- 1Faculty of Biomedical Sciences, Università della Svizzera italiana, Lugano, Switzerland
- 2Neuropsychological and Speech Therapy Unit, Neurocenter of Southern Switzerland, Ente Ospedaliero Cantonale (EOC), Lugano, Switzerland
The focus of this systematic review was to collect and align studies which analyze the functionality of theory of mind (TOM) in patients with mild cognitive impairments (MCI). Specifically, we identified 20 papers published between 2012 and 2022 which met inclusion criteria. Papers search, selection, and extraction followed the PRISMA guidelines. In order to summarize data from the papers, we used a narrative synthesis approach. Results in 18 of these 20 papers show that theory of mind (TOM) is impaired in all types of MCI patients—regardless of different etiology and diagnostic criteria. Only 2 out of 20 reported no significant differences in TOM performance between MCI patients and healthy control subjects. The review additionally aimed to bundle the variety of the type of tasks used by the author to assess multiple domains of TOM. This heterogeneity does not allow us to make a comprehensive comparison between the results, so we suggest the need to align the results using the same type of tests and TOM assessment. In the end, our work highlights the 2 neuropsychological studies which confirm more of our results; due to the objective approach adopted to investigate this topic, we suggest exploring this point of view more in future research.
Introduction
The ability to represent and attribute mental states to self and others—such as beliefs, emotions, desires, and knowledge—is called Theory of Mind (TOM) (Premack and Woodruff, 1978).
The first investigation about TOM dates to Premack and Woodruff (1978) and affirms that humans have the capacity to assume what others feels, believe, want, etc. and for those reasons, they infer states undeclared or not observable in a direct way and use these inferences to predict the behavior of others and their own, in an anticipatory way.
Theory of mind permits us to understand that others can have different values, beliefs, and feelings from ours. This kind of comprehension is a infinitely helpful in social interactions, because it allows us to make inferences on others' mental states and to deduce their behaviors (Premack and Woodruff, 1978).
TOM is not an innate ability; for this reason, Wimmer and Perner (1983) led studies in order to understand how children learn the representation of others' mental states and how they become able to differentiate them from the real world and their own. Thanks to the ideation of “false belief task” we know now that children acquire TOM abilities at around 4 years old. More recent studies, affirm that using more accurate methodology (for example, monitoring of gaze direction) children < 4 years old can also succeed in TOM tasks (Clements and Perner, 1994; Baillargeon et al., 2010).
In order to develop TOM abilities, there are several skills that each child should acquire first. The main skills are concept of attention, understanding others' intentions, and imitating others (Westby and Robinson, 2014; Bosco et al., 2017).
First, Baron-Cohen (1991) considers attention as a key concept and the real starting point to developing theory of mind, in particular regard for joint attention (when two people direct the attention to the same point/object, often pointing at it for more accuracy of direction).
Second, regarding intentionality, we mean the understanding that others' behaviors are directed toward a specific goal, which is determined by personal belief and desires (Dennett, 1983). Understanding that other's actions are driven by personal impulse, means being aware that everyone has their own desires, and so knowing how, attribute mental states. This ability is already developed in 2 year olds, not only in humans, but also in chimpanzees and orangutans (Call and Tomasello, 1998; Luchkina et al., 2018).
Last, the concept of imitating others means understanding that they have personal desires and beliefs. The first two concepts are fundamental to be able to copy other people, because it means realizing that people direct attention (to something or someone) in a motivated and personal way (drivers by personal desires).
As mentioned previously, already around 4 years old, children begin to consider the feelings and thoughts of others. Some researchers identified 5 phases of the development of TOM associated to a specific task to overcome (Wellman and Liu, 2004; Peterson et al., 2012).
Every phase consists of the ability to understand (Wellman and Liu, 2004; Peterson et al., 2012):
1. Wanting: Means realizing that others act in various ways in order to achieve different goals, influenced by their desires.
2. Thinking: Means realizing that others' actions are based on what they think could happen in a certain situation. The same situation could have different points of view because they are influenced by subjective beliefs.
3. Knowing: Seeing leads to knowing consists of the ability to recognize that others have different access to information. Often extra information is necessary to explain to other people what they have not seen or experienced.
4. False Beliefs: This concept refers to the ability to comprehend that other people may have incorrect beliefs that are not close to reality.
5. Hidden Feelings: Involves the ability to understand that sometimes others' emotions are masked. It means that it could happen that displayed emotions are different from real feelings.
TOM, according to several studies (Rossetto et al., 2018), is a multidimensional construct that includes different level of complexity; the attribution of intentions passed through the first order level of attribution, while the attribution of emotions concerns the second order level of attribution.
It is important to underline that TOM is often distinguished between cognitive and affective (Wang and Su, 2013): the ability to understand beliefs, intentions, and thoughts refers to cognitive TOM, differentiated from affective TOM that consists of thinking about their own or others' emotions and affect.
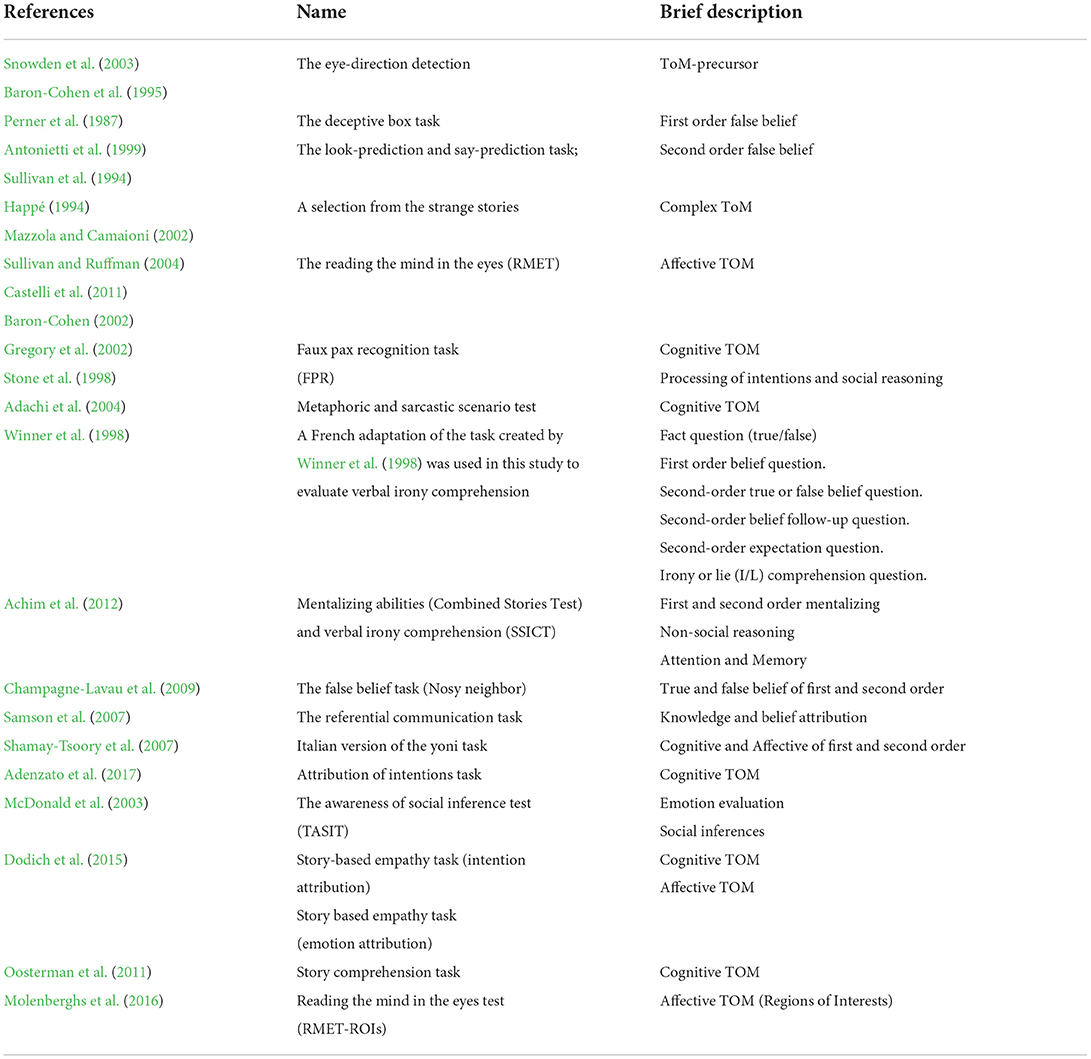
In this regard, there are several tests to assess TOM, such as the Reading the Mind in the Eyes Test (specific for affective TOM; Baron-Cohen et al., 2001), the Story Based Empathy Task (both for cognitive and affective TOM; Dodich et al., 2015) and the Faux Pas Recognition Test (specific for cognitive TOM; Stone et al., 1998) (for more complete information, see Table 2).
Different research showed that TOM can be impaired in several neurodegenerative disorders, such as in Alzheimer's Disease (as reported by Morese et al., 2018; Kessels et al., 2021; Morese and Palermo, 2022), Parkinson's Disease (Rossetto et al., 2018; Adenzato et al., 2019; Morese and Palermo, 2020), Frontotemporal Dementia—behavioral variant (Adenzato et al., 2010; Poletti et al., 2012; Orso et al., 2022), but also in cognitive disorders without dementia, such as Mild Cognitive Impairment (MCI). In this regard, several studies reported lower performance in many TOM tasks in MCI patients, compared to healthy populations (Baglio et al., 2012; Poletti and Bonuccelli, 2013; Moreau et al., 2015; Adenzato et al., 2019; Orso et al., 2022).
Mild Cognitive Impairment (MCI) is, by definition, considered a prodrome of dementia, a transitional phase between healthy aging and dementia (Anderson, 2019; Breton et al., 2019). In this phase patients have an increased risk of developing dementia in the following years, but their daily functioning is not yet impaired (Petersen, 1996a; Smith et al., 1996). The term MCI was first defined by Petersen et al. (1997) in order to describe this phase of transition, in which patients, as said before, do not meet criteria for dementia (Albert et al., 2011).
Also, in the DSM V (American Psychiatric Association, 2013), we can find a diagnostic category called “mild neurocognitive disorder (mild-NCD), to describe MCI. DSM V Diagnostic criteria meet the previously reported criteria (Petersen et al., 1997; Albert et al., 2011) and underline a mild cognitive decline in one or more domains (for example, executive function) that do not interfere with independence in daily activities, and it cannot be explained by a delirium context or by mental disorders (for example, depression or schizophrenia) (American Psychiatric Association, 2013).
Petersen (2016b) also provides us a distinction between 2 types of MCI: amnestic MCI (aMCI) and non-amnestic MCI (naMCI). Respectively, aMCI is related to deficits only in the memory domain, while naMCI is associated to deficits in single or multiple cognitive domains (for example, executive functions, language, memory, visuospatial abilities etc.), typically naMCI is prodromal of Alzheimer's disease (Albert et al., 2011).
Another specification that is important to report in MCI diagnosis is pathologic etiology on which cognitive decline depends, the main are: Alzheimer's Disease (AD-MCI), Parkinson's Disease (PD-MCI), Frontotemporal dementia (FTD-MCI), vascular disease (VaMCI), and Lewy body disease (MCI-LB) (Albert et al., 2011; Litvan et al., 2011; American Psychiatric Association, 2013).
Based on what was previously described, we performed a systematic review of the current literature to align and understand the current state of science on this topic; the aim of the present review is to deepen theory of mind in MCI patients. Since there is still a lot of ambiguity, this review can provide the opportunity to explore weaknesses and limitations on this argument and it can be a starting point for future research.
The goal of present review
This work is led by the necessity to align and collect studies that analyze social cognition in patients with MCI, in particular, regarding Theory of Mind. Generally, we know that social cognition is impaired in neuropsychological diseases (such as, frontotemporal dementia, Alzheimer's) (Morese et al., 2018; Palermo et al., 2020; Dodich et al., 2021), so we could expect that results in TOM tasks in patients with MCI might be insufficient compared to the control group. At the same time, we could also expect that TOM performance in MCI patients might be less impaired than performance in patients with severe neuropsychological disease (such as Alzheimer's).
Methods
The present systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, Moher et al., 2009). The method is currently available in the Open Science Framework (OSF, https://osf.io/hn7mc/).
Eligibility criteria
The focus of this systematic review was to collect studies which analyze the functionality of theory of mind (TOM) in patients with mild cognitive impairments (MCI).
The inclusion criteria were as follows:
• Studies must include populations with a diagnosis of mild cognitive impairment (MCI), evaluated by standardized diagnostic criteria of Petersen et al. (1997, 1999), Petersen and Negash (2008), Petersen (2016b) or DSM V criteria (American Psychiatric Association, 2013) or Winblad et al. (2004) or Albert (PD-MCI—Albert et al., 2011) or every cognitive impairment—without dementia—diagnosed with a validated cognitive test; for example, Dementia Rating Scale (DSR) (Mattis, 1988) or PD-MCI (Litvan et al., 2011).
• The medium age of the sample must be 60 years old, either male or female.
• We included all types of MCI: amnesic MCI (aMCI), non-amnesic MCI (naMCI), Parkinson's MCI (PD-MCI), Alzheimer's MCI (AD-MCI), Vascular MCI (VaMCI).
• We included studies that evaluated the domains of social cognition “theory of mind (TOM)”.
• Studies must include at least one clinical cognitive measurement for the social cognition domain analyzed (TOM).
The exclusion criteria were:
• Articles not in English were excluded.
• Meta-analysis, systematic reviews, single case studies, or other studies with a small sample (e.g., studies with < 10 participants) or only qualitative measurements, comments, books conference papers, letters, theses, and all studies not peer-reviewed were excluded.
Information sources
Search strategy
The search of the present study was conducted across Pubmed and Medline databases. For the MCI search strategy, we used the following terms: “MCI” OR “mild cognitive impairment”. The keywords were combined with the domain of social cognition: “theory of mind (TOM”) to produce the results.
Study selection
We only considered studies limited to humans and with a limited range period from January 2012 to May 2022. The reason behind this choice was that before 2012 we did not find significant studies which met our inclusion criteria (for example, before 2012 were published only studies which analyzed cognitive components and not social cognitive components related to MCI patients).
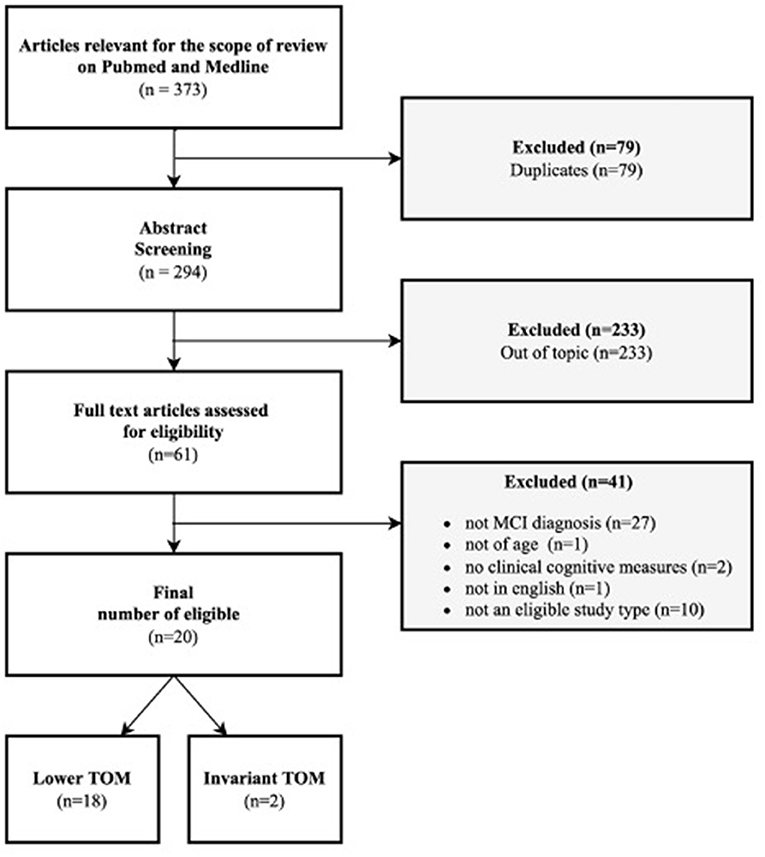
Moreover, meta-analysis, other systematic reviews, case studies, qualitative studies, or every study with a very small sample and without quantitative measurements were excluded from the present review. Initially, the papers included in the selection were 373, and then we excluded 79 duplicates. Reading title and abstract, from 294 articles we excluded 233 other articles out of the topic. Only 61 papers were considered eligible for the scope of the present review. Those 61 papers were further analyzed by reading the complete text, to discover if they respected inclusion criteria. At this point, another 41 articles were excluded because 27 did not have an MCI diagnosis (in line with inclusion criteria), 1 did not have a sample >60 years of age, another 2 did not have a clinical cognitive measurement to assess the domain selected for the present review (TOM), 1 was not in English and the last 10 were review or meta-analysis (as descripted previously in inclusion criteria). In the end, 20 articles were included in our review (see Figure 1).
Results
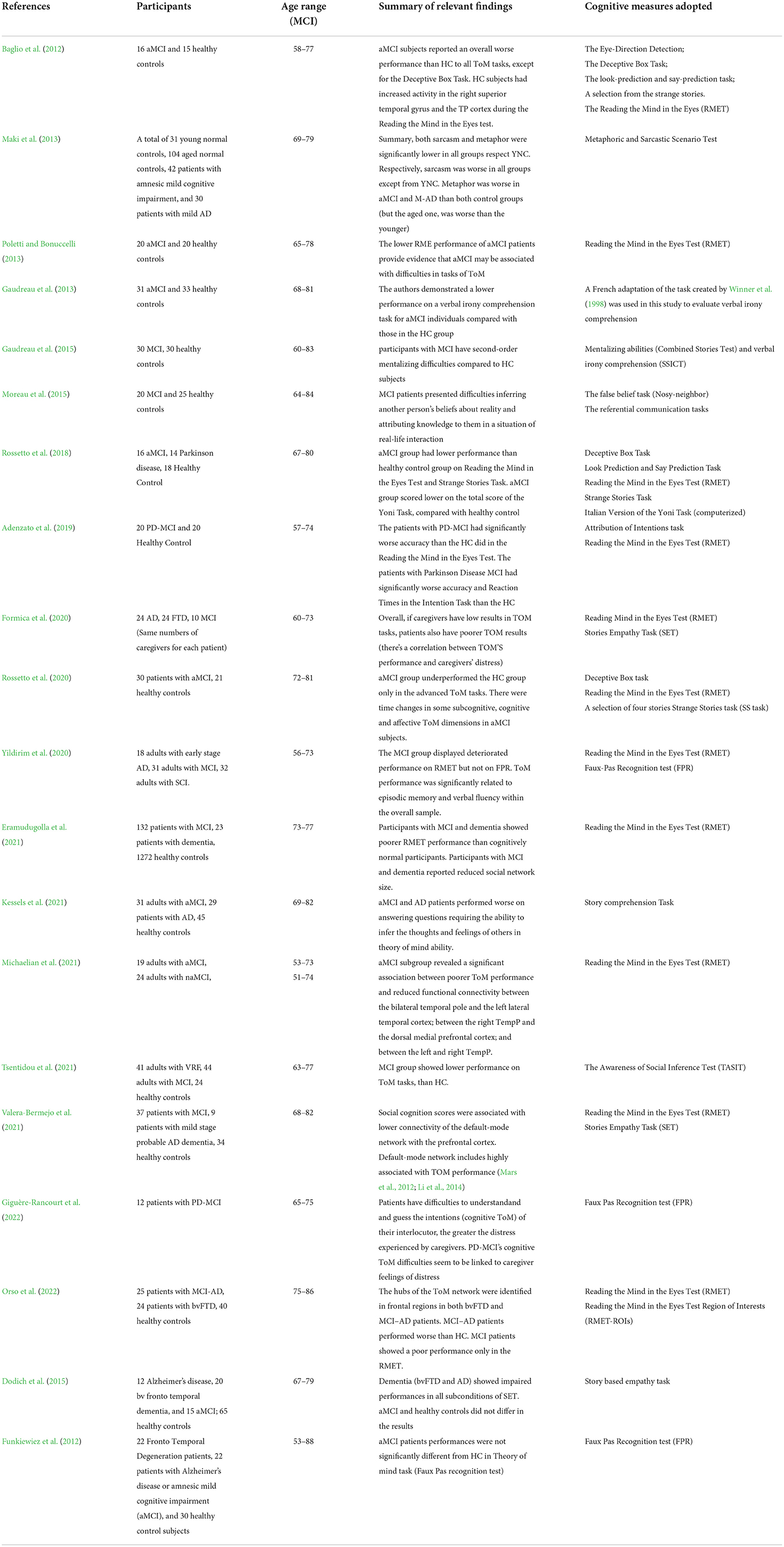
Overall, 20 studies were included in our review. Those studies examine the domain of theory of mind (TOM) (N = 20). As shown in the Table 1, in line with literature, most studies (N = 18) reported lower performance in Theory of Mind (TOM) than the control groups. Only 2 studies did not report significant differences.
Specifically, a study conducted by Baglio et al. (2012) on a sample of 16 amnesic MCI (aMCI) vs. healthy controls (HC), using a comprehensive battery of cognitive tasks (The Eye-Direction Detection; The Deceptive Box Task; The look-prediction and say-prediction task; A selection from the strange stories; The Reading the Mind in the Eyes test during fMRI—see the article for further and complete information), showed that aMCI subjects reported an overall worse performance than HC to all TOM tasks, except for the Deceptive Box Task (Perner et al., 1987; Happé, 1994; Sullivan et al., 1994; Baron-Cohen et al., 1995; Antonietti et al., 1999; Mazzola and Camaioni, 2002; Snowden et al., 2003; Sullivan and Ruffman, 2004; Castelli et al., 2011).
Another study (Maki et al., 2013), evaluated TOM with a different task (Metaphoric and Sarcastic Scenario Test—Adachi et al., 2004) on a sample of 31 young normal control (YNC), 104 aged normal controls (ANC), 42 patients with aMCI, and 30 patients with mild AD (M-AD). The results showed that both sarcasm and metaphor were significantly lower in all groups with respect to YNC. Respectively, sarcasm was worse in all groups except YNC. The metaphor was worse in aMCI and M-AD than in both control groups (but the aged one was worse than the younger). They used this task because sarcasm and metaphor could be compromised aspects in people with cognitive impairment. The most common task used to assess TOM is “The Reading the Mind in the Eyes test” (RMET; Baron-Cohen et al., 2001), for example, Poletti and Bonuccelli (2013) used this task on a sample of 20 aMCI vs. 20 HC, described a lower RMET performance of aMCI sample. Likewise, Gaudreau et al. (2013) with a similar sample (31 aMCI vs. 33 HC), used a test to evaluate verbal irony comprehension (Winner et al., 1998) and reported lower performances in the aMCI group. Also, Gaudreau et al. (2015) adopted a similar test for irony and mentalizing abilities (Achim et al., 2012) and reported the same results as the previous (difficulties in the second order of TOM in MCI sample).
Additional ways to evaluate TOM are “the False Belief Task” and the “Referential Communication Task” (Samson et al., 2007; Champagne-Lavau et al., 2009) used by Moreau et al. (2015) on a sample of 20 MCI; this study showed MCI patient had difficulties in others' beliefs and knowledge in a situation of real-life interaction compared to HC.
Rossetto et al. (2018), utilizing a large battery of tests (see Table 1), has discovered that a sample of 16 aMCI performed worse than the HC group on most tasks adopted. Adenzato et al. (2017) evaluated a sample of PD-MCI (n = 20) and showed a worse performance both in RMET and Attribution of Intention Task (Baron-Cohen et al., 2001; Adenzato et al., 2017).
Yildirim et al. (2020) conducted a study using the RME Test and the FPR Test (Faux Pas Recognition Test) (Baron-Cohen et al., 2001; Gregory et al., 2002) and discovered that the MCI group (n = 31) displayed deteriorated performance on RMET but not on FPR.
Rossetto et al. (2020) with a similar battery of tests used in a previous study (Rossetto et al., 2018), discovered that the aMCI group (n = 30) unperformed the HC only in the advanced TOM task (RMET and Strage Stories Task—Baron-Cohen et al., 2001; Happé, 1994; Mazzola and Camaioni, 2002). The RME Test (Baron-Cohen et al., 2001) was also used by Eramudugolla et al. (2021) on a sample of MCI (n = 132) and dementia patients (n = 23); the study displayed that MCI and dementia's groups showed a poorer RMET performance than HC group.
In line with the study of Moreau et al. (2015), Kessels et al. (2021), using the Story Comprehension Task (Oosterman et al., 2011) reported that aMCI (n = 31) and AD (n = 29) groups had difficulties inferring the thoughts and feelings of others.
Along the same line, Tsentidou et al. (2021) tested a sample of 31 adults with aMCI, 29 patients with AD, and 45 healthy controls and they discovered, using TASIT (The Awareness of Social Inference Test—McDonald et al., 2003), that performance on TOM tasks were worse in aMCI than in HC.
Two authors, included in the review, have reported a possible factor that could influence low results in TOM tasks (Formica et al., 2020; Giguère-Rancourt et al., 2022). The first of those studies included a sample of 10 MCI patients and the second study included a sample of 12 PD-MCI (with the same number of respective caregivers), and showed a possible correlation between low TOM performance in MCI patients and caregiver's distress. They assessed patient and caregivers with affective and cognitive TOM tasks (RMET, SET and FPR; Stone et al., 1998; Baron-Cohen et al., 2001; Gregory et al., 2002; Dodich et al., 2015), and both reported that MCI TOM difficulties seems to be linked to caregivers' distress.
Three studies adopted a neuropsychological approach, investigating the neural substrate of theory of mind.
First, Michaelian et al. (2021), on a sample of 43 adults with MCI (aMCI, n = 19; naMCI, n = 24) used RMET (Baron-Cohen et al., 2001) to assess TOM and functional magnetic resonance imaging to investigate the alterations in resting-state functional connectivity within the default mode network (DMN). The authors discovered that the aMCI group revealed a significant association between poorer TOM performance and reduced functional connectivity between the bilateral temporal pole (TempP) and the left lateral temporal cortex. The same phenomenon occurs between the right TempP and the dorsal medial prefrontal cortex and a decreased functional connectivity was reported also between the left and right TempP.
Valera-Bermejo et al. (2021) investigated TOM performance on a trial of 37 patients with MCI (mild stage with probable dementia, n = 9; healthy controls, n = 34) using the RME Test and the SET (Baron-Cohen et al., 2001; Dodich et al., 2015) showed that TOM scores were correlated with lower connectivity of the DMN with the prefrontal cortex.
In the end, also the most recent study of the present review (Orso et al., 2022), agrees with the previous studies. In fact, it is reported that MCI-AD patients (n = 25) showed a poorer performance in the RMET compared to HC (n = 40). Due to the adoption of the RMET ROIs (RMET Region of Interests—Molenberghs et al., 2016), the authors found that the neural regions involved in RMET are: left middle frontal gyrus, left middle temporal gyrus, and left superior frontal gyrus.
It is important to underline those two studies did not report significant differences between MCI patients and HC in TOM tasks. According to Funkiewiez et al. (2012) and Dodich et al. (2015) aMCI performances in cognitive tests used, were not significantly different from HC. Respectively, the first study assessed TOM with the ”Faux-pas Recognition test” (Stone et al., 1998; Gregory et al., 2002) on a sample of 11 aMCI. The latter used “Story-based Empathy task” (Dodich et al., 2015) on sample of 15 aMCI.
Discussion
We found that the overall evidence of reported results in TOM tasks are in line with the literature. Most of the studies reported that TOM impairment is related to all TOM phases (mentioned before) in both affective and cognitive TOM. Those results depend on the variety of tests used by the authors to investigate multiple domains of social cognition; in particular, out of 20 articles 19 different tests were used (see Table 2). This aspect is an advantage but, on the other hand, it is a limitation because it culminated in ambiguous results; for example, some studies reported lower TOM performance while investigating only the cognitive domain of TOM (Baglio et al., 2012; Maki et al., 2013; Moreau et al., 2015), yet there are studies that showed the same results using only affective tasks (Poletti and Bonuccelli, 2013; Yildirim et al., 2020; Eramudugolla et al., 2021; Michaelian et al., 2021; Orso et al., 2022). This heterogeneity in the adoption of the tests does not allow for overlapping results. Of the 20 studies, only half of the papers use a comprehensive battery of tasks, which permits us to explore TOM in a global way and to obtain more significative results than the authors who chose to adopt a narrower battery of tasks. Despite the methodological variety, the outcomes are all in accordance; social cognition, in particular regard with TOM, seems to be significantly impaired in all types of patients with mild cognitive impairment. Another aspect to be discussed is the composition of the sample, specifically, every paper has a different type of sample based on different etiology and diagnostic system for MCI (i.e., Pertersen or DSM V). For example, some studies have a sample of amnesic MCI (Baglio et al., 2012; Maki et al., 2013), others of Parkinson's Disease -MCI (Adenzato et al., 2019; Giguère-Rancourt et al., 2022) or Alzheimer's Disease -MCI (Valera-Bermejo et al., 2021; Orso et al., 2022). Despite the results being all along the same line, it could be interesting to divide and compare the results of MCI patients based on their etiology or diagnosis. In our review, this was not achievable given the few results on the topic, but it could be a good starting point for future research. Regarding the composition of the sample, we highlight that the present review included few subjects; consequently, the outcomes obtained could not be generalized and the magnitude of an association could be overestimated.
Last, it is important to underline that only 2 papers out of 20 did not reported significant differences in TOM performance between MCI patients and HC (Funkiewiez et al., 2012; Dodich et al., 2015). A possible explanation of these contradictory results is that both papers have small samples and the MCI groups were not considered as the mains focus of the studies (for example, in Funkiewiez et al., 2012, aMCI patients were used just as a control group). This could explain the different results.
The most recent studies (Valera-Bermejo et al., 2021; Orso et al., 2022), in addition to investigating the TOM through a cognitive approach, adopted also a neurological approach that described the neural substrate associated with low performance in TOM. These authors discovered that low performances in social cognition were correlated with lower connectivity of the default-mode-network (DMN—i.e., a set of brain regions in temporal, parietal, and frontal cortex involved in attention and complex cognition tasks, such as abstract thought; Smallwood et al., 2021) with the prefrontal cortex. Specifically, the prefrontal cortex is the damaged brain area in MCI patients. In conclusion, DMN is highly associated with TOM performance; this evidence provides a further demonstration of what was reported in most studies mentioned before (Table 2).
Conclusion
This systematic review analyzed the last 10 years of literature on theory of mind in MCI patients. MCI was diagnosed in several ways because there are different etiologies that could predict it (i.e., PD-MCI or AD-MCI). This could indicate a gap in the case of comparisons between studies.
Also, every author used different types of tasks to assess TOM (which measure all phases of TOM and both cognitive and affective TOM); this gave us a comprehensive view on this topic, but, on the other hand, this could be a limit if we make a comparison between results.
Our goal was to collect all results but align the results using the same diagnostic criteria, but the same type of tests could be advisable for future research.
Another limit of the sample was the small size for most of the studies and, consequently, the outcomes obtained could not be generalized and the magnitude of an association could be over-estimated.
Last, the neuropsychological approach should be more deeply explored because it could be another interesting point of view that might support the majority of results obtained up to now.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Author contributions
LS carried out part of the literature search, collected part of the studies, described part of the results, contributed to complete the table, and reviewed the final version manuscript. LM had the main contribution in the literature search and selection, created the draft, the two tables and the flow diagram, deals with defining and writing the method, wrote the abstract, the introduction and part of the results, of the discussion and the conclusion, and reviewed the references and the final version of the manuscript. AI elaborated the table and wrote part of the introduction, of the results, of the discussion and of the conclusion and reviewed the references and the final version of the manuscript. SR collected part of the literature search. MC and LR-C contributed to the collection and selection of the literature. GZ and EB contributed to the collected part of the literature search. All authors contributed to the article and approved the submitted version.
Funding
Open access funding provided by Università Della Svizzera Italiana.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Achim, A. M., Ouellet, R., Roy, M. A., and Jackson, P. L. (2012). Mentalizing in first-episode psychosis. Psychiatry Res. 196, 207–213. doi: 10.1016/j.psychres.2011.10.011
Adachi, T., Koeda, T., Hirabayashi, S., Maeoka, Y., Shiota, M., Wright, E. C., et al. (2004). The metaphor and sarcasm scenario test: a new instrument to help differentiate high functioning pervasive developmental disorder from attention deficit/hyperactivity disorder. Brain Develop. 26, 301–306. doi: 10.1016/S0387-7604(03)00170-0
Adenzato, M., Brambilla, M., Manenti, R., De Lucia, L., Trojano, L., Garofalo, S, et al. (2017). Gender differences in cognitive theory of mind revealed by transcranial direct current stimulation on medial prefrontal cortex. Sci. Rep. 7, 41219. doi: 10.1038/srep41219
Adenzato, M., Cavallo, M., and Enrici, I. (2010). Theory of mind ability in the behavioural variant of frontotemporal dementia: an analysis of the neural, cognitive, and social levels. Neuropsychologia 48, 2–12. doi: 10.1016/j.neuropsychologia.2009.08.001
Adenzato, M., Manenti, R., Enrici, I., Gobbi, E., Brambilla, M., Alberici, A., et al. (2019). Transcranial direct current stimulation enhances theory of mind in Parkinson's disease patients with mild cognitive impairment: a randomized, double-blind, sham-controlled study. Translat. Neurodegenerat. 8, 1–13. doi: 10.1186/s40035-018-0141-9
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Dementia J. Alzheimer's Associat. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
American Psychiatric Association (2013). “Cautionary statement for forensic use of DSM-5,” in Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington, DC: Author. Cautionary Statement.
Anderson, N. D. (2019). State of the science on mild cognitive impairment (MCI). CNS Spectrums 24, 78–87. doi: 10.1017/S1092852918001347
Antonietti, A., Liverta-Sempio, O., and Marchetti, A. (1999). Second-Order False Belief Tasks: Look-Prediction and Say-Prediction. Department of Psychology, Università Cattolica del Sacro Cuore, Milano, Italy.
Baglio, F., Castelli, I., Alberoni, M., Blasi, V., Griffanti, L., Falini, A., et al. (2012). Theory of mind in amnestic mild cognitive impairment: an FMRI study. J. Alzheimer's Dis. JAD 29, 25–37. doi: 10.3233/JAD-2011-111256
Baillargeon, R., Scott, R. M., and He, Z. (2010). False-belief understanding in infants. Trends Cognit. Sci. 14, 110–118. doi: 10.1016/j.tics.2009.12.006
Baron-Cohen S. (2002). The extreme male brain theory of autism. Trends Cogn. Sci. 6, 248–254. doi: 10.1016/s1364-6613(02)01904-6
Baron-Cohen, S. (1991). Precursors to a theory of mind: understanding attention in others. Natural theories of mind: evolution, development and simulation of everyday mindreading. Google Scholar 1, 233–251.
Baron-Cohen, S., Campbell, R., Karmiloff-Smith, A., Grant, J., and Walker, J. (1995). Are children with autism blind to the mentalistic significance of the eyes? Br. J. Dev. Psychol. 13, 379–398. doi: 10.1111/j.2044-835X.1995.tb00687.x
Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y., and Plumb, I. (2001). The “reading the mind in theeyes” test revised version: a study with normal adults, and adults with asperger syndrome orhigh-functioning autism. J. Child Psychol. Psychiatry 42, 241–251. doi: 10.1111/1469-7610.00715
Bosco, F. M., Parola, A., Valentini, M. C., and Morese, R. (2017). Neural correlates underlying the comprehension of deceitful and ironic communicative intentions. Cortex J. Devoted Study Nervous Syst. Behav. 94, 73–86. doi: 10.1016/j.cortex.2017.06.010
Breton, A., Casey, D., and Arnaoutoglou, N. A. (2019). Cognitive tests for the detection of mild cognitive impairment (MCI), the prodromal stage of dementia: meta-analysis of diagnostic accuracy studies. Int. J. Geriatric Psychiatry 34, 233–242. doi: 10.1002/gps.5016
Call, J., and Tomasello, M. (1998). Distinguishing intentional from accidental actions in orangutans (Pongo pygmaeus), chimpanzees (Pan troglodytes), and human children (Homo sapiens). J. Comparat. Psychol. 112, 192–206. doi: 10.1037/0735-7036.112.2.192
Castelli, I., Pini, A., Alberoni, M., Liverta-Sempio, O., Baglio F,Massaro, D., Marchetti, A., et al. (2011). Mapping levels of theory of mind in Alzheimer's disease: a preliminary study. Aging Ment. Health 15, 157–168. doi: 10.1080/13607863.2010.513038
Champagne-Lavau, M., Fossard, M., Martel, G., Chapdelaine, C., Blouin, G., Rodriguez, J. P., et al. (2009). Do patients with schizophrenia attribute mental states in a referential communication task? Cognit. Neuropsychiatry 14, 217–239. doi: 10.1080/13546800903004114
Clements, W. A., and Perner, J. (1994). Implicit understanding of belief. Cogn. Dev. 9, 377–395. doi: 10.1016/0885-2014(94)90012-4
Dennett, D. C. (1983). Intentional systems in cognitive ethology: the “Panglossian paradigm” defended. Behav. Brain Sci. 6, 343–390. doi: 10.1017/S0140525X00016393
Dodich, A., Cerami, C., Canessa, N., Crespi, C., Iannaccone, S., Marcone, A., et al. (2015). A novel task assessing intention and emotion attribution: Italian standardization and normative data of the Story-based Empathy Task. Neurol. Sci. Official J. Italian Neurol. Soc. Italian Soc. Clin. Neurophysiol. 36, 1907–1912. doi: 10.1007/s10072-015-2281-3
Dodich, A., Crespi, C., Santi, G. C., Cappa, S. F., and Cerami, C. (2021). Evaluation of discriminative detection abilities of social cognition measures for the diagnosis of the behavioral variant of frontotemporal dementia: a systematic review. Neuropsychol. Rev. 31, 251–266. doi: 10.1007/s11065-020-09457-1
Eramudugolla, R., Huynh, K., Zhou, S., Amos, J. G., and Anstey, K. J. (2021). Social cognition and social functioning in MCI and dementia in an epidemiological sample. J. Int. Neuropsychol. Soc. JINS 28, 1–12. doi: 10.1017/S1355617721000898
Formica, C., Bonanno, L., Todaro, A., Marra, A., Alagna, A., Corallo, F., et al. (2020). The role of mind theory in patients affected by neurodegenerative disorders and impact on caregiver burden. J. Clin. Neurosci. 78, 291–295. doi: 10.1016/j.jocn.2020.05.028
Funkiewiez, A., Bertoux, M., de Souza, L. C., Lévy, R., and Dubois, B. (2012). The SEA (Social cognition and Emotional Assessment): a clinical neuropsychological tool for early diagnosis of frontal variant of frontotemporal lobar degeneration. Neuropsychology 26, 81–90. doi: 10.1037/a0025318
Gaudreau, G., Monetta, L., Macoir, J., Laforce, R. Jr, Poulin, S., and Hudon, C. (2013). Verbal irony comprehension in older adults with amnestic mild cognitive impairment. Neuropsychology 27, 702–712. doi: 10.1037/a0034655
Gaudreau, G., Monetta, L., Macoir, J., Poulin, S., Laforce, R. Jr, and Hudon, C. (2015). Mental state inferences abilities contribution to verbal irony comprehension in older adults with mild cognitive impairment. Behav. Neurol. 2015, 685613. doi: 10.1155/2015/685613
Giguère-Rancourt, A., Plourde, M., Racine, E., Couture, M., Langlois, M., Dupr,é, N., et al. (2022). Altered theory of mind in Parkinson's disease and impact on caregivers: a pilot study. Canad. J. Neurol. Sci. Le J. Canad. Des Sci. Neurol. 49, 437–440. doi: 10.1017/cjn.2021.110
Gregory, C., Lough, S., Stone, V., Erzinclioglu, S., Martin, L., Baron-Cohen, S., et al. (2002). Theory of mind in patients withfrontal variant frontotemporal dementia and Alzheimer's disease: the-oretical and practical implications. Brain 125, 752–764. doi: 10.1093/brain/awf079
Happé F. G. (1994). An advanced test of theory of mind: understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J. Autism Develop. Disorders 24, 129–154. doi: 10.1007/BF02172093
Kessels, R., Waanders-Oude Elferink, M., and van Tilborg, I. (2021). Social cognition and social functioning in patients with amnestic mild cognitive impairment or Alzheimer's dementia. J. Neuropsychol. 15, 186–203. doi: 10.1111/jnp.12223
Li, W., Mai, X., and Liu, C. (2014). The default mode network and socialunderstanding of others: what do brain connectivity studies tell us. Front. Human Neurosci. 8, 74. doi: 10.3389/fnhum.2014.00074
Litvan, I., Aarsland, D., Adler, C. H., Goldman, J. G., Kulisevsky, J., Mollenhauer, B., et al. (2011). MDS task force on mild cognitive impairment in Parkinson's disease: critical review of PD-MCI. Movement Disorders Official J. Movement Disorder Soc. 26, 1814–1824. doi: 10.1002/mds.23823
Luchkina, E., Sommerville, J. A., and Sobel, D. M. (2018). More than just making it go: Toddlers effectively integrate causal efficacy and intentionality in selecting an appropriate causal intervention. Cognit. Develop. 45, 48–56. doi: 10.1016/j.cogdev.2017.12.003
Maki, Y., Yamaguchi, T., Koeda, T., and Yamaguchi, H. (2013). Communicative competence in Alzheimer's disease: metaphor and sarcasm comprehension. Am. J. Alzheimer's Dis. Other Dementias® 28, 69–74. doi: 10.1177/1533317512467677
Mars, R. B., Neubert, F. -X., Noonan, M. P., Sallet, J., Toni, I., and Rushworth, M. F. S. (2012). On the relationship between the “default mode network” and the “social brain”. Front. Human Neurosci. 6, 189. doi: 10.3389/fnhum.2012.00189
Mattis, S. (1988). Dementia Rating Scale: Professional Manual. Psychological Assessment Resources, Incorporated.
Mazzola, V., and Camaioni, L. (2002). Strane Storie: Versione Italiana a Cura Di Mazzola e Camaioni. Dipartimento di Psicologia Dinamica e Clinica. Università “La Sapienza”, Roma, Italy. Google Scholar.
McDonald, S., Flanagan, S., Rollins, J., and Kinch, J. (2003). TASIT: a new clinical tool for assessing social perception after traumatic brain injury. J. Head Trauma Rehabilitat. 18, 219–238. doi: 10.1097/00001199-200305000-00001
Michaelian, J. C., Duffy, S. L., Mowszowski, L., Guastella, A. J., McCade, D., McKinnon, A. C., et al. (2021). Poorer theory of mind in amnestic mild cognitive impairment is associated with decreased functional connectivity in the default mode network. J. Alzheimer's Dis. JAD 81, 1079–1091. doi: 10.3233/JAD-201284
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Prisma Group. (2009). Reprint-preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys. Ther. 89, 873–880. doi: 10.1136/bmj.b2535
Molenberghs, P., Johnson, H., Henry, J. D., and Mattingley, J. B. (2016). Understanding the minds of others: a neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 65, 276–291. doi: 10.1016/j.neubiorev.2016.03.020
Moreau, N., Rauzy, S., Bonnefoi, B., Reni,é, L., Martinez-Almoyna, L., Viallet, F., et al. (2015). Different patterns of theory of mind impairment in mild cognitive impairment. J. Alzheimer's Dis. 45, 581–597. doi: 10.3233/JAD-143021
Morese, R., and Palermo, S. (2020). Altruistic punishment and impulsivity in Parkinson's disease: a social neuroscience perspective. Front. Behav. Neurosci. 14, 102. doi: 10.3389/fnbeh.2020.00102
Morese, R., and Palermo, S. (2022). Feelings of loneliness and isolation: Social brain and social cognition in the elderly and Alzheimer's disease. Front. Aging Neurosci. 14, 896218. doi: 10.3389/fnagi.2022.896218
Morese, R., Stanziano, M., and Palermo, S. (2018). Commentary: metacognition and perspective-taking in alzheimer's disease: a mini-review. Front. Psychol. 9, 2010. doi: 10.3389/fpsyg.2018.02010
Oosterman, J. M., de Goede, M., Wester, A. J., van Zandvoort, M. J., and Kessels, R. P. (2011). Perspective taking in Korsakoff's syndrome: the role of executive functioning and task complexity. Acta Neuropsychiatrica 23, 302–308. doi: 10.1111/j.1601-5215.2011.00552.x
Orso, B., Lorenzini, L., Arnaldi, D., Girtler, N., Brugnolo, A., Doglione, E., et al. (2022). The role of hub and spoke regions in theory of mind in early Alzheimer's disease and frontotemporal dementia. Biomedicines 10, 544. doi: 10.3390/biomedicines10030544
Palermo, S., Carassa, A., and Morese, R. (2020). Editorial: perspective-taking, self-awareness and social cognition in neurodegenerative disorders, cerebral abnormalities and acquired brain injuries (ABI): a neurocognitive approach. Front. Psychol. 11, 614609. doi: 10.3389/fpsyg.2020.614609
Perner, J., Leekam, S. R., and Wimmer, H. (1987). Three-year-olds'difficulty with false belief: the case for a conceptual deficit. Br. J. Dev. Psychol. 5, 125–137. doi: 10.1111/j.2044-835X.1987.tb01048.x
Petersen, R., and Negash, S. (2008). Mild cognitive impairment: an overview. CNS Spectrums 13, 45–53. doi: 10.1017/S1092852900016151
Petersen, R. C. (1996a). “Disorders of memory,” in Office practice in neurology, eds M. A. Samuels and S. Feske (New York: Churchill Livingstone). vol. 138, p. 728–736.
Petersen, R. C. (2016b). Mild cognitive impairment. Continuum (Minneapolis, Minn.). 22(2 Dementia), 404–418. doi: 10.1212/CON.0000000000000313
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Kokmen, E., and Tangelos, E. G. (1997). Aging, memory, and mild cognitive impairment. Int. Psychogeriatrics 9, 65–69. doi: 10.1017/S1041610297004717
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. doi: 10.1001/archneur.56.3.303
Peterson, C. C., Wellman, H. M., and Slaughter, V. (2012). The mind behind the message: advancing theory-of-mind scales for typically developing children, and those with deafness, autism, or Asperger syndrome. Child Develop. 83, 469–485. doi: 10.1111/j.1467-8624.2011.01728.x
Poletti, M., and Bonuccelli, U. (2013). Alteration of affective Theory of Mind in amnestic mild cognitive impairment. J. Neuropsychol. 7, 121–131. doi: 10.1111/j.1748-6653.2012.02040.x
Poletti, M., Enrici, I., and Adenzato, M. (2012). Cognitive and affective Theory of Mind in neurodegenerative diseases: neuropsychological, neuroanatomical and neurochemical levels. Neurosci. Biobehav. Rev. 36, 2147–2164. doi: 10.1016/j.neubiorev.2012.07.004
Premack, D., and Woodruff, G. (1978). Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1, 515–526. doi: 10.1017/S0140525X00076512
Rossetto, F., Baglio, F., Massaro, D., Alberoni, M., Nemni, R., Marchetti, A., et al. (2020). Social cognition in rehabilitation context: different evolution of affective and cognitive theory of mind in mild cognitive impairment. Behav. Neurol. doi: 10.1155/2020/5204927
Rossetto, F., Castelli, I., Baglio, F., Massaro, D., Alberoni, M., Nemni, R., et al. (2018). Cognitive and affective theory of mind in mild cognitive impairment and Parkinson's disease: preliminary evidence from the italian version of the Yoni task. Develop. Neuropsychol. 43, 764–780. doi: 10.1080/87565641.2018.1529175
Samson, D., Apperly, I. A., and Humphreys, G. W. (2007). Error analyses reveal contrasting deficits in “theory of mind”: neuropsychological evidence from a 3-option false belief task. Neuropsychologia 45, 256–2569. doi: 10.1016/j.neuropsychologia.2007.03.013
Shamay-Tsoory, S. G., Aharon-Peretz, J., and Levkovitz, Y. (2007). The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophrenia research 90, 274–283. doi: 10.1016/j.schres.2006.09.020
Smallwood, J., Bernhardt, B. C., Leech, R., Bzdok, D., Jefferies, E., and Margulies, D. S. (2021). The default mode network in cognition: a topographical perspective. Nat. Rev. Neurosci. 22, 503–513. doi: 10.1038/s41583-021-00474-4
Smith, G. E, Petersen, R. C., Parisi, J. E., Ivnik, R. J., Kokmen, E., et al. (1996). Definition, course, and outcome of mild cognitive impairment. Aging Neuropsychol. Cognit. 3,141–147. doi: 10.1080/13825589608256619
Snowden, J. S., Gibbons, Z. C., Blackshaw, A., Doubleday, E., Thompson, J., Craufurd, D., et al. (2003). Social cognition in frontotemporal dementia and Huntington's disease. Neuropsychologia 41, 688–701. doi: 10.1016/S0028-3932(02)00221-X
Stone, V. E., Baron-Cohen, S., and Knight, R. T. (1998). Frontal lobecontributions to theory of mind. J. Cognit. Neurosci. 10, 640–656. doi: 10.1162/089892998562942
Sullivan, K., Zaitchik, D., and Tager-Flusberg, H. (1994). Preschoolers can attribute second-order beliefs. Dev. Psychol. 30, 395–402. doi: 10.1037/0012-1649.30.3.395
Sullivan, S., and Ruffman, T. (2004). Emotion recognition deficits in the elderly. Int. J. Neurosci. 114, 403–432. doi: 10.1080/00207450490270901
Tsentidou, G., Moraitou, D., and Tsolaki, M. (2021). Similar theory of mind deficits in community dwelling older adults with vascular risk profile and patients with mild cognitive impairment: the case of paradoxical sarcasm comprehension. Brain Sci. 11, 627. doi: 10.3390/brainsci11050627
Valera-Bermejo, J. M., De Marco, M., Mitolo, M., McGeown, W. J., and Venneri, A. (2021). Neuroanatomical and cognitive correlates of domain-specific anosognosia in early Alzheimer's disease. Cortex 129, 236–246. doi: 10.1016/j.cortex.2020.04.026
Wang, Z., and Su, Y. (2013). Age-related differences in the performance of theory of mind in older adults: a dissociation of cognitive and affective components. Psychol. Aging 28, 284–291. doi: 10.1037/a0030876
Wellman, H. M., and Liu, D. (2004). Scaling of theory-of-mind tasks. Child Develop. 75, 523–541. doi: 10.1111/j.1467-8624.2004.00691.x
Westby, C., and Robinson, L. (2014). A developmental perspective for promoting theory of mind. Topics Language Disorders 34, 362–383. doi: 10.1097/TLD.0000000000000035
Wimmer, H., and Perner, J. (1983). Beliefs about beliefs: representation and constraining function of wrong beliefs in young children's understandingof deception. Cognition 13, 103–128. doi: 10.1016/0010-0277(83)90004-5
Winblad, B., Palmer, K., Kivipelto, M., Jelic, V., Fratiglioni, L., Wahlund, L. O., et al. (2004). Mild cognitive impairment—beyond controversies, towards a consensus: report of the international working group on mild cognitive impairment. J. Int. Med. 256, 240–246. doi: 10.1111/j.1365-2796.2004.01380.x
Winner, E., Brownell, H., Happe, F., Blum, A., and Pincus, D. (1998). Distinguishing lies from jokes: theory of mind deficits and discourse interpretation in right hemisphere brain-damaged patients. Brain Language 62, 89–106. doi: 10.1006/brln.1997.1889
Keywords: theory of mind, mild cognitive impairment, social cognition, systematic review, tasks
Citation: Morellini L, Izzo A, Ceroni M, Rossi S, Zerboni G, Rege-Colet L, Biglia E and Sacco L (2022) Theory of mind in patients with mild cognitive impairment: A systematic review. Front. Psychol. 13:994070. doi: 10.3389/fpsyg.2022.994070
Received: 14 July 2022; Accepted: 16 August 2022;
Published: 18 October 2022.
Edited by:
Sara Palermo, University of Turin, ItalyReviewed by:
Elisabetta Groppo, San Paolo Hospital, ItalyLaura Zapparoli, University of Milano-Bicocca, Italy
Copyright © 2022 Morellini, Izzo, Ceroni, Rossi, Zerboni, Rege-Colet, Biglia and Sacco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucia Morellini, bHVjaWEubW9yZWxsaW5pQHVzaS5jaA==
 Lucia Morellini
Lucia Morellini Alessia Izzo
Alessia Izzo Martino Ceroni2
Martino Ceroni2 Stefania Rossi
Stefania Rossi Leonardo Sacco
Leonardo Sacco