- The National Clinical Research Center for Mental Disorders and Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital, Advanced Innovation Center for Human Brain Protection and Laboratory for Clinical Medicine, Capital Medical University, Beijing, China
Background: Aberrant cognition is one of the crucial symptoms of depression. However, whether the negative rumination participates in depression with melancholic features or anxious distress remains unclear.
Methods: In this study, we addressed this issue by compiling a questionnaire that can comprehensively measure the negative cognitive processing bias in depression. We also conducted an exhaustive analysis of its influencing factors, including the subtype of depression, age, gender, age of onset, family history of mental disorder, and education year.
Results: We found that depression increased negative attention bias, negative memory bias, negative interpretation bias, and negative rumination bias. Importantly, among the several dimensions of negative cognitive bias, negative rumination bias was more serious in the melancholic than anxious subgroup. Furthermore, Spearman rank correlation analysis showed that negative rumination bias correlates with family history and age of onset of depression.
Limitations: We mainly explored melancholic and anxiety subgroups and did not include other subtypes. Due to time constraints, we did not conduct long-term follow-ups or explore the neural mechanisms of the differences between depressive and anxious rumination.
Conclusion: Our results contribute to the existing literature on the psychological mechanisms underlying aberrant cognition in depression. These findings could provide guidance for clinical practice and individualized precision treatment of cognitive biases in major depressive disorder. Therefore, rumination-focused therapies would be tailored differently for melancholic versus anxious subgroups.
1 Introduction
Major depressive disorder (MDD) affects almost 350 million people globally, causing disability and a tremendous burden on families and society (Huang et al., 2019; Smith, 2014). However, MDD is a highly heterogeneous disorder (Uher et al., 2014; Zimmerman et al., 2015), with its pathophysiology remaining unclear. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), depression is divided into several subtypes. The specifier “with melancholic features” is characterized by profound hopelessness, despair, moroseness, or empty mood. Another specifier, “with anxious distress,” is defined as the presence of two or more of five anxious symptoms during depression (Uher et al., 2014). Recently, there has been increasing recognition of cognitive dysfunction as a core feature of depression. Patients with MDD having higher polygenic risk scores were more likely to exhibit melancholic features (Oliva et al., 2023). Melancholic patients were more severely depressed and characterized by worse cognitive performance in attention/working memory, visual learning, reasoning/problem-solving (Zaninotto et al., 2016), and information speed, decision speed, and reward-relevant emotional processing of happy expressions, even after co-varying for symptom severity. Anxious distress is known to have different neurobiological profiles compared to non-anxious depression regarding the hypothalamic–pituitary–adrenal axis function, structural and functional brain imaging findings, inflammation markers, and so on (Choi et al., 2020).
In MDD, aberrant cognition strongly contributes to functional impairments but is barely addressed in current therapies (Monroe and Harkness, 2022). The cognitive dysfunction is usually persistent and recurrent and significantly reduces the quality of life in patients with depression (Chamberlain and Sahakian, 2004). Indeed, negative cognitive biases may contribute to the maintenance of specific depression symptoms, such as sadness, hopelessness, guilt, pessimism, and indecision (Beevers et al., 2019). Beck’s cognitive bias theory of depression is that adverse life events result in negative automatic thinking; that is, MDD usually has cognitive distortion in perception, attention, memory, and reasoning (Kovacs and Beck, 1978; Pössel and Black, 2014). These negative biases are one of the core features of depression, including pessimistic views on self, environment, and future (Phillips et al., 2003). Indeed, depressed patients are more likely to recall negative autobiographical memories and rarely think of delighted times (Brittlebank et al., 1993). Korn et al. reported a lack of optimistic belief in depressed individuals, and this absence correlated with symptom severity (Korn et al., 2014). Depressive symptoms were positively and strongly correlated with negative automatic thoughts about self and moderately associated with dysfunctional attitudes among people living on the four continents.
The cognitive model of depression, including biased attention, rumination, memory, and dysfunctional schemas, has been strongly and consistently related to its onset and maintenance (Disner et al., 2011). In addition, rumination characterized by repetitive negative thinking is one of the risk factors for MDD. These negative thoughts usually have an intimate association with adverse outcomes in depression, including its onset, severity, and therapeutic efficacy (Wahl et al., 2019). Park and his colleagues demonstrated the association between higher rumination intensity and greater neural activity in frontoparietal regions responsible for cognitive control (Park et al., 2024). Moreover, the study reported the link between rumination and default mode network (DMN) activation, especially in the DMN core regions and the dorsal medial prefrontal cortex (Zhou et al., 2020). Since rumination is intertwined with the difficulty of regulating negative thoughts in MDD, the association between rumination and its subtypes has not been studied. The two most important characteristics of negatively biased cognition include pessimistic judgment bias (Douglas and Porter, 2010; Maniglio et al., 2014) and catastrophic reactions to negative feedback (Beats et al., 1996; Elliott et al., 1997). There is a study to examine the role of a negative interpretation bias in adolescent pain (Heathcote et al., 2016). In the cross-modal, the selective attentional bias occurs both in the engagement and the disengagement facets (Mao et al., 2020). Among the different subtypes of depression, patients with melancholic and anxious distress characteristics had higher morbidity and suicide risk than other subtypes, and negative bias was a prominent and common stress-coping pattern in the two subtypes (Smith et al., 2018; Sokol et al., 2022; Vinograd et al., 2020). Negative bias is one of the important psychopathological features of depression (Dai et al., 2019; Winer and Salem, 2016), which manifests differently in different subtypes. Previous studies have found that patients with anxious distress have significant negative attention and interpretation biases (Elgersma et al., 2018), and patients with melancholic traits have more significant negative memory and rumination biases (Marchetti et al., 2018). Therefore, negative memory and rumination bias are strongly associated with melancholic characteristics, and negative attention bias is closely related to anxious distress, but more evidence is needed. The negative cognitive processing bias questionnaire is used for the early warning and diagnosis of depression, and it could evaluate the extent of cognitive impairment in depressive patients.
However, whether the rumination is involved in the negative cognition bias in depression is not clear. Therefore, it is vital to comprehensively measure the negative cognitive processing bias in depression and analyze its influencing factors, including the subtypes of MDD, age, gender, age of onset, family history of mental disorder, education year, and so on.
2 Methods and materials
2.1 Participants and study setting
This is an observational, cross-sectional study. The inpatients aged 15–65 were recruited from 5 wards of the depression treatment center in Beijing Anding Hospital. Patients were enrolled using the continuous sampling method, as reported (Shi et al., 2023). While the continuous sampling method is common in observational studies, it may introduce selection bias. Inclusion and exclusion criteria were strictly followed to minimize selection bias. The patients diagnosed with major depressive disorder and who meet the subtype criteria of “with melancholic features” or “with anxious distress” according to DSM-5 were included. Patients with comorbid obsessive-compulsive disorder or substance abuse, or those unable to complete the questionnaire, were excluded. Besides, another group of subjects aged 15–65, with no previous history of mental illness, was recruited as a control group.
2.2 Assessment procedure
The depressed inpatients were required to complete the negative cognition processing bias questionnaire within 3 days after hospitalization. The people in the control group were also asked to complete the same questionnaire. The negative cognition processing bias questionnaire that fits Chinese culture was used in this program. It was formed after item analysis and factor analysis and has been tested nationwide in China. It has been proven reliable and valid (Miao et al., 2022). Cronbach’s alpha and McDonald’s omega showed good internal consistency reliability for this questionnaire, including the whole scale and its subdimensions (both α and ω = 0.866). Regarding its validity, the scores for both DAS (r = 0.551, p < 0.001) and BDI-II (r = 0.447, p < 0.001) were significantly correlated with the total score of the questionnaire and even its subdimensions. The four components of negative cognitive processing bias are negative attention bias, negative memory bias, negative interpretation bias, and negative rumination bias (Beal et al., 2023).
The demographic information, medical history, and disease characteristics of the MDD patients were recorded. The selected variables are as follows: subtypes of depression (with melancholic features or with anxious distress), gender, age, education year, age of onset, occupational status (employed/part-time/unemployed/student), family history of mental disorder, first episode or recurrence, and with or without psychotic symptoms. The study protocol was approved by the independent ethics committee of Beijing Anding Hospital.
2.3 Statistical analysis
The counting data were presented as means ± standard deviations (SD). The total score and the four different dimension score of the negative cognition processing bias were calculated. Homogeneity of variance and whether the data conform to normal distribution were tested. Gender differences were compared using a chi-squared test. An independent-samples t-test was used to analyze differences between the control and MDD groups, as well as the differences between the two subtypes with melancholic features or with anxious distress of MDD. Correlation analysis was used to explore the factors influencing the negative cognition processing bias. Non-parametric tests were performed on data that did not conform to normal distribution. Spearman’s rank correlation analysis was employed to identify the correlation between negative cognitive bias and age, age of onset, and years of education. The analysis was conducted using SPSS Statistics 26, with the level of significance set at 0.05, which is a two-tailed analysis.
3 Results
3.1 Demographic and clinical characteristics of the sample
In total, 154 subjects were included in the study. A total of 112 patients who fulfilled the inclusion criteria were recruited as the MDD group, with 56 depressed patients fitting the “with the melancholic feature” (Mel subgroup) and 56 fitting the “with anxious distress feature” (Anx subgroup). Additionally, 42 healthy subjects were recruited as a control group. The demographic and clinical characteristics of the subjects are presented in Table 1. The chi-squared test revealed that the sex distribution of MDD and the control group was not statistically significant (χ2 = 0.088, p = 0.767). The Mann–Whitney U test showed no significant statistical difference in age (Z = −0.708, p = 0.479) and education year (Z = 1.793, p = 0.073) between the MDD and control groups. The chi-squared test revealed that sex distribution (χ2 = 0.322, p = 0.570), recurrence (χ2 = 0.144, p = 0.705), family history (χ2 = 0.156, p = 0.693), and psychotic symptoms (χ2 = 0.019, p = 0.891) of the melancholic and anxious groups were not statistically significant. The Mann–Whitney U test showed no significant statistical difference in age (Z = 0.346, p = 0.729), age of onset (Z = −0.047, p = 0.963), time of onset (Z = 0.640, p = 0.522), and education year (Z = −0.819, p = 0.413) between the melancholic and anxious groups.
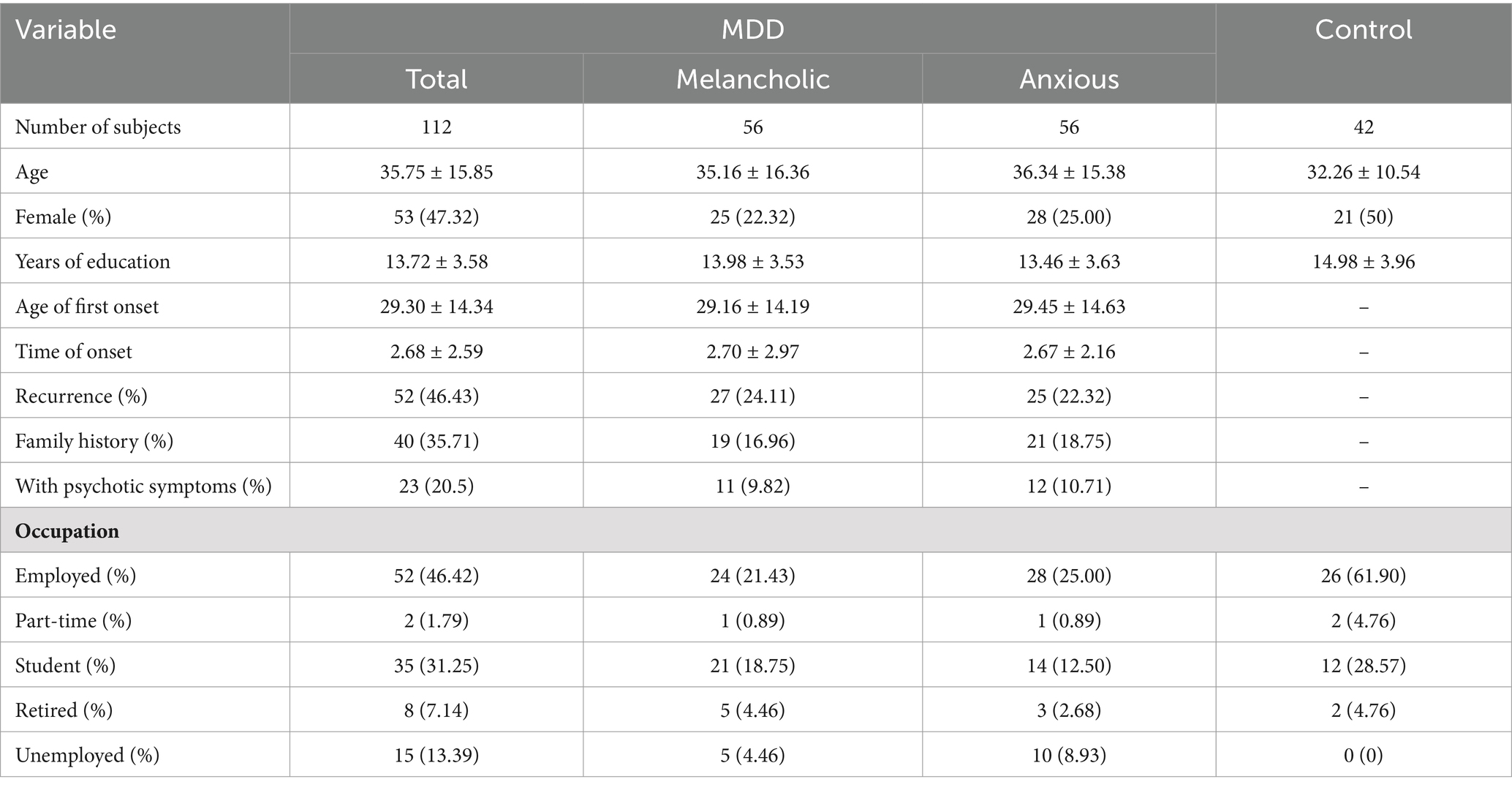
Table 1. Demographic and clinical characteristics of MDD, with melancholic features (Melancholic), with anxious distress (Anxious), and the control groups (Control).
3.2 Negative cognitive processing bias of the subjects
The total score and the scores of the four components of negative cognitive processing bias of the subjects are shown in Figure 1.
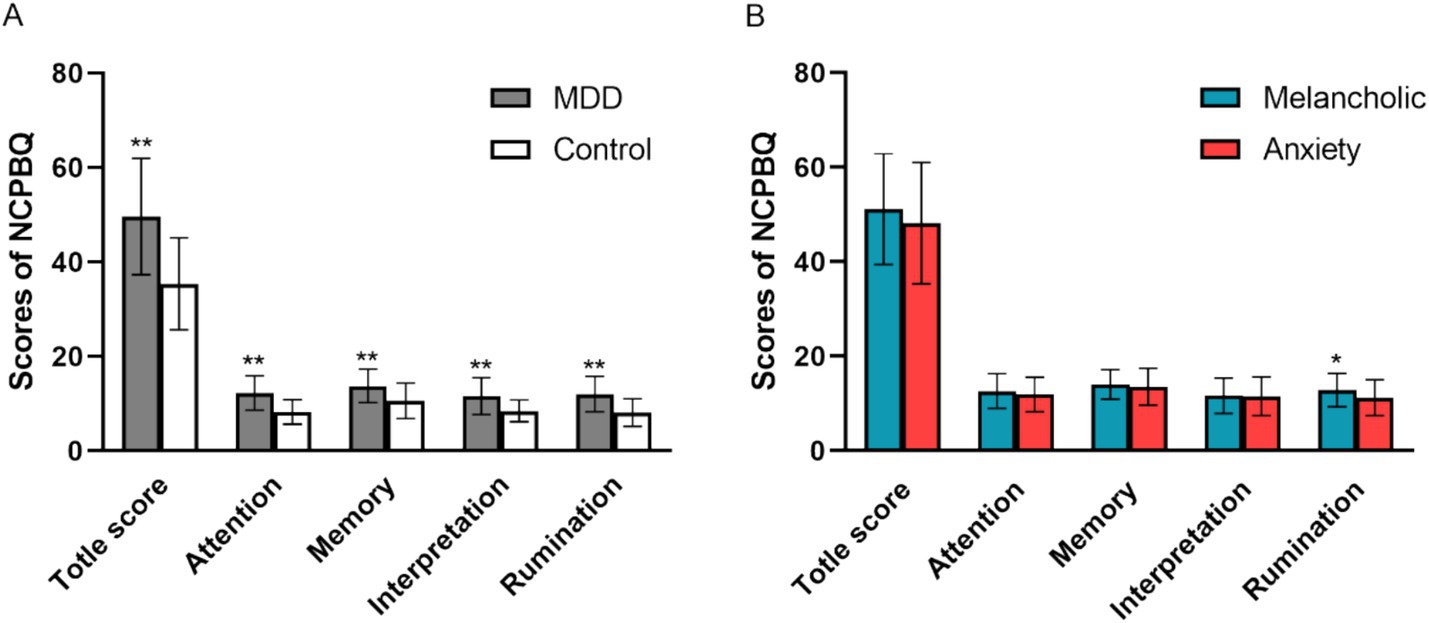
Figure 1. Total score and the subjects’ four components of negative cognitive processing bias. (A) Negative cognitive processing bias between MDD and Control. (B) Negative cognitive processing bias between two MDD subgroups of “with a melancholic feature” and “with anxious distress.” NCPBQ, negative cognition processing bias questionnaire; MDD, major depressive disorder. *p < 0.05, **p < 0.01.
3.2.1 Difference of negative cognitive processing bias between MDD and control groups
The Kolmogorov–Smirnov one-sample test (K-S normality test) showed that the total negative bias score, negative attention, negative memory, and negative rumination dimension data of the control group (n = 42) did not conform to normal distribution. The dimensions of negative memory, negative interpretation, and negative rumination in the depression group (n = 112) did not conform to the normal distribution. Therefore, non-parametric tests were performed to compare the differences between the two groups. The results showed that there were significant statistical differences in the total score of negative bias (Z = −6.038, p < 0.000) and each dimension of negative bias, such as negative attention (Z = −5.989, p < 0.000), negative interpretation (Z = −4.600, p < 0.000), negative memory (Z = −4.252, p < 0.000), and negative rumination (Z = −5.740, p < 0.000) between the depression group and the control group (independent sample Mann–Whitney U test) (Figure 1A).
3.2.2 Difference of negative cognitive processing bias between two MDD subgroups of “with the melancholic feature” and “with anxious distress”
The K-S one sample test showed that negative attentional bias and negative interpretation in the melancholic group did not conform to the normal distribution; therefore, the non-parametric test was performed to compare the differences between the two subgroups. Independent samples t-test showed a statistically significant difference in negative rumination bias between the melancholic and anxiety subgroups (t = 2.33, p = 0.022) (Figure 1B). The independent sample Mann–Whitney U test showed that there was no significant difference in negative interpretation and negative attention bias between the melancholic subgroup and the anxiety subgroup. Similarly, the independent samples t-test showed no significant difference in the total score and its component negative memory bias.
3.3 Clinical characteristics associated with the negative cognitive processing bias in patients with MDD
The Kolmogorov–Smirnov test showed that the data of age, age of onset, and education year did not conform to normal distribution. Spearman’s rank correlation analysis showed that negative rumination bias correlates with family history (ρ = −0.187, p = 0.049) and age of onset (ρ = −0.190, p = 0.045) (Figure 2). Moreover, age was correlated with the total score of negative bias (ρ = −0.245, p = 0.009), negative interpretation (ρ = −0.196, p = 0.038), and negative memory bias (ρ = −0.286, p = 0.002). Age of onset correlates with a total score of negative bias (ρ = −0.271, p = 0.004), negative memory bias (ρ = −0.286, p = 0.002), and negative interpretation (ρ = −0.229, p = 0.015). The educated year correlates with negative memory bias (ρ = −0.240, p = 0.011). There were no significant differences in negative bias among patients with the first episode or recurrence, as well as patients with or without psychotic symptoms.
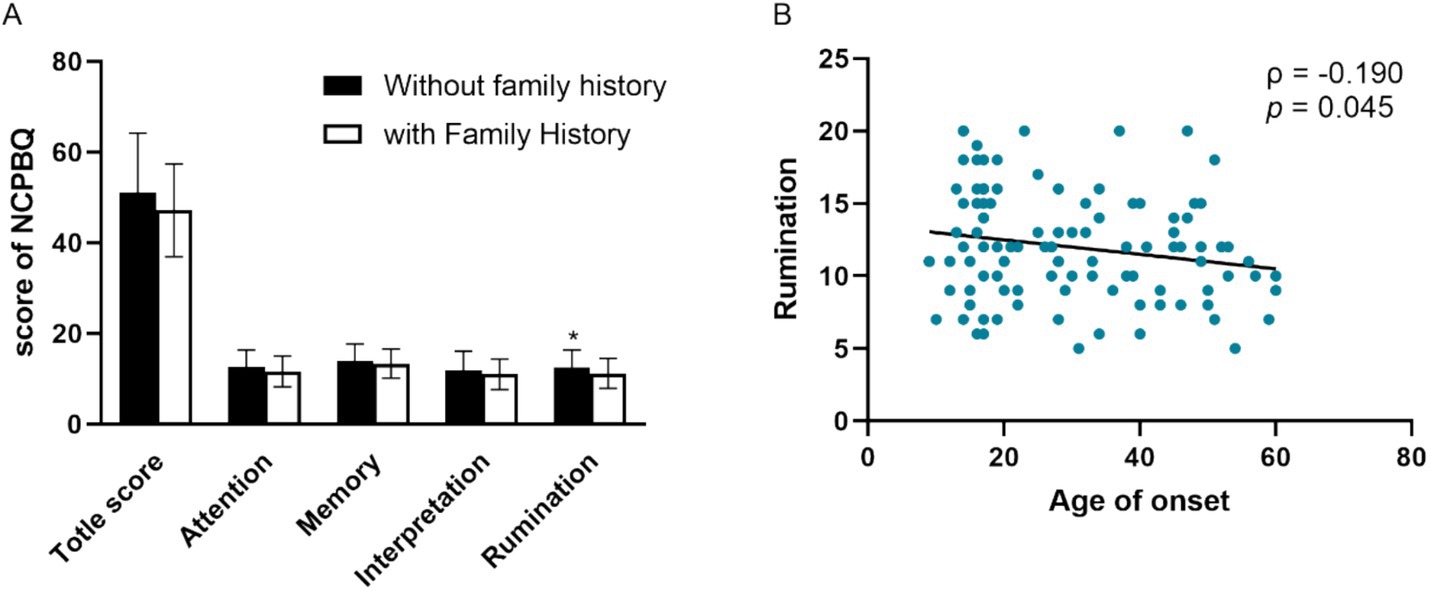
Figure 2. Clinical characters associated with the negative cognitive processing bias in patients with MDD. (A) Negative rumination bias correlates with family history. (B) Negative rumination bias correlates with age of onset. NCPBQ: negative cognition processing bias questionnaire. MDD: major depressive disorder. *p < 0.05.
4 Discussion
In the present study, we investigated the involvement of negative cognitive processing bias in major depressive disorder. We found that depression increased the total scores and each dimension of negative cognitive processing bias. Importantly, among the several dimensions of negative cognitive bias, rumination in the melancholic subgroup was more serious than in the anxiety subgroup. Furthermore, negative rumination bias correlates with family history and age of onset. Researchers suggest that decline in rumination may be a common feature following cognitive behavior therapy interventions. Moreover, rumination scores were associated with post-treatment mindfulness-based cognitive therapy. These findings could provide guidance for clinical practice and contribute to the understanding of cognitive biases in major depressive disorder. Therefore, rumination-focused therapies could be tailored differently for melancholic versus anxious subgroups. Our research provides evidence for individualized precision treatment.
Our finding that depression increased the negative cognitive processing bias is consistent with the results of a previous study (Li et al., 2022). It might be argued that healthy people deal with life events with a more positive attitude. However, negative processing bias is a cognitive trait that depressed patients prefer negative information. Similarly, the four components of negative cognitive processing bias, including negative attention bias, negative memory bias, negative interpretation bias, and negative rumination bias, were more serious than controls. Depression has two domains of biased cognition: pessimistic judgment bias and aberrant response to negative feedback (Noworyta et al., 2021). Abnormal neural processing and negative emotional bias are trait marks of depression (Li et al., 2022). Negative bias may be facilitated by the excessive ventral bottom-up negative emotion along with the incapability of the dorsal prefrontal top-down system (Noworyta et al., 2021).
Rumination is one of the crucial features of depression (Zhang et al., 2020). So far, patients are often treated homogeneously despite having heterogeneous symptoms with distinct underlying neural mechanisms. Treatment that is directly relevant to an individual patient’s subset of symptoms might more precisely and thus effectively aid in the alleviation of their specific symptoms. Our results showed that negative rumination bias in the melancholic subgroup is more serious than that in the anxiety subgroup. These results are consistent with previous studies. Nelson et al. proposed that ruminative thinking could distinguish melancholic from non-melancholic major depression. They found that ruminative thinking was present in 53% of patients with melancholia but only 11% of the non-melancholic patients. Rumination appears to be useful for making the diagnosis of melancholia and may facilitate the study of the psychobiology and treatment of this disorder (Nelson and Mazure, 1985). Patients who engage in rumination may focus more on adverse life events, gloomy emotions, and the possible terrible consequences and could not distract themselves from disruptive moods promptly. Then, rumination exacerbates and prolongs depressed symptoms in vulnerable individuals via the following mechanisms (Nolen-Hoeksema et al., 2008). Firstly, rumination intensifies pessimistic thoughts and biological responses to stress. Secondly, depression with a ruminative tendency leads to negative thinking and difficulty in solving problems effectively. Lastly, ruminative patients obtain less social support, which further increases depressive symptoms (Watkins, 2008), depressive episodes (Nolen-Hoeksema et al., 2008), and risk of relapse (Nolen-Hoeksema, 2000). Removing negative bias from patients with depression can modulate the abnormal physiological stress responses (Jopling et al., 2020). Depressed patients have more biological factors that cause more negative rumination. Moreover, Brooke et al. regard rumination as a mediator of metacognitive training for depression in older adults (Schneider et al., 2023). Mindfulness-based cognitive therapy led to decreased salience network connectivity to the lingual gyrus during a ruminative state, and this change mediated improvements in the ability to sustain and control attention to body sensations (van der Velden et al., 2023). Rumination symptoms may be alleviated over the course of antidepressant treatment (Segerberg et al., 2024). However, previous studies have not distinguished between melancholic and anxious subtypes. Therefore, rumination-focused therapies could be tailored differently for melancholic versus anxious subgroups. Our research can contribute to precise and individualized treatment of depression.
Our results demonstrated that rumination in the melancholic subgroup was more serious than in the anxiety subgroup. A tendency for higher CRP and adipokines was observed in atypical depression; increased IL-6 was found in melancholic depression (Křenek et al., 2023). Jessica et al. reported that a posterior cingulate dominant state results in less anticorrelation between the left dorsolateral prefrontal cortex/middle frontal gyrus and the left precuneus/posterior cingulate cortex in melancholic depression is expected to specifically relate to rumination symptoms (Taylor et al., 2022). The at-risk group of depression showed greater activation in two default mode network (DMN) regions, the medial prefrontal cortex and the inferior parietal lobule (IPL), after hearing criticism but not praise. Criticism-specific activation in the IPL was significantly correlated with rumination (Chou et al., 2023). Others confirm the suspected association between rumination and DMN activation, specifically implicating the DMN core regions and the dorsal medial prefrontal cortex subsystem (Zhou et al., 2020). These results are evidence of our research and provide a basis for the neural basis of rumination. However, some but not all studies showed that anxiety symptoms could predict suicide risk (Bjerkeset et al., 2008; Placidi et al., 2000) and worse outcomes with antidepressant treatment (Fava et al., 2008; Uher et al., 2011). Moreover, there were no significant differences among depressive rumination, but patients with bipolar disorder may have had more rumination on positive affect (Kovács et al., 2020).
Negative rumination bias correlates with family history. However, our results are contrary to previous reports. Individuals with depressive symptoms and those with a family history of depression were characterized by higher rumination than controls (Moretta and Messerotti Benvenuti, 2022). This inconsistency is related to the different references. We expect that there are more psychosocial factors in depression without a family history and more biological factors in patients with a family history. Thus, depressive rumination might have more correlations with psychology. Negative memory bias is a depressotypic process and might play a mechanistic role in the development of the co-occurrence of different psychiatric disorders (Duyser et al., 2020). Age of onset correlates with a total score of negative bias, negative memory bias, negative interpretation, and negative rumination bias. Late-life depression was slower to identify surprised faces and more likely to create negative statements in the interpretation task. There was no evidence of negative bias in memory or attention, but patients with late-life depression performed more poorly on the recall task (Baruch et al., 2021). In depression, age of onset was negatively correlated with emotion recognition. It has been suggested that rumination bias is due to underlying negative core beliefs that drive several cognitive processes.
This study provides new evidence of negative bias in depressed patients but did not show a global bias across cognitive domains. These findings may be relevant to patients in primary and secondary care suffering from depression, including inpatients and those presenting a high level of risk. However, we mainly explored melancholic and anxiety subgroups and did not include other subtypes. The exclusion of other depression subtypes (e.g., atypical depression) limits generalizability. Here, we analyzed clinical characteristics associated with the negative cognitive processing bias in patients with MDD. Due to time constraints, we did not conduct long-term follow-up and explore the neural mechanisms of the differences between depressive and anxious rumination. The minimum enrollment criterion is 15 years old. It is possible that the patient could have bipolar disorder later. However, psychiatric diagnosis is dynamic, and it is difficult to define whether it will progress to bipolar disorder. Future studies are needed to explore longitudinal changes in cognitive biases and their treatment responsiveness.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the independent ethics committee of Beijing Anding Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
H-lW: Writing – original draft. X-nS: Writing – review & editing. J-lZ: Writing – review & editing. QJ: Writing – original draft. WX: Writing – original draft. W-wD: Writing – original draft. Y-yZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82001418), the Beijing Municipal Administration of Hospitals Incubating Program (PX2021069), and the “Youth Program” of the Beijing Municipal Administration of Hospitals (QML20231904).
Acknowledgments
We thank Professor Tian-mei SI (Peking University) for helpful discussions and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baruch, N., Behrman, S., Wilkinson, P., Bajorek, T., Murphy, S. E., and Browning, M. (2021). Negative bias in interpretation and facial expression recognition in late life depression: A case control study. Int. J. Geriatr. Psychiatry 36, 1450–1459. doi: 10.1002/gps.5557
Beal, E. M., Slade, P., and Krahé, C. (2023). Cognitive processing biases associated with fear of childbirth. J. Anxiety Disord. 99:102761. doi: 10.1016/j.janxdis.2023.102761
Beats, B. C., Sahakian, B. J., and Levy, R. (1996). Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol. Med. 26, 591–603. doi: 10.1017/s0033291700035662
Beevers, C. G., Mullarkey, M. C., Dainer-Best, J., Stewart, R. A., Labrada, J., Allen, J. J. B., et al. (2019). Association between negative cognitive bias and depression: A symptom-level approach. J. Abnorm. Psychol. 128, 212–227. doi: 10.1037/abn0000405
Bjerkeset, O., Romundstad, P., and Gunnell, D. (2008). Gender differences in the association of mixed anxiety and depression with suicide. Br. J. Psychiatry 192, 474–475. doi: 10.1192/bjp.bp.107.045203
Brittlebank, A. D., Scott, J., Williams, J. M., and Ferrier, I. N. (1993). Autobiographical memory in depression: state or trait marker? Br. J. Psychiatry 162, 118–121. doi: 10.1192/bjp.162.1.118
Chamberlain, S. R., and Sahakian, B. J. (2004). Cognition in mania and depression: psychological models and clinical implications. Curr. Psychiatry Rep. 6, 451–458. doi: 10.1007/s11920-004-0010-3
Choi, K. W., Kim, Y. K., and Jeon, H. J. (2020). Comorbid anxiety and depression: clinical and conceptual consideration and Transdiagnostic treatment. Adv. Exp. Med. Biol. 1191, 219–235. doi: 10.1007/978-981-32-9705-0_14
Chou, T., Deckersbach, T., Dougherty, D. D., and Hooley, J. M. (2023). The default mode network and rumination in individuals at risk for depression. Soc. Cogn. Affect. Neurosci. 18:nsad032. doi: 10.1093/scan/nsad032
Dai, Q., Hu, L., and Feng, Z. (2019). Attentional bias modification reduces clinical depression and enhances attention toward happiness. J. Psychiatr. Res. 109, 145–155. doi: 10.1016/j.jpsychires.2018.11.024
Disner, S. G., Beevers, C. G., Haigh, E. A., and Beck, A. T. (2011). Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci. 12, 467–477. doi: 10.1038/nrn3027
Douglas, K. M., and Porter, R. J. (2010). Recognition of disgusted facial expressions in severe depression. Br. J. Psychiatry 197, 156–157. doi: 10.1192/bjp.bp.110.078113
Duyser, F. A., van Eijndhoven, P. F. P., Bergman, M. A., Collard, R. M., Schene, A. H., Tendolkar, I., et al. (2020). Negative memory bias as a transdiagnostic cognitive marker for depression symptom severity. J. Affect. Disord. 274, 1165–1172. doi: 10.1016/j.jad.2020.05.156
Elgersma, H. J., Koster, E. H. W., Tuijl, L. A., Hoekzema, A., Penninx, B. W. J. H., and Bockting, C. L. H. (2018). Attentional bias for negative, positive, and threat words in current and remitted depression. PLoS One 13:e0205154. doi: 10.1371/journal.pone.0205154
Elliott, R., Sahakian, B. J., Herrod, J. J., Robbins, T. W., and Paykel, E. S. (1997). Abnormal response to negative feedback in unipolar depression: evidence for a diagnosis specific impairment. J. Neurol. Neurosurg. Psychiatry 63, 74–82. doi: 10.1136/jnnp.63.1.74
Fava, M., Rush, A. J., Alpert, J. E., Balasubramani, G. K., Wisniewski, S. R., Carmin, C. N., et al. (2008). Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am. J. Psychiatry 165, 342–351. doi: 10.1176/appi.ajp.2007.06111868
Heathcote, L. C., Koopmans, M., Eccleston, C., Fox, E., Jacobs, K., Wilkinson, N., et al. (2016). Negative interpretation Bias and the experience of pain in adolescents. J. Pain 17, 972–981. doi: 10.1016/j.jpain.2016.05.009
Huang, Y., Wang, Y., Wang, H., Liu, Z., Yu, X., Yan, J., et al. (2019). Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry 6, 211–224. doi: 10.1016/s2215-0366(18)30511-x
Jopling, E., Gotlib, I. H., and LeMoult, J. (2020). Effects of working memory training on cognitive, affective, and biological responses to stress in major depression: A novel cognitive bias modification protocol. J. Affect. Disord. 265, 45–51. doi: 10.1016/j.jad.2020.01.007
Korn, C. W., Sharot, T., Walter, H., Heekeren, H. R., and Dolan, R. J. (2014). Depression is related to an absence of optimistically biased belief updating about future life events. Psychol. Med. 44, 579–592. doi: 10.1017/s0033291713001074
Kovacs, M., and Beck, A. T. (1978). Maladaptive cognitive structures in depression. Am. J. Psychiatry 135, 525–533. doi: 10.1176/ajp.135.5.525
Kovács, L. N., Takacs, Z. K., Tóth, Z., Simon, E., Schmelowszky, Á., and Kökönyei, G. (2020). Rumination in major depressive and bipolar disorder - a meta-analysis. J. Affect. Disord. 276, 1131–1141. doi: 10.1016/j.jad.2020.07.131
Křenek, P., Hořínková, J., and Bartečků, E. (2023). Peripheral inflammatory markers in subtypes and Core features of depression: A systematized review. Psychopathology 56, 403–416. doi: 10.1159/000528907
Li, L., Li, R., Shen, F., Wang, X., Zou, T., Deng, C., et al. (2022). Negative bias effects during audiovisual emotional processing in major depression disorder. Hum. Brain Mapp. 43, 1449–1462. doi: 10.1002/hbm.25735
Maniglio, R., Gusciglio, F., Lofrese, V., Belvederi Murri, M., Tamburello, A., and Innamorati, M. (2014). Biased processing of neutral facial expressions is associated with depressive symptoms and suicide ideation in individuals at risk for major depression due to affective temperaments. Compr. Psychiatry 55, 518–525. doi: 10.1016/j.comppsych.2013.10.008
Mao, N., Xia, L., Zhang, Q., Li, C., and Cui, L. (2020). Mechanisms of cross-modal selective attentional bias for negative faces of anger and disgust in high-trait anxiety individuals. Neuroreport 31, 879–884. doi: 10.1097/wnr.0000000000001455
Marchetti, I., Everaert, J., Dainer-Best, J., Loeys, T., Beevers, C. G., and Koster, E. H. W. (2018). Specificity and overlap of attention and memory biases in depression. J. Affect. Disord. 225, 404–412. doi: 10.1016/j.jad.2017.08.037
Miao, K., Liu, X., Zhang, X., Li, Y., Liao, X., Zhang, R., et al. (2022). Revision and psychometric properties of the negative cognitive processing bias scale. Front. Psych. 13:1013108. doi: 10.3389/fpsyt.2022.1013108
Monroe, S. M., and Harkness, K. L. (2022). Major depression and its recurrences: life course matters. Annu. Rev. Clin. Psychol. 18, 329–357. doi: 10.1146/annurev-clinpsy-072220-021440
Moretta, T., and Messerotti Benvenuti, S. (2022). Early indicators of vulnerability to depression: the role of rumination and heart rate variability. J. Affect. Disord. 312, 217–224. doi: 10.1016/j.jad.2022.06.049
Nelson, J. C., and Mazure, C. (1985). Ruminative thinking. A distinctive sign of melancholia. J. Affect. Disord. 9, 41–46. doi: 10.1016/0165-0327(85)90008-4
Nolen-Hoeksema, S. (2000). The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. J. Abnorm. Psychol. 109, 504–511. doi: 10.1037/0021-843X.109.3.504
Nolen-Hoeksema, S., Wisco, B. E., and Lyubomirsky, S. (2008). Rethinking rumination. Perspect. Psychol. Sci. 3, 400–424. doi: 10.1111/j.1745-6924.2008.00088.x
Noworyta, K., Cieslik, A., and Rygula, R. (2021). Neuromolecular underpinnings of negative cognitive Bias in depression. Cells 10:3157. doi: 10.3390/cells10113157
Oliva, V., Fanelli, G., Kasper, S., Zohar, J., Souery, D., Montgomery, S., et al. (2023). Melancholic features and typical neurovegetative symptoms of major depressive disorder show specific polygenic patterns. J. Affect. Disord. 320, 534–543. doi: 10.1016/j.jad.2022.10.003
Park, H., Kuplicki, R., Paulus, M. P., and Guinjoan, S. M. (2024). Rumination and over-recruitment of cognitive control circuits in depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 9, 800–808. doi: 10.1016/j.bpsc.2024.04.013
Phillips, M. L., Drevets, W. C., Rauch, S. L., and Lane, R. (2003). Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol. Psychiatry 54, 515–528. doi: 10.1016/s0006-3223(03)00171-9
Placidi, G. P., Oquendo, M. A., Malone, K. M., Brodsky, B., Ellis, S. P., and Mann, J. J. (2000). Anxiety in major depression: relationship to suicide attempts. Am. J. Psychiatry 157, 1614–1618. doi: 10.1176/appi.ajp.157.10.1614
Pössel, P., and Black, S. W. (2014). Testing three different sequential mediational interpretations of Beck's cognitive model of the development of depression. J. Clin. Psychol. 70, 72–94. doi: 10.1002/jclp.22001
Schneider, B. C., Veckenstedt, R., Karamatskos, E., Pinho, L. G., Morgado, B., Fonseca, C., et al. (2023). Negative cognitive beliefs, positive metacognitive beliefs, and rumination as mediators of metacognitive training for depression in older adults (MCT-silver). Front. Psychol. 14:1153377. doi: 10.3389/fpsyg.2023.1153377
Segerberg, T. S. S., Ozenne, B., Dam, V. H., Köhler-Forsberg, K., Jørgensen, M. B., Frokjaer, V. G., et al. (2024). Rumination in patients with major depressive disorder before and after antidepressant treatment. J. Affect. Disord. 360, 322–325. doi: 10.1016/j.jad.2024.05.135
Shi, X., Zhao, Y., Yang, H., Xu, X., Fang, Y., Yu, X., et al. (2023). Factors associated with hospitalization times and length of stay in patients with bipolar disorder. Front. Psych. 14:1140908. doi: 10.3389/fpsyt.2023.1140908
Smith, E. M., Reynolds, S., Orchard, F., Whalley, H. C., and Chan, S. W. (2018). Cognitive biases predict symptoms of depression, anxiety and wellbeing above and beyond neuroticism in adolescence. J. Affect. Disord. 241, 446–453. doi: 10.1016/j.jad.2018.08.051
Sokol, Y., Rosensweig, C., Levin, C., and Linzer, M. (2022). Anxiety and temporal self-appraisal: how people with anxiety evaluate themselves over time. J. Affect. Disord. 296, 309–314. doi: 10.1016/j.jad.2021.09.081
Taylor, J. E., Yamada, T., Kawashima, T., Kobayashi, Y., Yoshihara, Y., Miyata, J., et al. (2022). Depressive symptoms reduce when dorsolateral prefrontal cortex-precuneus connectivity normalizes after functional connectivity neurofeedback. Sci. Rep. 12:2581. doi: 10.1038/s41598-022-05860-1
Uher, R., Dernovsek, M. Z., Mors, O., Hauser, J., Souery, D., Zobel, A., et al. (2011). Melancholic, atypical and anxious depression subtypes and outcome of treatment with escitalopram and nortriptyline. J. Affect. Disord. 132, 112–120. doi: 10.1016/j.jad.2011.02.014
Uher, R., Payne, J. L., Pavlova, B., and Perlis, R. H. (2014). Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress. Anxiety 31, 459–471. doi: 10.1002/da.22217
van der Velden, A. M., Scholl, J., Elmholdt, E. M., Fjorback, L. O., Harmer, C. J., Lazar, S. W., et al. (2023). Mindfulness training changes brain dynamics during depressive rumination: A randomized controlled trial. Biol. Psychiatry 93, 233–242. doi: 10.1016/j.biopsych.2022.06.038
Vinograd, M., Williams, A., Sun, M., Bobova, L., Wolitzky-Taylor, K. B., Vrshek-Schallhorn, S., et al. (2020). Neuroticism and interpretive bias as risk factors for anxiety and depression. Clin. Psychol. Sci. 8, 641–656. doi: 10.1177/2167702620906145
Wahl, K., Ehring, T., Kley, H., Lieb, R., Meyer, A., Kordon, A., et al. (2019). Is repetitive negative thinking a transdiagnostic process? A comparison of key processes of RNT in depression, generalized anxiety disorder, obsessive-compulsive disorder, and community controls. J. Behav. Ther. Exp. Psychiatry 64, 45–53. doi: 10.1016/j.jbtep.2019.02.006
Watkins, E. R. (2008). Constructive and unconstructive repetitive thought. Psychol. Bull. 134, 163–206. doi: 10.1037/0033-2909.134.2.163
Winer, E. S., and Salem, T. (2016). Reward devaluation: dot-probe meta-analytic evidence of avoidance of positive information in depressed persons. Psychol. Bull. 142, 18–78. doi: 10.1037/bul0000022
Zaninotto, L., Solmi, M., Veronese, N., Guglielmo, R., Ioime, L., Camardese, G., et al. (2016). A meta-analysis of cognitive performance in melancholic versus non-melancholic unipolar depression. J. Affect. Disord. 201, 15–24. doi: 10.1016/j.jad.2016.04.039
Zhang, R., Kranz, G. S., Zou, W., Deng, Y., Huang, X., Lin, K., et al. (2020). Rumination network dysfunction in major depression: A brain connectome study. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 98:109819. doi: 10.1016/j.pnpbp.2019.109819
Zhou, H. X., Chen, X., Shen, Y. Q., Li, L., Chen, N. X., Zhu, Z. C., et al. (2020). Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage 206:116287. doi: 10.1016/j.neuroimage.2019.116287
Keywords: major depression, negative cognitive bias, subtype, melancholic, anxious distress
Citation: Wang H-l, Shi X-n, Zhao J-l, Jia Q, Xu W, Dun W-w and Zhao Y-y (2025) Negative rumination in depression subtypes with melancholic features and anxious distress. Front. Psychol. 16:1515500. doi: 10.3389/fpsyg.2025.1515500
Edited by:
Patricia Correa-Ghisays, Center for Biomedical Research in Mental Health Network (CIBERSAM), SpainReviewed by:
Zonglin Shen, The First Affiliated Hospital of Kunming Medical University, ChinaPau Soldevila-Matias, University of Valencia, Spain
Copyright © 2025 Wang, Shi, Zhao, Jia, Xu, Dun and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-ying Zhao, emhhb3lpbmd5aW5nMTAzMUAxNjMuY29t
 Hong-li Wang
Hong-li Wang Xiao-ning Shi
Xiao-ning Shi Ying-ying Zhao
Ying-ying Zhao