- 1Verily Life Sciences, South San Francisco, CA, United States
- 2Division of Digital Psychiatry, Department of Psychiatry, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, MA, United States
- 3Center for Health Sciences, Stanford Research Institute (SRI), Menlo Park, CA, United States
- 4Division of Pulmonary, Asthma, and Sleep Medicine, Department of Pediatrics, Stanford University School of Medicine, Palo Alto, CA, United States
- 5Department of Neurology, Radboud University Medical Center, Nijmegen, Netherlands
Introduction: This study evaluated the performance of a wrist-worn wearable, Verily Study Watch (VSW), in detecting key sleep measures against polysomnography (PSG).
Methods: We collected data from 41 adults without obstructive sleep apnea or insomnia during a single overnight laboratory visit. We evaluated epoch-by-epoch performance for sleep vs. wake classification, sleep stage classification and duration, total sleep time (TST), wake after sleep onset (WASO), sleep onset latency (SOL), sleep efficiency (SE), and number of awakenings (NAWK). Performance metrics included sensitivity, specificity, Cohen's kappa, and Bland-Altman analyses.
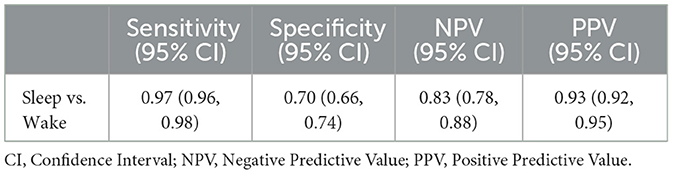
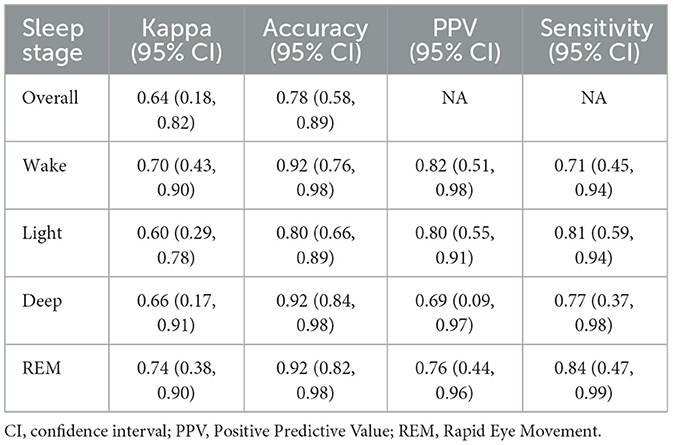
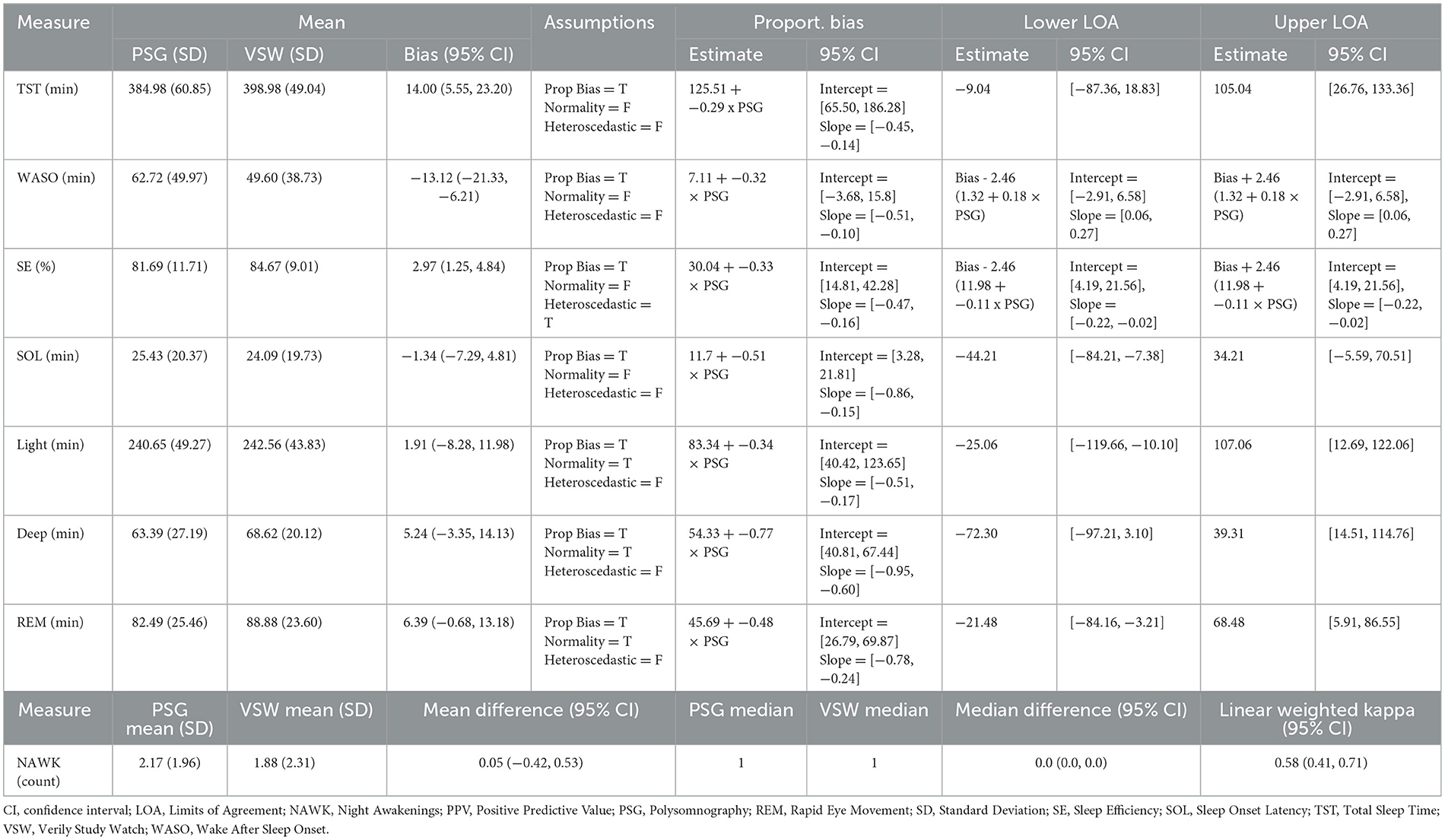
Results: Sensitivity and specificity (95% CIs) of sleep vs. wake classification were 0.97 (0.96, 0.98) and 0.70 (0.66, 0.74), respectively. Cohen's kappa (95% CI) for 4-class stage detection was 0.64 (0.18, 0.82). Most VSW sleep measures had proportional bias. The mean bias values (95% CI) were 14.0 min (5.55, 23.20) for TST, −13.1 min (−21.33, −6.21) for WASO, 2.97% (1.25, 4.84) for SE, −1.34 min (−7.29, 4.81) for SOL, 1.91 min (−8.28, 11.98) for light sleep duration, 5.24 min (−3.35, 14.13) for deep sleep duration, and 6.39 min (−0.68, 13.18) for REM sleep duration. Mean and median NAWK count differences (95% CI) were 0.05 (−0.42, 0.53) and 0.0 (0.0, 0.0), respectively.
Discussion: Results support applying the VSW to track overnight sleep measures in free-living settings. Registered at clinicaltrials.gov (NCT05276362).
Introduction
Characterizing sleep in a free-living setting provides valuable insights into physical and mental health. Changes in sleep may be key in the diagnosis of sleep disorders like insomnia and hypersomnia, and are clinically meaningful components for tracking mental and cardiovascular health, as well as other conditions (Parish, 2009; Freeman et al., 2020; Tobaldini et al., 2019; Young et al., 2008; Ahmadi et al., 2009; Hayashino et al., 2010). The gold standard for sleep assessment is lab-based polysomnography (PSG). However, PSG is resource intensive, challenging to administer and subject to intra- and inter-scorer variability, moreover, availability of PSG laboratories may be limited (Norman et al., 2000; Deutsch et al., 2006). It is also impractical for long-term surveillance, and may be prone to artifacts that affect representativeness, such as altered sleep patterns due to the novelty of a laboratory, and/or the discomfort of the electrode setting (Toussaint et al., 1995). Furthermore, while portable PSG tools do exist, they still have limited application in free-living environments or routine clinical care.
Wearable sensors, particularly wrist-worn devices, provide a promising avenue for sleep assessment in free-living settings. These devices are widely available, relatively inexpensive, comfortable to wear during sleep and include physiological sensors, such as photoplethysmogram (PPG) and accelerometer, that can be used for sleep monitoring (Imtiaz, 2021; de Zambotti et al., 2024). However, before utilizing wearable-based technology as a routine approach to monitor daily sleep, whether for care or for research purposes, it is important to conduct performance evaluation of devices and algorithms compared to a gold standard reference such as PSG. Furthermore, researchers now know the importance of conducting those analytical and clinical evaluations across diverse and representative populations, such as participants with different ages or skin tones, to increase confidence in the generalizability of the results (Colvonen et al., 2020; Baumert et al., 2023; Nelson et al., 2020).
This study evaluated the performance of the Verily Study Watch (VSW, a wrist-worn wearable) to monitor sleep in a diverse cohort of sleepers without obstructive sleep apnea (OSA) or elevated insomnia symptoms, by comparing VSW sleep measures against measures obtained from PSG-based labels. The VSW classifies every 30-second epoch into 4 sleep-related stages: wake, light sleep, deep sleep, and rapid eye movement (REM) sleep. These classifications enable the calculation of multiple sleep measures that provide information on the quantity and the quality of an individual's overnight sleep. In this study, the measures of interest were: total sleep time (TST), wake after sleep onset (WASO), sleep efficiency (SE), sleep onset latency (SOL), number of awakenings (NAWK), and duration of each sleep stage. Our main objective was to compare epoch-by-epoch VSW- against PSG- derived classification of sleep-vs.-wake state and of sleep stages. Additionally, we wanted to assess the VSW's accuracy for all computed sleep measures (listed above). Finally, we wanted to evaluate any potential variability in the performance of the VSW's sleep algorithm across demographic factors such as age, sex, body mass index (BMI), skin tone, and arm hair density.
Methods
Participants
The basic setup and eligibility for the study have been described elsewhere (Nelson et al., 2024). Eligible participants were between 18–80 years old, and did not have identified symptoms of sleep disorders based on the following criteria: obstructive sleep apnea 50 (OSA-50) scores <5; insomnia severity index (ISI) <8; Epworth Sleepiness Scale (ESS) scores <10; no evidence of sleep-disordered breathing at the PSG evaluation; and apnea-hypopnea index (AHI) threshold of 5 defining hypopnea as ≥30% reduction in airflow for ≥10 seconds associated with a ≥3% decrease in oxygen saturation or an arousal. Individuals were ineligible if they had a major medical or psychiatric condition, if they used supplemental oxygen, or were unwilling to cease use of therapy, such as continuous positive airway pressure or oral appliance for sleep-disordered breathing during the visit. Additional exclusion criteria included use of medications that affect sleep (e.g., hypnotics or antidepressants) or any sleep medications in the previous 24 h; pregnancy, lactation, or breastfeeding; having an implantable medical device; night-shift work; or travel over 3 time zones within 2 weeks prior to the study.
The study was approved by the WCG Institutional Review Board (20215892), and all participants provided informed consent.
This study was registered at clinicaltrials.gov (NCT05276362).
Data collection
For each participant, data were collected during a single overnight stay in a sleep laboratory at a single site (SRI; Menlo Park, California), between February 14th and September 1st, 2023. Participants slept in comfortable, sound-proof and temperature-controlled bedrooms. Standard PSG protocols were used for preparation, recording procedures, and instrument calibration (Nelson et al., 2024).
Study watch data
During their overnight visit, participants wore the VSW on their dominant wrist. This analysis was part of a larger study including two devices: the Verily Numetric Watch (VNW) (Nelson et al., 2024), in addition to the VSW. VSW is equipped with two sensors: a green-light PPG sensor, and a 3-axis accelerometer. Both sensors had a sampling frequency of 60 Hz (in the VNW, the PPG sensor consists of a green light emitter diode and two PPG signal channels and the sampling rate of the 3-axis accelerometer is 104 Hz).
The sleep stage classification algorithm consists of a deep convolutional neural network. The neural network model uses instantaneous pulse rate as input, which is calculated from the PPG signal, and predicts a probability distribution over the following 4 sleep stages every 30 seconds: wake, light sleep, deep sleep, and REM sleep. The model largely consists of regular convolutional layers that extract local features from the input, and additional dilated convolutional layers that are used to discover long-range temporal relationships across the length of the input. This model was initially trained using 10,000 nights of PSG-labeled data from the Sleep Heart Health Study (SHHS) and Multi-Ethnic Study of Atherosclerosis (MESA) public datasets (Sridhar et al., 2020). The algorithm was later modified to include accelerometer input as well, and was fine-tuned using a smaller polysomnography dataset collected at SRI, consisting of 30 nights of PSG-labeled data. This was a separate dataset from the one used for performance evaluation in this paper.
The overnight sleep measures, including TST, WASO, SE, SOL, NAWK, and sleep stage durations (Supplementary Table 1), for each participant were calculated using the VSW's predicted sleep stages, from the time the lights were turned off (“lights-off”) to the time lights were turned back on (“lights-on”). VSW start time was synced to the Lights Off time recorded on PSG to ensure alignment for analysis of simultaneously recorded signals, using procedures described elsewhere (Nelson et al., 2024; de Zambotti et al., 2019).
Reference data
Standard laboratory PSG sleep assessment including electroencephalography (EEG), submental electromyography and bilateral electrooculography was performed according to the American Academy of Sleep Medicine (AASM) guidelines. Leg movement, electrocardiography (ECG), respiratory, and oxygen saturation signals were also collected and used to confirm the absence of sleep disordered breathing. All recordings were performed using the Compumedics Grael® HD-PSG system (Compumedics, Abbotsford, Victoria, Australia). Two independent sleep scorers labeled every 30-second epoch of the PSG data by one of the following categories: wake, N1, N2, N3, and REM. Inter-rater reliability (Kappa) between the two scorers was 91%, and discrepancies were resolved by a third scorer.
For this analysis, PSG stages N1 and N2 were combined into a single light sleep category, and PSG N3 was termed deep sleep.
Similar to VSW, for each participant, the overnight sleep measures for PSG were calculated using the sleep scorer's stage labels from lights-off to lights-on.
Performance evaluation
Performance evaluation was done based on an existing standardization framework (Menghini et al., 2021).
We evaluated the epoch-by-epoch performance of VSW's sleep stage classification against PSG in two ways: (1) sleep vs. wake classification, using sleep as the positive class; and (2) 4-class (wake, light, deep, and REM) sleep stage classification. For the evaluation of sleep vs. wake classification, we estimated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). We calculated the 95% CI using cluster bootstrapping, and we accounted for the clustering of epochs within a participant using logistic mixed-effect regression models with the participant as random effect. For the 4-class stage classification, we used Cohen's kappa and accuracy along with their 95% bootstrapped CIs. Additionally we evaluated performance for each sleep stage by reporting Cohen's Kappa, accuracy, PPV, and sensitivity using the average method (Menghini et al., 2021). To obtain performance metrics on each sleep stage, the outcomes were dichotomized to the sleep stage of interest against all others. The average method calculates kappa for each individual participant and then averages out the kappa across all participants with their associated bootstrapped 95% CIs. All analyses were confined to the lights-off to lights-on period.
For evaluating the performance of all overnight sleep measures except NAWK, we performed the Bland Altman analysis, estimating the mean bias and lower and upper limits of agreement, testing for the assumptions of proportional bias, heteroscedasticity, and normality. For NAWK, we estimated the mean and median count difference and linearly weighted Cohen's kappa with their 95% CIs.
Finally, we evaluated all performance metrics across the participant subgroups, including age, sex, BMI, skin tone, arm hair index. For subgroups with insufficient number of samples (<10), we did not evaluate the performance.
All analyses were performed with R version 4.3.1 (2023-06-16).
Results
There were 41 adult participants (18 male, age range: 18–78 years) in this study. Participants had a diverse range of skin tones, BMI, and arm hair density (Supplementary Table 2).
VSW estimated sleep stages for a total of 38,796 epochs with data collected between lights-off and lights-on for each participant.
The sensitivity (95% CI) of the VSW in classifying sleep vs. wake was 0.97 (0.96, 0.98), specificity (95% CI) was 0.70 (0.66, 0.74), PPV (95% CI) was 0.93 (0.92, 0.95), and NPV (95% CI) was 0.83 (0.78, 0.88) (Table 1).
The accuracy (95% CI) of the VSW sleep algorithm in classifying all 4 sleep stages was 0.78 (0.58, 0.89), and the kappa (95% CI) was 0.64 (0.18, 0.82) (Table 2). There was variability in the performance across different sleep stages, with light sleep stage prediction having the lowest accuracy (Table 2), as there were instances of confusion between the light sleep stage and all other stages (Supplementary Table 3).
Mean bias and 95% CI values for all overnight sleep measures is shown in Table 3. Bland-Altman analyses (Figure 1) showed that all measures had significant proportional bias, with the VSW overestimating the measures at the lower end of the distribution, and underestimating them at the upper end, relative to the PSG. For all overnight sleep measures except the sleep stage durations, the assumption of normality was false, and for all measures except SE the assumption of homoscedasticity was true.
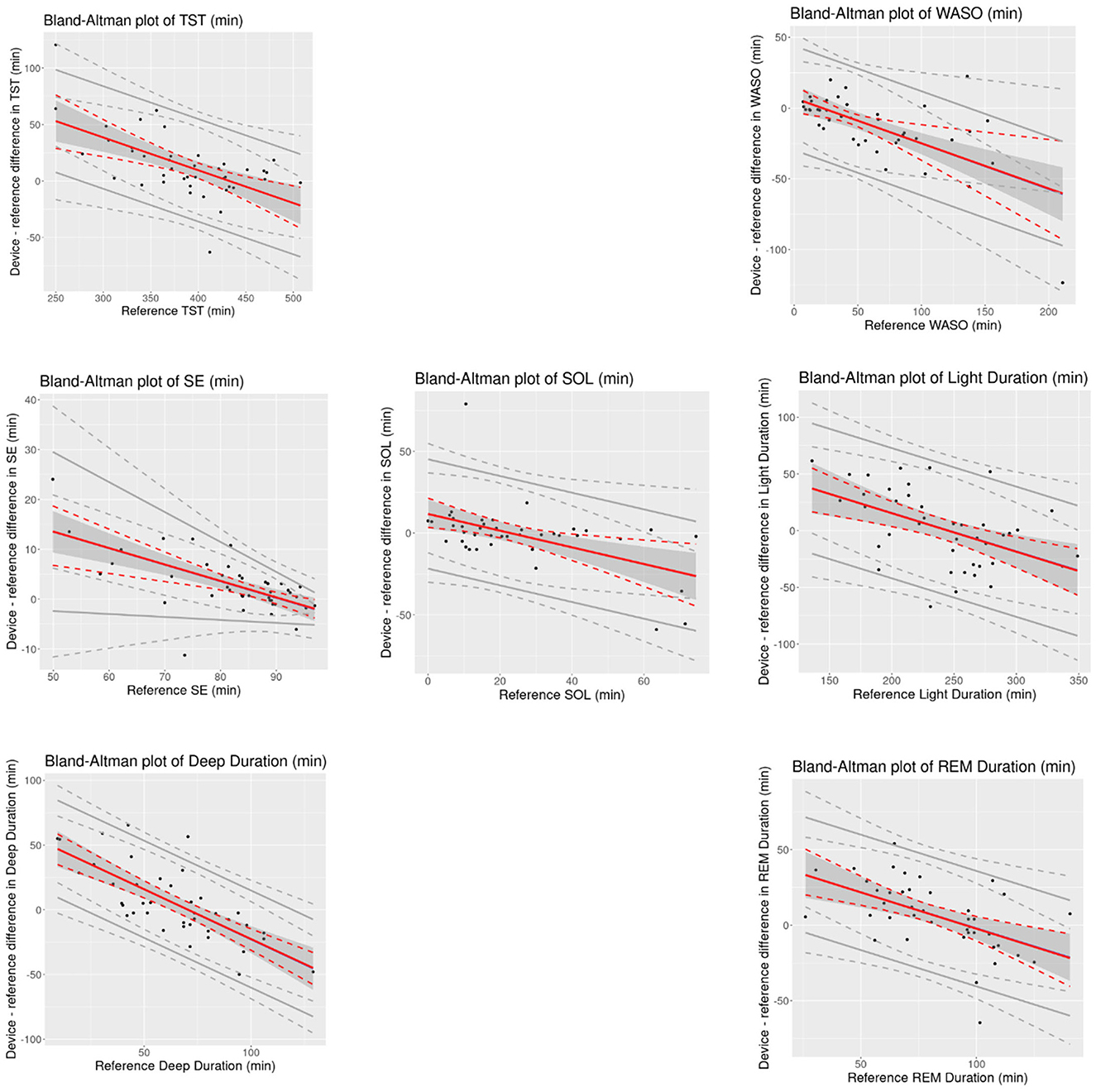
Figure 1. Bland-Altman plots of overnight sleep measures for the device (VSW) against the reference (PSG). Solid red lines indicate mean bias, dotted red lines indicate 95% CI of mean bias, solid gray lines indicate the 95% LOAs, and dotted gray lines indicate 95% CI of LOAs. Black dots are observations. CI, confidence interval; REM, Rapid Eye Movement; SD, Standard Deviation; SE, Sleep Efficiency; SOL, Sleep Onset Latency; TST, Total Sleep Time; WASO, Wake After Sleep Onset.
Performance of the VSW metrics across demographic subgroups of age, sex, BMI, skin tone, and arm hair density are reported (Supplementary Tables 4, 5) without formal statistical testing, due to small subgroup sample size.
Discussion
The results of this study show the ability of the VSW to capture information related to sleep quantity and quality, as well as the distribution of sleep stages across overnight periods in individuals without OSA or elevated insomnia symptoms. The sensitivity and specificity of the VSW in classifying sleep vs. wake were 0.97 and 0.70 respectively, and the Cohen's kappa for the 4-class stage classification was 0.64. This performance supports the application of the VSW to monitor overnight sleep in free-living settings.
As with other wearable sleep-wake detection devices (Pesonen and Kuula, 2018; de Zambotti et al., 2016; Miller et al., 2022), the sleep algorithm in this study was more likely to miss wake than sleep, as reflected in the higher sensitivity relative to specificity, and the positive and negative bias values for TST and WASO, respectively. When evaluating the performance of sleep monitoring devices, the AASM has established a range of “allowable differences,” based on actigraphy studies conducted in patients with specific sleep disorders (e.g., insomnia; Smith et al., 2018). The 95% CIs of the mean bias estimates for TST, WASO, SOL, and SE measured by the VSW were within those allowable difference ranges (40 min, 30 min, 30 min, and 5%, respectively). However, for the proportional mean bias estimates, which account for variations in bias over the range of measurement, 95% CIs exceeded these thresholds at lower and higher ends of the measurements (Figure 1). Nonetheless, applying the AASM standards to these results may require caution. Unlike the studies included in the AASM assessment, the present study excluded (via questionnaire) participants with symptoms of certain sleep disorders.
There are a few caveats to consider when interpreting our results. First, data collection for this study took place at a sleep laboratory, with standardized study boundaries and settings, such as lights-on/off to define the “in bed” time period when an individual is (in theory) set to sleep. Free-living environments are more organic and complex, and the generation of sleep measures in them may require additional layers of data. Following the prior example, defining “in bed” time may necessitate additional sensor readings, which then would be integrated into the derivation of the measures, particularly sleep stage classification and duration, or SOL. Second, the sleep stage classification had variable accuracy when compared to PSG, albeit on par with other wearable devices (Chinoy et al., 2021; Schyvens et al., 2024). Future research should continue to refine sleep stage classification to improve its accuracy and agreement with PSG.
Another caveat is that participants in this study were free of sleep-related diagnoses and symptoms (such as OSA or heightened insomnia symptoms). Participants with certain clinical conditions may manifest different patterns in their biological signals (e.g., pulse rate) and/or sleep architecture, which could complicate the sleep stage classification task. Future studies should evaluate the performance of VSW in real-world settings and in clinically relevant populations such as individuals with sleep disorders.
In summary, we evaluated the performance of the VSW and its algorithm to classify sleep vs. wake state and the four different sleep stages in sleepers without OSA or heightened insomnia symptoms, as well as a series of measures that illustrate the quantity and quality of overnight sleep. The results demonstrate the potential of VSW to classify sleep vs. wake states and sleep stages and compute overnight sleep measures when compared to gold-standard PSG measurements. These findings support further application of the VSW “sleep vs. wake” classification and all derived sleep metrics from this measure (TST, WASO, SOL, SE, and NAWK) for tracking the overnight sleep behaviors in individuals without OSA or heightened insomnia symptoms in free-living settings. The performance of VSW on individuals with more disrupted sleep such as OSA is yet to be evaluated.
Data availability statement
Data from this study are not available due to the nature of the program and participants did not consent for their data to be shared publicly. Further enquiries should be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the WCG Institutional Review Board (20215892). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SSa: Conceptualization, Writing – original draft, Writing – review & editing, Formal analysis. BN: Conceptualization, Writing – review & editing. PB: Formal analysis, Methodology, Writing – review & editing. NV: Methodology, Supervision, Writing – review & editing. HA: Data curation, Project administration, Writing – review & editing. MZ: Conceptualization, Writing – review & editing. FB: Data curation, Investigation, Project administration, Writing – review & editing. NA: Data curation, Investigation, Project administration, Writing – review & editing. NS: Data curation, Methodology, Writing – review & editing. SSS: Conceptualization, Writing – review & editing. SP: Project administration, Supervision, Writing – review & editing. ER: Methodology, Supervision, Writing – review & editing. RK: Conceptualization, Supervision, Writing – review & editing. SSh: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was sponsored by Verily Life Sciences.
Acknowledgments
Authors wish to acknowledge participants and study personnel that made the study possible.
Conflict of interest
SSa, BN, PB, NV, HA, NS, SSS, SP, ER, RK, and SSh report employment and equity ownership in Verily Life Sciences. MZ, FB, and NA received research funding through their institution from Verily Life Sciences for study execution.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frsle.2024.1481878/full#supplementary-material
References
Ahmadi, N., Shapiro, G. K., Chung, S. A., and Shapiro, C. M. (2009). Clinical diagnosis of sleep apnea based on single night of polysomnography vs. two nights of polysomnography. Sleep Breath. 13, 221–226. doi: 10.1007/s11325-008-0234-2
Baumert, M., Hartmann, S., and Phan, H. (2023). Automatic sleep staging for the young and the old - Evaluating age bias in deep learning. Sleep Med. 107, 18–25. doi: 10.1016/j.sleep.2023.04.002
Chinoy, E. D., Cuellar, J. A., Huwa, K. E., Jameson, J. T., Watson, C. H., Bessman, S. C., et al. (2021). Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep. 44:zsaa291 doi: 10.1093/sleep/zsaa291
Colvonen, P. J., DeYoung, P. N., Bosompra, N.-O. A., and Owens, R. L. (2020). Limiting racial disparities and bias for wearable devices in health science research. Sleep 43:zsaa159. doi: 10.1093/sleep/zsaa159
de Zambotti, M., Baker, F. C., Willoughby, A. R., Godino, J. G., Wing, D., Patrick, K., et al. (2016). Measures of sleep and cardiac functioning during sleep using a multi-sensory commercially-available wristband in adolescents. Physiol. Behav. 158, 143–149. doi: 10.1016/j.physbeh.2016.03.006
de Zambotti, M., Cellini, N., Goldstone, A., Colrain, I. M., and Baker, F. C. (2019). Wearable sleep technology in clinical and research settings. Med. Sci. Sports Exerc. 51, 1538–1557. doi: 10.1249/MSS.0000000000001947
de Zambotti, M., Goldstein, C., Cook, J., Menghini, L., Altini, M., Cheng, P., et al. (2024). State of the science and recommendations for using wearable technology in sleep and circadian research. Sleep 47:zsad325. doi: 10.1093/sleep/zsad325
Deutsch, P. A., Simmons, M. S., and Wallace, J. M. (2006). Cost-effectiveness of split-night polysomnography and home studies in the evaluation of obstructive sleep apnea syndrome. J. Clin. Sleep Med. 2, 145–153. doi: 10.5664/jcsm.26508
Freeman, D., Sheaves, B., Waite, F., Harvey, A. G., and Harrison, P. J. (2020). Sleep disturbance and psychiatric disorders. Lancet Psychiatry 7, 628–637. doi: 10.1016/S2215-0366(20)30136-X
Hayashino, Y., Yamazaki, S., Takegami, M., Nakayama, T., Sokejima, S., and Fukuhara, S. (2010). Association between number of comorbid conditions, depression, and sleep quality using the Pittsburgh Sleep Quality Index: results from a population-based survey. Sleep Med. 11, 366–371. doi: 10.1016/j.sleep.2009.05.021
Imtiaz, S. A. (2021). A systematic review of sensing technologies for wearable sleep staging. Sensors 21:1562. doi: 10.3390/s21051562
Menghini, L., Cellini, N., Goldstone, A., Baker, F. C., and de Zambotti, M. (2021). A standardized framework for testing the performance of sleep-tracking technology: step-by-step guidelines and open-source code. Sleep 44:zsaa170. doi: 10.1093/sleep/zsaa170
Miller, D. J., Sargent, C., and Roach, G. D. (2022). A validation of six wearable devices for estimating sleep, heart rate and heart rate variability in healthy adults. Sensors 22:6317. doi: 10.3390/s22166317
Nelson, B. W., Low, C. A., Jacobson, N., Areán, P., Torous, J., and Allen, N. B. (2020). Guidelines for wrist-worn consumer wearable assessment of heart rate in biobehavioral research. NPJ Digit. Med. 3:90. doi: 10.1038/s41746-020-0297-4
Nelson, B. W., Saeb, S., Barman, P., Verma, N., Allen, H., and de Zambotti, M. (2024) Performance evaluation of the Verily Numetric Watch sleep suite for digital sleep assessment against in-lab polysomnography. medRxiv. 10.1101/2024.09.10.24313425 .
Norman, R. G., Pal, I., Stewart, C., Walsleben, J. A., and Rapoport, D. M. (2000). Interobserver agreement among sleep scorers from different centers in a large dataset. Sleep 23, 901–908. doi: 10.1093/sleep/23.7.1e
Parish, J. M. (2009). Sleep-related problems in common medical conditions. Chest 135, 563–572. doi: 10.1378/chest.08-0934
Pesonen, A.-K., and Kuula, L. (2018). The validity of a new consumer-targeted wrist device in sleep measurement: an overnight comparison against polysomnography in children and adolescents. J. Clin. Sleep Med. 14, 585–591. doi: 10.5664/jcsm.7050
Schyvens, A. M., Van Oost, N. C., Aerts, J. M., Masci, F., Peters, B., Neven, A., et al. (2024). Accuracy of fitbit charge 4, garmin vivosmart 4, and WHOOP versus polysomnography: systematic review. JMIR Mhealth Uhealth 12:e52192. doi: 10.2196/52192
Smith, M. T., McCrae, C. S., Cheung, J., Martin, J. L., Harrod, C. G., Heald, J. L., et al. (2018). Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an american academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J. Clin. Sleep Med. 14, 1209–1230. doi: 10.5664/jcsm.7228
Sridhar, N., Shoeb, A., Stephens, P., Kharbouch, A., Shimol, D. B., Burkart, J., et al. (2020). Deep learning for automated sleep staging using instantaneous heart rate. NPJ Digit Med. 3:106. doi: 10.1038/s41746-020-0291-x
Tobaldini, E., Fiorelli, E. M., Solbiati, M., Costantino, G., Nobili, L., and Montano, N. (2019). Short sleep duration and cardiometabolic risk: from pathophysiology to clinical evidence. Nat. Rev. Cardiol. 16, 213–224. doi: 10.1038/s41569-018-0109-6
Toussaint, M., Luthringer, R., Schaltenbrand, N., Carelli, G., Lainey, E., Jacqmin, A., et al. (1995). First-night effect in normal subjects and psychiatric inpatients. Sleep 18, 463–469. doi: 10.1093/sleep/18.6.463
Keywords: sleep-wake detection, sleep stage, digital health measures, polysomnography, free-living, sleep detection accuracy, wearable technology
Citation: Saeb S, Nelson BW, Barman P, Verma N, Allen H, de Zambotti M, Baker FC, Arra N, Sridhar N, Sullivan SS, Plowman S, Rainaldi E, Kapur R and Shin S (2024) Performance of the Verily Study Watch for measuring sleep compared to polysomnography. Front. Sleep 3:1481878. doi: 10.3389/frsle.2024.1481878
Received: 16 August 2024; Accepted: 20 November 2024;
Published: 13 December 2024.
Edited by:
Ravi Gupta, All India Institute of Medical Sciences, Rishikesh, IndiaReviewed by:
Kaustav Kundu, Uttar Pradesh University of Medical Sciences, IndiaAbhishek Goyal, Graphic Era University, India
Copyright © 2024 Saeb, Nelson, Barman, Verma, Allen, de Zambotti, Baker, Arra, Sridhar, Sullivan, Plowman, Rainaldi, Kapur and Shin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sohrab Saeb, c29zYXRhQHZlcmlseS5jb20=
†These authors have contributed equally to this work and share first authorship
 Sohrab Saeb
Sohrab Saeb Benjamin W. Nelson1,2†
Benjamin W. Nelson1,2† Hannah Allen
Hannah Allen Massimiliano de Zambotti
Massimiliano de Zambotti Fiona C. Baker
Fiona C. Baker Shannon S. Sullivan
Shannon S. Sullivan