Explore article hub
- 1Center for Surface Chemistry and Catalysis, Katholieke Universiteit (KU) Leuven, Leuven, Belgium
- 2Solhyd N.V., Bierbeek, Belgium
- 3COOSHA Impact Venture, Boutersem, Belgium
An Editorial on the Frontiers in Science Lead Article
Photocatalytic water splitting for large-scale solar-to-chemical energy conversion and storage
Key points
- Hydrogen production from sunlight using innovative photocatalytic and photoelectrochemical systems offers decentralized, sustainable energy solutions with potential applications in remote, off-grid locations.
- Photocatalytic hydrogen production has the potential to transform clean cooking by reducing dependency on wood and charcoal in low-resource settings, addressing significant health and environmental challenges.
- Photocatalytic reactors could also be used to capture atmospheric carbon dioxide and perform artificial photosynthesis, mimicking processes found in nature producing green energy molecules.
Sunlight is the most abundant sustainable energy source available for our planet. The total power needed by humankind is less than 0.1% of what is available from the sun. By comparison, the potential of other energy sources, including wind, geothermal, tidal, renewable biomass, and hydropower, is hundreds of times smaller. Human beings currently consume most of their energy via historical sunlight accumulated and stored in fossil carbon sources, which has severe environmental repercussions. Theoretically, solar energy could easily replace fossil fuels as our main energy source, however, the technical challenges to extract and convert solar radiation into usable energy sources are formidable.
The main challenge with solar energy lies in its intermittency. Apart from the day and night and seasonal cycles, solar radiation also varies widely across different regions (i.e., more solar radiation reaches the equator than the poles). Another challenge is low solar flux density, which means the energy from sunlight is spread out over a large area. Consequently, collecting substantial amounts of solar energy requires significant space. Sunlight itself cannot be transported and needs to be converted into an energy vector to make it easier to move and stock. Electricity provides such an option, but its transportation via intercontinental cables is expensive. Chemicals in gaseous, liquid, and solid forms are more convenient to transport, as is currently done with natural gas, petroleum, and coal. However, if we aim to avoid the involvement of carbon atoms, hydrogen is the ideal molecule to be produced from sunlight. While transport of hydrogen gas needs strong compression and very low temperatures for liquefaction, converting it to ammonia (NH3) enables transport and storage of large volumes of hydrogen over long distances. NH3 can subsequently be cracked (decomposed) again to recover hydrogen, the desired fuel, as needed.
Solar-driven hydrogen production through water splitting has emerged as a feasible pathway for green energy generation. In their Frontiers in Science lead article, Hisatomi et al. (1) provide an in-depth discussion of the recent developments in green hydrogen production through photocatalytic water splitting. Currently, developments in this area are focused on scaling up hydrogen production via overall water splitting using photocatalysts.
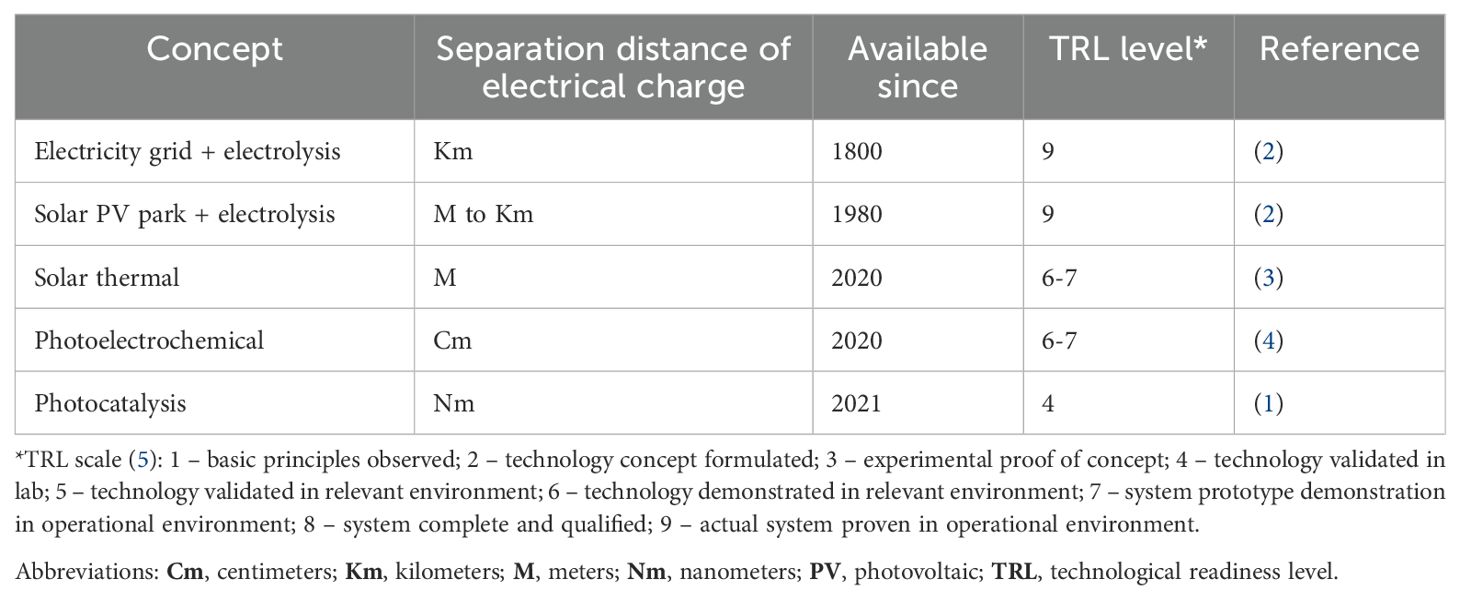
Before the emergence of photocatalysts for generating hydrogen, other methods were used to produce hydrogen from water. One of the oldest is electrolysis, which was eventually abandoned in favor of steam methane reforming but has now resurfaced as a key method for generating green hydrogen (Table 1).
The splitting of water molecules yields hydrogen gas, which is an industrially important chemical and energy vector, as well as oxygen gas, which is also needed in massive amounts in all kinds of industrial processes. Current electrolyzers have been growing increasingly powerful, ranging from megawatt (MW) to even gigawatt (GW) scale (6). Zero-carbon hydrogen can be produced if the electrolyzer is fueled via solar, wind, or nuclear energy. However, producing electricity solely through a photovoltaic power station is economically less attractive because of the handicap of the diurnal cycle providing no power to electrolyzers at night. Instead, solar thermal systems generating high temperatures for the catalytic or even spontaneous splitting of water molecules is an alternative emerging technology being demonstrated in regions with intense solar radiation. It is an example of a solar hydrogen generator not needing electricity as an intermediate energy vector.
Other types of devices that directly use sunlight are photoelectrochemical (PEC) and photocatalytic cells. The distinction between these technologies lies in the separation distance of electric charge generation within photovoltaic semiconductors and the catalytic sites for water splitting. In PEC cells, this distance can be several centimeters, while in photocatalytic systems, it is limited to the nanoscale. Both are emerging technologies that are gaining technological readiness (Table 1). Both systems are also intrinsically modular and autonomous.
PEC and photocatalytic systems offer the advantage of being decentralized, with less reliance on specific locations due to their disconnection from the electricity grid and lower water consumption. This flexibility makes an economically optimal location less critical than for large-scale electrolyzer systems. In contrast, electrolyzers require large volumes of water, which must be sourced either from seawater via desalination or from local freshwater supplies, often competing with other uses. This dependence on water availability, along with the need for access to harbors or pipelines for exporting the produced hydrogen, somewhat limits the location of electrolyzer-based hydrogen production plants.
Fresh water production by capturing atmospheric water vapor is another research field that is gaining momentum. An attractive option is to integrate an atmospheric water vapor collection function in PEC and photocatalytic systems, making them fully independent of local water resources and thereby providing a local solution to the water-energy nexus. These direct solar hydrogen production technologies can, in principle, be implemented anywhere, with access to sunlight as the only requirement. They are modular and useful at any scale. The solar-to-hydrogen (STH) efficiency of PEC hydrogen production systems can be very high when using illuminated photoelectrodes. Owing to the less efficient charge separation in state-of-the-art photocatalysis systems, the efficiencies are still lower, but the aim is to reach similar performances. High efficiency justifies working “at the rhythm of the sun” while producing hydrogen at a competitive price. In addition, the durability of materials is likely to be less of an issue as compared to electrodes and membranes of electrolyzers, which work at very high electric current densities. Small and medium in situ photocatalytic installations for local consumption at remote locations can be made safe by limiting the pressure and avoiding the need for transporting energy. This has been demonstrated by Histaomi et al. (1) in a 100 m2 photocatalyst array system for hydrogen production, utilizing photocatalyst sheets on panel reactors.
Hydrogen-producing devices based on photocatalysis offer a solution to the biggest technological challenges. Clean cooking is recognized by the World Health Organization (WHO) as the number one challenge to be solved (7). Approximately four billion people have access only to wood and charcoal as their household energy source. Daily exposure to smoke from wood fires, however, can result in serious health problems (8). This use of wood due to a lack of alternate sustainable energy sources also contributes significantly to deforestation across the Global South and contributes to global climate change, with roughly 2–7% of global greenhouse gas emissions resulting from the use of wood and charcoal as household energy sources (9). While improving the efficiency of cookstoves and reducing air-polluting emissions and the use of liquid petroleum gas should be considered, there is no obvious solution to this problem. However, hydrogen cookstoves fed with safe hydrogen gas produced locally by PEC and photocatalytic water splitting presents an imaginative disruptive clean technology, preserving nature, limiting carbon dioxide (CO2) emissions, and producing water vapor instead of polluting carbonaceous particulate matter.
Another exciting and promising development in solar-driven technologies is expanding their use to convert molecules other than water, such as CO2. Artificial photosynthesis captures atmospheric CO2 and transforms it into fuels or chemicals, creating a sustainable cycle that reduces emissions and provides renewable energy. This is considered the “holy grail” of renewable energy, since it could address both climate change and energy needs simultaneously. However, developing photocatalytic systems for CO2 may take decades to realize. Initial successes have been demonstrated by Hisatomi et al. (1) using photocatalytic sheets and exploring biomolecular catalysts.
In conclusion, the push for renewable energy, particularly solar-driven technologies such as photocatalytic hydrogen water splitting, is crucial for transitioning from fossil fuels and addressing climate change. Photocatalytic hydrogen production is key to energy sustainability because of the direct use of solar energy and its suitability for decentralized applications in regions where many people are currently living without access to clean energy sources. There are no fundamental limitations to STH technologies but further advancements in efficiency, material durability, and scalability are still needed, and supportive infrastructure and regulatory frameworks are essential. Nevertheless, the future for photocatalytic hydrogen production is bright and, with continuous developments, it can be expected to become an essential asset for future generations.
Statements
Author contributions
JM: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The author is the founder and a board member of SOLHYD N.V, a spin-off company of the Katholieke Universiteit (KU) Leuven, Belgium, which produces solar hydrogen panels, and is the founder and chairman of the board of directors of COOSHA Impact Venture. The companies were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hisatomi T, Wang Q, Zhang F, Ardo S, Reisner E, Nishiyama H, et al. Photocatalytic water splitting for large-scale solar-to-chemical energy conversion and storage. Front Sci (2024) 2:1411644. doi: 10.3389/fsci.2024.1411644
2. Smolinka T, Bergmann H, Garche J, Kusnezoff M. The history of water electrolysis from its beginnings to the present. In: Smolinka T, Garche J, editors. Electrochemical power sources: fundamentals, systems, and applications. Amsterdam: Elsevier (2022). 83–164. doi: 10.1016/C2018-0-05096-3
3. Safari F, Dincer I. A review and comparative evaluation of thermochemical water splitting cycles for hydrogen production. Energy Convers Manag (2020) 205:112182. doi: 10.1016/j.enconman.2019.112182
4. Lubbe F, Rongé J, Bosserez T, Martens JA. Golden hydrogen. Curr Opin Green Sust Chem (2023) 39:100732. doi: 10.1016/j.cogsc.2022.100732
5. Strazza C, Olivieri N, De Rose A, Stevens T, Peeters L, Tawil-Jamault D, et al. Technology readiness level – guidance principles for renewable energy technologies – final report. Luxembourg: Publications office of the European Union (2007). Available at: https://op.europa.eu/en/publication-detail/-/publication/d5d8e9c8-e6d3-11e7-9749-01aa75ed71a1
6. International Energy Agency. Electrolysers [online] (2023). Available at: https://www.iea.org/energy-system/low-emission-fuels/electrolysers
7. World Health Organization. Defining clean fuels and technologies [online]. Available at: https://www.who.int/tools/clean-household-energy-solutions-toolkit/module-7-defining-clean
8. World Health Organization. WHO guidelines for indoor air quality: household fuel combustion. Geneva: WHO (2014). Available at: https://iris.who.int/handle/10665/141496
9. van Dam J. The charcoal transition. Rome: Food and Agriculture Organization of the United Nations (2017). Available at: https://openknowledge.fao.org/items/86176899-1b4f-411d-8644-965b8cf83f3d
Keywords: electrolyzers, photoelectrochemical cells, photocatalytic systems, artificial photosynthesis, green hydrogen
Citation: Martens JA. The bright future of solar-driven hydrogen production. Front Sci (2024) 2:1532051. doi: 10.3389/fsci.2024.1532051
Received: 21 November 2024; Accepted: 22 November 2024;
Published: 03 December 2024.
Edited by:
Frontiers in Science Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2024 Martens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johan A. Martens, Sm9oYW4uTWFydGVuc0BrdWxldXZlbi5iZQ==
 Johan A. Martens
Johan A. Martens