- Normandie Univ, UNICAEN, PSL Université Paris, EPHE, INSERM, U1077, CHU de Caen, Centre Cyceron, Caen, France
Cognitive reserve and resilience refer to the set of processes allowing the preservation of cognitive performance in the presence of structural and functional brain changes. Investigations of these concepts have provided unique insights into the heterogeneity of cognitive and brain changes associated with aging. Previous work mainly relied on methods benefiting from a high spatial precision but a low temporal resolution, and thus the temporal brain dynamics underlying these concepts remains poorly known. Moreover, while spontaneous fluctuations of neural activity have long been considered as noise, recent work highlights its critical contribution to brain functions. In this study, we synthesized the current state of knowledge from magnetoencephalography (MEG) and electroencephalography (EEG) studies that investigated the contribution of maintenance of neural synchrony, and variability of brain dynamics, to cognitive changes associated with healthy aging and the progression of neurodegenerative disease (such as Alzheimer's disease). The reviewed findings highlight that compensations could be associated with increased synchrony of higher (>10 Hz) frequency bands. Maintenance of young-like synchrony patterns was also observed in healthy older individuals. Both maintenance and compensation appear to be highly related to preserved structural integrity (brain reserve). However, increased synchrony was also found to be deleterious in some cases and reflects neurodegenerative processes. These results provide major elements on the stability or variability of functional networks as well as maintenance of neural synchrony over time, and their association with individual cognitive changes with aging. These findings could provide new and interesting considerations about cognitive reserve, maintenance, and resilience of brain functions and cognition.
Introduction
Understanding the evolution of cognition as we age is crucial not only because it is intimately related to everyone's subjective experience, but to help describe and better understand cognition itself. Cognitive aging is a highly variable phenomenon, characterized by a decline in cognitive abilities that might lead to higher risk of pathological aging in some individuals, whereas others are able to remain efficient in most everyday tasks until an advanced age (Reuter-Lorenz and Park, 2014). Research on the variability of age-related changes across individuals aims at answering several questions, such as the factors associated with preserved cognitive functioning with age (the “why” question), or what differs between individuals with preserved vs. declined cognitive performance (the “how” question). While lifestyle activities associated with reduced risk of cognitive decline and pathological aging have been extensively investigated and specified (see Livingston et al., 2020, for a recent review), a mechanistic understanding of the neural bases underlying individual differences with advancing age remains to be further developed. Achieving this goal requires the use of highly sensitive methods, and the investigation on brain activity with high spatial and temporal resolutions.
The concept of cognitive reserve has originally been proposed to improve our understanding of mechanisms underlying sustained cognitive performance in spite of age-related and pathology-related brain changes (Stern, 2002). Over the last two decades, this concept has undergone several changes and developments (see Cabeza et al., 2018; Stern et al., 2020; Pascual-Leone and Bartres-Faz, 2021). The current concepts of maintenance, resistance, and resilience have been proposed to specify the heterogeneity brain and cognitive changes in face of aging and neurodegenerative disease. During healthy aging, maintenance (Nyberg et al., 2012) refers to the relative absence of brain changes with advancing age, whereas reserve (Stern, 2002) encompasses the compensatory processes allowing the preservation of cognitive performance in the presence of structural and functional brain changes. The concept of reserve involves two complementary aspects, namely, brain reserve (i.e., individual differences in brain size, number of synapses, etc.) and cognitive reserve (i.e., individual differences in the cognitive processes and brain networks recruited to perform a given task). With the progression of neurodegenerative disorders, resistance (Arenaza-Urquijo and Vemuri, 2018) defines the protection of brain reserve against alterations and the prevention of brain lesions and damages before their occurrence. Finally, resilience (Pascual-Leone and Bartres-Faz, 2021) concerns the processes involved in the compensation of changes occurring with the development of neurodegenerative disorders, explaining various degrees of symptomatology in the face of an equal pathological load. Some degree of overlap may exist between these concepts. As an example, compensatory adjustments (e.g., increased frontal recruitment) might be implemented in the presence of both age- and pathology-related changes, yet qualitative differences may also be observed between the adjustments implemented in response to healthy aging and pathology. A recent review of Pascual-Leone and Bartres-Faz (2021) specified these concepts and emphasized the need for individually based markers that could predict an individual's risk for disability and cognitive decline.
Neuroimaging studies have been central in the emergence and evolution of the concepts of reserve, maintenance, and resilience. Several decades of work aimed at investigating structural (i.e., gray matter, white matter) and functional (i.e., local activity, association between the activities of distinct brain regions) changes with age and pathology (e.g., Damoiseaux, 2017; Spreng and Turner, 2019). Recent work revealed that these dimensions are closely related, with large-scale brain functional network relying on a structural architecture of white matter fibers (e.g., Hinault et al., 2019; Suárez et al., 2020). Indeed, preserved brain integrity has been associated with preserved cognitive functioning. This work also identified compensatory adjustments in older adults (i.e., larger activations, bilateral recruitment) associated with similar cognitive performance to that of younger individuals (e.g., Cabeza et al., 2018). These studies mainly relied on high spatial resolution methods such as functional magnetic resonance imaging (fMRI), characterized by excellent spatial accuracy but low temporal resolution (in the order of seconds). Therefore, the temporal dynamics of brain networks and the influence of age and pathology on these dynamics remain poorly known. Investigating these dynamics with high temporal resolution methods (in the order of milliseconds) such as magnetoencephalography (MEG) or electroencephalography (EEG) is important because higher cognitive functions such as memory and cognitive control that are most affected by age and pathology involve multiple cognitive processes in rapid successions and short durations (e.g., Courtney and Hinault, 2021). Even when compensation may mask these changes in clinical or neuropsychological assessment, specifying dynamic network connectivity may reveal alterations in the stability of communications between brain regions or delays in the fast succession of connectivity patterns, providing a better understanding of the concepts of reserve, maintenance, resilience, and resistance.
The brain generates its own temporal structure, which is critical to the ways in which signals are routed, combined, and coordinated (e.g., Bao et al., 2015; Voytek and Knight, 2015). Brain rhythms present the particularity of being observed across species (e.g., Buzsáki, 2019), which suggest a major evolutionary advantage of rhythmic communications (e.g., Miller et al., 2018). Brain rhythms are distinguished in different frequency bands [delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz), gamma (30–45 Hz)]. MEG and EEG methods enable the investigation of these rhythms and dynamic connectivity patterns in humans (e.g., Baillet, 2017). Given the critical involvement of coordinated brain rhythms in higher-order cognitive processing, age-related changes in the dynamic of brain communications may act as a marker for cognitive decline in older adults. Such complex interplay across rhythms is highly sensitive to structural changes and the progression of neurodegenerative disorders (e.g., Gaubert et al., 2019; Courtney and Hinault, 2021) as even small changes of dynamic synchrony can lead to cognitive decline with aging (e.g., Hinault et al., 2020, 2021; Kumral et al., 2020).
In this study, we reviewed EEG and MEG studies in healthy older individuals and in patients to identify elements that shed new light on cognitive aging and pathology effects. We focused on three main patterns, namely, (1) compensatory or resilience adjustments when brain dynamics are altered, (2) maintenance of these dynamics in the face of aging or pathology, and (3) non-effective or maladaptive changes related to normal aging or pathology. These concepts have been extensively investigated with fMRI; we thus aimed at highlighting the complementary contribution of M/EEG findings. M/EEG can indeed detect between-group differences in the initiation, maintenance, or interruption of brain communication at the sub-second level, which is therefore highly sensitive to early disruptions associated with age and pathology. In this review, we first presented studies contributing to these concepts in healthy aging before presenting work on pathological aging. Given the work conducted in pathological aging, we focused on Alzheimer's disease (AD) and its preclinical stage the mild cognitive impairment (MCI). We concluded with recommendations and future directions that could guide and stimulate future studies. Such endeavor could lead to new cognitive stimulations strategies targeting brain rhythms and reduce the cognitive decline commonly associated with age.
Normal Aging
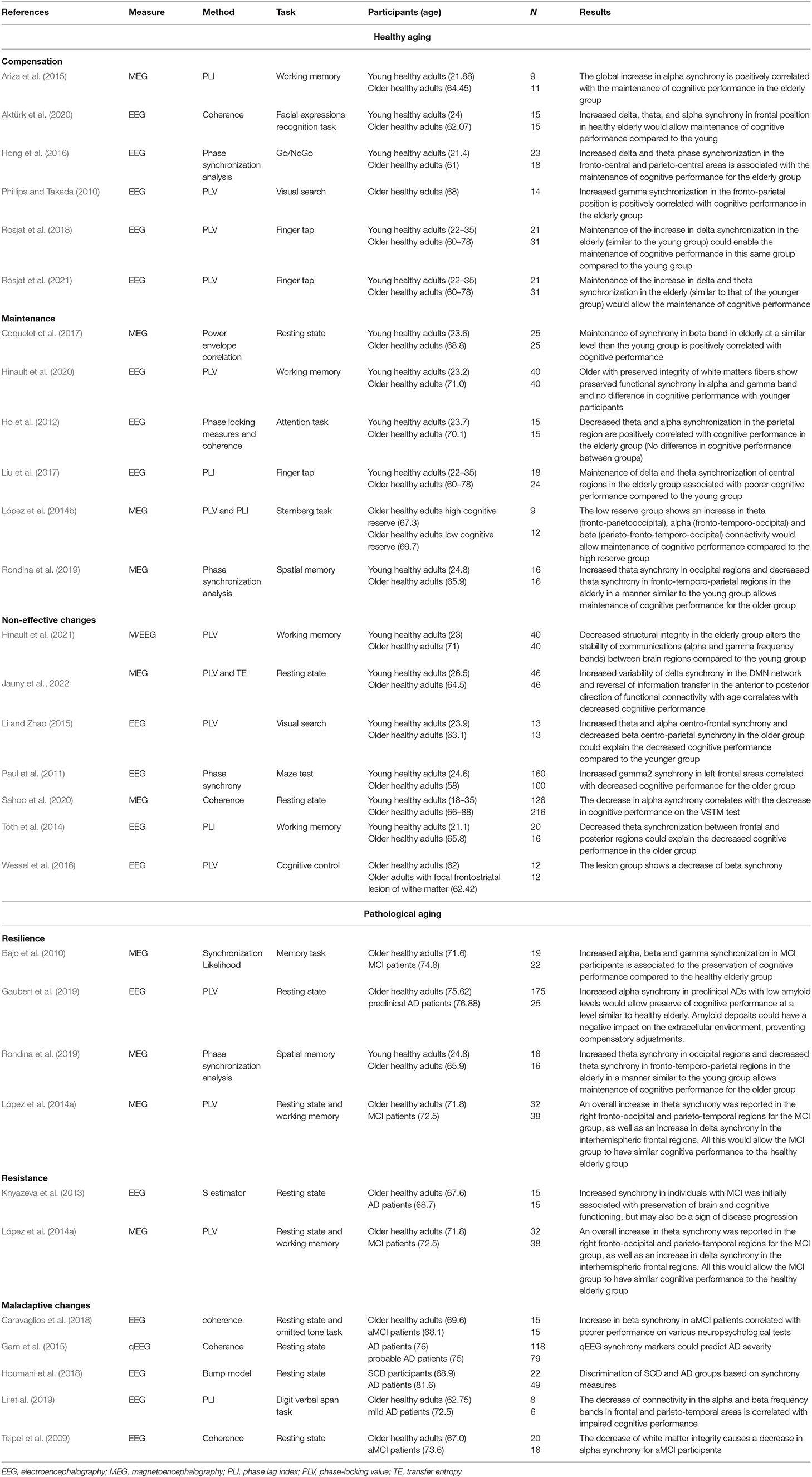
Nineteen M/EEG studies, including younger (20–30 years) and older (60–85 years) healthy participants, have been selected, investigating cognitive performance and neural synchrony data (see Table 1). In this study, we described three of the most commonly used techniques. First, the phase-locking value (PLV; Lachaux et al., 1999) measures whether the oscillatory phase of the activity of a group of neurons, in a certain frequency band, can transiently synchronize with those of another group of neurons. This transient phase locking has been associated with communications between neural groups (Fries, 2015). A second technique similar to this is the phase lag index (PLI; Stam et al., 2007), which measures the asymmetry of the distribution of phase differences between two signals. Finally, coherence (Nunez et al., 1997) measures the association of signals' amplitudes across brain regions. These measures and provides information about neural synchrony and the presence of functional connectivity between brain regions. The main results studied in this section are illustrated in Figure 1A.
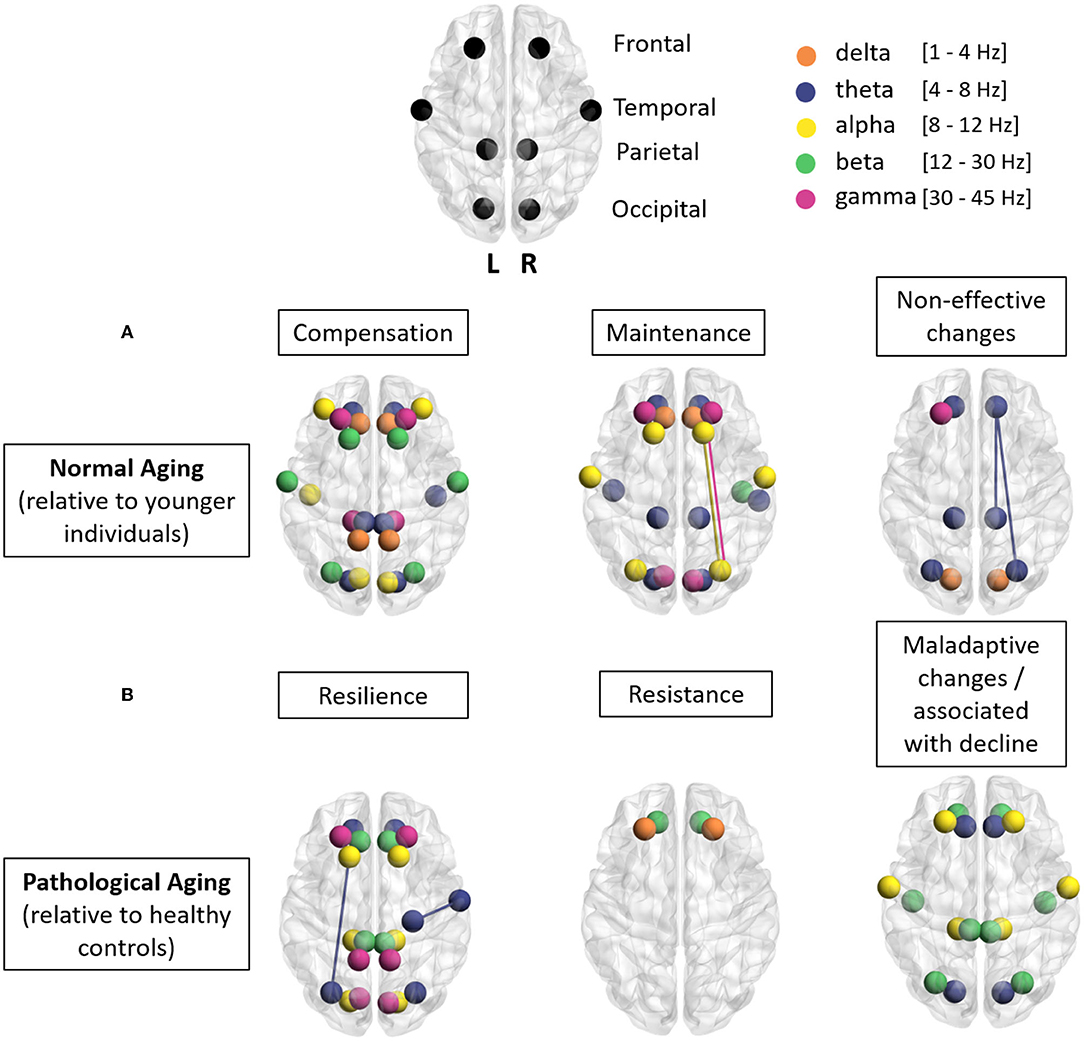
Figure 1. Illustration of the main results of this study: each point represents a brain region and each color a frequency band (orange, delta; blue, theta; yellow, alpha; green, beta; pink, gamma). (A) Normal aging. This row of three brains summarizes the main results found in this review (change in synchrony in different regions for different frequency bands relative to younger individuals) for the notions of compensation, maintenance, and non-effective changes in normal aging. (B) Pathological aging. This row of three brains summarizes the main results found in this review (change in synchrony in different regions for different frequency bands, relative to healthy controls) for the notions of resilience, resistance, and maladaptive changes in pathological aging. Using BrainNet-viewer tool (Xia et al., 2013).
Compensation
In older individuals with similar levels of cognitive performance as younger individuals, previous MRI studies have shown that older individuals frequently engage additional brain regions, as described in the Compensation Related Utilization of Neural Circuit Hypothesis (Reuter-Lorenz and Cappell, 2008) model. This increased recruitment of brain regions has been associated with cognitive performance and is referred to as compensation (Cabeza et al., 2018). Angel et al. (2010, 2011) investigated this phenomenon in EEG using event-related potential (ERP). They revealed larger bilateral activity in older individuals with higher cognitive performance. M/EEG studies critically identified that an increased synchronization of frontoparietal regions has been observed in older individuals showing preservation of cognitive functioning. Ariza et al. (2015) asked healthy young and elderly participants to perform an interference-based working memory task in which participants were asked to memorize a face/sound pair (a face was displayed and associated with a semantic attribute). The pair was followed by an interference phase during which a known face with a question about that person was presented to them. Finally, a recognition phase in which participants were asked to tell whether the presented pairs were the same as those presented in the encoding phase. This study reported a global increase in alpha synchrony (measured by PLV) in older individuals compared to younger individuals, whereas both groups showed similar cognitive performance. This increased alpha synchrony has only been observed during the interference period and has been interpreted as a compensatory mechanism in older individuals.
Using an inhibitory task, Hong et al. (2016) showed that an increase in phase synchrony of the delta and theta frequency bands localized at the frontocentral and parietocentral areas reflected a compensatory phenomenon in healthy older individuals (see also Phillips and Takeda, 2010, for similar findings in the gamma frequency band). Also, a posterior-anterior synchrony switch (increase in delta synchrony in anterior vs. posterior position) was also found to be compensatory for a facial emotion recognition task (Aktürk et al., 2020). These compensatory phenomena are found not only in cognitive tasks but also in motor tasks. For example, Rosjat et al. (2021) showed increases in delta and theta synchrony (measured by PLV) in healthy elderly in association with sustained motor performance at the same level as in young people (see also Rosjat et al., 2018).
Maintenance
Increased brain activity is not systematic, and it is important to note that some individuals do not need to implement these compensations, possibly because of higher cognitive reserve levels (Stern, 2009). López et al. (2014b), in a study comparing the global connectivity (with PLV and PLI) of older participants with higher or lower cognitive reserve, showed compensatory phenomena from the latter group only. Indeed, while cognitive performance was similar between low and high reserve participants, individuals with lower reserve showed an increase in theta, alpha, and beta synchrony in frontal, temporal, and occipital regions. This suggests that some individuals show a more efficient network functioning and therefore do not need to engage a more extended brain network. Relatedly, Ho et al. (2012) showed that lower theta and alpha synchrony levels (measured with coherence and phase-locking measures) in the parietal region were associated with similar performance than younger individuals.
Maintenance thus refers to the preservation of similar brain activity levels than younger individuals, allowing cognitive abilities to be relatively preserved with advancing age (Nyberg et al., 2012). MRI work showed, for example, that older individuals with little changes in functional activity during encoding, relative to younger adults, can sometimes perform better than older individuals with larger activation changes (Düzel et al., 2011). Studies on this phenomenon are, to our knowledge, rare. Rondina et al. (2019) investigated the preservation of brain dynamics with aging, using a spatial memory task where participants were asked to remember the spatial location of three objects seen separately. In the test phase, participants were then asked to determine whether the location of the objects was the same or different than in the memory phase. They revealed that older individuals showed a similar pattern of brain activations than young participants, with an increase in theta band oscillatory activity in occipital regions and a decrease in frontotemporal regions. The maintenance of this brain activity pattern was associated with similar cognitive performance between age groups. Also, in a visual attention task, young and older individuals showed a similar pattern of increased lower gamma frequency activity in the frontal region with increasing task difficulty (Phillips and Takeda, 2010). Moreover, Coquelet et al. (2017) investigated MEG phase synchrony at rest and reported maintenance of the electrophysiological connectome with age. Coquelet and colleagues observed similar patterns between age groups in the alpha and beta frequency bands. The maintenance of synchrony in the beta band was positively correlated with the maintenance of cognitive performance in older individuals.
This functional maintenance seems to critically depend on the maintenance of the underlying structures of the brain networks. Hinault et al. (2020) investigated the structure-function relationship between diffusion tensor imaging (DTI) and EEG data of neuronal synchrony (PLV) of young and old healthy participants. The authors used an arithmetic task, in which participants had to maintain, or update, arithmetic operations (addition, multiplication) in working memory to determine whether the proposed equation results (e.g., 8 × 4 = 36) were correct or incorrect. Preserved integrity of white matter fibers at a similar level to younger participants, in some older individuals, was associated with preserved functional synchrony in the alpha and gamma frequency bands, and similar cognitive performances between age groups. In particular, the inferior fronto-occipital tract was associated to functional coupling between the inferior frontal gyrus and the occipital lobe. These results also illustrate the strong association with the preservation of brain reserve, which would enable such maintenance (Stern, 2009).
Thus, the maintenance of functional synchrony with age (at rest and during task completion) is associated with the preservation of cognitive functions in healthy elderly individuals. This maintenance has mainly been observed between frontal and parietal regions. However, the preservation of this functional synchrony at a similar level to younger individuals is frequently associated with an increased synchrony in other brain regions. Liu et al. (2017) showed that a maintenance of delta and theta synchrony of central regions (measured with the PLI), in the absence of increased activity in other regions, was associated with decreased motor and cognitive performance. This suggests that maintenance is strongly linked to the presence of functional compensations. However, such increased brain activity could also be deleterious, reflecting network alteration and the presence of excitotoxic or neurodegenerative phenomena (Hillary and Grafman, 2017).
Non-Effective Changes
With advancing age, changes in brain function can be compensatory (as discussed above), but may also turn out to be ineffective. In an EEG study (Tóth et al., 2014), young and old healthy participants had to perform a working memory task consisting of remembering colored squares at certain locations. During the test phase, these squares remained at the same locations but could change in color. Participants had to determine whether squares were identical to those displayed during the encoding phase or not. Synchrony was determined with the PLI measure, and a specific decrease in theta synchrony between frontal and posterior sites was observed in healthy older adults compared to younger individuals (see also Li and Zhao, 2015). Importantly, this theta synchrony decrease has been associated with lower cognitive performance. Also, increased high gamma synchrony in the left frontal region during a maze task (visual planning task) has been shown to be negatively associated with performance (Paul et al., 2011).
In one of our studies (Jauny et al., 2022), we showed that maladaptive changes can be identified in resting-state activity. This study involved the Cam-CAN cohort (e.g., Shafto et al., 2014; Taylor et al., 2017) and changes in synchrony (with the PLV measure), as well as directed connectivity (with the entropy transfer measure, which provides a directed connectivity measure), were investigated. We observed a greater variability in brain networks' synchrony over time relative to younger individuals, mainly for the default mode network (this network is mainly activated when no task is proposed from the participant and plays a role in continuous environmental monitoring; Uddin et al., 2019) in the delta frequency band. Moreover, we showed that older individuals showed a revered dominant direction in information transfer, relative to younger individuals, with a significant increase in the anterior to posterior direction of functional connectivity. These changes were correlated with a decline in cognitive performance on tests measuring working memory, attention, and logical reasoning (also Sahoo et al., 2020). This reversal of the dominant information transfer direction could represent a failed compensation attempt. The increases in brain synchrony studied could also reflect excitotoxic processes (Hillary and Grafman, 2017; Mai et al., 2019), with increased activity preceding neuronal loss. Indeed, older individuals showing these hyperconnectivity phenomena had decreased cognitive performance.
As we have seen in these different sections, changes in synchrony vary over time and with task difficulty, and increased synchrony is not always beneficial. These changes appear to be related to changes in the level of white matter fibers integrity (Hinault et al., 2020, 2021). Indeed, the presence of white matter lesions has been associated with decreased brain synchrony (Wessel et al., 2016). In association with the influence of potentially preclinical excitotoxic processes, structural and functional changes could reflect the progression of neurodegenerative disorders.
Pathological Aging
In this section, we considered M/EEG study involving patients with AD and its prodromal stage called mild cognitive impairment. AD is the most common age-related pathology and is characterized by memory and cognitive control impairment (involving inhibition, planification, and mental flexibility; Baudic et al., 2006). The investigation of early AD stages such as the MCI stage is of significant interest because some individuals will later develop AD whereas others will remain stable or develop other pathologies such as frontotemporal or vascular dementia (e.g., Perez-Gonzalez et al., 2014; Jongsiriyanyong and Limpawattana, 2018). The reviewed studies were selected based on the same criteria as in the previous section on normal aging (see Table 1). The main results studied in this section are illustrated in Figure 1B.
Resilience
In line with the reserve concept developed in healthy older individuals, resilience refers to the processes involved in coping with the development of neurodegenerative disorders and the associated brain changes (Pascual-Leone and Bartres-Faz, 2021). Indeed, resilience allows, like reserve, the preservation of cognitive functions in spite of brain changes. Few studies have documented this phenomenon. Gaubert et al. (2019) compared resting-state EEG data (phase synchrony calculated using PLV and PLI) from patients with preclinical AD (at two AD progression stages) and control participants. They observed that the degree of neurodegeneration (measured by the level of amyloid beta load) impacted functional connectivity. Higher alpha functional connectivity in parieto-occipital sites was observed when amyloid load levels were low. This increase in alpha connectivity was interpreted as reflecting a compensatory phenomenon as patients showed normal cognitive performance (measured by the Free and Cued Selective Reminding Test, assessing episodic memory). In the presence of high amyloid loads, theta functional connectivity was decreased. It was suggested that amyloid deposits could negatively impact the extracellular environment, preventing compensatory adjustments. Moreover, Bajo et al. (2010) administered a memory task to healthy elderly MCI participants with similar performance levels than controls. They reported an increased interhemispheric gamma, beta, and alpha synchrony in MCI participants compared to controls. This increase in synchrony has been interpreted as reflecting compensatory adjustments in MCI individuals. The presence of interhemispheric activity is in line with the Hemispheric Asymmetry Reduction in Older (Cabeza et al., 2002) model. Finally, an overall increase in theta synchrony has been reported in fronto-occipital and right parietotemporal areas for MCI individuals during the performance of a mental arithmetic task, thus allowing the maintenance of cognitive performance (López et al., 2014a).
These resilience phenomena have been reported in patients with MCI, but may not always be necessary. Indeed, López et al. (2014b) showed that increases in synchrony were reduced in individuals with greater reserve.
Resistance
Resistance refers to the prevention of brain injury and damage before their occurrence (Pascual-Leone and Bartres-Faz, 2021). AD is characterized by advanced brain and cognitive impairments, being the results of years of neurodegenerative processes, and therefore resistance phenomenon can no longer be observed. However, investigating the earlier stages of this disease, López et al. (2014a) used a mental arithmetic task (mental subtraction) at two levels of difficulty (subtracting from one to one, being the easy level, and subtracting from three to three being the hard level). Patients with MCI and controls both showed similar cognitive performance. Furthermore, lower synchrony at rest and increased delta synchrony in frontal interhemispheric regions during task completion (measured with PLV) were similar across groups. Thus, there seems to be maintenance of certain brain functions in early AD stages. However, such maintenance of specific brain communications was associated with compensatory increases in synchrony in other brain regions in patients with MCI. The concept of resistance, alone, would therefore not allow the maintenance of cognitive performance in individuals at risk of developing a neurodegenerative disease. Furthermore, increased synchrony in individuals with MCI was initially associated with preservation of brain and cognitive functioning, but may also be a sign of disease progression (Knyazeva et al., 2013).
Maladaptive Changes
Despite the presence of maintained synchrony patterns and the ability of some individuals to compensate for disease-related brain damage, individuals with MCI also exhibit brain changes associated with impaired cognitive performance. For example, Caravaglios et al. (2018) showed that increases in beta synchrony in patients with MCI correlated with poorer performance on various neuropsychological tests (e.g., Trail Making Test, Rey Auditory Verbal Learning Test, and Semantic Verbal Fluency). Maladaptive changes thus occur and could be due to disturbances in the integrity of white matter fibers. Altered white matter integrity has indeed been shown to result in decreased alpha synchrony in individuals with MCI, particularly in parietal regions (Teipel et al., 2009). Synchrony analyses have also revealed decreased connectivity in frontal and parietotemporal alpha and beta activity, especially in interhemispheric couplings, associated with impaired cognitive performance (digit verbal span task; Li et al., 2019).
Thus, maladaptive changes appear to differ depending on AD progression level. Garn et al. (2015) revealed that qEEG markers such as decreased delta synchrony in parietal regions could have a predictive role in AD severity. Indeed, synchrony measures could discriminate between individuals with subjective cognitive decline (SCD; defined by a subjective complaint without cognitive impairment), MCI, and AD (Houmani et al., 2018). Using measures of synchrony (bump model) and complexity (epoch-based entropy), the authors discriminated patients with AD from patients with SCD and high specificity (91.6% accuracy, 100% specificity, and 87.8% sensitivity). Their results showed that patients with AD showed increased EEG synchrony in the theta band in frontal and occipital regions compared to patients with SCD. These differences could be explained by the degradation or lower cognitive reserve in patients with AD (Montemurro et al., 2021).
Discussion
Results reviewed here highlight the importance of considering the temporal dynamics of brain activity, and synchronized brain communications, to our understanding of cognitive reserve, resilience, and maintenance in healthy and pathological aging. Dynamic connectivity patterns must display both stability and flexibility as a function of the cognitive process involved and task context. While the stability of dynamics in the network engaged is important to process relevant information, flexibility is required to adjust to goal changes and to inhibit the processing of irrelevant information (e.g., Voytek and Knight, 2015). Cognitive decline during healthy and pathological aging can result from impairments of such dynamic network activity. While further investigations remain necessary, the present review summarizes current work on the association of sustained, increased, or altered neural synchrony across brain regions, with cognitive preservation or decline.
While reserve has been associated with increased frontal synchrony in the theta and alpha bands (Ariza et al., 2015; Hong et al., 2016), resilience has been associated with increased alpha, beta, and gamma synchrony (e.g., Bajo et al., 2010). It is important to note that not all changes are associated with a relative preservation of cognitive performance. Indeed, maladaptive changes have also been reported, such as the increased delta synchrony in posterior regions (Jauny et al., 2022). Findings suggest that (1) the neural mechanisms involved in both reserve and resilience could be partly similar, and (2) resilience could involve a stronger involvement of higher frequency bands relative to reserve. This difference could be determined by the degree of preservation of the structural network integrity, which is modulated by the pathology progression, leading to different compensation levels. Yet, whether differences across reserve and resilience are mainly of quantitative nature, or also qualitative, remains to be further clarified.
Maintenance-related patterns were also observed in healthy older individuals, with a relative absence of impairments in frontoparietal communication dynamics associated with preserved cognitive performance (Rondina et al., 2019; Hinault et al., 2020, 2021). The brain's structure-function interplay appears to be at the heart of the heterogeneity associated with maintenance during aging (Courtney and Hinault, 2021; Jauny et al., 2022). Indeed, maintenance was observed with preserved dynamics in the relative absence of integrity loss. Conversely, reserve-related adjustments are a necessity in the presence of reduced structural integrity. The inability of any of these two mechanisms will lead to cognitive decline. Additional longitudinal studies remain necessary to further specify the concepts of maintenance and resistance mechanisms. Another open question for future research lies in the distinction between hyperactivation or hyperconnectivity resulting from (1) failed compensatory attempts, although some degree of network reorganization has been implemented, and (2) excitotoxic processes associated with the progression of neurodegenerative disorders. Direct investigations, associated with longitudinal follow-up, will be necessary to further clarify this distinction.
While these findings are promising, they only reflect a fraction of M/EEG aging studies. Future studies in the field could integrate the following recommendations to further address the contribution of brain dynamics. First, while resting-state measurements are indicators of the potential for brain networks' reorganization in cognitively challenging situations, they cannot always inform on their actual implementation and association with specific cognitive processes. Although few studies were conducted to address these issues, we expect that dynamic stability and flexibility of task-related networks could provide critical elements regarding subsequent cognitive changes and the effect of cognitive stimulation programs. In line with recent questioning in the field (e.g., Finn and Bandettini, 2021), it also appears critical to question whether resting-state recording can precisely measure elements of cognitive reserve. Although changes can be observed in brain activity at rest, specific task-related delays in the implementation, maintenance, or interruption of communications across brain regions could be highly sensitive to age and pathology effects. Recent work (Babiloni et al., 2020; Güntekin et al., 2021) highlighted the clinical value of investigating oddball-related brain activity to detect pathology effects. Finally, from an epidemiological perspective, future studies should also consider middle-aged individuals (40–60 years) to clarify the association between changes in brain dynamics and later cognitive trajectories. Indeed, age-related brain changes occur throughout life and changes in older individuals (>65 years) may be the result of earlier brain changes. Also, it appears critical that future studies include a more diverse population, especially in terms of education level (de Oliveira et al., 2018; Ashby-Mitchell et al., 2020). Indeed, most of the reviewed studies considered populations with a high level of education, which limits the investigation of reserve and resilience mechanisms.
Many studies relied on methods that are blind to temporal changes, such as amplitude comparison following averaging of large time periods. This facilitates data analyses but could lead to missing important information. Hidden-Markov models (Tibon et al., 2021) or multiscale entropy (McIntosh et al., 2014) enable the specification of time-varying connectivity states, which could further our understanding of the heterogeneity of cognitive aging. Finally, the majority of the reviewed studies involved sensor-based analyses and brain connectivity methods, such as coherence analyses. While the reviewed work reveals important patterns associated with resilience and maintenance, they are prone to volume conduction effects (e.g., Brunner et al., 2016) and suffer from low spatial accuracy. A more generalized use of non-biased connectivity estimates (e.g., Allouch et al., 2022), associated with source reconstruction of M/EEG activity, could clarify some open questions and replicate the currently reported findings. This would also enable the investigation of the same networks and regions of interest as previous fMRI work (Pascual-Leone and Bartres-Faz, 2021) to specify their time course and dynamics.
Brain connectivity changes observed in M/EEG could differentiate individuals according to pathology stages (Houmani et al., 2018) in a non-invasive manner. These data could also help predict pathology progression. Specifying rhythmic brain communication changes with age and pathology could also guide direct modulations of brain rhythms through targeted stimulations. Recent work, such as Reinhart and Nguyen (2019), revealed the feasibility of short-term interventions using transcranial alternative current stimulation (application of a low-intensity current to the brain with electrodes to synchronize or desynchronize brain oscillations) on working memory performance in healthy older adults. Improved working memory performance (up to 50 min post-stimulation) has been reported in healthy older adults following frontotemporal theta stimulation relative to a sham condition. Several points, such as the long-term benefits of these interventions, together with the optimal number of sessions, remain to be specified. Such knowledge could lead to a new specific and neurophysiologically grounded intervention targeting disrupted brain communications and enhancing rhythms associated with successful compensation, suggesting an intense development of this research field in the coming years.
Author Contributions
GJ: investigation and writing. FE: supervision and review. TH: conceptualization, supervision, writing, and review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aktürk, T., Işoglu-Alkaç, Ü., Hanoglu, L., and Güntekin, B. (2020). Age related differences in the recognition of facial expression: evidence from EEG event-related brain oscillations. Int. J. Psychophysiol. 147, 244–256. doi: 10.1016/j.ijpsycho.2019.11.013
Allouch, S., Yochum, M., Kabbara, A., Duprez, J., Khalil, M., Wendling, F., et al. (2022). Mean-field modeling of brain-scale dynamics for the evaluation of eeg source-space networks. Brain Topogr. 35, 54–65. doi: 10.1007/s10548-021-00859-9
Angel, L., Fay, S., Bouazzaoui, B., and Isingrini, M. (2010). Individual differences in executive functioning modulate age effects on the ERP correlates of retrieval success. Neuropsychologia 48, 3540–3553. doi: 10.1016/j.neuropsychologia.2010.08.003
Angel, L., Fay, S., Bouazzaoui, B., and Isingrini, M. (2011). Two hemispheres for better memory in old age: role of executive functioning. J. Cogn. Neurosci. 23, 3767–3777. doi: 10.1162/jocn_a_00104
Arenaza-Urquijo, E. M., and Vemuri, P. (2018). Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology 90, 695–703. doi: 10.1212/WNL.0000000000005303
Ariza, P., Solesio-Jofre, E., Martínez, J. H., Pineda-Pardo, J. A., Niso, G., Maestú, F., et al. (2015). Evaluating the effect of aging on interference resolution with time-varying complex networks analysis. Front. Human Neurosci. 9, 255. doi: 10.3389/fnhum.2015.00255
Ashby-Mitchell, K., Willie-Tyndale, D., and Eldemire-Shearer, D. (2020). Proportion of dementia explained by five key factors in Jamaica. J. Alzheimer's Dis. 78, 603–609. doi: 10.3233/JAD-200601
Babiloni, C., Ferri, R., Noce, G., Lizio, R., Lopez, S., Soricelli, A., et al. (2020). Resting-state electroencephalographic delta rhythms may reflect global cortical arousal in healthy old seniors and patients with Alzheimer's disease dementia. Int. J. Psychophysiol. 158, 259–270. doi: 10.1016/j.ijpsycho.2020.08.012
Baillet, S. (2017). Magnetoencephalography for brain electrophysiology and imaging. Nat. Neurosci. 20, 327–339. doi: 10.1038/nn.4504
Bajo, R., Maestú, F., Nevado, A., Sancho, M., Gutiérrez, R., Campo, P., et al. (2010). Functional connectivity in mild cognitive impairment during a memory task: implications for the disconnection hypothesis. J. Alzheimer. Dis. 22, 183–193. doi: 10.3233/JAD-2010-100177
Bao, Y., Pöppel, E., Wang, L., Lin, X., Yang, T., Avram, M., et al. (2015). Synchronization as a biological, psychological and social mechanism to create common time: a theoretical frame and a single case study. PsyCh J. 4, 243–254. doi: 10.1002/pchj.119
Baudic, S., Barba, G. D., Thibaudet, M. C., Smagghe, A., Remy, P., and Traykov, L. (2006). Executive function deficits in early Alzheimer's disease and their relations with episodic memory. Arch. Clin. Neuropsychol. 21, 15–21. doi: 10.1016/j.acn.2005.07.002
Brunner, C., Billinger, M., Seeber, M., Mullen, T. R., Makeig, S., Lansky, P., et al. (2016). Volume conduction influences scalp-based connectivity estimates. Front. Comput. Neurosci. 10, 121. doi: 10.3389/fncom.2016.00121
Cabeza, R., Albert, M., Belleville, S., Craik, F. I. M., Duarte, A., Grady, C. L., et al. (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 19, 701–710. doi: 10.1038/s41583-018-0068-2
Cabeza, R., Anderson, N. D., Locantore, J. K., and McIntosh, A. R. (2002). Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage 17, 1394–1402. doi: 10.1006/nimg.2002.1280
Caravaglios, G., Castro, G., Muscoso, E. G., Crivelli, D., and Balconi, M. (2018). Beta responses in healthy elderly and in patients with amnestic mild cognitive impairment during a task of temporal orientation of attention. Clin. EEG Neurosci. 49, 258–271. doi: 10.1177/1550059416676144
Coquelet, N., Mary, A., Peigneux, P., Goldman, S., Wens, V., and De Tiège, X. (2017). The electrophysiological connectome is maintained in healthy elders: a power envelope correlation MEG study. Sci. Rep. 7, 1–10. doi: 10.1038/s41598-017-13829-8
Courtney, S. M., and Hinault, T. (2021). When the time is right: temporal dynamics of brain activity in healthy aging and dementia. Progress Neurobiol. 203, 102076. doi: 10.1016/j.pneurobio.2021.102076
Damoiseaux, J. S. (2017). Effects of aging on functional and structural brain connectivity. NeuroImage 160, 32–40. doi: 10.1016/j.neuroimage.2017.01.077
de Oliveira, F. F., de Almeida, S. S., Chen, E. S., Smith, M. C., Naffah-Mazzacoratti, M., and Bertolucci, P. (2018). Lifetime risk factors for functional and cognitive outcomes in patients with Alzheimer's disease. J. Alzheimer. Dis. 65, 1283–1299. doi: 10.3233/JAD-180303
Düzel, E., Schütze, H., Yonelinas, A. P., and Heinze, H. J. (2011). Functional phenotyping of successful aging in long-term memory: preserved performance in the absence of neural compensation. Hippocampus 21, 803–814. doi: 10.1002/hipo.20834
Finn, E. S., and Bandettini, P. A. (2021). Movie-watching outperforms rest for functional connectivity-based prediction of behavior. NeuroImage 235, 117963. doi: 10.1016/j.neuroimage.2021.117963
Fries, P. (2015). Rhythms for cognition: communication through coherence. Neuron 88, 220. doi: 10.1016/j.neuron.2015.09.034
Garn, H., Waser, M., Deistler, M., Benke, T., Dal-Bianco, P., Ransmayr, G., et al. (2015). Quantitative EEG markers relate to Alzheimer's disease severity in the Prospective Dementia Registry Austria (PRODEM). Clin. Neurophysiol. 126, 505–513. doi: 10.1016/j.clinph.2014.07.005
Gaubert, S., Raimondo, F., Houot, M., Corsi, M. C., Naccache, L., Sitt, J. D., et al. (2019). EEG evidence of compensatory mechanisms in preclinical Alzheimer's disease. Brain 142, 2096–2112. doi: 10.1093/brain/awz150
Güntekin, B., Aktürk, T., Arakaki, X., Bonanni, L., Percio, C. D., Edelmayer, R., et al. (2021). Are there consistent abnormalities in event-related EEG oscillations in patients with Alzheimer's disease compared to other diseases belonging to dementia? Psychophysiology 59, e13934. doi: 10.1111/psyp.13934
Hillary, F. G., and Grafman, J. H. (2017). Injured brains and adaptive networks: the benefits and costs of hyperconnectivity. Trends Cogn. Sci. 21, 385–401. doi: 10.1016/j.tics.2017.03.003
Hinault, T., Kraut, M., Bakker, A., Dagher, A., and Courtney, S. M. (2020). Disrupted neural synchrony mediates the relationship between white matter integrity and cognitive performance in older adults. Cereb. Cortex 30, 5570–5582. doi: 10.1093/cercor/bhaa141
Hinault, T., Larcher, K., Bherer, L., Courtney, S. M., and Dagher, A. (2019). Age-related differences in the structural and effective connectivity of cognitive control: a combined fMRI and DTI study of mental arithmetic. Neurobiol. Aging 82, 30–39. doi: 10.1016/j.neurobiolaging.2019.06.013
Hinault, T., Mijalkov, M., Pereira, J. B., Volpe, G., Bakke, A., and Courtney, S. M. (2021). Age-related differences in network structure and dynamic synchrony of cognitive control. NeuroImage 236, 118070. doi: 10.1016/j.neuroimage.2021.118070
Ho, M. C., Chou, C. Y., Huang, C. F., Lin, Y. T., Shih, C. S., Han, S. Y., et al. (2012). Age-related changes of task-specific brain activity in normal aging. Neurosci. Lett. 507, 78–83. doi: 10.1016/j.neulet.2011.11.057
Hong, X., Liu, Y., Sun, J., and Tong, S. (2016). Age-related differences in the modulation of small-world brain networks during a Go/NoGo task. Front. Aging Neurosci. 8, 100. doi: 10.3389/fnagi.2016.00100
Houmani, N., Vialatte, F., Gallego-Jutglà, E., Dreyfus, G., Nguyen-Michel, V. H., Mariani, J., et al. (2018). Diagnosis of Alzheimer's disease with electroencephalography in a differential framework. PLoS ONE 13, e0193607. doi: 10.1371/journal.pone.0193607
Jauny, G., Eustache, F, and Hinault, T. (2022). Connectivity dynamics and cognitive variability during aging. Neurobiol. Aging. doi: 10.1101/2022.01.26.477817. [Epub ahead of print].
Jongsiriyanyong, S., and Limpawattana, P. (2018). Mild cognitive impairment in clinical practice: a review article. Am. J. Alzheimer. Dis. Other Dement. 33, 500–507. doi: 10.1177/1533317518791401
Knyazeva, M. G., Carmeli, C., Khadivi, A., Ghika, J., Meuli, R., and Frackowiak, R. S. (2013). Evolution of source EEG synchronization in early Alzheimer's disease. Neurobiol. Aging 34, 694–705. doi: 10.1016/j.neurobiolaging.2012.07.012
Kumral, D., Sansal, F., Cesnaite, E., Mahjoory, K., Al, E., Gaebler, M., et al. (2020). BOLD and EEG signal variability at rest differently relate to aging in the human brain. NeuroImage 207, 116373. doi: 10.1016/j.neuroimage.2019.116373
Lachaux, J.-P., Rodriguez, E., Martinerie, J., and Varela, F. J. (1999). Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C
Li, L., and Zhao, D. (2015). Age-related inter-region EEG coupling changes during the control of bottom-up and top-down attention. Front. Aging Neurosci. 7, 223. doi: 10.3389/fnagi.2015.00223
Li, R., Nguyen, T., Potter, T., and Zhang, Y. (2019). Dynamic cortical connectivity alterations associated with Alzheimer's disease: an EEG and fNIRS integration study. NeuroImage Clin. 21, 101622. doi: 10.1016/j.nicl.2018.101622
Liu, L., Rosjat, N., Popovych, S., Wang, B. A., Yeldesbay, A., Toth, T. I., et al. (2017). Age-related changes in oscillatory power affect motor action. PLoS ONE 12, e0187911. doi: 10.1371/journal.pone.0187911
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
López, M. E., Aurtenetxe, S., Pereda, E., Cuesta, P., Castellanos, N. P., Bruña, R., et al. (2014b). Cognitive reserve is associated with the functional organization of the brain in healthy aging: a MEG study. Front. Aging Neurosci. 6, 125. doi: 10.3389/fnagi.2014.00125
López, M. E., Garcés, P., Cuesta, P., Castellanos, N. P., Aurtenetxe, S., Bajo, R., et al. (2014a). Synchronization during an internally directed cognitive state in healthy aging and mild cognitive impairment: a MEG study. Age 36, 1389–1406. doi: 10.1007/s11357-014-9643-2
Mai, G., Schoof, T., and Howell, P. (2019). Modulation of phase-locked neural responses to speech during different arousal states is age-dependent. NeuroImage. 189, 734–744. doi: 10.1016/j.neuroimage.2019.01.049
McIntosh, A. R., Vakorin, V., Kovacevic, N., Wang, H., Diaconescu, A., and Protzner, A. B. (2014). Spatiotemporal dependency of age-related changes in brain signal variability. Cereb. Cortex 24, 1806–1817. doi: 10.1093/cercor/bht030
Miller, E. K., Lundqvist, M., and Bastos, A. M. (2018). Working Memory 2.0. Neuron. 100, 463–475. doi: 10.1016/j.neuron.2018.09.023
Montemurro, S., Mondini, S., and Arcara, G. (2021). Heterogeneity of effects of cognitive reserve on performance in probable Alzheimer's disease and in subjective cognitive decline. Neuropsychology. 35, 876–888. doi: 10.1037/neu0000770
Nunez, P. L., Srinivasan, R., Westdorp, A. F., Wijesinghe, R. S., Tucker, D. M., Silberstein, R. B., et al. (1997). EEG coherency I: statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr. Clin. Neurophysiol. 103, 499–515. doi: 10.1016/S0013-4694(97)00066-7
Nyberg, L., Lövdén, M., Riklund, K., Lindenberger, U., and Bäckman, L. (2012). Memory aging and brain maintenance. Trends Cogn. Sci. 16, 292–305. doi: 10.1016/j.tics.2012.04.005
Pascual-Leone, A., and Bartres-Faz, D. (2021). Human brain resilience: a call to action. Ann. Neurol. 90, 336–349. doi: 10.1002/ana.26157
Paul, R. H., Clark, C. R., Lawrence, J., Goldberg, E., Williams, L. M., Cooper, N., et al. (2011). Age-dependent change in executive function and gamma 40 hz phase synchrony. J. Integr. Neurosci. 4, 63–76. doi: 10.1142/S0219635205000690
Perez-Gonzalez, J. L., Yanez-Suarez, O., Bribiesca, E., Cosío, F. A., Jiménez, J. R., and Medina-Bañuelos, V. (2014). Description and classification of normal and pathological aging processes based on brain magnetic resonance imaging morphology measures. J. Med. Imaging 1, 034002. doi: 10.1117/1.JMI.1.3.034002
Phillips, S., and Takeda, Y. (2010). Frontal-parietal synchrony in elderly EEG for visual search. Int. J. Psychophysiol. 75, 39–43. doi: 10.1016/j.ijpsycho.2009.11.001
Reinhart, R. M. G., and Nguyen, J. A. (2019). Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat. Neurosci. 22, 820–827. doi: 10.1038/s41593-019-0371-x
Reuter-Lorenz, P. A., and Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Curr. Direct. Psychol. Sci. 17, 177–182. doi: 10.1111/j.1467-8721.2008.00570.x
Reuter-Lorenz, P. A., and Park, D. C. (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 24, 355. doi: 10.1007/s11065-014-9270-9
Rondina, R., Olsen, R. K., Li, L., Meltzer, J. A., and Ryan, J. D. (2019). Age-related changes to oscillatory dynamics during maintenance and retrieval in a relational memory task. PLoS ONE 14, e211851. doi: 10.1371/journal.pone.0211851
Rosjat, N., Wang, B. A., Liu, L., Fink, G. R., and Daun, S. (2021). Stimulus transformation into motor action: dynamic graph analysis reveals a posterior-to-anterior shift in brain network communication of older subjects. Human Brain Mapp. 42, 1547–1563. doi: 10.1002/hbm.25313
Rosjat, N., Liu, L., Wang, B. A., Popovych, S., Tóth, T., Viswanathan, S., et al. (2018). Aging-associated changes of movement-related functional connectivity in the human brain. Neuropsychologia 117, 520–529. doi: 10.1016/j.neuropsychologia.2018.07.006
Sahoo, B., Pathak, A., Deco, G., Banerjee, A., and Roy, D. (2020). Lifespan associated global patterns of coherent neural communication. NeuroImage 216, 116824. doi: 10.1016/j.neuroimage.2020.116824
Shafto, M. A., Tyler, L. K., Dixon, M., Taylor, J. R., Rowe, J. B., Cusack, R., et al. (2014). The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 14, 204. doi: 10.1186/s12883-014-0204-1
Spreng, R. N., and Turner, G. R. (2019). The Aging Brain: Functional Adaptation Across Adulthood. Washington, DC: American Psychological Association, 9–43.
Stam, C. J., Nolte, G., and Daffertshofer, A. (2007). Phase lag index: assessment of functional connectivity from multi-channel EEG and MEG with diminished bias from common sources. Human Brain Mapp. 28, 1178. doi: 10.1002/hbm.20346
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. doi: 10.1017/S1355617702813248
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Stern, Y., Arenaza-Urquijo, E. M., Bartrés-Faz, D., Belleville, S., Cantilon, M., Chetelat, G., et al. (2020). Whitepaper: defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer. Dement. 16, 1305–1311. doi: 10.1016/j.jalz.2018.07.219
Suárez, L. E., Markello, R. D., Betzel, R. F., and Misic, B. (2020). Linking structure and function in macroscale brain networks. Trends Cogn. Sci. 24, 302–315. doi: 10.1016/j.tics.2020.01.008
Taylor, J. R., Williams, N., Cusack, R., Auer, T., Shafto, M. A., Dixon, M., et al. (2017). The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage 144, 262–269. doi: 10.1016/j.neuroimage.2015.09.018
Teipel, S. J., Pogarell, O., Meindl, T., Dietrich, O., Sydykova, D., Hunklinger, U., et al. (2009). Regional networks underlying interhemispheric connectivity: an EEG and DTI study in healthy ageing and amnestic mild cognitive impairment. Human Brain Mapp. 30, 2098–2119. doi: 10.1002/hbm.20652
Tibon, R., Tsvetanov, K. A., Price, D., Nesbitt, D., Can, C., and Henson, R. (2021). Transient neural network dynamics in cognitive ageing. Neurobiol. Aging 105, 217–228. doi: 10.1016/j.neurobiolaging.2021.01.035
Tóth, B., Kardos, Z., File, B., Boha, R., Stam, C. J., and Molnár, M. (2014). Frontal midline theta connectivity is related to efficiency of WM maintenance and is affected by aging. Neurobiol. Learn. Memory 114, 58–69. doi: 10.1016/j.nlm.2014.04.009
Uddin, L. Q., Yeo, B. T. T., and Spreng, R. N. (2019). Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 32, 926. doi: 10.1007/s10548-019-00744-6
Voytek, B., and Knight, R. T. (2015). Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol. Psychiatry 77, 1089–1097. doi: 10.1016/j.biopsych.2015.04.016
Wessel, J. R., Ullsperger, M., Obrig, H., Villringer, A., Quinque, E., Schroeter, M. L., et al. (2016). Neural synchrony indexes impaired motor slowing after errors and novelty following white matter damage. Neurobiol. Aging 38, 205–213. doi: 10.1016/j.neurobiolaging.2015.10.014
Keywords: aging, cognition, M/EEG, dementia, connectivity
Citation: Jauny G, Eustache F and Hinault TT (2022) M/EEG Dynamics Underlying Reserve, Resilience, and Maintenance in Aging: A Review. Front. Psychol. 13:861973. doi: 10.3389/fpsyg.2022.861973
Received: 25 January 2022; Accepted: 14 April 2022;
Published: 25 May 2022.
Edited by:
Renata Kochhann, Hospital Moinhos de Vento, BrazilReviewed by:
Tomasz Maciej Rutkowski, RIKEN Center for Advanced Intelligence Project (AIP), JapanFabricio Ferreira de Oliveira, Elysian Clinic, Brazil
Copyright © 2022 Jauny, Eustache and Hinault. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas Thierry Hinault, dGhvbWFzLmhpbmF1bHRAaW5zZXJtLmZy
 Gwendolyn Jauny
Gwendolyn Jauny Francis Eustache
Francis Eustache Thomas Thierry Hinault
Thomas Thierry Hinault