- 1Department of Psychology, University “G. d’Annunzio” of Chieti-Pescara, Chieti, Italy
- 2Department of Oncology and Hematology, Pescara General Hospital “Santo Spirito”, Pescara, Italy
- 3Department of Innovative Technologies in Medicine and Dentistry, University “G. d’Annunzio” of Chieti-Pescara, Chieti, Italy
Background: This cohort study aimed to explore whether and to what extent alexithymia would be associated with cardiovascular disease (CVD) risk over an estimated 10-year period, over and above established clinical cofactors (i.e., depressive symptoms, quality of life, sociodemographic, anthropometric, lifestyle, and biological data), in a low-risk population of blood donors.
Methods: A sample of 1,021 adult Italian blood donors (age 46.9 ± 8.39; 61.2% men) was consecutively recruited. The 10-year-CVD risk score was estimated using the CUORE risk score (CRS). Sociodemographic, lifestyle, anthropometric, biological, and psychological (i.e., quality of life, depressive symptoms, and alexithymia) CVD risk data were assessed using validated self-report measures or clinical records.
Results: As expected, most participants (78.5%) had a low CVD risk (CRS < 3%) and an overall low-risk profile for all the parameters. Compared with subjects at low risk of CVD (n = 911, 78.5%), those with high risk (i.e., rated ≥3 on CUORE risk assessment; n = 250, 21.5%) reported higher levels of alexithymia (p < 0.001). Subjects with higher alexithymia (n = 236, 23.1%) reported higher levels of psychosocial impairment, depressive symptoms, and biological risk variables for CVD. Alexithymia was significantly associated with 10-year CVD risk (OR = 1.02, 95% CI = 1.01–1.04, p = 0.009), even after adjusting for key sociodemographic and clinical risk variables.
Conclusion: Although limited by the cross-sectional design, this study is the first to show that alexithymia leads to a higher risk for 10-year CVD estimate in healthy subjects with low-risk profile, regardless of known biomarkers and traditional CVD risk factors.
1 Introduction
Cardiovascular disease (CVD) is the leading cause of premature mortality worldwide, accounting for more than 17 million estimated deaths per year (Lindstrom et al., 2022). To date, biomedical risk factors such as obesity (Franks et al., 2010), cholesterol (Burger et al., 2024), C-reactive protein (Prasad, 2024), blood-pressure level (Ettehad et al., 2016), and metabolic syndrome (MetS) (Kurniawan, 2024), as well as lifestyle (e.g., physical inactivity, smoking, alcohol consumption, unhealthy diet) and psychological variables (Bogle et al., 2016; Carola et al., 2024; Emdin et al., 2016; Rozanski, 2014) have been studied extensively. Among psychological variables, specifically depression has been largely investigated and associated with poor quality of life, higher healthcare costs, and increased risk of new cardiac events and mortality (Krittanawong et al., 2023), even when controlling for known risk factors (unhealthy lifestyle, body mass index [BMI], hypertension, diabetes, and socioeconomic status) (Gan et al., 2014; Meijer et al., 2011). It is notable that also minor depression (Freedland et al., 2016) and emotional distress—though at a subthreshold diagnostic level—may increase the risk of CVD morbidity and mortality (Lissåker et al., 2019; Norlund et al., 2018).
Alexithymia is one of the psychological factors investigated in CVD (e.g., Montisci et al., 2023; Sancassiani et al., 2023) and other medical conditions (Luminet et al., 2018). Alexithymia is a personality dimension defined as a deficit in the cognitive processing of emotions. It is defined by two higher-order factors, namely a deficit of affect awareness (difficulty in identifying and describing feelings) and operatory thinking (externally oriented thinking and poor imaginal process) (Taylor and Bagby, 2004). Current evidence shows that alexithymia may affect health in a number of ways, as it is associated with affective states (e.g., depressive symptoms), maladaptive behaviors (e.g., altered eating behavior), emotional dysregulation related psychopathology (through somatosensory amplification), post traumatic shutdown of emotions (e.g., acute reactions to illness), and altered autonomic, endocrine and immune activity leading to tissue damage (e.g., vulnerability to inflammatory processes) (for an extensive review, see Luminet et al., 2018). Alexithymia has been associated with known risk factors for cardiovascular disease such as biochemical and anthropometric alterations (e.g., high levels of cholesterol, triglyceride, glucose, BMI, resting sympathetic activity, heart rate, blood pressure, and blood pressure reactivity) and unhealthy behaviors (e.g., sedentary lifestyles, and poorer nutrition and glycemic control) (Aluja et al., 2020; Sancassiani et al., 2023). Furthermore, alexithymia was also found to be an independent factor increasing the risk of CVD death (Carta et al., 2022; Tolmunen et al., 2010; Vadini et al., 2019). More specifically, Carta et al. (2022) investigated the relationship between 10-year mortality and alexithymia among people with myocardial infarction. Among people with high alexithymia in 2011, a higher risk of early death in 2021 was found (RR = 5.75). In a prospective study, Vadini et al. (2019) found that alexithymia was an independent predictor of carotid plaques (OR = 4.93) at baseline and cardiovascular events (HR = 3.66) and mortality (HR = 3.93) at follow-up in patients with HIV. Tolmunen et al. (2010) have pointed out that subjects with high alexithymia have both higher BMI and systolic blood pressure, are more frequently smokers, perform less physical activity, and report a 2.3% increase in CVD mortality risk for each 1-point increase on the Toronto Alexithymia Scale. Other studies report similar results, thus providing further evidence of the role of alexithymia in triggering several CVD risk factors linked both to unhealthy lifestyle and to the autonomic alterations due to emotional dysregulation (Carta et al., 2013; Marchesi et al., 2015; Meloni et al., 2016; Montisci et al., 2023; Sancassiani et al., 2023). However, the role of alexithymia in CVD is still debated. For example, it remains unclear whether there is a significant association between behavioral and physiological CVD risk factors in healthy populations (Luminet et al., 2018; Montisci et al., 2023).
The present study aimed to explore whether alexithymia would be associated with increased CVD risk over an estimated 10-year period in a low-risk population of blood donors. Specifically, the aim of the study was twofold: (a) to investigate whether sociodemographic and anthropometric data, lifestyle, biological markers, and psychological characteristics would differ between subjects with high and low cardiovascular risk and between subjects with high and low levels of alexithymia; (b) to explore whether and to what extent alexithymia would be associated with increased CVD risk, over and above established clinical cofactors (i.e., depressive symptoms and quality of life, sociodemographic, anthropometric, lifestyle, and biological data). Blood donors are screened for good physical health and undergo blood donation strictly voluntarily, thus showing an active prosocial attitude. Although this was the first study investigating the association between alexithymia and CVD risk in a blood donor population, we expected that: (a) subjects with higher CVD risk would report higher alexithymia and subjects with higher levels of alexithymia would report higher CVD risk; (b) alexithymia would be associated with increased CVD risk over and above other relevant co-factors.
2 Materials and methods
2.1 Population and study design
In this cross-sectional study, a sample of N = 1,610 volunteer blood donors were consecutively enrolled at the Blood Transfusion Unit of the General Hospital of Pescara (Italy) between June 2015 and July 2017. All the participants were medically tested for major severe diseases and major contraindications for donating blood. The study protocol was administered during the medical examination to assess eligibility for blood donation. To maximize ecological validity, we used the standard inclusion criteria for blood donation: age range 35–69 with good physical health and a proactive social attitude. Subjects were excluded if they had certified medical or psychiatric morbidity (such as mental major impairment, drug or alcohol abuse), impairment in cognitive functions, were not fluent Italian speakers, or were pregnant.
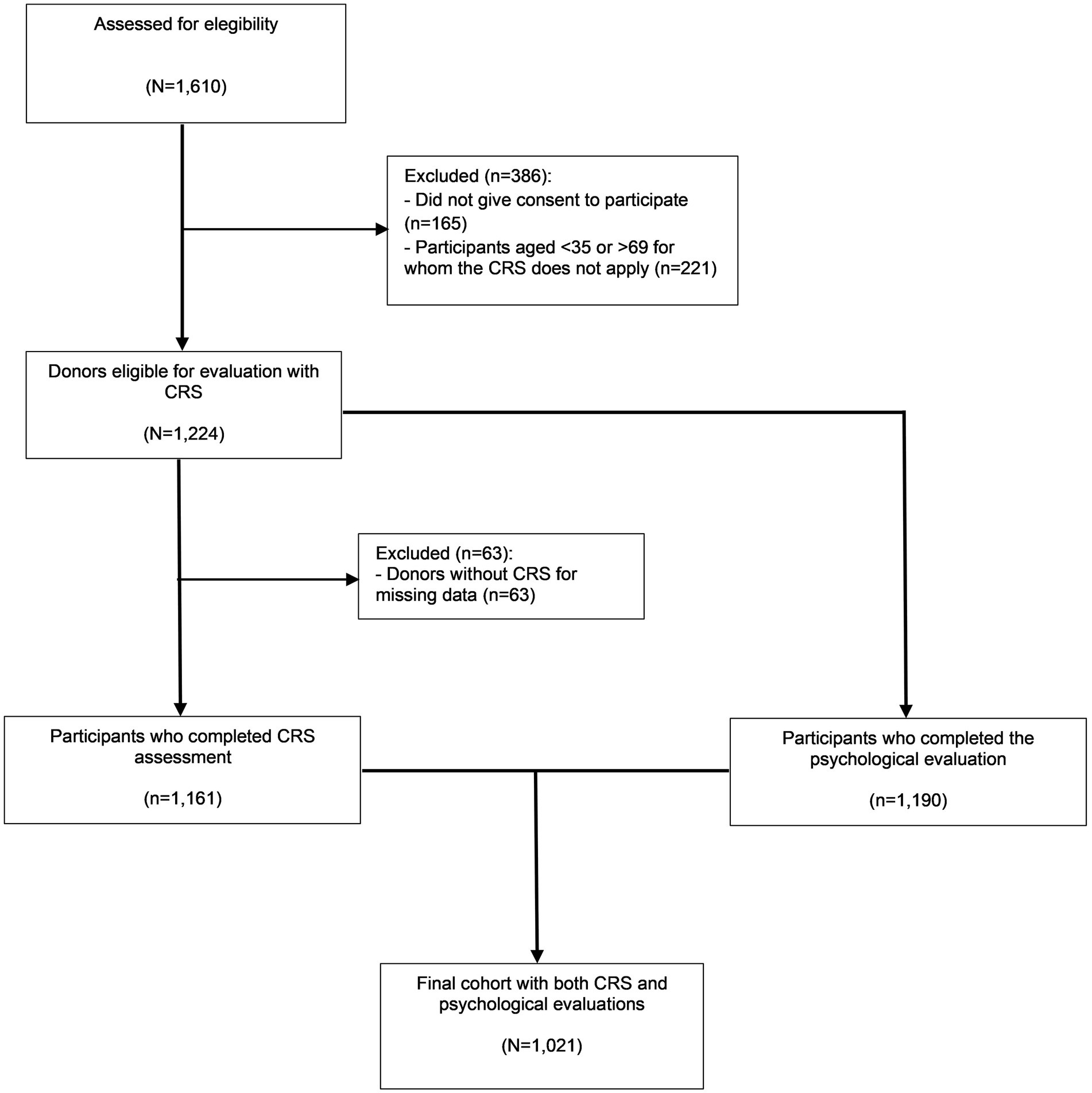
Figure 1 shows the flowchart of participation in the study. Of the 1,610 blood donors assessed for eligibility, 165 (10.2%) refused to participate for lack of time and 221 (13.7%) were of non-eligible age for evaluation with CRS, so they were excluded. Data were collected from 1,161 participants for the CRS assessment, from 1,190 participants for the psychological assessment, and from 1,021 participants for both assessments. Only the latter subjects entered the multivariate analysis. According to the sociodemographic variables, no significant difference was found between included and excluded participants.
The study protocol was ethically approved by the local institutional review board of the Oncology Hematology Department of Spirito Santo Hospital in Pescara. Written informed consent was obtained from the participants in accordance with the Declaration of Helsinki.
2.2 Measures
2.2.1 Socio-demographic data
Socio-demographic data (i.e., age, gender, years of education, occupation, marital status) were collected using an ad-hoc questionnaire.
The socioeconomic status (SES) was classified as low (manual workers and skilled/unskilled workers including farmers with primary education level [5 years]), middle (non-manual employees and clerks with secondary education level [8–12 years]), or high (professionals, executives, administrators, and entrepreneurs with tertiary level of education) status. Low education was defined as ≤13 years of school attendance (corresponding to compulsory education in Italy and to the <25th centile within the study sample) and high education as >13 years of school attendance.
2.2.2 Psychological factors
2.2.2.1 Alexithymia
Alexithymia was assessed using the 20-item Toronto Alexithymia Scale (TAS-20) which is rated on a 5-point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). In addition to the total, the TAS-20 yields score for difficulty in identifying feelings (DIF), difficulty in describing feelings (DDF), and externally oriented thinking (EOT). The scale is considered the standard measure for alexithymia given the internal reliability (Cronbach’s α coefficients of 0.80 to 0.83 for the total score) of its psychometric properties, the construct validity, and the factor structure (Bagby et al., 2020). Subjects scoring >60 are defined as highly alexithymic (Bressi et al., 1996; Taylor et al., 1999). The cut-off score of >60 used in clinical studies was found to be a restrictive threshold for our healthy population, with very few blood donors (7.5%) classified as alexithymic. Therefore, we divided the study population into two groups, high (HA) and low alexithymia (LA) groups, using the least restrictive cut-off corresponding to TAS-20 score > 49, a threshold previously employed in large non-clinical population studies (Grabe et al., 2010). Interestingly, this threshold in our cohort coincides with the 75th percentile of TAS-20 score. Furthermore, the TAS-20 > 49 threshold was used as a threshold particularly predictive of subclinical cardiometabolic risk factors in large population-based studies (Grabe et al., 2010; Vadini et al., 2019). Within the sample used in this analysis, Cronbach’s α was 0.83 for the total scale, 0.85 for the DIF, 0.70 for the DDF, and 0.56 for the EOT subscales.
2.2.2.2 Depressive symptoms
Depressive symptoms were assessed using the 21-item Beck Depression Inventory-II (BDI-II) (Beck et al., 1996), in which items are rated on a 4-point scale ranging from 0 to 3 and the total score ranges from 0 to 63. Higher scores correspond to greater severity. Prior research has indicated that this measure is internally consistent and reliable (Cronbach’s α coefficient of 0.89), has discriminant power, and correlates highly with other measures of depressive symptoms and depression-related constructs (for a review, see Whisman and Richardson, 2015). According to the guidelines, we used a score > 13 as a threshold for detecting depressive symptoms (Ghisi et al., 2006). Within the sample used in this analysis, Cronbach’s α was 0.86.
2.2.2.3 Health-related quality of life
Psychosocial functioning was assessed using the Short Form-12 Health Survey (SF-12) scale (Gandek et al., 1998). The SF-12 is a shortened version of the 36-item questionnaire (SF-36), proven to be more practical in large samples when an overall estimate of physical and mental health is needed (Apolone and Mosconi, 1998; Melville et al., 2003). The scale consists of 12 questions about both physical (PCS, physical component summary) and mental functioning (MCS, mental component summary). Higher scores indicate a better psychosocial functioning. The SF-12 has been used in various settings and study populations, thus it is considered a valid and reliable tool with a good internal reliability (Cronbach’s α coefficients of 0.72 to 0.89) (Andriopoulos et al., 2013; Rawal et al., 2013). Within the sample used in this analysis, Cronbach’s α was 0.83 for MCS and 0.88 for PCS.
2.2.3 Anthropometric, health and lifestyle behavior data
2.2.3.1 Blood pressure
Resting blood pressure (BP) was measured after 5 min in a seated position with an automated BP monitor using the oscillometric method. Both the systolic BP (SBP) ≥ 140 mmHg and the diastolic BP (DBP) ≥ 90 mmHg was measured using a standard mercury sphygmomanometer. A 2-min interval was allowed before the blood pressure measurement to control for signs of clinical alert and “white-coat”-like phenomena.
2.2.3.2 Smoking and physical activity
According to the CVD risk equations (Ferrario et al., 2005), smoking was defined as ≥1 cigarette per day and physical activity (PA) was defined as moderate to vigorous (≥150 min per week) according to the WHO (World Health Organization, 2010) recommendations.
2.2.3.3 Obesity
Height (cm), weight (kg), waist circumference (cm), and blood pressure were measured by trained occupational health nurses. The waist circumference was measured in the horizontal plane of the iliac crest superior border keeping the subject in a standing position after a full expiration. BMI was calculated as weight (kg) divided by the square of height (m2).
2.2.3.4 Metabolic syndrome
The metabolic syndrome (MetS) is broadly defined by a cluster of multiple metabolic abnormalities, including central obesity, hypertension, dyslipidemia, and insulin resistance. The criteria for MetS include 3+ of the following components: (1) elevated waist circumference (≥ 88 cm for women and ≥ 102 cm for men, (2) elevated TG (≥ 150 mg/dL) or being on treatment for dyslipidemia (statin and/or fibric acid derivative), (3) reduced HDL-C (< 40 mg/dL in men and < 50 mg/dL in women) or being on treatment for dyslipidemia (statin and/or fibric acid derivative), (4) elevated BP (systolic ≥ 130 and/or diastolic ≥ 85 mmHg) or antihypertensive drug treatment in patients with a history of hypertension), and (5) elevated FG (≥ 100 mg/dL) or drug treatment for elevated glucose level (Grundy et al., 2004).
2.2.4 Biological markers
Standard techniques were used for assessing fasting blood glucose (FBG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and triglycerides (TG). Fasting plasma homocysteine (Hcy) was measured by HemosIL™ Homocysteine assay, an automated, latex-enhanced immunoassay used for the quantitative determination of plasma Hcy (Instrumental Laboratory, Bedford, MA, USA). Lipoprotein(a) [Lp(a)] plasma concentrations were determined by IMMAGE® LPA immunonephelometric assay (IMMAGE LPA), performed on the IMMAGE® Immunochemistry System (Beckman Instrument, Inc., Galway, Ireland).
2.2.5 Ten-year cardiovascular risk estimate
The 10-year CV risk was assessed using the CV risk score (CRS) developed within the Italian project CUORE (Epidemiology and Prevention of Ischemic Heart Diseases) (Ferrario et al., 2005; Palmieri et al., 2006). The CUORE project, coordinated by the Italian National Institute of Health, is a prospective fixed-cohort study that includes representative cohorts from all the Italian regions. The CUORE project risk scores assess the likelihood of experiencing a first CV event over the following 10 years by evaluating the level of eight risk factors: age, gender, SBP, TC, HDL-C, diabetes mellitus, smoking habit, and use of antihypertensive medication. The method was validated in patients aged 35 to 69 years without previous major CV accidents. According to CRS, the subjects are classified into low-risk (CRS < 3.0%), moderate-risk (CRS = 3.0–19.9%), or high-risk (CRS ≥ 20.0%) groups. Since high CV risk is uncommon in blood donors, our population was divided into two groups: low-risk (<3%) and moderate-to-high risk (≥3%). This threshold identifies individuals for whom annual monitoring of CV risk factors is recommended.1 Data were collected using the software CUORE.exe, free downloadable from the CUORE project website.2
The CRS showed similar discrimination power compared with Framingham and European SCORE equations but is more appropriate for the Italian population to avoid risk overestimation in a population with a low incidence of cardiovascular disease (Donfrancesco et al., 2010). The Italian Ministry of Health recommended the CRS score for cardiovascular risk assessment of the general adult Italian population in primary prevention (Donfrancesco et al., 2010; Giampaoli et al., 2007).
2.3 Statistical analysis
Between-group differences were evaluated with Pearson’s chi-square and Student’s t-tests. Partial eta-square (η2) and Cohen’s d values were computed to assess the effect size (Cohen, 1988). Cohen’s d = 0–0.2 is considered trivial, 0.2–0.5 small, 0.5–0.8 moderate, and ≥ 0.8 large. Conversely, η2 = 0.01–0.05 is considered small, 0.06–0.14 moderate, and > 0.14 large. Univariate logistic regression analyses were performed to assess the risk of moderate CRS. The risk was synthesized as crude odds ratios (ORs) with 95% confidence intervals (95% CI). Binary logistic regression models were implemented to estimate the role of alexithymia in predicting the level of CRS, adjusting for clinical, lifestyle, and sociodemographic variables. The variables included in the logistic regression model were selected for their well-established link with cardiometabolic diseases. The CV risk variables excluded from the regression models were those that were already included in the computation of our dependent variable (CRS: 10-year CV prediction algorithm). The CRS was considered the dependent variable (dummy coded: 0 = low CRS; 1 = moderate-to-high CRS). The sociodemographic (Step 1), lifestyle (Step 2), anthropometric and biological (Step 3), and psychological (Step 4) data were entered as independent variables in separate blocks to determine how well each variable predicted the outcome. Four regression steps were processed and regression coefficients, confidence intervals (CI), odds ratio (OR), and p-values were estimated. A two-sided α level of 0.05 was considered statistically significant. The statistical analysis was performed using the software Stata version 14.1 (Stata Corp., College Station, Texas, USA).
3 Results
3.1 Characteristics of the sample
Subjects were prevalently males (61.1%), aged 46.9 years (SD = 8.39), married (77.2%), middle-class (84.3%), with a high education level (14.2 years, SD = 3.60) (Table 1). As a confirmation of the expected overall good health status of the participants, most of the subjects were nonsmokers (81.3%), half of them reported regular physical activity (56.4%) and presented anthropometric, CVD risk factors, and biomarkers indices (BMI, waist circumference, FBG, BP, TC, HDL-C, TG, and Hcy) within the normal range. Consistently, TAS-20, BDI-II, and SF-12 scored within the normal range (Table 2).
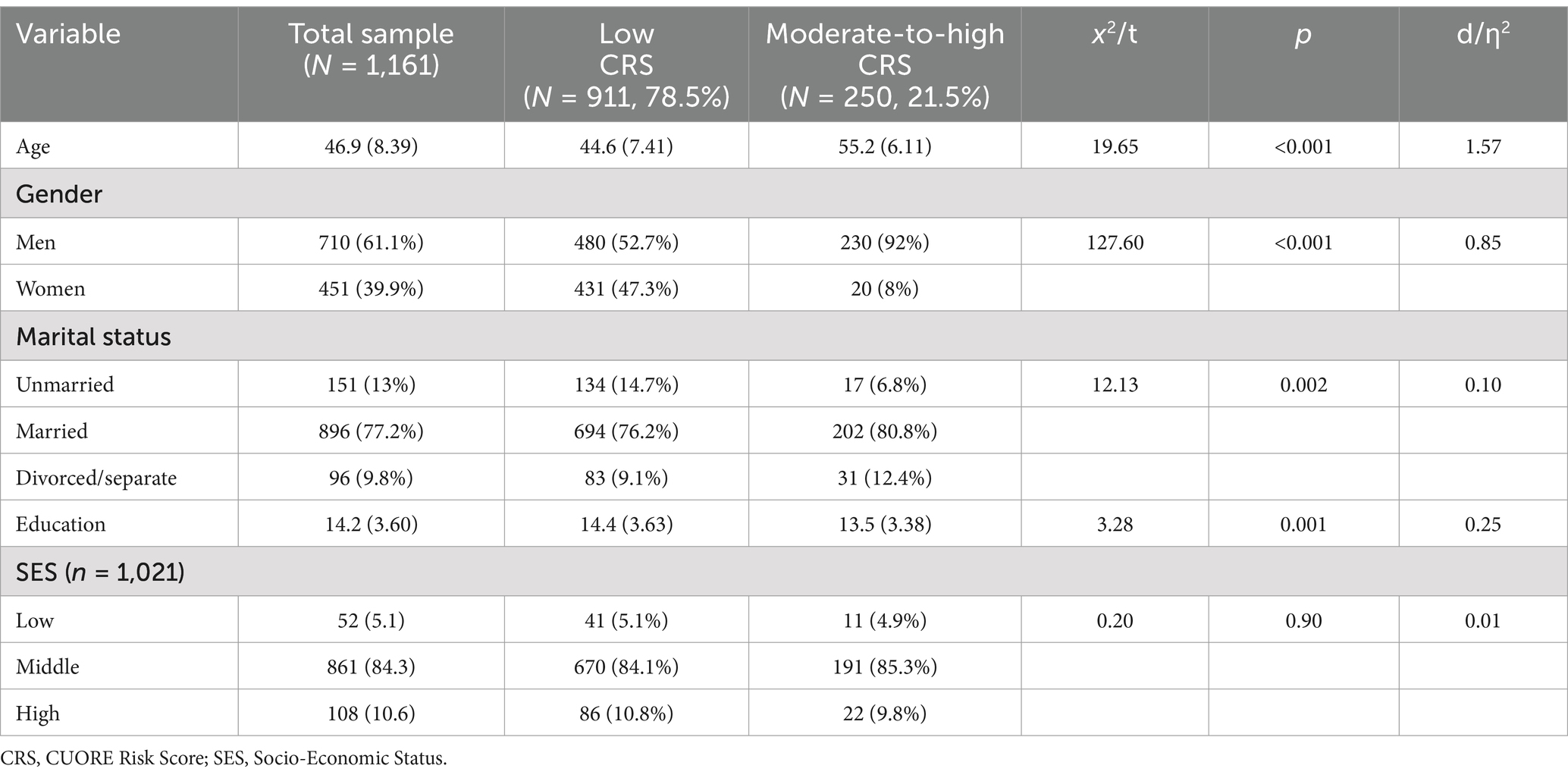
Table 1. Socio-demographic characteristics of the study sample stratified for estimated 10-year cardiovascular disease risk (CRS).
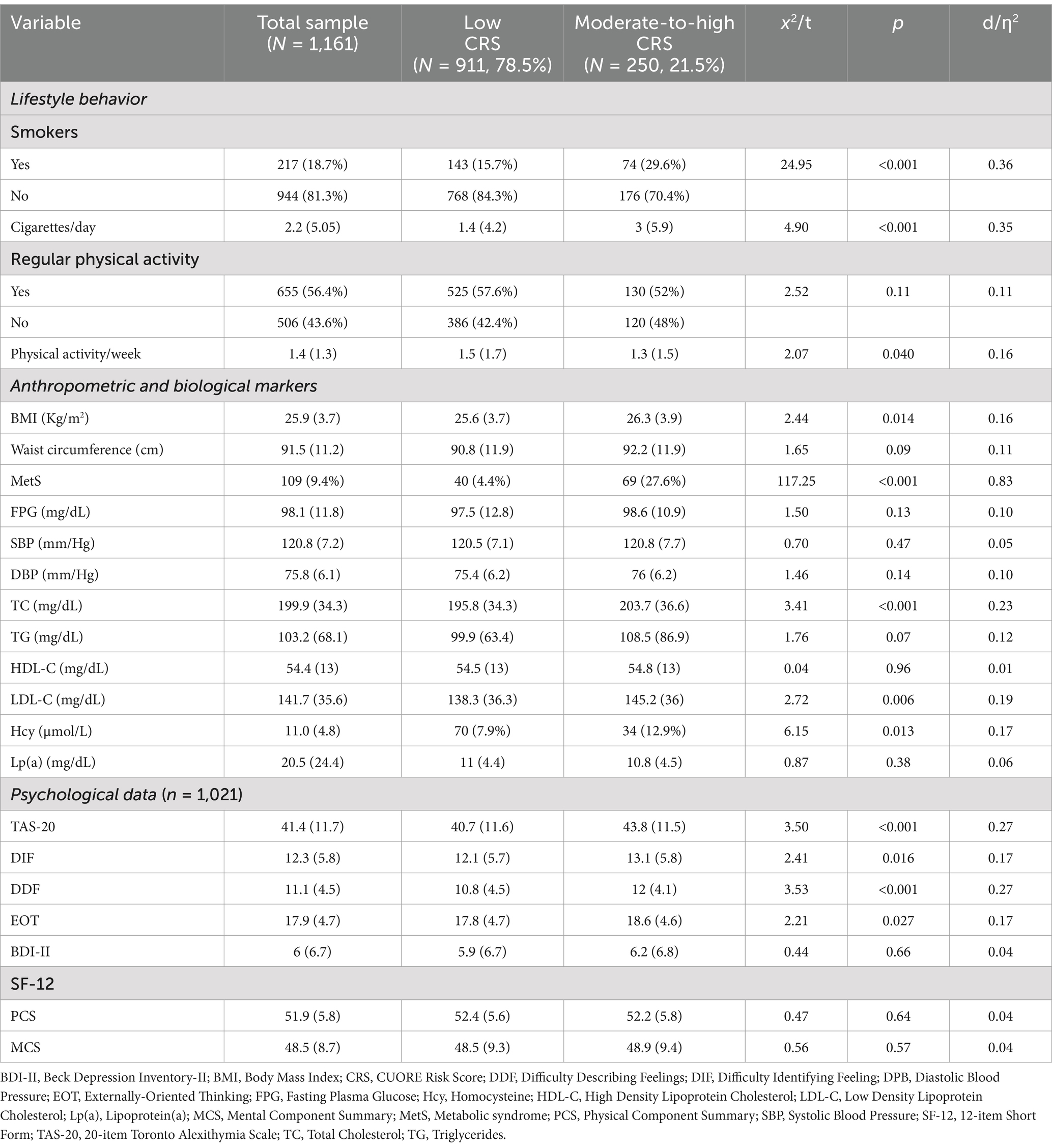
Table 2. Clinical characteristics of the study sample stratified for estimated 10-year cardiovascular disease risk (CRS).
3.2 Comparison between subjects with moderate-to-high and low CRS
As expected, more than three quarters of the participants had CRS ≤ 3% (n = 911, 78.5%), whereas nobody was classified at high CVD risk (CRS ≥ 20.0%). Subjects with higher CRS were prevalently older (d = 1.57, p < 0.001), men (d = 0.85, p < 0.001), unmarried (ƞ2 = 0.10, p = 0.002), and had lower education (d = 0.25, p < 0.001) (Table 1). As we expected, most of them were smokers (ƞ2 = 0.36, p < 0.001) and had higher anthropometric and biological risk factors (BMI, d = 0.16, p = 0.014; MetS, ƞ2 = 0.83, p < 0.001; TC, d = 0.23, p < 0.001; LDL-C, d = 0.19, p = 0.006; Hcy, d = 0.17, p = 0.017). Overall, all the participants showed a low CV risk (women: mean CRS = 1.0%, prevalence = 95%; men: mean CRS = 2.85%, prevalence = 67.5%), which was about 3 times lower when compared with the Italian general population (mean CRS in women was 3.0% and men 8.3%) (Palmieri et al., 2011).
By comparing the psychological factors between higher and lower CVD risk subjects, only alexithymia resulted significantly higher in the higher CVD risk subjects, though having a small effect size (TAS-20 total, d = 0.27, p < 0.001; and factor scales: DIF, d = 0.17, p = 0.016; DDF, d = 0.27, p < 0.001; EOT, d = 0.17, p = 0.027) (Table 2).
3.3 Comparison between subjects with higher (HA) and lower (LA) alexithymia
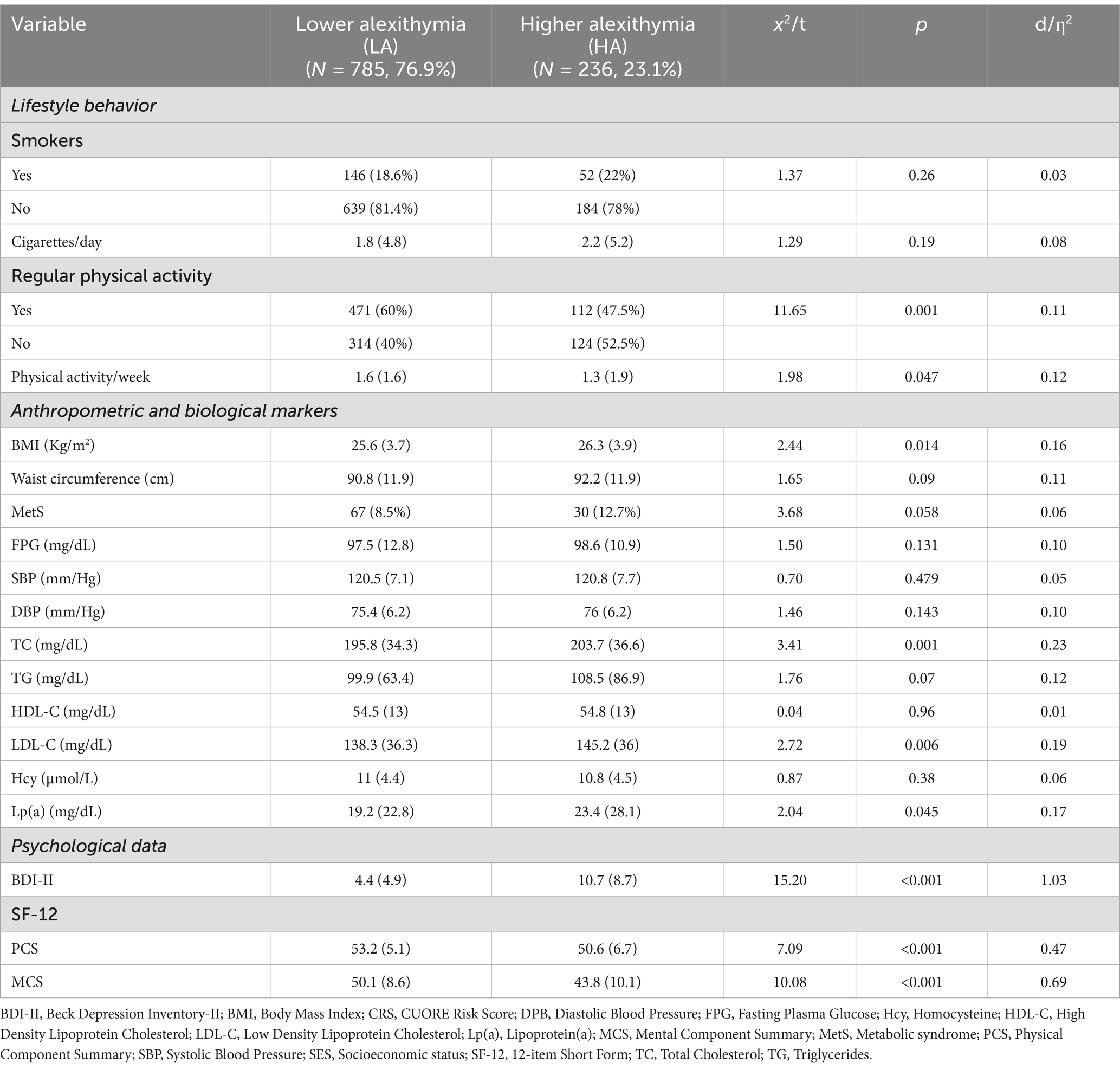
As expected, compared with LA, HA subjects were significantly older (d = 0.20, p = 0.002), less educated (d = 0.42, p < 0.001). Moreover, HA subjects were more prevalent in the lower socio-economic level and less in the higher socio-economic level (d = 0.17, p < 0.001). Gender and marital status were evenly distributed in the two groups. In addition, compared with LA, HA subjects had expectedly more depressive symptoms (d = 1.03, p < 0.001) and showed greater psychosocial impairment by considering both the components of the SF-12 (MCS, d = 0.69, p < 0.001; PCS, d = 0.47, p < 0.001) (Tables 3, 4).
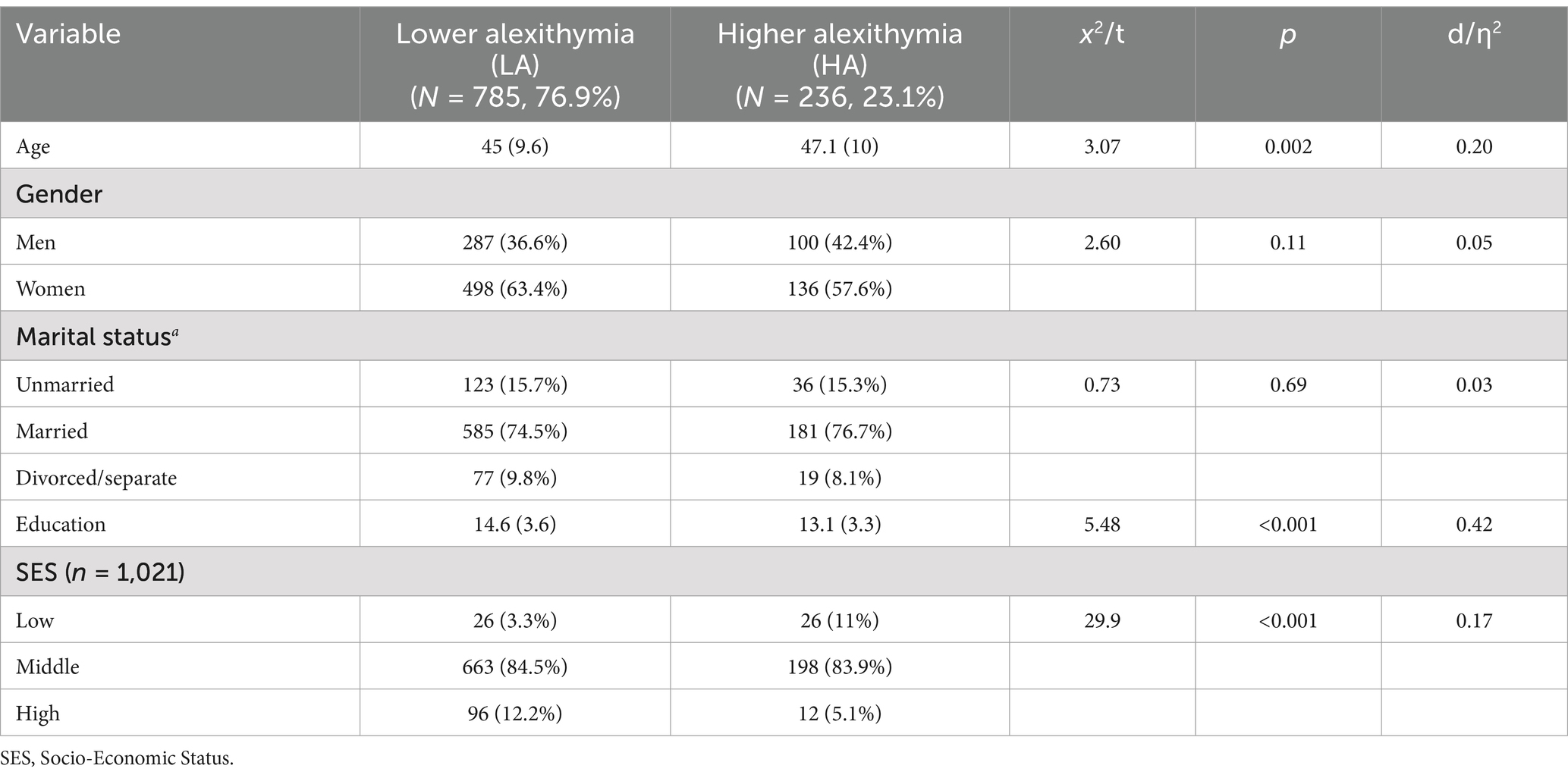
Table 3. Socio-demographic characteristics of the study population (N = 1,021), stratified for alexithymia.
Despite a small effect size, HA subjects had overall more unhealthy lifestyle behaviors (less physical activity and higher BMI, d = 0.11, p = 0.014, and d = 0.16, p < 0.001, respectively) and higher CVD risk factors such as TC (d = 0.23, p < 0.001), LDL-C (d = 0.19, p = 0.006), and Lp(a) (d = 0.17, p = 0.045) compared with LA subjects.
3.4 Predicting CVD risk from alexithymia
Table 5 shows binary logistic regression model with the 10-year CRS as a binary outcome criterion (low/high).
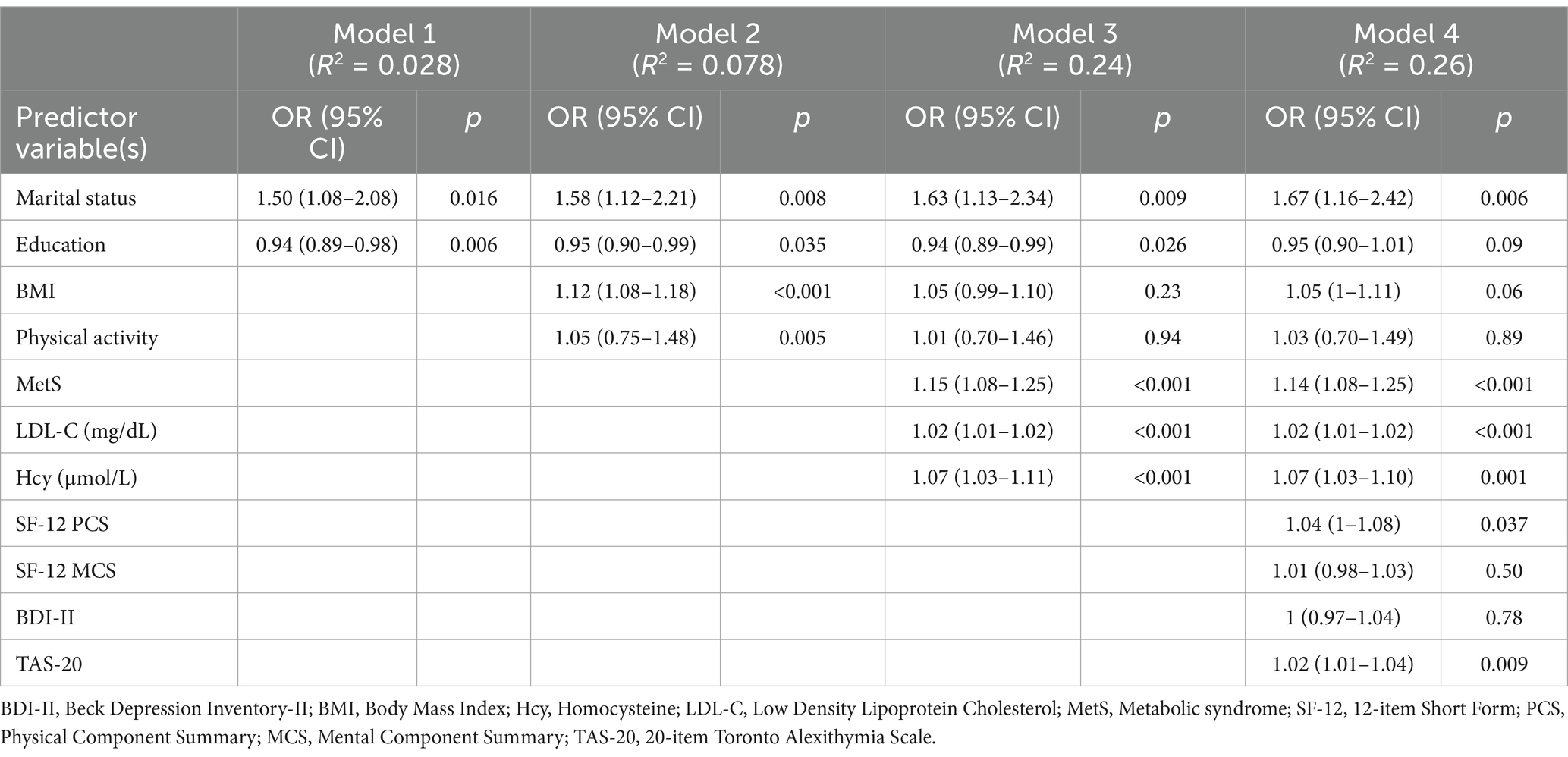
Table 5. Logistic regression model examining predictors of low and moderate-to-high 10-year cardiovascular disease risk (CRS) using the CUORE risk equation.
Sociodemographic (marital status, education level), lifestyle (BMI, physical education), biological (MetS, LDL-C, Hcy) and psychological (quality of life, depressive symptoms, alexithymia) risk factors for CVD were used as independent variables.
In the first step (R2 = 0.03), being married and having a lower education were significantly associated with higher CVD risk (ORs = 1.50 and 0.94, respectively). In the second step (R2 = 0.08), marital status and education remained significant predictors (ORs = 1.58 and 0.95, respectively), while BMI and physical activity showed a significant positive association with high CVD risk (ORs = 1.12 and 1.05, respectively). In the third step (R2 = 0.24), marital status (OR = 1.63), education (OR = 0.94), BMI (OR = 1.05), and physical activity (OR = 1.01) remained key factors. Additionally, MetS emerged as a significant predictor of higher CVD risk (OR = 1.15), along with LDL-C (OR = 1.02) and Hcy (OR = 1.07). When the psychological variables were added in the fourth step (R2 = 0.26), the TAS-20 (OR = 1.02) significantly predicted higher CVD risk along with physical component of SF-12 (OR = 1.04), marital status (OR = 1.67), MetS (OR = 1.14), LDL-C (OR = 1.02), and Hcy (OR = 1.07).
In other words, for each point increase on the TAS-20, the odds of having a higher CVD risk increased by 2% (Table 5).
4 Discussion
The present study investigated whether alexithymia was associated with increased estimated CVD risk over 10 years in a healthy, low-risk blood donor population. Specifically, we compared sociodemographic, lifestyle, biological, and psychological factors between groups with different levels of CVD risk and alexithymia, and we investigated whether alexithymia contributes to CVD risk independently of established risk factors. As expected, participants with a higher CVD risk were older, more likely to be male, unmarried, and had lower education. They were also more likely to smoke, have a higher BMI, and show indicators like MetS, elevated LDL cholesterol, and higher homocysteine levels. Among the psychological factors, only alexithymia was notably higher in participants with greater CVD risk. Moreover, after accounting for other risk factors, alexithymia still independently predicted CVD risk. In summary, we found that even in a healthy, low-risk population, higher alexithymia was associated with an increased likelihood of higher CVD risk, beyond what could be explained by traditional CVD risk factors (like BMI, lifestyle, and biological markers).
Recently, several studies have notably changed the notion of the heart as a mechanic organ beating through an internal pacemaker and pumping blood into vessels and arteries. The heart is embedded in a complex network of organs and functions including the immune, endocrine, and nervous systems. The so called “heart-brain axis” indicates a feedback control network including afferent, efferent, and local well-known neural circuits (such as the hypothalamic–pituitary axis triggering a neuro-hormonal cascade toward the autonomic nervous system) as well as brain circuits (such as the insular cortex, the anterior cingulate cortex (ACC), and the amygdala), (for a review see Fioranelli et al., 2018; Jha et al., 2019). Not surprisingly, therefore, mental disorders (particularly depression) and psychosocial stress have been repeatedly found as strictly linked in a bidirectional way to CVD onset, maintenance, and prognosis (Gaffey et al., 2022; O’Riordan et al., 2023). Studies have reported a connection through mediational factors such as social (unemployment, substance abuse, social isolation, and low education), biological (autonomic dysregulation, platelet factors, endothelial function, circulating neurohormones, insulin resistance, inflammatory cytokines), and behavioral mechanisms (obesity, smoking, low physical activity, poor diet, and medical noncompliance) both in large prospective surveys (Song et al., 2019) and in systematic reviews (Albasheer et al., 2024; Bradley and Rumsfeld, 2015; Gaffey et al., 2022). In addition, stimulation studies have documented the effects of cingulate electrical stimulation on autonomic and endocrine functions (Alexander et al., 2019; Koelsch et al., 2013) triggering altered emotional and behavioral responses such as the “cardiac signature of emotionality” (Koelsch et al., 2007).
The main strength of this study is the use of a large specific sample of individuals showing above average somatic and psychosocial health, as documented by the biological and psychological assessments. Thus, the subjects involved showed a baseline low-risk profile for CVD. The recruited subjects were middle-class, highly educated, with low exposure to known lifestyle (smoking, low physical activity) and biomedical (blood pressure, BMI, glycemia, cholesterol, triglycerides, homocysteine, lipoprotein(a), and metabolic syndrome) risk factors. Moreover, they had normal levels of alexithymia, depression, and psychosocial functioning. Nonetheless, HA subjects still presented a significantly higher risk for moderate CVD, as compared with the estimated 10-year CV risk score. More specifically, for every 1-point increase in the TAS-20 scores there was a significant and independent 2% increased risk of CVD over an estimated follow-up period of 10-years, even after adjusting for socio-demographic, established biomedical, and relevant psychological factors. In the fully adjusted model, although significant, only a small portion of variance was explained by the predicting variables. However, it is noteworthy that a personality construct (alexithymia) not directly associated with the biomedical outcome (CRS) showed a similar OR as the traditional biomedical and lifestyle risk factors (smoking, LDL, Hcy) or even higher (BMI, physical activity, depression) (e.g., O’Riordan et al., 2023). Furthermore, this result seems more surprising if one considers that the investigated sample was at a very low risk profile level from either the physiological, lifestyle, and psychological viewpoints.
Even though less investigated than other psychological constructs such as depression, alexithymia is proved to be involved in many mediational mechanisms underlying the health status. A poor cognitive processing of feelings affects negatively the regulation of the biological systems in the body (Luminet et al., 2018; Porcelli and Taylor, 2018). Alexithymia was found as a potential CV risk indicator in several studies, suggesting a connection with CV-related conditions as hypertension, atherosclerosis, CVD, and CV mortality both in general and clinical populations (e.g., Carta et al., 2022; Tolmunen et al., 2010; Vadini et al., 2019). In addition, several CV risk factors, such as sympathetic over-reactivity (Bermond et al., 2010), inflammation (Honkalampi et al., 2011), diabetes (Fares et al., 2019; Luca et al., 2015), obesity/MetS (Conti et al., 2019; Conti et al., 2021; Conti et al., 2023), and atherosclerosis (Grabe et al., 2010; Vadini et al., 2019) have been associated with a higher prevalence of alexithymia. In a series of experimental studies on healthy subjects, Koelsch et al. (2007, 2013) combined the use of electrocardiogram (ECG) with functional magnetic resonance imaging (fMRI) during the processing of emotional stimuli. The studies found a “cardiac signature of emotionality” involving a specific amplitude in the resting ECG correlating with brain activity in the amygdala and the hippocampus, with alexithymia scores, and with heart rate variability (HRV). Systematic reviews have shown that alexithymia may predict hypertension and subclinical atherosclerosis, may be a risk factor for early death in the long-term course of post-myocardial infarction, as well as may predispose to unhealthy behaviors including dysfunctional health care-seeking behavior in the event of adverse cardiac events (Montisci et al., 2023; Sancassiani et al., 2023).
Potential mechanisms underlying the relationship between alexithymia and CV risk are far from being definite. The CV network includes other biological systems such as the immune and the nervous (Fioranelli et al., 2018; Jha et al., 2019), thus there is some evidence that alexithymia may affect both systems. To this extent, some studies have shown that alexithymia is associated with increased inflammation and altered profiles of circulating cytokines, although no clear shift toward pro- or anti-inflammatory mediators has been demonstrated (Kano et al., 2018). In the Kuopio Depression Study, Honkalampi and colleagues (Honkalampi et al., 2011) found that alexithymia was associated with high-sensitive C-reactive protein (hsCRP) (a protein generated by the liver in response to IL-6 secretion by macrophages and T cells) as well as with adiponectin (a cytokine expressed in the adipose tissue). Individuals with major depression and CVD show a similar association pattern. Consistent with our findings, De Berardis et al. (2008) found significant connections between alexithymia, higher hsCRP, and altered serum lipid levels (TC, HDL, and LDL) in a group of drug-naïve major depressed outpatients. Altered neural activity in the limbic brain areas has been found in HA subjects and may constitute another potential mechanism linking cognitive processing and subjective experience of emotions to CV activity. Neuroimaging studies found out that altered functional activity of amygdala, insula, and ACC (Goerlich and Aleman, 2018; Moriguchi and Komaki, 2013) together with a reduced volume of dorsal ACC, left amygdala, and insula (Xu et al., 2018) may be considered the neural basis of alexithymia. Alexithymia may mirror a cluster of psychosocial, behavioral and biological risk factors that have synergistic effects on the individual health. Consistent with this hypothesis, even if presenting with a low-risk profile, our cohort of blood donors with HA reported relatively higher indices of unhealthy behaviors such as sedentary lifestyle, MetS, BMI, TC, HD, circulating Lp(a), as well as older age, lower education level, higher unemployment rate, lower socio-economic status, higher levels of depressive symptoms and overall psychosocial functioning. All those factors are considered alexithymia-related risk factors mediating between general health and CV mortality (Montisci et al., 2023; Sancassiani et al., 2023).
The results of our study have some limitations. First and foremost, the cross-sectional design does not allow addressing causality. Our data may indicate that alexithymia contributes to CVD risk, but a number of risk factors (low education, poor socio-economic status, depressive symptoms, and lower psychosocial functioning) may affect the individual ability to manage self-regulation. Longitudinal studies are needed to further assess those associations. Second, alexithymia may also be partly secondary to depression (Sagar et al., 2021). In our sample, a past or current diagnosis of major depression was not screened out at the enrollment stage, though alexithymia was then significantly associated with depressive symptoms and a poorer mental component of psychosocial functioning. Therefore, we cannot rule out that depression may play a relevant role in the association with CVD risk, even though adjusting for depressive symptoms did not decrease CRS risk score. Third, some variables that may mediate the relationship between alexithymia and CVD risk, such as psychopathology and social isolation, were not assessed. It has been suggested that alexithymia may affect CVD through social and emotional pathways rather than directly through behavioral or physiological factors (Montisci et al., 2023; Sancassiani et al., 2023). Our findings partly support these observations, although blood donors typically show healthy lifestyle and prosocial motivation (Li et al., 2015). In addition, adjusting for lifestyle factors did not change the predicting role of alexithymia. Finally, alexithymia was assessed using only a self-report scale, even though a multimethod assessment including facets of the constructs from other sources of data (as clinician ratings, by-proxy information, and implicit motives) could be preferred (Sekely et al., 2018). However, it is widely recognized that TAS-20 captures the core dimensions of the construct (impairment in experiencing and describing feelings) (Bagby et al., 2020) and shows overtime absolute and relative stability in the general population (Hiirola et al., 2017). Therefore, the self-report scale used in the study can be considered a valid and sound measure of alexithymia.
Despite these limitations, the present findings indicate that alexithymia is an independent strong predictor of CVD risk even in above average healthy individuals. Further research is needed to confirm longitudinally this association and to examine the underlying mechanisms. In addition, while previous studies have mainly focused on high-risk or clinical populations, our results showed that alexithymia could be an early indicator of cardiovascular disease risk, adding a psychological dimension to cardiovascular prevention efforts. Since alexithymia seems linked to cardiovascular disease risk even in low-risk samples, psychological factors such as emotion regulation could be important additions to traditional cardiovascular disease risk screenings. Further longitudinal research is needed to confirm this association and to examine the underlying mechanisms.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: the raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to cm9iZXJ0YS5sYW56YXJhQHVuaWNoLml0.
Ethics statement
The studies involving humans were approved by the Oncology Hematology Department of Spirito Santo Hospital in Pescara. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FV: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. RL: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. OI: Supervision, Writing – original draft. GA: Supervision, Writing – original draft. PP: Conceptualization, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The volunteers of the Pescara section of Italian Federation of Associations of Blood Donors (FIDAS), and all blood donors who have contributed to the realization of this survey, are gratefully acknowledged. We are heavily indebted to Dr. Anna Di Carlo, president of FIDAS Pescara, Abruzzo, Italy. The authors thanks Dr. Marzia Iasenza for helping with the English translation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Albasheer, O., Abdelwahab, S. I., Zaino, M. R., Altraifi, A. A. A., Hakami, N., El-Amin, E. I., et al. (2024). The impact of social isolation and loneliness on cardiovascular disease risk factors: a systematic review, meta-analysis, and bibliometric investigation. Sci. Rep. 14:12871. doi: 10.1038/s41598-024-63528-4
Alexander, L., Clarke, H. F., and Roberts, A. C. (2019). A focus on the functions of area 25. Brain Sci. 9:129. doi: 10.3390/brainsci9060129
Aluja, A., Malas, O., Urieta, P., Worner, F., and Balada, F. (2020). Biological correlates of the Toronto alexithymia scale (TAS-20) in cardiovascular disease and healthy community subjects. Physiol. Behav. 227:113151. doi: 10.1016/j.physbeh.2020.113151
Andriopoulos, P., Lotti-Lykousa, M., Pappa, E., Papadopoulos, A. A., and Niakas, D. (2013). Depression, quality of life, and primary care: a cross-sectional study. J. Epidemiol. Global Health 3, 245–252. doi: 10.1016/j.jegh.2013.06.004
Apolone, G., and Mosconi, P. (1998). The Italian SF-36 health survey. J. Clin. Epidemiol. 51, 1025–1036. doi: 10.1016/s0895-4356(98)00094-8
Bagby, R. M., Parker, J. D. A., and Taylor, G. J. (2020). Twenty-five years with the 20-item Toronto alexithymia scale. J. Psychosom. Res. 131:109940. doi: 10.1016/j.jpsychores.2020.109940
Beck, A., Steer, R., and Brown, G. K. (1996). Manual for the Beck depression inventory-II. TX: Psychological Corporation.
Bermond, B., Bierman, D. J., Cladder, M. A., Moormann, P. P., and Vorst, H. C. M. (2010). The cognitive and affective alexithymia dimensions in the regulation of sympathetic responses. Int. J. Psychophysiol. 75, 227–233. doi: 10.1016/j.ijpsycho.2009.11.004
Bogle, B. M., Ning, H., Mehrotra, S., Goldberger, J. J., and Lloyd-Jones, D. M. (2016). Lifetime risk for sudden cardiac death in the community. J. Am. Heart Assoc. 5:e002398. doi: 10.1161/JAHA.115.002398
Bradley, S. M., and Rumsfeld, J. S. (2015). Depression and cardiovascular disease. Trends Cardiovasc. Med. 25, 614–622. doi: 10.1016/j.tcm.2015.02.002
Bressi, C., Taylor, G., Parker, J., Bressi, S., Brambilla, V., Aguglia, E., et al. (1996). Cross-validation of the factor structure of the 20-item Toronto alexithymia scale: an Italian multicenter study. J. Psychosom. Res. 41, 551–559. doi: 10.1016/s0022-3999(96)00228-0
Burger, P. M., Dorresteijn, J. A. N., Koudstaal, S., Holtrop, J., Kastelein, J. J. P., Jukema, J. W., et al. (2024). Course of the effects of LDL-cholesterol reduction on cardiovascular risk over time: a meta-analysis of 60 randomized controlled trials. Atherosclerosis 396:118540. doi: 10.1016/j.atherosclerosis.2024.118540
Carola, V., Vincenzo, C., Di Vincenzo, G., Morale, C., Cecchi, V., and Nicolais, G. (2024). Psychological risk factors and cardiovascular disease. Front. Psychol. 15:1419731. doi: 10.3389/fpsyg.2024.1419731
Carta, M. G., Sancassiani, F., Bina, D., Licciardi, M., Cossu, G., Nardi, A. E., et al. (2022). Alexithymia is a determinant of early death in the long-term course of post-myocardial infarction. J. Public Health Res. 11:2803. doi: 10.4081/jphr.2022.2803
Carta, M. G., Sancassiani, F., Pippia, V., Bhat, K. M., Sardu, C., and Meloni, L. (2013). Alexithymia is associated with delayed treatment seeking in acute myocardial infarction. Psychother. Psychosom. 82, 190–192. doi: 10.1159/000341181
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. 2nd Edn. Hillsdale, NJ: Lawrence Erlbaum Associates Publishers.
Conti, C., di Francesco, G., Lanzara, R., Severo, M., Fumagalli, L., Guagnano, M. T., et al. (2019). Alexithymia and binge eating in obese outpatients who are starting a weight-loss program: a structural equation analysis. Eur. Eating Disord. Rev. 27, 628–640. doi: 10.1002/erv.2696.
Conti, C., Di Francesco, G., Severo, M., Lanzara, R., Richards, K., Guagnano, M. T., et al. (2021). Alexithymia and metabolic syndrome: the mediating role of binge eating. Eating Weight Disord. 26, 1813–1823. doi: 10.1007/s40519-020-00964-x
Conti, C., Di Nardo, M., Lanzara, R., Guagnano, M. T., Cardi, V., and Porcelli, P. (2023). Improvement in binge eating and alexithymia predicts weight loss at 9-month follow-up of the lifestyle modification program. Eat. Weight Disord. 28:30. doi: 10.1007/s40519-023-01560-5
de Berardis, D., Serroni, N., Campanella, D., Carano, A., Gambi, F., Valchera, A., et al. (2008). Alexithymia and its relationships with C-reactive protein and serum lipid levels among drug naïve adult outpatients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1982–1986. doi: 10.1016/j.pnpbp.2008.09.022
Donfrancesco, C., Palmieri, L., Cooney, M.-T., Vanuzzo, D., Panico, S., Cesana, G., et al. (2010). Italian cardiovascular mortality charts of the CUORE project: are they comparable with the SCORE charts? Eur. J. Cardiovasc. Prev. Rehabil. 17, 403–409. doi: 10.1097/HJR.0b013e328334ea70
Emdin, C. A., Odutayo, A., Wong, C. X., Tran, J., Hsiao, A. J., and Hunn, B. H. M. (2016). Meta-analysis of anxiety as a risk factor for cardiovascular disease. Am. J. Cardiol. 118, 511–519. doi: 10.1016/j.amjcard.2016.05.041
Ettehad, D., Emdin, C. A., Kiran, A., Anderson, S. G., Callender, T., Emberson, J., et al. (2016). Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 387, 957–967. doi: 10.1016/S0140-6736(15)01225-8
Fares, C., Bader, R., and Ibrahim, J.-N. (2019). Impact of alexithymia on glycemic control among Lebanese adults with type 2 diabetes. J. Diabetes Metab. Disord. 18, 191–198. doi: 10.1007/s40200-019-00412-3
Ferrario, M., Chiodini, P., Chambless, L. E., Cesana, G., Vanuzzo, D., Panico, S., et al. (2005). Prediction of coronary events in a low incidence population: assessing accuracy of the CUORE cohort study prediction equation. Int. J. Epidemiol. 34, 413–421. doi: 10.1093/ije/dyh405
Fioranelli, M., Bottaccioli, A. G., Bottaccioli, F., Bianchi, M., Rovesti, M., and Roccia, M. G. (2018). Stress and inflammation in coronary artery disease: a review psychoneuroendocrineimmunology-based. Front. Immunol. 9:2031. doi: 10.3389/fimmu.2018.02031
Franks, P. W., Hanson, R. L., Knowler, W. C., Sievers, M. L., Bennett, P. H., and Looker, H. C. (2010). Childhood obesity, other cardiovascular risk factors, and premature death. N. Engl. J. Med. 362, 485–493. doi: 10.1056/NEJMoa0904130
Freedland, K. E., Hesseler, M. J., Carney, R. M., Steinmeyer, B. C., Skala, J. A., Dávila-Román, V. G., et al. (2016). Major depression and long-term survival of patients with heart failure. Psychosom. Med. 78, 896–903. doi: 10.1097/PSY.0000000000000346
Gaffey, A. E., Gathright, E. C., Fletcher, L. M., and Goldstein, C. M. (2022). Screening for psychological distress and risk of cardiovascular disease and related mortality: a systematized review, meta-analysis, and case for prevention. J. Cardiopulmonary Rehabil. Preven. 42, 404–415. doi: 10.1097/HCR.0000000000000751
Gan, Y., Gong, Y., Tong, X., Sun, H., Cong, Y., Dong, X., et al. (2014). Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry 14:371. doi: 10.1186/s12888-014-0371-z
Gandek, B., Ware, J. E., Aaronson, N. K., Apolone, G., Bjorner, J. B., Brazier, J. E., et al. (1998). Cross-validation of item selection and scoring for the SF-12 health survey in nine countries. J. Clin. Epidemiol. 51, 1171–1178. doi: 10.1016/s0895-4356(98)00109-7
Ghisi, M., Flebus, G., Montano, A., Sanavio, E., and Sica, C. (2006). Beck depression inventory-second edition. Adattamento italiano: Manuale. Organizzazioni Speciali: Firenze.
Giampaoli, S., Palmieri, L., Donfrancesco, C., Panico, S., Vanuzzo, D., Pilotto, L., et al. (2007). Cardiovascular risk assessment in Italy: the CUORE project risk score and risk chart. Epidemiol. Biostat. Public Health 4:7. doi: 10.2427/5885.
Goerlich, K., and Aleman, A. (2018). “Neuroimaging studies of alexithymia” in Alexithymia: advances in research, theory, and clinical practice. eds. O. Luminet, R. M. Bagby, and G. J. Taylor (Cambridge: Cambridge University Press).
Grabe, H. J., Schwahn, C., Barnow, S., Spitzer, C., John, U., Freyberger, H. J., et al. (2010). Alexithymia, hypertension, and subclinical atherosclerosis in the general population. J. Psychosom. Res. 68, 139–147. doi: 10.1016/j.jpsychores.2009.07.015
Grundy, S. M., Cleeman, J. I., Merz, C. N. B., Brewer, H. B. J., Clark, L. T., Hunninghake, D. B., et al. (2004). Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J. Am. Coll. Cardiol. 44, 720–732. doi: 10.1016/j.jacc.2004.07.001
Hiirola, A. S., Pirkola, M., Karukivi, N., Markkula, R. M., Bagby, M., Joukamaa, A., et al. (2017). An evaluation of the absolute and relative stability of alexithymia over 11 years in a Finnish general population. J. Psychosom. Res. 95, 81–87. doi: 10.1016/j.jpsychores.2017.02.007
Honkalampi, K., Lehto, S. M., Koivumaa-Honkanen, H., Hintikka, J., Niskanen, L., Valkonen-Korhonen, M., et al. (2011). Alexithymia and tissue inflammation. Psychother. Psychosom. 80, 359–364. doi: 10.1159/000327583
Jha, M. K., Qamar, A., Vaduganathan, M., Charney, D. S., and Murrough, J. W. (2019). Screening and management of depression in patients with cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 1827–1845. doi: 10.1016/j.jacc.2019.01.041
Kano, M., Grabe, H. J., and Terock, J. (2018). “Genetic factors and endocrine and immune system functioning associated with alexithymia” in Alexithymia: advances in research, theory, and clinical practice. eds. O. Luminet, R. M. Bagby, and G. J. Taylor (Cambridge: Cambridge University Press).
Koelsch, S., Remppis, A., Sammler, D., Jentschke, S., Mietchen, D., Fritz, T., et al. (2007). A cardiac signature of emotionality. Eur. J. Neurosci. 26, 3328–3338. doi: 10.1111/j.1460-9568.2007.05889.x
Koelsch, S., Skouras, S., and Jentschke, S. (2013). Neural correlates of emotional personality: a structural and functional magnetic resonance imaging study. PLoS One 8:e77196. doi: 10.1371/journal.pone.0077196
Krittanawong, C., Maitra, N. S., Qadeer, Y. K., Wang, Z., Fogg, S., Storch, E. A., et al. (2023). Association of depression and cardiovascular disease. Am. J. Med. 136, 881–895. doi: 10.1016/j.amjmed.2023.04.036
Kurniawan, L. B. (2024). Triglyceride-glucose index as a biomarker of insulin resistance, diabetes mellitus, metabolic syndrome, and cardiovascular disease: a review. EJIFCC 35, 44–51
Li, S., Zhang, B., Guo, Y., and Zhang, J. (2015). The association between alexithymia as assessed by the 20-item Toronto alexithymia scale and depression: A meta-analysis. Psychiatry Res. 227, 1–9. doi: 10.1016/j.psychres.2015.02.006
Lindstrom, M., DeCleene, N., Dorsey, H., Fuster, V., Johnson, C. O., LeGrand, K. E., et al. (2022). Global burden of cardiovascular diseases and risks collaboration, 1990-2021. J. Am. Coll. Cardiol. 80, 2372–2425. doi: 10.1016/j.jacc.2022.11.001
Lissåker, C. T., Norlund, F., Wallert, J., Held, C., and Olsson, E. M. (2019). Persistent emotional distress after a first-time myocardial infarction and its association to late cardiovascular and non-cardiovascular mortality. Eur. J. Prev. Cardiol. 26, 1510–1518. doi: 10.1177/2047487319841475
Luca, A., Luca, M., Di Mauro, M., Palermo, F., Rampulla, F., and Calandra, C. (2015). Alexithymia more than depression influences glycaemic control of type 2 diabetic patients. J. Endocrinol. Investig. 38, 653–660. doi: 10.1007/s40618-015-0238-2
Luminet, O., Bagby, R.M., and Taylor, G. Alexithymia: advances in research theory and clinical practice. Cambridge: Cambridge University Press. (2018).
Marchesi, C., Ossola, P., Scagnelli, F., Mellini, L., Tonna, M., Ardissino, D., et al. (2015). The role of alexithymia in predicting incident depression in patients at first acute coronary syndrome. Compr. Psychiatry 62, 86–92. doi: 10.1016/j.comppsych.2015.06.013
Meijer, A., Conradi, H. J., Bos, E. H., Thombs, B. D., van Melle, J. P., and de Jonge, P. (2011). Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen. Hosp. Psychiatry 33, 203–216. doi: 10.1016/j.genhosppsych.2011.02.007
Meloni, L., Montisci, R., Pippia, V., Sancassiani, F., and Carta, M. G. (2016). Alexithymia affects the time from symptom onset to calling the emergency system in STEMI patients referred for primary PCI. Int. J. Cardiol. 219, 428–432. doi: 10.1016/j.ijcard.2016.06.038
Melville, M. R., Lari, M. A., Brown, N., Young, T., and Gray, D. (2003). Quality of life assessment using the short form 12 questionnaire is as reliable and sensitive as the short form 36 in distinguishing symptom severity in myocardial infarction survivors. Heart 89, 1445–1446. doi: 10.1136/heart.89.12.1445
Montisci, R., Sancassiani, F., Marchetti, M. F., Biddau, M., Carta, M. G., and Meloni, L. (2023). Alexithymia for cardiologists: a clinical approach to the patient. J. Cardiovasc. Med. 24, 392–395. doi: 10.2459/JCM.0000000000001487
Moriguchi, Y., and Komaki, G. (2013). Neuroimaging studies of alexithymia: physical, affective, and social perspectives. BioPsychoSocial Med. 7:8. doi: 10.1186/1751-0759-7-8
Norlund, F., Lissåker, C., Wallert, J., Held, C., and Olsson, E. M. (2018). Factors associated with emotional distress in patients with myocardial infarction: results from the SWEDEHEART registry. Eur. J. Prev. Cardiol. 25, 910–920. doi: 10.1177/2047487318770510
O’Riordan, A., Gallagher, S., and Howard, S. (2023). Type D personality and cardiovascular reactivity to acute psychological stress: a systematic review and meta-analysis. Division Thirty-Eight Health Psychology Membership Directory 42, 628–641. doi: 10.1037/hea0001328
Palmieri, L., Donfrancesco, C., Giampaoli, S., Trojani, M., Panico, S., Vanuzzo, D., et al. (2006). Favorable cardiovascular risk profile and 10-year coronary heart disease incidence in women and men: results from the Progetto CUORE. European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology and Prevention and Cardiac Rehabilitation and Exercise Physiology, Eur J Cardiovasc. Prev. Rehabil. 13, 562–570. doi: 10.1097/01.hjr.0000221866.27039.4b
Palmieri, L., Rielli, R., Demattè, L., Donfrancesco, C., Ciccarelli, P., De Sanctis Caiola, P., et al. (2011). CUORE project: implementation of the 10-year risk score. Eur. J. Cardiovasc. Prev. Rehabil. 18, 642–649. doi: 10.1177/1741826710389925
Porcelli, P., and Taylor, G. J. (2018) in Alexithymia and physical illness: A psychosomatic approach. In alexithymia. eds. O. Luminet, R. M. Bagby, and G. J. Taylor (Cambridge: Cambridge University Press), 105–126.
Prasad, K. (2024). Role of C-reactive protein, an inflammatory biomarker in the development of atherosclerosis and its treatment. Int. J. Angiol. 33, 271–281. doi: 10.1055/s-0044-1788296
Rawal, S., Atia, M., Nisenbaum, R., Paré, D. E., Joorden, S., and Hwang, S. W. (2013). Is "appearing chronically ill" a sign of poor health? A study of diagnostic accuracy. PLoS One 8:e79934. doi: 10.1371/journal.pone.0079934
Rozanski, A. (2014). Behavioral cardiology. J. Am. Coll. Cardiol. 64, 100–110. doi: 10.1016/j.jacc.2014.03.047
Sagar, R., Talwar, S., Desai, G., and Chaturvedi, S. K. (2021). Relationship between alexithymia and depression: a narrative review. Indian J. Psychiatry 63, 127–133. doi: 10.4103/psychiatry.IndianJPsychiatry_738_19
Sancassiani, F., Montisci, R., Meloni, L., Nardi, A. E., and Carta, M. G. (2023). Why is it important to assess and treat alexithymia in the cardiologic field? An overview of the literature. Clin. Pract. Epidemiol. Mental Health 19:e174501792307140. doi: 10.2174/17450179-v19-230810-2022-HT15-4764-1
Sekely, A., Bagby, R. M., and Porcelli, P. (2018). “Assessment of the alexithymia construct” in Alexithymia. eds. O. Luminet, R. M. Bagby, and G. J. Taylor (Cambridge: Cambridge University Press), 17–32.
Song, H., Fang, F., Arnberg, F. K., Mataix-Cols, D., Fernández de la Cruz, L., Almqvist, C., et al. (2019). Stress related disorders and risk of cardiovascular disease: population based, sibling controlled cohort study. BMJ 365:l1255. doi: 10.1136/bmj.l1255
Taylor, G. J., and Bagby, R. M. (2004). New trends in alexithymia research. Psychother. Psychosom. 73, 68–77. doi: 10.1159/000075537
Taylor, G.J., Bagby, R.M., and Parker, J.D.A. Disorders of affect regulation: alexithymia in medical and psychiatric illness. Cambridge: Cambridge University Press. (1999).
Tolmunen, T., Lehto, S. M., Heliste, M., Kurl, S., and Kauhanen, J. (2010). Alexithymia is associated with increased cardiovascular mortality in middle-aged Finnish men. Psychosom. Med. 72, 187–191. doi: 10.1097/PSY.0b013e3181c65d00
Vadini, F., Sozio, F., Madeddu, G., de Socio, G., Maggi, P., Nunnari, G., et al. (2019). Alexithymia predicts carotid atherosclerosis, vascular events, and all-cause mortality in human immunodeficiency virus-infected patients: an Italian multisite prospective cohort study. Open Forum Infect. Dis. 6:ofz331. doi: 10.1093/ofid/ofz331.
Whisman, M. A., and Richardson, E. D. (2015). Normative data on the Beck depression inventory--second edition (BDI-II) in college students. J. Clin. Psychol. 71, 898–907. doi: 10.1002/jclp.22188
World Health Organization (2010). Global recommendations on physical activity for health. Geneva: World Health Organization.
Keywords: alexithymia, blood donors, cardiovascular risk, quality of life, psychosomatic
Citation: Vadini F, Lanzara R, Iuliani O, Affaitati GP and Porcelli P (2024) Alexithymia and estimated 10-year cardiovascular disease risk in healthy adults: a community-based cross-sectional study. Front. Psychol. 15:1504143. doi: 10.3389/fpsyg.2024.1504143
Edited by:
Marialaura Di Tella, University of Turin, ItalyCopyright © 2024 Vadini, Lanzara, Iuliani, Affaitati and Porcelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberta Lanzara, cm9iZXJ0YS5sYW56YXJhQHVuaWNoLml0
 Francesco Vadini
Francesco Vadini Roberta Lanzara
Roberta Lanzara Ornella Iuliani2
Ornella Iuliani2 Piero Porcelli
Piero Porcelli