
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 09 April 2021
Sec. Neuropsychology
Volume 12 - 2021 | https://doi.org/10.3389/fpsyg.2021.650715
 Anna Lardone1
Anna Lardone1 Patrizia Turriziani2
Patrizia Turriziani2 Pierpaolo Sorrentino3
Pierpaolo Sorrentino3 Onofrio Gigliotta4
Onofrio Gigliotta4 Andrea Chirico1
Andrea Chirico1 Fabio Lucidi1
Fabio Lucidi1 Laura Mandolesi4*
Laura Mandolesi4*During the COVID-19 lockdown, individuals were forced to remain at home, hence severely limiting the interaction within environmental stimuli, reducing the cognitive load placed on spatial competences. The effects of the behavioral restriction on cognition have been little examined. The present study is aimed at analyzing the effects of lockdown on executive function prominently involved in adapting behavior to new environmental demands. We analyze non-verbal fluency abilities, as indirectly providing a measure of cognitive flexibility to react to spatial changes. Sixteen students (mean age 20.75; SD 1.34), evaluated before the start of the lockdown (T1) in a battery of psychological tasks exploring different cognitive domains, have been reassessed during lockdown (T2). The assessment included the modified Five-Point Test (m-FPT) to analyze non-verbal fluency abilities. At T2, the students were also administered the Toronto Alexithymia Scale (TAS-20). The restriction of behaviors following a lockdown determines increased non-verbal fluency, evidenced by the significant increase of the number of new drawings. We found worsened verbal span, while phonemic verbal fluency remained unchanged. Interestingly, we observed a significant tendency to use the left part of each box in the m-FPT correlated with TAS-20 and with the subscales that assess difficulty in describing and identifying feelings. Although our data were collected from a small sample, they evidence that the restriction of behaviors determines a leftward bias, suggesting a greater activation of the right hemisphere, intrinsically connected with the processing of non-verbal information and with the need to manage an emotional situation.
The lockdown imposed to contain the spreading of COVID-19 has made it difficult to maintain healthy habits, such as physical activity and social relations (excluding virtual ones), with consequences on psychological well-being and efficient cognitive functioning. A healthy and active lifestyle determines positive effects on cognition (Hötting and Röder, 2013), improving memory abilities and efficiency of attentional processes and executive-control processes (Colcombe and Kramer, 2003; Grego et al., 2005; Pereira et al., 2007; Winter et al., 2007; Chieffi et al., 2017). Moreover, the continuous interaction with the environment, the practice of physical exercise, and proper nutrition and sleep hygiene, as well as social connectedness, the perception of social support, and other social factors, reduce dysfunctional behaviors, as well as states such as depression and anxiety (Mandolesi et al., 2018; Brooks et al., 2020), improving the quality of life (e.g., Sherbourne and Stewart, 1991; Brown et al., 2012).
The scientific literature concerning similar pandemics, such as the SARS epidemic, demonstrated that quarantine negatively affects psychological well-being, leading to the development of post-traumatic stress symptoms (Reynolds et al., 2008; Castelli et al., 2020; Lardone et al., 2020). To this regard, there are many studies that document a strong relation between COVID-19 quarantine and the onset of stress or stress-related behaviors (Brooks et al., 2020; Lardone et al., 2020; Pisano et al., 2020; Zurlo et al., 2020) among other things, more observed in females (Mazza et al., 2020) and in those who are younger ( ≤ 40 years) (Xiong et al., 2020).
Although to a lesser extent, the effects of quarantine or social isolation on cognitive functioning are mainly documented in terms of behavioral strategies put into action to cope with the restriction period (Boss et al., 2015; for review, see Pera, 2020), recently, it was shown that social isolation determines a worsening of cognitive functioning in later life (Evans, 2019) and in clinical dementia (Tilvis et al., 2000). Accordingly, it is known that loneliness significantly increases the risk of developing dementia (Wilson et al., 2007). While isolation contributes to the acceleration of the aging processes and relates to global cognitive decline in the elderly, in young people, loneliness is correlated to the worsening of executive functions (Cacioppo and Hawkley, 2009), which are strongly affected by the environmental context (Montuori et al., 2019).
Generally, we can define the executive function as the cognitive processes that allow us to select goals; to identify and decide plans of action; to inhibit behaviors; to assume a different behavior in relation to a changing context; to filter interference; to direct, select, and maintain attention on a task; to anticipate the consequences of the actions of others; to reason and solve problems; and to keep the information that is being processed available (Eslinger et al., 1991). In this context, Cacioppo and Hawkley (2009), by means of a dichotic listening task, evidenced that attentional regulation was worse in lonely individuals as compared to non-lonely ones. Another study has evidenced a relation between social isolation and impairment in specific verbal tasks such as verbal fluency and backward digit span (Lara et al., 2019).
Somma and colleagues, analyzing the performances in different peripersonal spatial tasks of a sample of Italian university students before and during the COVID-19 lockdown, showed a significantly leftward bias in the lockdown period. In fact, they observed a tendency to start cancellation from a left-sided item in a cancellation task or to explore first a left-sided arm of a digitized radial arm maze, suggesting more pseudoneglect when behavior is constrained (Somma et al., 2021) and confirming the correlation between social isolation and worsening of the cognitive functioning. The pseudoneglect (Bowers and Heilman, 1980) is a neural pattern of right-hemisphere asymmetry concerning the frontoparietal brain network that plays an important role for orienting and controlling spatial attention (Corbetta et al., 2008; Bartolomeo and Malkinson, 2019). A relative hyperactivity of this attentional right networks might push spatial attention leftward (Gigliotta et al., 2017).
On the basis of the very little available evidence of the effects of the COVID-19 lockdown on cognitive functions in young people, we analyzed the cognitive functioning of a group of young participants before and toward the end of social confinement, in order to assess whether the restriction of behavior had affected their cognitive performance. In particular, we focused on the non-verbal aspects of cognition because during the lockdown period, the individuals were forced to remain at home, hence severely limiting the interaction with all other environmental stimuli, thus reducing the load on spatial competences. For this reason, our attention has focused on non-verbal fluency, which indirectly provides a measure of cognitive flexibility to react to spatial changes. Moreover, during this retest period, we also administered the Toronto Alexithymia Scale (TAS-20, Taylor et al., 1992) in order to detect a possible alexithymia, an affective-cognitive disorder in cognitive processing and emotional regulation (Taylor et al., 1997), often associated with psychopathological conditions (Di Tella and Castelli, 2013; Di Tella et al., 2015) and mainly characterized by difficulty in identifying feelings and in distinguishing between feelings and the bodily sensations of emotional arousal; by difficulty in describing subjective feelings; by restricted imaginative processes, as evidenced by a lack of imagination; and by a stimulus-bound, externally orientated cognitive style (Di Tella and Castelli, 2016).
It is possible to distinguish two types of alexithymia: primary alexithymia, a developmental phenomenon thought to be the result of genetic factors, and secondary alexithymia, thought to be a consequence of specific conditions as well as stress, chronic disease, or organic processes (e.g., brain trauma or stroke, in this case referring to organic alexithymia) (Freyberger, 1977; Spalletta et al., 2001; Messina et al., 2014).
Our hypothesis is that behavioral restriction affects graphic fluency abilities increasing a leftward bias, considering the graphic fluency as spatial competence. To this aim, we used a modified version of the Five-Point Test (m-FPT, Cattelani et al., 2011) that measures the ability of an individual to produce geometric drawings or unique figures within a given time interval, and although it is not a classic test for attention disorders, it allows us to evaluate a shift of the drawings toward the left side of the sheet. Furthermore, recognizing that non-verbal information is mainly processed by the right hemisphere and considering the lockdown period to be an emotionally charged situation, we hypothesize also an alteration of emotional regulation processes, mainly mediated by the right hemisphere, correlated to an increasing secondary alexithymia.
Sixteen female psychology and philosophy students from the University of Naples “Federico II” aged between 19 and 24 years (mean age 20.75; SD 1.34), previously evaluated before the start of the COVID-19 lockdown (T1) using a battery of psychological tasks exploring different cognitive domains, have been re-evaluated after roughly 40 days of lockdown (T2) to some of the previously administered tests and to TAS-20 to assess any difficulty in identifying and communicating their feelings and in externally oriented thinking. All tasks administered in both phases (T1 and T2) are reported in Table 1.
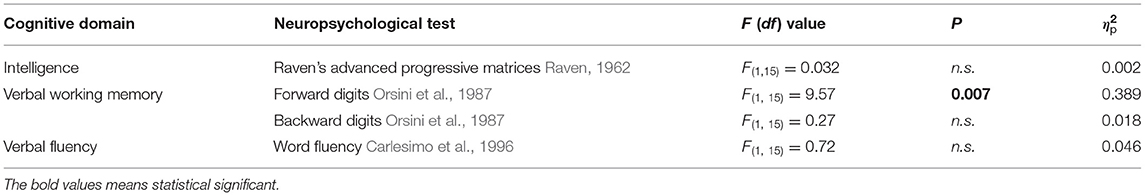
Table 1. Statistical comparisons between T1 and T2 by means of repeated-measures analyses of variance (ANOVAs) of neuropsychological assessment.
In particular, T1 refers to a period from February 17, 2020, to March 2, 2020, exactly 1 week before the start of the lockdown due to COVID-19 in Italy, while T2 refers to the last 2 weeks of quarantine, specifically from April 16, 2020, to May 2, 2020.
Selection criteria for participant recruitment included normal or corrected-to-normal vision and right-handedness. We included in the sample only students who declared that they did not contract COVID-19 and had no direct or indirect contact with a person affected by the virus. Another eligibility criteria concerned having a printer, with the re-test phase being completely carried out remotely via online meetings on the Microsoft® Teams platform. This condition implied the printing of the protocols. In addition, all participants were in good health and had no history of neurological or psychiatric illness. All students were voluntarily enrolled after written informed consent was obtained. The study was approved by the Local Ethics Committee of the University of Naples “Federico II” (protocol number: 12/2020) and was carried out in accordance with the Declaration of Helsinki. This cohort of students was part of a larger sample to which other tests were also administered, and the results are in the publication phase (Somma et al., 2021).
In order to evaluate the typical development of all the participants, Raven's Advanced Progressive Matrices (APM; Raven, 1962) were administered in T1 and T2 in digital version through the transposition of matrices on Google Modules. In addition to the assessment of intelligence, verbal working term memory abilities and verbal fluency were evaluated by means of forward and backward digits (Orsini et al., 1987) and word fluency (Carlesimo et al., 1996), respectively.
First developed by Regard et al. (1982), the Five-Point Test (FPT) is a highly reliable non-verbal measure of executive functioning, specifically evaluating the graphic figural fluency (Fernandez et al., 2009). In this study, we have used the m-FTP (Cattelani et al., 2011). In particular, the test measures the ability of an individual to produce geometric drawings or unique figures within a given time interval (3 min). It consists of an A4 sheet with 40 square-shaped matrices each with five dots inside, four of which are arranged at the vertices and one in the middle (Figure 1). The participants are required to connect two or more dots in each square with straight lines. Moreover, they must not repeat twice the same shape and must not draw lines that do not connect dots. This test allows us to analyze three subdomains of executive functions: flexibility, rule breaking, and strategic performance.
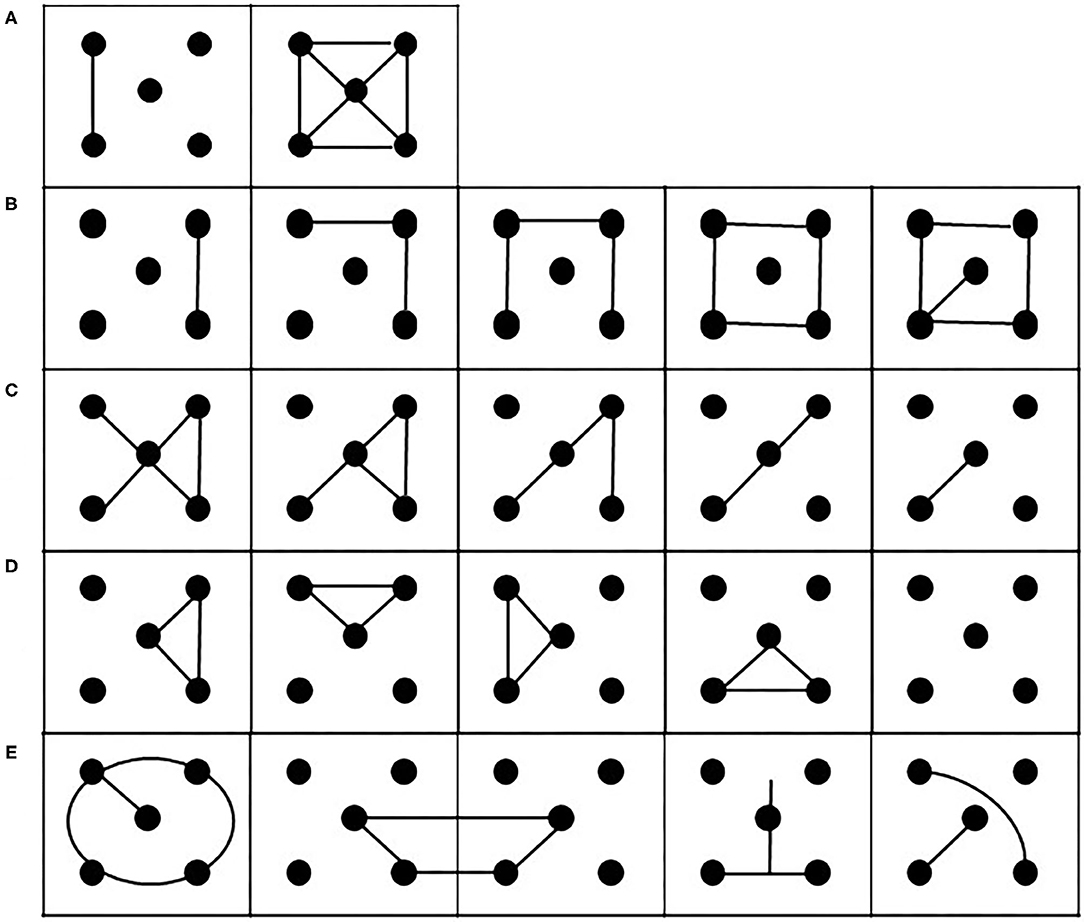
Figure 1. Examples of possible executions of the m-FPT. (A) Examples of the two solutions provided by the experimenter to illustrate how to perform the task. (B) strategies of addition or (C) subtraction elements. (D) Strategies of rotation of the patterns produced. (E) Examples of possible incorrect patterns.
The participant is asked: “See these five dots, now with this pencil you have to make several different figures until I say stop. Joining two or multiple dots in each square with a straight or straight line like this (the examiner shows two examples, Figure 1A). But be careful and remember these rules: you can use all the dots or just two, three or four; you don't have to repeat the figures; you must not draw lines that do not connect the dots.”
According to Cattelani et al., 2011, when a student finishes a page, the examiner quickly gives him/her a second sheet of paper while repositioning the first page so that the subject can easily see it.
The parameters evaluated were the following:
Total drawings: number of total drawings made in 3 min;
- Drawings with errors: number of drawings breaking the rules and or repeating previously drawn shapes;
- Error index: number of drawings with errors divided by the number of total drawings multiplied by 100;
- Number of unique drawings: calculated by subtracting the number of drawings with errors from the number of total drawings;
- Strategy index: number of drawings with strategy divided by number of unique drawings;
- Percentage of dots considered on the right: the number of dots on the right forming the drawing divided by the number of total dots multiplied by 100;
- Percentage of dots considered on the left: number of dots on the left forming the drawing divided by the number of total dots multiplied by 100; and
- Percentage of dots considered on the center: number of dots considered on the middle left forming the drawing divided by the number of total dots multiplied by 100.
The TAS (Taylor et al., 1992) and a subsequent 20-item form (TAS-20; Bagby et al., 1990) used in this study are the most used and most reliable self-assessment questionnaire for measuring alexithymia, an affective-cognitive disorder characterized by difficulty in identifying and describing owns emotions and in being interested in understanding those of others (Nemiah and Sifneos, 1970). The TAS-20 is made of three subscales, each capturing one of these aspects of the construct of alexithymia (Bressi et al., 1996): the Difficulty Describing Feelings subscale (DDF) consisted of five items (2, 4, 11, 12, and 17), the Difficulty Identifying Feeling subscale (DIF) consisted of seven items (1, 3, 6, 7, 9, 13, and 14), and the Externally Oriented Thinking subscale (EOT) measuring the tendency of individuals to focus their attention externally consisted of eight items (5, 8, 10, 15, 16, 18, 19, and 20).
In TAS-20, the subjects respond through a 5-point Likert scale, whereby 1 indicates strongly disagree and 5 indicates strongly agree. Items 4, 5, 10, 18, and 19 are negatively keyed. The total alexithymia score is the sum of the responses to all 20 items, while the score for each subscale factor is the sum of the responses to that subscale. Subjects with a score equal to or >61 are considered alexithymic, and those with a score equal to or <50 are considered non-alexithymic, with a borderline area between 50 and 60.
In phase T1 (1 week before the start of the COVID-19 lockdown), the participants have been tested in a quiet room of the University of Naples Federico II. In the room, there was a table with chairs, and the participant sat in front of the experimenter. Each participant was first administered the neuropsychological tasks and then the m-FPT, for a total of about 30 min.
In relation to social distancing measures, the second retest phase (T2, during the last 2 weeks of the quarantine) took place completely remotely via online meetings on the Microsoft® Teams platform, a unified communication and collaboration platform that combines chat, teleconferencing, content sharing, and application integration. This way, it was easy to administer all the tests used in T1, as well as the TAS-20 and the m-FPT. In fact, each student was provided with the test file in pdf, which was printed before the call by the experimenter. This way, the test took place under the full supervision of the experimenter. At the end of the test, the student took a picture of the page/pages with the drawings and sent it/them to the experimenter.
All statistical analyses were conducted with SPSS Statistics 22 software. For each comparison of T1 vs. T2, we used a repeated-measures analyses of variance (ANOVA). P values <0.05 were considered statistically significant.
Some data were tested by means of two-tailed Spearman's correlation analysis between the results of the TAS-20 and the increment of dots touched on the left at T2 in m-FPT and the worsening of forward digits, considering for both the Δ as the difference between T2 and T1.
The results are reported as F statistic (F), statistical significance (P), and bias effect size estimation ().
The statistical comparisons of the results obtained when comparing T1 vs. T2 on the previously described neuropsychological tests are shown in Table 1. We found a significant difference only for the forward digits test in which the participants exhibit a worsening in T2 (T1 = 6.5 ± 0.63; T2 = 5.9 ± 0.57).
The comparison between T1 and T2 revealed a significant difference between T1 and T2 in the total drawings parameter [F(1, 15) = 6.27; P: 0.024; = 0.295) as shown in Figure 2A]. A different pattern was observed in the comparison between T1 and T2 in relation to drawings with resulting errors similar to those in T1 and T2 [F(1, 15) = 0.24; P: 0.63; = 0.016]. This result is also reflected in the error index [F(1, 15) = 1.0; P: 0.33; = 0.016; Figure 2B] and in the number of unique drawings [F(1, 15) = 1.36; P: 0.78; = 0.019], which are not significantly different between T1 and T2. Also, in the strategy index, there are no significant differences between T1 and T2 [F(1, 15) = 1.01; P: 0.33; = 0.063, Figure 2C].
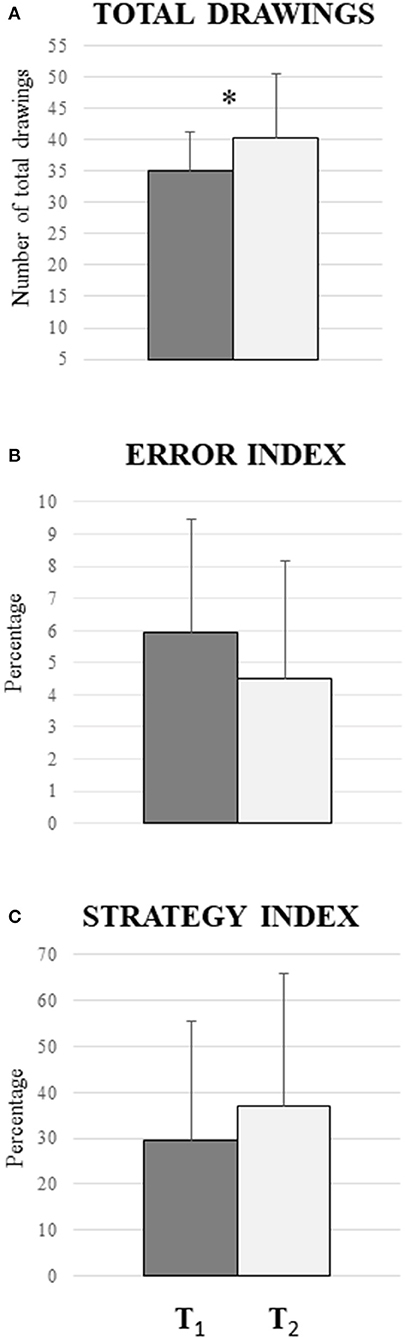
Figure 2. Comparison between the periods before the COVID-19 lockdown (T1) and during the COVID-19 lockdown (T2) in m-FPT. (A) Significant difference in total drawings parameter (P = 0.024): the participants produced more drawings during the quarantine. (B) No significant difference in the error index (P = 0.33), indicating that the percentage between the violations of the rule and the repeated drawings does not vary between the two phases. (C) No significant differences in the strategy index (P = 0.78): the result indicates that there are no changes in the increase of drawings with strategies. Vertical bars indicate SD.
Interestingly, as shown in Figure 3, when the percentage of dots forming the drawings (located either on the right or left or on the middle of each box in both phases) was considered, significant differences were found only for the percentage of dots on the left [F(1, 15) = 7.87; P: 0.01; = 0.34]. The percentage of dots on the right, on the other hand, was not significantly different [F(1, 15) = 2.04; P: 0.17; = 0.12]. Also, the percentage of dots on the middle remained unchanged [F(1, 15) = 0.03; P: 0.96; = 0.00].
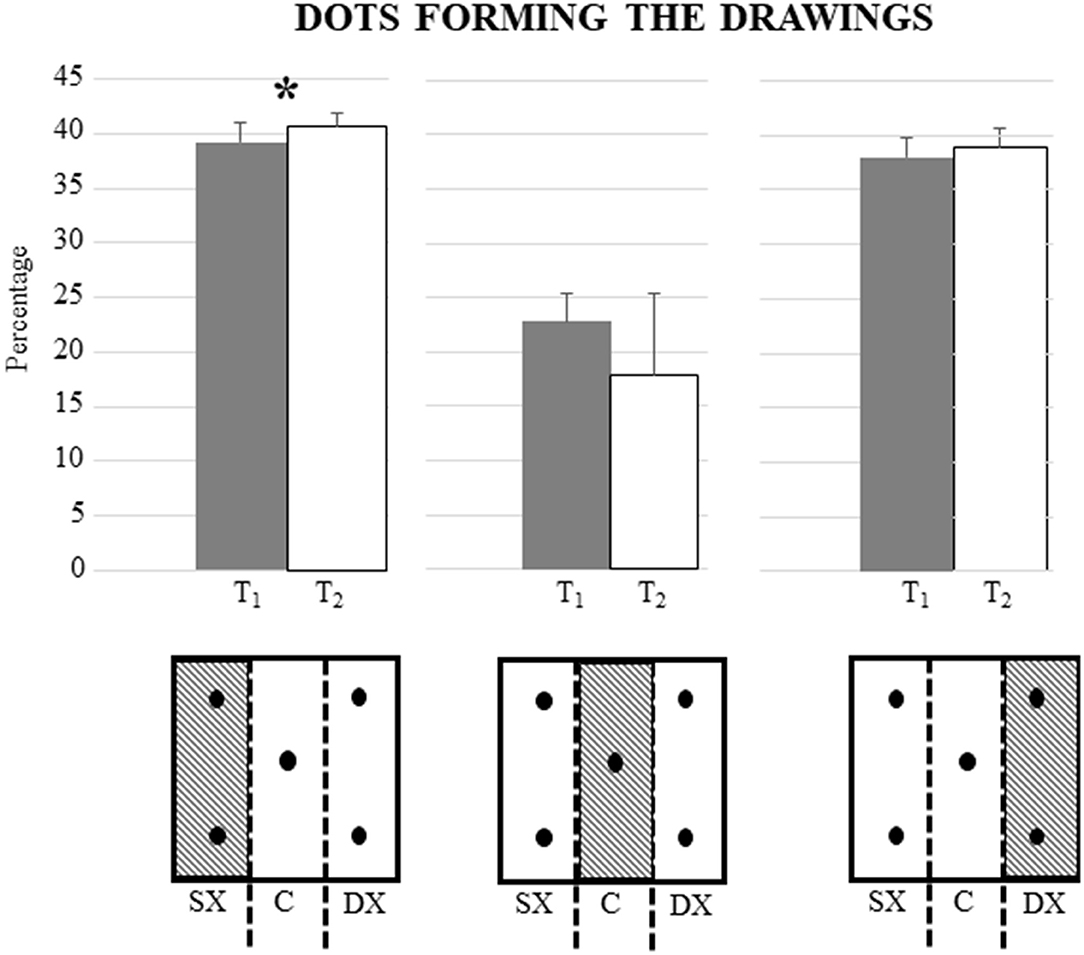
Figure 3. Differences in mean percentage of dots on the left, right, and middle of the boxes between the period before the COVID-19 lockdown (T1) and during the COVID-19 lockdown (T2). There is a significant difference only for the percentage of dots on the left the boxes forming the drawings (P = 0.01). Vertical bars indicate SD. In the lower part of the figure, there is a schematic representation of the division of the box into the left, middle, and right.
Among our 16 participants, only four were found to be alexithymic with an average score of 65. The rest of the group (12 students) obtained an average score of 53. Eight of these 12 students obtained an overall score that falls in the critical range of 50–60.
One-way ANOVA revealed a significant difference among three subscales (DDF, DIF, and EOT) [F(2, 45) = 18.44; P: 0.000]. Post hoc comparisons revealed that each subscale is different (DDF vs. DIF: P = 0.04; DDF vs. EOT: P = 0.000; DIF vs. EOT: P = 0.03).
To assess a potential relationship between the difficulty to identify and describe feelings, as well as externally oriented thinking, and the tendency of the m-FPT to shift to the left side at T2, a two-tailed Spearman correlation analysis was performed between the results of the TAS-20 and the increment of dots touched on the left at T2. Results showed a significant positive correlation between the bias to left and the TAS-20 (rS = 0.860, P = 0.000) (Figure 4). Then, correlations between the different TAS-20 subscales and bias to left in T2 were also conducted. Results showed significant correlations between the bias to the left and the DDF (rS = 0.796, P = 0.000) and the DIF (rS = 0.818, P = 0.000), while no significant correlation resulted when considering the EOT (rS = 0.144, P = 0.565).
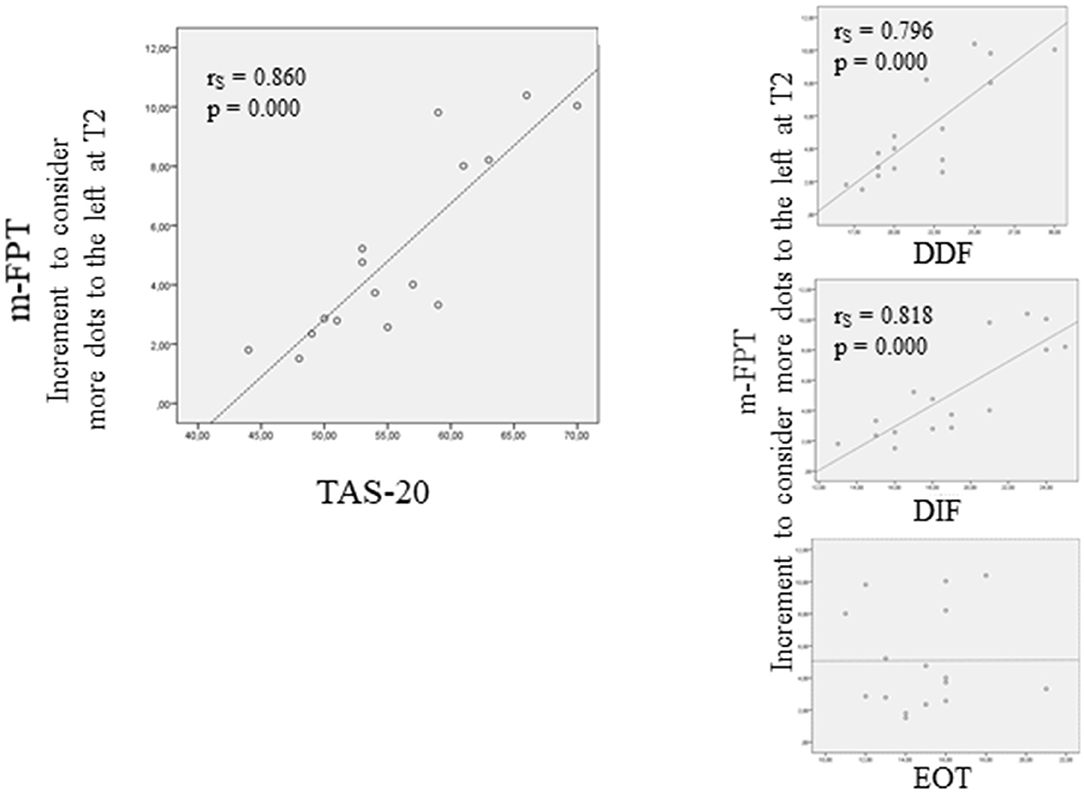
Figure 4. Correlation (Spearman rho) between and the tendency of the m-FPT to shift to the left side at T2 and the TAS-20. On the left side is shown the correlation between the difficulty to identify and describe feelings, as well as externally oriented thinking, and the increment of dots touched on the left at T2. On the right side of the figure is shown the correlations between the different TAS-20 subscales and bias to the left in T2.
To assess a potential relationship between the digits forward span and the left bias in m-FPT at T2, a two-tailed Spearman correlation analysis was performed. No significant correlation was evident (rS = −0.157, P = 0.561).
To assess a potential relationship between the difficulty to identify and describe feeling and to think externally and the worsening in span at T2, a two-tailed Spearman correlation analysis between the results of the TAS-20 and the worsening in span at T2 was performed. The results failed to show any significant correlation (rS = −0.161, P = 0.553). Similar results rose when the correlations were made between the different TAS-20 subscales and the worsening in span at T2 (DDF: rS = −0.110, P = 0.684; DIF: rS = −0.054, P = 0.440; EOT: rS = −0.208, P = 0.565) (Figure 4).
In the present study, we had the great opportunity to test executive functions in a group of students before and during the lockdown imposed by the Italian government, after roughly 40 days of enforced lockdown. This allowed us to compare their performances before (T1) and during the period of enforced behavioral restrictions (T2). In particular, we focused on the effects of the COVID-19 lockdown on non-verbal fluency abilities, which indirectly provide a measure of flexibility of the individual to react to spatial and environmental changes. To study this cognitive domain, we used the m-FPT, which allows us to detect a non-verbal measure of executive functioning and evaluates with a high degree of reliability the graphic figural fluency in a peripersonal space.
We observed that the period of behavioral restrictions determines an increase in spatial fluency ability. In fact, in T2, we see more drawings produced as compared to T1 (Figure 2A). This significant trend does not reflect itself in the other parameters evaluated, such as the error and strategy index (Figures 2B,C). A possible explanation could be that our sample is composed of typical-development students without cognitive deficits, as demonstrated by the normal values of the Raven matrices. Moreover, the overlapping scores of the Raven matrices in T1 and T2 suggest that the effects of behavioral restriction do not reflect a change in global cognitive functioning but, rather, concern specific cognitive domains. The worsening of the forward digits in T2 (Table 1) suggests that the verbal working memory could be specifically affected by the lockdown. A speculative remark might be based on the evidence that the working memory is strongly correlated to noradrenergic activity (Robertson, 2014), and living in a restricted environment, such as during a lockdown, might reduce activation and alertness and hence the noradrenergic tone, which in turn induces a worsening in the working memory performance. Unfortunately, since the second part of the test (T2) was carried out completely at a distance, we could not add the Corsi test (Spinnler and Tognoni, 1987) to the assessment of the spatial working memory, and hence, we could not investigate whether the worsening is specific for verbal working memory or also concerns the spatial domain.
However, the lockdown does not seem to have an effect on verbal fluency. The difference between the verbal domain (i.e., between verbal working memory which worsens during lockdown and verbal fluency which remains stable) could be explained by the fact that our participants are students and therefore continuously training verbal working memory by reading and repeating. Therefore, verbal fluency abilities continue to be exercised despite the lockdown imposing behavioral restrictions. In accordance with this hypothesis, several clinical evidences demonstrate that cognitive exercise increases the cognitive reserve (Stern, 2002; Mandolesi et al., 2017; Gelfo et al., 2018; Serra and Gelfo, 2019). In addition, it is to notice a worsening in forward digits test in T2 explainable for example with the fact that the social isolation determines an impairment in specific verbal tasks such as verbal fluency and backward digit span (Lara et al., 2019). This suggestion is also in accord with the “hemispheric activation model” (Kinsbourne, 1970; Bowers and Heilman, 1980), proposing that the distribution of attention in space is biased contralaterally to the more activated hemisphere. We can speculate that spatial processing activated the right hemisphere more strongly than the left language-dominant hemisphere. This resulted in attentional shifting attention toward the left hemispace.
Interestingly, in the m-FPT, there is a shift to the left side during the lockdown as compared to habitual living conditions (T1) (Figure 3). In fact, analyzing the percentage of dots touched on the right or left of each box of m-FPT, we observed an increment of the dots on the left of each box, suggesting thus an increment of pseudoneglect. These data are in agreement with a previous study conducted in the same experimental condition in which periods before and during COVID-19 lockdown are compared (Somma et al., 2021), which evidenced increased selective spatial attention to the left during lockdown in multiple peripersonal visuospatial tasks, such as cancellation task and a digitized version of the table radial arm maze task (Foti et al., 2020). The authors hypothesized that the stressful conditions experienced by the participants during the quarantine, as measured by the COVID-19 Student Stress Questionnaire (CSSQ; Zurlo et al., 2020), might have increased the activity of the right hemisphere attention networks (Somma et al., 2021), thus causing the observed leftward bias. Even more recently, in support of the hypothesis of greater activation of the right hemisphere in specific environmental conditions, Spreng et al. (2020) have observed consistent volumetric alterations in right inferior parietal and cingulo-opercular regions as well as in the dorsolateral prefrontal cortex in lonely individuals, thus supporting a neural model of loneliness. This evidence is in accordance with the hypothesis of an attentional right network driving spatial attention leftward (Gigliotta et al., 2017).
The greater involvement of the right hemisphere in conditions of behavioral restriction is compatible with the significant positive correlation between the tendency of the m-FPT to shift the attention to the left side and the score obtained by the TAS-20, which allows us to detect difficulties in identifying and describing feelings and in externally oriented thinking. Furthermore, by breaking down the TAS-20 into its three subscales and correlating them to the left increment in the m-FPT, we obtained positive correlations only for the difficulty to describe feelings and the difficulty to identify feelings subscales. These data suggest that the behavioral restriction determines a difficulty in the description and identification of feelings, which are mainly related to the cerebral networks of the right hemisphere. Constantly putting into action barrier gestures, such as disinfecting hands, wearing masks, and maintaining social distancing, might prevent people from fully focusing on the processing of feelings. Instead, the lack of correlation between the left increment in the m-FPT and the externally oriented thinking subscale could indicate that, even during the period of social restriction, one is oriented toward the outside and toward others precisely because humans are “ultra-social animals” (Tomasello, 2014).
In support of the idea that the spatial attentional bias is modulated by emotional states, we recall that patients with Parkinson's and individuals with post-traumatic stress disorder have pathological values at TAS-20, in fact being alexithymic (Salazar et al., 2019).
The strength is that this study is one of the few that investigated the effects of behavioral restriction in young people during the COVID-19 lockdown. However, there are at least three main limitations, which cannot be overcome but which warrant caution when interpreting the data.
Firstly, the low number of participants does not allow us to generalize completely the results. However, considering that the previous study by Somma et al. (2021) has analyzed a sample of almost 100 female students and found an increasing spatial pseudoneglect, we feel that a behavioral restriction of even a few days causes a leftward spatial attentional bias. Connected to this point, it should be noticed that m-FPT was not developed to study attentional disorders. However, the parameters we have added allow us to evaluate the shift in spatial attention. Another weakness concerns the limited cognitive evaluation made in the present study. We could not analyze all the cognitive domains because these subjects were tested in T1 for other purposes. With the COVID-19 lockdown, we continued to monitor these students to retest them (T2) with a similar modality. This meant that we could not use all the battery proposed in T1 also in T2.
The greatest drawback is that TAS-20 was administered only in T2. Not having the evaluation of the participants in T1, we cannot be sure in characterizing the emotional disorder as secondary alexithymia, although there are several evidences suggesting that relevant environmental factors and significative events occurring during the life determine secondary alexithymia (Messina et al., 2014).
In addition, the administration of TAS-20 only at T2 evidences a methodological issue. Surely, in a normal situation, rigor in research would have prevailed. However, being in a particular historical moment, we believe that any data and evidence must be documented and told.
The present data allow us to document that behavioral restrictions, even for a limited period of time, affects the processing of spatial information in terms of a left attention bias, allowing us to reflect on future intervention and support programs for other lockdowns, in which it will be necessary to study in depth and completely the effects on verbal and non-verbal cognitive functioning just as it will be more appropriate to consider the psychological effects that fall on emotional processing.
Future research must be directed toward the development of protocols capable of analyzing the effects of any lockdowns on all segments of the population, taking into account the different, and interrelated aspects that contribute to cognitive functioning, and must be directed toward the implementation of psychological support programs to avoid the onset of mental illness.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Local Ethics Committee of the University of Naples Federico II [Protocol number: 12/2020]. The participants provided their written informed consent to participate in this study.
AL, PS, AC, and OG tested participants. AL, PT, FL, and LM wrote the paper. All authors read, revised, and approved the final manuscript, designed research, and analyzed data and discussed data.
This research was supported by funding from the Foundation Jérôme Lejeune to L.M (N. 1567, 2016B) and from the Department of Humanities, University of Naples Federico II (Fondi ricerca dipartimentale 30% 2020 and 2021) to LM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Sara Iodice, Sara Telese, Maria Torromacco, Federica Somma, Antonietta Argiuolo, and Federica Vallone for participating in the recruitment of the sample. We sincerely thank all the students of the Department of Humanities of the Federico II University who underwent the test; without their contribution, this study would not have been possible.
Bagby, R. M., Taylor, G. J., Parker, J. D. A., and Loiselle, C. (1990). Cross-validation of the factor structure of the toronto alexithymia scale. J. Psychosomat. Res. 34, 47–51. doi: 10.1016/0022-3999(90)90007-Q
Bartolomeo, P., and Malkinson, T. S. (2019). Hemispheric lateralization of attention processes in the human brain. Curr. Opin. Psychol. 29, 90–96. doi: 10.1016/j.copsyc.2018.12.023
Boss, S., Galletta, D., Lowry, P. B., Moody, G. D., and Polak, P. (2015). What do systems users have to fear? Using fear appeals to engender threats and fear that motivate protective security behaviors. MIS Quarterly 39, 837–864. doi: 10.25300/MISQ/2015/39.4.5
Bowers, D., and Heilman, K. M. (1980). Pseudoneglect: effects of hemispace on a tactile line bisection task. Neuropsychologia 18, 491–498. doi: 10.1016/0028-3932(80)90151-7
Bressi, C., Taylor, G., Parker, J., Bressi, S., Brambilla, V., Aguglia, E., et al. (1996). Cross validation of the factor structure of the 20-item toronto alexithymia scale: an Italian multicenter study. J. Psychosomat. Res. 41, 551–559. doi: 10.1016/S0022-3999(96)00228-0
Brooks, S. K., Webster, R. K., Smith, L. E., Woodland, L., Wessely, S., Greenberg, N., et al. (2020). The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 395, 912–920. doi: 10.1016/S0140-6736(20)30460-8
Brown, K. M., Hoye, R., and Nicholson, M. (2012). Self-esteem, self-efficacy, and social connectedness as mediators of the relationship between volunteering and well-being. J. Soc. Service Res. 38, 468–483. doi: 10.1080/01488376.2012.687706
Cacioppo, J. T., and Hawkley, L. C. (2009). Perceived social isolation and cognition. Trends Cognit. Sci. 13, 447–454. doi: 10.1016/j.tics.2009.06.005
Carlesimo, G. A., Caltagirone, C., Gainotti, G., Fadda, L., Galassi, R., Lorusso, S., et al. (1996). The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 36, 378–384. doi: 10.1159/000117297
Castelli, L., Di Tella, M., Benfante, A., and Romeo, A. (2020). The Spread of COVID-19 in the Italian Population: anxiety, depression, and post-traumatic stress symptoms. Can. J. Psychiatr. 65, 731–732. doi: 10.1177/0706743720938598
Cattelani, R., Dal Sasso, F., Corsini, D., and Posteraro, L. (2011). The modified five-point test: normative data for a sample of Italian healthy adults aged 16–60. Neurol. Sci. 32, 595–601. doi: 10.1007/s10072-011-0489-4
Chieffi, S., Messina, G., Villano, I., Messina, A., Valenzano, A., Moscatelli, F., et al. (2017). Neuroprotective effects of physical activity: evidence from human and animal studies. Front. Neurol. 8:188. doi: 10.3389/fneur.2017.00188
Colcombe, S., and Kramer, A. F. (2003). Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol. Sci. 14, 125–130. doi: 10.1111/1467-9280.t01-1-01430
Corbetta, M., Patel, G., and Shulman, G. L. (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58, 306–324. doi: 10.1016/j.neuron.2008.04.017
Di Tella, M., and Castelli, L. (2013). Alexithymia e Fibromyalgia: clinical evidence. Front. Psychol. 4:909. doi: 10.3389/fpsyg.2013.00909
Di Tella, M., and Castelli, L. (2016). Alexithymia in chronic pain disorders. Curr. Rheumatol. Rep. 18:41. doi: 10.1007/s11926-016-0592-x
Di Tella, M., Castelli, L., Colonna, F., Fusaro, E., Toerta, R., Ardito, R. B., et al. (2015). Theory of mind and emotional functioning in fibromyalgia syndrome: an investigation of the relationship between social cognition and executive function. PLoS ONE 10:e0116542. doi: 10.1371/journal.pone.0116542
Eslinger, P. J., Warner, G. C., Grattan, L. M., and Easton, J. D. (1991). “Frontal lobe” utilization behavior associated with paramedian thalamic infarction. Neurology 41:450. doi: 10.1212/WNL.41.3.450
Evans, J. S. B. T. (2019). Reflections on reflection: the nature and function of type 2 processes in dual-process theories of reasoning. Think. Reas. 25, 383–415. doi: 10.1080/13546783.2019.1623071
Fernandez, A. L., Moroni, M. A., Carranza, J. M., Fabbro, N., and Lebowitz, B. K. (2009). Reliability of the five-point test. Clin. Neuropsychol. 23, 501–509. doi: 10.1080/13854040802279675
Foti, F., Sorrentino, P., Menghini, D., Montuori, S., Pesoli, M., Turriziani, P., et al. (2020). Peripersonal visuospatial abilities in williams syndrome analyzed by a table radial arm maze task. Front. Human Neurosci. 14:254. doi: 10.3389/fnhum.2020.00254
Freyberger, H. (1977). Supportive psychotherapy tecniques in primary and secondary alexithymia. Psichother. Psychosom. 28, 337–342. doi: 10.1159/000287080
Gelfo, F., Mandolesi, L., Serra, L., Sorrentino, G., and Caltagirone, C. (2018). The neuroprotective effects of experience on cognitive functions: evidence from animal studies on the neurobiological bases of brain reserve. Neuroscience 370, 218–235. doi: 10.1016/j.neuroscience.2017.07.065
Gigliotta, O., Malkinson, T. S., Miglino, O., and Bartolomeo, P. (2017). Pseudoneglect in visual search: Behavioral evidence and connectional constraints in simulated neural circuitry. Eneuro 4:ENEURO.0154-17. doi: 10.1523/ENEURO.0154-17.2017
Grego, F., Vallier, J. M., Collardeau, M., Rousseu, C., Cremieux, J., and Brisswalter, J. (2005). Influence of exercise duration and hydration status on cognitive function during prolonged cycling exercise. Int. J. Sports Med. 26, 27–33. doi: 10.1055/s-2004-817915
Hötting, K., and Röder, B. (2013). Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 37, 2243–2257. doi: 10.1016/j.neubiorev.2013.04.005
Kinsbourne, M. (1970). A model for the mechanism of unilateral neglect of space. Am. Neurol Ass. 95, 143–146.
Lara, E., Caballero, F. F., Rico-Uribe, L. A., Olaya, B., Haro, J. M., Ayuso-Mateos, J. L., et al. (2019). Are loneliness and social isolation associated with cognitive decline? Int. J. Geriatr. Psychiatr. 34, 1613–1622. doi: 10.1002/gps.5174
Lardone, A., Sorrentino, P., Giancamilli, F., Palombi, T., Simper, T., Mandolesi, L., et al. (2020). Psychosocial variables and quality of life during the COVID-19 lockdown: a correlational study on a convenience sample of young Italians. PeerJ 8:e10611. doi: 10.7717/peerj.10611
Mandolesi, L., Gelfo, F., Serra, L., Montuori, S., Polverino, A., Curcio, G., et al. (2017). Environmental factors promoting neural plasticity: insights from animal and human studies. Neural Plasticity 2017:7219461. doi: 10.1155/2017/7219461
Mandolesi, L., Polverino, A., Montuori, S., Foti, F., Ferraioli, G., Sorrentino, P., et al. (2018). Effects of physical exercise on cognitive functioning and wellbeing: biological and psychological benefits. Front. Psychol. 9:509. doi: 10.3389/fpsyg.2018.00509
Mazza, C., Ricci, E., Biondi, S., Colasanti, M., Ferracuti, S., Napoli, C., et al. (2020). A nationwide survey of psychological distress among italian people during the COVID-19 pandemic: immediate psychological responses and associated factors. Int. J. Environ. Res. Public Health 17:3165. doi: 10.3390/ijerph17093165
Messina, A., Beadle, J. N., and Paradiso, S. (2014). Towards a classification of alexithymia: primary, secondary and organic. J. Psychopathol. 20, 38–49. doi: 10.1055/s-0042-111913
Montuori, S., D'Aurizio, G., Foti, F., Liparoti, M., Lardone, A., Pesoli, M., et al. (2019). Executive functioning profiles in elite volleyball athletes: preliminary results by a sport-specific task switching protocol. Human Movement Sci. 63, 73–81. doi: 10.1016/j.humov.2018.11.011
Nemiah, J. C., and Sifneos, P. E. (1970). “Affect and fantasy in patients whit psychosomatic disorders,” in Modern Trends in Psychosomatic Medicine, ed O. Hill (London: Butterworths), 26–34.
Orsini, A., Grossi, D., Capitani, E., Laiacona, M., Papagno, C., and Vallar, G. (1987). Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Italian J. Neurol. Sci. 8, 537–548. doi: 10.1007/BF02333660
Pera, A. (2020). Cognitive, behavioral, and emotional disorders in populations affected by the COVID-19 outbreak. Front. Psychol. 11:2263. doi: 10.3389/fpsyg.2020.02263
Pereira, A. C., Huddleston, D. E., Brickman, A. M., Sosunov, A. A., Hen, R., McKhann, G. M., et al. (2007). An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 104, 5638–5643. doi: 10.1073/pnas.0611721104
Pisano, F., Giachero, A., Rugiero, C., Calati, M., and Marangolo, P. (2020). Does COVID-19 impact less on post-stroke aphasia? This is not the case. Front. Psychol. 30:564717. doi: 10.3389/fpsyg.2020.564717
Regard, M., Strauss, E., and Knapp, P. (1982). Children's production on verbal and non-verbal fluency tasks. Perceptual Motor Skills 55, 839–844. doi: 10.2466/pms.1982.55.3.839
Reynolds, D. L., Garay, J. R., Deamond, S. L., Moran, M. K., Gold, W., and Styra, R. (2008). Understanding, compliance and psychological impact of the SARS quarantine experience. Epidemiol. Infect. 136, 997–1007. doi: 10.1017/S0950268807009156
Robertson, I. H. (2014). A right hemisphere role in cognitive reserve. Neurobiol. Aging 35, 1375–1385. doi: 10.1016/j.neurobiolaging.2013.11.028
Salazar, R. D., Weizenbaum, E., Ellis, T. D., Earhart, G. M., Ford, M. P., Dibble, L. E., et al. (2019). Predictors of self-perceived stigma in Parkinson's disease. Parkinsonism Related Disord. 60, 76–80. doi: 10.1016/j.parkreldis.2018.09.028
Serra, L., and Gelfo, F. (2019). What good is the reserve? A translational perspective for the managing of cognitive decline. Neural Regeneration Res. 14:1219. doi: 10.4103/1673-5374.251328
Sherbourne, C. D., and Stewart, A. L. (1991). The MOS social support survey. Soc. Sci. Med. 32, 705–714. doi: 10.1016/0277-9536(91)90150-B
Somma, F., Bartolomeo, P., Vallone, F., Argiuolo, A., Cerrato, A., et al. (2021). Further to the left. Stress-induced increase of spatial pseudoneglect during the COVID-19 lockdown. Front. Psychol. 2020:954 doi: 10.31234/osf.io/xb954
Spalletta, G., Pasini, A., Costa, A., De Angelis, D., Ramundo, N., Paolucci, S., et al. (2001). Alexithymic features in stroke: effects of laterality and gender. Psychosom Med. 63, 944–950. doi: 10.1097/00006842-200111000-00013
Spinnler, H., and Tognoni, G. (1987). Taratura e standardizazione italiana di test neuropsicologici. Italian Neurol. Sci. 7, 1–19.
Spreng, R. N., Dimas, E., Mwilambwe-Tshilobo, L., Dagher, A., Koellinger, P., Nave, G., et al. (2020). The default network of the human brain is associated with perceived social isolation. Nat. Commun. 11:6393. doi: 10.1038/s41467-020-20039-w
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. doi: 10.1017/S1355617702813248
Taylor, G. J., Bagby, R. M., and Parker, J. D. (1992). The revised toronto alexithymia scale: some reliability, validity, and normative data. Psychother. Psychosomat. 57, 34–41. doi: 10.1159/000288571
Taylor, G. J., Bagby, R. M., and Parker, J. D. A. (1997). Disorders in Affect Regulation: Alexithymia in Medical and Psychiatric Illness. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511526831
Tilvis, R. S., Pitkala, K. H., Jolkkonen, J., and Strandberg, T. E. (2000). Social networks and dementia. Lancet 356, 77–78. doi: 10.1016/S0140-6736(05)73414-0
Tomasello, M. (2014). The ultra-social animal. Eur. J. Soc. Psychol. 44, 187–194. doi: 10.1002/ejsp.2015
Wilson, R. S., Krueger, K. R., Arnold, S. E., Schneider, J. A., Kelly, J. F., Barnes, L. L., et al. (2007). Loneliness and risk of Alzheimer disease. Arch. General Psychiatr. 64, 234–240. doi: 10.1001/archpsyc.64.2.234
Winter, B., Breitenstein, C., Mooren, F. C., Voelker, K., Fobker, M., Lechtermann, A., et al. (2007). High impact running improves learning. Neurobiol. Learn. Memory 87, 597–609. doi: 10.1016/j.nlm.2006.11.003
Xiong, J., Lipsitz, O., Nasri, F., Lui, L., Gill, H., Phan, L., et al. (2020). Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J. Affect. Disord. 277, 55–64. doi: 10.1016/j.jad.2020.08.001
Keywords: executive function, attention, cognition, coronavirus, quarantine, pandemic
Citation: Lardone A, Turriziani P, Sorrentino P, Gigliotta O, Chirico A, Lucidi F and Mandolesi L (2021) Behavioral Restriction Determines Left Attentional Bias: Preliminary Evidences From COVID-19 Lockdown. Front. Psychol. 12:650715. doi: 10.3389/fpsyg.2021.650715
Received: 07 January 2021; Accepted: 01 March 2021;
Published: 09 April 2021.
Edited by:
Sara Bottiroli, Giustino Fortunato University, ItalyReviewed by:
Lorys Castelli, University of Turin, ItalyCopyright © 2021 Lardone, Turriziani, Sorrentino, Gigliotta, Chirico, Lucidi and Mandolesi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mandolesi, bGF1cmEubWFuZG9sZXNpQHVuaW5hLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.