nevertheless, this is explained by the fact that the patient presented with a single lesion, without relapses or secondary masses, without clinical suspicion of FOP, POH or Albright hereditary osteodystrophy (AHO) and with a completely negative family history. Genetics in this setting is still largely inconclusive (23, 24).
In conclusion, even though a history of trauma or microtrauma is thought to be present in almost all non-hereditary cases of HO, with this report we underline the importance of considering this diagnosis even when the anamnestic feature of trauma is absent. In fact, this lesion could be identified as idiopathic. In addition, previous silent HO lesion may enter in a sort of proliferative phase even without evidence of trauma or other anamnestic risk factors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
DF and MB wrote the article and collected bibliography. VC and MM performed the surgery and were primarily involved during the follow up of the patient. APG performed the revision of the article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest..
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meyers C, Lisiecki J, Miller S, Levin A, Fayad L, Ding C, et al. Heterotopic ossification: a comprehensive review. JBMR Plus. (2019) 3(4):e10172. doi: 10.1002/jbm4.10172
2. Teasell RW, Mehta S, Aubut JL, Ashe MC, Sequeira K, MacAluso S, et al. A systematic review of the therapeutic interventions for heterotopic ossification after spinal cord injury. Spinal Cord. (2010) 48(7):512–21. doi: 10.1038/sc.2009.175
3. Riegler HF, Harris CM. Heterotopic bone formation after total hip arthroplasty. - Cerca con Google. https://www.google.com/search?q=Riegler+HF%2C+Harris+CM.+Heterotopic+bone+formation+after+total+hip+arthroplasty.&rlz=1C5CHFA_enIT859IT859&oq=Riegler+HF%2C+Harris+CM.+Heterotopic+bone+formation+after+total+hip+arthroplasty.&aqs=chrome..69i57.553j0j4&sourceid=chrome&ie=UTF-8 (Accessed December 17, 2020).
4. Hong CC, Nashi N, Hey HW, Chee YH, Murphy D. Clinically relevant heterotopic ossification after elbow fracture surgery: a risk factors study. Orthop Traumatol Surg Res. (2015) 101(2):209–13. doi: 10.1016/j.otsr.2014.10.021
5. Licini C, Farinelli L, Cerqueni G, Hosein A, Marchi S, Gigante A, et al. Heterotopic ossification in a patient with diffuse idiopathic skeletal hyperostosis: input from histological findings. Eur J Histochem. (2020) 64(4):317–22. doi: 10.4081/EJH.2020.3176
6. Davis G, Patel RP, Tan TL, Alijanipour P, Naik TU, Parvizi J. Ethnic differences in heterotopic ossification following total hip arthroplasty. Bone Jt J. (2016) 98-B(6):761–6. doi: 10.1302/0301-620X.98B6.36050
7. Slone HS, Walton ZJ, Daly CA, Chapin RW, Barfield WR, Leddy LR, et al. The impact of race on the development of severe heterotopic ossification following acetabular fracture surgery. Injury. (2015) 46(6):1069–73. doi: 10.1016/j.injury.2015.01.039
8. Observations on the induction of bone in soft tissues - PubMed. https://pubmed.ncbi.nlm.nih.gov/1090627/ (Accessed December 31, 2020).
9. Pignolo RJ, Shore EM. Circulating osteogenic precursor cells. Crit Rev Eukaryot Gene Expr. (2010) 20(2):171–80. doi: 10.1615/CritRevEukarGeneExpr.v20.i2.70
10. Bosch P, Musgrave D, Ghivizzani S, Latterman C, Day CS, Huard J. The efficiency of muscle-derived cell-mediated bone formation. Cell Transplant. (2000) 9(4):463–70. doi: 10.1177/096368970000900403
11. Yano M, Kawao N, Okumoto K, Tamura Y, Okada K, Kaji H. Fibrodysplasia ossificans progressiva-related activated activin-like kinase signaling enhances osteoclast formation during heterotopic ossification in muscle tissues. J Biol Chem. (2014) 289(24):16966–77. doi: 10.1074/jbc.M113.526038
12. Shore EM, Kaplan FS. Role of altered signal transduction in heterotopic ossification and fibrodysplasia ossificans progressiva. Curr Osteoporos Rep. (2011) 9(2):83–8. doi: 10.1007/s11914-011-0046-3
13. Hsieh HHS, Chung MT, Allen RM, Ranganathan K, Habbouche J, Cholok D, et al. Evaluation of salivary cytokines for diagnosis of both trauma-induced and genetic heterotopic ossification. Front Endocrinol. (2017) 8. doi: 10.3389/fendo.2017.00074
14. Agarwal S, Loder S, Brownley C, Cholok D, Mangiavini L, Li J, et al. Inhibition of Hif1α prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci U S A. (2016) 113(3):E338–47. doi: 10.1073/pnas.1515397113
15. Kaplan FS, Pignolo RJ, Shore EM. Granting immunity to FOP and catching heterotopic ossification in the Act. Semin Cell Dev Biol. (2016) 49:30–6. doi: 10.1016/j.semcdb.2015.12.013
16. Hudson SJ, Brett SJ. Heterotopic ossification - A long-term consequence of prolonged immobility. Crit Care. (2006) 10(6):174. doi: 10.1186/cc5091
17. Fournier DE, Kiser PK, Beach RJ, Dixon SJ, Séguin CA. Dystrophic calcification and heterotopic ossification in fibrocartilaginous tissues of the spine in diffuse idiopathic skeletal hyperostosis (DISH). Bone Res. (2020) 8(1). doi: 10.1038/s41413-020-0091-6
18. Hoda SA. Enzinger and Weiss’s soft tissue tumors, 6th edition. Adv Anat Pathol. (2014) 21(3):216. doi: 10.1097/pap.0000000000000020
19. Kransdorf MJ, Meis JM. From the archives of the AFIP. Extraskeletal osseous and cartilaginous tumors of the extremities. Radiographics. (1993) 13(4):853–84. doi: 10.1148/radiographics.13.4.8356273
20. Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic-pathologic correlation. Am J Roentgenol. (1991) 157(6):1243–8. doi: 10.2214/ajr.157.6.1950874
21. Popovic M, Agarwal A, Zhang L, Yip C, Kreder HJ, Nousiainen MT, et al. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta-analysis of published data. Radiother Oncol. (2014) 113(1):10–7. doi: 10.1016/j.radonc.2014.08.025
22. Chalidis B, Stengel D, Giannoudis PV. Early excision and late excision of heterotopic ossification after traumatic brain injury are equivalent: a systematic review of the literature. J Neurotrauma. (2007) 24(11):1675–86. doi: 10.1089/neu.2007.0342
23. Yang Z, Liu D, Guan R, Li X, Wang Y, Sheng B. Potential genes and pathways associated with heterotopic ossification derived from analyses of gene expression profiles. J Orthop Surg Res. (2021) 16(1):1–10. doi: 10.1186/S13018-021-02658-1/FIGURES/7
Keywords: heterotopic ossification, bone, foot, case report, children
Citation: Danya F, Marco B, Valentino C and Mario M (2022) Case Report: Unusual Heterotopic Ossification of the Hindfoot. Front. Surg. 9:917560. doi: 10.3389/fsurg.2022.917560
Received: 11 April 2022; Accepted: 9 May 2022;
Published: 7 June 2022.
Edited by:
Gianluca Testa P.O. Policlinico “Gaspare Rodolico” - Clinica Ortopedica - Via Santa Sofia, Catania, ItalyReviewed by:
Hui Lin, Nanchang University, ChinaChiara Arrigoni, Azienda Ospedaliera Nazionale SS. Antonio e Biagio e Cesare Arrigo, Italy
Copyright © 2022 Danya, Marco, Valentino, Mario and Antonio Pompilio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danya Falcioni c2NpZW56YS5jbGlub3J0b3BAZ21haWwuY29t
Specialty section: This article was submitted to Pediatric Orthopedics, a section of the journal Frontiers in Surgery
 Falcioni Danya2*
Falcioni Danya2*