Explore article hub
An Editorial on the Frontiers in Science Lead Article
Immune-mediated disease caused by climate change-associated environmental hazards: mitigation and adaptation
Key points
- Immune-mediated noncommunicable diseases (IMNCDs) account for an enormous and increasing burden of disease globally that is being exacerbated by climate change.
- Lived experiences of individuals affected by IMNCDs—including avoidable deaths among children caused by air pollution, poor housing, and allergies—offer a powerful approach for incentivizing and driving policy change.
- Synergies between climate mitigation/adaptation and health (preventing worsening health impacts and implementing climate-resilient and sustainable health systems) should address inequities in children’s health, with a focus on IMNCD prevention from conception through childhood.
Noncommunicable diseases (NCDs) increasingly dominate the global health burden, causing 41 million deaths each year (74% of all deaths). Of these, 77% occur in low- and middle-income countries, which are the least prepared for them (1). As Agache et al. point out in their lead article (2), a high proportion of NCDs are now known to be immune/inflammatory-mediated NCDs (IMNCDs, including common diseases such as hypertension, allergies, autoimmune diseases, type 1 diabetes, and dementia) for which there are no lasting cures. In almost all cases, active disease management involves controlling identified pathways and suppressing symptoms. However, compelling evidence now incriminates social and environmental determinants of health in initiating these disorders through gene–environmental interactions exacerbated by the disruptive effects of climate change (3).
While epidemiology remains the principal method for identifying disease trends, translating such data in a way that is understandable to politicians, physicians, and the public is challenging. Because the experiences of people in a wide range of real-world settings provide the substrate for epidemiology, drilling down to the lived experiences of individuals whose lives have been changed by IMNCDs is enlightening. Here, I use three examples to reveal the power of this approach for incentivizing and driving policy change in a field subject to complex interactions between biological, environmental, and social factors.
Air pollution—the hidden killer
Air pollution is one of the important drivers of adverse health, affecting not only the lungs, which are exposed to the highest concentrations of air pollutants throughout life, but almost every organ in the body. The experiences of Ella Adoo Kissi-Debrah, for example, offer salutary lessons. She was a 9-year-old London girl who, after almost 30 hospital admissions during her 30 months of asthma, tragically died on 15 February 2011 (4). A second inquest requested by her mother, Rosamund, conducted in November/December 2020 established that illegal levels of outdoor air pollution were a major factor in the onset and progression of her illness and subsequent asthma-related death. Ella was the first person to have air pollution listed as a cause of death on a death certificate in the United Kingdom. She had attended six leading London hospitals during her short life and yet not once was air pollution ever considered to be contributory to her devastating disease even though she lived only 30 meters from one of the city’s busiest and most polluted roads. A Prevention of Future Deaths (PFD) Report issued by the coroner highlighted the failings of public information systems and health practitioner knowledge and the failure of the government to comply with legal limits for air quality. Subsequent studies have shown the harmful effect of ambient air pollutants on asthma induction (5) and the beneficial effect of reducing such exposures on hospital admissions (6). Driven by evidence, the World Health Organization (WHO) recently lowered its health-related limit values for air pollution by particulate matter (PM)2.5 and nitrogen dioxide (NO2). Now, 97% of the urban population in the European Union is exposed to emissions exceeding these limits, and this is linked to up to 5 million premature deaths/year (7).
The scourge of poor housing and indoor air pollution
It has long been known that deprivation and poor housing are major determinants of poor health and that this effect begins from birth or even before. With climate change, we are familiar with the destructive influence of excess heat and flooding but less so with the insidious erosion of health by the indoor “breathed environment”. Awaab Ishak died in a small apartment in Rochdale, northwest England, on 21 December 2020, shortly after his second birthday. The autopsy found that Awaab’s airways were swollen to a degree that would compromise his breathing, and fungus was detected in his blood and lungs along with evidence of severe allergic granulomatous inflammation (8). Inspection of the apartment revealed extensive black mold on the walls and ceilings of the bathroom, kitchen, and Awaab’s bedroom caused by condensation from inadequate ventilation. Despite numerous appeals and requests by Awaab’s father, the social housing authority failed to deal with the issue or rehouse the family. The coroner said the death of the toddler, who suffered prolonged exposure to mold, should be a “defining moment” for the housing sector. A PFD Report was issued placing a duty upon landlords of social housing to rectify any recognized disrepair relating to damp and mold. Mold spores are ubiquitous and will only take hold if the temperature and humidity are suitable. Climate change is a key factor in creating these conditions of condensation and dampness in housing, especially in accommodation that has been “sealed” to conserve energy.
The allergy epidemic
While all IMNCDs continue to be on the increase, among the most rapid rises over the last two decades have been those involving allergy. For example, there has been a three-fold increase in the incidence of food-related hospital admissions for anaphylaxis in the United Kingdom (9), rising to five-fold for peanut anaphylaxis. Food allergy now affects 7% of children in the United Kingdom and 9% of those in Australia. On 17 July 2016, Natasha Ednan-Laperouse (aged 15 years) died from anaphylaxis while flying from the United Kingdom to Nice with her friend and father (10). Attempts to resuscitate her, including the use of two adrenaline EpiPens, sadly failed. From 2 months of age, Natasha was highly allergic and had her first anaphylactic reaction (to banana) aged 6 months. On that fateful day in 2016, prior to the departure of her flight from London, Natasha had purchased and eaten a baguette whose dough contained sesame seeds, to which she was extremely sensitive. The wrapper had no allergen advice as the baguette was made on the premises and contents labelling was not required by United Kingdom law. Following an inquest in 2018, the coroner issued a PFD Report directing urgent attention to mandatory ingredient labelling of food assembled in “local kitchens” and improvements to the needle length of adrenaline autoinjectors.
The power of narratives in health and risk messaging
What do these three tragic stories have in common other than having been caused by IMNCDs? First and foremost, each of these deaths was avoidable, yet—despite their desperate efforts—the parents were not in a position to effectively intervene to protect their children. Second, as the coroners’ PFD reports stated, these children died in circumstances where there was a lack of professional understanding of the seriousness of their conditions, their underlying causes, the necessity for communication between health and social service providers, and how the demise of these children could have been prevented by those with the appropriate leverage to do so. Third, in each case, the parents and families of these children have campaigned tirelessly and with great courage to ensure that the PFD recommendations are enacted, and, to their enormous credit, they continue to be leading lights advocating for change. Finally, the impacts of these heartrending stories on government, industry, and society at large are influencing policy changes (Ella’s Law, Awaab’s Law, and Natasha’s Law) that would never have occurred had it not been for the parents’ absolute commitment to turn personal tragedy into societal benefit.
While all three examples occurred in the United Kingdom, similar events are occurring in all countries and are increasing in frequency owing to climate change. Such narratives can be game-changing as an essential currency of human connection influencing both behavior and policy changes.
Identifying the overarching drivers of the increase in incidence and severity of IMNCDs
In their lead article, Agache et al. (2) raise the important point that from an evolutionary perspective, anthropogenic environmental changes may be too rapid for our immune system to adequately adapt. While anthropogenic changes to our environment and accompanying lifestyle changes are the principal drivers of upward disease trends in IMNCDs, the genetic architecture of immune diversity and its almost infinite capacity for being trained lies in evolutionary history—versions of genes being beneficial in one environment but not in in another. Barrie et al. (11) recently uncovered that the genetic risk for multiple sclerosis, a neuroinflammatory disorder, arose in Europe following the late Copper Age Yamnaya-related migration from the Pontic Steppe to Europe some 5,000 years ago, with immunogenetic variants most likely being positively selected as a result of pathogen challenges encountered with changes in diet, lifestyle, and population density. Another example is asthma (12) in which, of 51 asthma-associated non-human leukocyte antigen (HLA) susceptibility loci, 39 carry variants of Neanderthal lineage, many being shared across asthma, allergic rhinitis, eczema, and anaphylaxis.
While great advances have been made in managing established IMNCDs, their origins and the reasons for the rising trends remain a mystery. However, tracking the evolution of immune systems in the newborn over time has revealed remarkable dynamics between immune cells and proteins and their plasticity in early life (Figure 1). This occurs in response to the wide variety of microbes encountered at birth, in early childhood, and upon early-life imprinting by environmental exposures that influence the development of IMNCDs later in life. Such early life programming may even begin during intrauterine fetal development with maternal transfer of signals, both indirectly from the placenta or directly to the developing child via cord blood, to influence the expression of developmental genes through epigenetic mechanisms (Figure 1). Once born, the infant is confronted by microbes, initially from the mother and then from the wider environment, that may predispose them to or protect against IMNCDs. For example, environmental diversity and the composition of airway microbiota in 1-month-old asymptomatic infants shape the risk for the later development of asthma (13).
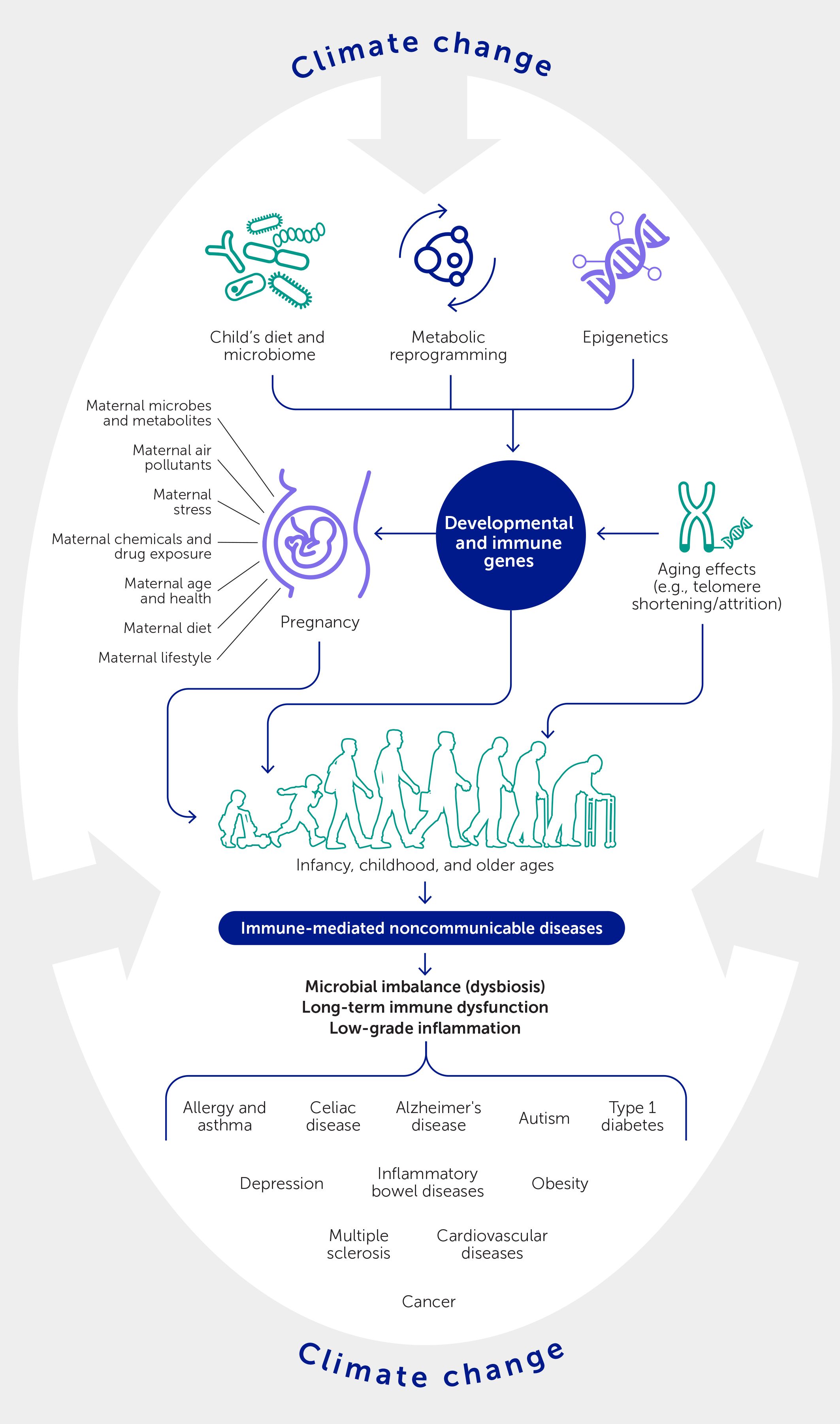
Figure 1 Maternal and early life influences on the emergence of later immune-mediated noncommunicable diseases. The intrauterine and early childhood environments play an important role in setting the stage for these disorders and at the same time reveal opportunities whereby environmental, dietary, and other interventions might interrupt the progression towards disease. Climate change will serve to amplify these processes at multiple interacting levels.
The epithelium, which protects enclosed organs, is a crucial determinant of the microbiome: a high proportion of susceptibility genes influencing various aspects of barrier function and repair are preferentially expressed at these sites in mucosal surfaces and the skin. As the three tragic examples above illustrate, childhood is a particularly vulnerable time when susceptibility and environmental exposures can converge, sometimes with devastating effects. In each of these cases, the epithelium was the interface between the internal milieu and the breathed environment—translating environmental exposures into disease: i.e., mucous metaplasia and delayed epithelial repair in Ella’s asthma, granulomas with fungal infection in Awaab’s lung disease, and food allergen sensitization through reduced skin, lung, and gut barrier functions in Natasha’s food-induced anaphylaxis.
One other mechanism recently shown to influence the expression of IMNCDs is trained immunity: the long-term epigenetic reprogramming of innate immune cells (monocytes, macrophages, and immune progenitor cells) in early life (Figure 1). While trained immunity is often beneficial—for example, the shaping of innate immune memory against infections (e.g., prolonged protective action of vaccines) via alterations in cellular metabolism (e.g., glycolysis and glutaminolysis, cholesterol synthesis)—its inappropriate expression has been linked to the emergence of multiple IMNCDs (14). Involvement of the innate immune response early in the evolution of IMNCDs opens up new preventative and therapeutic opportunities for developing targeted interventions, including environmental and dietary manipulations, that use epigenetic and metabolic pathways to redirect an individual’s immune system toward a less pathogenic configuration.
WHO states that “Two major global crises of our time, climate change and the epidemic of NCDs, are intertwined. They erode gains in health and development and the quality of life, hitting poor and marginalized people the hardest. Action to manage them both should be aligned in synergistic interventions that can address both” (1). In 2023, at the United Nations climate conference, COP28, the Declaration on Climate and Health (15) was made. It calls for policies to maximise health benefits from climate actions by strengthening synergies between climate mitigation/adaptation and health, both by preventing worsening health impacts and advocating for more climate-resilient and sustainable health systems. Such efforts need to prioritize addressing the inequities in children’s health, recognizing that IMNCD prevention should start from conception and continue throughout childhood to give every child the best start in life.
Statements
Author contributions
SH: Conceptualization, Investigation, Project administration, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study received funding from University of Southampton. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The author declares that the research was conducted in the absence of financial relationships that could be construed as a potential conflict of interest.
The author declares a current collaboration and past co-authorships with the lead article authors IAM, AD, CTH, IA, MJ, MA, TH, and CA.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Climate change and noncommunicable diseases: connections. Geneva: World Health Organization (2023). Available at: https://www.who.int/news/item/02-11-2023-climate-change-and-noncommunicable-diseases-connections.
2. Agache I, Akdis C, Akdis M, Al-Hemoud A, Annesi-Maesano I, Balmes J, et al. Immune-mediated disease caused by climate change-associated environmental hazards: mitigation and adaptation. Front Sci (2024) 1:1279192. doi: 10.3389/fsci.2023.1279192
3. Virolainen SJ, VonHandorf A, Viel KCMF, Weirauch MT, Kottyan LC. Gene-environment interactions and their impact on human health. Genes Immun (2023) 24(1):1–11. doi: 10.1038/s41435-022-00192-6
4. Dyer C. Air pollution from road traffic contributed to girl’s death from asthma, coroner concludes. BMJ (2020) 371:m4902. doi: 10.1136/bmj.m4902
5. Liu S, Jørgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, et al. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J (2021) 57(6):2003099. doi: 10.1183/13993003.03099-2020
6. Singh A, Morley GL, Coignet C, Leach F, Pope FD, Thomas GN, et al. Impacts of ambient air quality on acute asthma hospital admissions during the COVID-19 pandemic in Oxford City, UK: a time-series study. BMJ Open (2024) 14(1):e070704. doi: 10.1136/bmjopen-2022-070704
7. Lelieveld J, Haines A, Burnett R, Tonne C, Klingmüller K, Münzel T, et al. Air pollution deaths attributable to fossil fuels: observational and modelling study. BMJ (2023) 383:e077784. doi: 10.1136/bmj-2023-077784
8. Dyer C. Death of child from mould in home triggers questions over housing policies. BMJ (2022) 379:o2794. doi: 10.1136/bmj.o2794
9. Baseggio Conrado A, Ierodiakonou D, Gowland MH, Boyle RJ, Turner PJ. Food anaphylaxis in the United Kingdom: analysis of national data, 1998-2018. BMJ (2021) 372:n251. doi: 10.1136/bmj.n251
10. Natasha Allergy Research Foundation. Natasha’s story. Available at: https://www.narf.org.uk/natashas-allergies.
11. Barrie W, Yang Y, Irving-Pease EK, Attfield KE, Scorrano G, Jensen LT, et al. Elevated genetic risk for multiple sclerosis emerged in steppe pastoralist populations. Nature (2024) 625(7994):321–8. doi: 10.1038/s41586-023-06618-z
12. Day ASH, Gaulton KJ. A Neanderthal origin of genetic variants associated with asthma. bioRxiv (2023). doi: 10.1101/2023.10.30.564624
13. Thorsen J, Rasmussen MA, Waage J, Mortensen M, Brejnrod A, Bønnelykke K, et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat Commun (2019) 10(1):5001. doi: 10.1038/s41467-019-12989-7
14. Ochando J, Mulder WJM, Madsen JC, Netea MG, Duivenvoorden R. Trained immunity — basic concepts and contributions to immunopathology. Nat Rev Nephrol (2023) 19:23–37. doi: 10.1038/s41581-022-00633-5
15. World Health Organization. COP28 Declaration on Climate and Health. Geneva: World Health Organization (2023). Available at: https://www.who.int/publications/m/item/cop28-uae-declaration-on-climate-and-health.
Keywords: noncommunicable diseases, immunity, climate change, trained immunity, air pollutants, housing quality, food hypersensitivity
Citation: Holgate ST. The lived experience of immune-mediated noncommunicable diseases in relation to environmental change. Front Sci (2024) 2:1393167. doi: 10.3389/fsci.2024.1393167
Received: 28 February 2024; Accepted: 11 March 2024;
Published: 04 April 2024.
Edited by:
Frontiers in Science Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2024 Holgate. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephen T. Holgate, Uy5Ib2xnYXRlQHNvdG9uLmFjLnVr
 Stephen T. Holgate
Stephen T. Holgate