- 1Nara University of Education, Nara, Japan
- 2Osaka Shoin Women’s University, Higashi-osaka, Ōsaka, Japan
Since the time of Darwin, theories have been proposed on the origin and functions of music; however, the subject remains enigmatic. The literature shows that music is closely related to important human behaviours and abilities, namely, cognition, emotion, reward and sociality (co-operation, entrainment, empathy and altruism). Notably, studies have deduced that these behaviours are closely related to testosterone (T) and oxytocin (OXT). The association of music with important human behaviours and neurochemicals is closely related to the understanding of reproductive and social behaviours being unclear. In this paper, we describe the endocrinological functions of human social and musical behaviour and demonstrate its relationship to T and OXT. We then hypothesised that the emergence of music is associated with behavioural adaptations and emerged as humans socialised to ensure survival. Moreover, the proximal factor in the emergence of music is behavioural control (social tolerance) through the regulation of T and OXT, and the ultimate factor is group survival through co-operation. The “survival value” of music has rarely been approached from the perspective of musical behavioural endocrinology. This paper provides a new perspective on the origin and functions of music.
1. Introduction
From Aristotle (c. 335 B.C.) to Darwin (1874) and Spencer (1868), scholars have studied the origin and functions of music. From approximately 1900 to 1950, these authors were joined by comparative musicologist Sachs (1943), amongst others. This research was stalled by the anti-evolutionary environment that emerged in cultural anthropology after World War II (Wallin et al., 2000) but re-emerged at the end of the 20th century (Brown and Wallin, 2000; McDermott and Hauser, 2005; Trainor, 2015). Although there are several preliterate cultures, no culture is without music and language. Music is essential to human behaviour; however, its existence is a mystery, and there is no consensus regarding its origin and functions. Broadly, claims regarding this subject are divided into two categories: evolutionary adaptiveness (survival value) and evolutionary by-product (Pinker, 1997). Theories have ascribed a survival value to music, citing its benefits for social bonding and group cohesion (Brown, 2000), sexual selection (Darwin, 1874; Miller, 2000) or caregiving (mother–child interactions; Trehub and Trainor, 1998). However, these hypotheses are inconclusive (Beccacece et al., 2021).
These theoretical perspectives are based on the theory of evolution; however, most implemented approaches are based on psychology and sociology. The evolution and survival value of music have also been investigated from a biological perspective (e.g., Beccacece et al., 2021). Studies have found that music is closely related to important human behaviours and abilities, such as cognition, emotion, rewards and sociality (co-operation, synchronisation, empathy and altruism). Notably, the aforementioned behaviours are deeply linked to testosterone (T) and oxytocin (OXT). Neuropeptides [OXT and arginine vasopressin (AVP)] and steroid hormones (T and oestrogen) play central roles in the social lives of animals and humans (McCall and Singer, 2012), affecting cognition, emotions and motivation, which influence behaviour (van Honk et al., 2015). In both sexes, T and OXT influence reproductive behaviour and are vital in aspects of social behaviour, such as affiliation, social cognition, aggression, emotion and stress and anxiety. Indeed, they play a key role in psychiatric disorders that impair social communication and interaction, such as autism spectrum disorders, major depressive disorder, schizophrenia and personality disorders (Feldman, 2012; Brüne, 2016; Crespi, 2016; Bradley and Woolley, 2017; Dai et al., 2017; Bakker-Huvenaars et al., 2020). Furthermore, these diseases are related to musical behaviour (musicality and therapy; Foubert et al., 2017; Gassner et al., 2022). Why music is associated with important human behaviours and neurochemicals closely related to reproductive and social behaviours remains unclear. Also unclear is why music works for individuals with mental disorders that have no radical cure.
First, the neurochemical exploration of music (musical behavioural endocrinology) is in its early stages (Chanda and Levitin, 2013; Koshimori, 2019). Traditionally, research on music and endocrinology (biochemistry) has been conducted in the music therapy field because music can reduce stress. Moreover, investigations of the mechanism have demonstrated that a decrease in cortisol (C), also called the stress hormone, is responsible for reductions in stress (Koshimori, 2019). Since then, research has mainly focused on applying music for stress regulation using C as an indicator, as well as research on immune-related substances (Koshimori, 2019). As a research field, endocrinological research has until recently been conducted exclusively in music therapy. With the rapid development of higher brain function research using fMRI and PET in the 1990s, music started to gain attention from the perspective of clarifying emotions and creativity. Moreover, research using dopamine—a neurotransmitter—as an indicator of the relationship between reward and pleasure induced by music was conducted (Ferreri et al., 2019). Subsequently, the target substance was expanded to include endorphins and serotonin, and in recent years, research has centred on OXT as an indicator of sociability (affiliation).
Under these circumstances, few studies have examined the relationship between T and music, which plays critical role in human behaviour. Additionally, discussions from the viewpoint of origins and evolution have been scant (Fukui, 2001). Recently, research has demonstrated that listening to and playing music affects T and OXT and that music is closely related to social behaviours, such as co-operation, empathy and altruism (Fukui and Toyoshima, 2014); moreover, music has been approached from an evolutionary perspective (Harvey, 2020). However, despite the attention, fewer experimental studies have been conducted on OXT than on T (e.g., Bowling et al., 2022), and the origin or evolution of music from the perspective of the relationship between the two substances has not been investigated. Studies have shown that T and OXT have opposite effects on sociality and aggression (Crespi, 2016). Therefore, studies that analyse the relationship between T and OXT substances are necessary to understand the function and evolution of music. An approach solely based on a single substance is insufficient.
In this paper, we examine the endocrinological and psychological functions of human social and musical behaviours; demonstrate their relationship with T and OXT; and hypothesise that the emergence of music is linked to behavioural adaptations, which emerged as humans became increasingly social to ensure survival.
2. Human social behaviour, T and OXT
Humans are unique amongst the species with a social structure. As humans evolved, they formed social groups of various sizes and morphologies, based on the co-operation of genetically unrelated individuals (Fehr and Fischbacher, 2003). Cooperative behaviour has been observed in humans from the earliest stages of development (Tomasello, 2014; Grossmann, 2018), but its evolution remains unclear. Despite research on cooperative behaviour and altruism, their underlying molecular mechanisms and genetic basis remain unclear (Kalinowski et al., 2021).
However, the neuro-endocrinological study of social behaviour has been progressing (Soares et al., 2010; Trumble et al., 2015). Notably, hormones can serve as underlying mechanisms that influence behaviour in a functional manner. Understanding these proximate mechanisms might explain human psychology (Welling and Shackelford, 2019). Generally, hormones help in achieving specific behavioural goals in social contexts (Witczak et al., 2019). In both sexes, T and OXT influence reproductive behaviour and aspects of social behaviours such as co-operation (Feldman, 2012; McCall and Singer, 2012; Kurzban et al., 2015; Gangestad and Grebe, 2017; Gordon et al., 2017; Geniole and Carre, 2018; Servan et al., 2018).
Neuropeptides, such as OXT and arginine vasopressin (AVP) and steroid hormones T and oestrogen, play central roles in the social lives of animals and humans (van Anders et al., 2011; McCall and Singer, 2012). Neuropeptides and hormones affect cognition, emotions and motivation, all of which influence behaviour (McCall and Singer, 2012; van Honk et al., 2015). Endocrinological tradeoffs are required to maintain a balance between mating and rearing, the stability of the group between dominant and subordinate individuals, and simultaneous formation of multiple strong bonds between individuals (Witczak et al., 2019). T and OXT are involved in libido and nurturing and are key factors in understanding tradeoffs (Witczak et al., 2019). T is negatively related to sociality or tradeoffs, whereas OXT is positively related to sociality or tradeoffs (van Anders et al., 2011), with contradicting effects on the same cognition and behaviour (Holtfrerich et al., 2016). For example, the administration of T reduces the relation between the orbitofrontal cortex and amygdala and suppresses social behaviour, whereas OXT enhances the association with the amygdala and promotes social behaviour (Crespi, 2016). Furthermore, T and OXT interact (Gordon et al., 2017), having a notable effect on parental behaviour—maternal and paternal (Abraham and Feldman, 2018). Moreover, the interaction and increased rate between the two substances strongly correlate with behaviour (Jaeggi et al., 2015). Sex hormones such as T have organisational and activational effects on the human brain and can interact with the neurotransmitter system. These biological mechanisms can profoundly affect behaviour and the structural and functional regulation of the brain (Hornung et al., 2020).
T plays an important role in multiple physiological processes in the brain and can regulate the expression of specific genes by binding to the androgen receptor. Furthermore, T acts on neurotransmitter receptors and mediates non-genomic, neuro-active effects (Höfer et al., 2013). For example, T affects regulatory neurotransmitter systems in the brain, namely, key elements of dopaminergic and serotonergic neurotransmission (de Souza Silva et al., 2009). OXT exhibit strong functional binding with dopaminergic and opioid systems in the brain, the closely related neuropeptide hormone AVP and the steroid hormones T and oestrogen (van Anders et al., 2011).
As discussed in this section, certain aspects of T and OXT systems and their interaction have been documented. However, several questions remain (van Anders et al., 2011) regarding how these substances interact in the brain (Holtfrerich et al., 2018).
3. Musical behaviour as it relates to T and OXT
As the literature demonstrates, T and OXT are closely related to social cognition (Crespi, 2016). T is related to spatial abilities and memory (Vigil et al., 2016), although there are differences based on sex in its association with spatial–perceptual cognitive skills (Kimura, 2002; Voyer and Jansen, 2017). In women, high levels of T are associated with high skills in these areas, and in men, the opposite pattern has been observed (Gouchie and Kimura, 1991; Brosnan, 2006).
Musical ability, including composing, is related to the cognitive ability of spatial perception (Voyer and Jansen, 2017). Sex differences are also observed in musical ability studies; for example, females more easily recognise familiar melodies than males can (Miles et al., 2016). Musical perception and cognition are also influenced by T and OXT (Fukui and Toyoshima, 2008).
Hassler (1991) conducted the first investigation on the relation between musicality (composition) and T and deduced that they are strongly correlated and that this correlation differs based on sex (Hassler and Gupta, 1993). For male composers, lower T levels were associated with more highly-rated compositions, whereas amongst female composers, higher ratings were received by those with higher T values. T values are sex-dependent, but values are continuous, not discrete. There is an overlap in testosterone levels between men and women (Sudai, 2017). Following this line of evidence, Hassler (1991) concluded that an optimal level of T would promote creative musical behaviour, similar to the optimal level of T for cognitive function (Hogervorst et al., 2010; Holland et al., 2011). Because musical ability is a type of spatial–perceptual cognitive ability (Hassler, 1992), there is probably an optimal level between continuous T for men and women and may be at the bottom of normal male T range and at the top of normal female T range.
A weak-to-moderate but consistent association exists between OXT and creativity (e.g., novelty-seeking, extraversion, divergent thinking and problem solving; De Dreu et al., 2015). For vasopressin, a posterior pituitary hormone with a similar structure to that of OXT (they differ by two amino acids), an association between its receptor [arginine vasopressin receptor (AVPR)] and musical ability has been reported. The AVPR1A gene has one of the strongest links to musical activity and related behaviours, according to genome-wide linkage and association studies (Koshimori, 2019). In vertebrates (e.g., fish and birds), the vasopressin peptide signalling system is involved in musical signals (in review Ebstein et al., 2012), and an association between musicality and AVPR1A has been reported (Ukkola et al., 2009; Fukui and Toyoshima, 2013; Granot et al., 2013; Szyfter and Witt, 2020).
Research on the effects of music (both listening and playing) on T, OXT, and other substances is still in its infancy and will therefore take some time to elucidate.
To the best of our knowledge, the studies that have examined the relationship between T and OXT and music are listed in Table 1. According to this, at this time, there is only one study in which OXT was administered to humans and the relationship to music was examined. The study showed that when OXT was administered intranasally to performers, performance stress was alleviated. Although not in music, a study using the interpersonal finger-tapping paradigm showed that dyads administered OXT were more synchronised than dyads administered a placebo (Gebauer et al., 2016). However, there were no studies that administered T.
As already mentioned, musical ability is a type of spatial perceptual–cognitive ability, and the relationship between spatial perceptual–cognitive ability and T values is the same as that between musical talent (musical creativity) and T values. T values and talent are also known to have a sex-dependent inverse relationship. In males, low T values indicate high talent, whilst in females, conversely, high T values indicate high talent. To begin with, T levels in men and women are sex-dependent, and in healthy adults, T levels are said to be 5%–10% (1/10–1/20) of those in men when compared with blood testosterone levels. As we have already noted, several researchers have argued that somewhere in the difference between male and female T levels lies the optimal value for spatial perceptual–cognitive ability and musical talent.
Second, a relatively large number of studies have examined the relationship between music and T in healthy subjects, and the results are consistent. That is, music listening affects T values, but there are gender differences there. Particularly, music listening decreases T levels in men and increases them in women. The existence of sex differences in musical talent is also very interesting and will be discussed again in the Hypothesis section.
Conversely, the studies that have examined the relationship between OXT and music have not produced consistent results, so we are not in a position to say anything definitive about the effects of music. However, it is certain that music (both listening and playing) does affect OXT values.
4. Music, brain areas, sociality, T and OXT
Music has been consistently shown to activate a reward pathway similar to that associated with primary (food, sex, addictive drugs, friends and loved ones) and secondary reinforcers (money; e.g., Salimpoor et al., 2013). Simultaneously, it is deeply associated with brain regions of pro-sociality (co-operation, empathy, altruism; Harvey, 2020; Greenberg et al., 2021). Regions involved in the pleasure and emotion evoked by musical stimuli overlap with sociality and compare the limbic system (hippocampus, parahippocampal gyrus, amygdala and cingulate cortex), ventral striatum (nucleus accumbens), superior temporal gyrus, caudate nucleus, insular cortex, thalamus, orbitofrontal cortex, prefrontal cortex, dorsal prefrontal cortex, dorsolateral prefrontal cortex, inferior prefrontal cortex and supplementary motor cortex (in review Ferreri et al., 2019; Harvey, 2020).
T and OXT play an important role in emotion and are involved in activity in the aforementioned areas. Studies have shown that T is associated with limbic system activation, including the amygdala (e.g., Höfer et al., 2013). T can directly affect orbitofrontal function by interacting with local androgen receptors (Diekhof and Kraft, 2017). The circulation of T activates the androgen receptor and is a source of oestrogen for the brain (Juntti et al., 2010). Moreover, OXT exerts its effects by interacting with dopamine and the mesolimbic neurotransmitter system (Xiao et al., 2017). Brain structures affected by T comprise the hippocampus, hypothalamus, frontal cortex and cerebellum (Pillerová et al., 2021). Many of these areas are closely linked to the neural activity of OXT (Møller, 2021). Androgen receptors are found in the limbic, mesolimbic system and related areas. OXT receptors (OTR), vasopressin receptor 1a (V1aR) and vasopressin receptor 1b (V1bR) are expressed throughout the auditory and mesolimbic pathways (Johnson and Young, 2017). The ventral striatum/nucleus accumbens are the most relevant neural regions to music pleasure (Mueller et al., 2015). These structures are connected with the dopaminergic reward pathways, and dopamine has been suggested as relevant to music’s reward value (Blood and Zatorre, 2001; Ferreri et al., 2019). Subsequent findings have supported this hypothesis (Ferreri et al., 2019). Moreover, the dopamine system is regulated by T (Alarcón et al., 2017) and OXT (Gamal-Eltrabily and Manzano-García, 2018).
5. T and OXT are involved in social vocalisation
Vocalisation is an essential means of communication for mammals, from mice to humans, conveying important information on an individual’s reproductive, social and emotional state; location and identity: and the presence of food, relatives and predators (Tschida et al., 2019). Vocalisation is also central to music. As many theories on the origin of music suggest, music and language may be descendants of earlier forms of oral communication that disappeared amongst ancestral hominids (Leongómez et al., 2022). Many vocalisations can facilitate social interactions by reducing the uncertainty of the intentions and possible actions of the signaller. Such interactions help establish and maintain social bonds, contributing to successful reproduction (Cheney and Seyfarth, 2018).
Sex hormones are related to vocal behaviour in various species, including humans (Puts et al., 2016). Sex steroid hormones modulate vocal behaviour and regulate processing at various levels of the ascending auditory pathway (Caras, 2013).
According to studies on newborn vocalisation (babbling) and articulatory abilities in male and female children aged 5 months, T levels are related to auditory–vocal learning. Five-month T concentrations were negatively correlated to articulatory skills in babbling (Quast et al., 2016). Furthermore, there are sex differences in the perception of emotional prosody. Women are better at recognising emotional prosody than men (Lambrecht et al., 2014), and T may play an important role in this process (Fujisawa and Shinohara, 2011).
Humans have sex hormone receptors in their vocal folds, including the larynx (Kirgezen et al., 2017), and their development is closely related to T levels (Markova et al., 2016). Moreover, sex differences have been observed in emotional prosody recognition abilities. Adolescent females were more sensitive to emotional prosody than males. This finding suggests that sex differences for emotional prosody recognition emerge in adolescence, during which T levels become higher in males than females (Fujisawa and Shinohara, 2011), and that T is negatively correlated with aspects of social vocalisation.
Similar to T, OXT is involved in vocal processing in many animals, such as fish (Huffman et al., 2012), mice (Marlin et al., 2015) and humans (Theofanopoulou et al., 2017). However, unlike T, OXT positively correlates with social vocalisations. In humans, OXT positively correlates with maternal vocalisations, and maternal OXT levels during pregnancy positively correlate with motherese (Feldman, 2012), an infant-directed vocalisation (IDV; Fernald, 1992). Exposure to the mother’s voice increases daughters’ OXT levels (Seltzer et al., 2010). OXT administration in fathers also increases vocal synchronisation with their offspring (Weisman et al., 2014). OXT enhances the speaker’s vocal expression and social communication (Spengler et al., 2017). Although motherese has been associated with origins of music and language (Trehub, 2001; Falk, 2004), it is characterised by the exaggeration of vocal intonation (Saint-Georges et al., 2013; Adriaans and Swingley, 2017) and is not a form of music per se.
Vocal intonation transmits emotional information in many animals (Filippi et al., 2017), and thus the combination of emotional prosody and vocal expression may be at the origin of music (Darwin, 1874; Brown, 2000; Juslin and Laukka, 2003; Panksepp, 2009; Snowdon and Teie, 2010; Brown, 2017). Zimmermann et al. stated that the components of affective prosody in human speech and music have phylogenetic roots in non-human mammalian speech communication systems (Zimmermann et al., 2013). In summary, T is negatively correlated with synchrony and prosody, and OXT is positively correlated with these factors. OXT leads to increased synchrony (Gebauer et al., 2016; Josef et al., 2019), an important feature of music (and language). Similarly, prosody (pitch) is synonymous with musical melody.
6. T and OXT coordinate cooperative behaviour
Homo sapien is an ultra-social animal (Tomasello, 2014) and engages in cooperative behaviour regardless of the social group’s size. This unique ability to cooperate may have enabled humans to become Earth’s dominant species (de Waal, 2014).
From a neurobiological perspective, human pro-sociality has been suggested to be deeply rooted in neuroendocrine structures, with hormones regulating cooperative behaviour (Soares et al., 2010). These hormones include T and OXT, key neuromodulators of human social behaviour (van Honk et al., 2016; Geniole and Carre, 2018; Liu et al., 2019; Marsh et al., 2021).
Scientific investigations (particularly those involving neural imaging and physiological and psychological findings) have demonstrated that music plays a role in promoting co-operation within groups of genetically unrelated humans (Kniffin et al., 2017), altruistic behaviour (Fukui and Toyoshima, 2014) and social bonding (Savage et al., 2020). T and OXT play a role in cooperative behaviour and may therefore modulate co-operation in humans. Although studies have highlighted an association between OXT and social behaviour (Savage et al., 2020), few have examined the relationship between music and co-operation or altruism using OXT as an indicator.
OXT promotes synchronisation in the mother–child, father–child and other relationships (Feldman, 2017), whereas T inhibits synchronisation (Gordon et al., 2017). Rhythmic synchronisation is associated with cooperative, collaborative and pro-social behaviours (Cirelli, 2018). Furthermore, interpersonal behaviour synchronisation in infants increases pro-social behaviour (Tunçgenç et al., 2015). Movements synchronised with music promote cooperative behaviour in groups (McNeill, 1995) and increase group cohesion and social co-operation (Ellamil et al., 2016) amongst children (Kirschner and Tomasello, 2009; Cirelli, 2018). Music promotes communication (Spiro and Himberg, 2016) and triggers pro-social behaviour (Aucouturier and Canonne, 2017). It promotes the development of cognitive and motor skills and increases affinity and pro-sociality (Aucouturier and Canonne, 2017). Group singing can also increase intimacy and promote social cohesion (Pearce et al., 2016).
Empathy is a mechanism that causes altruistic behaviour (de Waal, 2012) and a central mechanism stimulated by emotions evoked by music (Juslin and Västfjäll, 2008). Studies on music and empathy have demonstrated that listening to and playing music promotes empathy (e.g., Fukui and Toyoshima, 2014). Furthermore, studies have found that music increases trust in others and promotes altruistic behaviour (Fukui and Toyoshima, 2014). When empathy was evoked, OXT and T levels were assumed to increase and decrease, respectively. Concentrations of OT were substantially lower with music than without it in highly empathic individuals. In a self-reported survey, highly empathic individuals reported that music enhanced their positive mood and increased their perception of being moved to a greater extent than low empathetic individuals did (Eerola et al., 2021).
The scope of the relationship between music and altruism was also shown using the dictator game (DG), an experimental measure of altruism commonly used in social psychology and economics wherein one participant (the dictator) receives a donation and then allocates it amongst the other anonymous participants (the recipients). In that experiment, DG was conducted with an in group (IG) and an out-group (OG) to determine whether participants listening to their preferred music (chill-inducing) affected altruistic behaviour (Fukui and Toyoshima, 2014). After listening to their preferred music, “dictators” increased the amount of money allocated to the recipient regardless of whether the recipient was in the IG or OG. The results indicate that music might encourage altruistic behaviour beyond ethnocentrism.
7. Hypothesis
Humans are hyper-social animals with distinctive cooperative behaviour relative to other species (Tomasello, 2014; Henrich and Muthukrishna, 2021), a high degree of social tolerance and a significant capacity for engaging in interpersonal assistance and collaboration (Tomasello, 2009).
As for co-operation, Darwin was troubled, because it runs counter to the theory of natural selection: the struggle for survival and the struggle for mating. The ability to cooperate emerged before advances in human cognition and culture (Hayes and Sanford, 2014).
Cooperative behaviour evolved and developed during the Pliocene and Pleistocene eras (approximately 5–10 million years ago) in specimens that branched from a common ancestor with chimpanzees (Meindl et al., 2018). These individuals experienced severe environmental selection pressure in an arid, unstable ecosystem, which contributed to the development and selection of cooperative traits for survival (Jetz and Rubenstein, 2011). Individuals with these abilities were selected via the evolutionary process.
The background explanation for this behavioural change is that the environmental selection pressure required males to invest in females. Examples of these behaviours include cooperative breeding (reproduction; Hrdy and Burkart, 2020; i.e., offspring of a bred female are cared for by others in the flock) and cooperative foraging (Tomasello, 2020; the flock or group collaboratively obtains food and other resources). Enabling these two co-operation types required new capabilities, especially (1) social tolerance and suppression of reactive aggression to help and cooperate with others and (2) cooperative–communicative abilities to enable co-operation.
Cooperative–communicative abilities may have co-evolved in humans and the coevolutionary relationship between vocal communication and group-level co-operation is not unique to humans of the ape lineage, but likely existed in the last common ancestor with chimpanzees (Mine et al., 2022).
These behavioural traits may have been caused by two endocrinological characteristics: reduced androgen responsiveness (reduced levels of circulating testosterone or reduced density of androgen receptors or decreased sensitivity in adults) and increased oxytocin activity (Cieri et al., 2014; Hare, 2017).
As aforementioned, T is generally correlated with aggression; thus, a decrease in T tends to decrease aggression. T is also negatively correlated with tolerance (sociability). Conversely, OXT generally has the opposite effects of T, increasing tolerance (emotional contagion, empathy) towards relatives.
The reduction of androgen during the process of humanisation has also been identified at the genetic level (the deletion of the androgen receptor). These genes were involved in steroid hormone signalling and neural function, and such deletions may have promoted brain size and upright walking and strengthened pair bonding (Reno, 2017). Aggressive, antisocial males with high T values are not expected to invest as fathers (Lovejoy, 2009; Potts, 2013; Clark and Henneberg, 2015), because high T values are negatively associated with paternal care (Abraham and Feldman, 2018) and vocalisations (Weisman et al., 2014) and were thus excluded from the selection (Lovejoy, 2009).
Moreover, T and OXT enabled the acquisition of other capabilities (cognition and vocalisation). T correlates with cognitive spatial abilities, and there is an optimal level between continuous T for men and women; OXT is also related to cognition, especially enhancing emotional cognition; and T has a negative effect on communication skills, whereas OXT has a positive effect. Thus, the action of both substances probably confers a high degree of cooperative–communicative abilities to the genus Homo. As aforementioned, there is an optimal level of T for spatial–perceptual cognitive ability, including musicality, and a presumption is that once T levels enter the optimal range, cognitive function improves. OXT is also involved in social information processing and memory (Guastella and MacLeod, 2012) and affects the recognition and retention of socially relevant information (Kinsley et al., 2015).
Babbling and IDV, a communication form that connects parents and their offspring, is universal in human language and may be unique to humans (Oller et al., 2019; Mehr et al., 2020). Babbling is rich in prosody. In IDV, mothers greatly change vocal timbre when speaking to their infants (Piazza et al., 2017). Moreover, IDV can be used by fathers and alloparents (Saint-Georges et al., 2013). This type of prosodic communication was probably also used amongst adults. Notably, IDVs have the requirements of music (pitch and rhythm) and can be referred to as primitive music (proto-music). Prosody is repeated to ensure that the intent is conveyed (rhythm) and that the range of intonation is increased (frequency modulation: melody). In addition, strength and weakness are added. These (rhythm, melody and dynamics) are the building blocks of music.
T and OXT are deeply involved in human Babbling-IDV-ADV (adult-directed vocalisation)-Prosodic vocalisation, T and OXT have an antagonistic effect on prosody and IDV is negatively correlated with T but positively correlated with OXT levels. The decrease in T and relative increase in OXT that occurred during the evolutionary process may have resulted in a quantitative increase in IDV and qualitative refinement, giving rise to proto-music.
Tone-rich proto-music (and features of modern music) results from a coordination of T and OXT, enabling emotional inflexion, synchronisation and the expression of empathy, facilitating cooperative breeding and alloparenting (paternal care), both of which are specific to human society.
The response of T levels to music differs by sex: a decrease in men and an increase in women, interestingly, the same changes that occur during love, which the process of pair bonding in humans (Marazziti and Canale, 2004). The decrease in males is probably a promotion of co-operation by suppressing reactive aggression. In interpreting the increase in females, the steroid/peptide theory provides (van Anders et al., 2011) a clue. It divides aggression into two types: antagonistic and protective aggression, both of which are associated with increased T. However, the aggression type depends on fluctuations in oxytocin. Whether this finding can be generalised remains unclear. If music increases OXT, the increased T in women can be interpreted as a correlate of protective aggression to protect their children and mates.
The difference due to sex in this reaction is considered the result of an ancestral survival strategy. In males, pro-social behaviour (co-operation) and pair bonding decrease T and increase OXT, whereas in females, T and OXT increase pro-social behaviour and pair bonding (Marazziti and Canale, 2004).
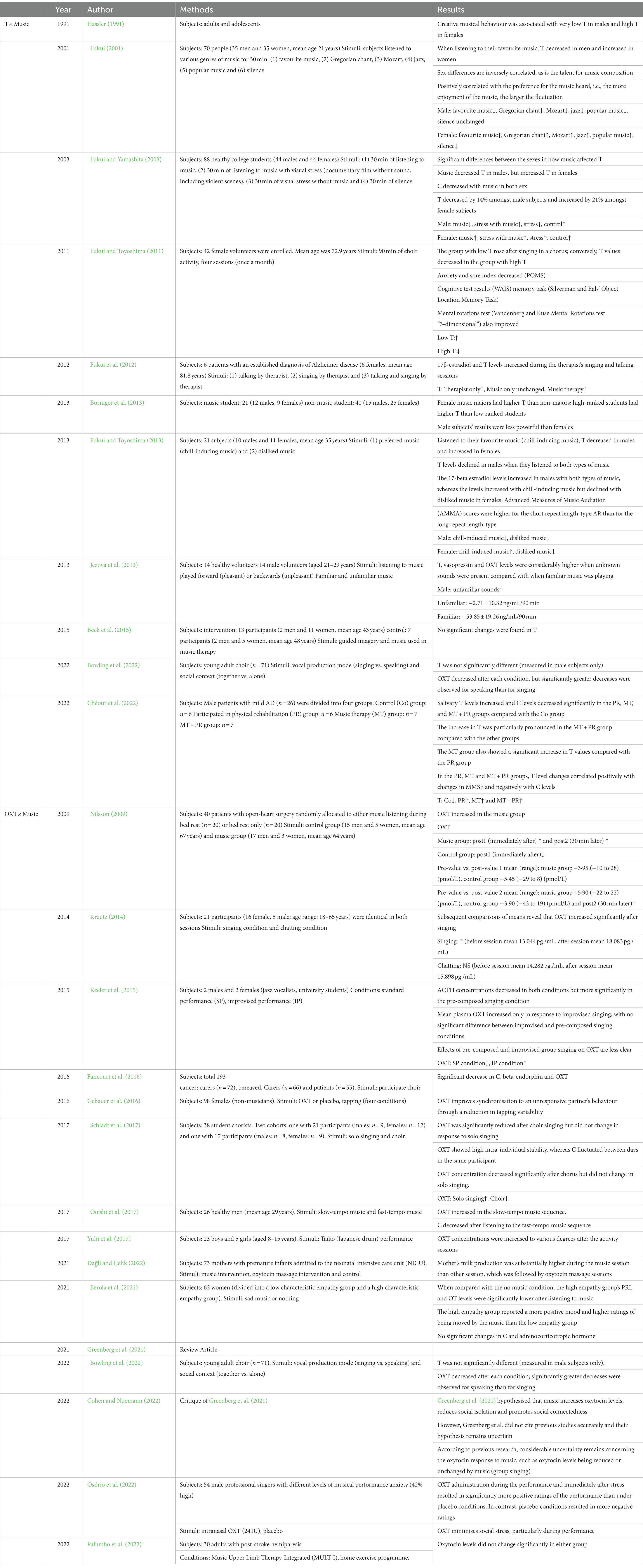
In summary, the proximate factor in the emergence of music is in behavioural control (aggression and social tolerance) through regulation of T and OXT, and the ultimate factor is group survival through co-operation. Music promotes co-operation and altruism, but it also promotes co-operation and altruism between groups, from mother and child to pairing and beyond (Figure 1).
8. Limitations and future research
The literature on music and endocrinology is still in its infancy. Although, this manuscript is based on research in the field of musical behavioural endocrinology, its qualitative approach may limit its effects in literature. The paucity of biochemical studies, especially of OXT, may limit the strength of our observations. T and OXT alone may not provide a comprehensive understanding of the endocrinological mechanisms of music. Future studies using fMRI, which can directly visualise neuronal activity, would be a powerful way to examine the effects of music and steroids on brain function.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HF conceived of the presented idea and developed the theory. KT investigate and verified the theory. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abraham, E., and Feldman, R. (2018). The neurobiology of human allomaternal care; implications for fathering, coparenting, and children's social development. Physiol. Behav. 193, 25–34. doi: 10.1016/j.physbeh.2017.12.034
Adriaans, F., and Swingley, D. (2017). Prosodic exaggeration within infant-directed speech: consequences for vowel learnability. J. Acoust. Soc. Am. 141, 3070–3078. doi: 10.1121/1.4982246
Alarcón, G., Cservenka, A., and Nagel, B. J. (2017). Adolescent neural response to reward is related to participant sex and task motivation. Brain Cogn. 111, 51–62. doi: 10.1016/j.bandc.2016.10.003
Aucouturier, J. J., and Canonne, C. (2017). Musical friends and foes: the social cognition of affiliation and control in improvised interactions. Cognition 161, 94–108. doi: 10.1016/j.cognition.2017.01.019
Bakker-Huvenaars, M. J., Greven, C. U., Herpers, P., Wiegers, E., Jansen, A., van der Steen, R., et al. (2020). Saliva oxytocin, cortisol, and testosterone levels in adolescent boys with autism spectrum disorder, oppositional defiant disorder/conduct disorder and typically developing individuals. Eur. Neuropsychopharmacol. 30, 87–101. doi: 10.1016/j.euroneuro.2018.07.097
Beccacece, L., Abondio, P., Cilli, E., Restani, D., and Luiselli, D. (2021). Human genomics and the biocultural origin of music. Int. J. Mol. Sci. 22:5397. doi: 10.3390/ijms22105397
Beck, B. D., Hansen, Å. M., and Gold, C. (2015). Coping with work-related stress through guided imagery and music (GIM): randomized controlled trial. J. Music. Ther. 52, 323–352. doi: 10.1093/jmt/thv011
Blood, A. J., and Zatorre, R. J. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. U. S. A. 98, 11818–11823. doi: 10.1073/pnas.191355898
Borniger, J. C., Chaudhry, A., and Muehlenbein, M. P. (2013). Relationships among musical aptitude, digit ratio and testosterone in men and women. PLoS One 8:e57637. doi: 10.1371/journal.pone.0057637
Bowling, D. L., Gahr, J., Ancochea, P. G., Hoeschele, M., Canoine, V., Fusani, L., et al. (2022). Endogenous oxytocin, cortisol, and testosterone in response to group singing. Horm. Behav. 139:105105. doi: 10.1016/j.yhbeh.2021.105105
Bradley, E. R., and Woolley, J. D. (2017). Oxytocin effects in schizophrenia: reconciling mixed findings and moving forward. Neurosci. Biobehav. Rev. 80, 36–56. doi: 10.1016/j.neubiorev.2017.05.007
Brosnan, M. J. (2006). Digit ratio and faculty membership: implications for the relationship between prenatal testosterone and academia. Br. J. Psychol. 97, 455–466. doi: 10.1348/000712605x85808
Brown, S. (2000). “The "Musilanguage" model of music evolution” in The origins of music. eds. N. L. Wallin, B. R. Merker, and S. Brown (Cambridge, Mass: MIT Press), 271–300.
Brown, S. (2017). A joint prosodic origin of language and music. Front. Psychol. 8:1894. doi: 10.3389/fpsyg.2017.01894
Brown, S. M. B., and Wallin, N. L. (2000). “An introduction to evolutionary musicology” in The origins of music. eds. N. L. Wallin, B. R. Merker, and S. Brown (Cambridge, Mass: MIT Press).
Brüne, M. (2016). On the role of oxytocin in borderline personality disorder. Br. J. Clin. Psychol. 55, 287–304. doi: 10.1111/bjc.12100
Caras, M. L. (2013). Estrogenic modulation of auditory processing: a vertebrate comparison. Front. Neuroendocrinol. 34, 285–299. doi: 10.1016/j.yfrne.2013.07.006
Chanda, M. L., and Levitin, D. J. (2013). The neurochemistry of music. Trends Cogn. Sci. 17, 179–193. doi: 10.1016/j.tics.2013.02.007
Cheney, D. L., and Seyfarth, R. M. (2018). Flexible usage and social function in primate vocalizations. Proc. Natl. Acad. Sci. U. S. A. 115, 1974–1979. doi: 10.1073/pnas.1717572115
Cieri, R. L., Churchill, S. E., Franciscus, R. G., Tan, J., and Hare, B. (2014). Craniofacial feminization, social tolerance, and the origins of behavioral modernity. Curr. Anthropol. 55, 419–443. doi: 10.1086/677209
Cirelli, L. K. (2018). How interpersonal synchrony facilitates early prosocial behavior. Curr. Opin. Psychol. 20, 35–39. doi: 10.1016/j.copsyc.2017.08.009
Clark, G., and Henneberg, M. (2015). The life history of Ardipithecus ramidus: A heterochronic model of sexual and social maturation. Anthropol. Rev. 78, 109–132. doi: 10.1515/anre-2015-0009
Cohen, A. J., and Neumann, I. D. (2022). A model for the social neuroscience of music production begins on a dubious note: Commentary on Greenberg et al. (2021). Am.Psychol. 77, 616–618. doi: 10.1037/amp0000973
Crespi, B. J. (2016). Oxytocin, testosterone, and human social cognition. Biol. Rev. Camb. Philos. Soc. 91, 390–408. doi: 10.1111/brv.12175
Chéour, S., Chéour, C., Kilani, C., Guemri, A., Zineddine, D., Khélifa, R., et al. (2022). Salivary testosterone and cortisol levels in tunisian elderly male patients with Mild Alzheimer’s disease. implications of musical therapy and/or physical rehabilitation. Front. Psychol. 13:839099. doi: 10.3389/fphys.2022.839099
Dağli, E., and Çelik, N. (2022). The effect of oxytocin massage and music on breast milk production and anxiety level of the mothers of premature infants who are in the neonatal intensive care unit: a self-controlled trial. Health Care Women Int. 43, 465–478. doi: 10.1080/07399332.2021.1947286
Dai, D., Li, Q. C., Zhu, Q. B., Hu, S. H., Balesar, R., Swaab, D., et al. (2017). Direct involvement of androgen receptor in oxytocin gene expression: possible relevance for mood disorders. Neuropsychopharmacology 42, 2064–2071. doi: 10.1038/npp.2017.76
De Dreu, C. K., Baas, M., and Boot, N. C. (2015). Oxytocin enables novelty seeking and creative performance through upregulated approach: evidence and avenues for future research. Wiley Interdiscip. Rev. Cogn. Sci. 6, 409–417. doi: 10.1002/wcs.1354
de Souza Silva, M. A., Mattern, C., Topic, B., Buddenberg, T. E., and Huston, J. P. (2009). Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. Eur. Neuropsychopharmacol. 19, 53–63. doi: 10.1016/j.euroneuro.2008.08.003
Diekhof, E. K., and Kraft, S. (2017). The association between endogenous testosterone level and behavioral flexibility in young men – evidence from stimulus-outcome reversal learning. Horm. Behav. 89, 193–200. doi: 10.1016/j.yhbeh.2017.02.006
Ebstein, R. P., Knafo, A., Mankuta, D., Chew, S. H., and Lai, P. S. (2012). The contributions of oxytocin and vasopressin pathway genes to human behavior. Horm. Behav. 61, 359–379. doi: 10.1016/j.yhbeh.2011.12.014
Eerola, T., Vuoskoski, J. K., Kautiainen, H., Peltola, H. R., Putkinen, V., and Schäfer, K. (2021). Being moved by listening to unfamiliar sad music induces reward-related hormonal changes in empathic listeners. Ann. N. Y. Acad. Sci. 1502, 121–131. doi: 10.1111/nyas.14660
Ellamil, M., Berson, J., Wong, J., Buckley, L., and Margulies, D. S. (2016). One in the dance: musical correlates of group synchrony in a real-world club environment. PLoS One 11:e0164783. doi: 10.1371/journal.pone.0164783
Falk, D. (2004). Prelinguistic evolution in early hominins: whence motherese? Behav. Brain Sci. 27, 491–503. discussion 503-483. doi: 10.1017/s0140525x04000111
Fancourt, D., Williamon, A., Carvalho, L. A., Steptoe, A., Dow, R., and Lewis, I. (2016). Singing modulates mood, stress, cortisol, cytokine and neuropeptide activity in cancer patients and carers. Ecancermedicalscience 10:631. doi: 10.3332/ecancer.2016.631
Fehr, E., and Fischbacher, U. (2003). The nature of human altruism. Nature 425, 785–791. doi: 10.1038/nature02043
Feldman, R. (2012). Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391. doi: 10.1016/j.yhbeh.2012.01.008
Feldman, R. (2017). The neurobiology of human attachments. Trends Cogn. Sci. 21, 80–99. doi: 10.1016/j.tics.2016.11.007
Fernald, A. (1992). “Meaningful melodies in mothers' speech to infants” in Nonverbal vocal communication: Comparative and developmental approaches (studies in emotion and social interaction). eds. H. Papousek and U. Jürgens (New York, NY: Cambridge University Press), 262–282.
Ferreri, L., Mas-Herrero, E., Zatorre, R. J., Ripolles, P., Gomez-Andres, A., Alicart, H., et al. (2019). Dopamine modulates the reward experiences elicited by music. Proc. Natl. Acad. Sci. U. S. A. 116, 3793–3798. doi: 10.1073/pnas.1811878116
Filippi, P., Congdon, J. V., Hoang, J., Bowling, D. L., Reber, S. A., Pašukonis, A., et al. (2017). Humans recognize emotional arousal in vocalizations across all classes of terrestrial vertebrates: evidence for acoustic universals. Proc. Biol. Sci. 284:20170990. doi: 10.1098/rspb.2017.0990
Foubert, K., Collins, T., and De Backer, J. (2017). Impaired maintenance of interpersonal synchronization in musical improvisations of patients with borderline personality disorder. Front. Psychol. 8:537. doi: 10.3389/fpsyg.2017.00537
Fujisawa, T. X., and Shinohara, K. (2011). Sex differences in the recognition of emotional prosody in late childhood and adolescence. J. Physiol. Sci. 61, 429–435. doi: 10.1007/s12576-011-0156-9
Fukui, H. (2001). Music and testosterone. A new hypothesis for the origin and function of music. Ann. N. Y. Acad. Sci. 930, 448–451. doi: 10.1111/j.1749-6632.2001.tb05767.x
Fukui, H., Arai, A., and Toyoshima, K. (2012). Efficacy of music therapy in treatment for the patients with Alzheimer's disease. Int. J. Alzheimers Dis. 2012:531646. doi: 10.1155/2012/531646
Fukui, H., and Toyoshima, K. (2008). Music facilitate the neurogenesis, regeneration and repair of neurons. Med. Hypotheses 71, 765–769. doi: 10.1016/j.mehy.2008.06.019
Fukui, H., and Toyoshima, K. (2011). “Music and steroids–music facilitates steroid–induced synaptic plasticity” in Steroids – clinical aspect. ed. H. Abduljabbar (London, UK: InTech)
Fukui, H., and Toyoshima, K. (2013). Influence of music on steroid hormones and the relationship between receptor polymorphisms and musical ability: a pilot study. Front. Psychol. 4:910. doi: 10.3389/fpsyg.2013.00910
Fukui, H., and Toyoshima, K. (2014). Chill-inducing music enhances altruism in humans. Front. Psychol. 5:1215. doi: 10.3389/fpsyg.2014.01215
Fukui, H., and Yamashita, M. (2003). The effects of music and visual stress on testosterone and cortisol in men and women. Neuro Endocrinol. Lett. 24, 173–180.
Gamal-Eltrabily, M., and Manzano-García, A. (2018). Role of central oxytocin and dopamine systems in nociception and their possible interactions: suggested hypotheses. Rev. Neurosci. 29, 377–386. doi: 10.1515/revneuro-2017-0068
Gangestad, S. W., and Grebe, N. M. (2017). Hormonal systems, human social bonding, and affiliation. Horm. Behav. 91, 122–135. doi: 10.1016/j.yhbeh.2016.08.005
Gassner, L., Geretsegger, M., and Mayer-Ferbas, J. (2022). Effectiveness of music therapy for autism spectrum disorder, dementia, depression, insomnia and schizophrenia: update of systematic reviews. Eur. J. Pub. Health 32, 27–34. doi: 10.1093/eurpub/ckab042
Gebauer, L., Witek, M. A. G., Hansen, N. C., Thomas, J., Konvalinka, I., and Vuust, P. (2016). Oxytocin improves synchronisation in leader-follower interaction. Sci. Rep. 6:38416. doi: 10.1038/srep38416
Geniole, S. N., and Carre, J. M. (2018). Human social neuroendocrinology: review of the rapid effects of testosterone. Horm. Behav. 104, 192–205. doi: 10.1016/j.yhbeh.2018.06.001
Gordon, I., Pratt, M., Bergunde, K., Zagoory-Sharon, O., and Feldman, R. (2017). Testosterone, oxytocin, and the development of human parental care. Horm. Behav. 93, 184–192. doi: 10.1016/j.yhbeh.2017.05.016
Gouchie, C., and Kimura, D. (1991). The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology 16, 323–334. doi: 10.1016/0306-4530(91)90018-o
Granot, R. Y., Uzefovsky, F., Bogopolsky, H., and Ebstein, R. P. (2013). Effects of arginine vasopressin on musical working memory. Front. Psychol. 4:712. doi: 10.3389/fpsyg.2013.00712
Greenberg, D. M., Decety, J., and Gordon, I. (2021). The social neuroscience of music: understanding the social brain through human song. Am. Psychol. 76, 1172–1185. doi: 10.1037/amp0000819
Grossmann, T. (2018). How to build a helpful baby: a look at the roots of prosociality in infancy. Curr. Opin. Psychol. 20, 21–24. doi: 10.1016/j.copsyc.2017.08.007
Guastella, A. J., and MacLeod, C. (2012). A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm. Behav. 61, 410–418. doi: 10.1016/j.yhbeh.2012.01.002
Hare, B. (2017). Survival of the friendliest: Homo sapiens evolved via selection for prosociality. Annu. Rev. Psychol. 68, 155–186. doi: 10.1146/annurev-psych-010416-044201
Harvey, A. R. (2020). Links between the neurobiology of oxytocin and human musicality. Front. Hum. Neurosci. 14:350. doi: 10.3389/fnhum.2020.00350
Hassler, M. (1991). Testosterone and musical talent. Exp. Clin. Endocrinol. 98, 89–98. doi: 10.1055/s-0029-1211105
Hassler, M. (1992). Creative musical behavior and sex hormones: musical talent and spatial ability in the two sexes. Psychoneuroendocrinology 17, 55–70. doi: 10.1016/0306-4530(92)90076-j
Hassler, M., and Gupta, D. (1993). Functional brain organization, handedness, and immune vulnerability in musicians and non-musicians. Neuropsychologia 31, 655–660. doi: 10.1016/0028-3932(93)90137-o
Hayes, S. C., and Sanford, B. T. (2014). Cooperation came first: evolution and human cognition. J. Exp. Anal. Behav. 101, 112–129. doi: 10.1002/jeab.64
Henrich, J., and Muthukrishna, M. (2021). The origins and psychology of human cooperation. Annu. Rev. Psychol. 72, 207–240. doi: 10.1146/annurev-psych-081920-042106
Höfer, P., Lanzenberger, R., and Kasper, S. (2013). Testosterone in the brain: neuroimaging findings and the potential role for neuropsychopharmacology. Eur. Neuropsychopharmacol. 23, 79–88. doi: 10.1016/j.euroneuro.2012.04.013
Hogervorst, E., Matthews, F. E., and Brayne, C. (2010). Are optimal levels of testosterone associated with better cognitive function in healthy older women and men? Biochim. Biophys. Acta 1800, 1145–1152. doi: 10.1016/j.bbagen.2009.12.009
Holland, J., Bandelow, S., and Hogervorst, E. (2011). Testosterone levels and cognition in elderly men: a review. Maturitas 69, 322–337. doi: 10.1016/j.maturitas.2011.05.012
Holtfrerich, S. K. C., Pfister, R., El Gammal, A. T., Bellon, E., and Diekhof, E. K. (2018). Endogenous testosterone and exogenous oxytocin influence the response to baby schema in the female brain. Sci. Rep. 8:7672. doi: 10.1038/s41598-018-26020-4
Holtfrerich, S. K., Schwarz, K. A., Sprenger, C., Reimers, L., and Diekhof, E. K. (2016). Endogenous testosterone and exogenous oxytocin modulate attentional processing of infant faces. PLoS One 11:e0166617. doi: 10.1371/journal.pone.0166617
Hornung, J., Lewis, C. A., and Derntl, B. (2020). Sex hormones and human brain function. Handb. Clin. Neurol. 175, 195–207. doi: 10.1016/B978-0-444-64123-6.00014-X
Hrdy, S. B., and Burkart, J. M. (2020). The emergence of emotionally modern humans: implications for language and learning. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 375:20190499. doi: 10.1098/rstb.2019.0499
Huffman, L. S., O'Connell, L. A., Kenkel, C. D., Kline, R. J., Khan, I. A., and Hofmann, H. A. (2012). Distribution of nonapeptide systems in the forebrain of an African cichlid fish, Astatotilapia burtoni. J Chem Neuroanat 44, 86–97. doi: 10.1016/j.jchemneu.2012.05.002
Jaeggi, A. V., Trumble, B. C., Kaplan, H. S., and Gurven, M. (2015). Salivary oxytocin increases concurrently with testosterone and time away from home among returning Tsimane' hunters. Biol. Lett. 11:20150058. doi: 10.1098/rsbl.2015.0058
Jetz, W., and Rubenstein, D. R. (2011). Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr. Biol. 21, 72–78. doi: 10.1016/j.cub.2010.11.075
Jezova, D., Hlavacova, N., Makatsori, A., Duncko, R., Loder, I., and Hinghofer-Szalkay, H. (2013). Increased anxiety induced by listening to unpleasant music during stress exposure is associated with reduced blood pressure and ACTH responses in healthy men. Neuroendocrinology 98, 144–150. doi: 10.1159/000354202
Johnson, Z. V., and Young, L. J. (2017). Oxytocin and vasopressin neural networks: implications for social behavioral diversity and translational neuroscience. Neurosci. Biobehav. Rev. 76, 87–98. doi: 10.1016/j.neubiorev.2017.01.034
Josef, L., Goldstein, P., Mayseless, N., Ayalon, L., and Shamay-Tsoory, S. G. (2019). The oxytocinergic system mediates synchronized interpersonal movement during dance. Sci. Rep. 9:1894. doi: 10.1038/s41598-018-37141-1
Juntti, S. A., Tollkuhn, J., Wu, M. V., Fraser, E. J., Soderborg, T., Tan, S., et al. (2010). The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66, 260–272. doi: 10.1016/j.neuron.2010.03.024
Juslin, P. N., and Laukka, P. (2003). Emotional expression in speech and music: evidence of cross-modal similarities. Ann. N. Y. Acad. Sci. 1000, 279–282. doi: 10.1196/annals.1280.025
Juslin, P. N., and Västfjäll, D. (2008). Emotional responses to music: the need to consider underlying mechanisms. Behav. Brain Sci. 31:559-575; discussion 575-621. doi: 10.1017/s0140525x08005293
Kalinowski, K., Kozłowska, A., Malesza, M., and Danel, D. P. (2021). Evolutionary origins of music. AR 84, 213–231. doi: 10.2478/anre-2021-0011
Keeler, J. R., Roth, E. A., Neuser, B. L., Spitsbergen, J. M., Waters, D. J., and Vianney, J. M. (2015). The neurochemistry and social flow of singing: bonding and oxytocin. Front. Hum. Neurosci. 9:518. doi: 10.3389/fnhum.2015.00518
Kimura, D. (2002). Sex hormones influence human cognitive pattern. Neuro Endocrinol. Lett. 23, 67–77.
Kinsley, C. H., Bales, K. L., Bardi, M., and Stolzenberg, D. S. (2015). Reproductive experiential regulation of cognitive and emotional resilience. Neurosci. Biobehav. Rev. 58, 92–106. doi: 10.1016/j.neubiorev.2015.05.015
Kirgezen, T., Sunter, A. V., Yigit, O., and Huq, G. E. (2017). Sex hormone receptor expression in the human vocal fold subunits. J. Voice 31, 476–482. doi: 10.1016/j.jvoice.2016.11.005
Kirschner, S., and Tomasello, M. (2009). Joint drumming: social context facilitates synchronization in preschool children. J. Exp. Child Psychol. 102, 299–314. doi: 10.1016/j.jecp.2008.07.005
Kniffin, K. M., Yan, J., Wansink, B., and Schulze, W. D. (2017). The sound of cooperation: musical influences on cooperative behavior. J. Organ. Behav. 38, 372–390. doi: 10.1002/job.2128
Koshimori, Y. (2019). “Neurochemical responses to music” in The Oxford handbook of music and the brain. eds. M. Thaut and D. A. Hodges. 1st ed (Oxford: Oxford University Press), 835.
Kreutz, G. (2014). Does singing facilitate social bonding? Music Med. 6, 51–60. doi: 10.47513/mmd.v6i2.180
Kurzban, R., Burton-Chellew, M. N., and West, S. A. (2015). The evolution of altruism in humans. Annu. Rev. Psychol. 66, 575–599. doi: 10.1146/annurev-psych-010814-015355
Lambrecht, L., Kreifelts, B., and Wildgruber, D. (2014). Gender differences in emotion recognition: impact of sensory modality and emotional category. Cogn. Emot. 28, 452–469. doi: 10.1080/02699931.2013.837378
Leongómez, J. D., Havlíček, J., and Roberts, S. C. (2022). Musicality in human vocal communication: an evolutionary perspective. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 377:20200391. doi: 10.1098/rstb.2020.0391
Liu, Y., Li, S., Lin, W., Li, W., Yan, X., Wang, X., et al. (2019). Oxytocin modulates social value representations in the amygdala. Nat. Neurosci. 22, 633–641. doi: 10.1038/s41593-019-0351-1
Lovejoy, C. O. (2009). Reexamining human origins in light of Ardipithecus ramidus. Science 326:74. doi: 10.1126/science.1175834
Marazziti, D., and Canale, D. (2004). Hormonal changes when falling in love. Psychoneuroendocrinology 29, 931–936. doi: 10.1016/j.psyneuen.2003.08.006
Markova, D., Richer, L., Pangelinan, M., Schwartz, D. H., Leonard, G., Perron, M., et al. (2016). Age-and sex-related variations in vocal-tract morphology and voice acoustics during adolescence. Horm. Behav. 81, 84–96. doi: 10.1016/j.yhbeh.2016.03.001
Marlin, B. J., Mitre, M., D'Amour, J. A., Chao, M. V., and Froemke, R. C. (2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504. doi: 10.1038/nature14402
Marsh, N., Marsh, A. A., Lee, M. R., and Hurlemann, R. (2021). Oxytocin and the neurobiology of prosocial behavior. Neuroscientist 27, 604–619. doi: 10.1177/1073858420960111
McCall, C., and Singer, T. (2012). The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat. Neurosci. 15, 681–688. doi: 10.1038/nn.3084
McDermott, J., and Hauser, M. (2005). The origina of music: innateness, uniqueness, and evolution. Music. Percept. 23, 29–59. doi: 10.1525/mp.2005.23.1.29
Mehr, S. A., Krasnow, M. M., Bryant, G. A., and Hagen, E. H. (2020). Origins of music in credible signaling. Behav. Brain Sci. 44:e60. doi: 10.1017/s0140525x20000345
Meindl, R. S., Chaney, M. E., and Lovejoy, C. O. (2018). Early hominids may have been weed species. Proc. Natl. Acad. Sci. U. S. A. 115, 1244–1249. doi: 10.1073/pnas.1719669115
Miles, S. A., Miranda, R. A., and Ullman, M. T. (2016). Sex differences in music: A female advantage at recognizing familiar melodies. Front. Psychol. 7:278. doi: 10.3389/fpsyg.2016.00278
Miller, G. (2000). “Evolution of human music through sexual selection” in The origins of music. eds. N. L. Wallin, B. R. Merker, and S. Brown (Cambridge, Mass: MIT Press)
Mine, J. G., Slocombe, K. E., Willems, E. P., Gilby, I. C., Yu, M., Thompson, M. E., et al. (2022). Vocal signals facilitate cooperative hunting in wild chimpanzees. Sci. Adv. 8:eabo5553. doi: 10.1126/sciadv.abo5553
Møller, M. (2021). Vasopressin and oxytocin beyond the pituitary in the human brain. Handb. Clin. Neurol. 180, 7–24. doi: 10.1016/b978-0-12-820107-7.00002-1
Mueller, K., Fritz, T., Mildner, T., Richter, M., Schulze, K., Lepsien, J., et al. (2015). Investigating the dynamics of the brain response to music: A central role of the ventral striatum/nucleus accumbens. NeuroImage 116, 68–79. doi: 10.1016/j.neuroimage.2015.05.006
Nilsson, U. (2009). Soothing music can increase oxytocin levels during bed rest after open-heart surgery: a randomised control trial. J. Clin. Nurs. 18, 2153–2161. doi: 10.1111/j.1365-2702.2008.02718.x
Oller, D. K., Griebel, U., Iyer, S. N., Jhang, Y., Warlaumont, A. S., Dale, R., et al. (2019). Language origins viewed in spontaneous and interactive vocal rates of human and bonobo infants. Front. Psychol. 10:729. doi: 10.3389/fpsyg.2019.00729
Ooishi, Y., Mukai, H., Watanabe, K., Kawato, S., and Kashino, M. (2017). Increase in salivary oxytocin and decrease in salivary cortisol after listening to relaxing slow-tempo and exciting fast-tempo music. PLoS One 12:e0189075. doi: 10.1371/journal.pone.0189075
Osório, F. L., Espitia-Rojas, G. V., and Aguiar-Ricz, L. N. (2022). Effects of intranasal oxytocin on the self-perception and anxiety of singers during a simulated public singing performance: A randomized, placebo-controlled trial. Front. Neurosci. 16:943578. doi: 10.3389/fnins.2022.943578
Panksepp, J. (2009). The emotional antecedents to the evolution of music and language. Music. Sci. 13, 229–259. doi: 10.1177/1029864909013002111
Palumbo, A., Aluru, V., Battaglia, J., Geller, D., Turry, A., Ross, M., et al. (2022). Music upper limb therapy-integrated provides a feasible enriched environment and 2 reduces post-stroke depression: A pilot randomized controlled trial. Am. J. Phys. Med. Rehabil. 101:937–946.. doi: 10.1097/PHM.0000000000001938
Pearce, E., Launay, J., van Duijn, M., Rotkirch, A., David-Barrett, T., and Dunbar, R. I. (2016). Singing together or apart: the effect of competitive and cooperative singing on social bonding within and between sub-groups of a university fraternity. Psychol. Music 44, 1255–1273. doi: 10.1177/0305735616636208
Piazza, E. A., Iordan, M. C., and Lew-Williams, C. (2017). Mothers consistently alter their unique vocal fingerprints when communicating with infants. Curr. Biol. 27, 3162–3167.e3. doi: 10.1016/j.cub.2017.08.074
Pillerová, M., Borbélyová, V., Hodosy, J., Riljak, V., Renczés, E., Frick, K. M., et al. (2021). On the role of sex steroids in biological functions by classical and non-classical pathways. An update. Front. Neuroendocrinol 62:100926. doi: 10.1016/j.yfrne.2021.100926
Potts, R. (2013). Hominin evolution in settings of strong environmental variability. Quat. Sci. Rev. 73, 1–13. doi: 10.1016/j.quascirev.2013.04.003
Puts, D. A., Hill, A. K., Bailey, D. H., Walker, R. S., Rendall, D., Wheatley, J. R., et al. (2016). Sexual selection on male vocal fundamental frequency in humans and other anthropoids. Proc. Biol. Sci. 283:20152830. doi: 10.1098/rspb.2015.2830
Quast, A., Hesse, V., Hain, J., Wermke, P., and Wermke, K. (2016). Baby babbling at five months linked to sex hormone levels in early infancy. Infant Behav. Dev. 44, 1–10. doi: 10.1016/j.infbeh.2016.04.002
Sachs, C. (1943). The rise of music in the ancient world, east and west. New York: W.W. Norton & Co.
Saint-Georges, C., Chetouani, M., Cassel, R., Apicella, F., Mahdhaoui, A., Muratori, F., et al. (2013). Motherese in interaction: at the cross-road of emotion and cognition? (A systematic review). PLoS One 8:e78103. doi: 10.1371/journal.pone.0078103
Salimpoor, V. N., van den Bosch, I., Kovacevic, N., McIntosh, A. R., Dagher, A., and Zatorre, R. J. (2013). Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 340, 216–219. doi: 10.1126/science.1231059
Savage, P. E., Loui, P., Tarr, B., Schachner, A., Glowacki, L., Mithen, S., et al. (2020). Music as a coevolved system for social bonding. Behav. Brain Sci. 44:e59. doi: 10.1017/s0140525x20000333
Schladt, T. M., Nordmann, G. C., Emilius, R., Kudielka, B. M., de Jong, T. R., and Neumann, I. D. (2017). Choir versus solo singing: effects on mood, and salivary oxytocin and cortisol concentrations. Front. Hum. Neurosci. 11:430. doi: 10.3389/fnhum.2017.00430
Seltzer, L. J., Ziegler, T. E., and Pollak, S. D. (2010). Social vocalizations can release oxytocin in humans. Proc. Biol. Sci. 277, 2661–2666. doi: 10.1098/rspb.2010.0567
Servan, A., Brunelin, J., and Poulet, E. (2018). The effects of oxytocin on social cognition in borderline personality disorder. Encéphale 44, 46–51. doi: 10.1016/j.encep.2017.11.001
Snowdon, C. T., and Teie, D. (2010). Affective responses in tamarins elicited by species-specific music. Biol. Lett. 6, 30–32. doi: 10.1098/rsbl.2009.0593
Soares, M. C., Bshary, R., Fusani, L., Goymann, W., Hau, M., Hirschenhauser, K., et al. (2010). Hormonal mechanisms of cooperative behaviour. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 365, 2737–2750. doi: 10.1098/rstb.2010.0151
Spengler, F. B., Scheele, D., Marsh, N., Kofferath, C., Flach, A., Schwarz, S., et al. (2017). Oxytocin facilitates reciprocity in social communication. Soc. Cogn. Affect. Neurosci. 12, 1325–1333. doi: 10.1093/scan/nsx061
Spiro, N., and Himberg, T. (2016). Analysing change in music therapy interactions of children with communication difficulties. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 371:20150374. doi: 10.1098/rstb.2015.0374
Sudai, M. (2017). The testosterone rule-constructing fairness in professional sport. J. Law Biosci. 4, 181–193. doi: 10.1093/jlb/lsx0004
Szyfter, K., and Witt, M. P. (2020). How far musicality and perfect pitch are derived from genetic factors? J. Appl. Genet. 61, 407–414. doi: 10.1007/s13353-020-00563-7
Theofanopoulou, C., Boeckx, C., and Jarvis, E. D. (2017). A hypothesis on a role of oxytocin in the social mechanisms of speech and vocal learning. Proc. Biol. Sci. 284:20170988. doi: 10.1098/rspb.2017.0988
Tomasello, M. (2014). The ultra-social animal. Eur. J. Soc. Psychol. 44, 187–194. doi: 10.1002/ejsp.2015
Tomasello, M. (2020). The adaptive origins of uniquely human sociality. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 375:20190493. doi: 10.1098/rstb.2019.0493
Trainor, L. J. (2015). The origins of music in auditory scene analysis and the roles of evolution and culture in musical creation. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 370:20140089. doi: 10.1098/rstb.2014.0089
Trehub, S. E. (2001). Musical predispositions in infancy. Ann. N. Y. Acad. Sci. 930, 1–16. doi: 10.1111/j.1749-6632.2001.tb05721.x
Trehub, S. E., and Trainor, L. (1998). Singing to infants: lullabies and play songs. Adv. Infancy Res. 12, 43–77.
Trumble, B. C., Jaeggi, A. V., and Gurven, M. (2015). Evolving the neuroendocrine physiology of human and primate cooperation and collective action. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 370:20150014. doi: 10.1098/rstb.2015.0014
Tschida, K., Michael, V., Takatoh, J., Han, B. X., Zhao, S., Sakurai, K., et al. (2019). A specialized neural circuit gates social vocalizations in the mouse. Neuron 103, 459–472.e4. doi: 10.1016/j.neuron.2019.05.025
Tunçgenç, B., Cohen, E., and Fawcett, C. (2015). Rock with me: the role of movement synchrony in infants' social and nonsocial choices. Child Dev. 86, 976–984. doi: 10.1111/cdev.12354
Ukkola, L. T., Onkamo, P., Raijas, P., Karma, K., and Järvelä, I. (2009). Musical aptitude is associated with AVPR1A-haplotypes. PLoS One 4:e5534. doi: 10.1371/journal.pone.0005534
van Anders, S. M., Goldey, K. L., and Kuo, P. X. (2011). The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology 36, 1265–1275. doi: 10.1016/j.psyneuen.2011.06.001
van Honk, J., Bos, P. A., Terburg, D., Heany, S., and Stein, D. J. (2015). Neuroendocrine models of social anxiety disorder. Dialogues Clin. Neurosci. 17, 287–293. doi: 10.31887/DCNS.2015.17.3/jhonk
van Honk, J., Will, G. J., Terburg, D., Raub, W., Eisenegger, C., and Buskens, V. (2016). Effects of testosterone administration on strategic gambling in poker play. Sci. Rep. 6:18096. doi: 10.1038/srep18096
Vigil, P., Del Rio, J. P., Carrera, B., ArAnguiz, F. C., Rioseco, H., and Cortes, M. E. (2016). Influence of sex steroid hormones on the adolescent brain and behavior: an update. Linacre Q. 83, 308–329. doi: 10.1080/00243639.2016.1211863
Voyer, D., and Jansen, P. (2017). Motor expertise and performance in spatial tasks: A meta-analysis. Hum. Mov. Sci. 54, 110–124. doi: 10.1016/j.humov.2017.04.004
Wallin, N. L., Origins of, M, Wallin, N. L., Merker, B. R., Brown, S. M., Institute of, B, et al. (2000). The origins of music. Cambridge, Massachusetts; London, England: The MIT Press.
Weisman, O., Zagoory-Sharon, O., and Feldman, R. (2014). Oxytocin administration, salivary testosterone, and father-infant social behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 49, 47–52. doi: 10.1016/j.pnpbp.2013.11.006
Welling, L. L. M., and Shackelford, T. K. (2019). “Integrating mechanisms and functions to understand behavior” in The Oxford handbook of evolutionary psychology and behavioral endocrinology. eds. L. L. M. Welling and T. K. Shackelford (New York: Oxford University Press) doi: 10.1093/oxfordhb/9780190649739.013.1
Witczak, L. R., Simmons, T. C., and Bales, K. L. (2019). “Social Bond Paradoxes” in The Oxford handbook of evolutionary psychology and behavioral endocrinology. eds. L. L. M. Welling and T. K. Shackelford (New York: Oxford University Press)
Xiao, L., Priest, M. F., Nasenbeny, J., Lu, T., and Kozorovitskiy, Y. (2017). Biased Oxytocinergic modulation of midbrain dopamine systems. Neuron 95, 368–384.e5. doi: 10.1016/j.neuron.2017.06.003
Yuhi, T., Kyuta, H., Mori, H. A., Murakami, C., Furuhara, K., Okuno, M., et al. (2017). Salivary oxytocin concentration changes during a group drumming intervention for maltreated school children. Brain Sci. 7:152. doi: 10.3390/brainsci7110152
Zimmermann, E., Leliveld, L., and Schehka, S. (2013). “Toward the evolutionary roots of affective prosody in human acoustic communication: A comparative approach to mammalian voices” in Evolution of emotional communication: from sounds in nonhuman mammals to speech and music in man. eds. E. Altenmüller, S. Schmidt, and E. Zimmermann (Oxford: Oxford University Press), 116–132. doi: 10.1093/acprof:oso/9780199583560.003.0008
Keywords: origin of music, testosterone, oxytocin, sociality, cooperation
Citation: Fukui H and Toyoshima K (2023) Testosterone, oxytocin and co-operation: A hypothesis for the origin and function of music. Front. Psychol. 14:1055827. doi: 10.3389/fpsyg.2023.1055827
Edited by:
Takako Fujioka, Stanford University, United StatesReviewed by:
Irun R. Cohen, Weizmann Institute of Science, IsraelShan Chen, Chongqing Technology and Business University, China
Copyright © 2023 Fukui and Toyoshima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hajime Fukui, ✉ ZnVrdWloQGNjLm5hcmEtZWR1LmFjLmpw
 Hajime Fukui
Hajime Fukui Kumiko Toyoshima
Kumiko Toyoshima