- 1Department of Cardiovascular and Metabolic Medicine, Institute of Life Course and Medical Sciences, Faculty of Health and Life Sciences, University of Liverpool, Liverpool, United Kingdom
- 2Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Heart and Chest Hospital, Liverpool, United Kingdom
- 3School of Psychology, Liverpool John Moores University, Liverpool, United Kingdom
Memory recall is subject to errors that can lead to the formation of false memories. Several factors affect memory processes, such as attention deficits or emotional distress. Additionally, cardiovascular diseases may lead to cognitive decline and memory loss, also increasing the occurrence of false events recall. Hypnosis has proved to affect the autonomic nervous system, positively impacting the cardiovascular response. Hypnosis has also been suggested as a tool to enhance memory and autobiographical events recall in both healthy and unhealthy individuals; however, this approach has led to several controversies. Particularly, the employment of hypnosis in autobiographical recall (hypnotic regression) has been accused of favoring the creation of false memories, leading to therapeutic fallacy. In this paper, we review the current literature on the mechanisms behind the creation of false memories and the role played by hypnosis in memory enhancement and false memory recall. The evidence here collected suggests that cardiovascular diseases affect brain health contributing to cognitive decline and memory impairments, also increasing the occurrence of false memories. Hypnosis induces an increase in parasympathetic activity and a decrease in sympathetic activity, suggesting a potential role in preventing some cardiovascular diseases, such as hypertension, which in turn may improve brain health. Additionally, hypnosis has been shown to have some effectiveness in enhancing memory functions, although contradictory findings reported by several studies make it difficult to draw proper conclusions. Hypnotic regression and guided imagery should be used with caution as they may unintentionally lead to false memory recall. Nevertheless, further studies are required to better understand the effects of hypnosis on the brain and the heart and how it can be used to enhance memory, especially in people with cognitive decline.
Introduction
Memory is the faculty of the mind that encodes, stores and retrieves information, and it is fundamental in the development of personal identity (Baddeley, 2013). While some degree of forgetting in general is part of normal memory function (Jasnow et al., 2012; Murayama et al., 2014; Williams et al., 2013), significant memory deficits may be related to age-related cognitive decline (e.g., dementia; Jahn, 2013), emotional or physical trauma (Van Der Kolk, 1998), or interference in memory processing due to poor mental health (such as depression, attention deficit, or emotional distress; Martinussen et al., 2005; Tyng et al., 2017). However, in contrast to forgetting an event, it is also possible to remember it incorrectly, typically leading to false memories (Martinussen et al., 2005).
Several theories have been proposed to explain false memory formation: of these, some attribute it to the way in which information is stored (fuzzy-trace theory; Reyna, 2013), and to the malleability of memory (construction hypothesis; Loftus, 1975). Additional factors may influence the formation of false memories, such as individual differences (e.g., having greater creativity or a tendency to dissociation; Dehon et al., 2008), social pressure (that increases the tendency to accept false events as true; Reysen, 2007), a history of trauma (that makes people more vulnerable to false memories; Zoellner et al., 2000), and sleep deprivation (that increases the chance of encoding false memories; Diekelmann et al., 2008). Moreover, it is also important to consider the influence that physiological factors have on memory, and how these affect memory recall (Birdsill et al., 2013). The importance of the cardiovascular system in satisfying the oxygen demand of the brain, thus influencing its general health, has been largely discussed in literature (brain-heart interaction; Chen et al., 2017), with cardiovascular diseases negatively affecting cognition and memory (e.g., hypertension, heart failure; Cannon et al., 2017; Feng et al., 2020; Habota et al., 2015; Kalaria et al., 2016; Ungvari et al., 2021).
Hypnosis is an altered state of consciousness characterized by focused attention, reduced peripheral awareness, and an increased tendency to respond to suggestions (Elkins et al., 2015). Hypnosis has been shown to affect the autonomic nervous system (De Benedittis, 2024) and to impact cardiovascular response (Emdin et al., 1996; Yüksel et al., 2013), which in turn may lead to a healthier brain (Kekecs et al., 2016; VandeVusse et al., 2010; Walker et al., 2017). Additionally, hypnosis has been suggested as a tool to enhance memory (hypermnesia) and facilitate memory recall (Mulligan, 2006). For example, hypnotic regression aims to recall the repressed memory of a traumatic experience that occurred in an earlier stage of life (Hunter and Eimer, 2012). However, this practice has been largely criticized, arguing that hypnosis may induce false memories (Bryant and Barnier, 1999) rather than recover forgotten ones, with serious implications in therapy and legal cases (such as the recall of past abuses that never occurred and that the patient now believes as true events; Hyman Jr and Loftus, 2001).
The aims of this narrative review are to: (i) give an overview of the mechanisms behind the formation of false memories, also highlighting the role played by the cardiovascular system, (ii) discuss how hypnosis impacts memory recall and false memories, and (iii) discuss the role of hypnosis in cardiovascular and cognitive functions and its implications for memory enhancement.
When memory fails: the construction of false memories
Broadly speaking, memory involves short-term processing of both visual and auditory information (working memory), and long-term memory (where information is stored for a long-term period; Baddeley, 2013). Long-term memory processes mainly consist of storage and recollection of information and previous experiences that can be easily verbalized (i.e., declarative memory; e.g., what I ate for breakfast), and of storage and retrieval of non-verbally articulated procedural information (i.e., non-declarative memory; e.g., how to ride a bicycle; Squire and Dede, 2015), generally in reference to the use of objects or to body movements. Declarative memory can be further subdivided into semantic memory (i.e., memory of facts and general knowledge) and episodic memory (i.e., memory of personal events; Greenberg and Verfaellie, 2010). The main cortical areas thought to play a role in memory processes are the prefrontal cortex, considered essential for working memory, complex thought and associative processes (Stuss and Benson, 2019), and the medial-temporal lobe (Davachi and Preston, 2014), including the parahippocampal gyrus (Luck et al., 2010). Subcortically, the hippocampus, also part of the medial-temporal-lobe, is critical for the acquisition and retention of declarative memories (Opitz, 2014), whereas the cerebellum and the basal ganglia are involved in procedural memory (Lee, 2014).
We have known since the work of Frederik Bartlett (Wagoner, 2017) in 1932 that memory does not provide an exact record of experience, but it is rather an adaptive constructive process, which produces errors, distortions, and illusions in order to preserve the functioning of memory itself (Brady et al., 2018; Schacter, 2013; Schacter et al., 2011). Memory is influenced by several factors, including prior knowledge, mood states and the environment in which learning takes place, which may affect the way in which a memory is acquired, stored and eventually reconstructed (El Sharkawy et al., 2008; Long et al., 2008). Attentional narrowing due to extreme stress and strong emotions affects the memory encoding process (Shields et al., 2017), increasing the occurrence of false memories (Kaplan et al., 2016; Van Damme et al., 2017). Episodic memory is more inclined to distortions than semantic memory (Straube, 2012).
Errors in the memory processes (which can lead to forgetting, incorrect recall, or source misattribution; Foley et al., 2015) can occur at the encoding, storage/consolidation, or retrieval stage (Straube, 2012). At the encoding stage, errors leading to false memories can be induced by visual imagery due to overlapping in the encoding of the imagined and perceived events (Gonsalves and Paller, 2000; Gonsalves et al., 2004). At the consolidation stage, errors leading to false memories are due to the interference of previous memories (retroactive interferences) and sleep deprivation (Diekelmann et al., 2008; Zaragoza et al., 2011). At the retrieval stage, errors leading to false memories are due to misinformation provided by retrieval clues (Brainerd and Reyna, 1998). Among false memories, confabulations occur as a compensatory mechanism to fill in gaps in one's memory (Fotopoulou, 2008). This can happen spontaneously (where the false memory is evoked without an external trigger) or be provoked (when the person is prompted to remember a specific event, e.g., a birthday; Kopelman, 1987). Confabulations can be related to several diseases (such as Korsakoff Syndrome, Alzheimer's disease, traumatic brain injury, etc.), but in some cases they can also occur in healthy individuals (Burgess, 1996). The mechanism underlying confabulations has been correlated with brain lesions (mainly located in the prefrontal cortex, frontal lobe and hypothalamus) and with dementia and psychiatric disorders (e.g., schizophrenia; Brown et al., 2017).
Anxiety and depression further increase the occurrence of errors during memory retrieval (Hertel and Brozovich, 2010). The experience of traumatic events may also lead to the formation of false memories, as people tend to remember more trauma that they really experienced (memory amplification effect; Oulton et al., 2016; Strange and Takarangi, 2015). The occurrence of false memories increases with aging, due to the decline in several regions within the medial temporal lobes and the prefrontal cortex (Dennis et al., 2008; Devitt and Schacter, 2016; Fandakova et al., 2018).
A meta-analysis (Kurkela and Dennis, 2016) of neuroimaging studies has shown that the medial superior frontal gyrus and left inferior parietal cortex may play a role in supporting false memory retrieval. Additionally, the weighting of previous knowledge during new memories acquisition by the medial prefrontal cortex may cause interferences during memory retrieval (memory distortion; Berkers et al., 2017). A recent study (Spets et al., 2021) has also shown differences in brain activity between men and women during false memory formation.
The deliberate induction of false memories in someone else's mind (memory implantation) has been deemed possible (Loftus et al., 2014; Loftus and Pickrell, 1995). Techniques to induce memory implantation have been used in cognitive psychology research to demonstrate how unreliable memory can be and to better understand the formation of false memories (Loftus and Pickrell, 1995). Key aspects in false memory formation are: (i) being exposed to misleading information or leading questions (construction hypothesis; Loftus, 1975), (ii) social influence (Reysen, 2007) and personal expectancy (Hirt et al., 1999), and (iii) imagination inflation (where imagining an event that never happened increases the confidence in the veracity of the event; Garry et al., 1996). Psychoactive drugs and sleep deprivation (Kloft et al., 2023), psychotherapeutic practices aiming at memory recall (Loftus, 1996), and hypnosis (Ofshe and Singer, 1994), especially when protracted for a long period of time, may lead to false memory fabrication (Scoboria et al., 2017).
“Give me your attention”: hypnosis and the brain
Hypnosis is an altered state of consciousness that can modulate both subjective experience (Rainville and Price, 2003) and physiological responses (Gruzelier, 1998). A high level of hypnotisability (that is: a high level of susceptibility to hypnosis; Rainville and Price, 2003; Vanhaudenhuyse et al., 2014) has been associated with the functional connectivity between the left dorsolateral prefrontal cortex and the dorsal anterior cingulate cortex (Faerman et al., 2024). Functional magnetic resonance (fMRI) under hypnosis has shown reduced connectivity between the executive control network, the default mode network, and the posterior cingulate cortex (Jiang et al., 2016) and a reduced activity of the dorsal anterior cingulate cortex (Jiang et al., 2016). Increased functional connectivity between the dorsolateral prefrontal cortex, the executive control network, and the insula in the salience network has also been observed (Jiang et al., 2016). Hypnotic states seem to induce a lower activation of the brainstem, of the right primary somatosensory cortex, and of the left and right insula when compared to wakefulness (Vanhaudenhuyse et al., 2009). Assessment of the hypnotic state during electroencephalography (EEG) has shown changes in brain oscillations, with increased theta band (indicating drowsiness) and changes in the gamma band (indicating problem solving, concentration; Jensen et al., 2015; Vanhaudenhuyse et al., 2014).
The use of hypnosis as therapy (known as hypnotherapy) seeks to induce a hypnotic state, which is then followed by suggestions aiming to positively modify a person's behavior (suggestion therapy; Karle and Boys, 1987) or to help them revive a repressed memory (regression therapy; Hunter, 2009) to correct maladaptive mental schemas (Alladin, 2013; Horowitz, 1988). To induce the hypnotic state, a hypnotic induction is generally used, which follows specific steps (Gruzelier, 1998) and may vary in length.
The applications of hypnosis in recalling memories cover different professional settings. Regression therapy (Hunter and Eimer, 2012) uses hypnosis to recall early life events which may have been purposely forgotten (repressed) as a defense mechanism to protect the self (Kramer, 2010). As these repressed memories may still operate outside of the person's conscious awareness, it is possible to experience maladaptive working schemas that may lead to a series of behavioral and mental problems (Mares, 2022). Helping the patient in recalling the forgotten event is expected to lead them to become aware of the memory, allowing for a rationalization of the event, acceptance, and eventual benefits to the person's mental health (Bateman et al., 2021). Hypnotic regression techniques have also been used to help eyewitnesses or crime victims in recalling memories of events; however, with several criticisms (Lynn et al., 2001; Winter, 2013). Additionally, hypnosis is used to enhance memory performance, usually employing post-hypnotic suggestions (suggestions made while the subject is in a hypnotic state, to be acted upon at some later time after the hypnosis session), such as hypnotic anchors (internal/external triggers—e.g., a gesture or a word—associated to a specific response; Schmidt et al., 2024b).
A forgetful heart: the role of the cardiovascular system in memory and cognition
The central nervous system exerts control over the autonomic and neurohumoral regulation of the cardiovascular system (top-down regulation; Tahsili-Fahadan and Geocadin, 2017). Alterations of the brain-heart axis, such as the ones occurring in the heart-stroke syndrome, can induce autonomic dysfunctions that affect heart rate variability and baroreceptor reflex sensitivity (Scheitz et al., 2018). Moreover, psychological stress has been shown to influence the onset of several cardiovascular diseases (Dar et al., 2019; Esler, 2017; Leo et al., 2023). Vice versa, the influence that the heart exerts on the brain (bottom-up regulation) is also recognized (Taylor et al., 2010; Wolters et al., 2018).
Memory and cognition are largely impacted by reduced cerebral blood flow (Birdsill et al., 2013). Cerebral perfusion is a function of cardiac output, arterial stiffness, and cerebral autoregulation (Moore and Jefferson, 2021). A lower cardiac output is associated with smaller gray matter in older adults (Park et al., 2017) and with worse cognitive performance (Sabayan et al., 2015). Patients with heart failure (HF) exhibit a reduced volume of the hippocampus, which deeply affects cognitive functions (Frey et al., 2021; Lu et al., 2022). Moreover, HF is associated with a 60% increased risk of developing dementia (Wolters et al., 2018). Arterial stiffness can impact brain health, as a greater stiffness of the aorta increases the pulsatile energy to the periphery, detrimentally impacting high blood flow organs such as the brain (Moore and Jefferson, 2021). An increase in the carotid-femoral pulse wave velocity (PWV—a measurement of arterial stiffness) is associated with a reduction in the total brain volume in older adults (Sabayan et al., 2015). Hypertension is a major risk factor in the development of dementia, inducing disruption in cerebral autoregulation (Carnevale et al., 2012; Scullin et al., 2017; Walker et al., 2017) and cerebral blood flow (Jennings et al., 2017). A 20 mmHg increase in systolic blood pressure is associated with a 62% increased risk of developing vascular dementia in people aged 30 to 50 years of age (Emdin et al., 2017). Cognitive impairments and dementia increase susceptibility to the occurrence of false memories (Malone et al., 2019; Watson et al., 2001).
Cognitive-attentional functions are negatively influenced by increased sympathetic activation, which reduces cognitive flexibility due to body arousal (Critchley et al., 2013). An increase in the resting heart rate has been associated with an increased risk of cognitive decline (Kim et al., 2022). Heart rate variability (HRV), which is the variation in time interval between heartbeats usually measured by an electrocardiogram (ECG; Shaffer and Ginsberg, 2017), has been suggested as a physiological correlate of cognitive functioning (Forte et al., 2019), with higher HRV linked to better cognitive performance, and lower HRV linked to worse cognitive function (Forte et al., 2019). High HRV increases control over memory and helps suppress unwanted memories (Gillie et al., 2014), while low HRV worsens performance in short and long-term verbal memory (Frewen et al., 2013). Possible explanations can be found in the relationship between resting HRV and active-inhibitory prefrontal-subcortical circuits, with a higher resting HRV related to increased activity of the executive brain regions (Forte et al., 2019; Thayer et al., 2012) and a lower resting HRV related to a hypoactive prefrontal regulation (Forte et al., 2019; Park and Thayer, 2014). A higher resting-state HRV has been associated with greater memory retrieval functions (Williams et al., 2019). On the contrary, subjects with a lower resting-state HRV have shown to be less capable of discriminating true from false memories (Feeling et al., 2021).
Cerebral hemorrhage can also affect episodic memory, leading to the occurrence of false memories formation (confabulations). Damages to the hippocampus or the temporal lobe can cause retrograde amnesia (Ketonis et al., 2024). Ischemic stroke is a strong risk factor in the development of dementia (Kuzma et al., 2018), due to lesions to cortical and subcortical areas that mediate executive functions (Kalaria et al., 2016). Confabulations have been commonly reported subsequent to traumatic brain injuries (Demery et al., 2001; Dockree et al., 2006) or other cerebrovascular incidents (Dalla Barba et al., 1997; DeLuca and Cicerone, 1991; Parand et al., 2020).
“Follow the beat”: how hypnosis affects the cardiovascular system
Hypnosis has been shown to affect the cardiovascular system in terms of HR and blood pressure (Figure 1). Several studies (Aubert et al., 2009; Bello et al., 2019; Boselli et al., 2018; De Benedittis, 2024; DeBenedittis et al., 1994; Diamond et al., 2007; Hippel et al., 2001; Kekecs et al., 2016; VandeVusse et al., 2010) have assessed the autonomic nervous system response during hypnosis, showing an increase in parasympathetic activity and a reduction in sympathetic activity. A randomized controlled study (Kekecs et al., 2016) conducted on 121 young adults showed that hypnosis was effective in reducing tonic sympathetic nervous activity (measured with skin conductance level) compared to the non-hypnosis control. Another study (DeBenedittis et al., 1994) conducted on 10 healthy subjects showed that hypnosis affects HRV, with increased parasympathetic activation and reduced sympathetic activity. A quasi experimental-study (VandeVusse et al., 2010) conducted on a sample of 30 healthy women showed that parasympathetic activity (changes in HRV) was enhanced during hypnosis. A study (Aubert et al., 2009) conducted on 12 healthy subjects undergoing ECG at rest and during hypnosis showed an enhanced parasympathetic activation (changes in HRV) during the hypnotic state. A quasi-experimental study (Bello et al., 2019) conducted on 15 healthy young men showed that hypnosis induces an increase in HRV. A study (Diamond et al., 2007) conducted on 10 healthy subjects showed that the high frequency of HRV is positively correlated with the depth of the hypnotic state. Another study (Hippel et al., 2001) conducted on 10 healthy subjects showed that hypnosis is effective in reducing sympathetic activity (changes in HRV). A prospective observational study (Boselli et al., 2018) conducted on 40 healthy subjects showed that hypnosis was effective in increasing the parasympathetic tone (assessed using the Analgesia/Nociception Index).
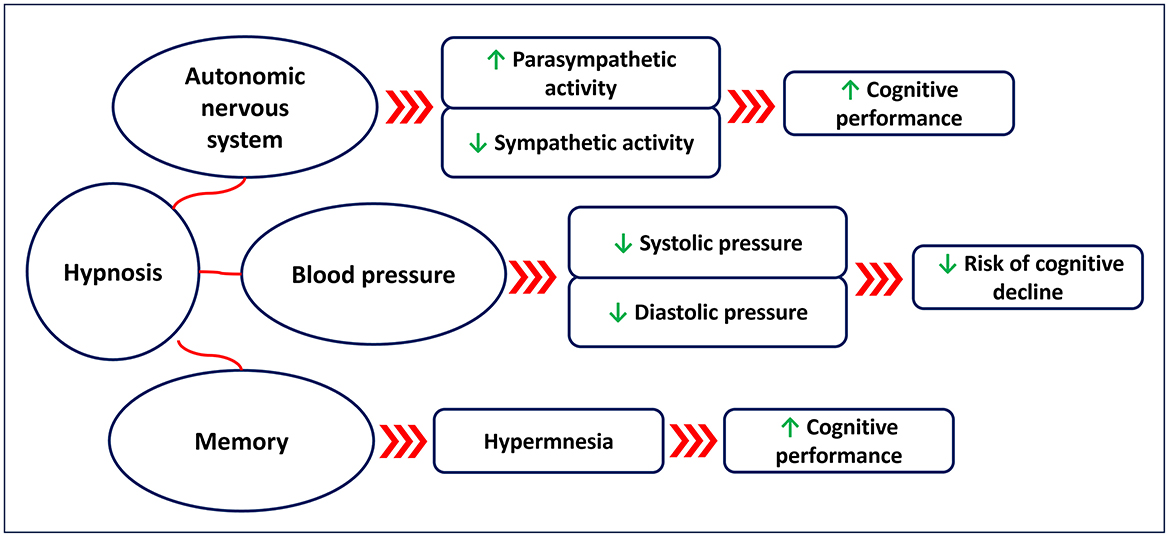
Figure 1. Suggested effects of hypnosis on the autonomic nervous system (changes in heart rate variability), blood pressure, and memory.
Hypnosis has also been shown to affect blood pressure. A study (Emdin et al., 1996) conducted on 10 highly hypnotisable subjects undergoing ECG and blood pressure monitoring during a 50 min hypnosis session showed that hypnosis induced bradycardia (P = 0.04) and a light increase in systolic (P = 0.01) and diastolic (P = 0.03) blood pressure. A randomized controlled pilot study (Raskin et al., 1999) conducted on 33 patients with hypertension, showed that self-hypnosis practiced twice a day for 1 month was effective in reducing diastolic blood pressure (P < 0.05) in the intervention group compared to the non-hypnosis control. A randomized controlled trial (Gay, 2007) conducted on 30 participants with mild essential hypertension, showed that 8 × 30 min sessions of hypnosis were effective in reducing diastolic blood pressure in the intervention group post-treatment (P < 0.0001) as well as at 6 (P < 0.003) and 12-month (P < 0.003) follow-up, compared to the non-hypnosis control. Reduction in systolic blood pressure was also observed in the intervention group post-treatment (P < 0.0003), and at 6 (P < 0.001) and 12-month (P < 0.001) follow-up, compared to the non-hypnosis control. A non-randomized control study (Holdevici and Crǎciun, 2013) conducted on 80 participants diagnosed with primary and secondary hypertension who completed an 8-month Ericksonian hypnosis treatment showed that hypnosis was effective in decreasing stress [Perceived Stress Scale (Cohen et al., 1983)—P = 0.003] and in improving quality of life [SF-36 (Ware and Sherbourne, 1992) —P < 0.05] of these patients post-intervention. However, the number of studies that have investigated the effects of hypnosis on hypertension is still low, and further research is needed to better clarify at what degree hypnosis is able to affect blood pressure, and the physiological mechanisms behind it.
“Now I remember!”: memory and hypnosis
Hypnosis has been suggested as a potential tool to enhance memory and learning. This may be partially explained by the effects that hypnosis has in reducing sympathetic activity (Aubert et al., 2009; Fernandez et al., 2021; Kekecs et al., 2016; Yüksel et al., 2013) and thus in favoring parasympathetic activity, with the latter related to better cognitive performance (Critchley et al., 2013). A randomized controlled study (Çetin et al., 2016) conducted on 70 healthy participants showed that hypnosis was more effective in vocabulary learning for a second language compared to the non-hypnotized control group. Another study (Nemeth et al., 2013) conducted on 14 university students showed that hypnosis has a positive effect on learning. A randomized controlled study (Lindeløv et al., 2017) conducted on 52 participants with brain injury showed the positive effects of a four-week hypnosis intervention (1 h/week) in improving memory performance in this population. Another randomized controlled study (Fligstein et al., 1998) conducted on 60 university students who were asked to recall the content of 60 slides at three recall periods showed that the hypnosis group recalled more correct items than the non-hypnosis group. A recent study (Schmidt et al., 2024a) conducted on 24 participants showed that a post-hypnotic anchor was effective in improving memory recall, with the effect also lasting at 1-week follow-up. A pilot study (Duff and Nightingale, 2005) conducted on seven patients with dementia reported a positive effect of hypnosis on memory after a 9-month intervention, with benefits also maintained at 12-month follow-up (Duff and Nightingale, 2006). Several studies have demonstrated the hypnotic hypermnesia effect in laboratory studies where individuals were administered hypnosis to help recall previous visually presented material (Crawford and Allen, 1983; Kunzendorf et al., 1987; McConkey and Kinoshita, 1988; Stager and Lundy, 1985). This seems to be especially true in highly hypnotisable individuals (Crawford and Allen, 1983). However, the findings of one (Stager and Lundy, 1985) of these studies reporting enhanced memory after hypnosis could not be replicated by another study (Lytle and Lundy, 1988). Moreover, several other studies have shown no effects of hypnosis in enhancing memory (Baker et al., 1983; Dasgupta et al., 1994; Dinges et al., 1992; Dywan, 1988; Nogrady et al., 1985; Putnam, 1979; Register and Kihlstrom, 1987). It has been argued that the hypermnesia noted in some studies involving hypnotic techniques is not induced by hypnosis per se but rather induced by the repeated retrieval effort (Erdelyi, 1994).
Conversely, hypnosis has also been suggested to induce functional amnesia, similar to the one observed in dissociative episodes (Kihlstrom, 1979, 1997). Post-hypnotic amnesia refers to the difficulty of a person in remembering the experience they had during hypnosis (Kihlstrom and Evans, 2014); however, this effect is reversible when a prearranged cue is present (Kihlstrom and Evans, 2014). Post-hypnotic amnesia seems to be associated with hypnotic-induced interferences in the temporal sequencing during the memory recall process (Kihlstrom and Evans, 2014), which may be partially explained by the decoupling of the dorsolateral prefrontal cortex from the default mode networks (Jiang et al., 2016). Two types of post-hypnotic amnesia have been suggested (Evans and Thorn, 1966): (i) post-hypnotic recall amnesia, where the individual is unable to recall the events that occurred during hypnosis, and (ii) post-hypnotic source amnesia, where the individual remembers the information presented during hypnosis, but is unable to recall how they learned it. Post-hypnotic amnesia seems to mainly affect explicit (declarative) memory rather than implicit (non-declarative) memory (David et al., 2000; Kihlstrom, 1997, 2021). In very high hypnotisable individuals, post-hypnotic amnesia can be induced for material learned either before or during hypnosis (Barnier et al., 2001; Bryant et al., 1999). In some individuals, post-hypnotic amnesia cannot be broken down (before its reversal) even when they are exposed to a videotape playback of the events that occurred during hypnosis (McConkey and Sheehan, 1981; McConkey et al., 1980).
Hypnotic age regression has been suggested as a tool to recover early childhood memories. However, the most obvious limitation of this approach is that it is difficult to evaluate whether the memory recalled by the subject is an accurate description of a real-life event or a fabrication (Lynn and Kirsch, 1996; Spanos et al., 1994). This is especially true when considering that memory recall itself is not an accurate process (Schacter et al., 2011), as already discussed. Research on hypnotic regression is scarce and mainly based on anecdotal evidence. A study (Fromm, 1970) published in 1969 presented the case of a patient who was successful in recovering repressed childhood language using hypnotic regression. Context and expectations toward hypnosis have been shown to influence the response in recalling autobiographical memories, also enhancing the subjects' confidence in the accuracy of the memory, especially in highly hypnotisable subjects (Green, 1999). Hypnotic regression has been, and still is, a source of controversy, as its applications have been deemed to potentially induce false memories (Ofshe and Singer, 1994), strongly affecting the outcome of the treatment (from both a therapeutic and a legal perspective—such as in alleged cases of abuse). It has been suggested that imagery and unintentional suggestions may lead to the formation of false memories (Arbuthnott et al., 2001; Strange and Takarangi, 2015) due to the source misattribution effect (Schacter, 1999). Additionally, hypnotic suggestions can inflate the person's confidence of the accuracy of their own (false) memories (imagery inflation), strongly affecting the rewriting of their memory (Heaps and Nash, 1999; Schacter, 1999; Wagstaff et al., 2004; Whitehouse et al., 1988). Some studies have argued that it is a high hypnotisability level rather than hypnosis per se that induces false memories (Barnier and McConkey, 1992; Bryant and Barnier, 1999; Sheehan et al., 1991). But opposing views have been presented as well (Wagstaff et al., 2011). Additionally, a study (Ready et al., 1997) on hypnotic memory recall has shown the negative impact of emotional distress (i.e., anxiety) in producing inaccurate memories, with highly anxious subjects more inclined to inaccurate recalling than non-anxious ones. Hypnotic suggestions have shown the potential to intentionally (Gravitz, 1994; Sheehan et al., 1984; Terrance et al., 2000) and unintentionally (Robin et al., 2018) modify memory recall. Pre-hypnotic warnings and post-hypnotic suggestions may partially reduce the occurrence of false memories (Wagstaff et al., 2008); however, this has been debated (Green et al., 1998).
Connecting the dots: the role of hypnosis on cardiovascular and cognitive functions and its implications for memory enhancement
The occurrence of false memories is related to a series of cognitive and physiological factors, each playing its part in fabricating a memory of an event that never occurred. Errors in the memory processes and emotional distress have an important role in false memory formation (Hertel and Brozovich, 2010; Straube, 2012). Social pressure, personal expectancy, misleading information, leading questions, and imagery inflation can all contribute to false memory formation (Garry et al., 1996; Hirt et al., 1999; Loftus, 1975; Reysen, 2007). In addition, psychoactive drugs and sleep deprivation can contribute to errors in the memory processes and to the occurrence of false memories (Kloft et al., 2023). Furthermore, cardiovascular factors associated with the interruption or reduction in blood flow to the brain (Birdsill et al., 2013) may lead to subsequent structural brain changes (Park et al., 2017), contributing to memory impairments and confabulation (DeLuca and Cicerone, 1991).
While hypnosis has been deemed as one of the causes of false memory fabrication, it is also true that it has shown relevant beneficial effects on both cognitive and cardiovascular functions. Hypnosis seems to positively affect the cardiovascular system with both direct and indirect effects. Direct effects relate to the influence of hypnosis on the autonomic nervous system (Aubert et al., 2009; Bello et al., 2019; Boselli et al., 2018; De Benedittis, 2024; DeBenedittis et al., 1994; Diamond et al., 2007; Hippel et al., 2001; Kekecs et al., 2016; VandeVusse et al., 2010), which regulates heart rate and blood pressure. Relaxation techniques have been shown to be effective in increasing parasympathetic activity while reducing sympathetic activity (Terathongkum and Pickler, 2004). During a hypnotic session, relaxation is usually accomplished during the initial phases of the hypnotic induction, where techniques such as progressive muscle relaxation and emphasis on focused breathing are employed (Karle and Boys, 2010). The decrease in sympathetic activity induced by hypnosis also mitigates the response of the cardiovascular system to emotional distress (Leo et al., 2024), with suggested positive effects on the heart and in reducing the incidence of conditions related to increased sympathetic activity and decreased parasympathetic activity (e.g., arrhythmias, hypertension). The indirect effects of hypnosis on the cardiovascular system relate to its contribution to inducing behavioral changes effectively, leading to the uptake of a healthier lifestyle (e.g., quitting smoking, reduced snacking, increased exercise adherence; Carmody et al., 2008; Delestre et al., 2022; Milling et al., 2018), therefore reducing the risk factors associated with cardiovascular diseases. Behavioral changes are often reached using hypnotic and post-hypnotic suggestions (e.g., ego-strengthening, anchoring; Karle and Boys, 2010). As brain health and cognitive performance depend on cardiovascular functions (such as adequate blood flow to the brain; Launer et al., 2015; Moroni et al., 2018), it seems clear that improved cardiovascular health can positively affect the brain, reducing the risk of early cognitive decline and memory deficit.
The role of hypnosis in improving cognitive functions has also been discussed, with contrasting results. The positive impact of hypnosis on cognitive functions may be related to several factors, such as increased relaxation and reduced sympathetic activity. Increased relaxation can improve focused attention and the recalling of episodic memory (Xu et al., 2014). Practices such as meditation and mindfulness, which share with hypnosis the focus on a relaxed state, have been shown to be effective in memory recalling and memory enhancement (Basso et al., 2019; Heeren et al., 2009; Subramanya and Telles, 2009), possibly suggesting that it is the relaxed state itself and not the technique per se that benefits memory. A decrease in sympathetic activity during the hypnotic state helps in reducing emotional distress that negatively affects memory functions (Shields et al., 2017). The use of imagery is a fundamental part of the hypnotic process, with imagery of emotional events capable of activating the autonomic nervous system in a similar way in which it is activated while experiencing the event in real life (Kosslyn et al., 2001). Guided imagery, when used as a tool to increase relaxation, may help improve memory recall. Self-imagining has been suggested as a potential tool to enhance memory in memory-impaired individuals (Grilli and Glisky, 2010; Raffard et al., 2016). However, several concerns about the use of hypnosis to enhance memory functions have also been raised. The hypermnesia induced by hypnosis reported by some studies (Crawford and Allen, 1983; Kunzendorf et al., 1987; McConkey and Kinoshita, 1988; Stager and Lundy, 1985) has been debated by other authors (Baker et al., 1983; Dasgupta et al., 1994; Dinges et al., 1992; Dywan, 1988; Nogrady et al., 1985; Putnam, 1979; Register and Kihlstrom, 1987), arguing that the observed enhancement of memory performance was more likely related to the repeated retrieval effort made by the participants (Erdelyi, 1994). Moreover, despite the fact that guided imagery is often used with the aim of reconstructing the memory of an event/situation (Arbuthnott et al., 2001), it can also negatively affect memory recall by facilitating the formation of false memories (Arbuthnott et al., 2001; Kealy and Arbuthnott, 2003; Paddock and Terranova, 2001), with realistic imagery more inclined to produce false memories compared to metaphoric imagery (Arbuthnott et al., 2001). Memory distortion during guided imagery is not exclusive to hypnosis but is common to several psychotherapeutic contexts where imagination is encouraged (Loftus, 1996; Lynn and Kirsch, 1996). Therefore, particular attention should be given to avoid leading the patient during the process when using guided imagery for memory recall during hypnosis or other psychotherapeutic approaches.
In summary, while the physiological effects of hypnosis may have a positive role on memory functions due to their beneficial impact on the cardiovascular system, the use of some hypnotic techniques, such as guided imagery, can increase the risk of developing false memories and should be employed with caution.
Conclusion and further directions
Human memory is not an exact record of past experiences but an adaptive process inclined to errors. Several factors may interfere with memory recall, potentially leading to incorrect memories. Factors such as emotional and physical stress can alter memory performance and recall. The brain-heart interaction is crucial in preserving brain health and improving cognitive processes. Health conditions affecting the heart can disrupt the balance between heart and brain processes, leading to cognitive decline and memory impairments. Additionally, alterations in the cardiovascular system may increase the occurrence of incorrect memory recall. Hypnosis has been shown to affect heart rate variability and blood pressure, suggesting a potential role in preventing cardiovascular diseases related to increased sympathetic activity and decreased parasympathetic activity. However, the paucity of evidence on the role that hypnosis has on the cardiovascular system leaves several unanswered questions. Contradictory findings on the role that hypnosis has on memory and cognitive processes make it difficult to draw proper conclusions. Moreover, guided imagery techniques used to enhance the recall of autobiographical events may lead to memory distortions (incorrect recall or source misattribution), and particular attention should be given to avoid unintentional hypnotic suggestions that could induce false memories. Cautions should be exerted when conducting regression therapy, and such an approach should be evaluated case by case.
The large heterogeneity in study design and hypnotic interventions of the studies reviewed in our paper may have been a critical factor for the differences in results reported in literature. Additionally, not all the examined studies have screened for hypnotisability level, a factor that may have contributed to the contradictory results.
Further research should be carried out to better define the effects of hypnosis on the brain and the cardiovascular system, as well as its impact on cognitive processes. Studies with robust design (e.g., randomized controlled studies) and bigger sample size should be conducted to test the efficacy of hypnosis in memory enhancement and investigate its potential beneficial effects in preventing cardiovascular diseases that increase the risk of cognitive decline (e.g., hypertension).
Author contributions
DGL: Conceptualization, Writing – original draft, Writing – review & editing. DB: Writing – review & editing. RP: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
DB is an editorial member of Frontiers in Psychology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alladin, A. (2013). Healing the wounded self: combining hypnotherapy with ego state therapy. Am. J. Clin. Hypn. 56, 3–22. doi: 10.1080/00029157.2013.796282
Arbuthnott, K. D., Arbuthnott, D. W., and Rossiter, L. (2001). Guided imagery and memory: implications for psychotherapists. J. Couns. Psychol. 48:123. doi: 10.1037/0022-0167.48.2.123
Aubert, A. E., Verheyden, B., Beckers, F., Tack, J., and Vandenberghe, J. (2009). Cardiac autonomic regulation under hypnosis assessed by heart rate variability: spectral analysis and fractal complexity. Neuropsychobiology 60, 104–112. doi: 10.1159/000239686
Baker, R. A., Haynes, B., and Patrick, B. S. (1983). Hypnosis, memory, and incidental memory. Am. J. Clin. Hypn. 25, 253–262. doi: 10.1080/00029157.1983.10404111
Barnier, A. J., Bryant, R. A., and Briscoe, S. (2001). Posthypnotic amnesia for material learned before or during hypnosis: explicit and implicit memory effects. Int. J. Clin. Exp. Hypn. 49, 286–304. doi: 10.1080/00207140108410079
Barnier, A. J., and McConkey, K. M. (1992). Reports of real and false memories: the relevance of hypnosis, hypnotizability, and context of memory test. J. Abnorm. Psychol. 101, 521–527. doi: 10.1037/0021-843X.101.3.521
Basso, J. C., McHale, A., Ende, V., Oberlin, D. J., and Suzuki, W. A. (2019). Brief, daily meditation enhances attention, memory, mood, and emotional regulation in non-experienced meditators. Behav. Brain Res. 356, 208–220. doi: 10.1016/j.bbr.2018.08.023
Bateman, A. W., Holmes, J., and Allison, E. (2021). Introduction to Psychoanalysis: Contemporary Theory and Practice. London: Routledge.
Bello, L. L. C., Tornés, A. A., Barthelemy, R. P., Figueroa, E. S., de la Paz Reyes, E., and Hechavarría, M. E. S. (2019). Heart rate variability in deep hypnosis. CorSalud 11:94. Available at: https://openurl.ebsco.com/EPDB%3Agcd%3A5%3A22458853/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A138116918&crl=c&link_origin=scholar.google.com
Berkers, R. M. W. J., van der Linden, M., de Almeida, R. F., Müller, N. C. J., Bovy, L., Dresler, M., et al. (2017). Transient medial prefrontal perturbation reduces false memory formation. Cortex 88, 42–52. doi: 10.1016/j.cortex.2016.12.015
Birdsill, A. C., Carlsson, C. M., Willette, A. A., Okonkwo, O. C., Johnson, S. C., Xu, G., et al. (2013). Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obesity 21, 1313–1320. doi: 10.1002/oby.20170
Boselli, E., Musellec, H., Martin, L., Bernard, F., Fusco, N., Guillou, N., et al. (2018). Effects of hypnosis on the relative parasympathetic tone assessed by ANI (Analgesia/Nociception Index) in healthy volunteers: a prospective observational study. J. Clin. Monit. Comput. 32, 487–492. doi: 10.1007/s10877-017-0056-5
Brady, T., Schacter, D. L., and Alvarez, G. (2018). The adaptive nature of false memories is revealed by gist-based distortion of true memories. PsyArXiv. doi: 10.31234/osf.io/zeg95
Brainerd, C. J., and Reyna, V. F. (1998). Fuzzy-trace theory and children's false memories. J. Exp. Child Psychol. 71, 81–129. doi: 10.1006/jecp.1998.2464
Brown, J., Huntley, D., Morgan, S., Dodson, K., and Cich, J. (2017). Confabulation: a guide for mental health professionals. Int. J. Neurol. Neurother. 4:70. doi: 10.23937/2378-3001/1410070
Bryant, R. A., and Barnier, A. J. (1999). Eliciting autobiographical pseudomemories: the relevance of hypnosis, hypnotizability and attributions. Int. J. Clin. Exp. Hypn. 47, 267–283. doi: 10.1080/00207149908410037
Bryant, R. A., Barnier, A. J., Mallard, D., and Tibbits, R. (1999). Posthypnotic amnesia for material learned before hypnosis. Int. J. Clin. Exp. Hypn. 47, 46–64. doi: 10.1080/00207149908410022
Burgess, P. W. (1996). Confabulation and the control of recollection. Memory 4, 359–412. doi: 10.1080/096582196388906
Cannon, J. A., Moffitt, P., Perez-Moreno, A. C., Walters, M. R., Broomfield, N. M., McMurray, J. J., et al. (2017). Cognitive impairment and heart failure: systematic review and meta-analysis. J. Card. Fail. 23, 464–475. doi: 10.1016/j.cardfail.2017.04.007
Carmody, T. P., Duncan, C., Simon, J. A., Solkowitz, S., Huggins, J., Lee, S., et al. (2008). Hypnosis for smoking cessation: a randomized trial. Nicotine Tob. Res. 10, 811–818. doi: 10.1080/14622200802023833
Carnevale, D., Mascio, G., D'Andrea, I., Fardella, V., Bell, R. D., Branchi, I., et al. (2012). Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension 60, 188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511
Çetin, Y., Çimen, O. A., and Yetkiner, Z. E. (2016). Using hypnosis to enhance learning second language vocabulary. Am. J. Clin. Hypn. 58, 399–410. doi: 10.1080/00029157.2015.1121373
Chen, Z., Venkat, P., Seyfried, D., Chopp, M., Yan, T., and Chen, J. (2017). Brain–heart interaction. Circ. Res. 121, 451–468. doi: 10.1161/CIRCRESAHA.117.311170
Cohen, S., Kamarck, T., and Mermelstein, R. (1983). A global measure of perceived stress. J. Health Soc. Behav. 24, 385–396. doi: 10.2307/2136404
Crawford, H. J., and Allen, S. N. (1983). Enhanced visual memory during hypnosis as mediated by hypnotic responsiveness and cognitive strategies. J. Exp. Psychol. Gen. 112:662. doi: 10.1037/0096-3445.112.4.662
Critchley, H. D., Eccles, J., and Garfinkel, S. N. (2013). “Interaction between cognition, emotion, and the autonomic nervous system,” in Handbook of Clinical Neurology, Vol. 117 (Elsevier), 59–77. doi: 10.1016/B978-0-444-53491-0.00006-7
Dalla Barba, G., Boiss,é, M.-F., Bartolomeo, P., and Bachoud-Lévi, A.-C. (1997). Confabulation following rupture of posterior communicating artery. Cortex 33, 563–570. doi: 10.1016/S0010-9452(08)70237-5
Dar, T., Radfar, A., Abohashem, S., Pitman, R. K., Tawakol, A., and Osborne, M. T. (2019). Psychosocial stress and cardiovascular disease. Curr. Treat Options Cardiovasc. Med. 21, 1–17. doi: 10.1007/s11936-019-0724-5
Dasgupta, A. M., Juza, D. M., White, G. M., and Maloney, J. F. (1994). Memory and hypnosis: a comparative analysis of guided memory, cognitive interview, and hypnotic hypermnesia. Imagin. Cogn. Pers. 14, 117–130. doi: 10.2190/M2MB-A6RD-2NEM-JKD1
Davachi, L., and Preston, A. (2014). “The medial temporal lobe and memory,” in The Cognitive Neurosciences, eds. M. S. Gazzaniga, and G. R. Mangun (The MIT Press). doi: 10.7551/mitpress/9504.003.0059
David, D., Brown, R., Pojoga, C., and David, A. (2000). The impact of posthypnotic amnesia and directed forgetting on implicit and explicit memory: new insights from a modified process dissociation procedure1. Int. J. Clin. Exp. Hypn. 48, 267–289. doi: 10.1080/00207140008415246
De Benedittis, G. (2024). Hypnotic modulation of autonomic nervous system (ANS) activity. Brain Sci. 14:249. doi: 10.3390/brainsci14030249
DeBenedittis, G., Cigada, M., Bianchi, A., Signorini, M. G., and Cerutti, S. (1994). Autonomic changes during hypnosis: a heart rate variability power spectrum analysis as a marker of sympatho-vagal balance. Int. J. Clin. Exp. Hypn. 42, 140–152. doi: 10.1080/00207149408409347
Dehon, H., Bastin, C., and Larøi, F. (2008). The influence of delusional ideation and dissociative experiences on the resistance to false memories in normal healthy subjects. Pers. Individ. Dif. 45, 62–67. doi: 10.1016/j.paid.2008.02.020
Delestre, F., Lehéricey, G., Estellat, C., Diallo, M. H., Hansel, B., and Giral, P. (2022). Hypnosis reduces food impulsivity in patients with obesity and high levels of disinhibition: HYPNODIET randomized controlled clinical trial. Am. J. Clin. Nutr. 115, 1637–1645. doi: 10.1093/ajcn/nqac046
DeLuca, J., and Cicerone, K. D. (1991). Confabulation following aneurysm of the anterior communicating artery. Cortex 27, 417–423. doi: 10.1016/S0010-9452(13)80036-6
Demery, J. A., Hanlon, R. E., and Bauer, R. M. (2001). Profound amnesia and confabulation following traumatic brain injury. Neurocase 7, 295–302. doi: 10.1093/neucas/7.4.295
Dennis, N. A., Kim, H., and Cabeza, R. (2008). Age-related differences in brain activity during true and false memory retrieval. J. Cogn. Neurosci. 20, 1390–1402. doi: 10.1162/jocn.2008.20096
Devitt, A. L., and Schacter, D. L. (2016). False memories with age: neural and cognitive underpinnings. Neuropsychologia 91, 346–359. doi: 10.1016/j.neuropsychologia.2016.08.030
Diamond, S. G., Davis, O. C., and Howe, R. D. (2007). Heart-rate variability as a quantitative measure of hypnotic depth. Int. J. Clin. Exp. Hypn. 56, 1–18. doi: 10.1080/00207140701672961
Diekelmann, S., Landolt, H.-P., Lahl, O., Born, J., and Wagner, U. (2008). Sleep loss produces false memories. PLoS ONE 3:e3512. doi: 10.1371/journal.pone.0003512
Dinges, D. F., Whitehouse, W. G., Orne, E. C., Powell, J. W., Orne, M. T., and Erdelyi, M. H. (1992). Evaluating hypnotic memory enhancement (hypermnesia and reminiscence) using multitrial forced recall. J. Exp. Psychol. Learn. Mem. Cogn. 18:1139. doi: 10.1037/0278-7393.18.5.1139
Dockree, P. M., O'Keeffe, F. M., Moloney, P., Bishara, A. J., Carton, S., Jacoby, L. L., et al. (2006). Capture by misleading information and its false acceptance in patients with traumatic brain injury. Brain 129, 128–140. doi: 10.1093/brain/awh664
Duff, S. C., and Nightingale, D. J. (2005). The efficacy of hypnosis in changing the quality of life in patients with dementia: a pilot-study evaluation. Eur. J. Clin. Hypn. 6, 20–29. Available at: https://psycnet.apa.org/record/2006-11342-003
Duff, S. C., and Nightingale, D. J. (2006). Long-term outcomes of hypnosis in changing the quality of life in patients with dementia. Eur. J. Clin. Hypn. 7:2. Available at: https://openurl.ebsco.com/EPDB%3Agcd%3A6%3A5589174/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A24402816&crl=c&link_origin=scholar.google.com
Dywan, J. (1988). The imagery factor in hypnotic hypermnesia. Int. J. Clin. Exp. Hypn. 36, 312–326. doi: 10.1080/00207148808410521
El Sharkawy, J., Groth, K., Vetter, C., Beraldi, A., and Fast, K. (2008). False memories of emotional and neutral words. Behav. Neurol. 19, 7–11. doi: 10.1155/2008/587239
Elkins, G. R., Barabasz, A. F., Council, J. R., and Spiegel, D. (2015). Advancing research and practice: the revised APA Division 30 definition of hypnosis. Int. J. Clin. Exp. Hypn. 63, 1–9. doi: 10.1080/00207144.2014.961870
Emdin, C. A., Anderson, S. G., Salimi-Khorshidi, G., Woodward, M., MacMahon, S., Dwyer, T., et al. (2017). Usual blood pressure, atrial fibrillation and vascular risk: evidence from 4.3 million adults. Int. J. Epidemiol. 46, 162–172. doi: 10.1093/ije/dyw053
Emdin, M., Santarcangelo, E. L., Picano, E., Raciti, M., Pola, S., Macerata, A., et al. (1996). Hypnosis effect on RR interval and blood pressure variability. Clin. Sci. 91, 36–36. doi: 10.1042/cs0910036supp
Erdelyi, M. H. (1994). Hypnotic hypermnesia: the empty set of hypermnesia. Int. J. Clin. Exp. Hypn. 42, 379–390. doi: 10.1080/00207149408409366
Esler, M. (2017). Mental stress and human cardiovascular disease. Neurosci. Biobehav. Rev. 74, 269–276. doi: 10.1016/j.neubiorev.2016.10.011
Evans, F. J., and Thorn, W. A. (1966). Two types of posthypnotic amnesia: recall amnesia and source amnesia. Int. J. Clin. Exp. Hypn. 14, 162–179. doi: 10.1080/00207146608412959
Faerman, A., Bishop, J. H., Stimpson, K. H., Phillips, A., Gülser, M., Amin, H., et al. (2024). Stanford hypnosis integrated with functional connectivity-targeted transcranial stimulation (SHIFT): a preregistered randomized controlled trial. Nat. Mental Health 2, 96–103. doi: 10.1038/s44220-023-00184-z
Fandakova, Y., Sander, M. C., Grandy, T. H., Cabeza, R., Werkle-Bergner, M., and Shing, Y. L. (2018). Age differences in false memory: the importance of retrieval monitoring processes and their modulation by memory quality. Psychol. Aging 33:119. doi: 10.1037/pag0000212
Feeling, N., Williams, D. P., Speller, L. F., Loftus, E. F., Koenig, J., and Thayer, J. F. (2021). Resting state heart rate variability and false memories. Int. J. Psychophysiol. 159, 17–22. doi: 10.1016/j.ijpsycho.2020.08.009
Feng, R., Rolls, E. T., Cheng, W., and Feng, J. (2020). Hypertension is associated with reduced hippocampal connectivity and impaired memory. EBioMedicine 61. Available at: https://www.thelancet.com/journals/ebiom/article/PIIS2352-3964(20)30458-8/fulltext. doi: 10.1016/j.ebiom.2020.103082
Fernandez, A., Urwicz, L., Vuilleumier, P., and Berna, C. (2021). Impact of hypnosis on psychophysiological measures: a scoping literature review. Am. J. Clin. Hypn. 64, 36–52. doi: 10.1080/00029157.2021.1873099
Fligstein, D., Barabasz, A., Barabasz, M., Trevisan, M. S., and Warner, D. (1998). Hypnosis enhances recall memory: a test of forced and non-forced conditions. Am. J. Clin. Hypn. 40, 297–305. doi: 10.1080/00029157.1998.10403441
Foley, M. A., Bays, R. B., Foy, J., and Woodfield, M. (2015). Source misattributions and false recognition errors: examining the role of perceptual resemblance and imagery generation processes. Memory 23, 714–735. doi: 10.1080/09658211.2014.925565
Forte, G., Favieri, F., and Casagrande, M. (2019). Heart rate variability and cognitive function: a systematic review. Front. Neurosci. 13:710. doi: 10.3389/fnins.2019.00710
Fotopoulou, A. (2008). False selves in neuropsychological rehabilitation: the challenge of confabulation. Neuropsychol. Rehabil. 18, 541–565. doi: 10.1080/09602010802083545
Frewen, J., Finucane, C., Savva, G. M., Boyle, G., Coen, R. F., and Kenny, R. A. (2013). Cognitive function is associated with impaired heart rate variability in ageing adults: the Irish longitudinal study on ageing wave one results. Clin. Auton. Res. 23, 313–323. doi: 10.1007/s10286-013-0214-x
Frey, A., Homola, G. A., Henneges, C., Mühlbauer, L., Sell, R., Kraft, P., et al. (2021). Temporal changes in total and hippocampal brain volume and cognitive function in patients with chronic heart failure—the COGNITION. MATTERS-HF cohort study. Eur. Heart J. 42, 1569–1578. doi: 10.1093/eurheartj/ehab003
Fromm, E. (1970). Age regression with unexpected reappearance of a repressed c3ildhood language. Int. J. Clin. Exp. Hypn. 18, 79–88. doi: 10.1080/00207147008415906
Garry, M., Manning, C. G., Loftus, E. F., and Sherman, S. J. (1996). Imagination inflation: imagining a childhood event inflates confidence that it occurred. Psychon. Bull. Rev. 3, 208–214. doi: 10.3758/BF03212420
Gay, M. C. (2007). Effectiveness of hypnosis in reducing mild essential hypertension: a one-year follow-up. Int. J. Clin. Exp. Hypn. 55, 67–83. doi: 10.1080/00207140600995893
Gillie, B. L., Vasey, M. W., and Thayer, J. F. (2014). Heart rate variability predicts control over memory retrieval. Psychol. Sci. 25, 458–465. doi: 10.1177/0956797613508789
Gonsalves, B., and Paller, K. A. (2000). Neural events that underlie remembering something that never happened. Nat. Neurosci. 3, 1316–1321. doi: 10.1038/81851
Gonsalves, B., Reber, P. J., Gitelman, D. R., Parrish, T. B., Mesulam, M. M., and Paller, K. A. (2004). Neural evidence that vivid imagining can lead to false remembering. Psychol. Sci. 15, 655–660. doi: 10.1111/j.0956-7976.2004.00736.x
Gravitz, M. A. (1994). Memory reconstruction by hypnosis as a therapeutic technique. Psychother. Theory Res. Pract. Train. 31:687. doi: 10.1037/0033-3204.31.4.687
Green, J. P. (1999). Hypnosis, context effects and the recall of early autobiographical memories. Int. J. Clin. Exp. Hypn. 47, 284–300. doi: 10.1080/00207149908410038
Green, J. P., Lynn, S. J., and Malinoski, P. (1998). Hypnotic pseudomemories, prehypnotic warnings, and the malleability of suggested memories. Appl. Cogn. Psychol. 12, 431–444.
Greenberg, D. L., and Verfaellie, M. (2010). Interdependence of episodic and semantic memory: evidence from neuropsychology. J. Int. Neuropsychol. Soc. 16, 748–753. doi: 10.1017/S1355617710000676
Grilli, M. D., and Glisky, E. L. (2010). Self-imagining enhances recognition memory in memory-impaired individuals with neurological damage. Neuropsychology 24:698. doi: 10.1037/a0020318
Gruzelier, J. (1998). A working model of the neurophysiology of hypnosis: a review of evidence. Contemp. Hypn. 15, 3–21. doi: 10.1002/ch.112
Habota, T., McLennan, S. N., Cameron, J., Henry, J. D., Ski, C. F., Thompson, D. R., et al. (2015). Prospective memory impairment in chronic heart failure. J. Int. Neuropsychol. Soc. 21, 183–192. doi: 10.1017/S1355617715000119
Heaps, C., and Nash, M. (1999). Individual differences in imagination inflation. Psychon. Bull. Rev. 6, 313–318. doi: 10.3758/BF03214120
Heeren, A., Van Broeck, N., and Philippot, P. (2009). The effects of mindfulness on executive processes and autobiographical memory specificity. Behav. Res. Ther. 47, 403–409. doi: 10.1016/j.brat.2009.01.017
Hertel, P. T., and Brozovich, F. (2010). Cognitive habits and memory distortions in anxiety and depression. Curr. Dir. Psychol. Sci.19, 155–160. doi: 10.1177/0963721410370137
Hippel, C. V., Hole, G., and Kaschka, W. P. (2001). Autonomic profile under hypnosis as assessed by heart rate variability and spectral analysis. Pharmacopsychiatry 34, 111–113. doi: 10.1055/s-2001-14279
Hirt, E. R., Lynn, S. J., Payne, D. G., Krackow, E., and McCrea, S. M. (1999). “Expectancies and memory: inferring the past from what must have been,” in How Expectancies Shape Experience, ed. I. Kirsch (American Psychological Association), 93–124. doi: 10.1037/10332-004
Holdevici, I., and Crǎciun, B. (2013). The role of ericksonian hypnosis in reducing essential and secondary hypertension. Procedia Soc. Behav. Sci. 78, 461–465. doi: 10.1016/j.sbspro.2013.04.331
Hunter, C. R., and Eimer, B. N. (2012). The Art of Hypnotic Regression Therapy: A Clinical Guide. Crown House Publishing.
Hunter, R. C. (2009). The five phases of regression therapy. Aust. J. Clin. Hypnother. Hypn. 30:14. Available at: https://openurl.ebsco.com/EPDB%3Agcd%3A16%3A26277776/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A42226458&crl=c&link_origin=scholar.google.com
Hyman Jr, I. E., and Loftus, E. F. (2001). “False childhood memories and eyewitness memory errors,” in Memory and Suggestibility in the Forensic Interview (Routledge), 77–98. doi: 10.4324/9781410602251-8
Jahn, H. (2013). Memory loss in Alzheimer's disease. Dialogues Clin. Neurosci. 15, 445–454. doi: 10.31887/DCNS.2013.15.4/hjahn
Jasnow, A. M., Cullen, P. K., and Riccio, D. C. (2012). Remembering another aspect of forgetting. Front. Psychol. 3:175. doi: 10.3389/fpsyg.2012.00175
Jennings, J. R., Heim, A. F., Sheu, L. K., Muldoon, M. F., Ryan, C., Gach, H. M., et al. (2017). Brain regional blood flow and working memory performance predict change in blood pressure over 2 years. Hypertension 70, 1132–1141. doi: 10.1161/HYPERTENSIONAHA.117.09978
Jensen, M. P., Adachi, T., and Hakimian, S. (2015). Brain oscillations, hypnosis, and hypnotizability. Am. J. Clin. Hypn. 57, 230–253. doi: 10.1080/00029157.2014.976786
Jiang, H., White, M. P., Greicius, M. D., Waelde, L. C., and Spiegel, D. (2016). Brain activity and functional connectivity associated with hypnosis. Cereb. Cortex 27, 4083–4093. doi: 10.1093/cercor/bhw220
Kalaria, R. N., Akinyemi, R., and Ihara, M. (2016). Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta. Mol. Basis Dis. 1862, 915–925. doi: 10.1016/j.bbadis.2016.01.015
Kaplan, R. L., Van Damme, I., Levine, L. J., and Loftus, E. F. (2016). Emotion and false memory. Emot. Rev. 8, 8–13. doi: 10.1177/1754073915601228
Karle, H. W. A., and Boys, J. H. (1987). Hypnotherapy: A Practical Handbook. Free Association. Available at: https://books.google.co.uk/books?id=OlZnQgAACAAJ
Karle, H. W. A., and Boys, J. H. (2010). Hypnotherapy: A Practical Handbook. Free Association Books. Available at: https://books.google.co.uk/books?id=rsRHbwAACAAJ
Kealy, K. L., and Arbuthnott, K. D. (2003). Phenomenal characteristics of co-created guided imagery and autobiographical memories. Appl. Cogn. Psychol. 17, 801–818. doi: 10.1002/acp.910
Kekecs, Z., Szekely, A., and Varga, K. (2016). Alterations in electrodermal activity and cardiac parasympathetic tone during hypnosis. Psychophysiology 53, 268–277. doi: 10.1111/psyp.12570
Ketonis, P. P., McClelland, T. Q., Parra, D., and Radvansky, G. A. (2024). Human retrograde amnesia and memory consolidation. Psychon. Bull. Rev. 1–13. doi: 10.3758/s13423-024-02567-4
Kihlstrom, J. F. (1979). Hypnosis and psychopathology: retrospect and prospect. J. Abnorm. Psychol. 88:459. doi: 10.1037/0021-843X.88.5.459
Kihlstrom, J. F. (1997). Hypnosis, memory and amnesia. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 352, 1727–1732. doi: 10.1098/rstb.1997.0155
Kihlstrom, J. F. (2021). Recognition in posthypnotic amnesia, revisited. Int. J. Clin. Exp. Hypn. 69, 383–410. doi: 10.1080/00207144.2021.1910827
Kihlstrom, J. F., and Evans, F. J. (2014). “Memory retrieval processes during posthypnotic amnesia,” in Functional Disorders of Memory (PLE: Memory) (London: Psychology Press), 179–218. doi: 10.4324/9781315794778
Kim, H. B., Jung, Y. H., and Han, H. J. (2022). Resting heart rate and cognitive decline: a meta-analysis of prospective cohort studies. J. Clin. Neurol. 18, 619–627. doi: 10.3988/jcn.2022.18.6.619
Kloft, L., Otgaar, H., Blokland, A., van Oorsouw, K., Schepers, J., Steinmeyer, S., et al. (2023). False memories in the field: Impact of substance intoxication and sleep restriction on false memory formation. J. Appl. Res. Mem. Cogn. 12:389. doi: 10.1037/mac0000055
Kopelman, M. D. (1987). Two types of confabulation. J. Neurol. Neurosurg. Psychiatry 50, 1482–1487. doi: 10.1136/jnnp.50.11.1482
Kosslyn, S. M., Ganis, G., and Thompson, W. L. (2001). Neural foundations of imagery. Nat. Rev. Neurosci. 2, 635–642. doi: 10.1038/35090055
Kramer, U. (2010). Coping and defence mechanisms: what's the difference?–Second act. Psychother. Theory Res. Pract. 83, 207–221. doi: 10.1348/147608309X475989
Kunzendorf, R. G., Lacourse, P., and Lynch, B. (1987). Hypnotic hypermnesia for subliminally encoded stimuli: state-dependent memory for “unmonitored” sensations. Imagin. Cogn. Pers. 6, 365–377. doi: 10.2190/CL03-4PEE-YXLF-DBM0
Kurkela, K. A., and Dennis, N. A. (2016). Event-related fMRI studies of false memory: an activation likelihood estimation meta-analysis. Neuropsychologia 81, 149–167. doi: 10.1016/j.neuropsychologia.2015.12.006
Kuzma, E., Lourida, I., Moore, S. F., Levine, D. A., Ukoumunne, O. C., and Llewellyn, D. J. (2018). Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 14, 1416–1426. doi: 10.1016/j.jalz.2018.06.3061
Launer, L. J., Lewis, C. E., Schreiner, P. J., Sidney, S., Battapady, H., Jacobs, D. R., et al. (2015). Vascular factors and multiple measures of early brain health: CARDIA brain MRI study. PLoS ONE 10:e0122138. doi: 10.1371/journal.pone.0122138
Lee, N. (2014). “The neurobiology of procedural memory,” in The Neurobiology of Learning (New York, NY: Routledge), 43–73.
Leo, D. G., Keller, S. S., and Proietti, R. (2024). “Close your eyes and relax”: the role of hypnosis in reducing anxiety, and its implications for the prevention of cardiovascular diseases. Front. Psychol. 15:1411835. doi: 10.3389/fpsyg.2024.1411835
Leo, D. G., Ozdemir, H., Lane, D. A., Lip, G. Y. H., Keller, S. S., and Proietti, R. (2023). At the heart of the matter: how mental stress and negative emotions affect atrial fibrillation. Front. Cardiovasc. Med. 10:1171647. doi: 10.3389/fcvm.2023.1171647
Lindeløv, J. K., Overgaard, R., and Overgaard, M. (2017). Improving working memory performance in brain-injured patients using hypnotic suggestion. Brain 140, 1100–1106. doi: 10.1093/brain/awx001
Loftus, E. F. (1975). Leading questions and the eyewitness report. Cogn. Psychol. 7, 560–572. doi: 10.1016/0010-0285(75)90023-7
Loftus, E. F. (1996). Memory distortion and false memory creation. J. Am. Acad. Psychiatry Law 24, 281–295.
Loftus, E. F., Coan, J. A., and Pickrell, J. E. (2014). “Manufacturing false memories using bits of reality,” in Implicit Memory and Metacognition (New York, NY: Psychology Press), 195–220. doi: 10.4324/9781315806136-8
Loftus, E. F., and Pickrell, J. E. (1995). The formation of false memories. Psychiatr. Ann. 25, 720–725. doi: 10.3928/0048-5713-19951201-07
Long, D. L., Prat, C., Johns, C., Morris, P., and Jonathan, E. (2008). The importance of knowledge in vivid text memory: an individual-differences investigation of recollection and familiarity. Psychon. Bull. Rev. 15, 604–609. doi: 10.3758/PBR.15.3.604
Lu, Z., Teng, Y., Wang, L., Jiang, Y., Li, T., Chen, S., et al. (2022). Abnormalities of hippocampus and frontal lobes in heart failure patients and animal models with cognitive impairment or depression: a systematic review. PLoS ONE 17:e0278398. doi: 10.1371/journal.pone.0278398
Luck, D., Danion, J.-M., Marrer, C., Pham, B.-T., Gounot, D., and Foucher, J. (2010). The right parahippocampal gyrus contributes to the formation and maintenance of bound information in working memory. Brain Cogn. 72, 255–263. doi: 10.1016/j.bandc.2009.09.009
Lynn, S. J., and Kirsch, I. I. (1996). Alleged alien abductions: false memories, hypnosis, and fantasy proneness. Psychol. Inq. 7, 151–155. doi: 10.1207/s15327965pli0702_8
Lynn, S. J., Neuschatz, J., Fite, R., and Kirsch, I. (2001). Hypnosis in the forensic arena. J. Forensic Psychol. Pract. 1, 113–122. doi: 10.1300/J158v01n01_08
Lytle, R. A., and Lundy, R. M. (1988). Hypnosis and the recall of visually presented material: a failure to replicate stager and lundy. Int. J. Clin. Exp. Hypn. 36, 327–335. doi: 10.1080/00207148808410522
Malone, C., Deason, R. G., Palumbo, R., Heyworth, N., Tat, M., and Budson, A. E. (2019). False memories in patients with mild cognitive impairment and mild Alzheimer's disease dementia: can cognitive strategies help? J. Clin. Exp. Neuropsychol. 41, 204–218. doi: 10.1080/13803395.2018.1513453
Mares, L. (2022). Unconscious processes in psychoanalysis, CBT, and schema therapy. J. Psychother. Integr. 32:443. doi: 10.1037/int0000276
Martinussen, R., Hayden, J., Hogg-Johnson, S., and Tannock, R. (2005). A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 44, 377–384. doi: 10.1097/01.chi.0000153228.72591.73
McConkey, K. M., and Kinoshita, S. (1988). The influence of hypnosis on memory after one day and one week. J. Abnorm. Psychol. 97:48. doi: 10.1037/0021-843X.97.1.48
McConkey, K. M., and Sheehan, P. W. (1981). The impact of videotape playback of hypnotic events on posthypnotic amnesia. J. Abnorm. Psychol. 90:46. doi: 10.1037/0021-843X.90.1.46
McConkey, K. M., Sheehan, P. W., and Cross, D. G. (1980). Post-hypnotic amnesia: seeing is not remembering. Br. J. Soc. Clin. Psychol. 19, 99–107. doi: 10.1111/j.2044-8260.1980.tb00934.x
Milling, L. S., Gover, M. C., and Moriarty, C. L. (2018). The effectiveness of hypnosis as an intervention for obesity: a meta-analytic review. Psychol. Conscious. Theory Res. Pract. 5:29. doi: 10.1037/cns0000139
Moore, E. E., and Jefferson, A. L. (2021). Impact of cardiovascular hemodynamics on cognitive aging. Arterioscler. Thromb. Vasc. Biol. 41, 1255–1264. doi: 10.1161/ATVBAHA.120.311909
Moroni, F., Ammirati, E., Rocca, M. A., Filippi, M., Magnoni, M., and Camici, P. G. (2018). Cardiovascular disease and brain health: focus on white matter hyperintensities. IJC Heart Vasc. 19, 63–69. doi: 10.1016/j.ijcha.2018.04.006
Mulligan, N. W. (2006). Hypermnesia and total retrieval time. Memory 14, 502–518. doi: 10.1080/09658210500513438
Murayama, K., Miyatsu, T., Buchli, D., and Storm, B. C. (2014). Forgetting as a consequence of retrieval: a meta-analytic review of retrieval-induced forgetting. Psychol. Bull. 140, 1383. doi: 10.1037/a0037505
Nemeth, D., Janacsek, K., Polner, B., and Kovacs, Z. A. (2013). Boosting human learning by hypnosis. Cereb. Cortex 23, 801–805. doi: 10.1093/cercor/bhs068
Nogrady, H., McConkey, K. M., and Perry, C. (1985). Enhancing visual memory: trying hypnosis, trying imagination, and trying again. J. Abnorm. Psychol. 94:195. doi: 10.1037/0021-843X.94.2.195
Ofshe, R. J., and Singer, M. T. (1994). Recovered-memory therapy and robust repression: influence and pseudomemories. Int. J. Clin. Exp. Hypn. 42, 391–410. doi: 10.1080/00207149408409367
Opitz, B. (2014). Memory function and the hippocampus. Hipp. Clin. Neurosci. 34, 51–59. doi: 10.1159/000356422
Oulton, J. M., Takarangi, M. K. T., and Strange, D. (2016). Memory amplification for trauma: investigating the role of analogue PTSD symptoms in the laboratory. J. Anxiety Disord. 42, 60–70. doi: 10.1016/j.janxdis.2016.06.001
Paddock, J. R., and Terranova, S. (2001). Guided visualization and suggestibility: effect of perceived authority on recall of autobiographical memories. J. Genet. Psychol. 162, 347–356. doi: 10.1080/00221320109597488
Parand, L., Niu, K., Yerstein, O., and Mendez, M. F. (2020). Fantastic thinking and frontal cerebrovascular disease. J. Neuropsychiatry Clin. Neurosci. 32, 201–203. doi: 10.1176/appi.neuropsych.19040086
Park, C. M., Williams, E. D., Chaturvedi, N., Tillin, T., Stewart, R. J., Richards, M., et al. (2017). Associations between left ventricular dysfunction and brain structure and function: findings from the SABRE (Southall and Brent Revisited) study. J. Am. Heart Assoc. 6:e004898. doi: 10.1161/JAHA.116.004898
Park, G., and Thayer, J. F. (2014). From the heart to the mind: cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Front. Psychol. 5:278. doi: 10.3389/fpsyg.2014.00278
Putnam, W. H. (1979). Hypnosis and distortions in eyewitness memory. Int. J. Clin. Exp. Hypn. 27, 437–448. doi: 10.1080/00207147908407577
Raffard, S., Bortolon, C., Burca, M., Novara, C., Gely-Nargeot, M.-C., Capdevielle, D., et al. (2016). Self-imagination can enhance memory in individuals with schizophrenia. Cogn. Neuropsychiatry 21, 168–181. doi: 10.1080/13546805.2016.1155438
Rainville, P., and Price, D. D. (2003). Hypnosis phenomenology and the neurobiology of consciousness. Int. J. Clin. Exp. Hypn. 51, 105–129. doi: 10.1076/iceh.51.2.105.14613
Raskin, R., Raps, C., Luskin, F., Carlson, R., and Cristal, R. (1999). Pilot study of the effect of self-hypnosis on the medical management of essential hypertension. Stress Med. 15, 243–247.
Ready, D. J., Bothwell, R. K., and Brigham, J. C. (1997). The effects of hypnosis, context reinstatement, and anxiety on eyewitness memory. Int. J. Clin. Exp. Hypn. 45, 55–68. doi: 10.1080/00207149708416106
Register, P. A., and Kihlstrom, J. F. (1987). Hypnotic effects on hypermnesia. Int. J. Clin. Exp. Hypn. 35, 155–170. doi: 10.1080/00207148708416051
Reyna, V. F. (2013). “Fuzzy-trace theory and false memory,” in Memory Distortions and Their Prevention (New York, NY: Psychology Press), 15–27.
Reysen, M. B. (2007). The effects of social pressure on false memories. Mem. Cogn. 35, 59–65. doi: 10.3758/BF03195942
Robin, F., Bonamy, J., and Ménétrier, E. (2018). Hypnosis and false memories. Psychol. Conscious. Theory Res. Pract. 5:358. doi: 10.1037/cns0000150
Sabayan, B., van Buchem, M. A., Sigurdsson, S., Zhang, Q., Harris, T. B., Gudnason, V., et al. (2015). Cardiac hemodynamics are linked with structural and functional features of brain aging: the age, gene/environment susceptibility (AGES)-Reykjavik Study. J. Am. Heart Assoc. 4:e001294. doi: 10.1161/JAHA.114.001294
Schacter, D. L. (1999). The seven sins of memory: insights from psychology and cognitive neuroscience. Am. Psychol. 54:182. doi: 10.1037/0003-066X.54.3.182
Schacter, D. L. (2013). Memory: sins and virtues. Ann. N.Y. Acad. Sci. 1303, 56–60. doi: 10.1111/nyas.12168
Schacter, D. L., Guerin, S. A., and Jacques, P. L. S. (2011). Memory distortion: an adaptive perspective. Trends Cogn. Sci. 15, 467–474. doi: 10.1016/j.tics.2011.08.004
Scheitz, J. F., Nolte, C. H., Doehner, W., Hachinski, V., and Endres, M. (2018). Stroke-heart syndrome: clinical presentation and underlying mechanisms. Lancet Neurol. 17, 1109–1120. doi: 10.1016/S1474-4422(18)30336-3
Schmidt, B., Böhmer, J., Schnuerch, M., Koch, T., and Michelmann, S. (2024a). Post-hypnotic suggestion improves confidence and speed of memory access with long-lasting effects. Acta Psychol. 245:104240. doi: 10.1016/j.actpsy.2024.104240
Schmidt, B., Rohleder, N., and Engert, V. (2024b). Post-hypnotic safety suggestion improves stress coping with long-lasting effects. Sci. Rep. 14:3548. doi: 10.1038/s41598-024-54071-3
Scoboria, A., Wade, K. A., Lindsay, D. S., Azad, T., Strange, D., Ost, J., et al. (2017). A mega-analysis of memory reports from eight peer-reviewed false memory implantation studies. Memory 25, 146–163. doi: 10.1080/09658211.2016.1260747
Scullin, M. K., Le, D. T., and Shelton, J. T. (2017). Healthy heart, healthy brain: hypertension affects cognitive functioning in older age. Transl. Issues Psychol. Sci. 3:328. doi: 10.1037/tps0000131
Shaffer, F., and Ginsberg, J. P. (2017). An overview of heart rate variability metrics and norms. Front. Public Health 5:258. doi: 10.3389/fpubh.2017.00258
Sheehan, P. W., Grigg, L., and McCann, T. (1984). Memory distortion following exposure to false information in hypnosis. J. Abnorm. Psychol. 93:259. doi: 10.1037/0021-843X.93.3.259
Sheehan, P. W., Statham, D., and Jamieson, G. A. (1991). Pseudomemory effects and their relationship to level of susceptibility to hypnosis and state instruction. J. Pers. Soc. Psychol. 60:130. doi: 10.1037/0022-3514.60.1.130
Shields, G. S., Sazma, M. A., McCullough, A. M., and Yonelinas, A. P. (2017). The effects of acute stress on episodic memory: a meta-analysis and integrative review. Psychol. Bull. 143:636. doi: 10.1037/bul0000100
Spanos, N. P., Burgess, C. A., and Burgess, M. F. (1994). Past-life identities, UFO abductions, and satanic ritual abuse: the social construction of memories. Int. J. Clin. Exp. Hypn. 42, 433–446. doi: 10.1080/00207149408409369
Spets, D. S., Karanian, J. M., and Slotnick, S. D. (2021). False memories activate distinct brain regions in females and males. Neuroimage 1:100043. doi: 10.1016/j.ynirp.2021.100043
Squire, L. R., and Dede, A. J. (2015). Conscious and unconscious memory systems. Cold Spring Harb. Perspect. Biol. 7:a021667. doi: 10.1101/cshperspect.a021667
Stager, G. L., and Lundy, R. M. (1985). Hypnosis and the learning and recall of visually presented material. Int. J. Clin. Exp. Hypn. 33, 27–39. doi: 10.1080/00207148508406633
Strange, D., and Takarangi, M. K. T. (2015). Memory distortion for traumatic events: the role of mental imagery [opinion]. Front. Psychiatry 6:27. doi: 10.3389/fpsyt.2015.00027
Straube, B. (2012). An overview of the neuro-cognitive processes involved in the encoding, consolidation, and retrieval of true and false memories. Behav. Brain Funct. 8, 1–10. doi: 10.1186/1744-9081-8-35
Stuss, D. T., and Benson, D. F. (2019). “The frontal lobes and control of cognition and memory,” in The Frontal Lobes Revisited (New York, NY: Psychology Press), 141–158. doi: 10.4324/9781315788975-8
Subramanya, P., and Telles, S. (2009). Effect of two yoga-based relaxation techniques on memory scores and state anxiety. BioPsychoSoc. Med. 3, 1–5. doi: 10.1186/1751-0759-3-8
Tahsili-Fahadan, P., and Geocadin, R. G. (2017). Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ. Res. 120, 559–572. doi: 10.1161/CIRCRESAHA.116.308446
Taylor, A. G., Goehler, L. E., Galper, D. I., Innes, K. E., and Bourguignon, C. (2010). Top-down and bottom-up mechanisms in mind-body medicine: development of an integrative framework for psychophysiological research. Explore 6, 29–41. doi: 10.1016/j.explore.2009.10.004
Terathongkum, S., and Pickler, R. H. (2004). Relationships among heart rate variability, hypertension, and relaxation techniques. J. Vasc. Surg. 22, 78–82. doi: 10.1016/j.jvn.2004.06.003
Terrance, C. A., Matheson, K., Allard, C., and Schnarr, J. A. (2000). The role of expectation and memory-retrieval techniques in the construction of beliefs about past events. Appl. Cogn. Psychol. 14, 361–378.
Thayer, J. F., Åhs, F., Fredrikson, M., Sollers, I. I. I. J. J, and Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 36, 747–756. doi: 10.1016/j.neubiorev.2011.11.009
Tyng, C. M., Amin, H. U., Saad, M. N., and Malik, A. S. (2017). The influences of emotion on learning and memory. Front. Psychol. 8:235933. doi: 10.3389/fpsyg.2017.01454
Ungvari, Z., Toth, P., Tarantini, S., Prodan, C. I., Sorond, F., Merkely, B., et al. (2021). Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat. Rev. Nephrol. 17, 639–654. doi: 10.1038/s41581-021-00430-6
Van Damme, I., Kaplan, R. L., Levine, L. J., and Loftus, E. F. (2017). Emotion and false memory: how goal-irrelevance can be relevant for what people remember. Memory 25, 201–213. doi: 10.1080/09658211.2016.1150489
Van Der Kolk, B. A. (1998). Trauma and memory. Psychiatry Clin. Neurosci. 52, S52–S64. doi: 10.1046/j.1440-1819.1998.0520s5S97.x
VandeVusse, L., Hanson, L., Berner, M. A., and Winters, J. M. W. (2010). Impact of self-hypnosis in women on select physiologic and psychological parameters. J. Obstet. Gynecol. Neonatal. Nurs. 39, 159–168. doi: 10.1111/j.1552-6909.2010.01103.x
Vanhaudenhuyse, A., Boly, M., Balteau, E., Schnakers, C., Moonen, G., Luxen, A., et al. (2009). Pain and non-pain processing during hypnosis: a thulium-YAG event-related fMRI study. Neuroimage 47, 1047–1054. doi: 10.1016/j.neuroimage.2009.05.031
Vanhaudenhuyse, A., Laureys, S., and Faymonville, M. E. (2014). Neurophysiology of hypnosis. Neurophysiol. Clin. 44, 343–353. doi: 10.1016/j.neucli.2013.09.006
Wagoner, B. (2017). “Frederic bartlett,” in The Routledge Handbook of Philosophy of Memory (London: Routledge), 537–545. doi: 10.4324/9781315687315-46
Wagstaff, G. F., Brunas-Wagstaff, J., Cole, J., Knapton, L., Winterbottom, J., Crean, V., et al. (2004). Facilitating memory with hypnosis, focused meditation, and eye closure. Int. J. Clin. Exp. Hypn. 52, 434–455. doi: 10.1080/00207140490889062
Wagstaff, G. F., Cole, J., Wheatcroft, J., Anderton, A., and Madden, H. (2008). Reducing and reversing pseudomemories with hypnosis. Contemp. Hypn. 25, 178–191. doi: 10.1002/ch.366
Wagstaff, G. F., Wheatcroft, J. M., and Jones, A. C. (2011). Are high hypnotizables especially vulnerable to false memory effects? A sociocognitive perspective. Int. J. Clin. Exp. Hypn. 59, 310–326. doi: 10.1080/00207144.2011.570658
Walker, K. A., Power, M. C., and Gottesman, R. F. (2017). Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr. Hypertens. Rep. 19, 1–16. doi: 10.1007/s11906-017-0724-3
Ware, J. E. Jr., and Sherbourne, C. D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483. doi: 10.1097/00005650-199206000-00002
Watson, J. M., Balota, D. A., and Sergent-Marshall, S. D. (2001). Semantic, phonological, and hybrid veridical and false memories in healthy older adults and in individuals with dementia of the Alzheimer type. Neuropsychology 15:254. doi: 10.1037/0894-4105.15.2.254
Whitehouse, W. G., Dinges, D. F., Orne, E. C., and Orne, M. T. (1988). Hypnotic hypermnesia: enhanced memory accessibility or report bias? J. Abnorm. Psychol. 97:289. doi: 10.1037//0021-843X.97.3.289
Williams, M., Hong, S. W., Kang, M.-S., Carlisle, N. B., and Woodman, G. F. (2013). The benefit of forgetting. Psychon. Bull. Rev. 20, 348–355. doi: 10.3758/s13423-012-0354-3
Williams, P. G., Cribbet, M. R., Tinajero, R., Rau, H. K., Thayer, J. F., and Suchy, Y. (2019). The association between individual differences in executive functioning and resting high-frequency heart rate variability. Biol. Psychol. 148:107772. doi: 10.1016/j.biopsycho.2019.107772
Winter, A. (2013). The rise and fall of forensic hypnosis. Stud. Hist. Philos. Biol. Biomed. Sci. 44, 26–35. doi: 10.1016/j.shpsc.2012.09.011
Wolters, F. J., Segufa, R. A., Darweesh, S. K., Bos, D., Ikram, M. A., Sabayan, B., et al. (2018). Coronary heart disease, heart failure, and the risk of dementia: a systematic review and meta-analysis. Alzheimers Dement. 14, 1493–1504. doi: 10.1016/j.jalz.2018.01.007
Xu, J., Vik, A., Groote, I. R., Lagopoulos, J., Holen, A., Ellingsen, Ø., et al. (2014). Nondirective meditation activates default mode network and areas associated with memory retrieval and emotional processing. Front. Hum. Neurosci. 8:86. doi: 10.3389/fnhum.2014.00086
Yüksel, R., Ozcan, O., and Dane, S. (2013). The effects of hypnosis on heart rate variability. Int. J. Clin. Exp. Hypn. 61, 162–171. doi: 10.1080/00207144.2013.753826
Zaragoza, M. S., Mitchell, K. J., Payment, K., and Drivdahl, S. (2011). False memories for suggestions: the impact of conceptual elaboration. J. Mem. Lang. 64, 18–31. doi: 10.1016/j.jml.2010.09.004
Keywords: false memories, hypnosis, memory, pseudo-memories, memory recall
Citation: Leo DG, Bruno D and Proietti R (2025) Remembering what did not happen: the role of hypnosis in memory recall and false memories formation. Front. Psychol. 16:1433762. doi: 10.3389/fpsyg.2025.1433762
Received: 16 May 2024; Accepted: 16 January 2025;
Published: 04 February 2025.
Edited by:
Claudio Lucchiari, University of Milan, ItalyReviewed by:
Erin Trifilio, University of Florida, United StatesFuting Zou, Brown University, United States
Copyright © 2025 Leo, Bruno and Proietti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Riccardo Proietti, UmljY2FyZG8uUHJvaWV0dGlAbGl2ZXJwb29sLmFjLnVr
†ORCID: Donato Giuseppe Leo orcid.org/0000-0002-0709-3073
Davide Bruno orcid.org/0000-0003-1943-9905
Riccardo Proietti orcid.org/0000-0003-4113-7030
 Donato Giuseppe Leo
Donato Giuseppe Leo Davide Bruno
Davide Bruno Riccardo Proietti
Riccardo Proietti