- Departamento de Psicologia, Laboratório de Neurociência do Comportamento, Pontifícia Universidade Católica do Rio de Janeiro, Rio de Janeiro, Brazil
To form a unified and coherent perception of the organism’s state and its relationship with the surrounding environment, the nervous system combines information from various sensory modalities through multisensory integration processes. Occasionally, data from two or more sensory channels may provide conflicting information. This is particularly evident in experiments using the mirror-guided drawing task and the mirror-box illusion, where there is conflict between positional estimates guided by vision and proprioception. This study combined two experimental protocols (the mirror-box and the mirror-guided drawing tasks) to examine whether the learned resolution of visuo-proprioceptive conflicts in the mirror-guided drawing task would improve proprioceptive target estimation of men and women during the mirror-box test. Our results confirm previous findings of visual reaching bias produced by the mirror-box illusion and show that this effect is progressively reduced by improvement in the mirror drawing task performance. However, this was only observed in women. We discuss these findings in the context of possible gender differences in multisensory integration processes as well as in embodiment.
1 Introduction
The concept of embodiment refers to the idea that cognitive processes are deeply rooted in the body’s interactions with the environment, and that perception is intrinsically linked to both sensory and motor processes (Fuchs and Schlimme, 2009; Craighero, 2014; Varela et al., 2016; Shapiro, 2019). From a more specific perspective, embodiment represents the subjective sensation associated with possessing and disposing of one’s own body (Longo et al., 2008; Medina et al., 2015). In this sense, several authors have shown the importance of research on embodiment for understanding the mechanisms of multisensory integration (Botvinick and Cohen, 1998; Holmes et al., 2004; Holmes and Spence, 2005; Miall and Cole, 2007; Longo et al., 2008; Otsuru et al., 2014; Diers et al., 2015; Medina et al., 2015; Vecchiato et al., 2015; Liu and Medina, 2017, 2018, 2021; Carey et al., 2019; Giroux et al., 2019; Leach and Medina, 2022; Ambron and Medina, 2023; Ding et al., 2023). Additionally, findings show the relevance of the sense of embodiment in various clinical applications, such as the treatment of “phantom pain” in amputees (Ramachandran et al., 1995; Schmalzl et al., 2013; Collins et al., 2018; Kundi and Spence, 2023), improvement of motor function after stroke (Altschuler et al., 1999; Thieme et al., 2018; Lee and Lee, 2019; Kundi and Spence, 2023), management of body image and eating disorders (Griffen et al., 2018), alleviation of motor symptoms associated with multiple sclerosis (Tekeoglu Tosun et al., 2021), and rehabilitation of complex regional pain syndrome (Al Sayegb et al., 2013).
The mirror therapy technique stands out as a powerful tool for understanding the processes related to multisensory integration and embodiment, particularly to those, but not limited to, the alleviation of symptoms associated with “phantom limb” pain (Ramachandran et al., 1995; Ramachandran and Rodgers-Ramachandran, 1996; Dohle et al., 2019). Amputee patients undergoing this therapy often report that the sight of the reflection of their remaining limb in motion or being stimulated is perceived as their missing limb - a phenomenon termed mirror visual feedback (Ramachandran et al., 1995; Ramachandran and Rodgers-Ramachandran, 1996; Deconinck et al., 2015). Despite the established effectiveness of the technique in clinical settings, the underlying mechanisms still need further elucidation (Dohle et al., 2019). Nevertheless, it is widely acknowledged that the process of embodiment during mirror feedback involves the integration of various sensory modalities such as kinesthesia (movement), touch, vision, and proprioception (Holmes et al., 2004). The latter encompasses sensory information from the joints, muscles, and tendons, contributing to our perception of body positioning and movement in space. It is important to note that, in the literature, the terms proprioception and kinesthesia are sometimes used interchangeably (Stillman, 2002), whereas for others, kinesthesia specifically denotes the perception of movement (Swanik et al., 2004; Swanik et al., 2002). There is also an understanding that kinesthesia is a subset of proprioception (Lephart et al., 1997; Myers et al., 1999). Yet, most commonly, proprioception is defined broadly, to include the sense of movement (Han et al., 2016; Tuthill and Azim, 2018; Blum et al., 2021; Moon et al., 2021), playing a pivotal role in the embodiment process during mirror therapy (Holmes et al., 2004; Holmes and Spence, 2005; Medina et al., 2015; Leach and Medina, 2022). Beyond its clinical applications, the concept underpinning mirror therapy serves as a valuable research tool for investigating visual-proprioceptive conflicts and expand our understanding of multisensory processes and embodiment in healthy individuals (Holmes et al., 2004; Holmes and Spence, 2005; Medina et al., 2015; Liu and Medina, 2021; Leach and Medina, 2022). For instance, discordant visual and proprioceptive-placement information significantly impacts the accuracy of target-reaching movements made with the unseen arm in non-clinical samples of young adults (visual capture; Holmes and Spence, 2005; Holmes et al., 2004).
The mirror drawing task stands as another common method to induce visuo-proprioceptive conflicts, wherein vision supersedes proprioception in resolving such conflicts (Lajoie et al., 1992; Balslev et al., 2004; Miall and Cole, 2007; Miall et al., 2021). A good illustration of this phenomenon is observed in the star-tracing drawing task where individuals must outline the reflected image of a six-pointed star, allowing for the assessment of how visual and proprioceptive information are integrated and modified through learning processes (Lajoie et al., 1992). Intriguingly, research has demonstrated that this ability is compromised in individuals afflicted with mirror agnosia and mirror ataxia (Ramachandran et al., 1997; Binkofski et al., 1999), whereas patients experiencing selective loss of proprioceptive afferent inputs show little to no visuo-proprioceptive conflicts (Lajoie et al., 1992; Balslev et al., 2004; Miall and Cole, 2007; Miall et al., 2021). In contrast, healthy individuals fully experience this conflict and must learn the new skill through visuomotor adaptation. This process occurs dynamically with the internal map recalibration, which iteratively resolves the visuo-proprioceptive conflict (Lajoie et al., 1992; Guedon et al., 1998; Balslev et al., 2004; Cressman and Henriques, 2009; Henriques and Cressman, 2012; Ruttle et al., 2016).
Visuomotor adaptation occurs when an individual is required to adjust their motor output in response to a mismatch between visual feedback and actual motor performance. This conflict is typically introduced experimentally by altering the visual representation of movement (Shadmehr and Mussa-Ivaldi, 1994; Redding and Wallace, 2006; Cressman and Henriques, 2009; Krakauer, 2009). As before mentioned, this kind of adaptation has been shown to occur in the mirror drawing task, where the participants are asked to trace a geometrical shape through its image in the mirror while the direct vision of the drawing hand is blocked (Lajoie et al., 1992; Milner, 1998; Balslev et al., 2004; Miall et al., 2021). After training, the brain adjusts the proprioceptive map of the body to align more closely with the altered visual feedback. This recalibration is thought to involve both short-term and long-term adjustments in sensory integration and neural plasticity, allowing the individual to perform the task more accurately as the brain recalibrates its sensory systems (Shadmehr and Krakauer, 2008; Cressman and Henriques, 2010; Ostry et al., 2010; Ruttle et al., 2016).
Research has demonstrated that men and women differ in visuomotor adaptation and in their ability to resolve visuo-proprioceptive conflicts. It is well established that men typically perform better in visuospatial and visuomotor tasks, and that sex differences also extend to the perception of visual illusions and body representation (Linn and Petersen, 1985; Viaud-Delmon et al., 1998; Peled et al., 2000; Barnett-Cowan et al., 2010; Cadieux et al., 2010; Egsgaard et al., 2011; Thakkar et al., 2011; Eshkevari et al., 2012; Ferri et al., 2014; Longo, 2022; Fioriti et al., 2024). Additionally, men and women differ in the prevalence of psychopathological conditions such as bulimia and anorexia nervosa, with women exhibiting up to 15 times the prevalence of anorexia compared to men (Qian et al., 2022). These disorders are strongly associated with multisensory integration deficits and body representation distortions, a phenomenon that also occurs in healthy individuals when exposed to discordant visual and proprioceptive information (Longo, 2022, 2023; Malighetti et al., 2022; Brizzi et al., 2023; Fusco et al., 2023; Navas-León et al., 2023). Thus, investigating visuo-proprioceptive conflicts through the lens of sex differences may offer valuable insights into the perceptual mechanisms underlying body representation distortions.
To further shed light on the specific roles of vision and proprioception in how the visuo-proprioceptive conflicts are resolved and given that previous findings show that visuomotor adaptation is followed by proprioceptive recalibration (Cressman and Henriques, 2009, 2010, 2011; Cressman et al., 2010; Henriques and Cressman, 2012; Flannigan et al., 2018), we integrated both the mirror box and the six-pointed star approaches to assess the visuo-proprioceptive recalibration in a sample of non-clinical participants. Furthermore, given previous findings suggesting sex differences in the perception of embodiment, visual illusions and visuomotor tasks (Linn and Petersen, 1985; Viaud-Delmon et al., 1998; Barnett-Cowan et al., 2010; Cadieux et al., 2010; Egsgaard et al., 2011), we also compared the performance of men and women.
2 Methods
2.1 Participants
Fifteen subjects (7 women and 8 men, mean age 42.3 ± 3.69 years) were recruited to participate in the study. All participants were right-handed, with no visual problems or with corrected vision and naïve to the purpose of the study. The study was approved by the Brazilian Ethics Committee (CEP/CONEP, # 63845022.3.0000.5281), ensuring that all procedures complied with ethical guidelines. All participants gave their informed consent prior the start of data collection.
2.2 Materials
A mirror box like the one used by Holmes et al. (2004) and Holmes and Spence (2005) was used for a task in which the participant should reach toward a target position with its hidden hand (reaching movement task; Holmes et al., 2004; Holmes and Spence, 2005). Briefly, a wooden parallelepiped (45 width × 45 length × 20 height, cm) without two opposite sidewalls was placed on a table (Supplementary Figure S1). The outward face of one of the remaining sidewalls had a slot to accommodate a removable mirror (45 cm × 30 cm). Three marks, only visible to the experimenter, were placed 5, 12, and 26 cm to the left of the mirror. The middle mark (12 cm) was considered the target position and the other two starting positions. To indicate the location of the target position to the participant, a cardboard with a downward arrow was positioned on the top surface of the parallelepiped (Supplementary Figure S1). A black cloth was used to cover the participant’s right arm and shoulder, occluding the view of their right hand.
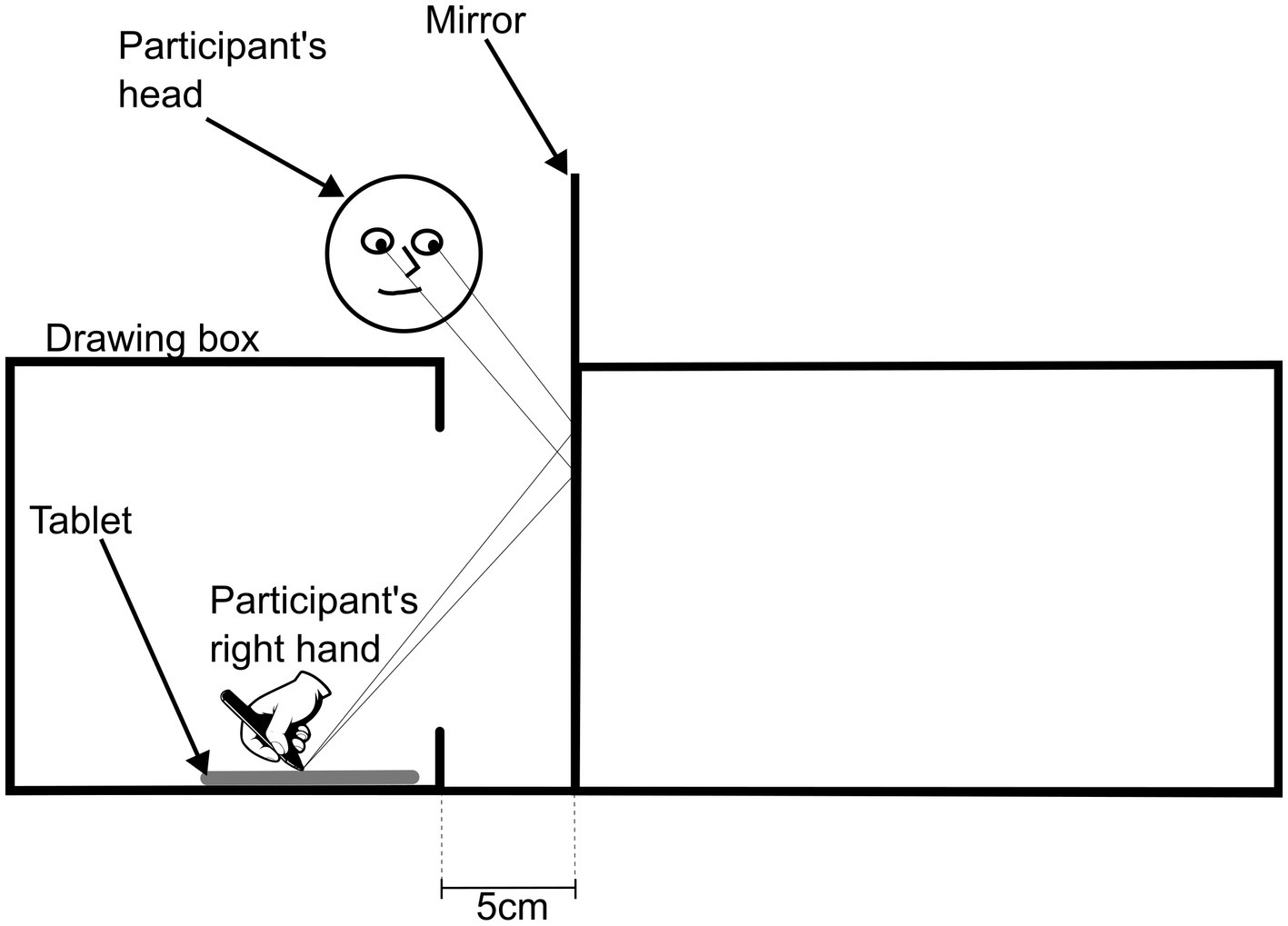
For the star-tracing task, a second wooden parallelepiped (45 width × 30 length × 20 height, cm), without two opposite sidewalls (30 length × 20 height, cm), and with a rectangular opening on one of the remaining sidewalls, was placed 5 cm to the right of the mirror (Supplementary Figure S2). A tablet (Samsung Galaxy Tab S6 Lite) was positioned inside the parallelepiped allowing the participant to see its reflection while preventing from looking directly at it (Supplementary Figure S2). An image of a six-pointed star (2,490 × 3,510 pixels, 300 dpi) was displayed on the tablet for the star tracing task (Supplementary Figures S2, S3).
2.3 Procedures
For the reaching movement task, the participant sat at the table facing the mirror box and was asked to put their left arm inside the mirror box and the index finger of the other arm 12 cm to the right of the mirror (Figure 1). The experimenter covered the participant’s left arm and shoulder with the black cloth and sat on the other side of the table facing the participant. Next, the experimenter placed the participant’s left index finger (the one inside the box) at either 5 or 26 cm starting positions. At this first stage, the mirror was not positioned in the slot (no-mirror condition) and the participant was instructed to tap both index fingers synchronously at a frequency of 170 BPM (2.83 Hz) defined by a metronome (Medina et al., 2015). After 6 s, the experimenter asked the participant to reach, with his or her left index finger, the target position inside the box (12 cm), which was indicated by the downward arrow sign on top of the box. Then the experimenter measured the distance (cm) from the target to the participant’s reached position (error). The task was repeated until 5 measurements were completed for each start position (5 and 26 cm) alternately, totaling 10 attempts. The same procedure and number of repetitions were done with the mirror inserted in the slot. While synchronously tapping the participant was instructed to keep looking at the reflection of their right hand on the mirror, which induced the mirror box illusion (mirror condition; Figure 1). On the first day, there was a training session to familiarize the participants with the task (Holmes and Spence, 2005). This was a short session, no longer than 5 min, which comprised the reaching task in the ‘no mirror’ condition and an exposure to the mirror-box illusion. Such session was useful to correct the way the participants executed the reaching movements. They were instructed to make an intuitively single and continuous movement, with no pauses.
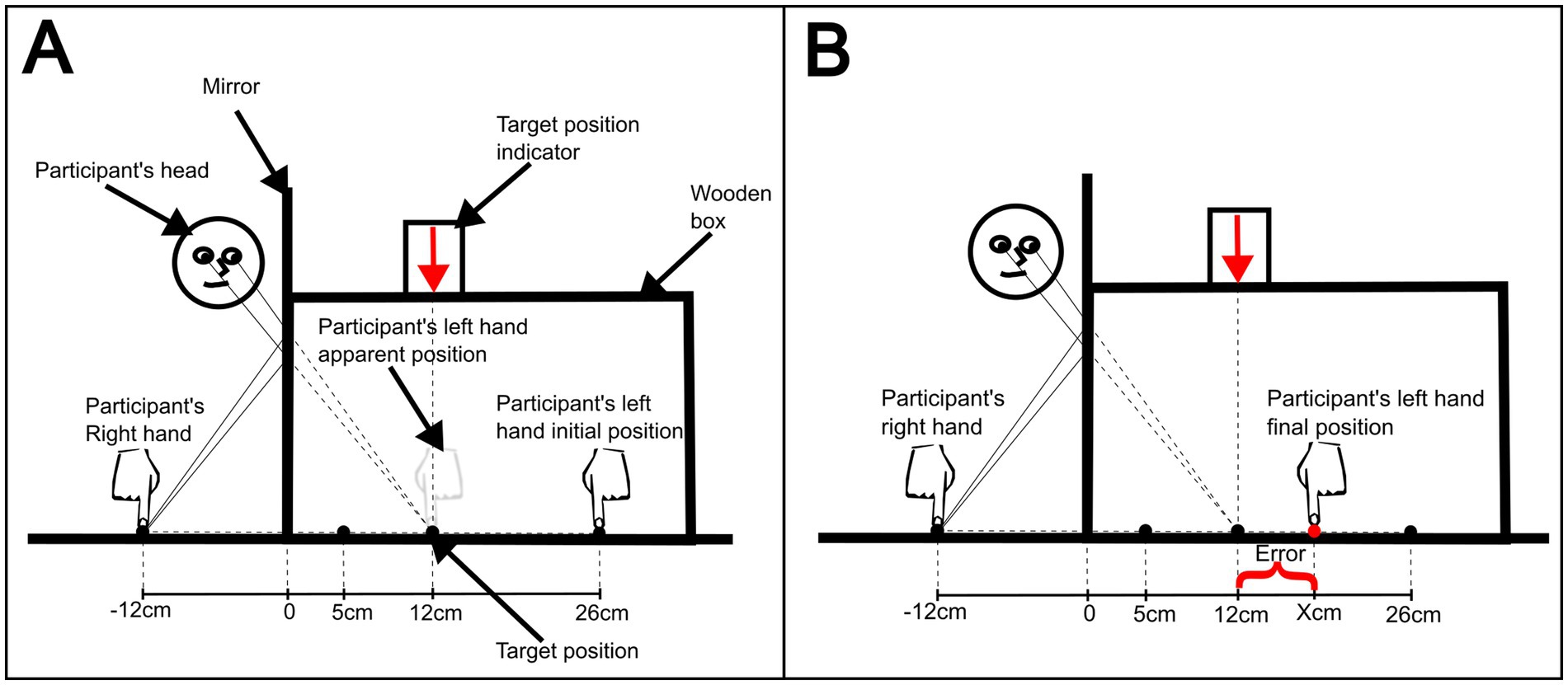
Figure 1. Schematic of target-reaching task, from the researcher’s perspective. (A) Initial task setup at the initial position of 26 cm. (B) Measurement of reaching error, after the participant’s reaching attempt.
Next, with the star-tracing setup in place, the participant was asked to draw the outline of the 6-pointed star looking at the reflection of the image (Figure 2). The experimenter positioned the tip of the pen at the top point of the star template and the participant had to choose whether they would like to outline the star in a counter-or clockwise direction, maintaining the same orientation in subsequent drawings. They were asked to complete the drawing the best way possible and in the shortest period. The participant was asked to repeat this sequence 10 times and, for each one, the image was captured, and the completion time recorded. Prior to the star-tracing task, each participant had to do the outlining of the star template three times with direct vision to the tablet.
After completing the star-tracing drawings, the participant repeated the protocol for the reaching target task in the ‘mirror’ condition and, right after, the same protocol, but in the ‘no-mirror’ condition (Supplementary Figure S4). The same sequence of experiments was repeated two more times, with a minimum interval of 24 h and a maximum of 72 h between sessions (36.8 ± 3.2 h).
2.4 Data analysis
Data analysis was performed using Python (version 3.10.10) and Jamovi software (version 2.2.5). Reaching task results were analyzed using repeated-measures ANOVA (rANOVA). The analysis included ‘sex’ (men and women) as the between-subject factor, while ‘mirror’ (mirror and no-mirror), ‘initial position’ (left hand at 5 or 26 cm), ‘reaching phase’ (before or after star-tracing task within a given day), and ‘session’ (first, second or third day of testing) were the within-subjects factor. Data from the star-tracing task were analyzed using a Python script (Supplementary materials). An accuracy index (Iacc) was calculated based on the number of pixels drawn within and outside the star’s template, as well as the total number of pixels.
where:
• is the total number of pixels drawn (within and outside the star outline).
• is the number of pixels drawn within the star outline limits.
• is the total number of pixels of a perfect outline (12 straight lines).
A second index was calculated to assess the speed of execution for the star-tracing task. This was done by comparing tracing times with participants looking to the mirror against those obtained with direct vision of the star template (i.e., not its reflection).
where:
• is the mean time (3 trials) to outline the star looking at it.
• is the time to outline the star’s reflection.
Indices of 0.0 indicate the lowest accuracy and speed values, while indices of 1.0 represent the highest accuracy and speed. To evaluate participants’ performance in the star-tracing task, a repeated-measures ANOVA (rANOVA) was conducted, with ‘sex’ as the between-subjects factor and ‘session’ (first, second or third day of testing) as the within-subject factor. The effect size for both rANOVA factors was calculated using partial eta squared (ηp2). Post hoc pairwise comparisons were adjusted for multiple comparisons using the Bonferroni-Holm method. Additionally, a correlation analysis was performed between the indices and errors made in the reaching movement task. All data are presented as mean ± SEM, unless otherwise specified. Statistical significance was set at p < 0.05.
3 Results
The five-way rANOVA for the reaching task showed a significant interaction among sex, mirror, initial position, reaching phase, and session [F(2, 146) = 4.079, p < 0.02, ηp2 = 0.053]. No significant four-way interactions were detected. We found significant three-way interactions among ‘sex’ × ‘mirror’ × ‘reaching phase’ [F(1, 73) = 4.777, p < 0.05, ηp2 = 0.061], ‘mirror’ × ‘initial position’ × ‘reaching phase’ [F(1, 73) = 4.989, p < 0.05, ηp2 = 0.064], and ‘initial position’ × ‘reaching phase’ × ‘session’ [F(2, 146) = 4.097, p < 0.05, ηp2 = 0.053]. Significant two-way interactions were also found among ‘sex’ × ‘mirror’ [F(1, 73) = 6.846, p < 0.05, ηp2 = 0.086], ‘sex’ × ‘session’ [F(2, 146) = 3.796, p < 0.05, ηp2 = 0.049], and ‘initial position’ × ‘session’ [F(2, 146) = 11.30038, p < 0.001, ηp2 = 0.134]. Finally, the main effects of ‘sex’ [F(1, 73) = 4.09, p < 0.05, ηp2 = 0.053], ‘mirror’ [F(1, 73) = 31.04, p < 0.001, ηp2 = 0.298], ‘initial position’ [F(1, 73) = 174.70, p < 0.001, ηp2 = 0.705], and ‘session’ [F(2,146) = 8.57, p < 0.001, ηp2 = 0.105] were detected. No other significant effects were found (all p-values >0.05). Post hoc comparisons revealed that the visual capture effect (Figure 3) occurred in all sessions of experiments, with all comparisons reaching p < 0.001 corrected by the Bonferroni-Hom method. Additionally, in the ‘mirror’ condition (with mirror), there were significant differences of reaching errors between men and women in the first session (1st day of testing), with women committing larger errors than man for the 26 cm ‘initial position’ (before mirror drawing: Mean Difference = 1.3 cm, pHolm < 0.05; after: Mean Difference = 1.7 cm, pHolm < 0.05). This significant difference disappeared in the next sessions since a significant decrease in reaching errors was found for women (26 cm ‘initial position’) from the first to third session (before mirror drawing: Mean Difference = 1.9 cm, pHolm < 0.05; after mirror drawing: Mean Difference = 2.1 cm, pHolm < 0.05). These results can be better visualized in Figure 4.
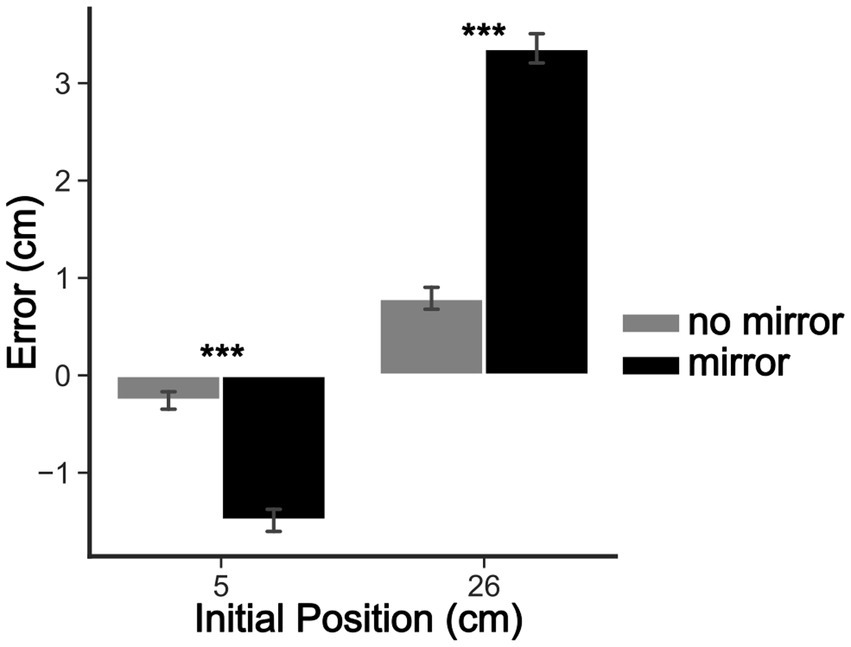
Figure 3. Visual capture effect. Bar graph depicting the mean target estimation errors made by participants in the reaching task for the mirror and no-mirror conditions. The x-axis shows the initial position of the hidden left hand in centimeters, and the y-axis shows the mean reaching errors in cm. Bars are means ± SEM. *p < 0.05; ***p < 0.001.
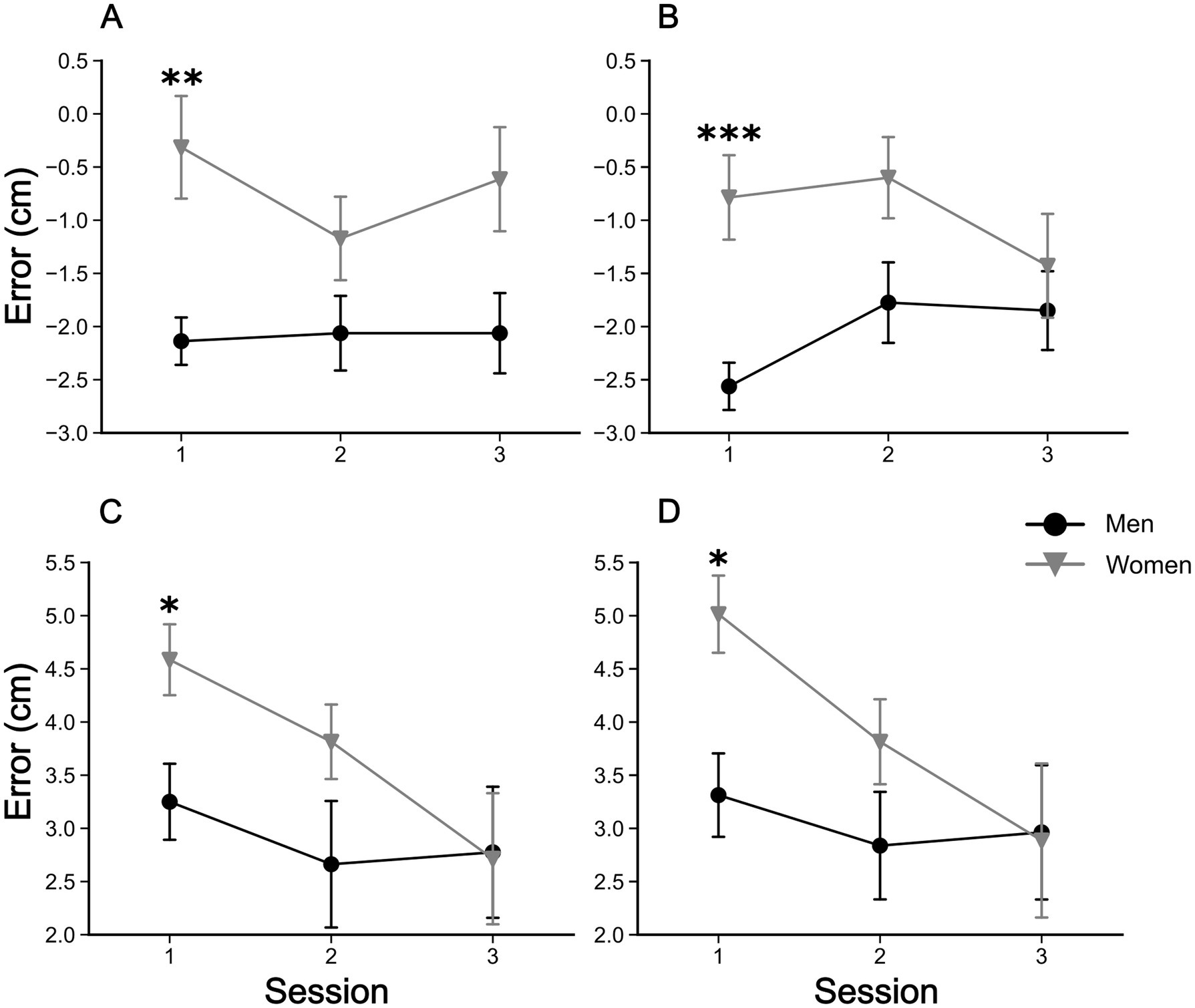
Figure 4. Evolution of reaching errors in the ‘mirror’ condition. Upper and lower panels show the errors for ‘initial position’ of 5 and 26 cm, respectively. Left and right panels show the errors for ‘reaching phase’ before and after the mirror drawing task, respectively. (A) Mean reaching errors for ‘reaching phase’ before the mirror drawing task, at the ‘initial position’ 5 cm. (B) Mean reaching errors for ‘reaching phase’ after the mirror drawing task, at the ‘initial position’ 5 cm. (C) Mean reaching errors for ‘reaching phase’ before the mirror drawing task, at the ‘initial position’ 26 cm. (D) Mean reaching errors for ‘reaching phase’ after the mirror drawing task, at the ‘initial position’ 26 cm. Symbols are means ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001.
The two-way rANOVA for the accuracy index revealed a significant the interaction for ‘sex’ × ‘session’ [F(2,296) = 24.2, p < 0.001, ηp2 = 0.141], as well as for the main effects of ‘sex’ [F(1,148) = 43.2, p < 0.001, ηp2 = 0.226], and ‘session’ [F(2,296) = 195.6, p < 0.001, ηp2 = 0.569]. For the speed index we found no significant interaction between ‘sex’ and ‘session. However, significant effects were found for ‘sex’ [F(1,148) = 23.1, p < 0.001, ηp2 = 0.135] and ‘session’ [F(2,296) = 132.61, p < 0.001, ηp2 = 0.473]. Although both sexes showed a clear improvement in both accuracy and speed in the mirror drawing task over testing sessions, the overall performance of women was always worse compared to that observed for men (Figures 5A,B). For representative man and woman mirror drawing performances, please see Supplementary Figure S5. Moreover, women showed the largest improvement in mirror drawing accuracy (Figure 5).
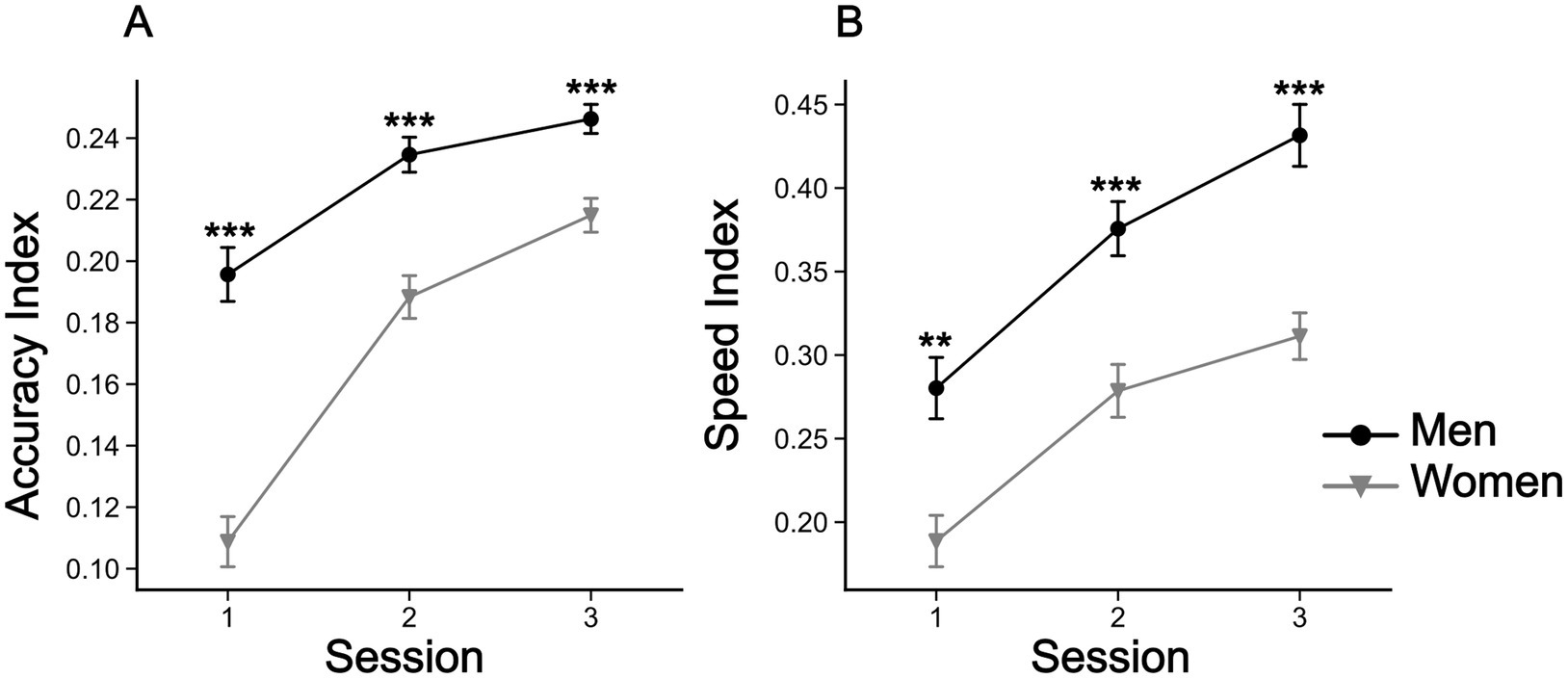
Figure 5. Evolution of accuracy and speed indices for the mirror drawing task. (A) Mean accuracy indices for men and women in the mirror drawing task across sessions. (B) Mean speed indices for men and women in the mirror drawing task across sessions. Symbols are means ± SEM. **p < 0.01, and ***p < 0.001.
Having found significant differences in performance over sessions and between sexes for both reaching target and mirror drawing tasks, we next investigated possible relationships between reaching errors and mirror drawing indices. Since no significant effect for ‘reaching phase’ [F(1,73) = 0.578, p = 0.449, ηp2 = 0.008], nor interaction between ‘reaching phase’ × ‘session’ [F(2,146) = 0.361, p = 0.697, ηp2 = 0.005] was found, reaching target errors before and after mirror drawing within each session were aggregated as average errors. Due to the aim of the study and space limitations, only correlations for the mirror condition are showed (Figure 6). Interestingly, correlations involving accuracy indices (Figures 6A,C) were found to be significant for women (5 cm, r = −0.456, p < 0.05; 26 cm = −0.462, p < 0.05), but not for men (5 cm, r = −0.305, p = 0.15; 26 cm, r = −0.264, p = 0.21). In contrast, we have found no statistical significance for correlations between speed index values and reaching errors (Figures 6B,D), except for men in the farthest reaching errors (Figure 6D, men, r = −0.504, p < 0.05; women, r = −0.184, p = 0.43).
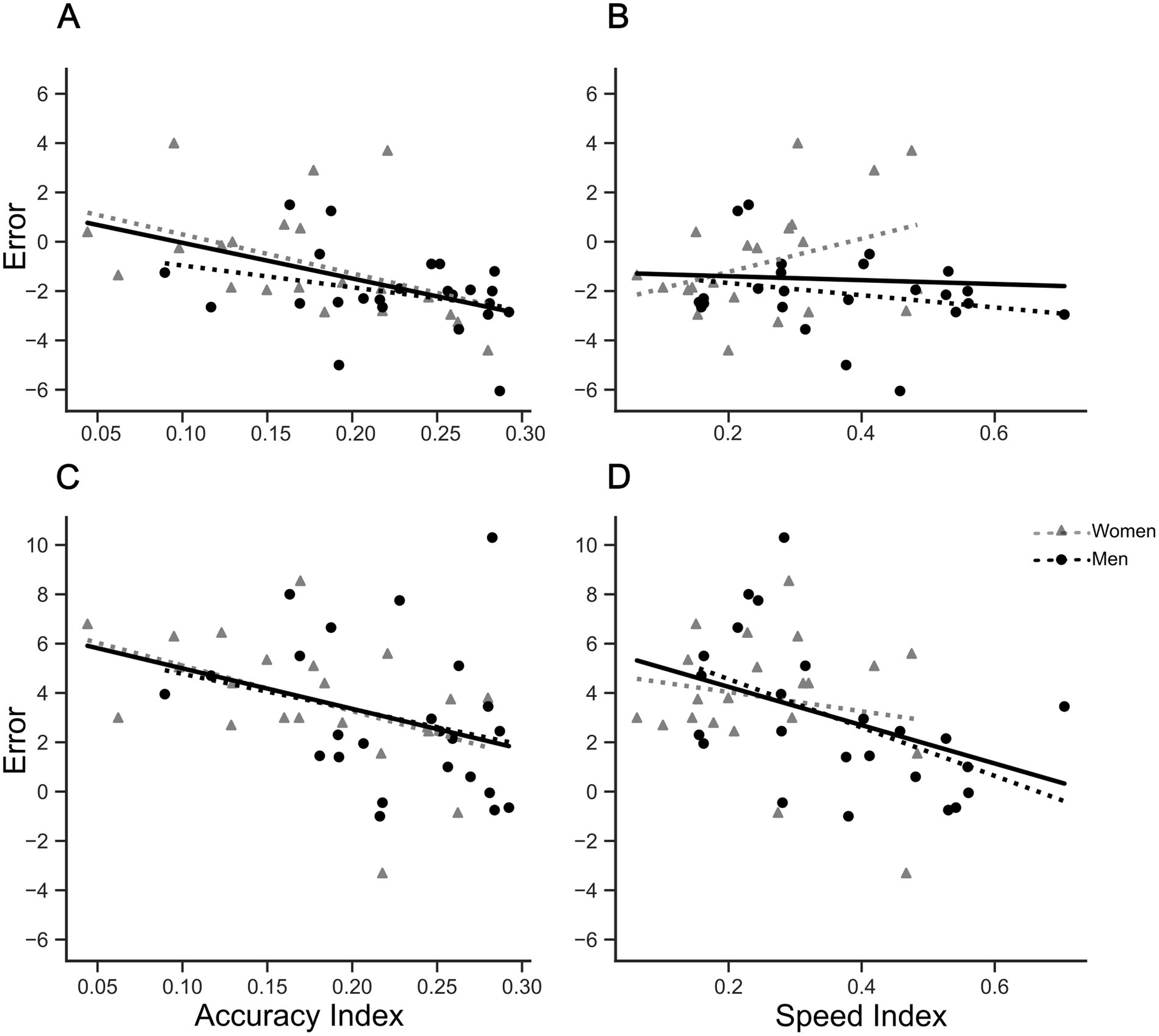
Figure 6. Correlations between reaching errors in ‘mirror’ condition and mirror drawing indices. Upper and lower panels show scatter plots and tendency lines for ‘initial position’ of 5 and 26 cm, respectively. Left and right panels show the scatter plot and tendency lines for mirror drawing accuracy and speed indices, respectively. (A) Correlation between reaching errors at 5 cm ‘initial position’ and Accuracy Indices. Women: r = −0.456, p < 0.05; Men: No significant correlation. (B) Correlation between reaching errors at 5 cm ‘initial position’ and Speed Indices. No significant correlations found for women and men. (C) Correlation between reaching errors at 26 cm ‘initial position’ and Accuracy Indices. Women: r = −0.462, p < 0.05; Men: No significant correlation. (D) Correlation between reaching errors at 26 cm ‘initial position’ and Speed Indices. Women: No significant correlation. Men: r = −0.504, p < 0.05. Solid and traced lines represent linear fits. Accuracy and speed indices showed a positive correlation of 0.579 (p < 0.001).
4 Discussion
Our results support previous studies showing that the mirror box illusion alters participants’ perception of the hidden hand’s position. Given that participants consistently committed greater mean reaching errors in the ‘mirror’ condition, and that such errors were systematically made through smaller reaching movements, we can state that the perceived hidden hand’s initial position was shifted toward the visually informed location, in accordance with the visual capture phenomenon reported by other researchers (Holmes et al., 2004; Holmes and Spence, 2005; Medina et al., 2015; Liu and Medina, 2018). This effect was evident in both men and women during the mirror-box illusion, supporting the notion that the brain integrates visual and proprioceptive information in a statistical way and that the visual capture results from this integrative process in presence of proprioceptive drift. Since the precision (reliability) of proprioceptive inputs are diminished because of its greater variance, which in turn is induced by the blocked vision of the performing hand, vision’s relative weight is increased, creating the visual bias (Wann and Ibrahim, 1992; Van Beers et al., 1999; Ernst and Banks, 2002; Brown et al., 2003; Ernst and Bülthoff, 2004; Holmes and Spence, 2005; Guerraz et al., 2012). The novelty of our findings is showing that this embodiment effect was progressively mitigated in women by the introduction of the star-tracing task. In line with previous research on visuomotor adaptation (Cressman and Henriques, 2009, 2015; Cressman et al., 2010; Salomonczyk et al., 2011; Henriques and Cressman, 2012; Ruttle et al., 2016; Flannigan et al., 2018; Bouchard and Cressman, 2021; Wijeyaratnam et al., 2022), our results indicate that training-induced recalibration can affect subsequent tasks, supporting the idea that recalibration is not task-specific but can be applied to other contexts involving similar sensory conflicts. Yet, this was only true for women and if we consider errors associated to the furthest distance from the target. One limitation of our study was to not include a control group, that would not be a participant in the star-tracing task. However, this issue was overcome by the comparison between the reaching errors in the ‘mirror’ and ‘no mirror’ conditions. If the improvement in the reaching errors was to be attributed to the participants learning how to better perform in the reaching task, we should have observed a significant reduction in the ‘no mirror’ condition as well, which was not true.
Visuo-proprioceptive conflicts impacting the accuracy of target-reaching movements made with the unseen hand (Holmes et al., 2004; Holmes and Spence, 2005; Medina et al., 2015; Liu and Medina, 2018) can also be influenced by one’s embodiment experience (Medina et al., 2015; Liu and Medina, 2017, 2018). Another limitation of our study is that we did not investigate embodiment measures associated with the subjective experience of the mirror-box illusion and how it is related to visuo-proprioceptive recalibration. We would expect that embodiment measures should decrease after the visuo-proprioceptive recalibration, which would reinforce the association between multisensory integration processes and body ownership distortions.
Lajoie et al. (1992) showed in their seminal paper that visual-proprioceptive conflicts occur in the mirror star-tracing task. It was demonstrated that a patient with severe proprioceptive deficits outperformed controls from the outset, while controls only achieved similar performances after resolving visuo-proprioceptive conflicts through mirror star-tracing practice. This suggests the necessity of implicit motor learning to recalibrate their visual-proprioceptive spatial representation. This understanding is supported by another study in which TMS was used to disrupt the proprioceptive processing of participants, thereby improving their performance in mirror-guided tasks (Balslev et al., 2004). Moreover, it was later demonstrated that at the beginning of mirror-guided tasks, where visual-proprioceptive conflicts are stronger, there is significant suppression of primary somatosensory cortex activation by prefrontal cortex inputs, which wanes with task repetition (Bernier et al., 2009).
A key aspect of our study was the focus on sex differences in visuo-proprioceptive integration. As outlined in the introduction, previous research has shown that men and women differ in their ability to resolve sensory conflicts, particularly in tasks that involve visuospatial and visuomotor skills (Linn and Petersen, 1985; Viaud-Delmon et al., 1998; Barnett-Cowan et al., 2010; Cadieux et al., 2010; Egsgaard et al., 2011; Fioriti et al., 2024). In alignment with these results, we show that women experienced higher levels of visuo-proprioceptive conflict at first, but also exhibited greater adaptation after training on the star-tracing task. This is confirmed by the significant reduction in the reaching errors and the greater improvement of women found in the star-tracing. In this task they improved the initial mean accuracy index (Iacc) by 90%, in contrast with an increase of 20% for men, while the speed index showed a similar improvement for both sexes (50% for women and 54% for men). Finally, reinforcing this finding, the correlation analysis showed that the reaching errors and the accuracy index are significantly and negatively correlated only for women, adding up to the evidence for a visuomotor adaptation followed by a decrease in the visuo-proprioceptive conflict experienced by women.
The observed sex differences in visuo-proprioceptive conflicts and recalibration also have implications for understanding perceptual distortions in body representation, particularly in the context of eating disorders. As stated earlier, women are disproportionately affected by disorders such as bulimia and anorexia nervosa, which are associated with multisensory integration deficits and distorted body representation (Eshkevari et al., 2012; Malighetti et al., 2020; Longo, 2022; Qian et al., 2022). Our findings suggest that women are more susceptible to visual-proprioceptive conflicts, but that they can adapt to match the levels experienced by men. Research on interventions for eating disorders and its related body representation distortion, such as mirror therapy and virtual reality therapies (Griffen et al., 2018; Malighetti et al., 2020; Matamala-Gomez et al., 2021; Riva et al., 2021; Navas-León et al., 2024), suggest that the greater magnitude of visuo-proprioceptive recalibration observed in women may also have therapeutic implications, as interventions aimed at improving multisensory integration could be tailored to leverage this result.
By using a protocol that combines mirror-box reaching target (Holmes et al., 2004; Holmes and Spence, 2005) and mirror star-tracing tasks (mirror drawing; Lajoie et al., 1992), we demonstrate that resolving visual-proprioceptive conflicts in the latter improves women’s performance in reaching target estimations. However, it is important to note that the improvement in performance in reaching the target from the farthest position is noticeable only across the three sessions of the experiment, not within the same day. This suggests that recalibration of visual-proprioceptive target estimation depends on the performance improvement observed in the mirror star-tracing task over time, indicating a cognitive process involving long-term consolidation of implicit memory (Salomonczyk et al., 2011; Tibi et al., 2013; Voges et al., 2015; Maksimovic and Cressman, 2018; Neville and Cressman, 2018; Hadjiosif et al., 2023). Previous studies demonstrating improvement in mirror star-tracing task performance have shown that this change relies on implicit memory consolidation (Corkin, 1968, 2002; Shadmehr et al., 1998). Furthermore, the observation that this improvement in performance in reaching target estimation was evident only in women also calls for further investigation. While embodiment differences between men and women have been previously demonstrated (Egsgaard et al., 2011), the understanding of the underlying mechanisms remains elusive. Nevertheless, we cannot dismiss the potential clinical applications of visual-proprioceptive conflict mitigation, especially in psychiatric conditions such as eating disorders, where heightened embodiment and multisensory integration deficits are well documented (Eshkevari et al., 2012; Keizer et al., 2014; Zopf et al., 2016; Serino and Dakanalis, 2017; Malighetti et al., 2020; Matamala-Gomez et al., 2021; Riva et al., 2021; Navas-León et al., 2024).
In conclusion, this study provides novel insights into how visuo-proprioceptive recalibration generalizes across tasks and highlights the importance of considering sex differences in multisensory integration. Our results suggest that women may experience greater visuo-proprioceptive conflicts, but also that they can adapt to match men’s levels, which has potential implications not only for understanding perceptual distortions in healthy participants, but also for clinical populations, such as those with eating disorders. By investigating the mechanisms underlying these differences, we can develop more targeted interventions for individuals with multisensory integration deficits.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Ave Regina de Azevedo Silva, Universidade Católica de Petrópolis—UCP/RJ. (CEP/CONEP, # 63845022.3.0000.5281). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AM: Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Visualization. JL-F: Formal analysis, Funding acquisition, Supervision, Writing – review & editing. TK: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
To all the volunteers who participated in the experiments for their time and support of this study. We acknowledge the use of ChatGPT-4o for assisting in debugging code and reviewing grammar and punctuation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2024.1462934/full#supplementary-material
References
Al Sayegb, S., Filén, T., Johansson, M., Sandström, S., Stiewe, G., and Butler, S. (2013). Mirror therapy for complex regional pain syndrome (CRPS)-a literature review and an illustrative case report. Scand. J. Pain 4, 200–207. doi: 10.1016/J.SJPAIN.2013.06.002
Altschuler, E. L., Wisdom, S. B., Stone, L., Foster, C., Galasko, D., Llewellyn, D. M. E., et al. (1999). Rehabilitation of hemiparesis after stroke with a mirror. Lancet 353, 2035–2036. doi: 10.1016/S0140-6736(99)00920-4
Ambron, E., and Medina, J. (2023). Examining constraints on embodiment using the Anne Boleyn illusion. J. Exp. Psychol. Hum. Percept. Perform. 49, 877–892. doi: 10.1037/XHP0001125
Balslev, D., Christensen, L. O. D., Lee, J. H., Law, I., Paulson, O. B., and Miall, R. C. (2004). Enhanced accuracy in novel mirror drawing after repetitive transcranial magnetic stimulation-induced proprioceptive deafferentation. J. Neurosci. 24, 9698–9702. doi: 10.1523/JNEUROSCI.1738-04.2004
Barnett-Cowan, M., Dyde, R. T., Thompson, C., and Harris, L. R. (2010). Multisensory determinants of orientation perception: task-specific sex differences. Eur. J. Neurosci. 31, 1899–1907. doi: 10.1111/J.1460-9568.2010.07199.X
Bernier, P. M., Burle, B., Vidal, F., Hasbroucq, T., and Blouin, J. (2009). Direct evidence for cortical suppression of somatosensory afferents during visuomotor adaptation. Cereb. Cortex 19, 2106–2113. doi: 10.1093/CERCOR/BHN233
Binkofski, F., Buccino, G., Dohle, C., Seitz, R. J., and Freund, H. J. (1999). Mirror agnosia and mirror ataxia constitute different parietal lobe disorders. Ann. Neurol. 46, 51–61. doi: 10.1002/1531-8249(199907)46:1<51::AID-ANA9>3.0.CO;2-Q
Blum, K. P., Versteeg, C., Sombeck, J., Chowdhury, R. H., and Miller, L. E. (2021). Proprioception: a sense to facilitate action. Somatosensory Feedback Neuroprosthetics, 41–76. doi: 10.1016/B978-0-12-822828-9.00017-4
Botvinick, M., and Cohen, J. (1998). Rubber hands “feel” touch that eyes see [8]. Nature 391:756. doi: 10.1038/35784
Bouchard, J. M., and Cressman, E. K. (2021). Intermanual transfer and retention of visuomotor adaptation to a large visuomotor distortion are driven by explicit processes. PLoS One 16:e0245184. doi: 10.1371/journal.pone.0245184
Brizzi, G., Sansoni, M., Di Lernia, D., Frisone, F., Tuena, C., and Riva, G. (2023). The multisensory mind: a systematic review of multisensory integration processing in anorexia and bulimia nervosa. J. Eat. Disord. 11:204. doi: 10.1186/s40337-023-00930-9
Brown, L. E., Rosenbaum, D. A., and Sainburg, R. L. (2003). Movement speed effects on limb position drift. Experiment. Brain Res. 153, 266–274. doi: 10.1007/s00221-003-1601-7
Cadieux, M. L., Barnett-Cowan, M., and Shore, D. I. (2010). Crossing the hands is more confusing for females than males. Exp. Brain Res. 204, 431–446. doi: 10.1007/s00221-010-2268-5
Carey, M., Crucianelli, L., Preston, C., and Fotopoulou, A. (2019). The effect of visual capture towards subjective embodiment within the full body illusion. Sci. Rep. 9:2889. doi: 10.1038/s41598-019-39168-4
Collins, K. L., Russell, H. G., Schumacher, P. J., Robinson-Freeman, K. E., O’Conor, E. C., Gibney, K. D., et al. (2018). A review of current theories and treatments for phantom limb pain. J. Clin. Invest. 128, 2168–2176. doi: 10.1172/JCI94003
Corkin, S. (1968). Acquisition of motor skill after bilateral medial temporal-lobe excision. Neuropsychologia 6, 255–265. doi: 10.1016/0028-3932(68)90024-9
Corkin, S. (2002). What’s new with the amnesic patient H.M.? Nat. Rev. Neurosci. 3, 153–160. doi: 10.1038/NRN726
Craighero, L. (2014). The role of the motor system in cognitive functions. Routledge Handbook Embodied Cognit, 51–58. doi: 10.4324/9781315775845-14
Cressman, E. K., and Henriques, D. Y. P. (2009). Sensory recalibration of hand position following visuomotor adaptation. J. Neurophysiol. 102, 3505–3518. doi: 10.1152/jn.00514.2009
Cressman, E. K., and Henriques, D. Y. P. (2010). Reach adaptation and proprioceptive recalibration following exposure to misaligned sensory input. J. Neurophysiol. 103, 1888–1895. doi: 10.1152/jn.01002.2009
Cressman, E. K., and Henriques, D. Y. P. (2011). Motor adaptation and proprioceptive recalibration. Prog. Brain Res. 191, 91–99. doi: 10.1016/B978-0-444-53752-2.00011-4
Cressman, E. K., and Henriques, D. Y. P. (2015). Generalization patterns for reach adaptation and proprioceptive recalibration differ after visuomotor learning. J. Neurophysiol. 114, 354–365. doi: 10.1152/jn.00415.2014
Cressman, E. K., Salomonczyk, D., and Henriques, D. Y. P. (2010). Visuomotor adaptation and proprioceptive recalibration in older adults. Exp. Brain Res. 205, 533–544. doi: 10.1007/s00221-010-2392-2
Deconinck, F. J. A., Smorenburg, A. R. P., Benham, A., Ledebt, A., Feltham, M. G., and Savelsbergh, G. J. P. (2015). Reflections on mirror therapy: a systematic review of the effect of mirror visual feedback on the brain. Neurorehabil. Neural Repair 29, 349–361. doi: 10.1177/1545968314546134
Diers, M., Kamping, S., Kirsch, P., Rance, M., Bekrater-Bodmann, R., Foell, J., et al. (2015). Illusion-related brain activations: a new virtual reality mirror box system for use during functional magnetic resonance imaging. Brain Res. 1594, 173–182. doi: 10.1016/j.brainres.2014.11.001
Ding, L., Sun, Q., Jiang, N., He, J., and Jia, J. (2023). The instant effect of embodiment via mirror visual feedback on electroencephalogram-based brain connectivity changes: a pilot study. Front. Neurosci. 17:1138406. doi: 10.3389/fnins.2023.1138406
Dohle, C., Altschuler, E., and Ramachandran, V. S. (2019). Mirror therapy. Multisensory Percept, 449–461. doi: 10.1016/B978-0-12-812492-5.00020-6
Egsgaard, L. L., Petrini, L., Christoffersen, G., and Arendt-Nielsen, L. (2011). Cortical responses to the mirror box illusion: a high-resolution EEG study. Exp. Brain Res. 215, 345–357. doi: 10.1007/s00221-011-2902-x
Ernst, M. O., and Banks, M. S. (2002). Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415, 429–433. doi: 10.1038/415429A
Ernst, M. O., and Bülthoff, H. H. (2004). Merging the senses into a robust percept. Trends Cogn. Sci. 8, 162–169. doi: 10.1016/j.tics.2004.02.002
Eshkevari, E., Rieger, E., Longo, M. R., Haggard, P., and Treasure, J. (2012). Increased plasticity of the bodily self in eating disorders. Psychol. Med. 42, 819–828. doi: 10.1017/S0033291711002091
Ferri, F., Costantini, M., Salone, A., Di Iorio, G., Martinotti, G., Chiarelli, A., et al. (2014). Upcoming tactile events and body ownership in schizophrenia. Schizophr. Res. 152, 51–57. doi: 10.1016/J.SCHRES.2013.06.026
Fioriti, C. M., Martell, R. N., Daker, R. J., Malone, E. P., Sokolowski, H. M., Green, A. E., et al. (2024). Examining the interplay between the cognitive and emotional aspects of gender differences in spatial processing. J. Intelligence 12. doi: 10.3390/jintelligence12030030
Flannigan, J. C., Posthuma, R. J., Lombardo, J. N., Murray, C., and Cressman, E. K. (2018). Adaptation to proprioceptive targets following visuomotor adaptation. Exp. Brain Res. 236, 419–432. doi: 10.1007/s00221-017-5141-y
Fuchs, T., and Schlimme, J. E. (2009). Embodiment and psychopathology: a phenomenological perspective. Curr. Opin. Psychiatry 22, 570–575. doi: 10.1097/YCO.0b013e3283318e5c
Fusco, G., Ciccarone, S., Petrucci, M., Cozzani, B., Vercelli, G., Cotugno, A., et al. (2023). Altered processing of conflicting body representations in women with restrictive anorexia nervosa. Psychol. Res. 87, 1696–1709. doi: 10.1007/s00426-022-01788-3
Giroux, M., Barra, J., Barraud, P. A., Graff, C., and Guerraz, M. (2019). From embodiment of a point-light display in virtual reality to perception of One’s own movements. Neuroscience 416, 30–40. doi: 10.1016/j.neuroscience.2019.07.043
Griffen, T. C., Naumann, E., and Hildebrandt, T. (2018). Mirror exposure therapy for body image disturbances and eating disorders: a review. Clin. Psychol. Rev. 65, 163–174. doi: 10.1016/J.CPR.2018.08.006
Guedon, O., Gauthier, G., Vercher, J. L., Blouin, J., and Cole, J. (1998). Adaptation in visuomanual tracking depends on intact proprioception. J. Mot. Behav. 30, 234–248. doi: 10.1080/00222899809601339
Guerraz, M., Provost, S., Narison, R., Brugnon, A., Virolle, S., and Bresciani, J. P. (2012). Integration of visual and proprioceptive afferents in kinesthesia. Neuroscience 223, 258–268. doi: 10.1016/j.neuroscience.2012.07.059
Hadjiosif, A. M., Morehead, J. R., and Smith, M. A. (2023). A double dissociation between savings and long-term memory in motor learning. PLoS Biol. 21:e3001799. doi: 10.1371/JOURNAL.PBIO.3001799
Han, J., Waddington, G., Adams, R., Anson, J., and Liu, Y. (2016). Assessing proprioception: a critical review of methods. J. Sport Health Sci. 5, 80–90. doi: 10.1016/j.jshs.2014.10.004
Henriques, D. Y. P., and Cressman, E. K. (2012). Visuomotor adaptation and proprioceptive recalibration. J. Mot. Behav. 44, 435–444. doi: 10.1080/00222895.2012.659232
Holmes, N. P., Crozier, G., and Spence, C. (2004). When mirrors lie: “visual capture” of arm position impairs reaching performance. Cogn. Affect. Behav. Neurosci. 4, 193–200. doi: 10.3758/CABN.4.2.193
Holmes, N. P., and Spence, C. (2005). Visual bias of unseen hand position with a mirror: spatial and temporal factors. Exp. Brain Res. 166, 489–497. doi: 10.1007/S00221-005-2389-4
Keizer, A., Smeets, M. A. M., Postma, A., van Elburg, A., and Dijkerman, H. C. (2014). Does the experience of ownership over a rubber hand change body size perception in anorexia nervosa patients? Neuropsychologia 62, 26–37. doi: 10.1016/J.NEUROPSYCHOLOGIA.2014.07.003
Krakauer, J. W. (2009). Motor learning and consolidation: the case of visuomotor rotation. Adv. Exp. Med. Biol. 629, 405–421. doi: 10.1007/978-0-387-77064-2_21
Kundi, M. K., and Spence, N. J. (2023). Efficacy of mirror therapy on lower limb motor recovery, balance and gait in subacute and chronic stroke: a systematic review. Physiother. Res. Int. 28:e1997. doi: 10.1002/PRI.1997
Lajoie, Y., Paillard, J., Teasdale, N., Bard, C., Fleury, M., Forget, R., et al. (1992). Mirror drawing in a deafferented patient and normal subjects: visuoproprioceptive conflict. Neurology 42, 1104–1106. doi: 10.1212/WNL.42.5.1104
Leach, W. T., and Medina, J. (2022). Understanding components of embodiment: evidence from the mirror box illusion. Conscious. Cogn. 103:103373. doi: 10.1016/J.CONCOG.2022.103373
Lee, D., and Lee, G. (2019). Effect of afferent electrical stimulation with mirror therapy on motor function, balance, and gait in chronic stroke survivors: a randomized controlled trial. Eur. J. Phys. Rehabil. Med. 55, 442–449. doi: 10.23736/S1973-9087.19.05334-6
Lephart, S. M., Pincivero, D. M., Giraldo, J. L., and Fu, F. H. (1997). The role of proprioception in the management and rehabilitation of athletic injuries. Am. J. Sports Med. 25, 130–137. doi: 10.1177/036354659702500126
Linn, M. C., and Petersen, A. C. (1985). Emergence and characterization of sex differences in spatial ability: a meta-analysis. Child Dev. 56, 1479–1498. doi: 10.2307/1130467
Liu, Y., and Medina, J. (2017). Influence of the body schema on multisensory integration: evidence from the Mirror box illusion. Sci. Rep. 7:5060. doi: 10.1038/s41598-017-04797-0
Liu, Y., and Medina, J. (2018). Integrating multisensory information across external and motor-based frames of reference. Cognition 173, 75–86. doi: 10.1016/j.cognition.2018.01.005
Liu, Y., and Medina, J. (2021). Visuoproprioceptive conflict in hand position biases tactile localization on the hand surface. J. Exp. Psychol. Hum. Percept. Perform. 47, 344–356. doi: 10.1037/XHP0000893
Longo, M. R. (2022). Distortion of mental body representations. Trends Cogn. Sci. 26, 241–254. doi: 10.1016/j.tics.2021.11.005
Longo, M. R. (2023). Motor adaptation and distorted body representations. Trends Cogn. Sci. 27:9. doi: 10.1016/j.tics.2022.10.006
Longo, M. R., Schüür, F., Kammers, M. P. M., Tsakiris, M., and Haggard, P. (2008). What is embodiment? A psychometric approach. Cognition 107, 978–998. doi: 10.1016/j.cognition.2007.12.004
Maksimovic, S., and Cressman, E. K. (2018). Long-term retention of proprioceptive recalibration. Neuropsychologia 114, 65–76. doi: 10.1016/j.neuropsychologia.2018.04.009
Malighetti, C., Chirico, A., Serino, S., Cavedoni, S., Matamala-Gomez, M., Stramba-Badiale, C., et al. (2020). Manipulating body size distortions and negative body-related memories in patients with anorexia nervosa: a virtual reality-based pilot study. Ann. Rev. Cyber Therapy Telemed. 18, 177–181.
Malighetti, C., Sansoni, M., Gaudio, S., Matamala-Gomez, M., Di Lernia, D., Serino, S., et al. (2022). From virtual reality to regenerative virtual therapy: some insights from a systematic review exploring inner body perception in anorexia and bulimia nervosa. J. Clin. Med. 11. doi: 10.3390/jcm11237134
Matamala-Gomez, M., Maselli, A., Malighetti, C., Realdon, O., Mantovani, F., and Riva, G. (2021). Virtual body ownership illusions for mental health: a narrative review. J. Clin. Med. 10. doi: 10.3390/jcm10010139
Medina, J., Khurana, P., and Coslett, H. B. (2015). The influence of embodiment on multisensory integration using the mirror box illusion. Conscious. Cogn. 37, 71–82. doi: 10.1016/j.concog.2015.08.011
Miall, R. C., Afanasyeva, D., Cole, J. D., and Mason, P. (2021). The role of somatosensation in automatic visuo-motor control: a comparison of congenital and acquired sensory loss. Exp. Brain Res. 239, 2043–2061. doi: 10.1007/s00221-021-06110-y
Miall, R. C., and Cole, J. (2007). Evidence for stronger visuo-motor than visuo-proprioceptive conflict during mirror drawing performed by a deafferented subject and control subjects. Exp. Brain Res. 176, 432–439. doi: 10.1007/s00221-006-0626-0
Milner, B. (1998). “Brenda Milner” in The history of neuroscience in autobiography. ed. L. R. Squire (San Diego, CA, USA and London, UK: Academic Press, a division of Harcourt Brace & Company), 276–305.
Moon, K. M., Kim, J., Seong, Y., Suh, B. C., Kang, K. J., Choe, H. K., et al. (2021). Proprioception, the regulator of motor function. BMB Rep. 54, 393–402. doi: 10.5483/BMBRep.2021.54.8.052
Myers, J. B., Guskiewicz, K. M., Schneider, R. A., and Prentice, W. E. (1999). Proprioception and neuromuscular control of the shoulder after muscle fatigue. J. Athl. Train. 34, 362–367
Navas-León, S., Morales Márquez, L., Sánchez-Martín, M., Crucianelli, L., Bianchi-Berthouze, N., Borda-Mas, M., et al. (2023). Exploring multisensory integration of non-naturalistic sounds on body perception in young females with eating disorders symptomatology: a study protocol. J. Eat. Disord. 11:28. doi: 10.1186/s40337-023-00749-4
Navas-León, S., Tajadura-Jiménez, A., Motrico, E., Morales, L., Borda-Mas, M., Almeda, N., et al. (2024). Understanding and treating body image disturbances in eating disorders through body illusion interventions: a scoping review protocol. Syst. Rev. 13:65. doi: 10.1186/s13643-024-02458-8
Neville, K. M., and Cressman, E. K. (2018). The influence of awareness on explicit and implicit contributions to visuomotor adaptation over time. Exp. Brain Res. 236, 2047–2059. doi: 10.1007/s00221-018-5282-7
Ostry, D. J., Darainy, M., Mattar, A. A. G., Wong, J., and Gribble, P. L. (2010). Somatosensory plasticity and motor learning. J. Neurosci. 30, 5384–5393. doi: 10.1523/JNEUROSCI.4571-09.2010
Otsuru, N., Hashizume, A., Nakamura, D., Endo, Y., Inui, K., Kakigi, R., et al. (2014). Sensory incongruence leading to hand disownership modulates somatosensory cortical processing. Cortex 58, 1–8. doi: 10.1016/j.cortex.2014.05.005
Peled, A., Ritsner, M., Hirschmann, S., Geva, A. B., and Modai, I. (2000). Touch feel illusion in schizophrenic patients. Biol. Psychiatry 48, 1105–1108. doi: 10.1016/S0006-3223(00)00947-1
Qian, J., Wu, Y., Liu, F., Zhu, Y., Jin, H., Zhang, H., et al. (2022). An update on the prevalence of eating disorders in the general population: a systematic review and meta-analysis. Eat. Weight Disord. 27, 415–428. doi: 10.1007/s40519-021-01162-z
Ramachandran, V. S., Altschuler, E. L., and Hillyer, S. (1997). Mirror agnosia. Proc. R. Soc. B Biol. Sci. 264, 645–647. doi: 10.1098/rspb.1997.0091
Ramachandran, V. S., and Rodgers-Ramachandran, D. (1996). Synaesthesia in phantom limbs induced with mirrors. Proc. R. Soc. B Biol. Sci. 263, 377–386. doi: 10.1098/rspb.1996.0058
Ramachandran, V. S., Rogers-Ramachandran, D., and Cobb, S. (1995). Touching the phantom limb. Nature 377, 489–490. doi: 10.1038/377489a0
Redding, G. M., and Wallace, B. (2006). Prism adaptation and unilateral neglect: review and analysis. Neuropsychologia 44, 1–20. doi: 10.1016/j.neuropsychologia.2005.04.009
Riva, G., Malighetti, C., and Serino, S. (2021). Virtual reality in the treatment of eating disorders. Clin. Psychol. Psychother. 28, 477–488. doi: 10.1002/cpp.2622
Ruttle, J. E., Cressman, E. K., Hart, B. M., and Henriques, D. Y. P. (2016). Time course of reach adaptation and proprioceptive recalibration during visuomotor learning. PLoS One 11:e0163695. doi: 10.1371/journal.pone.0163695
Salomonczyk, D., Cressman, E. K., and Henriques, D. Y. P. (2011). Proprioceptive recalibration following prolonged training and increasing distortions in visuomotor adaptation. Neuropsychologia 49, 3053–3062. doi: 10.1016/j.neuropsychologia.2011.07.006
Schmalzl, L., Ragnö, C., and Ehrsson, H. H. (2013). An alternative to traditional mirror therapy: illusory touch can reduce phantom pain when illusory movement does not. Clin. J. Pain 29, e10–e18. doi: 10.1097/AJP.0B013E3182850573
Serino, S., and Dakanalis, A. (2017). Bodily illusions and weight-related disorders: clinical insights from experimental research. Ann. Phys. Rehabil. Med. 60, 217–219. doi: 10.1016/J.REHAB.2016.10.002
Shadmehr, R., Brandt, J., and Corkin, S. (1998). Time-dependent motor memory processes in amnesic subjects. J. Neurophysiol. 80, 1590–1597. doi: 10.1152/JN.1998.80.3.1590
Shadmehr, R., and Krakauer, J. W. (2008). A computational neuroanatomy for motor control. Exp. Brain Res. 185, 359–381. doi: 10.1007/s00221-008-1280-5
Shadmehr, R., and Mussa-Ivaldi, F. A. (1994). Adaptive representation of dynamics during learning of a motor task. J. Neurosci. 14, 3208–3224. doi: 10.1523/jneurosci.14-05-03208.1994
Stillman, B. C. (2002). Making sense of proprioception: the meaning of proprioception, kinaesthesia and related terms. Physiotherapy 88, 667–676. doi: 10.1016/S0031-9406(05)60109-5
Swanik, C. B., Lephart, S. M., and Rubash, H. E. (2004). Proprioception, kinesthesia, and balance after total knee arthroplasty with cruciate-retaining and posterior stabilized prostheses. J. Bone Joint Surg. Am. 86, 328–334. doi: 10.2106/00004623-200402000-00016
Swanik, K. A., Lephart, S. M., Swanik, C. B., Lephart, S. P., Stone, D. A., and Fu, F. H. (2002). The effects of shoulder plyometric training on proprioception and selected muscle performance characteristics. J. Shoulder Elb. Surg. 11, 579–586. doi: 10.1067/mse.2002.127303
Tekeoglu Tosun, A., Ipek, Y., Razak Ozdincler, A., and Saip, S. (2021). The efficiency of mirror therapy on drop foot in multiple sclerosis patients. Acta Neurol. Scand. 143, 545–553. doi: 10.1111/ane.13385
Thakkar, K. N., Nichols, H. S., McIntosh, L. G., and Park, S. (2011). Disturbances in body ownership in schizophrenia: evidence from the rubber hand illusion and case study of a spontaneous out-of-body experience. PLoS One 6:e27089. doi: 10.1371/JOURNAL.PONE.0027089
Thieme, H., Morkisch, N., Mehrholz, J., Pohl, M., Behrens, J., Borgetto, B., et al. (2018). Mirror therapy for improving motor function after stroke. Cochrane Database Syst. Rev. 2018:CD008449. doi: 10.1002/14651858.CD008449.pub3
Tibi, R., Eviatar, Z., and Karni, A. (2013). Fact retrieval and memory consolidation for a movement sequence: bidirectional effects of “unrelated” cognitive tasks on procedural memory. PLoS One 8:e80270. doi: 10.1371/JOURNAL.PONE.0080270
Tuthill, J. C., and Azim, E. (2018). Proprioception. Curr. Biol. 28, R194–R203. doi: 10.1016/j.cub.2018.01.064
Van Beers, R. J., Sittig, A. C., and Denier Van Der Gon, J. J. (1999). Integration of proprioceptive and visual position-information: an experimentally supported model. J. Neurophysiol. 81, 1355–1364. doi: 10.1152/jn.1999.81.3.1355
Varela, F. J., Thompson, E., Rosch, E., and Kabat-Zinn, J. (2016). The embodied mind: cognitive science and human experience. Embodied Mind Cognit. Sci. Hum. Exp. 1, 171–173. doi: 10.29173/CMPLCT8718
Vecchiato, G., Jelic, A., Tieri, G., Maglione, A. G., De Matteis, F., and Babiloni, F. (2015). Neurophysiological correlates of embodiment and motivational factors during the perception of virtual architectural environments. Cogn. Process. 16, 425–429. doi: 10.1007/s10339-015-0725-6
Viaud-Delmon, I., Ivanenko, Y. P., Berthoz, A., and Jouvent, R. (1998). Sex, lies and virtual reality. Nat. Neurosci. 1, 15–16. doi: 10.1038/215
Voges, C., Helmchen, C., Heide, W., and Sprenger, A. (2015). Ganzfeld stimulation or sleep enhance long term motor memory consolidation compared to normal viewing in saccadic adaptation paradigm. PLoS One 10:e0123831. doi: 10.1371/JOURNAL.PONE.0123831
Wann, J. P., and Ibrahim, S. F. (1992). Does limb proprioception drift? Exp. Brain Res. 91, 162–166. doi: 10.1007/BF00230024
Wijeyaratnam, D. O., Chua, R., and Cressman, E. K. (2022). Changes in movement control processes following Visuomotor adaptation. J. Mot. Behav. 54, 113–124. doi: 10.1080/00222895.2021.1921687
Keywords: multisensory integration, visuo-proprioceptive conflict, embodiment, mirror box illusion, mirror drawing, star-tracing, visuo-proprioceptive recalibration, visuomotor adaptation
Citation: de Melo AB, Landeira-Fernandez J and Krahe TE (2024) Women show enhanced proprioceptive target estimation through visual-proprioceptive conflict resolution. Front. Psychol. 15:1462934. doi: 10.3389/fpsyg.2024.1462934
Edited by:
Luigi F. Cuturi, University of Messina, ItalyReviewed by:
Silvia Zanchi, Italian Institute of Technology (IIT), ItalyAnna Maria Berti, University of Turin, Italy
Copyright © 2024 de Melo, Landeira-Fernandez and Krahe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Eichenberg Krahe, dGVrcmFoZUBwdWMtcmlvLmJy
 Anderson Barcelos de Melo
Anderson Barcelos de Melo Jesus Landeira-Fernandez
Jesus Landeira-Fernandez Thomas Eichenberg Krahe
Thomas Eichenberg Krahe