
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol., 20 October 2022
Sec. Psychology for Clinical Settings
Volume 13 - 2022 | https://doi.org/10.3389/fpsyg.2022.977264
This article is part of the Research TopicEmpowering of Assessment Techniques in Clinical Health PsychologyView all 4 articles
 Tonia Samela1,2*
Tonia Samela1,2* Giorgia Cordella2
Giorgia Cordella2 Valeria Antinone2
Valeria Antinone2 Paride Sarandrea3
Paride Sarandrea3 Anna Rita Giampetruzzi3
Anna Rita Giampetruzzi3 Damiano Abeni1
Damiano Abeni1Objectives: To measure general psychopathology in dermatologic outpatients using the Symptom-Checklist-K-9 (SCL-K-9); to investigate whether the SCL-K-9 is able to categorize patients with and without significant non-psychotic disorders; and to perform a single-item analysis of the SCL-K-9, with a focus on gender differences.
Methods: Cross-sectional study on consecutive dermatological patients. We used two self-administered questionnaires to assess general psychopathology symptoms: General Health Questionnaire-12 (GHQ-12) and SCL-K-9. Sociodemographic information was collected with standardized forms. The performance of the SCL-K-9 in classifying patients according to their current emotional distress severity was assessed using a ROC procedure. Finally, we measured differences in scores obtained among women and men in SCL-K-9 single items.
Results: A total of 292 patients were studied (71.2% women). We observed statistically significant differences in SCL-K-9 total mean scores and in most single items among genders. We found that it would be more appropriate to use gender-specific cut-offs when using SCL-K-9 to screen dermatological patients for general psychopathology.
Conclusion: The SCL-K-9, with its compact format could provide, in a short time, a wide range of information related to critical areas that challenge the mental health of patients with skin diseases.
Different dermatologic conditions are chronic, relapsing, difficult to manage and characterized by a wide variety of symptoms. Although skin conditions, even the severe ones, are not often directly life-threatening, it has been reported that dermatology patients are at greater risk of experiencing mental illness than the general population (Picardi et al., 2000; Huilaja et al., 2018; Konstantinou and Konstantinou, 2019), and the co-existence of psychological distress and chronic skin conditions is well established in the literature (Sampogna et al., 2004; Picardi et al., 2006a; Konstantinou and Konstantinou, 2019; Jafferany et al., 2020; Mento et al., 2020; Finlay et al., 2021).
Several studies are also stressing the observation that women sustain a greater burden of illness compared to men with skin diseases (Ständer et al., 2013; Napolitano et al., 2020), or autoimmune diseases with skin manifestations (Ngo et al., 2014) or other chronic conditions (August and Sorkin, 2010; Kim et al., 2011; Kim and Kim, 2018).
For these reasons a general personality and psychopathology evaluation should be considered a crucial part of the multidisciplinary assessment for dermatologic issues (Panebianco et al., 2018), and it would also be advisable to pay greater attention to gender differences in the process of evaluation.
However, as confirmed by a multi-center study conducted across 13 countries in Europe, dermatologists would tend to underestimate psychiatric symptoms in a significant group of dermatologic patients (Dalgard et al., 2018), for reasons that could be related to the limited availability of time and to the high number of patients attending the clinical units daily.
Several tools have been validated in order to measure psychological distress in dermatologic outpatients, with consistent results (Lewis and Finlay, 2004; Picardi et al., 2004; Prinsen et al., 2010; Sampogna et al., 2013); therefore, quick and manageable self-reported screening tools are available, and could be useful for clinicians in order to identify patients who need a more in-depth psychological assessment procedure, and possibly counselling and support.
The Symptom Checklist-90-Revised (SCL-90-R; Derogatis and Savitz, 2000) is a commonly used self-rating measure, composed by ninety items, for assessing general psychopathology in different populations (Schauenburg and Strack, 1999; Hardt et al., 2000; Bianciardi et al., 2021; Castelo-Branco et al., 2021; Melchior et al., 2022; Timman and Arrindell, 2022). Additionally, this tool has demonstrated a factorial invariance (i.e., the concept that postulates that the psychometric properties of a questionnaire, used either by multiple groups or by the same group over time, have to be identical to ensure an unbiased comparison of factor means) across genders (Derogatis and Cleary, 1977).
Since the 1980s, several short forms of the SCL-90-R have been proposed to provide a more manageable tool (Prinz et al., 2013). Among these versions, the Symptom-Checklist-K-9 (SCL-K-9), composed of the nine items of the SCL-90-R (i.e., #24, #28, #31, #34, #43, #57, #58, #75, #77) that exhibit the highest item-total correlation, was proposed as an efficient screening tool representing all of the original symptom subscales of the SCL-90-R (Klaghofer and Brähler, 2001). It was also validated in the German (Klaghofer and Brähler, 2001) and Ukrainian (Sereda and Dembitskyi, 2016) general populations, and in Italian overweight and obese patients seeking weight-loss treatment (Imperatori et al., 2020). Furthermore, Petrowski et al. (2019), also confirmed results of factorial invariance of the scale across gender groups in representative German population (Petrowski et al., 2019).
Therefore, the SCL-K-9 could be considered a suitable and valid tool that could be used within the time restraints that preclude the use of the full-length form in the busy daily clinical practice.
To the best of our knowledge, no study has ever applied this tool to measure general psychopathology in dermatologic patients during the routine clinical activities. Thus, the aims of the present study were: (i) to measure general psychopathology in dermatologic outpatients using SCL-K-9; (ii) to investigate, using a receiver operating characteristic (ROC) curve procedure, whether the SCL-K-9 is able to categorize patients with and without significant non-psychotic disorders (i.e., serious emotional distress; Piccinelli et al., 1993) using the GHQ-12 as the standard test; and (iii) to perform a single-item analysis of the SCL-K-9, with a focus on gender differences.
This is a cross-sectional, single-center observational study. The research has been conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethical Committee. Data were collected from October 7, 2021 to May 9, 2022 at the dermatological research hospital IDI-IRCCS, Rome, Italy. Specifically, consecutive patients were enrolled in the Dermatological Day-Hospital Unit, the inpatient Dermatological Clinic, and the Outpatient Dermatological Unit of IDI-IRCCS. Study patients had to satisfy the following inclusion criteria: (i) ≥18 years old; (ii) a clinical diagnosis of a chronic or recurrent skin condition; and (iv) signed written informed consent. Exclusion criteria were: (i) inability to understand Italian; (ii) inability to understand the questionnaires; and (iii) any diagnosed major neuro-psychiatric disorder (i.e., diagnosed major depressive disorder, bipolar disorders, schizophrenia, attention deficit disorder, eating disorders, addictions, mild–severe cognitive impairment, and epilepsy or seizures).
The Physician Global Assessment (PGA) is a 5-point scoring system used to assess disease severity (Pascoe et al., 2015).
After enrolment, the Italian version of the SCL-K-9, and the 12-item General Health Questionnaire (GHQ-12) were distributed to all patients. Both tools are self-report.
The SCL-K-9, as already described above, is the 9-item screener for global psychological symptom severity (Imperatori et al., 2020). Items are scored on 5-point Likert scale, with range “0” to “4,” investigating the severity of nine main psychopathological dimensions over the week before the survey, including: somatic symptoms, interpersonal sensitivity, obsessive–compulsive behaviors, anxiety and depressive symptoms, hostility, phobic symptoms, paranoid tendencies, and psychoticism. This scale yields a global severity index (GSI) as a measure of general psychopathology severity, with higher scores reflecting higher levels of psychopathological distress as well as a greater severity of self-reported symptoms.
The GHQ-12 is a self-report questionnaire that measures psychological distress and may detect current non-psychotic psychiatric disorders (Piccinelli et al., 1993). GHQ-12 scores were computed both through the dichotomous scoring method (0–0–1–1), and Likert scoring method (Picardi et al., 2000) in order to detect “GHQ-cases” (i.e., a GHQ score ≥ 7 is indicative of the presence of probable and clinically relevant depressive syndrome; Picardi et al., 2004, 2005a,b). It is a validated tool widely used in dermatological research (Abeni et al., 2002; Picardi et al., 2004). In this study the GHQ-12 “case” status has been used as the standard measure to evaluate SCL-K-9 performance.
Pain perception was assessed through the Visual Analog Scale for pain (VAS). VAS is a psychometric instrument designed to assess the level of pain related to symptom severity in patients, and it is used to achieve a statistically measurable and reproducible classification of symptom severity and disease control (Price et al., 1983). We used the 10 cm scale, with 0–10 as possible range.
Patients were informed about the aim of the study and, after signing the written informed consent form, they completed the sociodemographic (i.e., gender, age, height and weight in order to assess body mass index, dermatologic diagnosis, duration of the disease, educational level expressed in school-years, marital status, employment) and the psychological self-report measures in a standardized form.
The study sample corresponds to the actual number of patients seen during the study period who agreed to participate to the study; being a screening-based study, no sample size calculation was performed.
Categorical variables were described as number and percentage, and continuous variable were firstly classified in different levels and then described as discrete (Table 1).
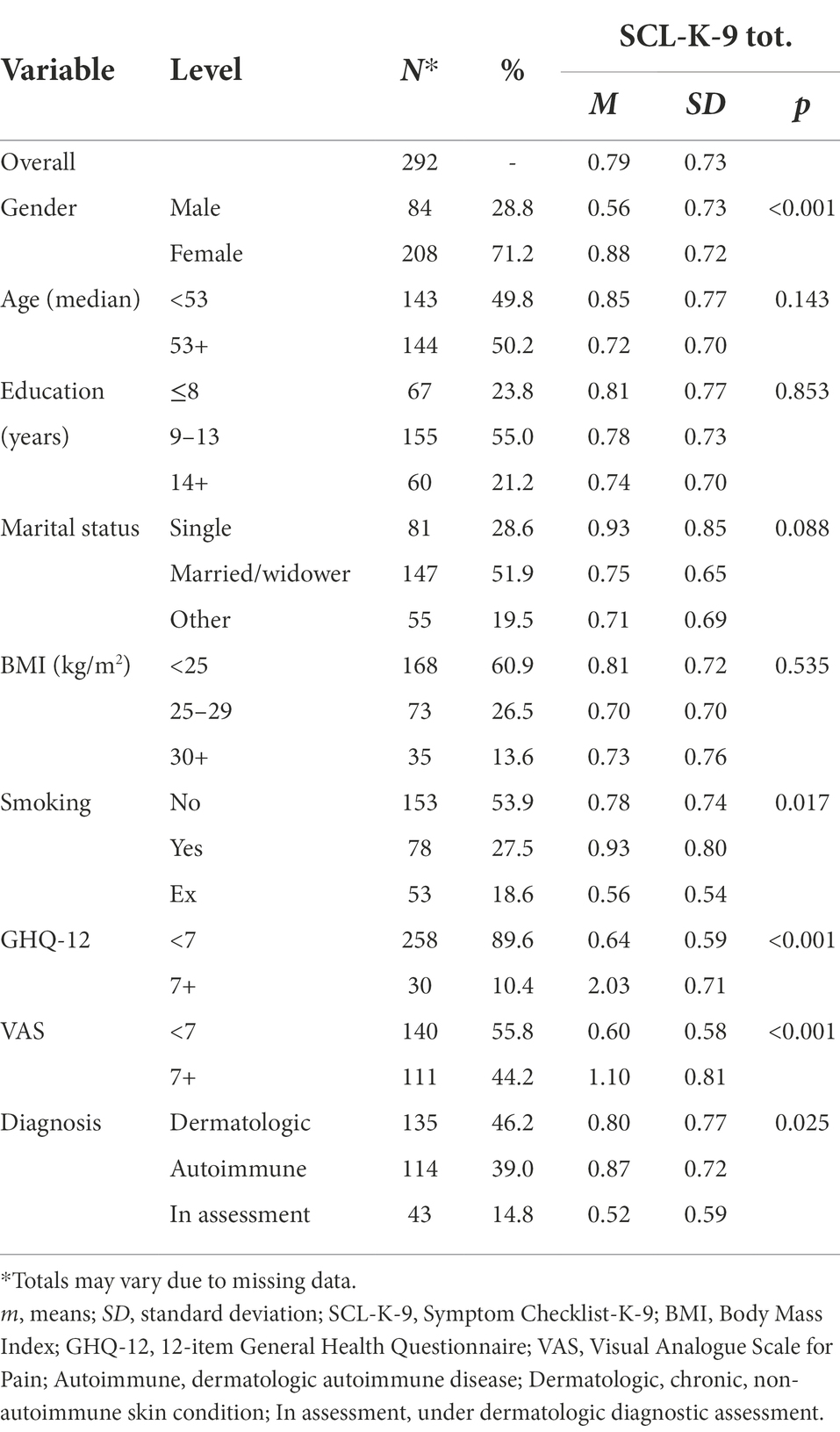
Table 1. Sociodemographic and clinical features of the study participants, with SCL-K-9 total mean scores.
Reliability was assessed using Cronbach alpha for all scales. To measure differences in scores obtained among women and men in SCL-K-9 single items and GSI, the mean and standard deviation scores were computed for each item, separately for males and females (Figure 1). Spearman’s correlation coefficient was used to measure correlation between the instruments’ total scores.
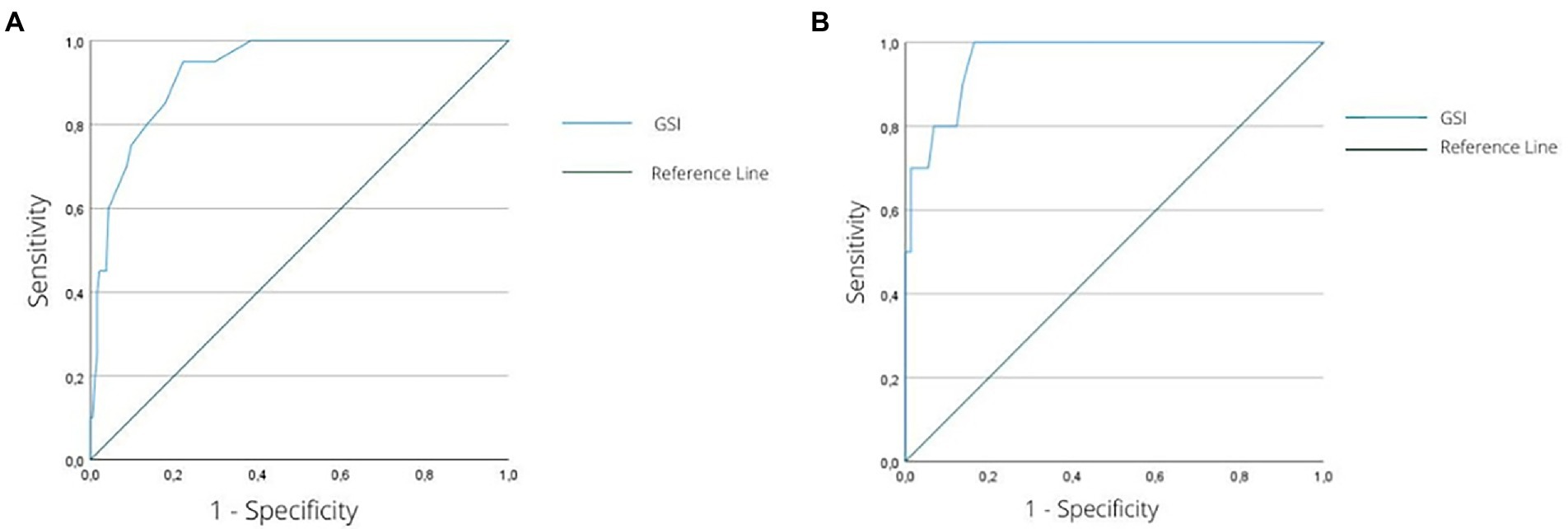
Figure 1. (A) ROC curve graph for the ability of the GSI to discriminate female individuals with significant psychological distress levels (GHQ-12 score 7+) from those without a significant level of psychological distress (GHQ-12 < 7). (B) ROC curve graph for the ability of the GSI to discriminate male individuals with significant psychological distress levels (GHQ-12 score 7+) from those without a significant level of psychological distress (GHQ-12 < 7). ROC, receiver operating characteristic; GSI, global severity index of the Symptom Checklist-K-9; GHQ-12, 12-item General Health Questionnaire.
To evaluate the performance of the SCL-K-9 in classifying patients according to their current emotional distress severity, we performed a ROC test procedure (Ruopp et al., 2008). A ROC curve is a bidimensional description of test performances (Fawcett, 2006), with the main outcome variable being the area under the ROC curve (i.e., AUC), which reflects the probability that a randomly sampled respondent would be correctly categorized (Centor and Schwartz, 1985). The AUC value represents the overall accuracy of the instrument in indexing a sample (Swets, 1988), where values of ≥0.70 are classified as satisfactory. Additionally, the Youden Index (Youden, 1950) was calculated in order to detect the maximum thresholds of both sensitivity (i.e., the proportion of individuals who have the target condition and receive positive test results) and specificity (i.e., the proportion of individuals without the target condition who receive negative test results). The same procedure was also conducted by distinguishing the scores among women and men for the purpose of verifying the presence of different gender-specific cut-offs (Table 2).
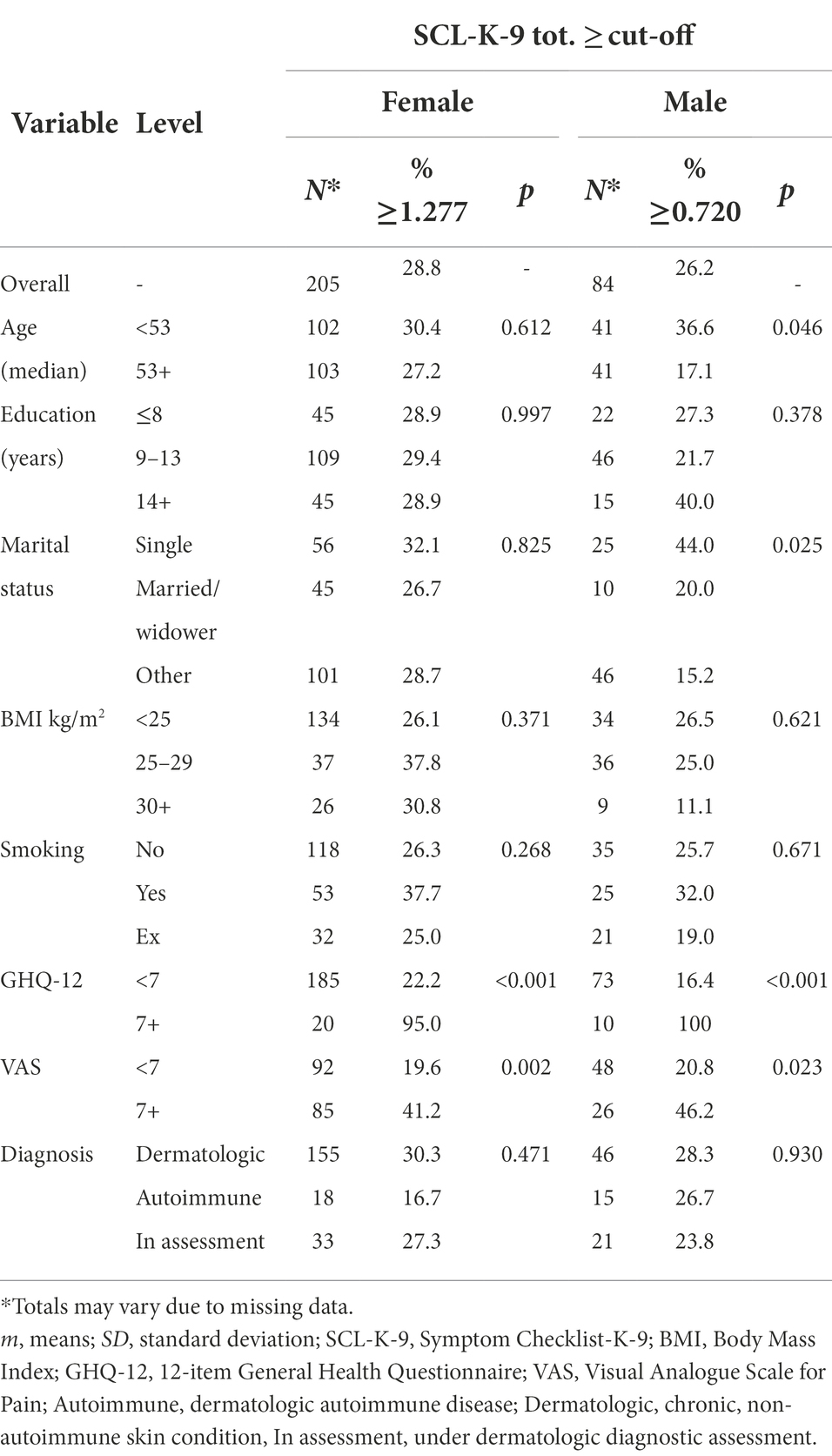
Table 2. Proportion of participants, separately for women and men, who reach the SCL-K-9 proposed cut-offs.
Finally, a SCL-K-9 single-item analysis was performed to portray the presence of psychopathological symptoms detected among women and men. The mean values of these two independent groups were then compared, using the non-parametrical Mann–Whitney U-test, to determine whether there was statistical evidence that the population means are significantly different (Table 2).
The results were considered significant if p < 0.05. Spearman r coefficients were reported as measures of association. Spearman r coefficients <0.10 are considered to be a negligible or null effect, r between 0.10 and 0.30 a small effect, r between 0.30 and 0.50 a medium effect, and r ≥ 0.50 a large effect (Cohen, 1988). All statistical analyses were run under IBM® Statistical Package for Social Science (SPSS), version 28.0 for Windows.
Two hundred and ninety-two patients took part in the study. There were 208 women (71.2%) and 84 men (28.7%); the median age was 53 years, and 40.1% had overweight or obesity (i.e., BMI ≥ 25); 27.5% were currently smoking. At the time of enrollment 46.2% of the sample had a non-autoimmune skin condition, 39.0% had a dermatologic autoimmune disease, and 14.8% definitely had a skin condition, but were still undergoing further diagnostic assessment. Concerning clinical features, 11.3% had a GHQ-12 score ≥ 7; 44.2% had a VAS score ≥ 7. The mean GSI score was 0.79 (SD ± 0.73). Other socio-demographic features of the sample are summarized in Table 1, which also shows the mean GSI scores in the different levels of the main variables of interest.
We observed a large and statistically significant difference in GSI mean scores among genders (0.88, for women vs. 0.56 for men; p < 0.001). Significant differences in GSI scores were also found in scores below and above the GHQ-12 cut-off (0.64 mean score for GHQ-12 < 7 vs. 2.03 mean score for GHQ-12 7+; p < 0.001). Moreover, the GSI scores were significantly different in pain perception (i.e., VAS), with 0.60 mean score for VAS <7 points and 1.10 mean score for VAS 7+ points (p < 0.001). The comparison in GSI score according to diagnosis was also significantly different: patients with a dermatologic-autoimmune disease reported a higher GSI score compared to patients with dermatologic disease, and to patients undergoing diagnostic assessment (i.e., 0.87, 0.80, 0.52 GSI mean score respectively; p = 0.025).
In the present sample Cronbach’s α were 0.87 for SCL-K-9 nine items, and 0.88 for GHQ-12 twelve items; therefore, the study measures can be considered reliable. Correlations between these two measures (i.e., GSI and GHQ-12 total Likert 0–36 score) were significantly and strongly associated (r = 0.68, p < 0.001), but did not overlap. Thus, it is possible to hypothesize that GHQ-12 and SCL-K-9 do not measure the same construct, detecting different forms of psychological distress in dermatological setting.
The ROC curve analysis showed that both the GSI score for women (area under the ROC curve = 0.92, 95%; confidence interval [0.88, 0.97], p < 0.001; Figure 1) and the GSI score for men (area under the ROC curve = 0.96, 95%; confidence interval [0.92, 1.00], p < 0.001; Figure 2) accurately classified patients with relevant psychological distress.
Particularly, for women, a score of 1.277 or higher on the GSI (Youden index = 0.73) categorized individuals with a sensitivity of 0.95 (95% of all the individuals with GHQ-12 > 7 were correctly detected) and a specificity of 0.78 (22% of patients were incorrectly identified as having a relevant psychological distress). The same procedure conducted for men revealed that a score of 0.720 of higher on the GSI score (Youden index = 0.84) classified patients with relevant psychological distress (i.e., GHQ-12 ≥ 7) with a sensitivity of 1.00 (100% of all the individuals with GHQ-12 ≥ 7 were correctly detected) and a specificity of 0.84 (16% of patients were incorrectly identified as having relevant psychological distress).
According to the new proposed cut-offs, 28.8% of women and 26.2% of men were above the GSI cut-offs. Among women, the significant differences were observed for GHQ-12 and VAS (i.e., 95.0% vs. 22.2%; 41.2% vs. 19.6%, respectively). Among men, in addition to GHQ-12 and VAS (i.e., 100.0% vs. 16.4%; 46.2% vs. 20.8%, respectively), younger (36.6% vs. 17.1%) and single patients (44.0%) had significantly higher proportions of above cut-off GSI score. The full description of sociodemographic features of the sample according to SCL-K-9 proposed cut-offs reported in Table 2.
The single-item analysis, using the non-parametrical Mann–Whitney U-test, showed that all the SCL-K-9 symptoms mean values were higher in women than in men. Specifically, women reported significantly higher scores indicative of psychological distress, in the symptomatic areas of hostility (p = 0.032), obsessive–compulsive symptoms (p = 0.004), depressive symptoms (p < 0.001), interpersonal sensitivity (p = 0.003), anxiety symptoms (p < 0.001), somatization symptoms (p < 0.001), and psychoticism (p = 0.020). Higher scores, among women, even if not statistically significant, were also observed in paranoid ideation symptoms (p = 0.115), and phobic anxiety symptoms (p = 0.066; Figure 2 provides a graphical representation of these differences between mean scores).
The SCL-90-R (Derogatis, 2017) is one of the most internationally used questionnaire to assess psychological distress, especially in clinical practice (Parker et al., 1990; Weathers et al., 1996; Prunas et al., 2012; Gül et al., 2015; Christensen et al., 2016). However, it is a time-consuming questionnaire, and this usually is a serious problem in non-psychiatric hospital settings, in which there is not much time available for the patient’s psychological assessment. Therefore, short versions were developed to overcome this problem (Müller et al., 2010). One of these is the SCL-K-9 version (Klaghofer and Brähler, 2001). The major purpose of the current research was to measure, for the first time, the general psychopathology in a sample of patients with different type of skin diseases using the Italian adaptation of SCL-K-9 (Imperatori et al., 2020), with a focus on gender differences. From our results, among patients with skin diseases, women had higher levels of psychological distress than men, as previously reported in the literature, with data from different assessment tools (Finzi et al., 2007; Mina et al., 2015; Masood et al., 2016). This greater vulnerability experienced by women compared to men seems to have multifactorial and unclear reasons (Zachariae et al., 2004). For example, as asserted by Picardi et al. (2001b), in women, but not in men, the prevalence of psychiatric morbidity was higher in patients with lesions on visible parts of the body. According to the authors, the increased psychological vulnerability of female patients might be related to a higher impact of changes in body image on self-esteem compared to men (Picardi et al., 2001b). Particularly, the skin plays an essential role throughout the life cycle not only as a sensory organ, but even in socialization processes. So, skin appearance can affect body image and self-esteem through processes of stigmatization and lasting emotions like shame (Lahousen et al., 2016; Hawro et al., 2017). This interpretation is supported by other studies that stressed the concept that women are more invested in appearance (Pingitore et al., 1997) and less satisfied with their body image compared to men (Smith et al., 1999), but a more extensive investigation is needed to better understand sociological, anthropological, and psychological reasons beyond these observations.
Because of these previously reported significant differences in psychopatologic symptoms among women and men, confirmed by our own findings in the present study, it seems appropriate to us to have identified two different GSI cut-offs, which may be of help in detecting gender-specific psychopathology risk among dermatologic patients. Previous studies tested the SCL-K-9 in different clinical settings. For example, Goetzmann et al. (2009) investigated the psychological processing of transplantation in lung recipients and, among others psychometric tools, they used the German version of SCL-K-9; and Crössmann et al. (2010) used SCL-K-9 to evaluate an intervention to reduce psychopathology and improve quality of life in patients with an implantable cardioverter defibrillator. However, to the best of our knowledge, no research has focused on the investigation of the SCL-K-9 performance among dermatological patients, and on evaluating gender differences when measuring psychopathology with the SCL-K-9.
As for the ROC curve methodology, Imperatori et al., used the Italian version of the SCL-K-9 to discriminate between overweight/obese patients with and without significant binge eating disorder. The authors identified a cut-off of 0.83 or higher to categorize individuals as having binge eating disorder (Imperatori et al., 2020). Bianciardi et al. (2021) used the Italian SCL-K-9 to assess the level of psychopathology in women eligible for bariatric surgery, and identified a different cut-off (i.e., 0.50 or higher to identify patients with at least one psychiatric disorder from those without any psychiatric disorder) (Bianciardi et al., 2021). However, all participants of both studies were females.
One of the most used questionnaires to assess minor psychiatric disorders and to screen for depression symptomatology in dermatology is the GHQ-12 (Picardi et al., 2001a), for which a cut-off score of >7 has been used to identify symptoms of depressive disorders (Picardi et al., 2004). However, this questionnaire does not permit the ability to investigate other psychopathological issues that are detectable in dermatological patients and that can significantly impair their quality of life (Graham, 1955; Gupta and Gupta, 2013; Dalgard et al., 2015; Peters, 2016). For this reason, a SCL-K-9 single-item analysis has been performed, to highlight specific contents related to the psychological distress reported by patients (Klaghofer and Brähler, 2001; Imperatori et al., 2020), differentiating them according to gender.
As conceptualized by Derogatis and Cleary (1977), the Hostility dimension of SCL-90 (item #24) and first item of the SCL-K-9, reflects tendencies of externalized anger and aggressiveness; the Obsessive–compulsive dimension of SCL-90 (item #28), second SCL-K-9 item, reflects the tendency to worry and control the external and internal environments; the Depression dimension of SCL-90 (#31), third item of SCL-K-9, refers to depressive symptomatology (low or discouraged mood resulting from disappointment or imaginary or real loss); the Interpersonal sensitivity dimension of SCL-90 (#34), fourth SCL-K-9 item, is consistent with the traditional notion of the “inferiority complex,” and highlights feelings of personal inadequacy, self-deprecation, and acute self-consciousness; the Paranoia dimension of SCL-90 (#43), fifth SCL-K-9 item, closely reflects tendencies of suspiciousness; the Anxiety dimension of SCL-90 (#57), sixth SCL-K-9 item, leads to physical sensation and cognitive tendencies of being frightened and fearful; the Somatization dimension of SCL-90 (#58), seventh item of SCL-K-9, has a primary focus on psychological distress that arises from perceptions of bodily dysfunction; the Phobic Anxiety dimension of SCL-90 (#75), eighth SCL-K-9 item, has been designed to reflect the “agoraphobic” symptomatology; the Psychoticism dimension (#77), the last SCL-K-9 item, attempts to capture the symptoms of interpersonal distance and alienation.
As already noted, women reported significantly higher scores indicative of psychological distress than men, especially in some symptomatic areas. For this reason, and particularly in women who reach the SCL-K-9 cut-off, a more in-dept psychological assessment should be carried out, to evaluate levels of hostility, interpersonal sensitivity, obsessive–compulsive traits, depression, anxiety, and somatization symptoms, as well as psychoticism that could be detected but not fully identified as assessed through a brief-screening tool. These issues experienced by patients could affect general well-being and functioning, often causing suffering, isolation, and a worse adherence to treatments (Renzi et al., 2002; Picardi et al., 2006b; Yélamos et al., 2015) It is important to note some issues limiting the generalizability of our findings. First, the ratio among women and men in the sample was quite unbalanced; however, it reflects the greater access of women to Dermatology Units than men. To this respect, it is interesting to note that the Italian version of the SCL-K-9 had never been used, before the present study, to detect psychopathologic symptoms in men. Second, even though we excluded people reporting the presence of a major neuropsychiatric diagnosis, no a priori psychological evaluation has been carried out on our sample.
In conclusion, even if the SCL-K-9 is a screening and not a diagnostic tool, with its compact format could provide, in a short time, a wide range of information related to critical areas that challenge the mental health of patients with skin diseases. Moreover, it can allow to increase attention to this key area of concern that is often overlooked in dermatologic patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Ethical Committee of dermatological research hospital IDI-IRCCS, Rome, Italy. The patients/participants provided their written informed consent to participate in this study.
TS, DA, GC, and VA: Conceptualization. TS, GC, and PS: Enrollment and data curation. TS and DA: Methodology and formal analysis. DA, AG, and VA: Project administration and supervision. TS: Writing - original draft. TS, DA, GC, VA, and AG: Writing - review and editing. All authors discussed the results and contributed to the final manuscript.
This study was supported in part by “Progetto Ricerca Corrente 2020–2021” of the Italian Ministry of Health.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abeni, D., Picardi, A., Pasquini, P., Melchi, C. F., and Chren, M. M. M. M. (2002). Further evidence of the validity and reliability of the Skindex-29: an Italian study on 2, 242 dermatological outpatients. Dermatology 204, 43–49. doi: 10.1159/000051809
August, K. J., and Sorkin, D. H. (2010). Marital status and gender differences in managing a chronic illness: the function of health-related social control. Soc. Sci. Med. 71, 1831–1838. doi: 10.1016/j.socscimed.2010.08.022
Bianciardi, E., Gentileschi, P., Niolu, C., Innamorati, M., Fabbricatore, M., Contini, L. M., et al. (2021). Assessing psychopathology in bariatric surgery candidates: discriminant validity of the SCL-90-R and SCL-K-9 in a large sample of patients. Eat. Weight Disord. 26, 2211–2218. doi: 10.1007/s40519-020-01068-2
Castelo-Branco, C., Ribera Torres, L., Gómez-Gil, E., Uribe, C., and Cañizares, S. (2021). Psychopathological symptoms in Spanish subjects with gender dysphoria. A cross-sectional study. Gynecol. Endocrinol. 37, 534–540. doi: 10.1080/09513590.2021.1913113
Centor, R. M., and Schwartz, J. S. (1985). An evaluation of methods for estimating the area under the receiver operating characteristic (ROC) curve. Med. Decis. Mak. 5, 149–156. doi: 10.1177/0272989x8500500204
Christensen, J., Fisker, A., Lykke Mortensen, E., Raabæk Olsen, L., Steen Mortensen, O., Hartvigsen, J., et al. (2016). Comparison of mental distress in patients with low back pain and a population based referent group. Physiotherapy 102, e182–e183. doi: 10.1016/j.physio.2016.10.218
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. New York, NY, USA: Routledge.
Crössmann, A., Schulz, S. M., Kühlkamp, V., Ritter, O., Neuser, H., Schumacher, B., et al. (2010). A randomized controlled trial of secondary prevention of anxiety and distress in a German sample of patients with an implantable cardioverter defibrillator. Psychosom. Med. 72, 434–441. doi: 10.1097/PSY.0b013e3181d9bcec
Dalgard, F. J., Gieler, U., Tomas-Aragones, L., Lien, L., Poot, F., Jemec, G. B. E., et al. (2015). The psychological burden of skin diseases: a cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Investig. Dermatol. 135, 984–991. doi: 10.1038/jid.2014.530
Dalgard, F. J., Svensson, Å., Gieler, U., Tomas-Aragones, L., Lien, L., Poot, F., et al. (2018). Dermatologists across Europe underestimate depression and anxiety: results from 3635 dermatological consultations. Br. J. Dermatol. 179, 464–470. doi: 10.1111/bjd.16250
Derogatis, L.R. (2017). “Symptom Checklist-90-Revised, brief Symptom Inventory, and BSI-18.” (New York, NY, USA: Routledge/Taylor & Francis Group), 599–629.
Derogatis, L. R., and Cleary, P. A. (1977). Factorial invariance across gender for the primary symptom dimensions of the SCL-90. Br. J. Soc. Clin. Psychol. 16, 347–356. doi: 10.1111/j.2044-8260.1977.tb00241.x
Derogatis, L. R., and Savitz, K. L. (2000). “The SCL–90–R and brief symptom inventory (BSI) in primary care,” in Handbook of Psychological Assessment in Primary care Settings (Mahwah, NJ, USA: Lawrence Erlbaum Associates Publishers), 297–334.
Fawcett, T. (2006). An introduction to ROC analysis. Pattern Recogn. Lett. 27, 861–874. doi: 10.1016/j.patrec.2005.10.010
Finlay, A. Y., Chernyshov, P. V., Tomas Aragones, L., Bewley, A., Svensson, A., Manolache, L., et al. (2021). Methods to improve quality of life, beyond medicines. Position statement of the European academy of dermatology and venereology task force on quality of life and patient oriented outcomes. J. Eur. Acad. Dermatol. Venereol. 35, 318–328. doi: 10.1111/jdv.16914
Finzi, A., Colombo, D., Caputo, A., Andreassi, L., Chimenti, S., Vena, G., et al. (2007). Psychological distress and coping strategies in patients with psoriasis: the PSYCHAE study. J. Eur. Acad. Dermatol. Venereol. 21, 1161–1169. doi: 10.1111/j.1468-3083.2007.02079.x
Goetzmann, L., Irani, S., Moser, K. S., Schwegler, K., Stamm, M., Spindler, A., et al. (2009). Psychological processing of transplantation in lung recipients: a quantitative study of organ integration and the relationship to the donor. Br. J. Health Psychol. 14, 667–680. doi: 10.1348/135910708X399447
Graham, D. T. (1955). Cutaneous vascular reactions in Raynaud's disease and in states of hostility, anxiety, and depression. Psychosom. Med. 17, 200–207. doi: 10.1097/00006842-195505000-00004.343
Gül, A. I., Özkırış, M., Aydin, R., Şimşek, G., and Saydam, L. (2015). Coexistence of anxiety sensitivity and psychiatric comorbidities in patients with chronic tinnitus. Neuropsychiatr. Dis. Treat. 11, 413–418. doi: 10.2147/ndt.S77786
Gupta, M. A., and Gupta, A. K. (2013). Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J. Psychosom. Res. 75, 55–59. doi: 10.1016/j.jpsychores.2013.01.015
Hardt, J., Gerbershagen, H. U., and Franke, P. (2000). The symptom check-list, SCL-90-R: its use and characteristics in chronic pain patients. Eur. J. Pain 4, 137–148. doi: 10.1053/eujp.2000.0162
Hawro, M., Maurer, M., Weller, K., Maleszka, R., Zalewska-Janowska, A., Kaszuba, A., et al. (2017). Lesions on the back of hands and female gender predispose to stigmatization in patients with psoriasis. J. Am. Acad. Dermatol. 76, 648–654.e2. doi: 10.1016/j.jaad.2016.10.040
Huilaja, L., Tiri, H., Jokelainen, J., Timonen, M., and Tasanen, K. (2018). Patients with hidradenitis Suppurativa have a high psychiatric disease burden: a Finnish Nationwide registry study. J. Investig. Dermatol. 138, 46–51. doi: 10.1016/j.jid.2017.06.020
Imperatori, C., Bianciardi, E., Niolu, C., Fabbricatore, M., Gentileschi, P., Di Lorenzo, G., et al. (2020). The symptom-checklist-K-9 (SCL-K-9) discriminates between overweight/obese patients with and without significant binge eating pathology: psychometric properties of an Italian version. Nutrients 12:674. doi: 10.3390/nu12030674
Jafferany, M., Ferreira, B. R., Abdelmaksoud, A., and Mkhoyan, R. (2020). Management of psychocutaneous disorders: a practical approach for dermatologists. Dermatol. Ther. 33:e13969. doi: 10.1111/dth.13969
Kim, I. L. H., Chun, H., and Kwon, J.-W. (2011). Gender differences in the effect of obesity on chronic diseases among the elderly Koreans. J. Korean Med. Sci. 26, 250–257. doi: 10.3346/jkms.2011.26.2.250
Kim, Y. S., and Kim, N. (2018). Sex-gender differences in irritable bowel syndrome. J. Neurogastroenterol. Motil. 24, 544–558. doi: 10.5056/jnm18082
Klaghofer, R., and Brähler, E. (2001). Konstruktion und Teststatistische Prüfung einer Kurzform der SCL-90–R. [construction and test statistical evaluation of a short version of the SCL-90–R]. Z. Klin. Psychol. Psychiatr. Psychother. 49, 115–124.
Konstantinou, G. N., and Konstantinou, G. N. (2019). Psychiatric comorbidity in chronic urticaria patients: a systematic review and meta-analysis. Clin. Transl. Allergy 9:42. doi: 10.1186/s13601-019-0278-3
Lahousen, T., Kupfer, J., Gieler, U., Hofer, A., Linder, M. D., and Schut, C. (2016). Differences between psoriasis patients and skin-healthy controls concerning appraisal of touching, Shame and Disgust. Acta Derm. Venereol. 96, 78–82. doi: 10.2340/00015555-2373
Lewis, V., and Finlay, A. Y. (2004). 10 years experience of the dermatology life quality index (DLQI). J. Investig. Dermatol. Symp. Proc. 9, 169–180. doi: 10.1111/j.1087-0024.2004.09113.x
Masood, A., Masud, Y., and Mazahir, S. (2016). Gender differences in resilience and psychological distress of patients with burns. Burns 42, 300–306. doi: 10.1016/j.burns.2015.10.006
Melchior, C., Algera, J., Colomier, E., Törnblom, H., Simrén, M., and Störsrud, S. (2022). Food avoidance and restriction in irritable bowel syndrome: relevance for symptoms, quality of life and nutrient intake. Clin. Gastroenterol. Hepatol. 20, 1290–1298.e4. doi: 10.1016/j.cgh.2021.07.004
Mento, C., Rizzo, A., Muscatello, M. R. A., Zoccali, R. A., and Bruno, A. (2020). Negative emotions in skin disorders: a systematic review. Int. J. Psychol. Res. 13, 71–86. doi: 10.21500/20112084.4078
Mina, S., Jabeen, M., Singh, S., and Verma, R. (2015). Gender differences in depression and anxiety among atopic dermatitis patients. Indian J. Dermatol. 60:211. doi: 10.4103/0019-5154.152564
Müller, J. M., Postert, C., Beyer, T., Furniss, T., and Achtergarde, S. (2010). Comparison of eleven short versions of the symptom checklist 90-revised (SCL-90-R) for use in the assessment of general psychopathology. J. Psychopathol. Behav. Assess. 32, 246–254. doi: 10.1007/s10862-009-9141-5
Napolitano, M., Mastroeni, S., Fusari, R., Mazzanti, C., Ciccone, D., Pallotta, S., et al. (2020). The international hidradenitis Suppurativa severity score system (IHS4) is a valuable tool to assess gender-associated differences. Eur. J. Dermatol. 30, 201–203. doi: 10.1684/ejd.2020.3743
Ngo, S. T., Steyn, F. J., and McCombe, P. A. (2014). Gender differences in autoimmune disease. Front. Neuroendocrinol. 35, 347–369. doi: 10.1016/j.yfrne.2014.04.004
Panebianco, A., Francesca, S., Maria Luisa, I., Luciano, S., Elisabetta, A., Valeria, A., et al. (2018). A screening programme for dermatologists as a guide to request psychological consultation in routine clinical practice. Eur. J. Dermatol. 28, 326–331. doi: 10.1684/ejd.2018.3316
Parker, J. C., Buckelew, S. P., Smarr, K. L., Buescher, K. L., Beck, N. C., Frank, R. G., et al. (1990). Psychological screening in rheumatoid arthritis. J. Rheumatol. 17, 1016–1021.
Pascoe, V. L., Enamandram, M., Corey, K. C., Cheng, C. E., Javorsky, E. J., Sung, S. M., et al. (2015). Using the physician global assessment in a clinical setting to measure and track patient outcomes. JAMA Dermatol. 151, 375–381. doi: 10.1001/jamadermatol.2014.3513
Peters, E. M. J. (2016). Stressed skin? – a molecular psychosomatic update on stress-causes and effects in dermatologic diseases. J. Dtsch. Dermatol. Ges. 14, 233–252; quiz 253. doi: 10.1111/ddg.12957
Petrowski, K., Schmalbach, B., Kliem, S., Hinz, A., and Brähler, E. (2019). Symptom-checklist-K-9: norm values and factorial structure in a representative German sample. PLoS One 14, –e0213490. doi: 10.1371/journal.pone.0213490
Picardi, A., Abeni, D., Mazzotti, E., Fassone, G., Lega, I., Ramieri, L., et al. (2004). Screening for psychiatric disorders in patients with skin diseases: a performance study of the 12-item general health questionnaire. J. Psychosom. Res. 57, 219–223. doi: 10.1016/S0022-3999(03)00619-6
Picardi, A., Abeni, D., Melchi, C. F., Puddu, P., and Pasquini, P. (2000). Psychiatric morbidity in dermatological outpatients: an issue to be recognized. Br. J. Dermatol. 143, 983–991. doi: 10.1046/j.1365-2133.2000.03831.x
Picardi, A., Abeni, D., and Pasquini, P. (2001a). Assessing psychological distress in patients with skin diseases: reliability, validity and factor structure of the GHQ-12. J. Eur. Acad. Dermatol. Venereol. 15, 410–417. doi: 10.1046/j.1468-3083.2001.00336.x
Picardi, A., Abeni, D., Renzi, C., Braga, M., Puddu, P., and Pasquini, P. (2001b). Increased psychiatric morbidity in female outpatients with skin lesions on visible parts of the body. Acta Derm. Venereol. 81, 410–414. doi: 10.1080/000155501317208345
Picardi, A., Adler, D. A., Abeni, D., Chang, H., Pasquini, P., Rogers, W. H., et al. (2005a). Screening for depressive disorders in patients with skin diseases: a comparison of three screeners. Acta Derm. Venereol. 85, 414–419. doi: 10.1080/00015550510034966
Picardi, A., Mazzotti, E., and Pasquini, P. (2006a). Prevalence and correlates of suicidal ideation among patients with skin disease. J. Am. Acad. Dermatol. 54, 420–426. doi: 10.1016/j.jaad.2005.11.1103
Picardi, A., Pasquini, P., Abeni, D., Fassone, G., Mazzotti, E., and Fava, G. A. (2005b). Psychosomatic assessment of skin diseases in clinical practice. Psychother. Psychosom. 74, 315–322. doi: 10.1159/000086323
Picardi, A., Porcelli, P., Pasquini, P., Fassone, G., Mazzotti, E., Lega, I., et al. (2006b). Integration of multiple criteria for psychosomatic assessment of dermatological patients. Psychosomatics 47, 122–128. doi: 10.1176/appi.psy.47.2.122
Piccinelli, M., Bisoffi, G., Bon, M., Cunico, L., and Tansella, M. (1993). Validity and test-retest reliability of the Italian version of the 12-item GHQ in general practice: a comparison between three scoring methods. Compr. Psychiatry 34, 198–205. doi: 10.1016/0010-440X(93)90048-9
Pingitore, R., Spring, B., and Garfield, D. (1997). Gender differences in body satisfaction. Obes. Res. 5, 402–409. doi: 10.1002/j.1550-8528.1997.tb00662.x
Price, D. D., McGrath, P. A., Rafii, A., and Buckingham, B. (1983). The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 17, 45–56. doi: 10.1016/0304-3959(83)90126-4
Prinsen, C. A. C., Lindeboom, R., Sprangers, M. A. G., Legierse, C. M., and De Korte, J. (2010). Health-related quality of life assessment in dermatology: interpretation of skindex-29 scores using patient-based anchors. J. Investig. Dermatol. 130, 1318–1322. doi: 10.1038/jid.2009.404
Prinz, U., Nutzinger, D. O., Schulz, H., Petermann, F., Braukhaus, C., and Andreas, S. (2013). Comparative psychometric analyses of the SCL-90-R and its short versions in patients with affective disorders. BMC Psychiatry 13:104. doi: 10.1186/1471-244X-13-104
Prunas, A., Sarno, I., Preti, E., Madeddu, F., and Perugini, M. (2012). Psychometric properties of the Italian version of the SCL-90-R: a study on a large community sample. Eur. Psychiatry 27, 591–597. doi: 10.1016/j.eurpsy.2010.12.006
Renzi, C., Picardi, A., Abeni, D., Agostini, E., Baliva, G., Pasquini, P., et al. (2002). Association of Dissatisfaction with Care and Psychiatric Morbidity with Poor Treatment Compliance. Arch. Dermatol. 138, 337–342. doi: 10.1001/archderm.138.3.337
Ruopp, M. D., Perkins, N. J., Whitcomb, B. W., and Schisterman, E. F. (2008). Youden index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom. J. 50, 419–430. doi: 10.1002/bimj.200710415
Sampogna, F., Picardi, A., Chren, M. M., Melchi, C. F., Pasquini, P., Masini, C., et al. (2004). Association between poorer quality of life and psychiatric morbidity in patients with different dermatological conditions. Psychosom. Med. 66, 620–624. doi: 10.1097/01.psy.0000132869.96872.b2
Sampogna, F., Spagnoli, A., Di Pietro, C., Pagliarello, C., Paradisi, A., Tabolli, S., et al. (2013). Field performance of the Skindex-17 quality of life questionnaire: a comparison with the Skindex-29 in a large sample of dermatological outpatients. J. Investig. Dermatol. 133, 104–109. doi: 10.1038/jid.2012.244
Schauenburg, H., and Strack, M. (1999). Measuring psychotherapeutic change with the symptom checklist SCL 90 R. Psychother. Psychosom. 68, 199–206. doi: 10.1159/000012333
Sereda, Y., and Dembitskyi, S. (2016). Validity assessment of the symptom checklist SCL-90-R and shortened versions for the general population in Ukraine. BMC Psychiatry 16:300. doi: 10.1186/s12888-016-1014-3
Smith, D. E., Thompson, J. K., Raczynski, J. M., and Hilner, J. E. (1999). Body image among men and women in a biracial cohort: the CARDIA study. Int. J. Eat. Disord. 25, 71–82. doi: 10.1002/(sici)1098-108x(199901)25:1<71::aid-eat9>3.0.co;2-3
Ständer, S., Stumpf, A., Osada, N., Wilp, S., Chatzigeorgakidis, E., and Pfleiderer, B. (2013). Gender differences in chronic pruritus: women present different morbidity, more scratch lesions and higher burden. Br. J. Dermatol. 168, 1273–1280. doi: 10.1111/bjd.12267
Swets, J. A. (1988). Measuring the accuracy of diagnostic systems. Science 240, 1285–1293. doi: 10.1126/science.3287615
Timman, R., and Arrindell, W. A. (2022). A very short symptom Checklist-90-R version for routine outcome monitoring in psychotherapy; the SCL-3/7. Acta Psychiatr. Scand. 145, 397–411. doi: 10.1111/acps.13396
Weathers, F. W., Litz, B. T., Keane, T. M., Herman, D. S., Steinberg, H. R., Huska, J. A., et al. (1996). The utility of the SCL-90-R for the diagnosis of war-zone related posttraumatic stress disorder. J. Trauma. Stress. 9, 111–128. doi: 10.1007/BF02116837
Yélamos, O., Ros, S., and Puig, L. (2015). Improving patient outcomes in psoriasis: strategies to ensure treatment adherence. Psoriasis (Auckl) 5, 109–115. doi: 10.2147/PTT.S54070
Youden, W. J. (1950). Index for rating diagnostic tests. Cancer 3, 32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3
Keywords: skin diseases, patient-reported measures, psychological screening tool, gender differences, psychology in clinical settings
Citation: Samela T, Cordella G, Antinone V, Sarandrea P, Giampetruzzi AR and Abeni D (2022) The use of SCL-k-9 to measure general psychopathology in women and men with skin conditions. Front. Psychol. 13:977264. doi: 10.3389/fpsyg.2022.977264
Received: 24 June 2022; Accepted: 15 September 2022;
Published: 20 October 2022.
Edited by:
Barbara Colombo, Champlain College Neuroscience Lab, United StatesReviewed by:
Giorgia Varallo, University of Parma, ItalyCopyright © 2022 Samela, Cordella, Antinone, Sarandrea, Giampetruzzi and Abeni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tonia Samela, dC5zYW1lbGFAaWRpLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.