- 1Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, St. Louis, MO, United States
- 2Division of Urology, Department of Surgery, Washington University School of Medicine, St. Louis, MO, United States
- 3The Empowerment Network, St. Louis, MO, United States
Background: Many individuals undergoing cancer treatment experience substantial financial hardship, often referred to as financial toxicity (FT). Those undergoing prostate cancer treatment may experience FT and its impact can exacerbate disparate health outcomes. Localized prostate cancer treatment options include: radiation, surgery, and/or active surveillance. Quality of life tradeoffs and costs differ between treatment options. In this project, our aim was to quantify direct healthcare costs to support patients and clinicians as they discuss prostate cancer treatment options. We provide the transparent steps to estimate healthcare costs associated with treatment for localized prostate cancer among the privately insured population using a large claims dataset.
Methods: To quantify the costs associated with their prostate cancer treatment, we used data from the Truven Health Analytics MarketScan Commercial Claims and Encounters, including MarketScan Medicaid, and peer reviewed literature. Strategies to estimate costs included: (1) identifying the problem, (2) engaging a multidisciplinary team, (3) reviewing the literature and identifying the database, (4) identifying outcomes, (5) defining the cohort, and (6) designing the analytic plan. The costs consist of patient, clinician, and system/facility costs, at 1-year, 3-years, and 5-years following diagnosis.
Results: We outline our specific strategies to estimate costs, including: defining complex research questions, defining the study population, defining initial prostate cancer treatment, linking facility and provider level related costs, and developing a shared understanding of definitions on our research team.
Discussion and next steps: Analyses are underway. We plan to include these costs in a prostate cancer patient decision aid alongside other clinical tradeoffs.
Introduction
“Financial toxicity” (FT) is the personal financial burden faced by those undergoing cancer treatment, specifically the harms associated with this burden (Yousuf, 2016). Any individual with cancer may experience FT, including those with prostate cancer. In the US, prostate cancer is the most commonly diagnosed cancer in men and the second leading cause of cancer-specific mortality (Siegel et al., 2022). For patients with localized prostate cancer, the type of treatment they choose contributes to their susceptibility to FT, with radiation and surgery often having greater direct costs, financial burden, and variability over time (Imber et al., 2020; Stone et al., 2021). Patients experiencing FT are more likely to report nonadherence to medication, inability to afford prescription drugs, and forgoing mental health services, doctor’s visits, and medical tests (Knight et al., 2018). FT is associated with disparate health outcomes and lower quality of life (Yousuf, 2016).
Survival is similar for non-metastatic, localized prostate cancer across treatment options (i.e., radiation, surgery, active surveillance), but patients must weigh quality of life tradeoffs (e.g., distress, urinary incontinence, erectile dysfunction) during this preference-sensitive decision (Bill-Axelson et al., 2014; Lamers et al., 2017; Sanda et al., 2018). Providing cost estimates of the cost burden associated with different prostate cancer treatment pathways alongside clinical tradeoffs can support this decision; cost can be a substantial quality-of-life tradeoff that is often not discussed or not precisely known to patients during decision-making (Politi et al., 2021). There is a growing call regarding the importance of including direct and indirect cost information in shared-decision making conversations for prostate cancer as nonmetastatic treatment outcomes are generally similar and costs can help inform patients as they weigh their options (Ubel et al., 2013; Politi et al., 2023). Direct costs include insurance related fees (e.g., co-pays, co-insurance) and indirect costs include the often unforeseen costs (e.g., loss of work, absenteeism, presenteeism). Even with interest from patients and clinicians, cost conversations can be difficult to navigate(Kelly et al., 2015) due to the multidimensional nature of costs, impacting material, behavioral, and psychosocial domains (Tucker-Seeley and Thorpe, 2019). Discussing cost burden with patients upfront can enable patients to consider potential tradeoffs, seek financial assistance early on in their care, and thus potentially reduce future costs and the burden of care (Ubel et al., 2013; George et al., 2021).
In this paper, we aimed to quantify direct care costs and the associated financial burden for patients aged 18–63 years diagnosed with localized prostate cancer as the first step. We plan to incorporate this data into shared decision making materials and support patients as they consider which treatment option is right for them. Cost information will help patients consider both side effects and financial burden when they make decisions about their treatment among different treatment options. In this paper, we outline the steps involved in estimating direct costs following a prostate cancer diagnosis using insurance claims data. This report outlines lessons learned and recommendations for other researchers conducting similar analyses.
Methods
Step 1: identifying the problem
This research question arose from an existing project, evaluating a prostate cancer treatment decision aid that includes relative cost information led by a member of the research team (Politi et al., 2021). Formative interviews identified a gap in cost information for those making decisions about prostate cancer treatment options and their clinicians. Clinicians wanted to know more about these costs and patients and caregivers wanted to share more about the impact of immediate and downstream direct and indirect costs on their life (Politi et al., n.d.). Consequently, our research question was informed by the clinical, research, community and patient partners engaged in this formative work. Our research team prioritized engaging with these partners throughout the duration of this project. We knew this complex problem would also require the expertise of a multidisciplinary team as it spans patient care, clinical decision making, patient-centered communication, and economic evaluation.
Step 2: engaging a multidisciplinary team
To develop a multidisciplinary collaborative team, the team met to discuss the research question and identify a potential funding source prior to approaching other team members. We invited a community collaborator and leader of a prostate cancer community-based organization to join our core research team and engage with local and regional community partners to incorporate their perspectives on costs and their impact on patients. We also engaged an urologist with clinical expertise on prostate cancer treatment, a health economist with expertise in cost analyses using administrative claims data, and a community-engaged researcher and leader of a local cancer center. We identified the need for expertise in these specific disciplines because of the complexities of calculating costs incorporating the clinical, economic, and community perspectives. To ensure our questions were clinically relevant and our operational definitions were accurate, a practicing urologic surgeon scientist helped generate and review the treatment definitions, billing and procedure codes, and define the clinical context. The health economist with expertise in estimating patient direct costs and large claims databases has worked extensively with data scientists on the institutional informatics team to oversee the analyses. The community-engaged researchers with expertise in health disparities provided important perspectives on the disproportionate experiences of FT by those from socially, economically, and racially marginalized groups. A postdoctoral trainee with expertise in cancer survivorship to support focusing on the impact of cost upfront and through survivorship over time. With this team of content and research experts, we also identified a research coordinator with extensive experience in clinical decision support informed by billing and procedure codes to oversee the administrative aspects of this project.
Step 3: reviewing the literature and identifying the database
Through engaging our multidisciplinary team, we identified, reviewed, and selected the codes to extract, with this process occurring over multiple phases. First, the research team reviewed existing literature to identify procedure and billing codes. This involved reviewing peer reviewed literature and guidelines. Second, we reviewed the procedure codes included in the Fair Health Consumer1 prostate cancer shared decision making cost tool. Third, we reviewed the National Library of Medicine’s Value Set Authority Center (VSAC; https://vsac.nlm.nih.gov/) and the Unified Medical Language System (UMLS; https://www.nlm.nih.gov/research/umls/index.html). The research team compiled these resources, reviewed them together, and confirmed the procedure set we would use in this project. Our clinical team member, a practicing urologic surgeon, led iterative review and selection of procedure codes and discussed with clinical partners, including a radiation oncologist with experience working in claims data, when there were uncertainties about which to include. Our final code set is included in Table 1.
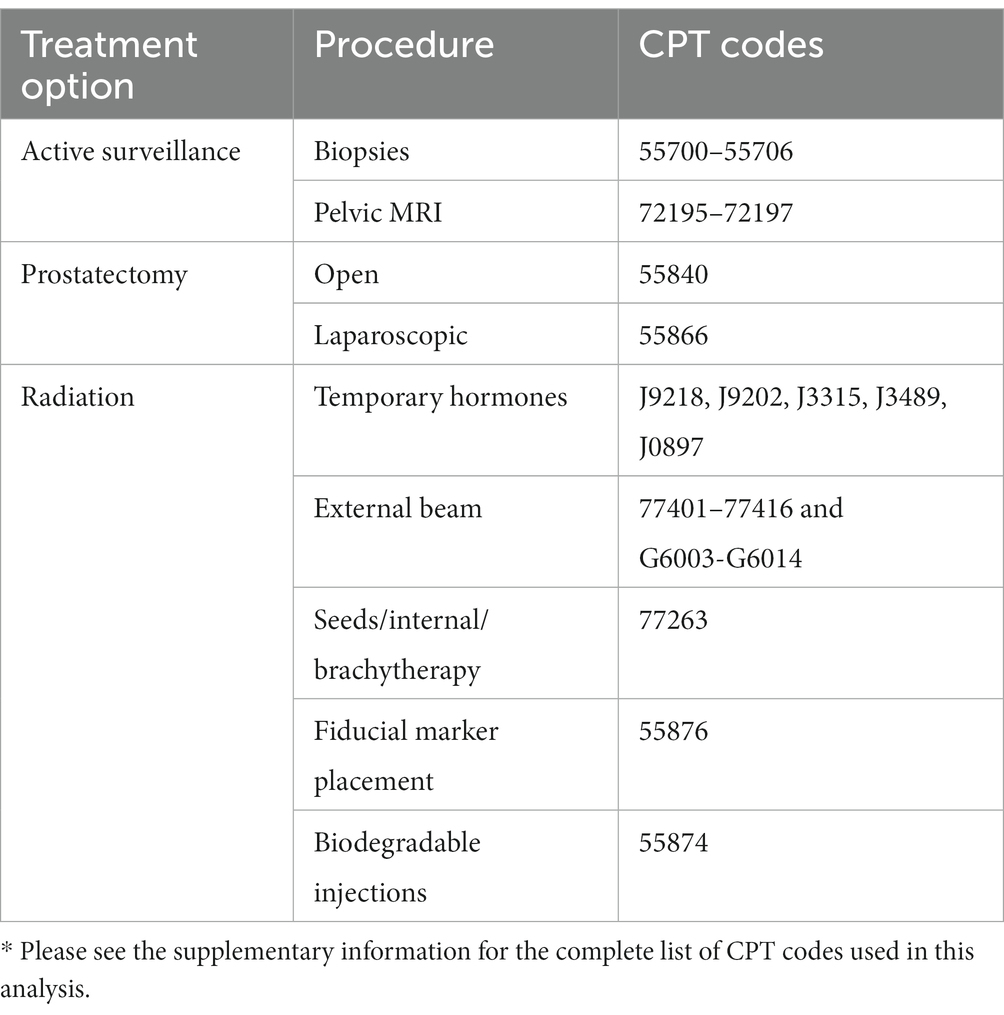
Table 1. Summarized list of treatment options, procedures, and CPT codes for the 3 treatment types.*
Based on this review, our research team decided to use data from the Truven Health Analytics MarketScan Commercial Claims and Encounters, including MarketScan Medicaid, (MarketScan). We selected MarketScan because of the inclusion of variables needed for our research question and to conduct analyses, national representativeness of a privately insured population, extant literature using MarketScan for similar analyses on financial burden in cancer survivors, and the availability and expertise within our institution. While the median age for prostate cancer is 66 years, over 170 million people under 65 years are covered by private health insurance (National Health Statistics Reports, 2021). Specifically, there were 224,733 new prostate cancer cases diagnosed in the US in 2019, and 37% of those cases were among men aged 45–64 years (Prostate Cancer Incidence by Stage at Diagnosis, 2023). These cost estimates will be relevant to this large group of people. Individuals with private insurance often spend more on care, have more medical debt, and report that costs impact care access (Wray et al., 2021). Those under 65 years are often exposed to more variable costs and cost estimates may be particularly relevant to this population.
Step 4: identifying outcomes
Based on the findings from our initial work and literature review, the research team identified that treatment-related costs can occur over time, and a single time point would be unable to capture the costs across a trajectory of prostate cancer care. Thus, we quantified these costs cumulatively at 1 year, 3 years, and 5 years. Estimating costs at multiple time points would provide a better estimate of patient costs over time (Eldefrawy et al., 2013; Gustavsen et al., 2020). Using the MarketScan database for data extraction, we created an analyzable dataset to estimate the patient, clinician, and system/facility costs. Initially, our goal was to estimate these costs for patients with localized prostate cancer. Ideally, localized prostate cancer would be defined by Gleason, PSA, or tumor staging data, but these variables are not available in the MarketScan, despite the many strengths that prompted us to choose to use this database. Considering this limitation, we chose to use the metastatic vs. non-metastatic variable to define our cohort of interest.
Step 5: defining the cohort
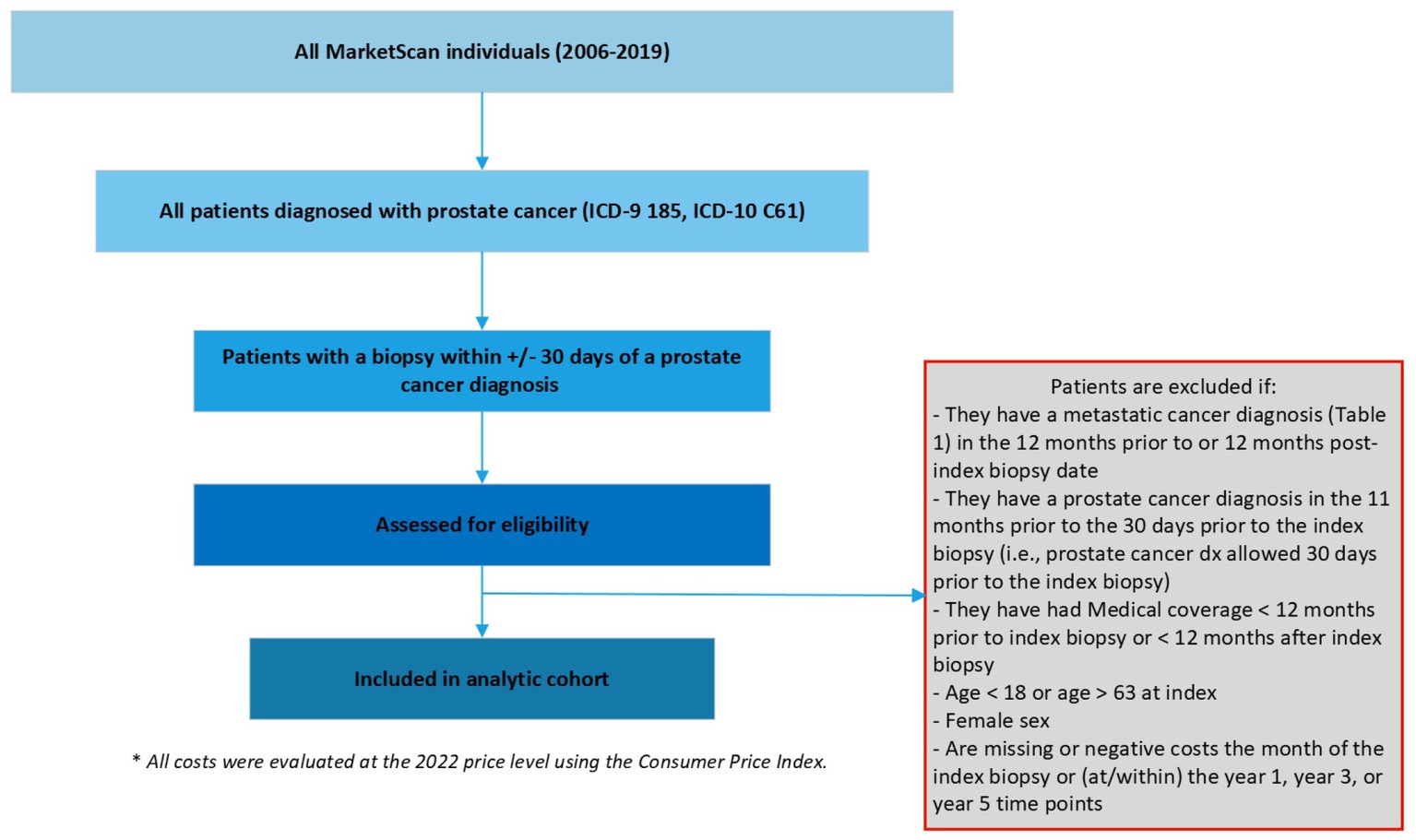
We defined localized prostate cancer as being diagnosed with prostate cancer and the absence of metastatic diseases using International Classification of Diseases, Ninth/Tenth Revision, Clinical Modification (ICD-9/10-CM). We first included patients with at least 2 outpatient codes at least 30-days apart or one inpatient prostate cancer diagnosis (ICD-9185, ICD-10 C61) between 2006 and 2019 (the most updated data at the time of study). Among these patients, the date of diagnosis (index date hereafter) was defined as the date of the first biopsy within +/− 30 days of a prostate cancer diagnosis, as biopsy is needed to determine a diagnosis and the dates of biopsy and diagnosis may lag administratively. Patients without a date of diagnosis were excluded. Additional exclusion criterion included patients: (1) with a secondary cancer diagnosis other than prostate cancer, (2) with a metastatic cancer diagnosis in the 12-months prior to or post the index date, (3) with a prostate cancer diagnosis in the 11-months prior to the index date, (4) with medical coverage <12-months prior to index date or < X-year after index date since this indicates incomplete cost data, where X = 1, 3, or 5 (i.e., the duration of the target cumulative cost of interest), (5) age < 18 years or age > 63 years at index (for the concern of incomplete data due to Medicare eligibility), (6) female sex, and (7) missing or negative costs within the duration of the target cost due to administrative data entry errors (see Figure 1).
Defining the treatment groups
We defined a patient’s initial treatment decision as the first treatment codes present within the 12-months following their index biopsy code. We categorized patients into 3 groups based on their initial treatment choice following diagnosis: active surveillance, surgery, and/or radiation using the Current Procedural Terminology (CPT) codes (see Table 1). Surgery included laparoscopic and open prostatectomy. Radiation included external beam and seeds/internal/brachytherapy. Active surveillance was defined as having no surgery or radiation codes within 12-months of the index date. Specifically, within 12-months following diagnosis, if a patient did not have treatment codes for either surgery or radiation, the patient was considered to have selected active surveillance. We estimated the cost associated with this treatment and all follow-up costs within the 5-year period, including other potential treatments (i.e., surgery, radiation). This approach captures all treatment related costs associated with their initial treatment decision.
Step 6: designing the analytic plan
The analytic plan was finalized as the research team refined our research question and defined our variables. The analytic plan was an iterative process and refined as the team identified the data available in the database, the variables of interest, and our overall research objectives. Our team decided we would quantify total costs at 1, 3, 5 years following diagnosis and aggregate these costs across those time points. Total costs were evaluated from the healthcare sector’s perspective, including patients’ out-of-pocket cost and cost paid by third party payers. Costs pooling from different years were evaluated at the 2022 price level using the Consumer Price Index Medical Care Component (Consumer Price Index (CPI), 2023). For unadjusted analysis, we plan to average costs across patients receiving each treatment option to calculate mean costs for each treatment pathway. For adjusted analyses, the distribution of the total costs at different years will be visually examined. Appropriate statistical analyses will be determined and performed using these total costs as the outcome variables with covariates including patient characteristics (e.g., age, comorbidities), insurance types, geographical region where they received treatments, and treatments that they received. Total costs by initial treatment decision will be predicted based on the estimated regression at the three time periods based on patient characteristics, insurance type, geographical region, and a combination of treatment options.
Results
Lessons learned and strategies
A summary of lessons learned, strategies, and examples is described below in detail and summarized in Table 2.
Complex research questions across multiple disciplines
Our team met frequently and worked together to translate our research questions across discipline specific language, including across oncology, urology, public health, economics, data science, decision science, psychology, occupational therapy, and community engaged research. Initially our meetings were 60-min every other week, but we increased the frequency to meeting for 30-min twice a week. While this increased frequency can be demanding to the research team members, we found as the momentum of our project started to increase, we needed rapid feedback and to update the team on progress. We did cancel meetings if they were not needed and corresponded over email to update the team. We also shared detailed meeting minutes to keep all team members apprised of updates. Through our frequent, brief meetings, we refined our analytic plan, and we were able to ask questions in real time to address and translate discipline specific jargon and assumptions, and ultimately agree on our analytic process. We then created a draft analysis plan to circulate with the research team to elicit additional input from our team members. Through this process, we incorporated scientific, medical, and community perspectives to refine our questions and define a clinically meaningful cohort within the larger dataset. This is an ongoing process as new information emerges or challenges arise, yet our goal is to identify these issues early and often so we can address them in a way that aligns with the research question, data, science, clinical relevance, and patient experiences.
Defining the cohort
Due to the aforementioned limitations of unavailable data to define localized prostate cancer in the MarketScan database, we worked with our team to identify which metrics/measures exist and how to feasibly extract them from the database. Through this process, our team elected to use metastatic vs. non-metastatic cancer diagnosis to help define localized prostate cancer. This distinction comes with additional considerations, including how to identify the non-metastatic cohort and when to exclude metastatic cases (e.g., upfront, at a certain period of time). We selected the time parameters to provide the framework needed to verify the confirmed prostate cancer records. Primarily, we determined that the date of a patient’s initial biopsy would serve as the index date. 12-months before the prostate cancer index date no ICD codes for prostate cancer beyond 30-days from the index date and if both biopsy and diagnostic codes are present within 1-month, we will consider this patient to be diagnosed with prostate cancer. Limitations to this approach include those patients who may have had a biopsy outside of what was captured in the MarketScan database, but the research team evaluated the tradeoffs between a smaller sample size and a well-defined patient cohort (patients diagnosed with non-metastatic prostate cancer) and erred on the side of caution to include confirmed prostate cancer records in our analysis.
Identifying procedure, billing codes, and patient treatment decision making
Our team reviewed existing databases to cross-reference resources for ICD-9/10 and CPT code identification. This included FairHealth, and the National Library of Medicine’s Value Set Authority Center (VSAC) and the Unified Medical Language System (UMLS). Specifically, we needed to define what the operational definition would be for active surveillance as it is the absence of a discrete treatment event, rather a cluster of treatment events over time. At what time point can we determine the patient has chosen active surveillance? Our research team defined selecting active surveillance as a prostate cancer diagnosis and the absence of surgery or radiation related codes within the 12-months post index diagnosis (i.e., first biopsy). Our team decided this timeframe was a clinically meaningful timeframe in which you would expect a patient to initiate and commence their initial treatment plan.
Defining treatment decisions
For those categorized as selecting active surveillance, if the patient transitioned to another treatment type as defined by the presence of treatment-related codes (i.e., surgery, radiation; Table 1) after 12-months, we included these patient records since we are interested in capturing all treatment related costs associated with their initial treatment decision. This approach allowed us to estimate the overall costs (initial costs and follow-up costs) associated with following treatment paths: (1) first electing active surveillance treatment (at 1-year, 3-years, and 5-years), (2) first electing surgery treatment (at 1-year, 3-years, and 5-years), and (3) first electing radiation treatment (at 1-year, 3-years, and 5-years).
Linking facility and provider level related costs using treatment variables
Using CPT codes to identify the procedures lead to a discrepancy between the number of people with facility costs versus the number with provider costs. To avoid a systematic missing of facility costs, our research team considered: (1) either providing the ICD-9/10 procedure codes for treatments that would logically be done during an inpatient admission (especially the surgical procedures) or (2) linking facility claims to provider surgery claims based on dates (+/− 1 day). We decided to use approach 2 because each case would likely vary and approach 2 would be more inclusive of all associated costs.
Shared understanding of definitions and documentation of research questions
One of our team’s main challenges has been reaching a shared understanding of the definitions and criteria for our analytic plan. We have adopted several strategies to help enhance communication and achieve consensus among our multi-disciplinary team. Primarily, we have conducted frequent, short meetings to ensure that all team members are updated and to create space to troubleshoot issues and misconceptions. In addition, we have created a centralized location for all files and realized the importance of regularly updating documents and operational definitions that are iteratively adjusted. We have also identified the importance of sending team-wide email updates after any modification is made to the analytic plan, cohort definitions, or inclusion and exclusion criteria. Not all team members are able to be present at each meeting, so regular email updates have also been a critical method for communicating changes and maintaining consensus.
Discussion and next steps
Cost analyses are currently underway. We will be estimating total costs at 1-year, 3-years, and 5-years following diagnosis. In parallel, we are conducting semi-structured interviews among Black prostate cancer survivors and their caregivers to characterize the role of direct and indirect costs during their prostate cancer treatment through lived experiences. We are planning to include these direct and indirect costs in a prostate cancer patient decision aid and test this decision aid among patients with localized prostate cancer.
Limitations
This research approach is not without limitations. First, active surveillance and watchful waiting are very different treatment types philosophically and in practice, but it is challenging to differentiate these approaches using claims data as the billing records may appear to be the same. Therefore, our multidisciplinary research team agreed upon using 12-months as the timeframe to suggest a patients’ selection of active surveillance. However, this could include some watchful waiting patients, which has the potential to artificially lower cost estimates. Additionally, MarketScan data does not include data on PSA, Gleason score, or tumor staging data. While this limited our ability to define low risk prostate cancer, the overall goal of the analysis is to better understand treatment-specific costs. Selecting a prostate cancer treatment pathway is a preference-sensitive decision, and therefore it is still important to include this information. MarketScan only includes claims data for those who are insured (including those eligible for Medicaid and Medicare), precluding those who are uninsured from our analysis. Together with a lack of race or ethnicity data, our analysis is not able to consider health equity. To address this, our larger research project includes a second aim where we will conduct qualitative interviews with Black men with prostate cancer to identify and further explore the direct and indirect costs associated with their treatment. We are also continually learning new information about our approach and analysis and identifying challenges. With new information and challenges, we will make informed decisions for how to proceed with the input of our team. Thus, our final analytic plan will be reported at the end of this research project. Our goal for this paper is to rapidly translate our methods and strategies to other researchers grappling with similar questions in an effort to facilitate academic discourse and increase transparency.
Conclusions
Leveraging the expertise of a multidisciplinary team can help to identify the essential factors needed to estimate patient-related costs. These are complex research questions that evolve iteratively as additional information is uncovered through identifying the variables and clarifying the analytic plan. As we finalize our decision aid with cost information from this work, we will engage with clinical, patient, caregiver, community and decision science partners to review the presentation of information and identify supports needed to implement in routine care. We will prioritize recruiting from socially and economically marginalized populations to evaluate how the inclusion of costs may support decision making because of the disproportionate financial burden experiences by these populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AH: conceptualization, methodology, investigation, writing (original draft, review, editing), resources, supervision, and funding acquisition. HR: investigation, writing (original draft, review, editing), and visualization. S-HC: conceptualization, methodology, investigation, writing (review, editing), supervision, and funding acquisition. AL’H: investigation, writing (review, editing). EK and MP: conceptualization, methodology, writing (review, editing), and funding acquisition. BD and RW-J: conceptualization, writing (review, editing), and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This project was funded, in part, by The Implementation Science Centers in Cancer Control (ISC3; Beau Biden Cancer Moonshot Initiative, NCI P50 CA244431), Washington University and Siteman Cancer Center Institutional matching funds, and formative research was funded by Robert Wood Johnson Foundation grant number 77292.
Acknowledgments
We would like to thank Mellve A. Shahid, Sr. and The Empowerment Network, Inc. (TEN) for their partnership.
Conflict of interest
RW-J was employed by The Empowerment Network Inc., United States. MP was a consultant for UCB Biopharma in 2022 on a topic unrelated to this manuscript.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other funding agencies.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1176843/full#supplementary-material
Footnotes
References
Bill-Axelson, A., Holmberg, L., Garmo, H., Rider, J. R., Taari, K., Busch, C., et al. (2014). Radical prostatectomy or watchful waiting in early prostate Cancer. N. Engl. J. Med. 370, 932–942. doi: 10.1056/NEJMoa1311593
Consumer Price Index (CPI). Databases: U.S. Bureau of Labor Statistics (2023). Available at: https://www.bls.gov/cpi/data.htm
Eldefrawy, A., Katkoori, D., Abramowitz, M., Soloway, M. S., and Manoharan, M. (2013). Active surveillance vs. treatment for low-risk prostate cancer: a cost comparison. Urol. Oncol. 31, 576–580. doi: 10.1016/j.urolonc.2011.04.005
George, N., Grant, R., James, A., Mir, N., and Politi, M. C. (2021). Burden associated with selecting and using health insurance to manage care costs: results of a qualitative study of nonelderly Cancer survivors. Med Care Res Rev MCRR. 78, 48–56. doi: 10.1177/1077558718820232
Gustavsen, G., Gullet, L., Cole, D., Lewine, N., and Bishoff, J. T. (2020). Economic burden of illness associated with localized prostate cancer in the United States. Future Oncol Lond Engl. 16, 4265–4277. doi: 10.2217/fon-2019-0639
Imber, B. S., Varghese, M., Ehdaie, B., and Gorovets, D. (2020). Financial toxicity associated with treatment of localized prostate cancer. Nat. Rev. Urol. 17, 28–40. doi: 10.1038/s41585-019-0258-3
Kelly, R. J., Forde, P. M., Elnahal, S. M., Forastiere, A. A., Rosner, G. L., and Smith, T. J. (2015). Patients and physicians can discuss costs of Cancer treatment in the clinic. J. Oncol. Pract. 11, 308–312. doi: 10.1200/JOP.2015.003780
Knight, T. G., Deal, A. M., Dusetzina, S. B., Muss, H. B., Choi, S. K., Bensen, J. T., et al. (2018). Financial toxicity in adults with Cancer: adverse outcomes and noncompliance. J. Oncol. Pract. 14, e665–e673. doi: 10.1200/JOP.18.00120
Lamers, R. E. D., Cuypers, M., de Vries, M., van de Poll-Franse, L. V., Ruud Bosch, J. L. H., and Kil, P. J. M. (2017). How do patients choose between active surveillance, radical prostatectomy, and radiotherapy? The effect of a preference-sensitive decision aid on treatment decision making for localized prostate cancer. Urol Oncol Semin Orig Investig. 35, 37.e9–37.e17. doi: 10.1016/j.urolonc.2016.09.007
National Health Statistics Reports (2021). Number 159, 2021;(159). Available at: https://www.cdc.gov/nchs/data/nhsr/nhsr159-508.pdf
Politi, M. C., Forcino, R. C., Parrish, K., Durand, M. A., O’Malley, A. J., and Elwyn, G. (2021). Cost talk: protocol for a stepped-wedge cluster randomized trial of an intervention helping patients and urologic surgeons discuss costs of care for slow-growing prostate cancer during shared decision-making. Trials 22:422. doi: 10.1186/s13063-021-05369-4
Politi, M. C., Forcino, R. C., Parrish, K., Durand, M. A., O’Malley, A. J., Moses, R., et al. (n.d.). The impact of adding cost information to a conversation aid to support shared decision making about low-risk prostate cancer treatment: results of a stepped-wedge cluster randomized trial. Health Expect.
Politi, M. C., Housten, A. J., Forcino, R. C., Jansen, J., and Elwyn, G. (2023). Discussing cost and value in patient decision aids and shared decision making: a call to action. MDM Policy Pract. 8:238146832211486. doi: 10.1177/23814683221148651
Prostate Cancer Incidence by Stage at Diagnosis. United States—2001−2019 | CDC [Internet] (2023) cited 2023 May 31. Available at: https://www.cdc.gov/cancer/uscs/about/data-briefs/no34-prostate-cancer-incidence-2001-2019.htm
Sanda, M. G., Cadeddu, J. A., Kirkby, E., Chen, R. C., Crispino, T., Fontanarosa, J., et al. (2018). Clinically localized prostate Cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J. Urol. 199, 990–997. doi: 10.1016/j.juro.2018.01.002
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33. doi: 10.3322/caac.21708
Stone, B. V., Laviana, A. A., Luckenbaugh, A. N., Huang, L. C., Zhao, Z., Koyama, T., et al. (2021). Patient-reported financial toxicity associated with contemporary treatment for localized prostate Cancer. J. Urol. 205, 761–768. doi: 10.1097/JU.0000000000001423
Tucker-Seeley, R. D., and Thorpe, R. J. (2019). Material-psychosocial-behavioral aspects of financial hardship: a conceptual model for Cancer prevention. The Gerontologist 59, S88–S93. doi: 10.1093/geront/gnz033
Ubel, P. A., Abernethy, A. P., and Zafar, S. Y. (2013). Full disclosure — out-of-pocket costs as side effects. N. Engl. J. Med. 369, 1484–1486. doi: 10.1056/NEJMp1306826
Wray, C. M., Khare, M., and Keyhani, S. (2021). Access to care, cost of care, and satisfaction with care among adults with private and public health insurance in the US. JAMA Netw. Open 4:e2110275. doi: 10.1001/jamanetworkopen.2021.10275
Keywords: prostate cancer, financial toxicity, cost estimates, multidisciplinary collaboration, shared decision making (SDM), quality of life
Citation: Housten AJ, Rice HE, Chang S-H, L'Hotta AJ, Kim EH, Drake BF, Wright-Jones R and Politi MC (2023) Financial burden of men with localized prostate cancer: a process paper. Front. Psychol. 14:1176843. doi: 10.3389/fpsyg.2023.1176843
Edited by:
Salene M. W. Jones, Fred Hutchinson Cancer Research Center, United StatesReviewed by:
Aaron Falchook, Memorial Healthcare System, United StatesGil Bar-Sela, Technion Israel Institute of Technology, Israel
Copyright © 2023 Housten, Rice, Chang, L’Hotta, Kim, Drake, Wright-Jones and Politi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashley J. Housten, YWhvdXN0ZW5Ad3VzdGwuZWR1
 Ashley J. Housten
Ashley J. Housten Hannah E. Rice
Hannah E. Rice Su-Hsin Chang1
Su-Hsin Chang1 Allison J. L'Hotta
Allison J. L'Hotta Bettina F. Drake
Bettina F. Drake