- 1Department of Psychiatry, Wuhan Mental Health Center, Wuhan, Hubei, China
- 2Department of Psychiatry, Wuhan Hospital for Psychotherapy, Wuhan, Hubei, China
- 3Psychiatric Rehabilitation Department, Wuhan Mental Health Center, Wuhan, Hubei, China
- 4Psychiatric Rehabilitation Department, Wuhan Hospital for Psychotherapy, Wuhan, Hubei, China
- 5Department of Psychiatry, Suizhou Hospital, Hubei University of Medicine, Suizhou, Hubei, China
Objective: Major depressive disorder (MDD) is associated with suicidal attempts (SAs) among adolescents, with suicide being the most common cause of mortality in this age group. This study explored the predictive utility of support vector machine (SVM)-based analyses of amplitude of low-frequency fluctuation (ALFF) results as a neuroimaging biomarker for aiding the diagnosis of MDD with SA in adolescents.
Methods: Resting-state functional magnetic resonance imaging (rs-fMRI) analyses of 71 first-episode, drug-naive adolescent MDD patients with SA and 54 healthy control individuals were conducted. ALFF and SVM methods were used to analyze the imaging data.
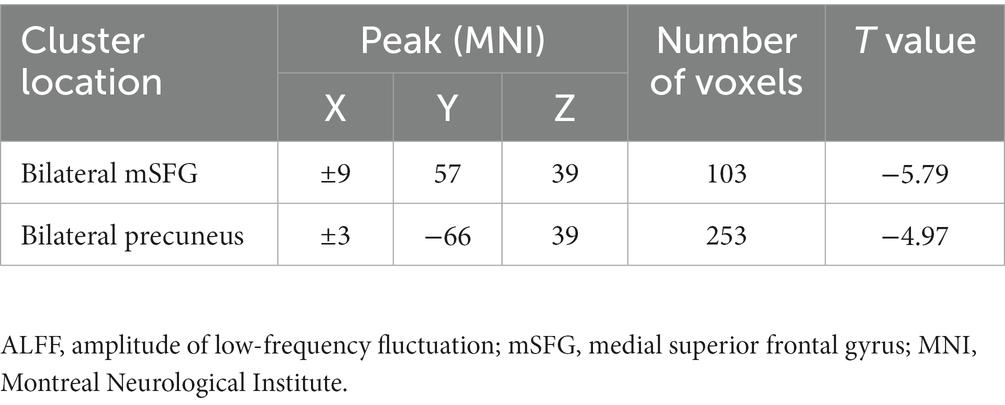
Results: Relative to healthy control individuals, adolescent MDD patients with a history of SAs showed reduced ALFF values in the bilateral medial superior frontal gyrus (mSFG) and bilateral precuneus. These lower ALFF values were also negatively correlated with child depression inventory (CDI) scores while reduced bilateral precuneus ALFF values were negatively correlated with Suicidal Ideation Questionnaire Junior (SIQ-JR) scores. SVM analyses showed that reduced ALFF values in the bilateral mSFG and bilateral precuneus had diagnostic accuracy levels of 76.8% (96/125) and 82.4% (103/125), respectively.
Conclusion: Adolescent MDD patients with a history of SA exhibited abnormal ALFF. The identified abnormalities in specific brain regions may be involved in the pathogenesis of this condition and may help identify at-risk adolescents. Specifically, reductions in the ALFF in the bilateral mSFG and bilateral precuneus may be indicative of MDD and SA in adolescent patients.
1. Introduction
Major depressive disorder (MDD) is an extremely debilitating neuropsychiatric disease that causes characteristic and often severe emotional dysregulation (Wu et al., 2020). MDD is strongly associated with age and adolescents in the United States exhibit lifetime and 12-month MDD prevalence rates of 11 and 7.5%, respectively (Avenevoli et al., 2015). Adolescents suffering from MDD are also at risk of a range of other comorbid psychiatric conditions, suicide attempts (SAs; Doruk Camsari et al., 2019), substance abuse, and reduced social skills (Nardi et al., 2013); thus, the disease is a major focus of clinical psychiatry and public health-focused research throughout the world (Chen et al., 2020). MDD is the most common disorder present in adolescents that commit or attempt suicide (Nock et al., 2013), with suicide remaining the second most common driver of mortality among individuals 10–19 years of age (Breslin et al., 2020). Despite the severe toll that adolescent MDD can have on patients and those around them, the neurophysiological basis for this condition is not completely understood.
A range of magnetic resonance imaging (MRI) strategies have been used to assess patients with MDD. These include functional MRI (fMRI), magnetic resonance spectroscopy, structural MRI, and diffusion tensor imaging (Gao et al., 2021). Of these, fMRI offers value as a safe, noninvasive, reproducible tool that can aid in diagnosing disease through the assessment of minute shifts in blood oxygenation-level-dependent (BLOD) MRI signaling linked to brain activity (Gore, 2003). fMRI includes both resting-state (rs-fMRI) and task-based formats (Gao et al., 2021), with rs-fMRI performed in a quiet setting while subjects have their eyes closed and are not performing any tasks. Research by Biswal et al. suggests that the spontaneous resting-state BLOD signals observed under these conditions are reflective of basal neuronal activity (Biswal et al., 1995). Accordingly, rs-fMRI offers value as a means of examining spontaneous brain function and detecting any abnormalities or aberrant functional connectivity within the central nervous system (Biswal et al., 1995; Biswal, 2012). Unlike task-based fMRI analyses, rs-fMRI permits the evaluation of individuals not performing complex tasks and is thus better suited to the assessment of individuals unable to complete cognitively demanding tasks due to neurological or psychiatric disorders (Takamura and Hanakawa, 2017). For these reasons, fMRI had been used to study patients with a range of diseases and disorders, including depression (Guo et al., 2022), bipolar disorder (Vargas et al., 2013), schizophrenia (Whitfield-Gabrieli et al., 2009), epilepsy (Gao et al., 2022b), attention deficit hyperactivity disorder, abnormal brain development (Jolles et al., 2011), migraine (Wang et al., 2022), mild cognitive impairment (Gao et al., 2022c), and Parkinson’s Disease (Mi et al., 2021).
The two parameters that are most commonly calculated from BLOD signals recorded during rs-fMRI analyses include the amplitude of low-frequency fluctuation (ALFF) and functional connectivity (FC) (Zhang et al., 2014). Of these, ALFF is an indicator of the intensity of spontaneous local neuronal activity under these basal conditions. Shifts in spontaneous brain activity are assessed by fast Fourier transform of time-series data into the frequency domain and then examining the average amplitude from 0.01 to 0.08 Hz to compare changes in BOLD signals (Zang et al., 2007). Unlike FC, ALFF is a frequency-specific signal that is related to oscillatory phenomena, and directly reflects the intensity of spontaneous neural activity in a given region of the brain. While some studies have explored ALFF values in MDD patients, there is limited analysis of the results using support vector machine (SVM) techniques. SVM machine learning algorithms classify high-dimensional data points through the maximization of margins between classes (Pereira et al., 2009). SVM methods are frequently employed in psychiatric and neurological settings due to their high degree of classification accuracy and ability to process high-dimensional data (Gaonkar et al., 2015; Li et al., 2021).
Here, an SVM approach was employed to identify brain regions showing differences in ALFF values between adolescent MDD patients with a history of SA and healthy control individuals. The relationships between these values and patient depression and suicide scale scores were also assessed, and the utility of these changes in ALFF values as a neuroimaging biomarker of MDD with SA in adolescents was examined.
2. Materials and methods
2.1. Participants
A total of 71 first-episode drug-naïve adolescent depression patients were recruited from the Department of Psychiatry of Wuhan Mental Health Center. Patients were diagnosed with depression by two experienced psychiatrists based on DSM-IV criteria. To be eligible for study inclusion, patients had to be 7–17 years of age, right-handed, meet the diagnostic criteria for an acute episode of depression, have a history of SA within the past 14 days, be free of serious physical illnesses, be free of the alcohol and/or substance abuse or dependence, and be free of other Axis I disorders including schizophrenia, bipolar disorder, and substance-induced mood disorders. In addition, 54 age- and sex-matched healthy control individuals were recruited from the Wuhan Mental Health Center medical examination center. These controls were right-handed, had no history or family history of psychiatric disorders, and were free of any severe physical illness. All participants provided written informed consent for study participation. The Ethics Committee of Wuhan Mental Health Center approved this research, which was conducted in accordance with the guidelines of the Declaration of Helsinki.
SA was defined as any self-destructive behavior intended to terminate one’s own life that did not result in death (O’Carroll et al., 1996; Li et al., 2021). The patients included in this study were confirmed to have a history of SA through interviews with experienced psychiatrists, who also collected relevant details including the numbers of SAs and the dates on which they had occurred. When ambiguous results were obtained, the psychiatrists also made inquiries with the parents or clinicians of that patient to confirm these results. The Suicidal Ideation Questionnaire Junior (SIQ-JR; Keane et al., 1996) scale was conducted on the same day as the rs-fMRI to evaluate the severity of suicidal ideation, while the child depression inventory (CDI; Akimana et al., 2019) was used to assess depression severity.
2.2. Image acquisition
An Achieva 3 T MRI scanner (Philips, The Netherlands) was used for all imaging acquisition. The rs-fMRI data were preprocessed using MATLAB DPARSF software (Wang et al., 2017), as previously described (Gao et al., 2022a). Further details are provided in the Supplementary material.
2.3. ALFF analysis
ALFF analyses were performed with Rest software1 as reported previously (Zou et al., 2008). Further details are provided in the Supplementary material.
2.4. Classification analysis
SVM methods were used to evaluate the ability of ALFF values in specific brain regions to differentiate between MDD patients with a history of SA and healthy control individuals. The SVM analysis was conducted using the LIBSVM package in MATLAB; further information is provided in the Supplementary material.
2.5. Statistical analysis
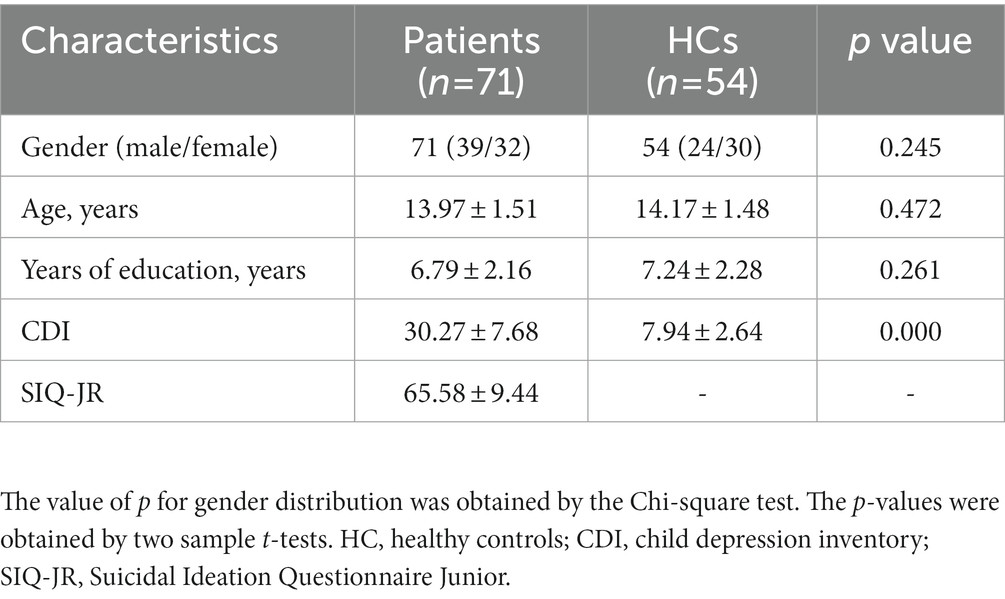
Age, CDI scores, and years of education were compared between MDD patients and control individuals using two-sample t-tests, whereas gender distributions were compared with Chi-square tests. SPSS 22.0 was used for statistical analyses. Correlations between abnormal ALFF values and specific clinical findings were assessed via Pearson correlation analyses. p < 0.05 were considered significant.
A voxel-by-voxel covariance analysis of individual whole-brain ALFF maps was used to detect differences between the two study cohorts. Analyzed covariates included age, years of education, and framewise displacement. REST was used for the GRF correction of results at p < 0.01 (cluster significance: p < 0.01, voxel significance: p < 0.001).
3. Results
3.1. Participant characteristics
A total of 71 first-episode MDD patients with a history of recent SAs were enrolled in the study, together with 54 healthy control individuals were enrolled. The clinical and demographic characteristics of the participants are shown in Table 1. No significant differences in age, sex, or education level were observed between the groups.
3.2. ALFF differences between groups
Differences in ALFF values were compared between MDD patients and controls using two-sample t-tests, revealing significantly lower ALFF values in the bilateral medial superior frontal gyrus (mSFG) and bilateral precuneus of MDD patients with a history of SA compared with the controls (Figure 1; Table 2).
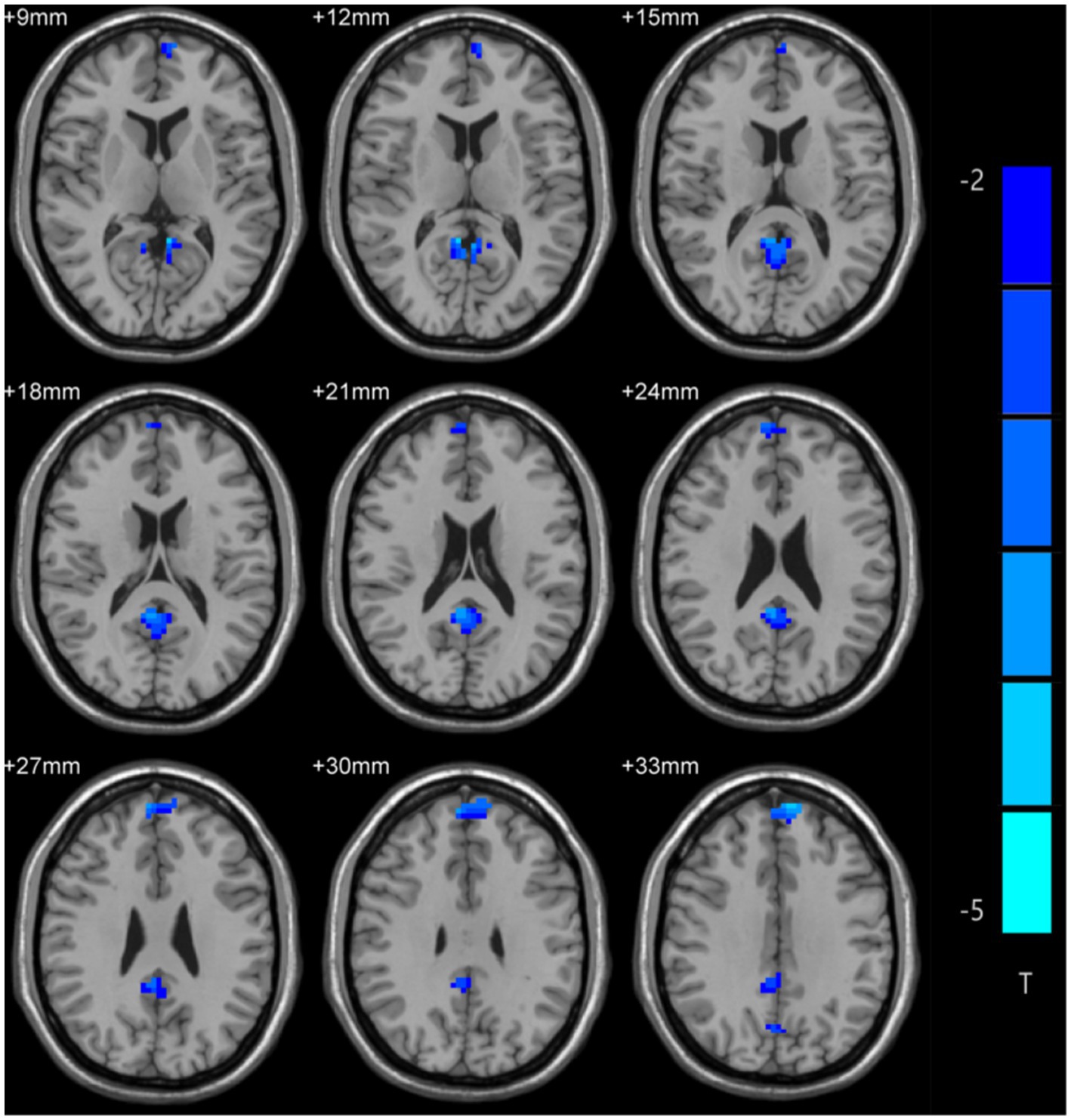
Figure 1. Amplitude of low-frequency fluctuation (ALFF) differences across groups. Blue color denotes low ALFF, and the darker the color, the lower the ALFF value of the brain area.
3.3. SVM results
An SVM approach was used to analyze decreases in the ALFF values of the bilateral mSFG and bilateral precuneus in MDD patients with a history of SA. This showed that reduced ALFF values in the bilateral precuneus offered the highest diagnostic accuracy of 82.4% (103/125), with a sensitivity and specificity of 91.5% (65/71) and 70.4% (38/54), respectively (Figure 2). The accuracy value for the reduced ALFF values in the bilateral mSFG was 76.8% (96/125) (data not shown).
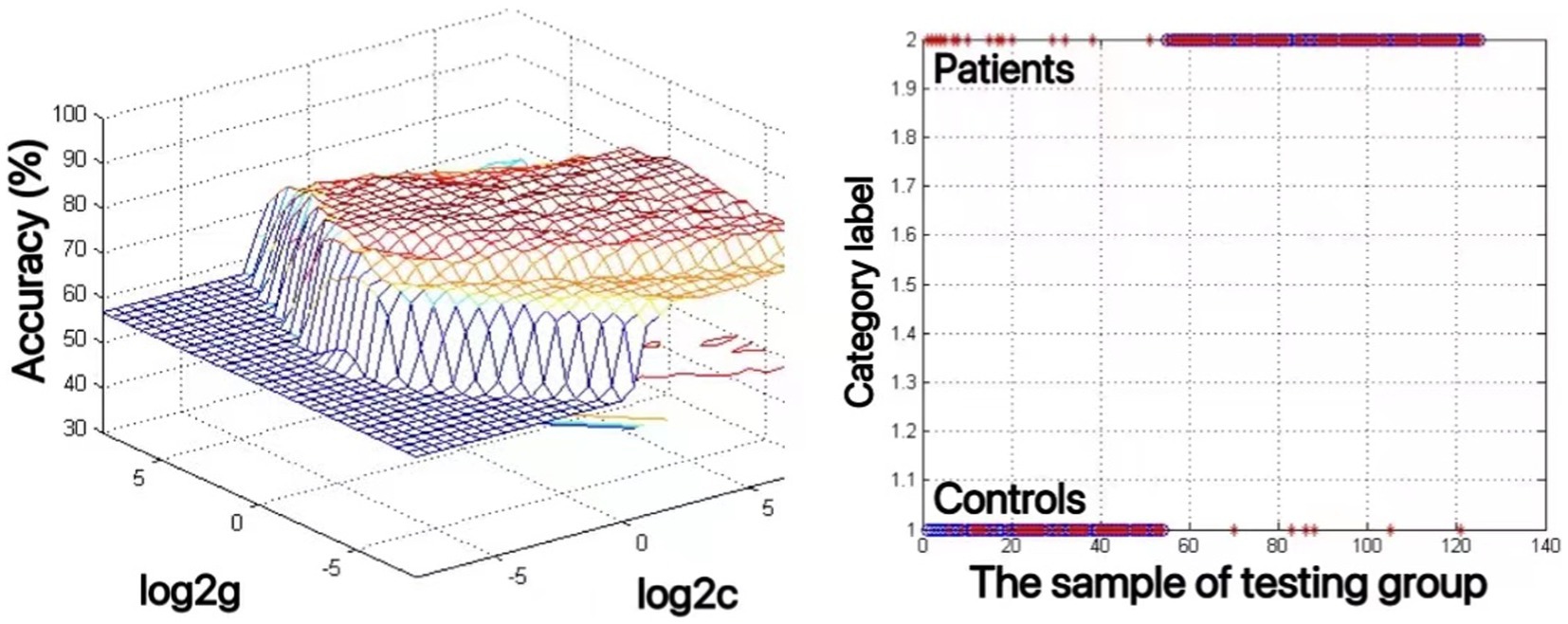
Figure 2. The use of decreased ALFF values in the bilateral precuneus to differentiate adolescents with major depressive disorder with suicidal attempts from healthy controls. Visualization of classifications through support vector machine (SVM) techniques using the ALFF values in the bilateral precuneus. Left: SVM parameters selection result of 3D view; Right: Classification map of the ALFF values in the bilateral precuneus.
3.4. Correlation results
The associations between ALFF values and other clinical variables in MDD patients were assessed through Pearson correlation analyses. This revealed the ALFF values in the bilateral mSFG and bilateral precuneus were negatively correlated with CDI scores (Figure 3), while there was also a negative correlation between ALFF values in the bilateral precuneus and SIQ-JR scores (Figure 4). ALFF values were not significantly correlated with patient age or years of education (data not shown).
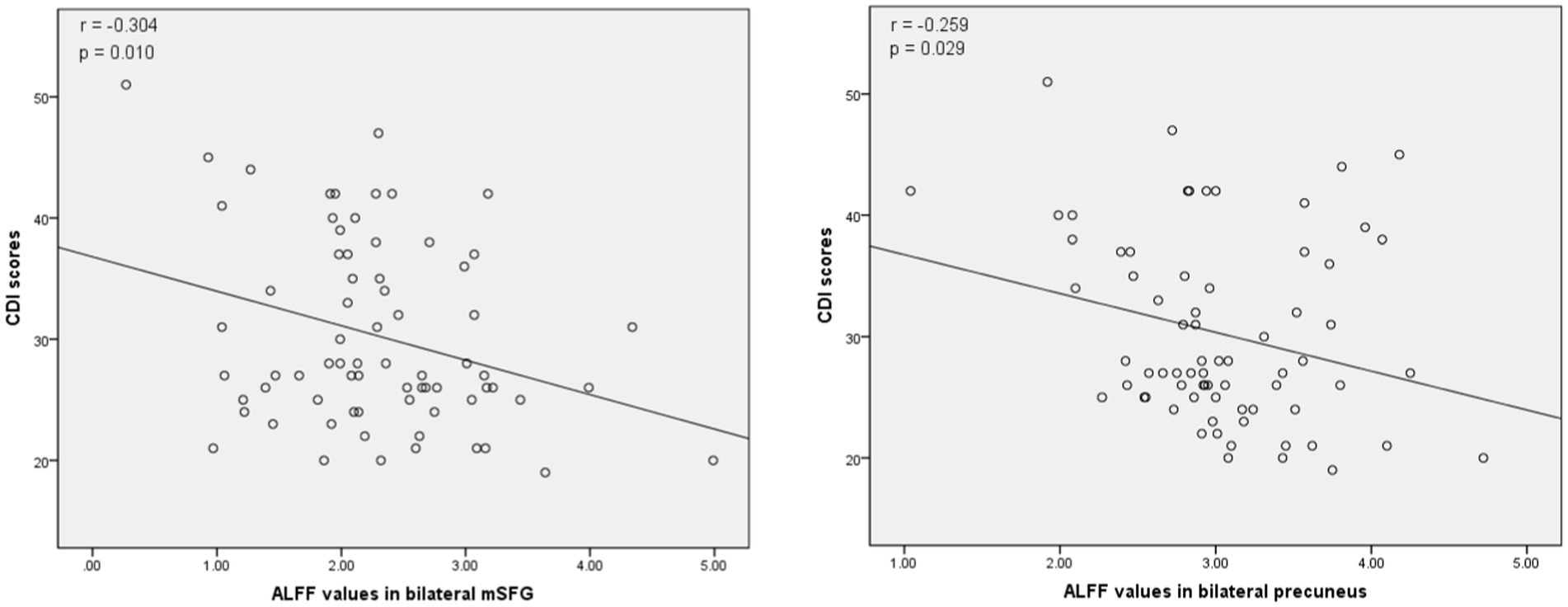
Figure 3. Correlations between abnormal ALFF and child depression inventory (CDI) scores. Left: Negative correlation between the ALFF values in the bilateral medial superior frontal gyrus (mSFG) and CDI scores. Right: Negative correlation between the ALFF values in the bilateral precuneus and CDI scores.
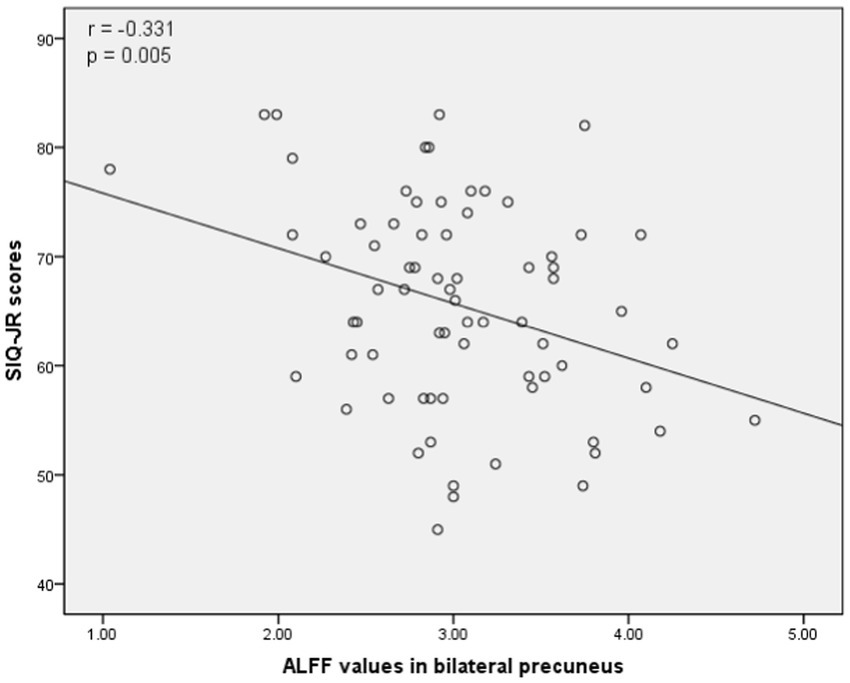
Figure 4. Correlations between abnormal ALFF and Suicidal Ideation Questionnaire Junior (SIQ-JR) scores. A negative correlation was found between the ALFF values in the bilateral precuneus and SIQ-JR scores.
4. Discussion
This is the first report of the use of an SVM technique to detect alterations in rs-fMRI-derived ALFF values in adolescent MDD patients with a history of SA compared with healthy controls. Significant reductions in the ALFF values were found in the mSFG and precuneus of MDD patients compared with the controls. Further analysis using SVM methods confirmed that these neuroimaging alterations may be of diagnostic value for the identification of adolescents with MDD.
The prefrontal cortex is positioned anteriorly to the premotor and motor portions of the frontal lobe (Lewis, 2004) and includes the mSFG, orbital SFG, and dorsolateral SFG (Liu et al., 2021). The default mode network (DMN) consists of an interconnected series of regions in the brain that exhibits higher levels of activity under resting conditions than during task completion. The mSFG is a DMN hub (Franco et al., 2009) and has been reported to be associated with aberrant neurological activity in patients with MDD (Guo et al., 2018). The mSFG also serves as an essential region of the brain necessary for emotional processing, executive function, the detection of causality, and for sequence learning (Van Overwalle, 2009). Previous studies have explored the association between the mSFG and MDD, with Peng et al., for example, reporting increased mSFG gray matter volume in these patients (Peng et al., 2016) while Liu et al. reported an association between dysfunction of the right mSFG and the cognitive processing of negative emotion in MDD (Liu et al., 2021). Here, ALFF values in the bilateral mSFG were found to be lower in MDD patients. In line with these results, a case–control rs-fMRI study of first-episode drug-naïve adolescent MDD patients showed lower ALFF values in the left medial frontal lobe (Gong et al., 2014). However, one voxel-based meta-analysis found higher ALFF values in the SFG in MDD patients than in controls (Gong et al., 2020). These differences may be linked to variations in study cohorts, duration of illness, medication use, and scanning parameters. For example, while higher bilateral SFG ALFF values were reported in early-onset depression, reduced values were found in late-onset depression (Guo et al., 2013). Another analysis that focused on non-suicidal self-harm also observed significantly lower bilateral mSFG ALFF values in adolescents suffering from MDD (Huang et al., 2021). Moreover, lower ALFF values in the right ventral medial frontal gyrus were reported in MDD patients with a history of SA compared with non-suidical patients (Fan et al., 2013). This may suggest a close relationship between ALFF values in the frontal gyrus and the risk of self-harm or suicide in MDD. Here, the SVM results indicated a 76.8% accuracy in the use of the these values to differentiate between adolescent patients with MDD and a history of SA and healthy controls. Correlation analysis showed that the mSFG ALFF values were negatively correlated with the and CDI scores but were unrelated to the SIQ-JR scores. Changes in the ALFF values in the mSFG may thus be closely related to the pathophysiology of MDD and/or suicidal ideation in these patients.
The precuneus has a unique anatomical location within the posteromedial parietal cortex buried in the interhemispheric fissure. It is thus rarely injured in isolation and it is particularly challenging to study (Cavanna and Trimble, 2006). The functions of the precuneus include self-awareness, cognition, autobiographical memory, and visuospatial processing (Zhang and Li, 2012; Tanglay et al., 2021). Much like the mSFG, the precuneus functions as a core DMN hub, and prior work suggests a close relationship with MDD. For example, in one rs-fMRI study, ALFF values in the left precuneus were found to be abnormal in individuals affected by social anxiety disorders and to be negatively correlated with the clinical symptoms experienced by these patients (Yuan et al., 2018). Lower ALFF values in the right precuneus have also been reported in MDD, which were found to remain below those of healthy controls even after remission, suggesting that ALFF values may represent a valuable biomarker for MDD (Wang et al., 2020). Consistently, other studies have proposed the application of ALFF as a diagnostic neuroimaging biomarker of MDD in both adolescent and adult populations (Gong et al., 2014; Li et al., 2018; Gong et al., 2020). Patients with depression accompanied by suicidal tendencies also exhibit reductions in right precuneus FC compared with controls (Shu et al., 2022). Consistent with these findings, the present study found that adolescent MDD patients with a history of SA showed lower ALFF values in the precuneus relative to healthy controls, and negative correlations were observed between the precuneus ALFF values and both CDI and SIQ-JR scores. The accuracy of the SVM results was 82.4%, indicating that the precuneus may represent a key mediator of MDD and SA pathogenesis. A reduced precuneus ALFF value should thus be explored as a neuroimaging biomarker for the diagnosis of MDD with suicidal tendencies in adolescents.
5. Summary and conclusion
MDD and suicidality represent growing public health problems throughout the world, especially among adolescents. Despite this pressing issue, the current understanding of MDD in adolescents is less understood compared with adults. The advent of novel neuroimaging technologies has led to the establishment of rs-fMRI as a valuable noninvasive means of aiding patient diagnosis in clinical settings. ALFF values, in particular, enable researchers to detect abnormalities in spontaneous brain activity through the monitoring of brain energy metabolism (Jing et al., 2013). Here, an SVM algorithm, which is commonly used for data analysis in biomedical contexts, was employed, demonstrating the value of reduced ALFF values in the bilateral mSFG and bilateral precuneus as neuroimaging biomarkers capable of identifying adolescent MDD patients with a history of SA.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Wuhan Mental Health Center. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
YZ: investigation, resources, data analysis, manuscript writing and submitting. YS: investigation, resources, software, manuscript writing. CC: investigation, data curtion, writing-review. SY: data curtion, writing-review and editing. MC: conceptualization, methodology. TL: project administration, supervision. All authors contributed to the article and approved the submitted version.
Funding
The investigation was supported by the Students’ Mental Health Network Project (SMHN) and the grant from the Wuhan Municipal Health Commission (Grant No.WG17D02).
Acknowledgments
Sincere appreciation to all patients and colleagues for their generous support of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2023.1146944/full#supplementary-material
Footnotes
References
Akimana, B., Abbo, C., Balagadde-Kambugu, J., and Nakimuli-Mpungu, E. (2019). Prevalence and factors associated with major depressive disorder in children and adolescents at the Uganda cancer institute. BMC Cancer 19:466. doi: 10.1186/s12885-019-5635-z
Avenevoli, S., Swendsen, J., He, J. P., Burstein, M., and Merikangas, K. R. (2015). Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J. Am. Acad. Child Adolesc. Psychiatry 54, 37–44.e2. doi: 10.1016/j.jaac.2014.10.010
Biswal, B. B. (2012). Resting state fMRI: a personal history. NeuroImage 62, 938–944. doi: 10.1016/j.neuroimage.2012.01.090
Biswal, B., Yetkin, F. Z., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Breslin, K., Balaban, J., and Shubkin, C. D. (2020). Adolescent suicide: what can pediatricians do? Curr. Opin. Pediatr. 32, 595–600. doi: 10.1097/MOP.0000000000000916
Cavanna, A. E., and Trimble, M. R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain J. Neurol. 129, 564–583. doi: 10.1093/brain/awl004
Chen, L. C., Chen, Y. H., Bai, Y. M., Chen, T. J., Chen, M. H., and Su, T. P. (2020). Antidepressant resistance in adolescents with major depressive disorder: a nationwide longitudinal study. J. Affect. Disord. 262, 293–297. doi: 10.1016/j.jad.2019.11.038
Doruk Camsari, D., Lewis, C. P., Sonmez, A. I., Nandakumar, A. L., Gresbrink, M. A., Daskalakis, Z. J., et al. (2019). Transcranial magnetic stimulation markers of antidepressant treatment in adolescents with major depressive disorder. Int. J. Neuropsychopharmacol. 22, 435–444. doi: 10.1093/ijnp/pyz021
Fan, T., Wu, X., Yao, L., and Dong, J. (2013). Abnormal baseline brain activity in suicidal and non-suicidal patients with major depressive disorder. Neurosci. Lett. 534, 35–40. doi: 10.1016/j.neulet.2012.11.032
Franco, A. R., Pritchard, A., Calhoun, V. D., and Mayer, A. R. (2009). Interrater and intermethod reliability of default mode network selection. Hum. Brain Mapp. 30, 2293–2303. doi: 10.1002/hbm.20668
Gao, Y., Tong, X., Hu, J., Huang, H., Guo, T., Wang, G., et al. (2022a). Decreased resting-state neural signal in the left angular gyrus as a potential neuroimaging biomarker of schizophrenia: an amplitude of low-frequency fluctuation and support vector machine analysis. Front. Psych. 25:949512. doi: 10.3389/fpsyt.2022.949512
Gao, Y., Wang, X., Xiong, Z., Ren, H., Liu, R., Wei, Y., et al. (2021). Abnormal fractional amplitude of low-frequency fluctuation as a potential imaging biomarker for first-episode major depressive disorder: A resting-state fMRI study and support vector machine analysis. Front. Neurol. 12:751400. doi: 10.3389/fneur.2021.751400
Gao, Y., Xiong, Z., Wang, X., Ren, H., Liu, R., Bai, B., et al. (2022b). Abnormal degree centrality as a potential imaging biomarker for right temporal lobe epilepsy: A resting-state functional magnetic resonance imaging study and support vector machine analysis. Neuroscience 487, 198–206. doi: 10.1016/j.neuroscience.2022.02.004
Gao, Y., Zhao, X., Huang, J., Wang, S., Chen, X., Li, M., et al. (2022c). Abnormal regional homogeneity in right caudate as a potential neuroimaging biomarker for mild cognitive impairment: A resting-state fMRI study and support vector machine analysis. Front. Aging Neurosci. 14:979183. doi: 10.3389/fnagi.2022.979183
Gaonkar, B., Shinohara, R. T., and Davatzikos, C., Alzheimers Disease Neuroimaging Initiative (2015). Interpreting support vector machine models for multivariate group wise analysis in neuroimaging. Med. Image Anal. 24, 190–204. doi: 10.1016/j.media.2015.06.008
Gong, Y., Hao, L., Zhang, X., Zhou, Y., Li, J., Zhao, Z., et al. (2014). Case-control resting-state fMRI study of brain functioning among adolescents with first-episode major depressive disorder. Shanghai Arch. Psychiatry 26, 207–215. doi: 10.3969/j.issn.1002-0829.2014.04.004
Gong, J., Wang, J., Qiu, S., Chen, P., Luo, Z., Wang, J., et al. (2020). Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl. Psychiatry 10:353. doi: 10.1038/s41398-020-01036-5
Gore, J. C. (2003). Principles and practice of functional MRI of the human brain. J. Clin. Invest. 112, 4–9. doi: 10.1172/JCI19010
Guo, W., Cui, X., Liu, F., Chen, J., Xie, G., Wu, R., et al. (2018). Increased anterior default-mode network homogeneity in first-episode, drug-naive major depressive disorder: a replication study. J. Affect. Disord. 225, 767–772. doi: 10.1016/j.jad.2017.08.089
Guo, W. B., Liu, F., Xun, G. L., Hu, M. R., Guo, X. F., Xiao, C. Q., et al. (2013). Reversal alterations of amplitude of low-frequency fluctuations in early and late onset, first-episode, drug-naive depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 40, 153–159. doi: 10.1016/j.pnpbp.2012.08.014
Guo, X., Wang, W., Kang, L., Shu, C., Bai, H., Tu, N., et al. (2022). Abnormal degree centrality in first-episode medication-free adolescent depression at rest: A functional magnetic resonance imaging study and support vector machine analysis. Front. Psychiatry. 13:926292. doi: 10.3389/fpsyt.2022.926292
Huang, Q., Xiao, M., Ai, M., Chen, J., Wang, W., Hu, L., et al. (2021). Disruption of neural activity and functional connectivity in adolescents with major depressive disorder who engage in non-suicidal self-injury: a resting-state fMRI study. Front. Psych. 12:571532. doi: 10.3389/fpsyt.2021.571532
Jing, B., Liu, C. H., Ma, X., Yan, H. G., Zhuo, Z. Z., Zhang, Y., et al. (2013). Difference in amplitude of low-frequency fluctuation between currently depressed and remitted females with major depressive disorder. Brain Res. 1540, 74–83. doi: 10.1016/j.brainres.2013.09.039
Jolles, D. D., van Buchem, M. A., Crone, E. A., and Rombouts, S. A. (2011). A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb. Cortex 21, 385–391. doi: 10.1093/cercor/bhq104
Keane, E. M., Dick, R. W., Bechtold, D. W., and Manson, S. M. (1996). Predictive and concurrent validity of the suicidal ideation questionnaire among American Indian adolescents. J. Abnorm. Child Psychol. 24, 735–747. doi: 10.1007/BF01664737
Lewis, D. A. (2004). Structure of the human prefrontal cortex. Am. J. Psychiatry 161:1366. doi: 10.1176/appi.ajp.161.8.1366
Li, G., Rossbach, K., Zhang, A., Liu, P., and Zhang, K. (2018). Resting-state functional changes in the precuneus within first-episode drug-naive patients with MDD. Neuropsychiatr. Dis. Treat. 14, 1991–1998. doi: 10.2147/NDT.S168060
Li, X. Y., Tabarak, S., Su, X. R., Qin, Z., Chai, Y., Zhang, S., et al. (2021). Identifying clinical risk factors correlate with suicide attempts in patients with first episode major depressive disorder. J. Affect. Disord. 295, 264–270. doi: 10.1016/j.jad.2021.08.028
Li, X., Yang, C., Xie, P., Han, Y., Su, R., Li, Z., et al. (2021). The diagnosis of amnestic mild cognitive impairment by combining the characteristics of brain functional network and support vector machine classifier. J. Neurosci. Methods 363:109334. doi: 10.1016/j.jneumeth.2021.109334
Liu, S., Ma, R., Luo, Y., Liu, P., Zhao, K., Guo, H., et al. (2021). Facial expression recognition and ReHo analysis in major depressive disorder. Front. Psychol. 12:688376. doi: 10.3389/fpsyg.2021.688376
Mi, T. M., Zhang, W., Li, Y., Liu, A. P., Ren, Z. L., and Chan, P. (2021). Altered functional segregated sensorimotor, associative, and limbic cortical-striatal connections in Parkinson’s disease: an fMRI investigation. Front. Neurol. 12:720293. doi: 10.3389/fneur.2021.720293
Nardi, B., Francesconi, G., Catena-Dell’osso, M., and Bellantuono, C. (2013). Adolescent depression: clinical features and therapeutic strategies. Eur. Rev. Med. Pharmacol. Sci. 17, 1546–1551.
Nock, M. K., Green, J. G., Hwang, I., McLaughlin, K. A., Sampson, N. A., Zaslavsky, A. M., et al. (2013). Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiat. 70, 300–310. doi: 10.1001/2013.jamapsychiatry.55
O’Carroll, P. W., Berman, A. L., Maris, R. W., Moscicki, E. K., Tanney, B. L., and Silverman, M. M. (1996). Beyond the tower of babel: a nomenclature for suicidology. Suicide Life Threat. Behav. 26, 237–252.
Peng, W., Chen, Z., Yin, L., Jia, Z., and Gong, Q. (2016). Essential brain structural alterations in major depressive disorder: a voxel-wise meta-analysis on first episode, medication-naive patients. J. Affect. Disord. 199, 114–123. doi: 10.1016/j.jad.2016.04.001
Pereira, F., Mitchell, T., and Botvinick, M. (2009). Machine learning classifiers and fMRI: a tutorial overview. NeuroImage 45, S199–S209. doi: 10.1016/j.neuroimage.2008.11.007
Shu, Y., Wu, G., Bi, B., Liu, J., Xiong, J., and Kuang, L. (2022). Changes of functional connectivity of the subgenual anterior cingulate cortex and precuneus after cognitive behavioral therapy combined with fluoxetine in young depressed patients with suicide attempt. Behav. Brain Res. 417:113612. doi: 10.1016/j.bbr.2021.113612
Takamura, T., and Hanakawa, T. (2017). Clinical utility of resting-state functional connectivity magnetic resonance imaging for mood and cognitive disorders. J. Neural Transm. (Vienna) 124, 821–839. doi: 10.1007/s00702-017-1710-2
Tanglay, O., Young, I. M., Dadario, N. B., Briggs, R. G., Fonseka, R. D., Dhanaraj, V., et al. (2021). Anatomy and white-matter connections of the precuneus. Brain Imaging Behav. 16, 574–586. doi: 10.1007/s11682-021-00529-1
Van Overwalle, F. (2009). Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 30, 829–858. doi: 10.1002/hbm.20547
Vargas, C., López-Jaramillo, C., and Vieta, E. (2013). A systematic literature review of resting state network--functional MRI in bipolar disorder. J. Affect. Disord. 150, 727–735. doi: 10.1016/j.jad.2013.05.083
Wang, Q., Gao, Y., Zhang, Y., Wang, X., Li, X., Lin, H., et al. (2022). Decreased degree centrality values as a potential neuroimaging biomarker for migraine: A resting-state functional magnetic resonance imaging study and support vector machine analysis. Front. Neurol. 13:1105592. doi: 10.3389/fneur.2022.1105592
Wang, M., Ju, Y., Lu, X., Sun, J., Dong, Q., Liu, J., et al. (2020). Longitudinal changes of amplitude of low-frequency fluctuations in MDD patients: a 6-month follow-up resting-state functional magnetic resonance imaging study. J. Affect. Disord. 276, 411–417. doi: 10.1016/j.jad.2020.07.067
Wang, L., Li, X., Li, K., Su, Y., Zeng, Y., Zhang, Q., et al. (2017). Mapping the effect of escitalopram treatment on amplitude of low-frequency fluctuations in patients with depression: a resting-state fMRI study. Metab. Brain Dis. 32, 147–154. doi: 10.1007/s11011-016-9871-5
Whitfield-Gabrieli, S., Thermenos, H. W., Milanovic, S., Tsuang, M. T., Faraone, S. V., McCarley, R. W., et al. (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 106, 1279–1284. doi: 10.1073/pnas.0809141106
Wu, F., Tu, Z., Sun, J., Geng, H., Zhou, Y., Jiang, X., et al. (2020). Abnormal functional and structural connectivity of amygdala-prefrontal circuit in first-episode adolescent depression: a combined fMRI and DTI study. Front. Psych. 10:983. doi: 10.3389/fpsyt.2019.00983
Yuan, C., Zhu, H., Ren, Z., Yuan, M., Gao, M., Zhang, Y., et al. (2018). Precuneus-related regional and network functional deficits in social anxiety disorder: a resting-state functional MRI study. Compr. Psychiatry 82, 22–29. doi: 10.1016/j.comppsych.2017.12.002
Zang, Y. F., He, Y., Zhu, C. Z., Cao, Q. J., Sui, M. Q., Liang, M., et al. (2007). Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29, 83–91. doi: 10.1016/j.braindev.2006.07.002
Zhang, S., and Li, C. S. (2012). Functional connectivity mapping of the human precuneus by resting state fMRI. NeuroImage 59, 3548–3562. doi: 10.1016/j.neuroimage.2011.11.023
Zhang, X., Zhu, X., Wang, X., Zhu, X., Zhong, M., Yi, J., et al. (2014). First-episode medication-naive major depressive disorder is associated with altered resting brain function in the affective network. PLoS One 9:e85241. doi: 10.1371/journal.pone.0085241
Keywords: amplitude of low-frequency fluctuation, major depressive disorder, suicidal attempts, resting-state fMRI, support vector machine
Citation: Zhou Y, Song Y, Chen C, Yan S, Chen M and Liu T (2023) Abnormal amplitude of low-frequency fluctuation values as a neuroimaging biomarker for major depressive disorder with suicidal attempts in adolescents: A resting-state fMRI and support vector machine analysis. Front. Psychol. 14:1146944. doi: 10.3389/fpsyg.2023.1146944
Edited by:
Yujun Gao, Wuhan University, ChinaReviewed by:
Chang Liu, The Fourth Hospital of Harbin Medical University, ChinaLiang Liang, Xinjiang Medical University, China
Copyright © 2023 Zhou, Song, Chen, Yan, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mo Chen, ✉ Y2hlbm1vMDY2QGh1c3QuZWR1LmNu; Tao Liu, ✉ MTkwMjEwNTY0N0BxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Yang Zhou
Yang Zhou Yu Song3,4†
Yu Song3,4† Shu Yan
Shu Yan Mo Chen
Mo Chen