- 1Department of Anesthesiology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Anesthesiology, West China Hospital of Sichuan University, Chengdu, China
Background: This study aimed to determine the efficacy and safety of celecoxib for pain management after total knee arthroplasty (TKA).
Methods: PubMed, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched to identify eligible randomized controlled trials (RCTs) that compared celecoxib with a placebo in term of pain control efficacy after TKA. Primary outcomes included pain scores at 24, 48, and 72 h after TKA. Secondary outcomes included the active range of motion (ROM) at 24, 48,72 h, and 7 days postoperatively, morphine consumption over 72 h after TKA, incidence of postoperative nausea and vomiting (PONV), and total blood loss after surgery. Data analysis was conducted using RevMan version 5.3.
Results: Five RCTs involving 593 participants were included in the study. Compared with a placebo, celecoxib significantly reduced visual analog scale (VAS) scores at rest at 24 h [mean difference (MD) = −0.72; 95% confidence interval (CI), −1.27 to −0.17; I2 = 82%; P = 0.01], 48 h (MD = −1.51; 95% CI, −2.07 to −0.95; I2 = 0%; P < 0.00001), and 72 h (MD = −1.30; 95% CI, −2.07 to −0.54; I2 = 82%; P = 0.0009) after TKA, decreased morphine consumption over postoperative 72 h (MD = −0.73; 95% CI, −0.96 to −0.51; I2 = 96%; P < 0.00001), and increased active ROM at 48 h (MD = 13.23; 95% CI, 7.79 to 18.67; I2 = 0%; P < 0.00001), 72 h (MD = 6.52; 95% CI, 4.95 to 8.10; I2 = 68%; P < 0.00001), and 7 days (MD = 7.98; 95% CI, 3.64 to 12.31; I2 = 68%; P = 0.0003) after the operation. No significant difference was found in the active ROM at 24 h (MD = 7.60; 95% CI, −6.14 to 21.34; I2 = 94%; P = 0.28) and the incidence of PONV after surgery [risk ratio (RR) = 0.66; 95% CI, 0.40 to 1.09; I2 = 0%; P = 0.11].
Conclusion: The administration of celecoxib is an effective and safe strategy for postoperative analgesia after TKA.
Introduction
Total knee arthroplasty (TKA) is associated with moderate to severe postoperative pain, which contributes to the demand for analgesics and the inability to participate in postoperative rehabilitation, which leads to delayed recovery and hospital discharge (1–5). The primary goal of postoperative analgesia is to reduce both pain and the need for opioid prescriptions, thereby reducing the rate of occurrence of opioid-related adverse events. The current strategies for postoperative pain after TKA include administration of oral analgesics, periarticular injections of local anesthetics, peripheral nerve blocks, and multimodal pain regimens (6).
Celecoxib is a selective cyclooxygenase (COX)-2 inhibitor and an effective analgesic for acute postoperative pain (7). One recent study showed that early administration of celecoxib after TKA significantly reduced early visual analog scale (VAS) pain scores, improved sleep quality, and active knee flexion angles (8). However, Stepan et al. reported that celecoxib had no effect on postoperative pain and opioid consumption in patients who underwent hand surgery (9). Therefore, it is worthwhile to conduct a systematic review and meta-analysis to synthesize evidence regarding the effect of celecoxib on postoperative pain management after TKA.
Methods
This systematic review and meta-analysis was conducted in accordance with the PRISMA guidelines (10).
Search Strategy
Two authors (XG and DZ) independently searched PubMed, the Cochrane Central Register of Controlled Trials (CENTRAL), EMBASE, and Web of Science from the first record to April 30, 2021. The search strategy was conducted using the following keywords: celecoxib, postoperative pain, and TKA. The reference lists of relevant studies were also checked to identify other potentially eligible studies.
Eligibility Criteria
The inclusion criteria were as follows: (a) the studies were randomized controlled trials (RCTs); (b) the participants of the studies were adult patients scheduled to undergo primary TKA due to degenerative arthritis of the knee joint; (c) the studies focused on the comparison between celecoxib and placebo for pain management of TKA; and (d) any of the following outcomes of interest were reported: perioperative VAS scores, range of motion (ROM), morphine consumption, and side effects (e.g., nausea, vomiting, and blood loss).
Data Exaction and Outcome Measures
The following information was extracted: author, number of patients, age, sex, body mass index, duration of surgery, type of anesthesia, and outcomes. Additional data were obtained from the corresponding authors via e-mail, as necessary. Disagreements were resolved by discussion with another author. The primary outcomes were pain scores at postoperative 24, 48, and 72 h. Secondary outcomes included the ROM at postoperative 24, 48 h, and 7 days; morphine consumption over 72 h postoperatively, the incidence of postoperative nausea and vomiting (PONV), and the estimated blood loss.
Pain scores were evaluated using the VAS (VAS 0, no pain; VAS 10, the worst unbearable pain). When continuous data were presented using the median and range, we attempted to contact the corresponding author to obtain the original data. If there was no response, the median and range were converted to the mean and standard deviation (11). And two intervention groups in one study were merged into a single intervention group (12). If pain scores were not reported at rest or during movement, we tried to contact the corresponding author; if there was no response, scores were assumed to be pain scores at rest.
Study Selection
Two authors (XG and DZ) independently reviewed the studies identified. The full text of the relevant articles was reviewed after screening their titles and abstracts. Disagreements were resolved by discussion with another author (SZ).
Risk of Bias Assessment
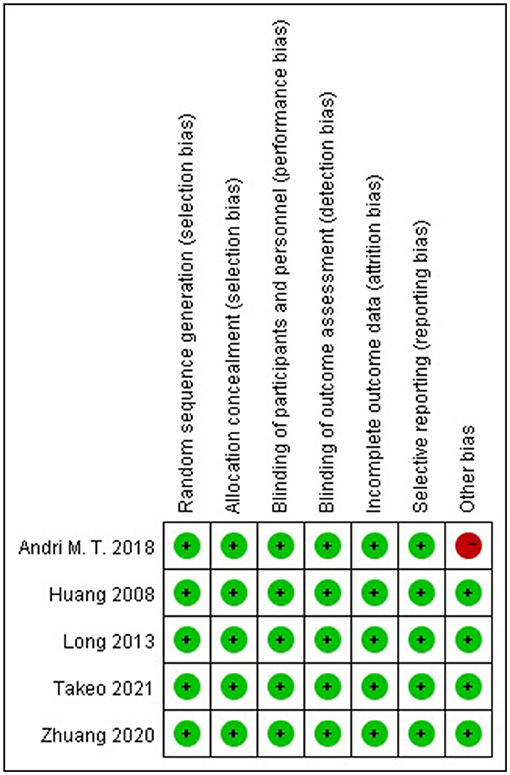
Two authors (XG and DZ) independently assessed the quality of included studies using the Cochrane Collaboration's tool (12), which comprises seven items: (a) random sequence generation (selection bias), (b) allocation concealment (selection bias), (c) blinding of participants and personnel (performance bias), (d) blinding of outcome assessment (detection bias), (e) incomplete outcome data (attrition bias), (f) selective reporting (reporting bias), and (g) other bias. The estimated risk bias for each item was rated as “low risk,” “unclear risk,” or “high risk.” Disagreements were resolved by discussion with another author (SZ).
Statistical Analysis
All statistical analyses were performed using RevMan version 5.3. Mean differences (MD) and 95% confidence intervals (CIs) were used to construct forest plots of continuous data. Risk ratios (RRs) and 95% CIs were used to construct forest plots of dichotomous data. A random-effects model was used for all analyses due to clinical heterogeneity. I2-value was used to determine the level of heterogeneity. And significant heterogeneity was considered when the I2 was > 50%. The threshold for statistical significance was set at p < 0.05.
Results
Characteristics of the Included Studies
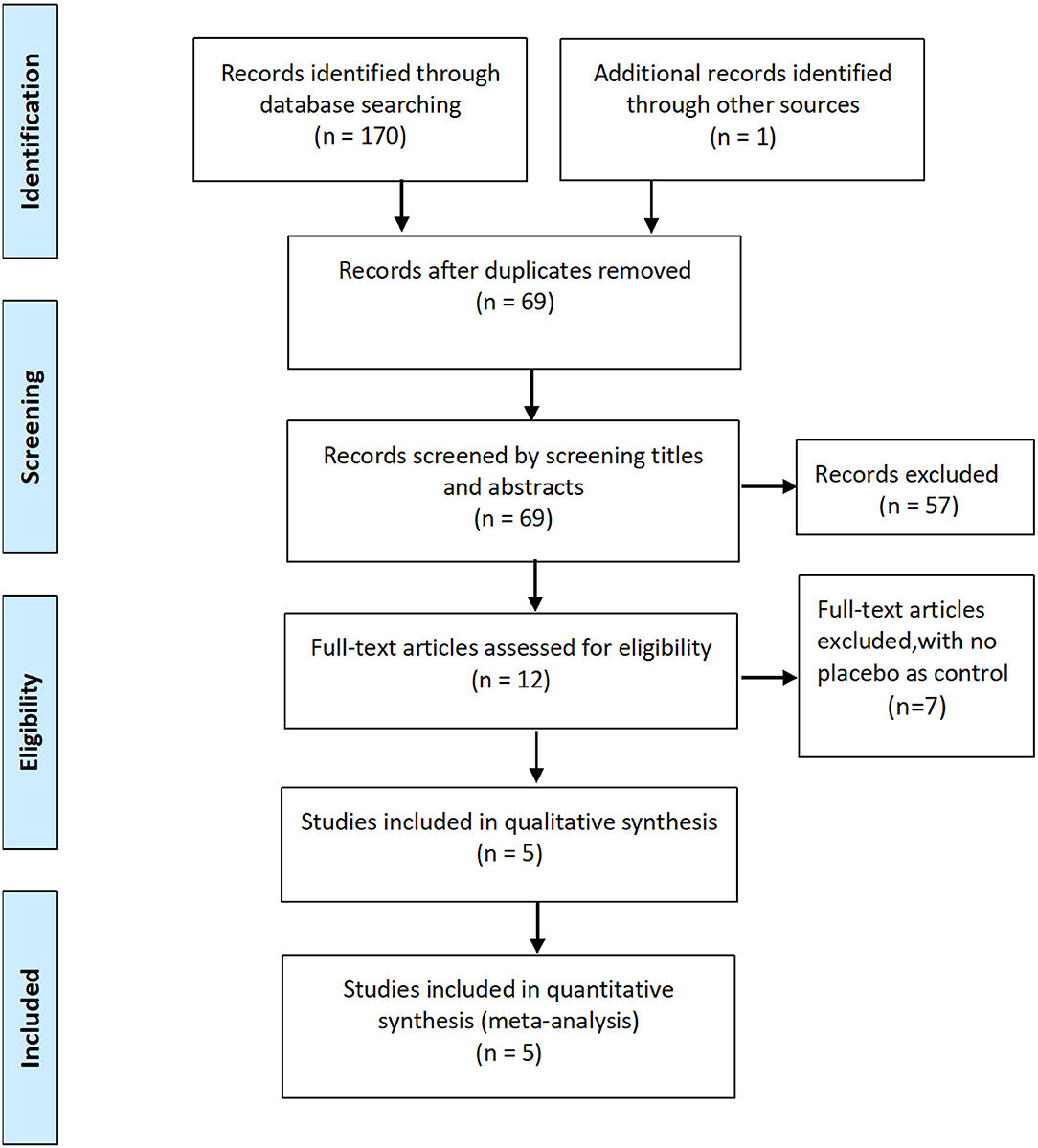
Initially, 170 potentially relevant studies were identified, of which 91 duplicates were removed. Of the 79 records left, 67 were excluded after screening the titles and abstracts, leaving 12 potentially relevant studies for full-text review. Finally, five RCTs involving 593 patients were included in the meta-analysis (8, 13–16).
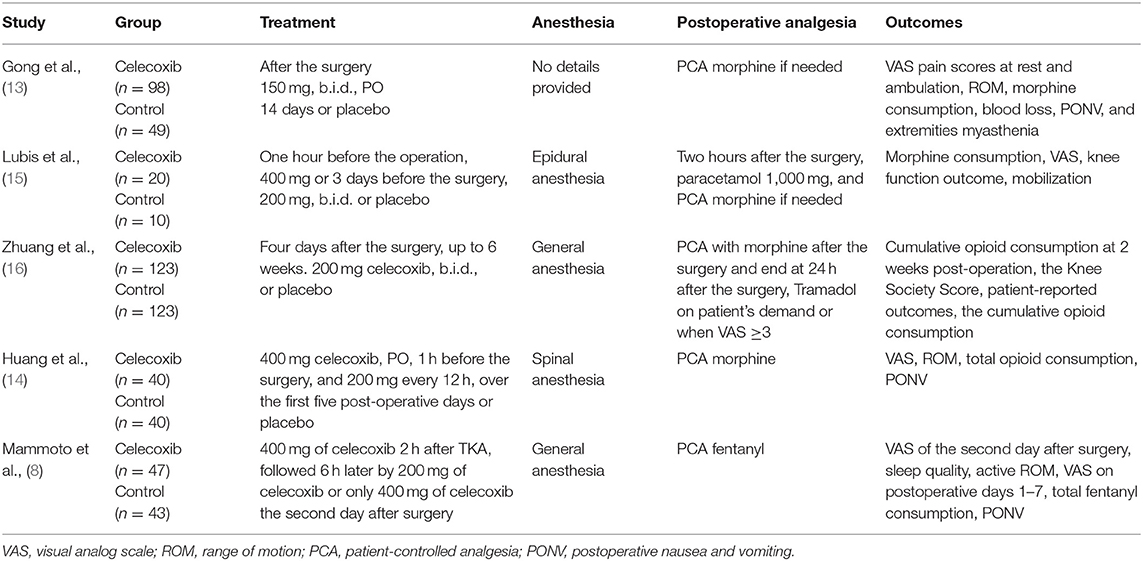
The characteristics of the included RCTs are listed in Table 1. The flow diagram of the study selection process is shown in Figure 1. The risk of bias of the included studies is shown in Figure 2.
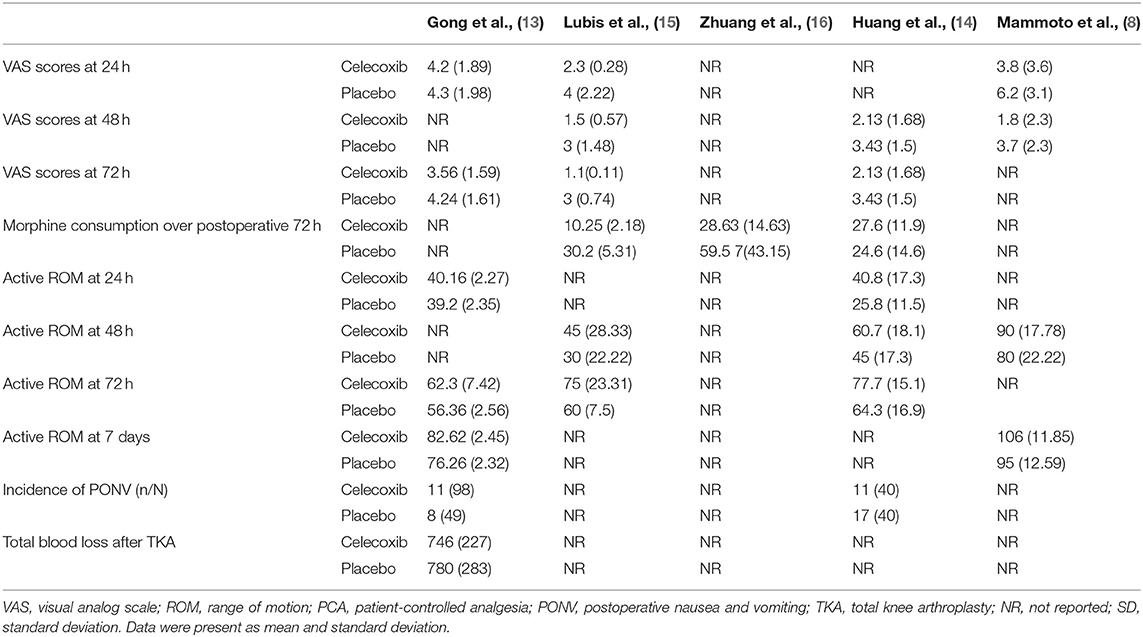
The included studies, which were performed in China, Japan and Indonesia, were published between 2008 and 2021. Among the five included studies, three reported VAS scores at 24 h after TKA (8, 13, 14), three reported VAS scores at 48 h after TKA (8, 13, 16), three reported VAS scores at 72 h after TKA (13, 14, 16), three reported morphine consumption over 72 h postoperatively, (13, 15, 16), two reported active ROM at 24 h after TKA (14, 16), three reported active ROM at 48 h after TKA (8, 13, 16), three (13, 14, 16) reported active ROM at postoperative 72 h, two reported active ROM at 7 days after TKA (8, 14), two (14, 16) reported the incidence of PONV, and one (14) reported total blood loss after TKA. The main outcomes of the included studies are summarized Table 2.
Results of the Meta-Analyses
VAS Scores at Rest at Postoperative 24 h
Three studies (8, 13, 14) reported VAS scores at rest at postoperative 24 h. Pooled results showed a significant reduction in VAS scores at rest at 24 h in the celecoxib group compared with the placebo group (MD = −0.72; 95% CI, −1.27 to −0.17; I2 = 82%; P = 0.01) (Figure 3A).

Figure 3. Forest plot for the meta-analysis of VAS scores at rest at postoperative (A) 24 h, (B) 48 h, and (C) 72 h.
VAS Scores at Rest at Postoperative 48 h
Three studies (8, 13, 16) reported VAS scores at rest at postoperative 48 h. Pooled results showed a significant reduction in VAS scores at rest at 48 h in the celecoxib group compared with the placebo group (MD = −1.51; 95% CI, −2.07 to −0.95; I2 = 0%; P < 0.00001) (Figure 3B).
VAS Scores at Rest at Postoperative 72 h
Three studies (13, 14, 16) reported VAS scores at rest at postoperative 72 h. Pooled results showed a significant reduction in VAS scores at rest at 72 h in the celecoxib group compared with the placebo group (MD = −1.30; 95% CI, −2.07 to −0.54; I2 = 82%; P = 0.0009) (Figure 3C).
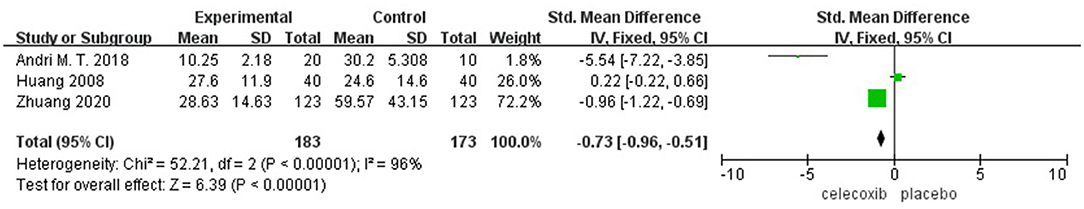
Morphine Consumption Over Postoperative 72 h
Three studies (13, 15, 16) reported morphine consumption over 72 h postoperatively. Pooled results showed a significant reduction in morphine consumption over 72 h in the celecoxib group compared with the placebo group (MD = −0.73; 95% CI, −0.96 to −0.51; I2 = 96%; P < 0.00001) (Figure 4).
Active ROM at Postoperative 24 h
Two studies (14, 16) reported active ROM at 24 h postoperatively. No significant difference was found in the active ROM at 24 h in the celecoxib group compared with the placebo group (MD = 7.60; 95% CI, −6.14 to 21.34; I2 = 94%; P = 0.28) (Figure 5A).
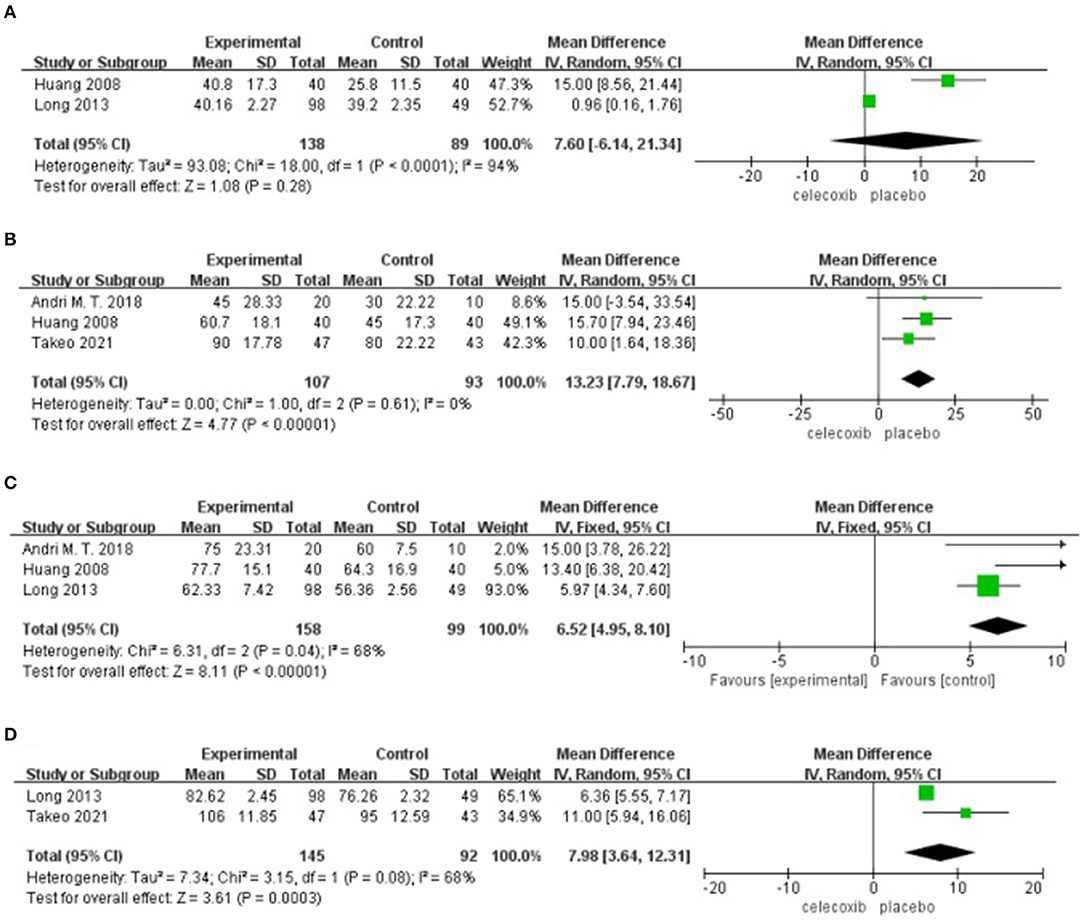
Figure 5. Forest plot for the meta-analysis of active ROM at postoperative (A) 24 h, (B) 48 h, (C) 72 h, and (D) 7 days.
Active ROM at Postoperative 48 h
Three studies (8, 13, 16) reported active ROM at 48 h postoperatively. Pooled results showed a significant increase in the active ROM at 48 h in the celecoxib group compared with the placebo group (MD = 13.23; 95% CI, 7.79 to 18.67; I2 = 0%; P < 0.00001) (Figure 5B).
Active ROM at Postoperative 72 h
Three studies (13, 14, 16) reported active ROM at 72 h postoperatively. Pooled results showed a significant increase in the active ROM at 72 h in the celecoxib group compared with the placebo group (MD = 6.52; 95% CI, 4.95 to 8.10; I2 = 68%; P < 0.00001) (Figure 5C).
Active ROM at Postoperative 7 Days
Two studies (8, 14) reported active ROM on postoperative day 7. Pooled results showed a significant increase in the active ROM at 7 days in the celecoxib group compared with the placebo group (MD = 7.98; 95% CI, 3.64 to 12.31; I2 = 68%; P = 0.0003) (Figure 5D).
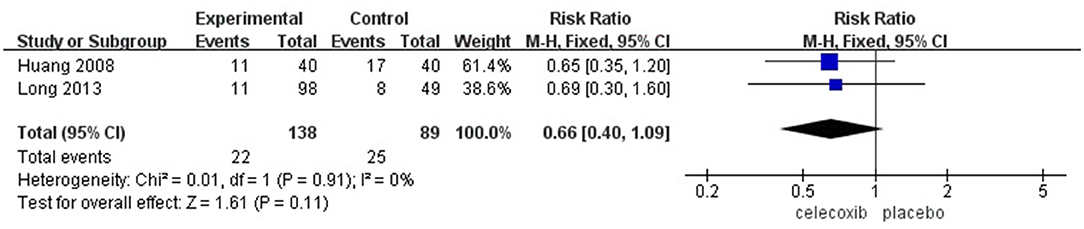
The Incidence of PONV
Two studies (14, 16) reported the incidence of PONV. No significant difference was found in the incidence of PONV in the celecoxib group compared with the placebo group (RR = 0.66; 95% CI, 0.40 to 1.09; I2 = 0%; P = 0.11) (Figure 6).
Total Blood Loss After TKA
One study (14) reported total blood loss after TKA. No significant difference was found in total blood loss in the celecoxib group compared with the placebo group (MD = −34.0; 95% CI, −125.10 to 57.10; P = 0.46).
Publication Bias
Funnel plots for publication bias could not be reliably tested because the number of included studies was small.
Discussion
This meta-analysis showed that the perioperative use of celecoxib at a dose of 200–400 mg can reduce the intensity of postoperative pain at rest at 24, 48, and 72 h after TKA. Morphine consumption over 72 h postoperatively was also significantly reduced in the celecoxib group. Moreover, celecoxib significantly improved the postoperative active ROM at 48, 72 h, and 7 days after TKA. However, celecoxib had no significant effect on PONV and blood loss.
Enhanced recovery after surgery (ERAS), first proposed by Kehlet (17), is widely used in most surgical fields. This study aimed to optimize traditional perioperative care to reduce postoperative morbidity, shorten the length of hospital stay, and accelerate postoperative recovery (17). Because of the weight-bearing characteristics of the knee joint and the high demand for functional exercise after surgery, TKA has been regarded as the most painful type of orthopedic surgery (18). Furthermore, most of the patients who underwent TKA belong to middle-aged and elderly populations; therefore, the excessive use of morphine may have easily induced addiction and caused obvious side effects, especially in elderly patients with cardiopulmonary diseases (19). Therefore, pain management for patients undergoing TKA remains difficult and has attracted wide attention (13).
Celecoxib has been proven to be beneficial for pain control in various orthopedic surgical interventions (20). Patients receiving 300 mg of celecoxib twice daily for 14 days after TKA have significantly decreased postoperative VAS scores on ambulation, reduced morphine consumption, and improved postoperative ROM without experiencing an increased estimated postoperative blood loss (14). In another study, a decrease in opioid consumption, lower occurrence of adverse events, lower VAS pain scores, and higher Knee Society Scores and EQ-5D scores were associated with celecoxib use for TKA (15).
The VAS score is the most common assessment tool for pain intensity. As the primary outcome in our meta-analysis, data on VAS scores showed that celecoxib was associated with effective pain relief, which is consistent with the findings of previous studies (20). Meanwhile, a meta-analysis involving 548 patients who underwent arthroscopy revealed that the administration of celecoxib (200 or 400 mg) before surgery reduced pain intensity without increasing the incidence of PONV (21). Our results indicate that the perioperative use of celecoxib can significantly reduce VAS scores at rest at 24, 48, and 72 h after TKA. Therefore, we recommend the perioperative use of celecoxib as routine analgesia for TKA in the absence of any contraindication.
Early mobilization after TKA may contribute to better outcomes, such as lower morbidity and shorter hospital length of stay (22). Thus, active ROM assessment is an important indicator for evaluating postoperative recovery after TKA. However, many factors may affect postoperative mobilization, such as age, comorbidities, muscle strength, and psychological factors (15). Our meta-analysis revealed that the administration of celecoxib significantly increased active ROM at postoperative 48 h and 7 days postoperatively but not at 24 h, which may be due to early postoperative edema; however, further studies are needed to verify this.
Nausea and vomiting are among the most common postoperative complications, which may lead to dehydration, electrolyte imbalance, and prolonged hospital stay (23). The recommended pharmacologic antiemetics for PONV include 5-hydroxytryptamine (5-HT3) receptor antagonists, such as antagonists, dolasetron and corticosteroids, antihistamines, and anticholinergics (24). The perioperative administration of celecoxib decreased the incidence of PONV due to pain relief and reduced the consumption of morphine after TKA; however, our results showed that celecoxib had no significant effects on the incidence of PONV. Further studies with larger sample sizes are needed to confirm this.
Nevertheless, the results of several out outcomes from included studies were not inconsistent with our pooled results. For example, one study (13) showed that there was no difference in VAS scores at rest at postoperative 24 h between the placebo and celecoxib group, which may be due to the timing of administration. In another study (14), no difference in active ROM at postoperative 48 h between the placebo and celecoxib group which may be influenced by the small sample. Therefore, our pooled results regarding some outcomes may be underestimated by several included studies. Large sample and well-designed RCTs are needed.
This meta-analysis has several limitations. First, only five studies were included, and the sample size was relatively small. Particularly, one study only had a sample size of 30. Second, there was significant heterogeneity among the included studies, which might have resulted from different surgical procedures, doses, and timing of celecoxib use. Therefore, the pooled results from these included studies should be treated with caution. Large samples and multicenter RCTs are still needed to determine the optimal strategy of celecoxib use for postoperative pain after TKA.
Conclusions
This meta-analysis demonstrated that perioperative use of celecoxib decreased pain scores at 24, 48, and 72 h postoperatively and increased active ROM at 48, 72 h, and 7 days postoperatively. We therefore recommend the routine perioperative use of celecoxib as a part of multimodal analgesia after TKA to enhance postoperative recovery.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XG and DZ designed and conceived the study, performed the statistical analysis, and drafted the manuscript. SZ and XZ performed the systematic review, study selection, statistical analysis, and preparation of the article for publication. XL and LJ contributed to data extraction and quality assessment. All authors participated in writing the manuscript, preparing the figures, have read, and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Berend ME, Berend KR, Lombardi AV Jr. Advances in pain management: game changers in knee arthroplasty. Bone Joint J. (2014) 96-B:7–9. doi: 10.1302/0301-620x.96b11.34514
2. Buvanendran A, Kroin JS, Tuman KJ, Lubenow TR, Elmofty D, Moric M, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: a randomized controlled trial. Jama. (2003) 290:2411–8. doi: 10.1001/jama.290.18.2411
3. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. (2011) 93:1938–43. doi: 10.2106/JBJS.9320icl
4. Maheshwari AV, Blum YC, Shekhar L, Ranawat AS, Ranawat CS. Multimodal pain management after total hip and knee arthroplasty at the Ranawat Orthopaedic Center. Clin Orthop Relat Res. (2009) 467:1418–23. doi: 10.1007/s11999-009-0728-7
5. Ranawat AS, Ranawat CS. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J Arthroplasty. (2007) 22:12–5. doi: 10.1016/j.arth.2007.05.040
6. Elmallah RK, Chughtai M, Khlopas A, Newman JM, Stearns KL, Roche M, et al. Pain control in total knee arthroplasty. J Knee Surg. (2018) 31:504–13. doi: 10.1055/s-0037-1604152
7. Clemett D, Goa KL. Celecoxib: a review of its use in osteoarthritis, rheumatoid arthritis and acute pain. Drugs. (2000) 59:957–80. doi: 10.2165/00003495-200059040-00017
8. Mammoto T, Fujie K, Taguchi N, Ma E, Shimizu T, Hashimoto K. Short-Term effects of early postoperative celecoxib administration for pain, sleep quality, and range of motion after total knee arthroplasty: a randomized controlled trial. J Arthroplasty. (2021) 36:526–31. doi: 10.1016/j.arth.2020.08.018
9. Stepan JG, London DA, Osei DA, Boyer MI, Dardas AZ, Calfee RP. Perioperative celecoxib and postoperative opioid use in hand surgery: a prospective cohort study. J Hand Surg Am. (2018) 43:346–53. doi: 10.1016/j.jhsa.2017.11.001
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9, w264. doi: 10.7326/0003-4819-151-4-200908180-00135
11. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
12. Higgins J, Green, S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. The Cochrane Collaboration (2013).
13. Gong L, Dong JY, Li ZR. Effects of combined application of muscle relaxants and celecoxib administration after total knee arthroplasty (TKA) on early recovery: a randomized, double-blind, controlled study. J Arthroplasty. (2013) 28:1301–5. doi: 10.1016/j.arth.2012.10.002
14. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty - a randomized, controlled study. BMC Musculoskelet Disord. (2008) 9:77. doi: 10.1186/1471-2474-9-77
15. Lubis AMT, Rawung RBV, Tantri AR. Preemptive analgesia in total knee arthroplasty: comparing the effects of single dose combining celecoxib with pregabalin and repetition dose combining celecoxib with pregabalin: double-blind controlled clinical trial. Pain Res Treat. (2018) 2018:3807217. doi: 10.1155/2018/3807217
16. Zhuang Q, Tao L, Lin J, Jin J, Qian W, Bian Y, et al. Postoperative intravenous parecoxib sodium followed by oral celecoxib post total knee arthroplasty in osteoarthritis patients (PIPFORCE): a multicentre, double-blind, randomised, placebo-controlled trial. BMJ Open. (2020) 10:e030501. doi: 10.1136/bmjopen-2019-030501
17. Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. (1997) 78:606–17. doi: 10.1093/bja/78.5.606
18. Thomazeau J, Rouquette A, Martinez V, Rabuel C, Prince N, Laplanche JL, et al. Acute pain Factors predictive of post-operative pain and opioid requirement in multimodal analgesia following knee replacement. Eur J Pain. (2016) 20:822–32. doi: 10.1002/ejp.808
19. Prommer E, Ficek B. Management of pain in the elderly at the end of life. Drugs Aging. (2012) 29:285–305. doi: 10.2165/11599210-000000000-00000
20. Meunier A, Lisander B, Good L. Effects of celecoxib on blood loss, pain, and recovery of function after total knee replacement: a randomized placebo-controlled trial. Acta Orthop. (2007) 78:661–7. doi: 10.1080/17453670710014365
21. Wan R, Li P, Jiang H. The efficacy of celecoxib for pain management of arthroscopy: a meta-analysis of randomized controlled trials. Medicine (Baltimore). (2019) 98:e17808. doi: 10.1097/md.0000000000017808
22. Miner AL, Lingard EA, Wright EA, Sledge CB, Katz JN. Knee range of motion after total knee arthroplasty: how important is this as an outcome measure? J Arthroplasty. (2003) 18:286–94. doi: 10.1054/arth.2003.50046
23. Apfel CC, Heidrich FM, Jukar-Rao S, Jalota L, Hornuss C, Whelan RP, et al. Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth. (2012) 109:742–53. doi: 10.1093/bja/aes276
Keywords: total knee arthroplasty, celecoxib, meta-analysis, pain management, randomized controlled trials
Citation: Geng X, Zhou S, Zhang X, Liu X, Cheng X, Jiang L and Zhang D (2022) The Efficacy and Safety of Celecoxib for Pain Management After Total Knee Arthroplasty: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Surg. 9:791513. doi: 10.3389/fsurg.2022.791513
Received: 08 October 2021; Accepted: 03 January 2022;
Published: 28 January 2022.
Edited by:
Jaimo Ahn, University of Michigan, United StatesReviewed by:
Alessandro Vittori, Bambino Gesù Children's Hospital (IRCCS), ItalyUlf Gunnarsson, Umeå University, Sweden
Copyright © 2022 Geng, Zhou, Zhang, Liu, Cheng, Jiang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihua Jiang, Z2VkYTY2JiN4MDAwNDA7MTI2LmNvbQ==; Donghang Zhang, emhhbmdkaHNjdSYjeDAwMDQwOzE2My5jb20=
 Xiaoyuan Geng1
Xiaoyuan Geng1 Donghang Zhang
Donghang Zhang