
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Psychol. , 16 March 2022
Sec. Neuropsychology
Volume 13 - 2022 | https://doi.org/10.3389/fpsyg.2022.789377
This article is part of the Research Topic The Nocebo Effect and its Consequences for Clinical Trials and Clinical Practice View all 15 articles
 Giacomo Rossettini1,2†
Giacomo Rossettini1,2† Andrea Colombi1†
Andrea Colombi1† Elisa Carlino3
Elisa Carlino3 Mattia Manoni1
Mattia Manoni1 Mattia Mirandola2
Mattia Mirandola2 Andrea Polli4,5,6
Andrea Polli4,5,6 Eleonora Maria Camerone1,7‡
Eleonora Maria Camerone1,7‡ Marco Testa1*‡
Marco Testa1*‡This Perspective adapts the ViolEx Model, a framework validated in several clinical conditions, to better understand the role of expectations in the recovery and/or maintenance of musculoskeletal (MSK) pain. Here, particular attention is given to the condition in which dysfunctional expectations are maintained despite no longer being supported by confirmatory evidence (i.e., belief—lifting the arm leads to permanent tendon damage; evidence—after the patient lifts the arm no tendon damage occurs). While the ViolEx Model suggests that cognitive immunization strategies are responsible for the maintenance of dysfunctional expectations, we suggest that such phenomenon can also be understood from a Bayesian Brain perspective, according to which the level of precision of the priors (i.e., expectations) is the determinant factor accounting for the extent of priors’ updating (i.e., we merge the two frameworks, suggesting that highly precise prior can lead to cognitive immunization responses). Importantly, this Perspective translates the theory behind these two frameworks into clinical suggestions. Precisely, it is argued that different strategies should be implemented when treating MSK pain patients, depending on the nature of their expectations (i.e., positive or negative and the level of their precision).
Musculoskeletal (MSK) pain is ranked at the top of non-communicable diseases (Safiri et al., 2021), representing a profound burden for all socioeconomic and healthcare systems worldwide (Briggs et al., 2020). Although most MSK pain states have a good prognosis, there is a substantial proportion of patients who do not show spontaneous remission or do not respond favorably to first-line interventions and usual care, thus developing long-lasting symptoms, disabilities, and participation loss (Blyth et al., 2019).
The management of these patients is challenging because their subjective complaints (i.e., level of disability) rarely correlate with clinical and radiological findings (i.e., structural impairments; Rondoni et al., 2017; Tonosu et al., 2017; Viceconti et al., 2020). Thus, the lack of an identifiable pathology observed in various MSK diseases (i.e., low back pain and fibromyalgia) can have clinical implications. On the one hand, patients may repetitively seek care, thus adopting unhelpful health-seeking behaviors. For example, they may contact various health care providers (Ng et al., 2020), overuse health services (Sajid et al., 2021), request complementary and alternative medicine (Setchell et al., 2021), and misuse drugs (Ashaye et al., 2018). On the other, clinicians risk to invalidate patients’ experience (De Ruddere et al., 2013), offer contradictory explanations about their condition (Bunzli et al., 2013; Mannion et al., 2013), and generic diagnoses (Yunus, 2007). As a result, patients often experience negative emotions and adopt unhelpful coping strategies (i.e., catastrophic thinking, avoidance of movement), which are, per se, capable of worsening their clinical conditions and foster symptoms persistence (Bunzli et al., 2015; Darlow, 2016). Moreover, they may develop negative expectations about the course of their illness and the likely outcomes (Kravvariti et al., 2018, 2021; Thomaidou et al., 2021).
Negative expectations impact MSK pain (Hallegraeff et al., 2012; Geurts et al., 2017; Hayden et al., 2019; Fishbain and Pulikal, 2020; Mohamed et al., 2020), playing a significant role in transitioning from acute to persistent pain (Manai et al., 2019), and maintaining symptoms (Blasini et al., 2017; Klinger et al., 2017). Moreover, they can bias symptom perception (Handley et al., 2013; Bagaric et al., 2021) and reduce treatment effectiveness (Colloca et al., 2018; Corsi et al., 2019), inducing nocebo-related effects (Petrie and Rief, 2019; Benedetti et al., 2020). Within the MSK context, nocebo-related effects refer to those negative responses that follow treatment administration (i.e., painkillers, manual therapy, and therapeutic exercises) associated with a negative expectation (Rossettini et al., 2020a). Investigating patients’ beliefs and expectations represent a priority for clinicians treating MSK pain (Lewis and O'Sullivan, 2018; Caneiro et al., 2020; Lin et al., 2020; Cook et al., 2021; Hutting et al., 2021; Lewis et al., 2021). However, such practice is not routinely implemented in clinical practice (Rossettini et al., 2019, 2020c). As emerged in previous surveys, clinicians involved in MSK care report difficulties in managing patients’ expectations and avoid nocebo-related effects (Palese et al., 2019a; Cadorin et al., 2020; Rossettini et al., 2020b; Bisconti et al., 2021). This lack highlights the need for clinicians to have a framework that they can apply in everyday practice.
This Perspective has two aims. First, to provide clinicians with a better understanding of why some MSK patients hold on to their negative expectations. To this end, we will adapt the ViolEx Model (Kube et al., 2020) and the Bayesian brain hypothesis (Büchel et al., 2014). Second, we suggest some key strategies that clinicians can use to help patients update their dysfunctional expectations based on the theoretical frameworks discussed.
When patients receive a MSK treatment, they can either “get what they expect” or “not get what they expect.” The ViolEx Model offers an interesting description of the possible outcomes and consequences of such expectations match/mismatch (Kube et al., 2020). The starting point of this model is that individuals develop expectations that are based on their own experiences: if a patient with neck pain has a negative past experience with therapeutic exercises, it is likely that this patient will have negative expectations regarding such treatment in the future. Moreover, expectations are also shaped by personality traits: that is, neuroticism, pessimism, and trait anxiety have been associated with a tendency to expect worse outcomes in situations perceived as threatening (Barlow et al., 2014). On the whole, these expectations produce an internal model of “if A-then-B,” that is “if I do this exercise (A) then I will experience side-effects (B).” When the internal model is consolidated, three different scenarios can occur. In the first scenario, reality matches the internal model and expectations are confirmed and reinforced: it means that the patient performs the exercise, the exercise produces side effects leading to a consolidation and reinforcement of negative expectations. The consequence is that the patient will learn that the treatment produces negative effects and he/she will therefore seek a different intervention in the future (Figure 1A; expectation confirmation).

Figure 1. The ViolEx Model and the Bayesian Brain: from theory to clinical practice in musculoskeletal (MSK) pain. The ViolEx Model (A) and the Bayesian brain (B,C). Image (A) is a schematization of the ViolEx Model showing different outcomes depending on whether the violation of expectations is followed by immunization or change, resulting in either expectations maintenance or expectations updating, respectively. Image (B) is an example in which a prior with a high level of certainty is considered reliable and therefore undergoes minimal updating, while the interpretation of the sensory data is shifted toward the prior, resulting in a biased percept. Image (C) is an example in which a prior with a low level of certainty is updated to better fit sensory data, resulting in a posterior which is a better proxy of the sensory information. Examples of clinical scenarios (D–F). Image (D) shows a typical clinical situation in which the clinician asks the patient with MSK pain to perform a basic exercise (lift the arm), while the patient is reluctant to do it due to their negative expectations (i.e., pain/injury). The patient finally agrees and lifts the arm, without facing negative consequences. Such situation may result in two different outcomes: in (E) the positive experience associated with the exercise leads to the violation of the patient’s negative expectation with an update (low prior); whereas in (F) the positive experience associated with the exercise is not sufficient to violate the patient’s negative expectation, thus maintaining the previous experience (high prior).
In the second scenario, reality does not match the internal model and expectations are violated: it means that the patient performs the exercise and does not experience negative side effects. Thus, two possible outcomes can occur: expectations can be updated, based on the newly acquired information (i.e., the patient experiences that the prior negative expectations toward the exercise was wrong and learns to no longer be worried about such treatment; Figure 1A; expectation violation followed by an update), or they can be maintained, despite the disproving evidence (i.e., despite treatment intake is not followed by the predicted negative consequences, the patient persists in believing that the exercise is likely to be followed by negative side effects; Figure 1A; expectation violation followed by dysfunctional beliefs maintenance).
Out of these three scenarios, the third is the problematic one. The ViolEx Model explains this last scenario introducing the concept of “cognitive immunization,” which indicates the engagement of strategies adapted to reappraise new information in such a way that the discrepancy between the evidence and the prior expectation is reduced, contributing to the maintenance of negative beliefs, despite the occurrence of conflicting events (Rief and Petrie, 2016; Rief and Joormann, 2019; Kube et al., 2019b).
Recently, the ViolEx Model has given interesting insights into clinical conditions such as depression (Kube et al., 2017; Rief and Joormann, 2019). Precisely, patients suffering from depression have been shown to maintain negative beliefs, even when presented with positive evidence disconfirming their prior negative beliefs (i.e., the example of Figure 1A; expectation violation followed by dysfunctional beliefs maintenance; Korn et al., 2014; Liknaitzky et al., 2017; Everaert et al., 2018). Interestingly, Kube et al. (2019a,b) have successfully demonstrated that by delivering instructions either promoting or discouraging cognitive immunization, it was possible to enhance negative expectations maintenance or to reduce them, respectively, demonstrating that cognitive immunization is likely to underlie dysfunctional expectations maintenance. Another interesting finding is that healthy individuals have been shown to have a positive bias, meaning that they are less likely to update positive priors if presented with contradictory negative evidence (Sharot et al., 2007, 2011, 2012; Garrett and Sharot, 2017), yet, such bias is abolished in the presence of perceived threat, in which case they become more responsive to the newly acquired negative evidence, updating their expectations (Sharot et al., 2007, 2011, 2012; Garrett and Sharot, 2017). This could be an important finding given that MSK pain patients report high threat perception linked to their experience of pain (Ochsner et al., 2006; Turk and Wilson, 2010); accordingly, this could indicate that MSK pain patients (similarly to depressed ones), become more susceptible to negative evidence, promoting the maintenance of dysfunctional expectations. Accordingly, it has been shown that patients with somatization syndrome (Rief et al., 2006) and MSK pain (Traeger et al., 2019; Barbari et al., 2021; Barth et al., 2021; Cashin et al., 2021; Cheung and Soundy, 2021; Jones et al., 2021) do not often use positive reassurance and education to update their negative dysfunctional beliefs and expectations.
Overall, previous research has shown the ViolEx model to be a valuable framework to better understand dysfunctional expectations maintenance in some clinical populations; therefore, we suggest that such model should be used to understand pain in MSK patients. In the daily practice clinicians often see MSK patients maintaining their dysfunctional expectations even if positive and reassuring evidence are provided (i.e., ViolEx Model; Figure 1A, scenario three). A good example is the case of patients that expect that their shoulder would break if they lift their arm (Figure 1D). When patients manage to fully lift their arm under the clinician supervision, and realize that their shoulder does not snap, the positive scenario (interiorizing that they can lift their arm without any negative consequences; Figure 1E) is less likely than the negative one represented by the “cognitive immunization” strategies (interpreting the event as lucky or as an exception to the rule; Figure 1F). Although this model is yet to be empirically tested upon MSK patients, it is likely to suggest that dysfunctional expectations maintenance and cognitive immunization strategies are recurrent in this clinical population.
Although cognitive immunization explains why dysfunctional expectations are maintained, it is yet to be understood why some patients implement such strategies to protect their negative beliefs and why others do not, updating their negative expectations with new positive evidence. We suggest that such differences in updating responses can be understood, at least to some extent, from a Bayesian perspective.
From a Bayesian perspective, our brain is conceptualized as a predictor machine that generates predictions, known as priors, about the expected sensory inputs. The integration between the prior and the sensory input results in a posterior (the percept), which can be more or less influenced by the prior and by the sensory data, depending on their level of precision (i.e., data encoded as probabilistic representatations; Friston, 2008, 2010; Büchel et al., 2014; Seymour and Mancini, 2020). Within this framework, a prior with high precision is considered reliable and, therefore, will exert greater influence on the interpretation of the incoming sensory input, resulting in a posterior (i.e., percept) which is biased toward the prior (Figure 1B). Differently, a prior with low precision will be considered unreliable, and therefore, will be given less consideration when interpreting the incoming sensory input, resulting in a posterior (i.e., percept) that is a better proxy of the sensory data (Figure 1C). Consider a sensory input which does not match the prior; if the prior has higher precision this is likely to result in a smaller prediction error (PE) (i.e., since the percept is biased toward the prior, there will be less discrepancy between the prior and the percept), compared to a prior with less precision (i.e., since the percept is a better proxy of the sensory information there will be more discrepancy between the prior and the percept), resulting in greater prior updating in the latter case (Friston, 2008, 2010; Büchel et al., 2014; Seymour and Mancini, 2020).
With this Bayesian model in mind, it is possible to better understand the second and third scenarios of the ViolEx Model discussed in the previous section (Figure 1A; Kube et al., 2020). Precisely, expectation violation followed by update could be attributed to one’s having a prior with low precision which is updated accordingly with the newly acquired evidence (i.e., according to this view, the patient who is shown that lifting their arm does not lead to their shoulder to break and therefore updates such dysfunctional belief does not have a highly confident negative prior).
Differently, expectation violation followed by the maintenance of the dysfunctional belief would be understood as the consequence of one’s having a highly precise prior which is considered highly reliable, and therefore, the newly acquired disconfirmatory evidence is not sufficient to disproof and update such strong prior. Indeed, the attribution of high certainty to such prior can motivate the engagement of higher-order cognitive strategies, such as cognitive immunization (Kube et al., 2017). For example, MSK patients that, during a clinical session, manage to lift their arm without any negative consequences to their shoulder but still belief that lifting the arm will eventually lead to their shoulder to break, are likely to have a highly precise prior and might discard the positive evidence (success in lifting the arm) which might be classified as an exception instead of the rule (example of cognitive immunization; Figure 1F).
As we have suggested here, the Bayesian framework can give further insights into the mechanisms of expectations updating described by the ViolEx Model. Yet, it is important to highlight that these two accounts differ in one major aspect. While the ViolEx model is cognitivist in nature; that is, it is premised on the existence of cognitive states called “expectations” and is concerned with the relation between expectations and symptoms independently of discussions of neuronal processes (Kube et al., 2020), the Bayesian brain hypothesis (also called “predictive processing”) is a theory of brain function, not a cognitivist theory. From a Bayesian brain perspective, “expectations” are probabilistic predictions about the body and the world that are encoded at the level of neuronal populations. Indeed, the validity of Bayesian brain as a scientific theory rests on the actual existence of priors and PE at the neuronal level (Downey, 2018; Seymour and Mancini, 2020). However, although the Bayesian perspective does not assume that a positive expectation communicated to the patient from the clinician on a conscious level translates directly into a prior with higher precision, this cannot be excluded. In the case of pain, new research is successfully applying the Bayesian framework to the cognitive domain, exploring whether priors are translated directly at the conscious level (Mancini et al., 2021). Accordingly, it has recently been shown that humans can explicitly predict the likelihood of incoming pain intensities in a way that is consistent with Bayesian inference (i.e., not only conscious predictions were measured but also the conscious confidence of such predictions; Mancini et al., 2021). The possibility that the Bayesian brain hypothesis can extend to the description of cognitive functioning is indeed exciting, yet further research is needed before drawing such conclusions.
A crucial point, that is the second aim of this perspective, is to use these models to create clinical strategies to treat dysfunctional patients’ expectations in order to maximize treatment effectiveness, avoiding nocebo-related effects. In the following section, we offer a clinical framework for assessing and addressing MSK patients’ expectations aimed to avoid nocebo-related effects during all phases of the therapeutic encounter (i.e., history taking, physical examination, and therapeutic administration; Palese et al., 2019b; Rossettini et al., 2020a; Thomson and Rossettini, 2021).
Since expectations and priors can critically change patients’ perception and adherence to a clinical treatment, their assessment and management are crucial steps in the clinical practice (Figure 2).
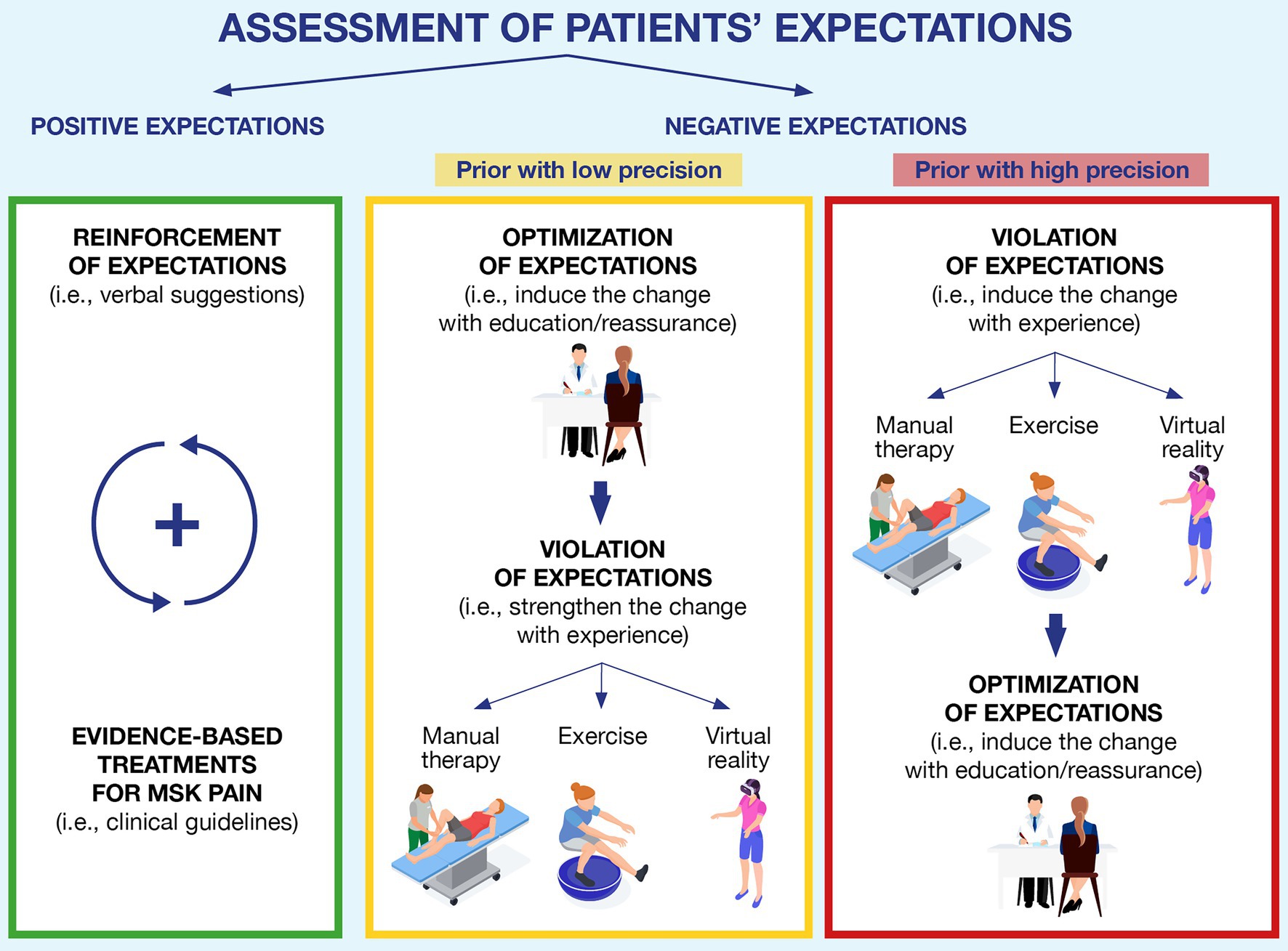
Figure 2. A clinical framework to assess and address patients’ expectations in musculoskeletal pain. The figure depicts the three typical scenarios that can occur in clinical practice. Green block: the patient shows positive expectations toward the rehabilitation process, and the clinician can reinforce them by providing verbal suggestions and confirming such expectations with the recommended evidence-based treatments; Yellow block: if negative expectations are detected during the initial assessment, but they have low precision, the clinician can optimize them through education and reassurance and subsequently try to violate such expectations by exposing patients to experience (using manual therapy, exercise, virtual reality, or a combination of them); and Red block: if high-precision expectations are detected, we suggest starting by exposing patients to experiences that could challenge patients’ expectations, and if this outcome is achieved, it can be reinforced through verbal suggestions (education or reassurance).
In the clinical encounter, clinicians can start using open questions (i.e., “What do you expect from this therapy?”; “How do you expect the course of this condition will be?”; Laferton et al., 2017; Rossettini et al., 2018) or specific questionnaires self-completed by the patients (i.e., the EXPECT Questionnaire, the Expectation for Treatment Scale; Jones et al., 2016; Barth et al., 2019) to assess both the direction (i.e., positive or negative) of expectations and the strength of the patients’ priors (i.e., high or low precision). This first step is important because depending on patients’ expectations, different strategies can be used. If expectations are positive, the clinician should reinforce them through verbal suggestions associated with evidence-based treatments for the MSK pain (Rossettini et al., 2018). On the other hand, if the expectations are negative, the clinician has two different strategies to address them: optimization or violation (Peerdeman et al., 2016; Kube et al., 2018).
In MSK pain, patient education and reassurance are examples of optimizations (Louw et al., 2016; Bulow et al., 2021), adopted in clinical settings, to provide information, reconceptualize beliefs and facilitate patients’ ability to cope with their condition (Watson et al., 2019). Instead, manual therapy (i.e., mobilization with movement and symptom modification procedure), therapeutic exercise (i.e., active range of motion tasks), and virtual reality (i.e., immersive scenario; Geneen et al., 2017; Gumaa and Rehan Youssef, 2019; Ahern et al., 2020; Bordeleau et al., 2021; Brea-Gomez et al., 2021; Satpute et al., 2021; Tsokanos et al., 2021) are examples of successful violation strategies commonly adopted to reduce pain and disability (Zusman, 2013a,b; Rabey et al., 2017; Bialosky et al., 2018; Geri et al., 2019; Cerritelli et al., 2021). From a Bayesian perspective, both optimization and violation can be considered as bottom-up inputs that clinicians can offer to patients to challenge the negative expectations of the patient, facilitating their updating (Friston, 2012). While optimization aims to modify one’s priors by working at a high cognitive level (i.e., providing a new understanding of the pain by explaining how it works; Doering et al., 2018), violation strategies challenge one’s dysfunctional beliefs by providing first-hand disconfirmatory evidence with experience (Craske et al., 2018), which in turn can be used to update the negative priors. Since there are currently no criteria to guide the clinicians on which strategy to use first (optimization first or violation first; Peerdeman et al., 2016; Kube et al., 2018), we suggest a clinically oriented choice which should depend on the level of precision of patients’ priors (as assessed during the clinical encounter).
Let us consider a patient that is scared of squatting because they have once read on social media that squatting repeatedly can ruin the cartilage of their knee. The clinician assesses the strength of patient’s expectations (i.e., the clinician discovers that the patient is aware that social media are full of fake news and is aware that the information about squatting and cartilage damage might not be true) and establishes that the negative expectations are likely to have low precision. In this case, we suggest that clinicians could use optimization strategies first (i.e., reducing unrealistic beliefs about possible side effects through education; Doering et al., 2018) and then violation strategies (Craske et al., 2018). By doing so, expectations are first challenged at the cognitive level via optimization, while violation is used at a later stage to further challenge dysfunctional expectations with experience. Since patients’ expectations are not so rooted, we would expect them to update easily, resulting in observable positive changes sooner rather than later (i.e., within a session or after a reduced number of treatments).
Instead, consider patients having negative expectations with high precision (i.e., believing that their cervical disk herniation is a severe condition limiting all neck movements). When clinicians understand that such negative expectations are firmly rooted in the patients’ minds (i.e., the patient that knows, mainly through word-of-mouth, several people whose herniation got worse because they were too active and sporty, and is therefore convinced that movement is bad for this type of condition), we suggest inverting the two strategies (violation-optimization) to avoid ruining the therapeutic alliance. If clinicians insist on telling patients information that goes against their strong expectations (i.e., optimization), patients might start losing trust in the clinicians—in other words, telling patients things that they do not want to hear can be counterproductive (Laferton et al., 2017; Rossettini et al., 2018). Instead, we propose focusing on building trust (i.e., listening to the patient, without, at first, saying things that are directly in contrast with their view), meanwhile using violation strategies so that patients can disproof their expectations for themselves (e.g., providing pain-free experiences with manual therapy, exercise, and virtual reality). Later, when patients are more open to hearing information against their initial beliefs, clinicians can use optimization strategies to further promote and strengthen priors updating (Craske et al., 2018; Schemer et al., 2019). Worth mentioning is that in the case of firmly rooted expectations (i.e., where the patient implements strategies such as cognitive immunization), patients might require more evidence before successfully updating their priors. In this situation, clinicians should consider offering a higher number of disconfirming trials (i.e., repeating violation strategies more times than usual or offering the patient more treatment sessions; Gatzounis et al., 2021; Hilleke et al., 2021).
Despite some preliminary research suggests that the Bayesian framework (Owens et al., 2018; Ongaro and Kaptchuk, 2019; Kaptchuk et al., 2020) and the ViolEx model (Kube et al., 2020; Panitz et al., 2021) are good fit for describing pain processing and symptoms persistence, some open questions remained unresolved.
First, it is crucial to understand how clinicians can translate the strategies used to modulate patient’s expectations within the specific context of MSK pain (Caneiro et al., 2021). So far, researchers have investigated the modulation of expectations mainly in mental health (i.e., anxiety and depression) or medical conditions (i.e., cancer and coronary heart disease; Peerdeman et al., 2016; Kube et al., 2018), compared to MSK pain (Barth et al., 2021). Therefore, the interplay between direct experiences (i.e., previous healthcare exposures), social and cultural influences (i.e., peers and media), individual differences (i.e., personality traits and genetic factor), and expectations (Panitz et al., 2021) in patients presenting different MSK pain mechanism (i.e., nociceptive, neuropathic, and nociplastic) represents a challenge for future studies (Rossettini and Testa, 2018).
Second, it is crucial to investigate if it is possible to change patient’s expectations permanently. Even if expectations can change in a specific situation (i.e., “I did not feel pain in my back when bending over on this occasion”), this modification does not necessarily translate into a general and long-lasting change (i.e., “Every time I will bend over, I will not feel pain in the back”; Schemer et al., 2019; Riecke et al., 2020). Furthermore, if the patient has negative expectations with very high priors, it could be difficult to change them quickly (Kube et al., 2020). Therefore, we need future studies to investigate if expectations can change for long periods (Bromberg-Martin and Sharot, 2020; Camerone et al., 2021a,b) and if they are generalized to different MSK pain conditions.
Third, it is necessary to understand the optimal PE magnitude needed to update patient expectations. According to the recent scientific literature, patients ignore very small PEs and avoid to update their expectations as often the cognitive costs of the change outweigh the benefits (Linton et al., 2012; Panitz et al., 2021; Pinquart et al., 2021). Furthermore, even substantial PE can be considered an exception to the rule and thus discarded without changing expectations (Linton et al., 2012; Panitz et al., 2021; Pinquart et al., 2021). Therefore, future studies should identify the magnitude of PE capable to challenge the patients’ negative expectations in MSK pain.
Managing patients’ expectations continues to represent a challenge in MSK pain. Clinicians should choose wisely if, when and how to challenge patients’ negative expectations, considering whether the benefits of avoiding nocebo-related effects outweigh the risks of eroding the therapeutic alliance and having drop-outs. Based on the theoretical frameworks here presented (ViolEx Model and Bayesian Brain Hypothesis), we suggest that clinicians could use the strength of patients’ expectations as an indicator to decide when to directly challenge patients’ negative expectations (i.e., optimization), or when to start by challenging their beliefs indirectly (i.e., violation), avoiding damages to the therapeutic alliance.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
GR, AC and MT designed and supervised the writing of the paper. GR, AC, ECam, ECar, MMan, MMir, AP and MT participated in drafting and revising the different versions of the article. All authors contributed to the article and approved the submitted version.
GR leads education programs on placebo, nocebo effects and contextual factors in healthcare to under- and post-graduate students along with private CPD courses.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahern, M. M., Dean, L. V., Stoddard, C. C., Agrawal, A., Kim, K., Cook, C. E., et al. (2020). The effectiveness of virtual reality in patients with spinal pain: a systematic review and meta-analysis. Pain Pract. 20, 656–675. doi: 10.1111/papr.12885
Ashaye, T., Hounsome, N., Carnes, D., Taylor, S. J. C., Homer, K., Eldridge, S., et al. (2018). Opioid prescribing for chronic musculoskeletal pain in UK primary care: results from a cohort analysis of the COPERS trial. BMJ Open 8:e019491. doi: 10.1136/bmjopen-2017-019491
Bagaric, B., Jokic-Begic, N., and Jokic, C. S. (2021). The nocebo effect: a review of contemporary experimental research. Int. J. Behav. Med. doi: 10.1007/s12529-021-10016-y [Epub ahead of print].
Barbari, V., Storari, L., Maselli, F., and Testa, M. (2021). Applicability of pain neuroscience education: where are we now? J. Back Musculoskelet. Rehabil. 34, 511–520. doi: 10.3233/BMR-200091
Barlow, D. H., Ellard, K. K., Sauer-Zavala, S., Bullis, J. R., and Carl, J. R. (2014). The origins of neuroticism. Perspect. Psychol. Sci. 9, 481–496. doi: 10.1177/1745691614544528
Barth, J., Kern, A., Luthi, S., and Witt, C. M. (2019). Assessment of patients' expectations: development and validation of the expectation for treatment scale (ETS). BMJ Open 9:e026712. doi: 10.1136/bmjopen-2018-026712
Barth, J., Muff, S., Kern, A., Zieger, A., Keiser, S., Zoller, M., et al. (2021). Effect of briefing on acupuncture treatment outcome expectations, pain, and adverse side effects among patients with chronic low back pain: a randomized clinical trial. JAMA Netw. Open 4:e2121418. doi: 10.1001/jamanetworkopen.2021.21418
Benedetti, F., Frisaldi, E., Barbiani, D., Camerone, E., and Shaibani, A. (2020). Nocebo and the contribution of psychosocial factors to the generation of pain. J. Neural Transm. 127, 687–696. doi: 10.1007/s00702-019-02104-x
Bialosky, J. E., Beneciuk, J. M., Bishop, M. D., Coronado, R. A., Penza, C. W., Simon, C. B., et al. (2018). Unraveling the mechanisms of manual therapy: modeling an approach. J. Orthop. Sports Phys. Ther. 48, 8–18. doi: 10.2519/jospt.2018.7476
Bisconti, M., Venturin, D., Bianco, A., Capurso, V., and Giovannico, G. (2021). Understanding contextual factors effects and their implications for Italian physiotherapists: findings from a national cross-sectional study. Healthcare 9:689. doi: 10.3390/healthcare9060689
Blasini, M., Corsi, N., Klinger, R., and Colloca, L. (2017). Nocebo and pain: an overview of the psychoneurobiological mechanisms. Pain Rep. 2:e585. doi: 10.1097/PR9.0000000000000585
Blyth, F. M., Briggs, A. M., Schneider, C. H., Hoy, D. G., and March, L. M. (2019). The global burden of musculoskeletal pain-where to from here? Am. J. Public Health 109, 35–40. doi: 10.2105/AJPH.2018.304747
Bordeleau, M., Stamenkovic, A., Tardif, P. A., and Thomas, J. (2021). The use of virtual reality in back pain rehabilitation: a systematic review and meta-analysis. J. Pain 23, 175–195. doi: 10.1016/j.jpain.2021.08.001
Brea-Gomez, B., Torres-Sanchez, I., Ortiz-Rubio, A., Calvache-Mateo, A., Cabrera-Martos, I., Lopez-Lopez, L., et al. (2021). Virtual reality in the treatment of adults with chronic low back pain: a systematic review and meta-analysis of randomized clinical trials. Int. J. Environ. Res. Public Health 18:11806. doi: 10.3390/ijerph182211806
Briggs, A. M., Shiffman, J., Shawar, Y. R., Akesson, K., Ali, N., and Woolf, A. D. (2020). Global health policy in the 21st century: challenges and opportunities to arrest the global disability burden from musculoskeletal health conditions. Best Pract. Res. Clin. Rheumatol. 34:101549. doi: 10.1016/j.berh.2020.101549
Bromberg-Martin, E. S., and Sharot, T. (2020). The value of beliefs. Neuron 106, 561–565. doi: 10.1016/j.neuron.2020.05.001
Büchel, C., Geuter, S., Sprenger, C., and Eippert, F. (2014). Placebo analgesia: a predictive coding perspective. Neuron 81, 1223–1239. doi: 10.1016/j.neuron.2014.02.042
Bulow, K., Lindberg, K., Vaegter, H. B., and Juhl, C. B. (2021). Effectiveness of pain neurophysiology education on musculoskeletal pain: a systematic review and meta-analysis. Pain Med. 22, 891–904. doi: 10.1093/pm/pnaa484
Bunzli, S., Smith, A., Schutze, R., and O'sullivan, P. (2015). Beliefs underlying pain-related fear and how they evolve: a qualitative investigation in people with chronic back pain and high pain-related fear. BMJ Open 5:e008847. doi: 10.1136/bmjopen-2015-008847
Bunzli, S., Watkins, R., Smith, A., Schutze, R., and O'sullivan, P. (2013). Lives on hold: a qualitative synthesis exploring the experience of chronic low-back pain. Clin. J. Pain 29, 907–916. doi: 10.1097/AJP.0b013e31827a6dd8
Cadorin, L., Rossettini, G., Testa, M., Geri, T., and Palese, A. (2020). The awareness of contextual factors, placebo and nocebo effects among nursing students: findings from a cross-sectional study. Nurse Educ. Pract. 42:102670. doi: 10.1016/j.nepr.2019.102670
Camerone, E. M., Piedimonte, A., Testa, M., Wiech, K., Vase, L., Zamfira, D. A., et al. (2021a). The effect of temporal information on placebo analgesia and nocebo hyperalgesia. Psychosom. Med. 83, 43–50. doi: 10.1097/PSY.0000000000000882
Camerone, E. M., Wiech, K., Benedetti, F., Carlino, E., Job, M., Scafoglieri, A., et al. (2021b). 'External timing' of placebo analgesia in an experimental model of sustained pain. Eur. J. Pain 25, 1303–1315. doi: 10.1002/ejp.1752
Caneiro, J. P., Roos, E. M., Barton, C. J., O'sullivan, K., Kent, P., Lin, I., et al. (2020). It is time to move beyond 'body region silos' to manage musculoskeletal pain: five actions to change clinical practice. Br. J. Sports Med. 54, 438–439. doi: 10.1136/bjsports-2018-100488
Caneiro, J. P., Smith, A., Bunzli, S., Linton, S., Moseley, G. L., and O'sullivan, P. (2021). From fear to safety: a roadmap to recovery from musculoskeletal pain. Phys. Ther. 102:pzab271. doi: 10.1093/ptj/pzab271
Cashin, A. G., Lee, H., Traeger, A. C., Hubscher, M., Skinner, I. W., and Mcauley, J. H. (2021). Feeling reassured after a consultation does not reduce disability or healthcare use in people with acute low back pain: a mediation analysis of a randomised trial. J. Physiother. 67, 197–200. doi: 10.1016/j.jphys.2021.06.007
Cerritelli, F., Chiera, M., Abbro, M., Megale, V., Esteves, J., Gallace, A., et al. (2021). The challenges and perspectives of the integration between virtual and augmented reality and manual therapies. Front. Neurol. 12:700211. doi: 10.3389/fneur.2021.700211
Cheung, L., and Soundy, A. (2021). The impact of reassurance on musculoskeletal (MSK) pain: a qualitative review. Behav. Sci. 11:150. doi: 10.3390/bs11110150
Colloca, L., Corsi, N., and Fiorio, M. (2018). The interplay of exercise, placebo and nocebo effects on experimental pain. Sci. Rep. 8:14758. doi: 10.1038/s41598-018-32974-2
Cook, C. E., Denninger, T., Lewis, J., Diener, I., and Thigpen, C. (2021). Providing value-based care as a physiotherapist. Arch. Physiother. 11:12. doi: 10.1186/s40945-021-00107-0
Corsi, N., Emadi Andani, M., Sometti, D., Tinazzi, M., and Fiorio, M. (2019). When words hurt: verbal suggestion prevails over conditioning in inducing the motor nocebo effect. Eur. J. Neurosci. 50, 3311–3326. doi: 10.1111/ejn.14489
Craske, M. G., Hermans, D., and Vervliet, B. (2018). State-of-the-art and future directions for extinction as a translational model for fear and anxiety. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 373:20170025. doi: 10.1098/rstb.2017.0025
Darlow, B. (2016). Beliefs about back pain: the confluence of client, clinician and community. Int. J. Osteopath. Med. 20, 53–61. doi: 10.1016/j.ijosm.2016.01.005
De Ruddere, L., Goubert, L., Stevens, M., De, C. W. A. C., and Crombez, G. (2013). Discounting pain in the absence of medical evidence is explained by negative evaluation of the patient. Pain 154, 669–676. doi: 10.1016/j.pain.2012.12.018
Doering, B. K., Glombiewski, J. A., and Rief, W. (2018). Expectation-focused psychotherapy to improve clinical outcomes. Int. Rev. Neurobiol. 138, 257–270. doi: 10.1016/bs.irn.2018.02.004
Downey, A. (2018). Predictive processing and the representation wars: a victory for the eliminativist (via fictionalism). Synthese 195, 5115–5139. doi: 10.1007/s11229-017-1442-8
Everaert, J., Bronstein, M. V., Cannon, T. D., and Joormann, J. (2018). Looking through tinted glasses: depression and social anxiety are related to both interpretation biases and inflexible negative interpretations. Clin. Psychol. Sci. 6, 517–528. doi: 10.1177/2167702617747968
Fishbain, D. A., and Pulikal, A. (2020). Can patient expectations of returning to work documented before, during, or at the end of treatment predict actual return to work post-treatment? An evidence-based structured systematic review. Pain Med. 21, 3034–3046. doi: 10.1093/pm/pnaa093
Friston, K. (2008). Hierarchical models in the brain. PLoS Comput. Biol. 4:e1000211. doi: 10.1371/journal.pcbi.1000211
Friston, K. (2010). The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11, 127–138. doi: 10.1038/nrn2787
Friston, K. (2012). The history of the future of the Bayesian brain. NeuroImage 62, 1230–1233. doi: 10.1016/j.neuroimage.2011.10.004
Garrett, N., and Sharot, T. (2017). Optimistic update bias holds firm: three tests of robustness following Shah et al. Conscious. Cogn. 50, 12–22. doi: 10.1016/j.concog.2016.10.013
Gatzounis, R., Den Hollander, M., and Meulders, A. (2021). Optimizing long-term outcomes of exposure for chronic primary pain from the lens of learning theory. J. Pain 22, 1315–1327. doi: 10.1016/j.jpain.2021.04.012
Geneen, L. J., Moore, R. A., Clarke, C., Martin, D., Colvin, L. A., and Smith, B. H. (2017). Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst. Rev. 4:CD011279. doi: 10.1002/14651858.CD011279.pub2
Geri, T., Viceconti, A., Minacci, M., Testa, M., and Rossettini, G. (2019). Manual therapy: exploiting the role of human touch. Musculoskelet. Sci. Pract. 44:102044. doi: 10.1016/j.msksp.2019.07.008
Geurts, J. W., Willems, P. C., Lockwood, C., Van Kleef, M., Kleijnen, J., and Dirksen, C. (2017). Patient expectations for management of chronic non-cancer pain: a systematic review. Health Expect. 20, 1201–1217. doi: 10.1111/hex.12527
Gumaa, M., and Rehan Youssef, A. (2019). Is virtual reality effective in orthopedic rehabilitation? A systematic review and meta-analysis. Phys. Ther. 99, 1304–1325. doi: 10.1093/ptj/pzz093
Hallegraeff, J. M., Krijnen, W. P., Van Der Schans, C. P., and De Greef, M. H. G. (2012). Expectations about recovery from acute non-specific low back pain predict absence from usual work due to chronic low back pain: a systematic review. J. Phys. 58, 165–172. doi: 10.1016/S1836-9553(12)70107-8
Handley, I. M., Fowler, S. L., Rasinski, H. M., Helfer, S. G., and Geers, A. L. (2013). Beliefs about expectations moderate the influence of expectations on pain perception. Int. J. Behav. Med. 20, 52–58. doi: 10.1007/s12529-011-9203-4
Hayden, J. A., Wilson, M. N., Riley, R. D., Iles, R., Pincus, T., and Ogilvie, R. (2019). Individual recovery expectations and prognosis of outcomes in non-specific low back pain: prognostic factor review. Cochrane Database Syst. Rev. 2019:CD011284. doi: 10.1002/14651858.CD011284.pub2
Hilleke, M., Lang, T., and Helbig-Lang, S. (2021). Match or mismatch? The impact of expected fear on experienced fear during exposure. Clin. Psychol. Sci. Pract. 28, 148–160. doi: 10.1037/cps0000005
Hutting, N., Caneiro, J. P., Ong'wen, O. M., Miciak, M., and Roberts, L. (2021). Patient-centered care in musculoskeletal practice: key elements to support clinicians to focus on the person. Musculoskelet. Sci. Pract. 57:102434. doi: 10.1016/j.msksp.2021.102434
Jones, S. M., Lange, J., Turner, J., Cherkin, D., Ritenbaugh, C., Hsu, C., et al. (2016). Development and validation of the EXPECT questionnaire: assessing patient expectations of outcomes of complementary and alternative medicine treatments for chronic pain. J. Altern. Complement. Med. 22, 936–946. doi: 10.1089/acm.2016.0242
Jones, C. M. P., Shaheed, C. A., Ferreira, G. E., Kharel, P., Christine Lin, C.-W., and Maher, C. G. (2021). Advice and education provide small short-term improvements in pain and disability in people with non-specific spinal pain: a systematic review. J. Phys. 67, 263–270. doi: 10.1016/j.jphys.2021.08.014
Kaptchuk, T. J., Hemond, C. C., and Miller, F. G. (2020). Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ 370:m1668. doi: 10.1136/bmj.m1668
Klinger, R., Blasini, M., Schmitz, J., and Colloca, L. (2017). Nocebo effects in clinical studies: hints for pain therapy. Pain Rep. 2:e586. doi: 10.1097/PR9.0000000000000586
Korn, C. W., Sharot, T., Walter, H., Heekeren, H. R., and Dolan, R. J. (2014). Depression is related to an absence of optimistically biased belief updating about future life events. Psychol. Med. 44, 579–592. doi: 10.1017/S0033291713001074
Kravvariti, E., Kitas, G. D., Mitsikostas, D. D., and Sfikakis, P. P. (2018). Nocebos in rheumatology: emerging concepts and their implications for clinical practice. Nat. Rev. Rheumatol. 14, 727–740. doi: 10.1038/s41584-018-0110-9
Kravvariti, E., Kotsani, M., Mitsikostas, D. D., and Sfikakis, P. P. (2021). Nocebo phenomena may be enhanced in aging: implications for clinical practice. Maturitas 143, 10–16. doi: 10.1016/j.maturitas.2020.07.011
Kube, T., Glombiewski, J. A., Gall, J., Touissant, L., Gartner, T., and Rief, W. (2019a). How to modify persisting negative expectations in major depression? An experimental study comparing three strategies to inhibit cognitive immunization against novel positive experiences. J. Affect. Disord. 250, 231–240. doi: 10.1016/j.jad.2019.03.027
Kube, T., Glombiewski, J. A., and Rief, W. (2018). Using different expectation mechanisms to optimize treatment of patients with medical conditions: a systematic review. Psychosom. Med. 80, 535–543. doi: 10.1097/PSY.0000000000000596
Kube, T., Rief, W., and Glombiewski, J. A. (2017). On the maintenance of expectations in major depression—investigating a neglected phenomenon. Front. Psychol. 8:9. doi: 10.3389/fpsyg.2017.00009
Kube, T., Rief, W., Gollwitzer, M., Gartner, T., and Glombiewski, J. A. (2019b). Why dysfunctional expectations in depression persist—results from two experimental studies investigating cognitive immunization. Psychol. Med. 49, 1532–1544. doi: 10.1017/S0033291718002106
Kube, T., Rozenkrantz, L., Rief, W., and Barsky, A. (2020). Understanding persistent physical symptoms: conceptual integration of psychological expectation models and predictive processing accounts. Clin. Psychol. Rev. 76:101829. doi: 10.1016/j.cpr.2020.101829
Laferton, J. A., Kube, T., Salzmann, S., Auer, C. J., and Shedden-Mora, M. C. (2017). Patients' expectations regarding medical treatment: a critical review of concepts and their assessment. Front. Psychol. 8:233. doi: 10.3389/fpsyg.2017.00233
Lewis, J., and O'sullivan, P. (2018). Is it time to reframe how we care for people with non-traumatic musculoskeletal pain? Br. J. Sports Med. 52, 1543–1544. doi: 10.1136/bjsports-2018-099198
Lewis, J. S., Stokes, E. K., Gojanovic, B., Gellatly, P., Mbada, C., Sharma, S., et al. (2021). Reframing how we care for people with persistent non-traumatic musculoskeletal pain. Suggestions for the rehabilitation community. Physiotherapy 112, 143–149. doi: 10.1016/j.physio.2021.04.002
Liknaitzky, P., Smillie, L. D., and Allen, N. B. (2017). Out-of-the-blue: depressive symptoms are associated with deficits in processing inferential expectancy-violations using a novel cognitive rigidity task. Cogn. Ther. Res. 41, 757–776. doi: 10.1007/s10608-017-9853-x
Lin, I., Wiles, L., Waller, R., Caneiro, J. P., Nagree, Y., Straker, L., et al. (2020). Patient-centred care: the cornerstone for high-value musculoskeletal pain management. Br. J. Sports Med. 54, 1240–1242. doi: 10.1136/bjsports-2019-101918
Linton, S. J., Boersma, K., Vangronsveld, K., and Fruzzetti, A. (2012). Painfully reassuring? The effects of validation on emotions and adherence in a pain test. Eur. J. Pain 16, 592–599. doi: 10.1016/j.ejpain.2011.07.011
Louw, A., Zimney, K., Puentedura, E. J., and Diener, I. (2016). The efficacy of pain neuroscience education on musculoskeletal pain: a systematic review of the literature. Physiother. Theory Pract. 32, 332–355. doi: 10.1080/09593985.2016.1194646
Manai, M., Van Middendorp, H., Veldhuijzen, D. S., Huizinga, T. W. J., and Evers, A. W. M. (2019). How to prevent, minimize, or extinguish nocebo effects in pain: a narrative review on mechanisms, predictors, and interventions. Pain Rep. 4:e699. doi: 10.1097/PR9.0000000000000699
Mancini, F., Zhang, S., and Seymour, B. (2021). Learning the statistics of pain: computational and neural mechanisms. bioRxiv [Preprint]. doi: 10.1101/2021.10.21.465270
Mannion, A. F., Wieser, S., and Elfering, A. (2013). Association between beliefs and care-seeking behavior for low back pain. Spine 38, 1016–1025. doi: 10.1097/BRS.0b013e31828473b5
Mohamed, W. J. M., Joseph, L., Canby, G., Paungmali, A., Sitilertpisan, P., and Pirunsan, U. (2020). Are patient expectations associated with treatment outcomes in individuals with chronic low back pain? A systematic review of randomised controlled trials. Int. J. Clin. Pract. 74:e13680. doi: 10.1111/ijcp.13680
Ng, F., Smith, G. D., Ma, C. C., and Li, L. W. (2020). “Health seeking behaviour: doctor shopping,” in Primary Care Revisited: Interdisciplinary Perspectives for a New Era. eds. B. Y. F. Fong, V. T. S. Law, and A. Lee (Singapore: Springer Singapore).
Ochsner, K. N., Ludlow, D. H., Knierim, K., Hanelin, J., Ramachandran, T., Glover, G. C., et al. (2006). Neural correlates of individual differences in pain-related fear and anxiety. Pain 120, 69–77. doi: 10.1016/j.pain.2005.10.014
Ongaro, G., and Kaptchuk, T. J. (2019). Symptom perception, placebo effects, and the Bayesian brain. Pain 160, 1–4. doi: 10.1097/j.pain.0000000000001367
Owens, A. P., Allen, M., Ondobaka, S., and Friston, K. J. (2018). Interoceptive inference: from computational neuroscience to clinic. Neurosci. Biobehav. Rev. 90, 174–183. doi: 10.1016/j.neubiorev.2018.04.017
Palese, A., Cadorin, L., Testa, M., Geri, T., Colloca, L., and Rossettini, G. (2019a). Contextual factors triggering placebo and nocebo effects in nursing practice: findings from a national cross-sectional study. J. Clin. Nurs. 28, 1966–1978. doi: 10.1111/jocn.14809
Palese, A., Rossettini, G., Colloca, L., and Testa, M. (2019b). The impact of contextual factors on nursing outcomes and the role of placebo/nocebo effects: a discussion paper. Pain Rep. 4:e716. doi: 10.1097/PR9.0000000000000716
Panitz, C., Endres, D., Buchholz, M., Khosrowtaj, Z., Sperl, M. F., Mueller, E., et al. (2021). A revised framework for the investigation of expectation update versus maintenance in the context of expectation violations: the ViolEx 2.0 model. Front. Psychol. 2:726432. doi: 10.3389/fpsyg.2021.726432
Peerdeman, K. J., Van Laarhoven, A. I. M., Keij, S. M., Vase, L., Rovers, M. M., Peters, M. L., et al. (2016). Relieving patients' pain with expectation interventions: a meta-analysis. Pain 157, 1179–1191. doi: 10.1097/j.pain.0000000000000540
Petrie, K. J., and Rief, W. (2019). Psychobiological mechanisms of placebo and nocebo effects: pathways to improve treatments and reduce side effects. Annu. Rev. Psychol. 70, 599–625. doi: 10.1146/annurev-psych-010418-102907
Pinquart, M., Endres, D., Teige-Mocigemba, S., Panitz, C., and Schutz, A. C. (2021). Why expectations do or do not change after expectation violation: a comparison of seven models. Conscious. Cogn. 89:103086. doi: 10.1016/j.concog.2021.103086
Rabey, M., Hall, T., Hebron, C., Palsson, T. S., Christensen, S. W., and Moloney, N. (2017). Reconceptualising manual therapy skills in contemporary practice. Musculoskelet. Sci. Pract. 29, 28–32. doi: 10.1016/j.msksp.2017.02.010
Riecke, J., Rief, W., Vlaeyen, J. W. S., and Glombiewski, J. A. (2020). Generalizability of harm and pain expectations after exposure in chronic low back pain patients. Eur. J. Pain 24, 1495–1504. doi: 10.1002/ejp.1604
Rief, W., Heitmuller, A. M., Reisberg, K., and Ruddel, H. (2006). Why reassurance fails in patients with unexplained symptoms--an experimental investigation of remembered probabilities. PLoS Med. 3:e269. doi: 10.1371/journal.pmed.0030269
Rief, W., and Joormann, J. (2019). Revisiting the cognitive model of depression: the role of expectations. Clin. Psychol. Eur. 1:32605. doi: 10.32872/cpe.v1i1.32605
Rief, W., and Petrie, K. J. (2016). Can psychological expectation models be adapted for placebo research? Front. Psychol. 7:1876. doi: 10.3389/fpsyg.2016.01876
Rondoni, A., Rossettini, G., Ristori, D., Gallo, F., Strobe, M., Giaretta, F., et al. (2017). Intrarater and inter-rater reliability of active cervical range of motion in patients with nonspecific neck pain measured with technological and common use devices: a systematic review with meta-regression. J. Manip. Physiol. Ther. 40, 597–608. doi: 10.1016/j.jmpt.2017.07.002
Rossettini, G., Camerone, E. M., Carlino, E., Benedetti, F., and Testa, M. (2020a). Context matters: the psychoneurobiological determinants of placebo, nocebo and context-related effects in physiotherapy. Arch. Physiother. 10:11. doi: 10.1186/s40945-020-00082-y
Rossettini, G., Carlino, E., and Testa, M. (2018). Clinical relevance of contextual factors as triggers of placebo and nocebo effects in musculoskeletal pain. BMC Musculoskelet. Disord. 19:27. doi: 10.1186/s12891-018-1943-8
Rossettini, G., Geri, T., Palese, A., Marzaro, C., Mirandola, M., Colloca, L., et al. (2020b). What physiotherapists specialized in orthopedic manual therapy know about nocebo-related effects and contextual factors: findings from a national survey. Front. Psychol. 11:582174. doi: 10.3389/fpsyg.2020.582174
Rossettini, G., Latini, T. M., Palese, A., Jack, S. M., Ristori, D., Gonzatto, S., et al. (2020c). Determinants of patient satisfaction in outpatient musculoskeletal physiotherapy: a systematic, qualitative meta-summary, and meta-synthesis. Disabil. Rehabil. 42, 460–472. doi: 10.1080/09638288.2018.1501102
Rossettini, G., Palese, A., Geri, T., Mirandola, M., Tortella, F., and Testa, M. (2019). The knowledge of contextual factors as triggers of placebo and nocebo effects in patients with musculoskeletal pain: findings from a national survey. Front. Psychol. 10:478. doi: 10.3389/fpsyt.2019.00478
Rossettini, G., and Testa, M. (2018). Manual therapy RCTs: should we control placebo in placebo control? Eur. J. Phys. Rehabil. Med. 54, 500–501. doi: 10.23736/S1973-9087.17.05024-9
Safiri, S., Kolahi, A. A., Cross, M., Hill, C., Smith, E., Carson-Chahhoud, K., et al. (2021). Prevalence, deaths, and disability-adjusted life years due to musculoskeletal disorders for 195 countries and territories 1990-2017. Arthritis Rheum. 73, 702–714. doi: 10.1002/art.41571
Sajid, I. M., Parkunan, A., and Frost, K. (2021). Unintended consequences: quantifying the benefits, iatrogenic harms and downstream cascade costs of musculoskeletal MRI in UK primary care. BMJ Open Qual. 10:e001287. doi: 10.1136/bmjoq-2020-001287
Satpute, K., Reid, S., Mitchell, T., Mackay, G., and Hall, T. (2021). Efficacy of mobilization with movement (MWM) for shoulder conditions: a systematic review and meta-analysis. J. Man. Manip. Ther. 30, 13–32. doi: 10.1080/10669817.2021.1955181
Schemer, L., Körfer, K., and Glombiewski, J. A. (2019). Evaluation of exposure instructions to pain: should therapist focus on fear reduction or expectation violation? Cogn. Ther. Res. 44, 697–708. doi: 10.1007/s10608-019-10070-7
Setchell, J., Costa, N., Abrosimoff, M., and Hodges, P. W. (2021). Exploring why people with back pain use the pain management strategies they do: is research looking in the wrong places? Pain Med. 22, 2298–2306. doi: 10.1093/pm/pnab246
Seymour, B., and Mancini, F. (2020). Hierarchical models of pain: inference, information-seeking, and adaptive control. NeuroImage 222:117212. doi: 10.1016/j.neuroimage.2020.117212
Sharot, T., Guitart-Masip, M., Korn, C. W., Chowdhury, R., and Dolan, R. J. (2012). How dopamine enhances an optimism bias in humans. Curr. Biol. 22, 1477–1481. doi: 10.1016/j.cub.2012.05.053
Sharot, T., Korn, C. W., and Dolan, R. J. (2011). How unrealistic optimism is maintained in the face of reality. Nat. Neurosci. 14, 1475–1479. doi: 10.1038/nn.2949
Sharot, T., Riccardi, A. M., Raio, C. M., and Phelps, E. A. (2007). Neural mechanisms mediating optimism bias. Nature 450, 102–105. doi: 10.1038/nature06280
Thomaidou, M. A., Peerdeman, K. J., Koppeschaar, M. I., Evers, A. W. M., and Veldhuijzen, D. S. (2021). How negative experience influences the brain: a comprehensive review of the neurobiological underpinnings of nocebo hyperalgesia. Front. Neurosci. 15:652552. doi: 10.3389/fnins.2021.652552
Thomson, O. P., and Rossettini, G. (2021). ‘Don't focus on the finger, look at the moon’—the importance of contextual factors for clinical practice and research. Int. J. Osteopath. Med. 40, 1–3. doi: 10.1016/j.ijosm.2021.06.001
Tonosu, J., Oka, H., Higashikawa, A., Okazaki, H., Tanaka, S., and Matsudaira, K. (2017). The associations between magnetic resonance imaging findings and low back pain: a 10-year longitudinal analysis. PLoS One 12:e0188057. doi: 10.1371/journal.pone.0188057
Traeger, A. C., Lee, H., Hubscher, M., Skinner, I. W., Moseley, G. L., Nicholas, M. K., et al. (2019). Effect of intensive patient education vs placebo patient education on outcomes in patients With acute low back pain: a randomized clinical trial. JAMA Neurol. 76, 161–169. doi: 10.1001/jamaneurol.2018.3376
Tsokanos, A., Livieratou, E., Billis, E., Tsekoura, M., Tatsios, P., Tsepis, E., et al. (2021). The efficacy of manual therapy in patients with knee osteoarthritis: a systematic review. Medicina 57:696. doi: 10.3390/medicina57070696
Turk, D. C., and Wilson, H. D. (2010). Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr. Pain Headache Rep. 14, 88–95. doi: 10.1007/s11916-010-0094-x
Viceconti, A., Camerone, E. M., Luzzi, D., Pentassuglia, D., Pardini, M., Ristori, D., et al. (2020). Explicit and implicit own's body and space perception in painful musculoskeletal disorders and rheumatic diseases: a systematic scoping review. Front. Hum. Neurosci. 14:83. doi: 10.3389/fnhum.2020.00083
Watson, J. A., Ryan, C. G., Cooper, L., Ellington, D., Whittle, R., Lavender, M., et al. (2019). Pain neuroscience education for adults with chronic musculoskeletal pain: a mixed-methods systematic review and meta-analysis. J. Pain 20, 1140.e1–1140.e22. doi: 10.1016/j.jpain.2019.02.011
Yunus, M. B. (2007). Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin. Arthritis Rheum. 36, 339–356. doi: 10.1016/j.semarthrit.2006.12.009
Zusman, M. (2013a). Associative memory for movement-evoked chronic back pain and its extinction with musculoskeletal physiotherapy. Phys. Ther. Rev. 13, 57–68. doi: 10.1179/174328808X251948
Keywords: nocebo effects, contextual factors, pain, musculoskeletal, physiotherapy, expectation, predictive brain, placebo effects
Citation: Rossettini G, Colombi A, Carlino E, Manoni M, Mirandola M, Polli A, Camerone EM and Testa M (2022) Unraveling Negative Expectations and Nocebo-Related Effects in Musculoskeletal Pain. Front. Psychol. 13:789377. doi: 10.3389/fpsyg.2022.789377
Received: 04 October 2021; Accepted: 24 February 2022;
Published: 16 March 2022.
Edited by:
Luana Colloca, University of Maryland, Baltimore, United StatesReviewed by:
John Raglin, Indiana University, United StatesCopyright © 2022 Rossettini, Colombi, Carlino, Manoni, Mirandola, Polli, Camerone and Testa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Testa, bWFyY28udGVzdGFAdW5pZ2UuaXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.