- 1Department of Urology Surgery, Harbin Medical University Cancer Hospital, Harbin, China
- 2Heilongjiang Province Public Security Department Ankang Hospital, Addiction Treatment Centre, Harbin, China
- 3Medical Department, Harbin Medical University Cancer Hospital, Harbin, China
- 4Nursing Department, Harbin Medical University Cancer Hospital, Harbin, China
Background: Anxiety and depression are commonly recognized and prognostically relevant in cancer patients. The aim of this study was to explore the 3-year longitudinal changes in anxiety and depression, their risk factors, and prognostic value in patients with bladder cancer.
Methods: Hospital Anxiety and Depression Scale for anxiety (HADS-A) and depression (HADS-D) scores of 120 postoperative bladder cancer patients and 100 healthy controls (HCs) were assessed. Additionally, the HADS-A and HADS-D scores of bladder cancer patients were determined at 1 year, 2 years, and 3 years post surgery.
Results: HADS-A score (7.7 ± 3.0 vs. 4.8 ± 2.6), anxiety rate (38.3% vs. 9.0%), HADS-D score (7.7 ± 3.3 vs. 4.3 ± 2.6), depression rate (40.0% vs. 11.0%), as well as anxiety degree and depression degree, were all increased in bladder cancer patients compared with HCs (all P < 0.001). Besides, the HADS-A score gradually increased from baseline to 3 years (P = 0.004), while the anxiety rate, HADS-D score, and depression rate did not change significantly (all P > 0.050). Gender, tumor size, marriage status, hypertension, diversity, and lymph node (LN) metastasis were associated with anxiety or depression in patients with bladder cancer (all P < 0.050). Anxiety was associated with shortened overall survival (OS) (P = 0.024) but did not link with disease-free survival (DFS) (P = 0.201); depression was not correlated with either DFS or OS (both P > 0.050).
Conclusion: The prevalence and severity of anxiety and depression are high in patients with bladder cancer, which are influenced by gender, tumor features, marriage status, and hypertension; in addition, their correlation with survival is relatively weak.
Introduction
Bladder cancer is the 10th most common cancer worldwide, with 570,000 new cases and 210,000 deaths in 2020 (1–3). Transurethral resection of bladder tumor (TURBT) is the gold standard for definitive diagnosis and the standard treatment for non-muscle-invasive bladder cancer (NMIBC), while radical cystectomy with lymph node dissection is the primary surgical regimen for patients with muscle-invasive or advanced bladder cancer (4–6). Although the 5-year survival for patients with resectable bladder cancer is estimated to be between 35% and 69%, many patients will encounter postoperative complications (such as urinary tract infection, frequent urination, cachexia, etc.) after surgeries; meanwhile, they will also suffer from mental disorders (including anxiety, depression, suicidality, etc.), which will ultimately impair their quality of life (7–10).
Anxiety and depression are the most common psychological problems observed in patients with postoperative bladder cancer and have been frequently reported in previous studies (11–14). For instance, a systematic review exhibited that the prevalence of anxiety (ranges from 12.5% to 71.3%) and depression (ranges from 4.7% to 78%) in bladder cancer patients varies in different regions and countries (11). Another study disclosed that more than half of bladder cancer patients were recognized with anxiety and depression after treatment; meanwhile, mental and psychological disorders led to poor clinical outcomes (12). However, most relevant studies were cross-sectional or had a short follow-up duration (less than 1 year), let alone the long-term follow-up.
Hence, this study detected the anxiety and depression status during a 3-year follow-up period with the aim of exploring their longitudinal changes, risk factors, and predictive value for survival in patients with bladder cancer.
Methods
Subjects
This prospective study serially included bladder cancer patients who underwent tumor resection by TURBT or radical cystectomy (by means of open surgery, laparoscope, or robot-assisted laparoscope) from January 2016 to March 2018; the surgical regimens depended on patient's pathological type (NMIBC or MIBC). The inclusion criteria were as follows: (1) pathologically diagnosed with bladder cancer; (2) more than 18 years old; (3) received TURBT or radical cystectomy; (4) had no difficulty in completing the evaluation of anxiety and depression using the Hospital Anxiety and Depression Scale (HADS); (5) willing to comply with the follow-up schedule. The patients who met the following conditions were excluded: (1) having complications with a severe mental disorder, such as bipolar disorder and schizophrenia; (2) having severe cognitive impairment; (3) who were concomitant with other malignancies; (4) who were pregnant or lactating women. Besides, during the same period, healthy subjects who came to the hospital for physical examinations were enrolled in the study as health controls (HCs). The inclusion criteria for HCs were: (1) having no abnormities in physical examinations; (2) having matched age and gender to bladder cancer patients; (3) having no severe mental disorders or severe cognitive impairments; (4) those without a prior history of cancers or other malignant diseases; (5) those who were non-pregnant and non-lactating. The exclusion criteria for bladder cancer patients were also suitable for HCs. The study was permitted by the Ethics Committee. All patients in this study signed the informed consent.
Assessment of Anxiety and Depression
The HADS for anxiety (HADS-A) score and the HADS for depression (HADS-D) score were used to access the anxiety status and depression status of all subjects, respectively. For patients with bladder cancer, the assessments were carried out on the day of discharge from the hospital (baseline), at 1 year (±1 month) after surgery, 2 years (±1 month) after surgery, and 3 years (±1 month) after surgery. For HCs, the assessments were performed after enrollment. The maximum score of HADS-A or HADS-D was 21, with a higher score indicating a severer anxiety or depression status. Specifically, the HADS-A or HADS-D was divided into four grades: 0–7, no anxiety or no depression; 8–10, mild status; 11–14, moderate status; 15–21, severe status (15).
Evaluation of Survival Data
All patients with bladder cancer were followed up through outpatient visits for at least 3 years until March 2021. During the follow-up period, the disease status of patients was recorded. Totally, 23 (19.2%) patients were lost to follow-up, and 14 (11.2%) patients died. Based on the follow-up data, disease-free survival (DFS), overall survival (OS), and cancer-specific survival (CSS) were imputed.
Statistical Analysis
Statistical analysis and figure plotting were performed using SPSS V.22.0 (IBM Corp., USA) and GraphPad Prism V.6.0 (GraphPad Software Inc., USA), respectively. Differences in anxiety and depression status between the two groups were analyzed using the Student's t-test, Mann–Whitney U test, or Chi-square test. Changes in anxiety and depression status over time were determined using the repeated ANOVA test or Cochran's Q test. Correlations of clinical features with anxiety and depression status were assessed using the Chi-square test or Fisher's exact test. Independence risk factors of anxiety status and depression status were determined using forward-stepwise multivariate logistic regression analysis with all potential parameters included. Correlation of DFS, OS, and CSS with anxiety and depression status was presented using the Kaplan–Meier method and evaluated using the log-rank test. A score of P < 0.05 was considered significant.
Results
Clinical Features of Bladder Cancer Patients
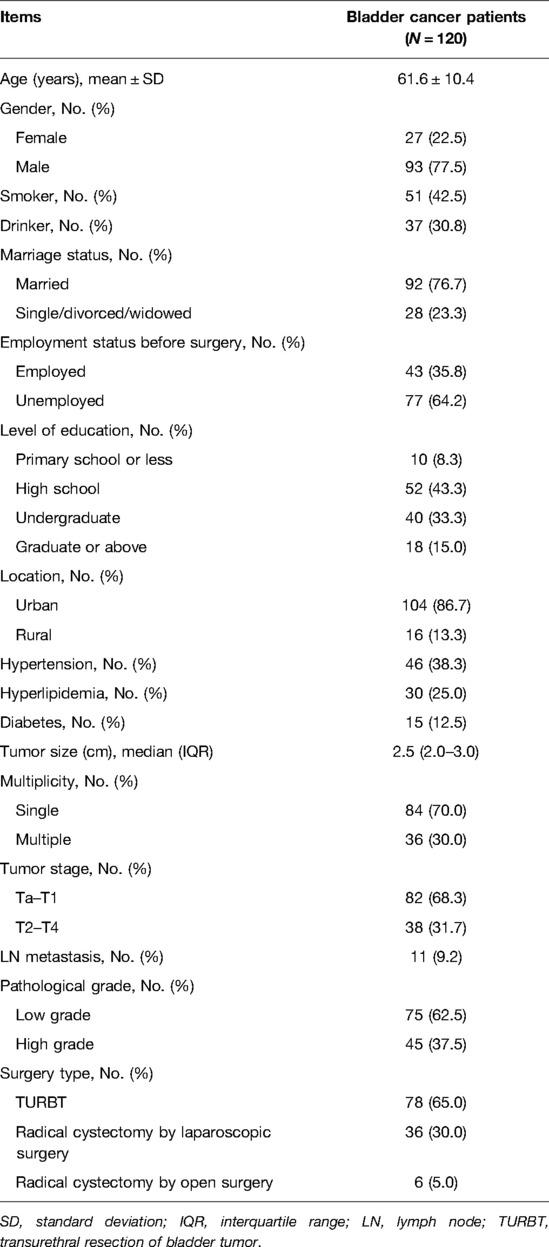
In this study, a total of 120 bladder cancer patients and 100 HCs were recruited. The mean age of the 120 patients with bladder cancer was 61.6 ± 10.4 years, of whom 27 (22.5%) were females, and 93 (77.5%) were males (Table 1). Moreover, the median tumor size was 2.5 (interquartile range (IQR): 2.0–3.0) cm; furthermore, 84 (70.0%) patients were diagnosed with a single tumor, whereas the remaining 36 (30.0%) patients were diagnosed with multiple tumors. In terms of the tumor stage, 82 (68.3%) patients were defined as Ta–T1 stage, and 38 (31.7%) patients were assessed as T2–T4 stage. Furthermore, 11 (9.2%) patients were found to have lymph node (LN) metastasis. In terms of the pathological grade, 75 (62.5%) patients were classified as low grade; meanwhile, 45 (37.5%) patients were classified as high grade. With regard to the surgery type, 78 (65.0%), 36 (30.0%), and 6 (5.0%) patients received TURBT, radical cystectomy by laparoscopic surgery, and radical cystectomy by open surgery, accordingly. Additionally, the detailed clinical features of the patients with bladder cancer are listed in Table 1.
Comparison of Anxiety and Depression Status Between Bladder Cancer Patients and HCs
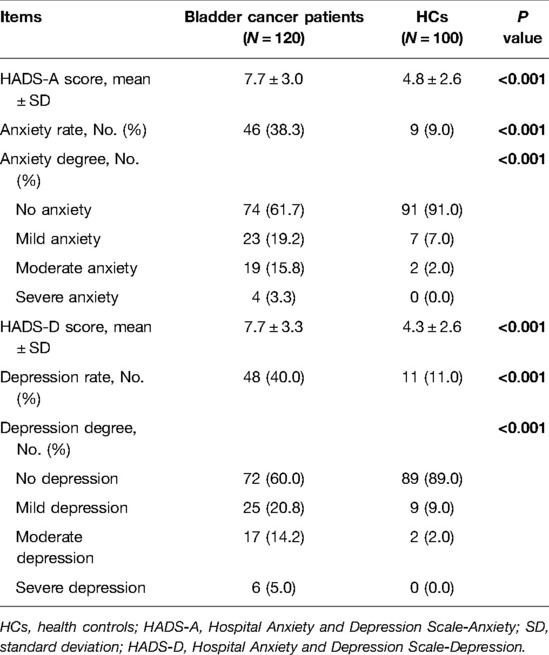
Patients with bladder cancer exhibited aggravated anxiety and depression compared with HCs (Table 2). More specifically, both the mean HADS-A scores (7.7 ± 3.0 vs. 4.8 ± 2.6) and the anxiety rates (38.3% vs. 9.0%) were elevated in bladder cancer patients compared with HCs (both P < 0.001). Besides, anxiety was more severe in patients with bladder cancer than in HCs (P < 0.001). Similarly, the mean HADS-D scores (7.7 ± 3.3 vs. 4.3 ± 2.6) and depression rates (40.0% vs. 11.0%) were increased in patients with bladder cancer than in HCs (both P < 0.001). Also, depression was more severe in patients with bladder cancer than in HCs (P < 0.001).
Longitudinal Changes of Anxiety and Depression in Bladder Cancer Patients
The HADS-A scores of patients with bladder cancer gradually increased from baseline to 3 years (P = 0.004, Figure 1A); meanwhile, the anxiety rate also disclosed an increasing trend from baseline to 3 years (but lacked statistical significance) (P = 0.054, Figure 1B). In detail, the HADS-A scores at baseline, 1, 2, and 3 years were 7.7 ± 3.0, 7.8 ± 3.3, 8.1 ± 2.9, and 8.5 ± 3.2, respectively; meanwhile, the anxiety rates at baseline, 1, 2, and 3 years were 38.3%, 41.4%, 44.1%, and 48.2%, correspondingly. There was no difference in HADS-D scores (P = 0.131, Figure 1C) at baseline, 1, 2, and 3 years in patients with bladder cancer, as well as in the depression rate (P = 0.818, Figure 1D). In detail, the HADS-D scores at baseline, 1, 2, and 3 years were 7.7 ± 3.3, 7.5 ± 3.1, 7.9 ± 3.1, and 8.1 ± 3.1, respectively; meanwhile, the depression rates at baseline, 1, 2, and 3 years were 40.0%, 37.1%, 41.2%, and 43.4%, correspondingly.
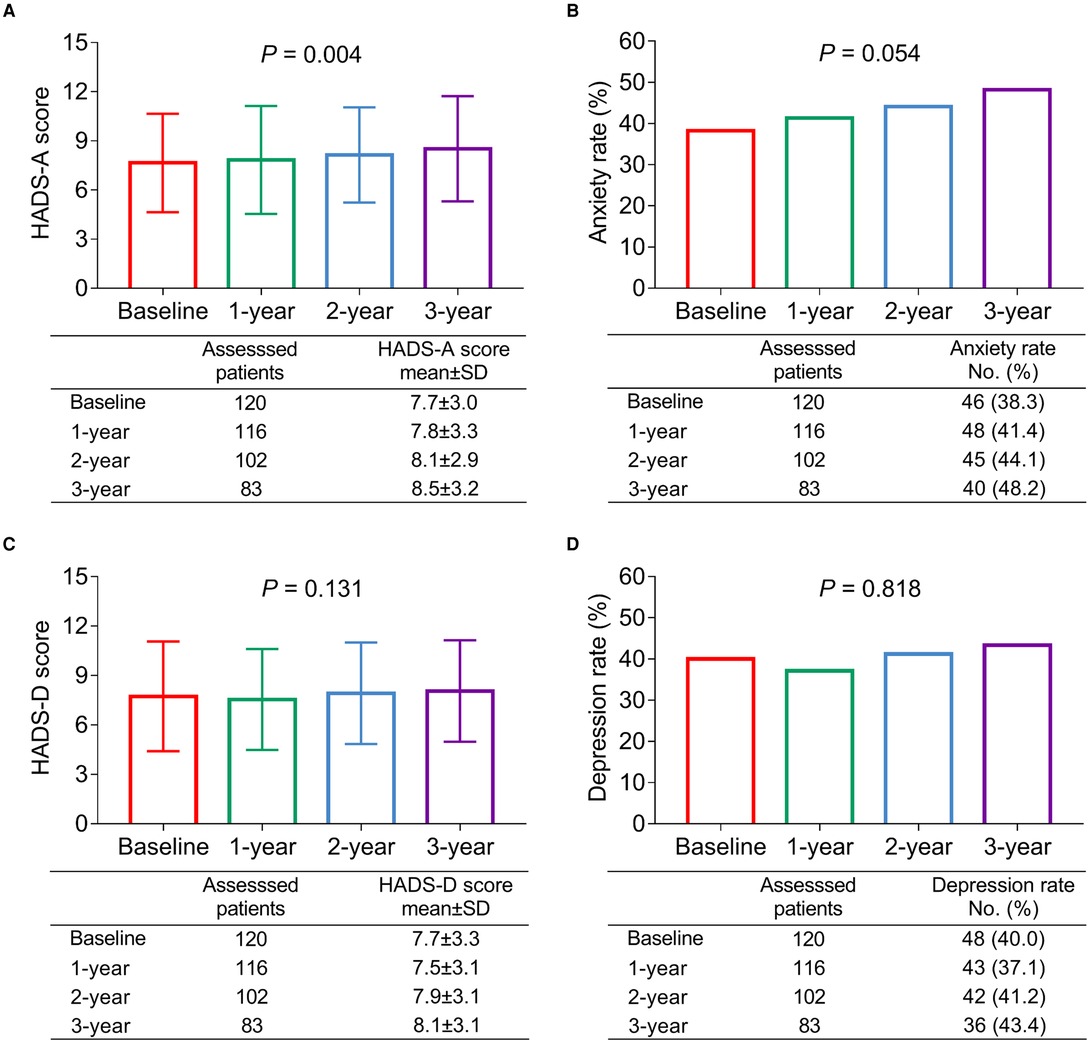
Figure 1. Anxiety gradually aggravated, while depression status was not changed from baseline to 3 years in patients with bladder cancer. The longitudinal changes of the HADS-A score (A); anxiety rate (B); HADS-D score (C); and depression rate (D) during 3-year follow-up duration in patients with bladder cancer.
Correlation of Clinical Features with Anxiety and Depression in Bladder Cancer Patients
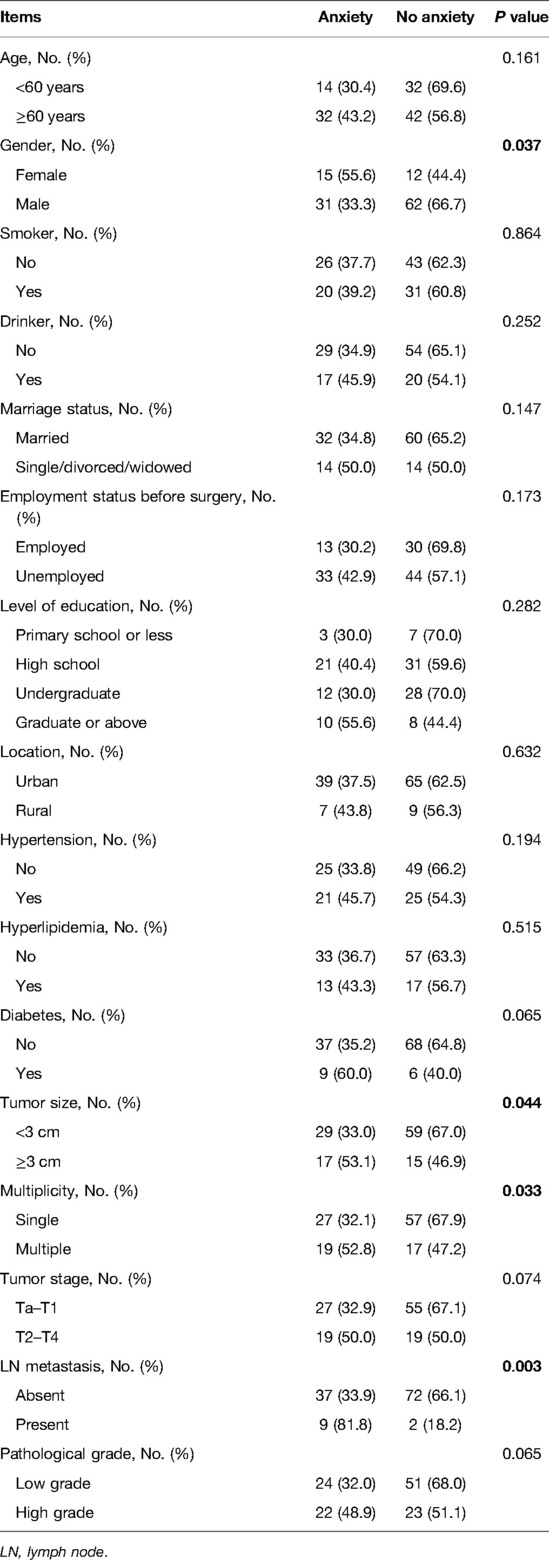
Female (vs. male) (P = 0.037), tumor size ≥3 cm (vs. <3 cm) (P = 0.044), multiple tumors (vs. single) (P = 0.033), and LN metastasis (vs. absent) (P = 0.003) were associated with elevated anxiety rate in patients with bladder cancer (Table 3). However, age, smoking, drinking, marriage status, employment status before surgery, level of education, location, hypertension, hyperlipidemia, diabetes, tumor stage, and pathological grade were not linked with anxiety (all P > 0.050).
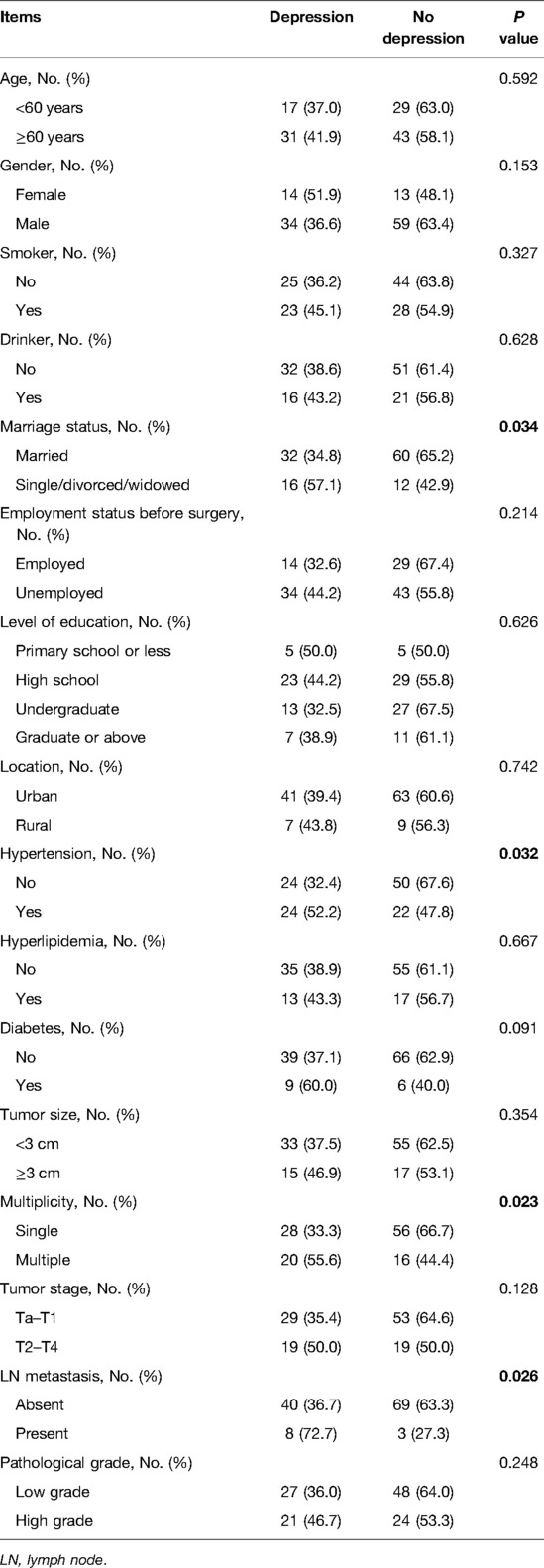
Besides, single/divorced/widowed (vs. married) (P = 0.034), hypertension (vs. no) (P = 0.032), multiple tumors (vs. single) (P = 0.023), and LN metastasis (vs. absent) (P = 0.026) were correlated with an increased depression rate in patients with bladder cancer (Table 4). Nevertheless, age, gender, smoking, drinking, employment status before surgery, level of education, location, hyperlipidemia, diabetes, tumor size, tumor stage, and pathological grade were not related to depression (all P > 0.050).
Further, forward-stepwise multivariate logistic regression analyses were conducted to investigate the independent risk factors of anxiety and depression in bladder cancer patients. Diabetes (vs. no) (odds ratio (OR): 3.528, P = 0.035), multiple tumors (vs. single) (OR: 2.612, P = 0.028), and LN metastasis (vs. absent) (OR: 10.252, P = 0.005) were independently linked with the occurrence of anxiety; meanwhile, multiple tumors (vs. single) (OR: 2.500, P = 0.028) and LN metastasis (vs. absent) (OR: 4.600, P = 0.034) were also independently associated with the occurrence of depression in bladder cancer patients (Supplementary Table 1).
With regard to the correlation between surgery type and HADS score among bladder cancer patients, it was observed that the HADS-A score at baseline was varied among patients who received TURBT, radical cystectomy by laparoscopic surgery, and radical cystectomy by open surgery (P = 0.029); in detail, the respective HADS-A score at baseline was highest in patients treated with radical cystectomy by open surgery (10.5 ± 1.5), followed by patients who received radical cystectomy by laparoscopic surgery (8.0 ± 3.2), and lowest in patients treated with TURBT (7.3 ± 2.9) (Supplementary Table 2). While the HADS-A score at 1, 2, and 3 years and the HADS-D score at baseline, 1, 2, and 3 years showed no difference among patients with different surgery types (all P < 0.050), they only disclosed a trend that they were highest in patients with radical cystectomy by open surgery, followed by patients with radical cystectomy by laparoscopic surgery, and lowest in patients with TURBT.
Correlation of Anxiety and Depression at Baseline with Survival in Bladder Cancer Patients
Anxiety at baseline was not linked with cumulative DFS (P = 0.201, Figure 2A), whereas it was associated with shortened cumulative OS (P = 0.024, Figure 2B) in patients with bladder cancer. In terms of depression at baseline, it was correlated with neither cumulative DFS (P = 0.240, Figure 3A) nor OS (P = 0.173, Figure 3B) in bladder cancer patients.
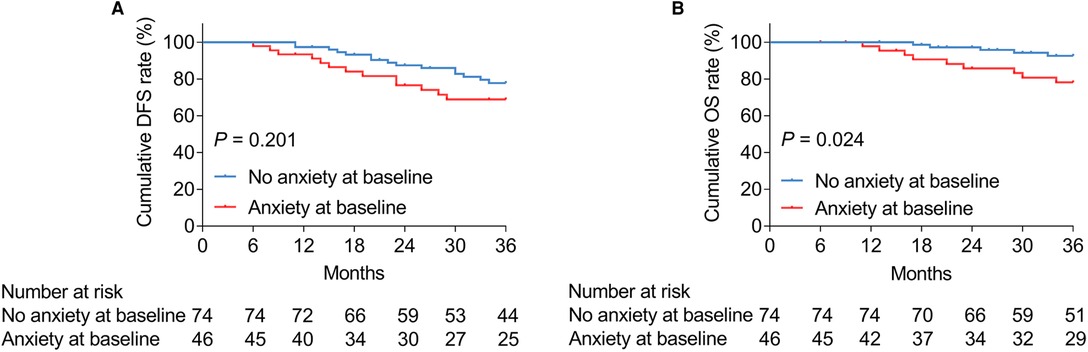
Figure 2. Anxiety at baseline was related to shortened OS in patients with bladder cancer. Correlation of anxiety at baseline with cumulative DFS (A) and OS (B) in patients with bladder cancer.
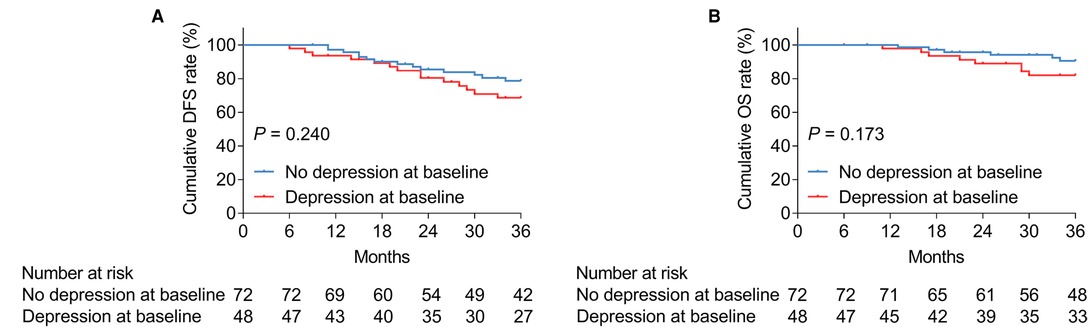
Figure 3. Depression at baseline was not linked with DFS or OS in patients with bladder cancer. Correlation of depression at baseline with cumulative DFS (A) and OS (B) in patients with bladder cancer.
Among 14 death cases, 12 cases were assessed as cancer-related deaths. Moreover, anxiety at baseline was related to reduced CSS in patients with bladder cancer (P = 0.025, Supplementary Figure 1A), whereas depression at baseline was not associated with CSS (P = 0.180, Supplementary Figure 1B).
Discussion
There is a growing trend of bladder cancer patients to undergo psychological disorders (such as anxiety and depression) after surgical treatment, as has been demonstrated (13, 16–19). For instance, a previous study indicated that many bladder cancer patients who received radical cystectomy suffered from anxiety and depression during the perioperative period, with a prevalence rate of 34% (16). However, the previous studies were primarily cross-sectional, and only a few had a short-term follow-up period, let alone the long-term follow-up. The current study showed that their anxiety status, depression status, and incidence rates were all elevated in bladder cancer patients than HCs. Besides, there was an increasing trend of anxiety status from baseline to 3 years in bladder cancer patients, whereas there was no difference in depression. The possible reasons might be as follows: (1) Patients tend to panic about cancer and worry about their lives, and the negative emotions would cause anxiety and depression. (2) Patients with bladder cancer usually encounter postoperative complications (such as urinary tract infection, frequent urination, etc.) after surgical treatment, which would aggravate their anxiety and depression (19). Therefore, combining these two explanations, patients with bladder cancer have a higher prevalence and severity of anxiety and depression than HCs. (3) Those postoperative patients initially feel relieved after surgical treatment, while they would be worried about the recurrent over time, and then their anxiety would gradually increase during the 3-year follow-up period. Therefore, it is necessary to provide nursing, health education, and adaptability improvement for patients with recurrence to relieve them of anxiety and depression.
Apart from investigating the detailed anxiety and depression status of bladder cancer patients, this study found that the gender female (vs. male), tumor size ≥3 cm (vs. <3 cm), multiple tumors (vs. single), and LN metastasis (vs. absent) were associated with an elevated anxiety rate, whereas single/divorced/widowed (vs. married), hypertension (vs. no), multiple tumors (vs. single), and LN metastasis (vs. absent) were correlated with an increased depression rate. Probable explanations might be as follows: (1) It was observed that females were more likely to encounter anxiety during the hormonal flux period, whereas testosterone in males could be protective against anxiety (20, 21). Thus, females (vs. males) were correlated with an increased anxiety rate in patients with bladder cancer. (2) Single/divorced/widowed patients were more likely to feel lonely and unsupported, which would lead to depression (22, 23). Therefore, single/divorced/widowed was linked with depression in patients with bladder cancer. (3) The previous study showed that antihypertensive medication regimens might cause depression (24). Hence, hypertension was associated with depression in patients with bladder cancer. (4) Patients with a large tumor size, multiple tumors, or LN metastasis often suffered from limited treatment efficacy and worse clinical outcomes, which made them more anxious and depressed (25). Thus, tumor size ≥3 cm (vs. <3 cm) was related to elevated anxiety; meanwhile, multiple tumors (vs. single) and LN metastasis (vs. absent) were correlated with increased anxiety as well as depression in patients with bladder cancer. Additionally, the HADS-A score was highest in patients treated with radical cystectomy by open surgery, followed by patients who receive radical cystectomy by laparoscopic surgery, and lowest in patients treated with TURBT. While the HADS-A score at 1, 2, and 3 years, and the HADS-D score at baseline, 1, 2, and 3 years showed no difference among patients with different surgery types, they only disclosed a trend that they were highest in patients with radical cystectomy by open surgery, followed by patients with radical cystectomy by laparoscopic surgery, and lowest in patients with TURBT. The possible explanation for this might be as follows: Radical cystectomy by open surgery tended to cause more injuries than the other two surgery types, which would lead to more serious anxiety and depression, while its impairment was obvious only in a short term. Hence, the difference in long-term HADS-A and HADS-D scores among patients with different surgery types was not obvious.
In terms of the correlation of anxiety and depression with survival in bladder cancer patients, the previous studies exhibited that bladder cancer patients with post-treatment anxiety and depression tend to have worse OS and cancer-specific survival (9, 12). Unlike the present study, which found that anxiety at baseline was not associated with DFS but with shortened OS, depression at baseline was not correlated with either DFS or OS in patients with bladder cancer. The possible reason for this might be as follows: Anxiety and depression affected patients compliance with treatment to some extent, whereas the survival was more directly influenced by treatment efficacy (such as surgical treatment and pharmacotherapy, etc.), tumor stage, and other factors. Therefore, the correlation of anxiety and depression with survival in bladder cancer patients was relatively weak.
There were some limitations in the current study. First, the sample size was relatively small, which would result in weak statistical power. Second, the mental status of recurrent patients was usually worse than that of newly diagnosed patients, which deserved further study. Third, the anxiety and depression status of those patients prior to surgical treatment was undetected.
In conclusion, the prevalence and severity of anxiety and depression are high in patients with bladder cancer, which are influenced by gender, tumor features, marriage status, and hypertension; in addition, their correlation with survival is relatively weak.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Harbin Medical University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GC conceptualized the study; MG and WW contributed to data acquisition; MG, XK, and GC contributed to data analysis; MG, YL, and WW drafted the manuscript; XK and GC revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Haiyan Foundation of Harbin Medical University Cancer Hospital (NO.JJQN 2020-23) and Haiyan Foundation of Harbin Medical University Cancer Hospital (NO.JJMS 2022-14).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.893249/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. (2020) 324:1980–91. doi: 10.1001/jama.2020.17598
2. Liu X, Jiang J, Yu C, Wang Y, Sun Y, Tang J, et al. Secular trends in incidence and mortality of bladder cancer in China, 1990–2017: a joinpoint and age-period-cohort analysis. Cancer Epidemiol. (2019) 61:95–103. doi: 10.1016/j.canep.2019.05.011
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Babjuk M, Burger M, Comperat EM, Gontero P, Mostafid AH, Palou J, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) – 2019 update. Eur Urol. (2019) 76:639–57. doi: 10.1016/j.eururo.2019.08.016
5. Witjes JA, Bruins HM, Cathomas R, Comperat EM, Cowan NC, Gakis G, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. (2021) 79:82–104. doi: 10.1016/j.eururo.2020.03.055
6. Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. (2020) 70:404–23. doi: 10.3322/caac.21631
7. Pfail JL, Small AC, Cumarasamy S, Galsky MD. Real world outcomes of patients with bladder cancer: effectiveness versus efficacy of modern treatment paradigms. Hematol Oncol Clin North Am. (2021) 35:597–612. doi: 10.1016/j.hoc.2021.01.005
8. Lokeshwar SD, Press BH, Nie J, Klaassen Z, Kenney PA, Leapman MS. Cachexia and bladder cancer: clinical impact and management. Curr Opin Support Palliat Care. (2021) 15:260–5. doi: 10.1097/SPC.0000000000000580
9. Pham H, Torres H, Sharma P. Mental health implications in bladder cancer patients: a review. Urol Oncol. (2019) 37:97–107. doi: 10.1016/j.urolonc.2018.12.006
10. Vartolomei L, Vartolomei MD, Shariat SF. Bladder cancer: depression, anxiety, and suicidality among the highest-risk oncology patients. Eur Urol Focus. (2020) 6:1158–61. doi: 10.1016/j.euf.2019.10.008
11. Vartolomei L, Ferro M, Mirone V, Shariat SF, Vartolomei MD. Systematic review: depression and anxiety prevalence in bladder cancer patients. Bl Cancer. (2018) 4:319–26. doi: 10.3233/BLC-180181
12. Jazzar U, Yong S, Klaassen Z, Huo J, Hughes BD, Esparza E, et al. Impact of psychiatric illness on decreased survival in elderly patients with bladder cancer in the United States. Cancer. (2018) 124:3127–35. doi: 10.1002/cncr.31404
13. Oserowsky A, Anwar T, Lough C, Golzy M, Murray KS. The significant role of depression in elderly patients with bladder cancer. Eur Urol Open Sci. (2021) 33:11–8. doi: 10.1016/j.euros.2021.08.007
14. Li M, Wang L. The associations of psychological stress with depressive and anxiety symptoms among Chinese bladder and renal cancer patients: the mediating role of resilience. PLoS One. (2016) 11:e0154729. doi: 10.1371/journal.pone.0154729
15. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
16. Palapattu GS, Haisfield-Wolfe ME, Walker JM, BrintzenhofeSzoc K, Trock B, Zabora J, et al. Assessment of perioperative psychological distress in patients undergoing radical cystectomy for bladder cancer. J Urol. (2004) 172:1814–7. doi: 10.1097/01.ju.0000141245.08456.1a
17. Mansson A, Al Amin M, Malmstrom PU, Wijkstrom H, Abol Enein H, Mansson W. Patient-assessed outcomes in Swedish and Egyptian men undergoing radical cystectomy and orthotopic bladder substitution–a prospective comparative study. Urology. (2007) 70:1086–90. doi: 10.1016/j.urology.2007.07.071
18. Benner C, Greenberg M, Shepard N, Meng MV, Rabow MW. The natural history of symptoms and distress in patients and families following cystectomy for treatment of muscle invasive bladder cancer. J Urol. (2014) 191:937–42. doi: 10.1016/j.juro.2013.10.101
19. Yang YL, Liu L, Li MY, Shi M, Wang L. Psychological disorders and psychosocial resources of patients with newly diagnosed bladder and kidney cancer: a cross-sectional study. PLoS One. (2016) 11:e0155607. doi: 10.1371/journal.pone.0155607
20. McHenry J, Carrier N, Hull E, Kabbaj M. Sex differences in anxiety and depression: role of testosterone. Front Neuroendocrinol. (2014) 35:42–57. doi: 10.1016/j.yfrne.2013.09.001
21. Kiely KM, Brady B, Byles J. Gender, mental health and ageing. Maturitas. (2019) 129:76–84. doi: 10.1016/j.maturitas.2019.09.004
22. Kawata Y, Maeda M, Sato T, Maruyama K, Wada H, Ikeda A, et al. Association between marital status and insomnia-related symptoms: findings from a population-based survey in Japan. Eur J Public Health. (2020) 30:144–9. doi: 10.1093/eurpub/ckz119
23. Brown CR, Hambleton IR, Sobers-Grannum N, Hercules SM, Unwin N, Nigel Harris E, et al. Social determinants of depression and suicidal behaviour in the Caribbean: a systematic review. BMC Public Health. (2017) 17:577. doi: 10.1186/s12889-017-4371-z
24. Hamam MS, Kunjummen E, Hussain MS, Nasereldin M, Bennett S, Miller J. Anxiety, depression, and pain: considerations in the treatment of patients with uncontrolled hypertension. Curr Hypertens Rep. (2020) 22:106. doi: 10.1007/s11906-020-01117-2
Keywords: anxiety and depression, bladder cancer, longitudinal change, risk factors, survival
Citation: Guo M, Li Y, Wang W, Kang X and Chen G (2022) Variation of Anxiety and Depression During a 3-Year Period as Well as Their Risk Factors and Prognostic Value in Postoperative Bladder Cancer Patients. Front. Surg. 9:893249. doi: 10.3389/fsurg.2022.893249
Received: 10 March 2022; Accepted: 21 June 2022;
Published: 19 July 2022.
Edited by:
Dmitry Enikeev, I.M. Sechenov First Moscow State Medical University, RussiaReviewed by:
Liliana Vartolomei, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaAndrey O Morozov, I.M. Sechenov First Moscow State Medical University, Russia
Copyright © 2022 Guo, Li, Wang, Kang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guiyun Chen eXVueW91OTQ3ODMyQDE2My5jb20=
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Meiling Guo1
Meiling Guo1 Guiyun Chen
Guiyun Chen