
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 15 July 2022
Sec. Neuropsychology
Volume 13 - 2022 | https://doi.org/10.3389/fpsyg.2022.898326
This article is part of the Research Topic The Nocebo Effect and its Consequences for Clinical Trials and Clinical Practice View all 15 articles
 Hartmuth Nowak1*
Hartmuth Nowak1* Alexander Wolf1
Alexander Wolf1 Tim Rahmel1
Tim Rahmel1 Guenther Oprea1
Guenther Oprea1 Lisa Grause1
Lisa Grause1 Manuela Moeller2
Manuela Moeller2 Katharina Gyarmati3
Katharina Gyarmati3 Corinna Mittler3
Corinna Mittler3 Alexandra Zagler4
Alexandra Zagler4 Katrin Lutz5
Katrin Lutz5 Johannes Loeser3
Johannes Loeser3 Thomas Saller4
Thomas Saller4 Michael Tryba2,6
Michael Tryba2,6 Michael Adamzik1
Michael Adamzik1 Ernil Hansen5
Ernil Hansen5 Nina Zech5
Nina Zech5Postoperative nausea and vomiting (PONV) are one of the most adverse events after general anesthesia, a distressing experience, and pose a risk to the patient. Despite advances in drug prophylaxis and PONV treatment, the incidence remains high and additional non-pharmacological treatments are needed. In this post hoc analysis of a recently published double-blind multicenter randomized controlled trial on the efficacy of intraoperative therapeutic suggestions on postoperative opioid dosage, we analyzed the effects of intraoperative therapeutic suggestions on PONV. We focus on patients with a high risk of PONV (Apfel risk score of 3–4) and distinguished early (first two postoperative hours) and delayed PONV (2–24 h). A total of 385 patients with a moderate or high risk for PONV were included. The incidence of early and delayed PONV was reduced (22.7–18.3 and 29.9–24.1%, respectively), without statistical significance, whereas in high-risk patients (n = 180) their incidence was nearly halved, 17.2 vs. 31.2% (p = 0.030) and 20.7 vs. 34.4% (p = 0.040), corresponding to a number needed to treat of 7 to avoid PONV. In addition, there was a significant reduction in PONV severity. In a multivariate logistic regression model, assignment to the control group (OR 2.2; 95% CI: 1.1–4.8) was identified as an independent predictor of the occurrence of early PONV. Our results indicate that intraoperative therapeutic suggestions can significantly reduce the incidence of PONV in high-risk patients. This encourages the expansion of therapeutic suggestions under general anesthesia, which are inexpensive and virtually free of side effects.
Clinical Trial Registration: German Clinical Trials Register, https://drks.de, registration number: DRKS00013800.
Since postoperative nausea and vomiting (PONV) are major adverse events after surgery under general anesthesia (Cohen et al., 1994; Apfel et al., 1999), effective interventions, which are able to reduce the incidence of PONV, have always been the subject of anesthesiologic research (Gan et al., 2020). In addition to the fact that PONV is a very distressing experience (Myles et al., 2000), it can have a direct impact on the patient’s outcome. The appearance of PONV poses a risk of severe complications such as suture dehiscence, aspiration, pneumonia, dehydration, hydroelectrolytic changes, esophageal rupture, and increased intracranial pressure (Bremner and Kumar, 1993; Schumann and Polaner, 1999; Samuels, 2013).
Early PONV within the first two postoperative hours with a relationship to volatile anesthetics can be distinguished from delayed PONV (Apfel et al., 2002). Despite advances in drug prophylaxis and treatment of PONV, the incidence remains high and is reported to be up to 30% in all postoperative patients and up to 80% in high-risk patients, which can be predicted by the presence of 3 or 4 factors of the Apfel simplified risk score, which include: female sex, non-smoking status, history of PONV or motion sickness, and/or use of postoperative opioids (Apfel et al., 1999). Therefore, in addition to drug treatment, non-pharmacological measures must also be considered to effectively reduce the incidence of PONV. One possible approach used in the past is the application of perioperative therapeutic suggestions, i.e., given pre- or postoperatively in hypnosis (Holler et al., 2021), or under general anesthesia intraoperatively. Suggestions are defined as verbal or non-verbal messages that the receiver involuntarily accepts and follows (Varga, 2013) and might therefore affect behavior, emotions, and autonomous body functions. Their effects can not only be subjectively recorded, but objectively measured and quantified (Zech et al., 2020, 2022). It is observed that even under general anesthesia, the central auditory pathway remains intact (Madler et al., 1991), and the perception of sounds and words is not interrupted (Hudetz, 2008; Sanders et al., 2012). However, several randomized controlled trials conducted on the effects of verbal suggestions given during general anesthesia in the past could only show very heterogeneous results (Rosendahl et al., 2016). These trials were small, heterogeneous in design, and conducted mainly in the 1990s and therefore did not reflect the current management of general anesthesia and PONV prophylaxis.
A recently published double-blind multicenter randomized controlled trial on the efficacy of intraoperative therapeutic suggestions showed a positive effect on postoperative opioid dosage and pain within the first 24 h after surgery, while for the incidence of PONV no differences were observed (Nowak et al., 2020). This study included patients at high and moderate risk for PONV. However, especially high-risk patients need a multimodal therapy approach to prevent PONV (Gan et al., 2020). Therefore, the question of whether intraoperative therapeutic suggestions influence PONV in these patients is of great interest and may have an impact on PONV management since therapeutic suggestions promise to be side-effect-free. Therefore, we conducted this post hoc analysis on the effect of intraoperative therapeutic suggestions on PONV after general anesthesia in patients with a high risk of PONV.
Parts of this study were recently published and reported on the effects of therapeutic suggestions during general anesthesia on postoperative pain and opioid use (Nowak et al., 2020). This study was registered in the German Clinical Trials Register (registration number DRKS00013800, registration date 26th January 2018). In a double-blind randomized, placebo-controlled trial in 5 tertiary care hospitals in Germany, patients were included between the ages of 18 and 70 who underwent elective surgery requiring general anesthesia with a planned duration of 1–3 h and a risk of PONV, defined by an Apfel risk score (Apfel et al., 1999) of two or more points. Exclusion criteria were an American Society of Anesthesiologist (ASA) score of ≥4 (Owens et al., 1978), requirement for postoperative mechanical ventilation, or the use of regional anesthesia. Eligible patients were included after written informed consent.
The study was approved by the Ethics Committee of the Ruhr-University Bochum Medical Faculty, Bochum, Germany (Chairman Prof. Dr. M. Zenz, approval No. 17-5957-BR) on 15th May 2017.
Patients were randomly assigned in a 1:1 ratio to intervention or control group. After induction of general anesthesia, patients in the intervention group listened to an Audio File containing background music and therapeutic suggestions, based on hypnotherapeutic principles, which included direct and indirect positive messages (see Figure 1). The tape was continuously played during surgery over earphones. At the end of surgery, a different file was presented to prepare the patients for emergence from anesthesia. Patients in the control group listened to a blank Audio File. For details see Nowak et al. (2020).
General anesthesia was performed as balanced anesthesia with volatile anesthetics (sevoflurane, isoflurane, or desflurane) at a minimum alveolar concentration of 1.0 ± 0.2 and repeated opioid administration. The depth of anesthesia was controlled by electroencephalography-based monitoring (Bispectral Index, Medtronic, Meerbusch, Germany, or Narcotrend, Narcotrend Group, Hannover, Germany), with a target index of 40–60. Both, a strict range of MAC above 0.8 and anesthesia depth monitoring, guarantee exclusion of inadequate anesthesia (Merikle and Daneman, 1996; Messina et al., 2016). Postoperative pain therapy was nurse or patient-controlled, according to local protocols. Before surgery, patients’ susceptibility to verbal suggestions was tested using a modified Harvard Group 5-item Hypnotic Susceptibility (HGSHS-5:G) (Riegel et al., 2021), and the level of anxiety was tested using the State Trait Anxiety Inventory (STAI-S) (Marteau and Bekker, 1992).
The risk of PONV was assessed by the preoperative Apfel risk score (Apfel et al., 1999). Only patients with medium or high risk of PONV (2–4 points) were eligible for study inclusion. Pharmacological PONV prophylaxis was administered before general anesthesia induction or intraoperatively according to local standards. These included, among others, dexamethasone, ondansetron, droperidol, metoclopramide, or dimenhydrinate. After surgery, the incidence of PONV was evaluated in the recovery room (first 2 h) and 24 h after extubation (normal ward). The severity of PONV was assessed using the simplified PONV impact scale (0–6), described by Myles and Wengritzky (2012). Treatment of PONV was performed again with dexamethasone, ondansetron, droperidol, metoclopramide, dimenhydrinate, or a combination. Antiemetic milligram equivalents (AMEs) were calculated for the comparability of various antiemetics (AME = ondansetron × 4 + dexamethasone × 4 + droperidol × 1.25 + metoclopramide × 20 + dimenhydrinate × 50) (Apfel et al., 2004a).
Sample size calculation was carried out on the primary outcome (postoperative opioid use), which was based on a recent meta-analysis (Rosendahl et al., 2016). Based on a 1:1 randomization ratio with an assumed effect size of 0.3, we calculated a total of 368 patients to obtain 80% power to detect a difference in postoperative opioid dosage at a two-sided α level of 0.05. Baseline characteristics and outcomes were analyzed as follows: continuous variables are presented as mean ± standard deviation (SD) for normally distributed variables and median and interquartile range (IQR; 25th and 75th percentiles) for non-normally distributed variables. Categorical variables are expressed as frequency and percentage. Comparison of continuous variables between groups was performed using a parametric Student’s t-test or a non-parametric Mann–Whitney U, respectively. Categorical variables were compared using Pearson’s Chi-square test and by calculation of the number needed to treat (NNT). In addition, the resulting risk differences including the 95% confidence intervals were calculated. In contrast to the previously published data of this study we performed, for better assessment of non-normally distributed variables in the outcome analysis, a bootstrapping method with resampling and calculated the means, SD, and 95% confidence intervals. For further analysis, we post hoc formed a subgroup of patients at high risk of PONV, defined by a preoperative Apfel score of 3–4. For the assessment of the joint effect of therapeutic suggestions and potential confounding factors in this subgroup, a logistic regression analysis was performed with single and multiple predictors. These included: assignment to the control group, preoperative Apfel score, dose of intraoperative antiemetics and opioids, type of surgery, and duration of surgery. Finally, a multivariable restricted model was built by using stepwise backward elimination. Furthermore, since the severity of PONV and the application of antiemetics are not independent variables, their correlation was analyzed represented by the Spearman coefficient. Statistical analysis was performed with The R Project for Statistical Computing 4.0.4 (The R Foundation for Statistical Computing, Vienna, Austria). Graphical representations of the results were created with GraphPad Prism 8.1 (GraphPad Software, San Diego, CA, United States). A two-sided p-value of less than 0.05 was considered statistically significant.
In total, 400 patients were recruited and randomized from January to December 2018 and 385 of them were analyzed in the per-protocol analysis (191 in intervention and 194 in control group), see Figure 2. The subgroup of patients with a high risk of PONV is formed by 180 patients, 87 patients in the intervention and 93 patients in the control group. Table 1 presents the baseline characteristics. Almost all parameters for the entire cohort, as well as for the high-risk subcohort, were evenly distributed between both groups. Only the distribution of the types of surgery in high-risk patients was uneven, with a higher proportion of intraabdominal surgeries in the control group and a resulting lower proportion of other types of surgery. None of the patients reported remembering to wear headphones or listening to music or verbal suggestions. No side effects were observed.
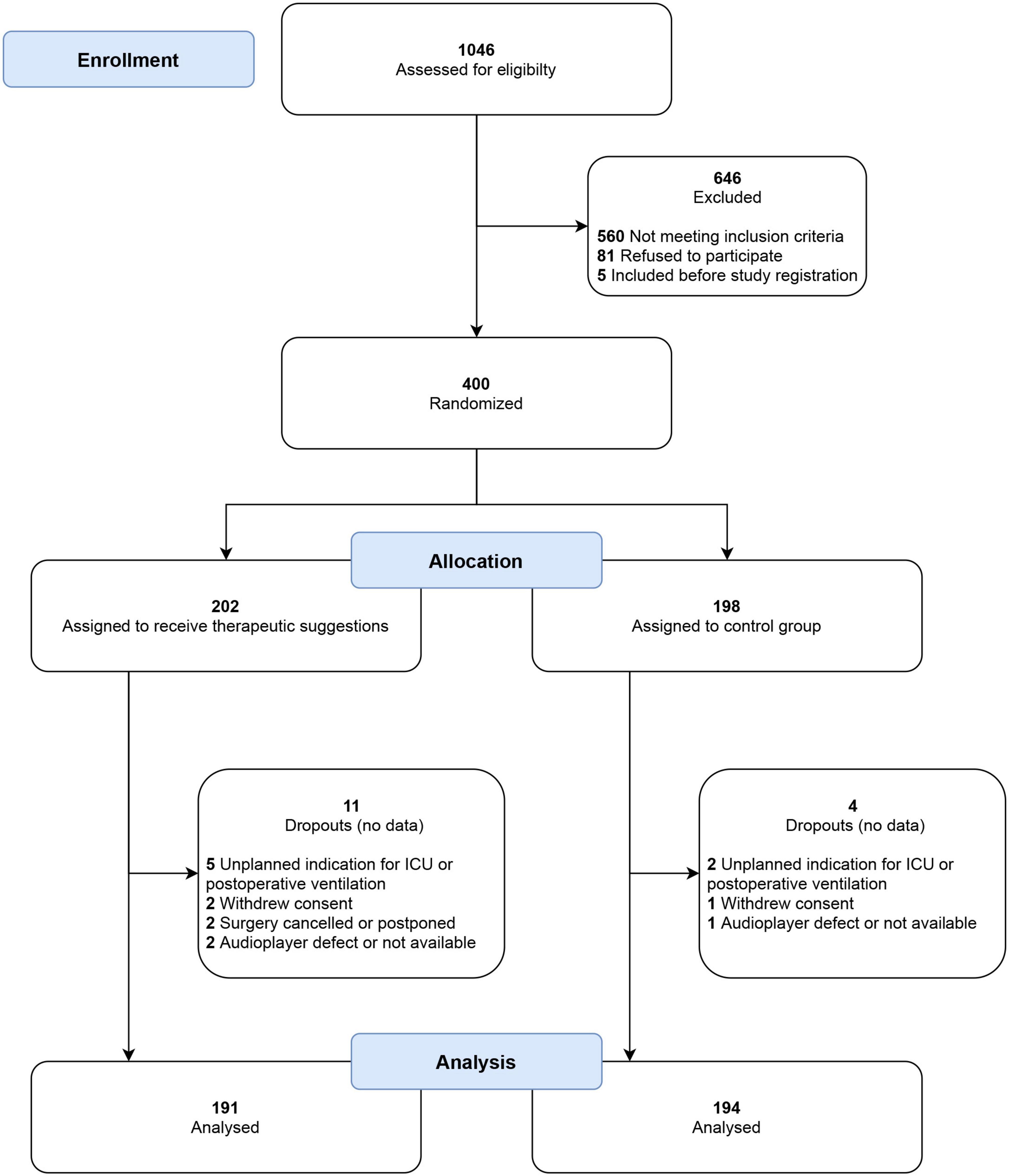
Figure 2. CONSORT flow chart of patient recruitment. No postoperative data were collected for dropouts, and they were excluded from analysis before unblinding of the study. ICU, intensive care unit.
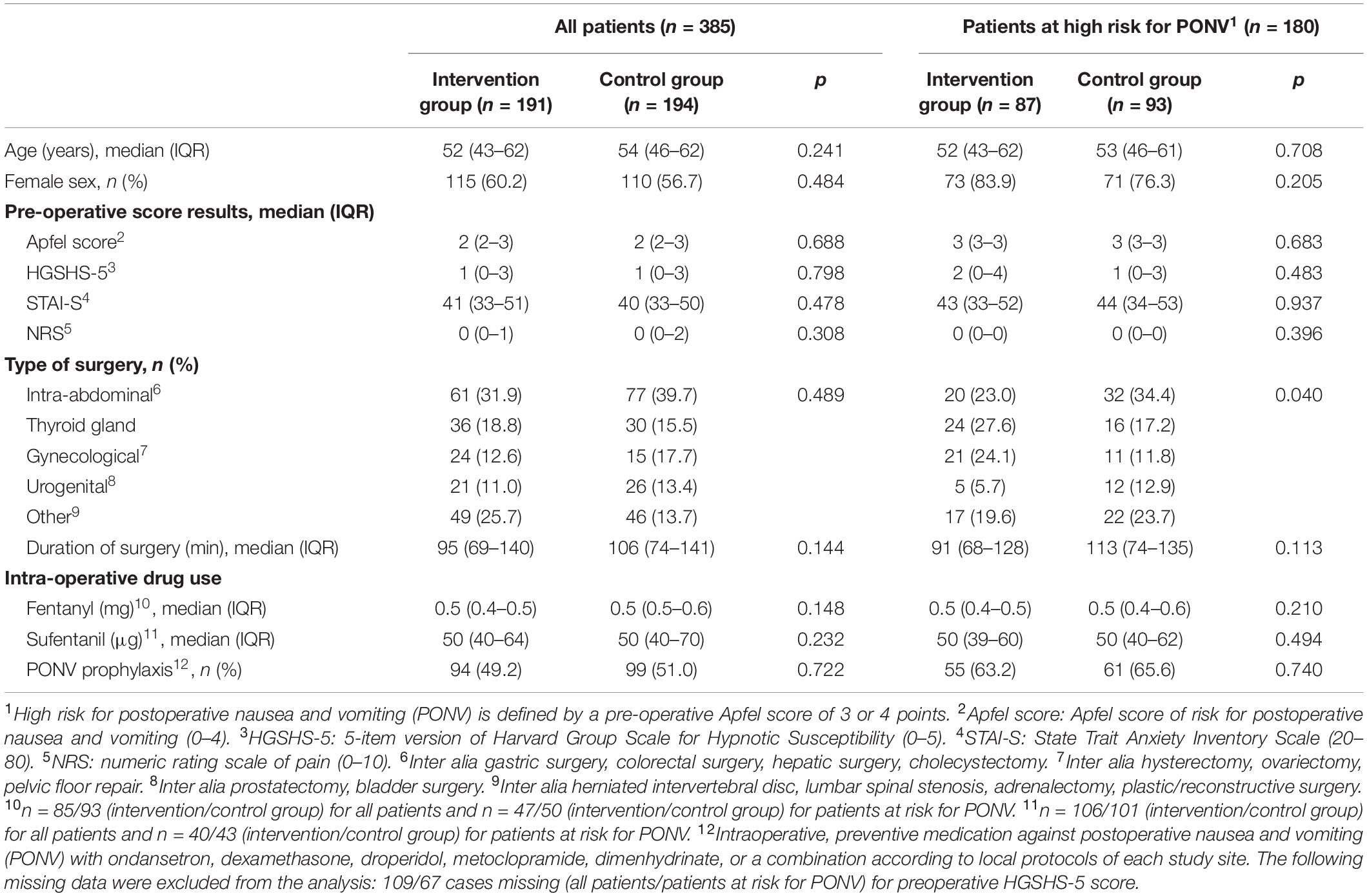
Table 1. Baseline characteristics for all patients and subgroup of patients at high risk for postoperative nausea and vomiting, defined by pre-operative Apfel-score of 3 or 4.
Outcomes are presented in Table 2. In the cohort of all patients no significant differences were observed in the incidence or severity of PONV. If the focus is set on patients at high risk for PONV, intraoperative therapeutic suggestions showed a marked effect. The incidence of both early and delayed PONV was significantly reduced by 45 or 40%, respectively (Figure 3). This corresponds to the number of patients needed to treat (NNT) of approximately 7 to avoid one case of early or delayed PONV. For high-risk patients, the PONV impact score was reduced by the intervention by approximately 50% within the first 2 or 24 h.
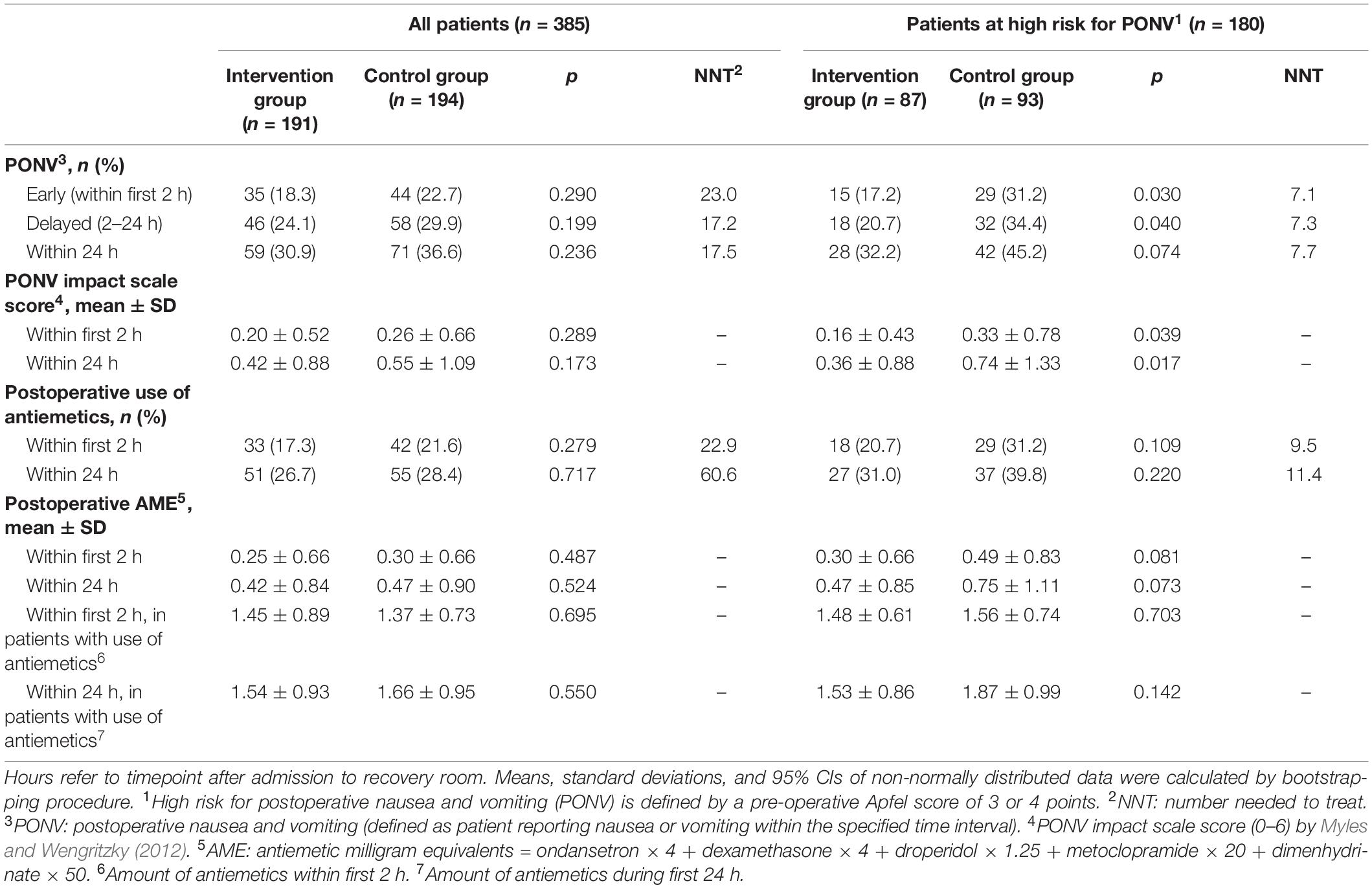
Table 2. Outcome variables for all patients and subgroup of patients at high risk for postoperative nausea and vomiting, defined by a pre-operative Apfel-score of 3 or 4.
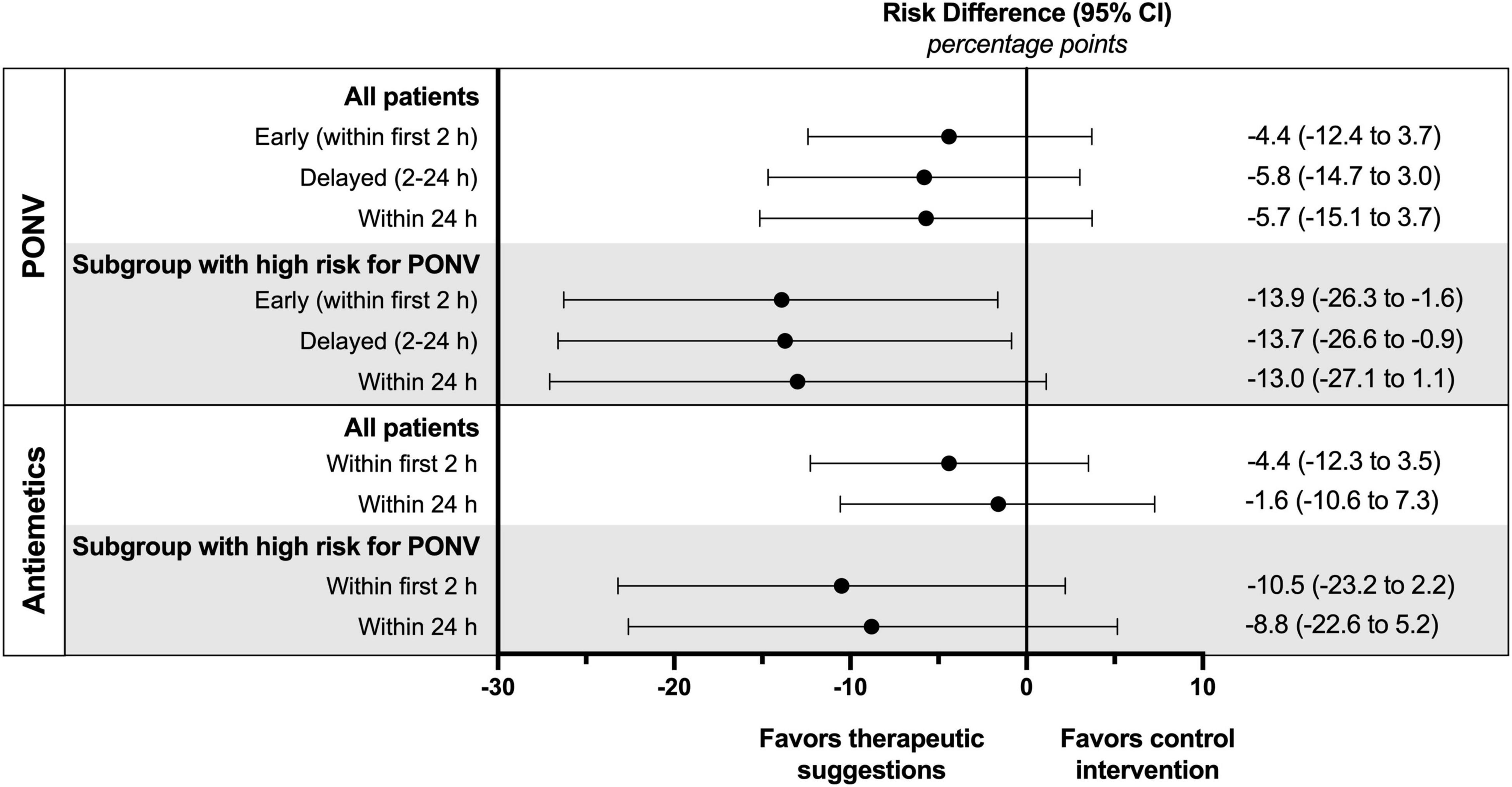
Figure 3. Absolute risk differences of PONV incidence and postoperative use of antiemetics for all patients and subgroup of patients with a high risk for PONV (defined by an Apfel score of 3 or 4).
In patients with a high risk of PONV therapeutic suggestions resulted in an absolute risk reduction for the use of postoperative antiemetics of 11% within the first 2 h and 9% within 24 h, although not reaching statistical significance (Table 2 and Figure 3). The corresponding NNT was about 10. There was also a trend to a lower dose of postoperative antiemetics by one-third in the intervention group.
In the control group, a small to intermediate but significant correlation between the PONV score and the total antiemetics dose (intraoperative and postoperative) was observed for both time periods. With the intervention, this correlation decreased (Table 3). Furthermore, in patients who developed PONV, the correlation between PONV and the use of antiemetic lost statistical significance.
Several factors associated with PONV were tested using logistic regression analysis (Table 4). In the univariate testing, the assignment to the control group had an impact on both early and delayed PONV. Further significant confounders were the preoperative Apfel risk score and intraabdominal surgery for early PONV, and the dose of postoperative opioids for delayed PONV. In the restricted multivariable model, assignment to the control group, preoperative Apfel score, and intraabdominal surgery were confirmed as independent predictors for the development of early PONV. For delayed PONV, only the PONV risk score and the postoperative dose of opioids remained predictors of statistical significance.
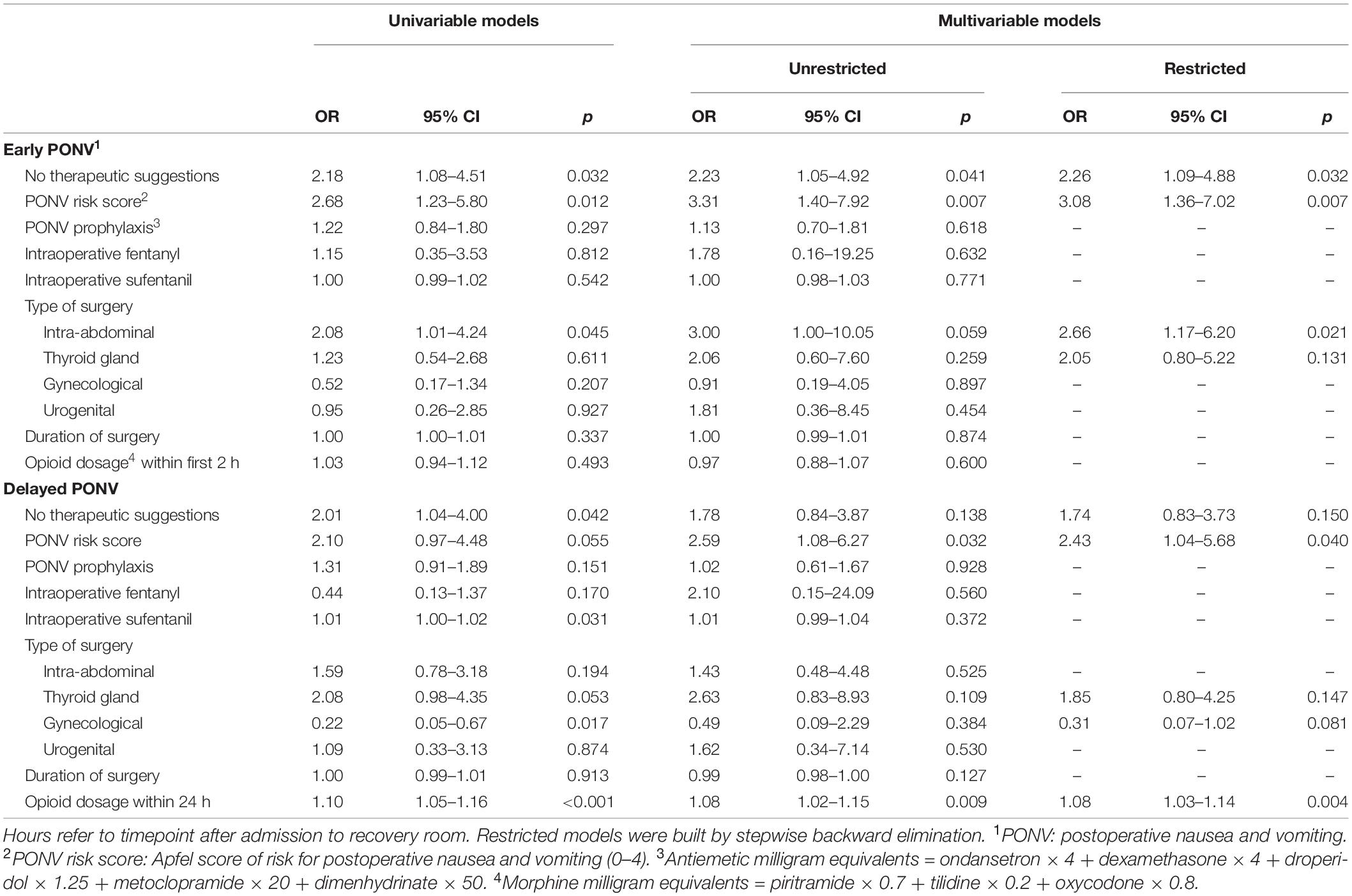
Table 4. Logistic regression model for single and multiple predictors of postoperative nausea and vomiting in patients with high risk (Apfel score 3–4) (n = 180).
Postoperative nausea and vomiting is one of the most common adverse events after surgery under general anesthesia and has a profound impact on patient comfort and satisfaction (Myles et al., 2000). Patients are often more compromised by PONV than by postoperative pain (Simanski et al., 2001). Therefore, in addition to pharmacological options, effective non-pharmacological prophylaxis and treatments are urgently needed to reduce the incidence of PONV, especially in high-risk patients with a dramatically high incidence between 61 and 79% (Gan et al., 2020).
Our intervention was able to significantly reduce the incidence of both, “early” and “delayed” PONV in patients at high risk. Furthermore, PONV severity was halved by the intervention. However, the mean intensity of PONV was low, probably because more than half of these patients, although at risk, did not develop PONV. The low incidence and severity might be attributable to the wide use of pharmacological PONV prophylaxis in this study and to an accompanying placebo effect. The demand for antiemetics was reduced by approximately one-third. In general, whenever a dose is observed, the proportion of patients treated must also be considered. In our study, the number of patients with demand for antiemetics was also reduced by one-third. However, in these patients, the difference in the total antiemetic dose between the intervention and control groups diminished. Therefore, the main reason for the observed dose reduction was probably the decreased number of patients with demand for antiemetic treatment.
The use of antiemetics affects the severity of PONV, and the severity of PONV triggers the use and dose of antiemetics. Therefore, both factors must be considered. The intervention in our study affected and reduced the correlation of these two entities. A possible interpretation of these results is the induction of tolerance against PONV by intraoperative positive suggestions, where an identical dose of antiemetics results in lower manifestations of PONV in the intervention group, and a comparable severity of PONV leads to a lower requirement for antiemetic treatment. This development of tolerance by a change in the perception, the impact and the significance of nausea is one possible basis of the observed effects. Others are an antiemetic effect of the suggestions, including images of physiological functions (the idea of appetite or of a flow downward), or interference with the generation of nausea. In contrast to awake interventions the suggestions were given during surgery, i.e., at the time of surgery and anesthesia that might be responsible for the development of nausea.
The risk of PONV in adults is influenced by many different patient-specific and surgery-related factors, e.g., female sex, history of PONV, motion sickness, non-smoking status, young age, duration of surgery/anesthesia, and specific types of intraabdominal surgery (Apfel et al., 1999, 2004b,2012; Sinclair et al., 1999). Furthermore, anesthesia-related predictors of PONV include volatile anesthetics, nitrous oxide, and postoperative use of opioids, while these factors are dose and duration dependent (Apfel et al., 2002; Breitfeld et al., 2003; Habib et al., 2006; Myles et al., 2007; Peyton and Wu, 2014). As a result, current guidelines define different prophylactic measurements depending on the individual risk of PONV. These include, in addition to pharmacological antiemetic prophylaxis, the avoidance of volatile anesthetics and nitrous oxide, and instead the use of total intravenous anesthesia (TIVA) (Gan et al., 2020). Despite differentiated prophylactic therapy approaches, a large number of especially high-risk patients still suffer from PONV (Rusch et al., 2010), with a request for multimodal approaches that also include non-pharmacological interventions (Gan et al., 2020).
In the present study, assignment to the control group was a significant determinator of PONV in high-risk patients, which subsequently proved that intraoperative therapeutic suggestions are a promising intervention against PONV. Early PONV within the first 2 h after surgery was affected by the affiliation of the study group, the preoperative Apfel PONV risk score, and the type of surgery, namely intraabdominal operations. Especially the low impact of intraoperative opioid dose and duration of surgery for the incidence of early PONV is unexpected, as the respective dose of inhalational anesthetics was previously described as a determinator (Apfel et al., 2002). In contrast to the first 2 h after surgery, where patients were in a very controlled setting in the recovery room, delayed PONV was only affected by postoperative opioid dose. These findings may be attributable to the circumstances in the postoperative setting after discharge from the recovery room to the normal ward, where many other possible confounders occur that have not been recorded or evaluated. As both state anxiety and hypnotic susceptibility did not differ in the two groups, these parameters were not included in the multivariate analysis, and no conclusion on their impact can be drawn
Several different medical interventions have been tested and reported in the effort to prevent PONV (Gan et al., 2020). To avoid one case of PONV after isoflurane anesthesia, six patients would have to receive TIVA (Visser et al., 2001). Pharmacological antiemetic prophylaxis with ondansetron has a NNT of 6 for the prevention of vomiting and 7 for nausea, respectively (Tramer et al., 1997). In addition, non-pharmacological means including acupuncture were found effective (Lee et al., 2015). However, mainly antiemetics have found their way into the everyday clinical practice of PONV prophylaxis established in the meantime.
The effect of communication techniques on PONV has also been evaluated, for instance, by studies with perioperative hypnotherapy in awake patients (Kekecs et al., 2014) and under the rather special condition of general anesthesia (Rosendahl et al., 2016). In a meta-analysis of Rosendahl et al. (2016), only 3 out of 21 included trials showed a positive effect on the incidence of PONV. However, the overall effect identified therapeutic suggestions to significantly reduce PONV. Since these former studies were conducted mainly in the 1980s and 1990s without routine PONV prophylaxis, incidence, and severity of PONV were higher. However, our study in patients after PONV prophylaxis demonstrated an even higher effect with a NNT of 7.
In general, the comparison of the various pharmacological and non-pharmacological attempts of PONV prophylaxis shows that the effect of therapeutic suggestions in our study is of a comparable magnitude with much lower costs, effort, and side effects. It should be noted that in clinical practice often a combination of different treatments is necessary to achieve a sufficient antiemetic effect and that the result is additive (Weibel et al., 2021). In our study, almost all patients had received pharmacological PONV prophylaxis. However, the effect was measured against a control group with only antiemetic drugs. Thus, it can be assumed that the observed reduction in PONV is a direct consequence of the intervention tested. Therefore, therapeutic suggestions could provide an inexpensive and safe possibility for supplementation of PONV prophylaxis.
This study has several limitations. First of all, this is a post hoc analysis of an original study, therefore, our findings should be tested in a prospective, sufficiently powered study. Moreover, the role of other contributing factors than therapeutic suggestions remain unclear – for example, positive effects may also be expected from background music. Although regularly positive effects of music on pain and anxiety can be observed, mainly with treatment in awake patients (Cepeda et al., 2006; Hole et al., 2015; Kuhlmann et al., 2018), evidence for an antiemetic effect is missing (Stoicea et al., 2015). Furthermore, a beneficial effect can also be expected from shielding the ears from intraoperative noise and careless talk, including negative suggestions (Hansen and Zech, 2019). However, this was applicable to both groups in our study.
The perception of words under general anesthesia was not unexpected, as evidence has been gathered that the auditory pathway is preserved during anesthesia (Madler et al., 1991; Hudetz, 2008). Moreover, the phenomenon of “intraoperative awareness” has been described regularly (Millar and Watkinson, 1983; Ghoneim and Block, 1992; Schwender et al., 1994). However, our results cannot be explained by a few patients reacting like in “intraoperative awareness” with an incidence of only 0.1–0.2% for explicit recall (Sanders et al., 2012) and a few percent for implicit memory (Fu et al., 2021; Linassi et al., 2021). Therefore, auditory impressions that a patient perceives under general anesthesia must be critically questioned, since conversations and noises in the operating room can have a negative influence on patients and should be avoided (Hansen and Zech, 2019).
With regard to the mechanisms responsible for the observed responses, we consider a reduced resistance to suggestions after loss of critical, rational thinking and an access to the subconscious to be responsible. This parallels the mechanism of hypnosis that is characterized by a depression of the dorsolateral frontal cortex (DLPFC) (Dienes and Hutton, 2013), and shows similar beneficial effects of suggestions in surgical patients (Kekecs et al., 2014). The difference between low incidences of “intraoperative awareness” and the high incidence of perception in this study may be explained by the content. While it is random in explicit memory (thoughtless conversations in the operating room) and neutral in experiments of implicit memory (test texts), it is characterized by meaning in the application of therapeutic communication before or during surgery. In experiments with intraoperative simulation of a ventilation incident, 8 out of 10 patients had an implicit memory or reaction (Levinson, 1965). While the reports on “intraoperative awareness” with its low incidences did not lead to a general change in the behavior in the operating rooms over all those years, hopefully the present demonstration of intraoperative perception will.
Intraoperative therapeutic suggestions were demonstrated to affect postoperative pain and request for analgesics (Nowak et al., 2020), as well as PONV and use of antiemetics as reported here. The high efficacy of the tested intervention compared to previous trials might be attributed to the specific text of the suggestions. Negative words and negations such as “no nausea” were avoided. Instead, “increased comfort,” appetite and pleasurable food intake after surgery were addressed. The suggestions presented intraoperatively dealt with items such as support, care, and self-healing power. From a text addressing such general topics of well-being, further effects can be expected and should be studied. Some interesting parameters that cannot be monitored and measured so fast and easily might be affected concurrently, such as wound healing, homeostasis, or immune surveillance, but also could be addressed more specifically.
We consider the addressing of themes of meaning essential for the observed effects, namely accompaniment, contact, comfort, confidence, information, control, instructions, respect, safety, and healing (Hansen and Zech, 2019). Constructing placebo effects as a mechanism of action is difficult since generation of expectations under general anesthesia has not been described yet. However, it has been suggested to better call the placebo effect a “meaning response” as well (Moerman and Jonas, 2002). Actually, response to meaning could be the common basis of hypnosis, therapeutic communication and placebo effects. The melody of the voice and the perception of a caring person close may play a role in addition.
Our results encourage the use of therapeutic suggestions under general anesthesia, especially since it is an inexpensive intervention that is virtually free of side effects. They should not be limited to taped recordings favorable for standardized study conditions but stimulate personal talk to patients and wider application of positive and therapeutic communication also in awake patients. The demonstrated positive effects of therapeutic suggestions even under general anesthesia should stimulate further research and application in other patients during impaired wakefulness, such as during resuscitation or intensive care, “touching the unconscious in the unconscious.”
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Ruhr-University Bochum Medical Faculty, Bochum, Germany. The patients/participants provided their written informed consent to participate in this study.
HN: writing of the manuscript, data analysis, and critical revision of the manuscript. AW and TR: data analysis and critical revision of the manuscript. GO: study conception and design, study center supervision, data collection, and critical revision of the manuscript. LG, MM, KG, CM, AZ, and KL: data collection, analysis and interpretation, and critical revision of the manuscript. JL, TS, and MT: study center supervision, data collection, analysis and interpretation, and critical revision of the manuscript. MA: study supervision and critical revision of the manuscript. EH and NZ: study conception and design, development and taping of the intervention text, study supervision, writing of the manuscript and data interpretation, and critical revision of the manuscript. All authors approved the final version to be published and agreed to be accountable for all aspects of the work.
We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the patients for participation in the study; and Anita Jung (hypnotherapist, Austin, TX, United States) for English proofreading of the tested texts for intraoperative suggestions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2022.898326/full#supplementary-material
Apfel, C. C., Heidrich, F. M., Jukar-Rao, S., Jalota, L., Hornuss, C., Whelan, R. P., et al. (2012). Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br. J. Anaesth. 109, 742–753. doi: 10.1093/bja/aes276
Apfel, C. C., Korttila, K., Abdalla, M., Kerger, H., Turan, A., Vedder, I., et al. (2004a). A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N. Engl. J. Med. 350, 2441–2451. doi: 10.1056/NEJMoa032196
Apfel, C. C., Kranke, P., and Eberhart, L. H. (2004b). Comparison of surgical site and patient’s history with a simplified risk score for the prediction of postoperative nausea and vomiting. Anaesthesia 59, 1078–1082. doi: 10.1111/j.1365-2044.2004.03875.x
Apfel, C. C., Kranke, P., Katz, M. H., Goepfert, C., Papenfuss, T., Rauch, S., et al. (2002). Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br. J. Anaesth. 88, 659–668. doi: 10.1093/bja/88.5.659
Apfel, C. C., Laara, E., Koivuranta, M., Greim, C. A., and Roewer, N. (1999). A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 91, 693–700. doi: 10.1097/00000542-199909000-00022
Breitfeld, C., Peters, J., Vockel, T., Lorenz, C., and Eikermann, M. (2003). Emetic effects of morphine and piritramide. Br. J. Anaesth. 91, 218–223. doi: 10.1093/bja/aeg165
Bremner, W. G., and Kumar, C. M. (1993). Delayed surgical emphysema, pneumomediastinum and bilateral pneumothoraces after postoperative vomiting. Br. J. Anaesth. 71, 296–297. doi: 10.1093/bja/71.2.296
Cepeda, M. S., Carr, D. B., Lau, J., and Alvarez, H. (2006). Music for pain relief. Cochrane Database Syst. Rev. 2:CD004843. doi: 10.1002/14651858.CD004843.pub2
Cohen, M. M., Duncan, P. G., DeBoer, D. P., and Tweed, W. A. (1994). The postoperative interview: assessing risk factors for nausea and vomiting. Anesth. Analg. 78, 7–16. doi: 10.1213/00000539-199401000-00004
Dienes, Z., and Hutton, S. (2013). Understanding hypnosis metacognitively: rTMS applied to left DLPFC increases hypnotic suggestibility. Cortex 49, 386–392. doi: 10.1016/j.cortex.2012.07.009
Fu, V. X., Sleurink, K. J., Janssen, J. C., Wijnhoven, B. P. L., Jeekel, J., and Klimek, M. (2021). Perception of auditory stimuli during general anesthesia and its effects on patient outcomes: a systematic review and meta-analysis. Can. J. Anaesth. 68, 1231–1253. doi: 10.1007/s12630-021-02015-0
Gan, T. J., Belani, K. G., Bergese, S., Chung, F., Diemunsch, P., Habib, A. S., et al. (2020). Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth. Analg. 131, 411–448. doi: 10.1213/ANE.0000000000004833
Ghoneim, M. M., and Block, R. I. (1992). Learning and consciousness during general anesthesia. Anesthesiology 76, 279–305. doi: 10.1097/00000542-199202000-00018
Habib, A. S., Chen, Y. T., Taguchi, A., Hu, X. H., and Gan, T. J. (2006). Postoperative nausea and vomiting following inpatient surgeries in a teaching hospital: a retrospective database analysis. Curr. Med. Res. Opin. 22, 1093–1099. doi: 10.1185/030079906X104830
Hansen, E., and Zech, N. (2019). Nocebo effects and negative suggestions in daily clinical practice - Forms, impact and approaches to avoid them. Front. Pharmacol. 10:77. doi: 10.3389/fphar.2019.00077
Hole, J., Hirsch, M., Ball, E., and Meads, C. (2015). Music as an aid for postoperative recovery in adults: a systematic review and meta-analysis. Lancet 386, 1659–1671. doi: 10.1016/S0140-6736(15)60169-6
Holler, M., Koranyi, S., Strauss, B., and Rosendahl, J. (2021). Efficacy of hypnosis in adults undergoing surgical procedures: a meta-analytic update. Clin. Psychol. Rev. 85:102001. doi: 10.1016/j.cpr.2021.102001
Hudetz, A. G. (2008). Are we unconscious during general Anesthesia? Int. Anesthesiol. Clin. 46, 25–42. doi: 10.1097/AIA.0b013e3181755db5
Kekecs, Z., Nagy, T., and Varga, K. (2014). The effectiveness of suggestive techniques in reducing postoperative side effects: a meta-analysis of randomized controlled trials. Anesth. Analg. 119, 1407–1419. doi: 10.1213/ANE.0000000000000466
Kuhlmann, A. Y. R., de Rooij, A., Kroese, L. F., van Dijk, M., Hunink, M. G. M., and Jeekel, J. (2018). Meta-analysis evaluating music interventions for anxiety and pain in surgery. Br. J. Surg. 105, 773–783. doi: 10.1002/bjs.10853
Lee, A., Chan, S. K., and Fan, L. T. (2015). Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database Syst. Rev. 11:CD003281. doi: 10.1002/14651858.CD003281.pub4
Levinson, B. W. (1965). States of awareness during general anaesthesia. Preliminary communication. Br. J. Anaesth. 37, 544–546. doi: 10.1093/bja/37.7.544
Linassi, F., Obert, D. P., Maran, E., Tellaroli, P., Kreuzer, M., Sanders, R. D., et al. (2021). Implicit memory and Anesthesia: a systematic review and meta-analysis. Life 11:850. doi: 10.3390/life11080850
Madler, C., Keller, I., Schwender, D., and Poppel, E. (1991). Sensory information processing during general anaesthesia: effect of isoflurane on auditory evoked neuronal oscillations. Br. J. Anaesth. 66, 81–87. doi: 10.1093/bja/66.1.81
Marteau, T. M., and Bekker, H. (1992). The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 31(Pt 3), 301–306. doi: 10.1111/j.2044-8260.1992.tb00997.x
Merikle, P. M., and Daneman, M. (1996). Memory for unconsciously perceived events: evidence from anesthetized patients. Conscious. Cogn. 5, 525–541. doi: 10.1006/ccog.1996.0031
Messina, A. G., Wang, M., Ward, M. J., Wilker, C. C., Smith, B. B., Vezina, D. P., et al. (2016). Anaesthetic interventions for prevention of awareness during surgery. Cochrane Database Syst. Rev. 10:CD007272. doi: 10.1002/14651858.CD007272.pub2
Millar, K., and Watkinson, N. (1983). Recognition of words presented during general anaesthesia. Ergonomics 26, 585–594. doi: 10.1080/00140138308963377
Moerman, D. E., and Jonas, W. B. (2002). Deconstructing the placebo effect and finding the meaning response. Ann. Intern. Med. 136, 471–476. doi: 10.7326/0003-4819-136-6-200203190-00011
Myles, P. S., Leslie, K., Chan, M. T., Forbes, A., Paech, M. J., Peyton, P., et al. (2007). Avoidance of nitrous oxide for patients undergoing major surgery: a randomized controlled trial. Anesthesiology 107, 221–231. doi: 10.1097/01.anes.0000270723.30772.da
Myles, P. S., and Wengritzky, R. (2012). Simplified postoperative nausea and vomiting impact scale for audit and post-discharge review. Br. J. Anaesth. 108, 423–429. doi: 10.1093/bja/aer505
Myles, P. S., Williams, D. L., Hendrata, M., Anderson, H., and Weeks, A. M. (2000). Patient satisfaction after anaesthesia and surgery: results of a prospective survey of 10,811 patients. Br. J. Anaesth. 84, 6–10. doi: 10.1093/oxfordjournals.bja.a013383
Nowak, H., Zech, N., Asmussen, S., Rahmel, T., Tryba, M., Oprea, G., et al. (2020). Effect of therapeutic suggestions during general anaesthesia on postoperative pain and opioid use: multicentre randomised controlled trial. BMJ 371:m4284. doi: 10.1136/bmj.m4284
Owens, W. D., Felts, J. A., and Spitznagel, E. L. Jr. (1978). ASA physical status classifications: a study of consistency of ratings. Anesthesiology 49, 239–243.
Peyton, P. J., and Wu, C. Y. (2014). Nitrous oxide-related postoperative nausea and vomiting depends on duration of exposure. Anesthesiology 120, 1137–1145. doi: 10.1097/ALN.0000000000000122
Riegel, B., Tonnies, S., Hansen, E., Zech, N., Eck, S., Batra, A., et al. (2021). German Norms of the Harvard Group Scale of Hypnotic Susceptibility, Form A (HGSHS:A) and Proposal of a 5-Item Short-Version (HGSHS-5:G). Int. J. Clin. Exp. Hypn. 69, 112–123. doi: 10.1080/00207144.2021.1836645
Rosendahl, J., Koranyi, S., Jacob, D., Zech, N., and Hansen, E. (2016). Efficacy of therapeutic suggestions under general anesthesia: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 16:125. doi: 10.1186/s12871-016-0292-0
Rusch, D., Eberhart, L. H., Wallenborn, J., and Kranke, P. (2010). Nausea and vomiting after surgery under general anesthesia: an evidence-based review concerning risk assessment, prevention, and treatment. Dtsch. Arztebl. Int. 107, 733–741. doi: 10.3238/arztebl.2010.0733
Samuels, J. D. (2013). Perioperative nausea and vomiting: much ado about nothing? Aesthet. Plast. Surg. 37, 634–635. doi: 10.1007/s00266-013-0068-6
Sanders, R. D., Tononi, G., Laureys, S., and Sleigh, J. W. (2012). Unresponsiveness not equal unconsciousness. Anesthesiology 116, 946–959. doi: 10.1097/ALN.0b013e318249d0a7
Schumann, R., and Polaner, D. M. (1999). Massive subcutaneous emphysema and sudden airway compromise after postoperative vomiting. Anesth. Analg. 89, 796–797. doi: 10.1097/00000539-199909000-00050
Schwender, D., Kaiser, A., Klasing, S., Peter, K., and Poppel, E. (1994). Midlatency auditory evoked potentials and explicit and implicit memory in patients undergoing cardiac surgery. Anesthesiology 80, 493–501. doi: 10.1097/00000542-199403000-00004
Simanski, C., Waldvogel, H. H., and Neugebauer, E. (2001). [Postoperative nausea and vomiting (PONV). Clinical significance, basic principles, prevention and therapy]. Chirurg 72, 1417–1426. doi: 10.1007/s001040170005
Sinclair, D. R., Chung, F., and Mezei, G. (1999). Can postoperative nausea and vomiting be predicted? Anesthesiology 91, 109–118. doi: 10.1097/00000542-199907000-00018
Stoicea, N., Gan, T. J., Joseph, N., Uribe, A., Pandya, J., Dalal, R., et al. (2015). Alternative therapies for the prevention of postoperative nausea and vomiting. Front. Med. 2:87. doi: 10.3389/fmed.2015.00087
Tramer, M. R., Reynolds, D. J., Moore, R. A., and McQuay, H. J. (1997). Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials. Anesthesiology 87, 1277–1289. doi: 10.1097/00000542-199712000-00004
Varga, K. (2013). Suggestive techniques connected to medical interventions. Interv. Med. Appl. Sci. 5, 95–100. doi: 10.1556/IMAS.5.2013.3.1
Visser, K., Hassink, E. A., Bonsel, G. J., Moen, J., and Kalkman, C. J. (2001). Randomized controlled trial of total intravenous anesthesia with propofol versus inhalation anesthesia with isoflurane-nitrous oxide: postoperative nausea with vomiting and economic analysis. Anesthesiology 95, 616–626. doi: 10.1097/00000542-200109000-00012
Weibel, S., Schaefer, M. S., Raj, D., Rucker, G., Pace, N. L., Schlesinger, T., et al. (2021). Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: an abridged Cochrane network meta-analysis. Anaesthesia 76, 962–973. doi: 10.1111/anae.15295
Zech, N., Scharl, L., Seemann, M., Pfeifer, M., and Hansen, E. (2022). Nocebo effects of clinical communication and placebo effects of positive suggestions on respiratory muscle strength. Front. Psychol. 13:825839. doi: 10.3389/fpsyg.2022.825839
Keywords: general anesthesia, hypnotherapy, patient communication, postoperative nausea and vomiting, therapeutic suggestions
Citation: Nowak H, Wolf A, Rahmel T, Oprea G, Grause L, Moeller M, Gyarmati K, Mittler C, Zagler A, Lutz K, Loeser J, Saller T, Tryba M, Adamzik M, Hansen E and Zech N (2022) Therapeutic Suggestions During General Anesthesia Reduce Postoperative Nausea and Vomiting in High-Risk Patients – A Post hoc Analysis of a Randomized Controlled Trial. Front. Psychol. 13:898326. doi: 10.3389/fpsyg.2022.898326
Received: 17 March 2022; Accepted: 16 June 2022;
Published: 15 July 2022.
Edited by:
Martina Amanzio, University of Turin, ItalyReviewed by:
Chris Apfel, SageMedic Corp., United StatesCopyright © 2022 Nowak, Wolf, Rahmel, Oprea, Grause, Moeller, Gyarmati, Mittler, Zagler, Lutz, Loeser, Saller, Tryba, Adamzik, Hansen and Zech. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hartmuth Nowak, aGFydG11dGgubm93YWtAcnVoci11bmktYm9jaHVtLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.