
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 20 April 2021
Sec. Psychology for Clinical Settings
Volume 12 - 2021 | https://doi.org/10.3389/fpsyg.2021.633959
This article is part of the Research Topic Psychological Sleep Studies: New Insights to Support and Integrate Clinical Practice Within the Healthcare System View all 14 articles
Objective: Previous research suggests a positive association between pain, depression and sleep. In this study, we investigate how sleep correlates with varying levels of pain and depression in nursing home (NH) patients with dementia.
Materials and methods: Cross-sectional study (n = 141) with sleep-related data, derived from two multicenter studies conducted in Norway. We included NH patients with dementia according to the Mini-Mental State Examination (MMSE ≤ 20) from the COSMOS trial (n = 46) and the DEP.PAIN.DEM trial (n = 95) whose sleep was objectively measured with actigraphy. In the COSMOS trial, NH patients were included if they were ≥65 years of age and with life expectancy >6 months. In the DEP.PAIN.DEM trial, patients were included if they were ≥60 years and if they had depression according to the Cornell Scale for Depression in Dementia (CSDD ≥ 8). In both studies, pain was assessed with the Mobilization-Observation-Behavior-Intensity-Dementia-2 Pain Scale (MOBID-2), and depression with CSDD. Sleep parameters were total sleep time (TST), sleep efficiency (SE), sleep onset latency (SOL), wake after sleep onset (WASO), early morning awakening (EMA), daytime total sleep time (DTS) and time in bed (TiB). We registered use of sedatives, analgesics, opioids and antidepressants from patient health records and adjusted for these medications in the analyses.
Results: Mean age was 86.2 years and 76.3% were female. Hierarchical regressions showed that pain was associated with higher TST and SE (p < 0.05), less WASO (p < 0.01) and more DTS (p < 0.01). More severe dementia was associated with more WASO (p < 0.05) and TiB (p < 0.01). More severe depression was associated with less TST (p < 0.05), less DTS (p < 0.01) and less TiB (p < 0.01). Use of sedative medications was associated with less TiB (p < 0.05).
Conclusion: When sleep was measured with actigraphy, NH patients with dementia and pain slept more than patients without pain, in terms of higher total sleep time. Furthermore, their sleep efficiency was higher, indicating that the patients had more sleep within the time they spent in bed. Patients with more severe dementia spent more time awake during the time spent in bed. Furthermore, people with more severe depression slept less at daytime and had less total sleep time Controlling for concomitant medication use did not affect the obtained results.
Approximately 50 million people are living with dementia worldwide, and every year 10 million new cases are registered (World Health Organization [WHO], 2019). Sleep disturbances, like difficulties in initiating sleep, frequent awakenings, excessive daytime sleepiness and nighttime wandering are often seen in people with dementia (McCleery et al., 2016; Webster et al., 2020a). However, to estimate the prevalence of sleep disturbances in people with dementia is difficult due to differences in assessment methods and because of the patients’ reduced communicative ability (Blytt et al., 2017).
A recent systematic review and meta-analysis conducted by Webster et al. (2020b) found a 20% pooled prevalence of clinically significant sleep disturbances in people with dementia and a 70% prevalence of sleep disturbances as measured with actigraphy. Furthermore, Webster et al. (2020a) found that sleep disturbances negatively affect physical and psychological well-being in people with dementia. In the NH setting, sleep disturbances also caused disruption for other patients and increased stress among staff (Webster et al., 2020a). Previously conducted studies have found that sleep disturbances in dementia are associated with reduced quality of life and impaired daytime functioning (Kyle et al., 2010), increased family burden and earlier nursing home (NH) admission, thereby increasing the social and economic costs for society (McCrae et al., 2016; Livingston et al., 2017).
Pain is a subjective experience that is difficult to assess for people with dementia, due to reduced capacity to give valid self-report (Blytt et al., 2017). When assessed by proxy-rater measurements, moderate to severe pain is found in approximately 40–60% of NH patients with dementia (Achterberg et al., 2010; Hemmingsson et al., 2018; Wagatsuma et al., 2020). Reduced quality of life, increased agitation and anxiety are all associated with pain in NH patients (Ballard et al., 2009). In addition, approximately 50% of people with dementia experience symptoms of depression – a condition that may reduce their quality of life and accelerate the decline in cognition and daily functioning (Enache et al., 2011).
Previous research has found a reciprocal relationship between pain and depression in people without dementia (Bair et al., 2003; Goldenberg, 2010; Kroenke et al., 2011). This relationship is known as the “pain-depression dyad,” indicating that the conditions often coexist, respond to similar treatment and share common signal pathways (Chopra and Arora, 2014). In NH patients, Erdal et al. (2017) found that reduced pain intensity was associated with subsequent reduction of depressive symptoms, regardless of analgesic or antidepressant use. These associations were found in patients with mild, moderate and severe cognitive impairment. However, results on the primary outcome measures
in an analgesic intervention trial did not support a direct causal relationship between pain and increased depression in NH patients with dementia (Erdal et al., 2018).
There is also considerable evidence for a reciprocal relationship between pain and sleep in people without dementia. Previous research suggests that poor sleep may affect the perception of pain (Sivertsen et al., 2015), but also that pain in itself can be a possible cause of poor sleep (Sivertsen et al., 2015; Flo et al., 2017). There is some evidence suggesting that depression may be a central underlying mechanism in the relationship between pain and sleep in people without cognitive impairment (Ravyts et al., 2018). Furthermore, pain may lead to depression, which in turn may affect the patient’s sleep (Nicassio et al., 2012; Flo et al., 2017). By extension people with dementia and depression may be lying in bed, experiencing trouble falling asleep or maintaining sleep because they are in pain (Blytt, 2019). However, there is still a lack of evidence regarding this relationship in people with dementia (Flo et al., 2017).
To our knowledge, there are no previous studies investigating how sleep is associated with pain and depression in NH patients with dementia. Given the frequency and clinical implications of sleep disturbances for the patients, co-residents and staff, new knowledge regarding the relationship between pain, depression and sleep in this patient group is valuable. Consequently, we aimed to investigate the association between sleep, pain, depression and dementia in NH patients. We hypothesized that a) more pain was associated with poorer sleep; b) more severe dementia was associated with poorer sleep; and c) more severe depression was associated with poorer sleep. Because medical treatment for pain, depression and sleep disorders may also impact sleep parameters, analyses were adjusted for the use of analgesic, antidepressant and sedative-hypnotic drugs.
The study was based on sleep-related data derived from two independent multicenter studies conducted in Norway: the COSMOS trial (n = 46) and the DEP.PAIN.DEM trial (n = 95). The COSMOS (Communication, Systematic pain assessment and treatment, Medication review, Organization of activities, and Safety) trial is a multicenter cluster-randomized controlled trial over 4 months with follow-up at month 9. The study was conducted from January 2014 to December 2015. It aimed to improve the quality of life among NH patients through a multicomponent intervention (Husebo et al., 2015). A total of 765 patients were invited to participate in the COSMOS study, and 545 participants from 67 NH units were included (Husebø et al., 2019). The COSMOS trial used the following inclusion and exclusion criteria: NH patients ≥65 years of age and life expectancy >6 months.
The DEP.PAIN.DEM trial is a multicenter, placebo-controlled randomized clinical trial over 13 weeks. The study was conducted in Norway from August 2014 to September 2016. The main aim of the DEP.PAIN.DEM trial was to investigate the efficacy of pain treatment on depression in people with dementia and depression. Patients were included if they were long-term NH patients ≥60 years with >4 week stay at inclusion, dementia according to the Mini-Mental State Examination (MMSE score ≤ 20) and depression according to the Cornell Scale for Depression in Dementia (CSDD score ≥ 8). The sleep subproject of DEP.PAIN.DEM included 106 patients from 47 NHs (Blytt et al., 2018a).
Patients with Parkinson disorder or any form of paralysis in the arms or upper body were excluded from actigraphy registrations. In the present study, we included patients who had valid actigraphy measurements and dementia according to MMSE (≤20) from both the DEP.PAIN.DEM and the COSMOS trials, which implies that we used data collected at baseline (week 0) before any intervention was conducted.
In total, 141 patients from the COSMOS (n = 46) and the DEP.PAIN.DEM (n = 95) trials had valid actigraphy measurements and dementia according to MMSE and were included in the present study. Some of the respondents did not have complete datasets for all variables (see Table 1 for the number of observations for the different variables). Mean age was 86.2 years and 76.3% were female.
In the data collection process, the patient’s medical decision-making capacity was discussed with the patient’s primary nurse at the NH. Efforts were made to adjust the information for patients who had reduced capacity to give consent (MMSE score from 16 to 19). In addition, the researchers telephoned all the eligible patients’ legal guardians. If the legal guardians gave presumed consent on behalf of the patient, they received written and oral information together with a consent form that they signed and mailed back. The DEP.PAIN.DEM study was approved by the Regional Ethics Committee (REC-West 2013/1474). The study’s clinical trial number is NCT02267057. The COSMOS trial was also approved by the Regional Committee for Medical and Health Research Ethics, West Norway (REK 2013/1765) and registered at www.clinicaltrials.gov (NCT02238652).
In both studies, sleep was measured with Actiwatch Spectrum (Philips Respironics) for seven consecutive days.
NH patients may be quite inactive and in order to enhance the possibility that movement would be detected we placed the actigraphs on the patients’ dominant wrist. Prior studies have found no discrepancy between data gathered from actigraphs placed on different locations (Sadeh et al., 1994; Jean-Louis et al., 1997). To enable better scoring of the actual time patients spent in bed, NH staff was instructed to register bedtimes and rise times by pushing the event button on the actigraph (light off at night and light on in the morning). Instructions were given both verbally and on a written instruction that was placed above the nightstand in the patients’ rooms and in the call room.
The Actiware 6 (Respironics) scoring program and validated algorithm was used for sleep/wake scoring. An actigraph consists of an accelerometer and memory storage (Sivertsen et al., 2006). Based on differences in movements associated with wakefulness and sleep, an estimate of sleep-wake schedules is provided (Sivertsen et al., 2006). We used medium sensitivity since this is most commonly used., and sleep/waking status was determined for each one-minute epoch. A skilled technician scored all activity protocols. A standardized hierarchical approach was applied to set rest intervals for the actigraphy data, using a) event markers when possible; or b) light and activity data; or c) light or activity data. If there was clear differentiation between active and rest periods, alternatives b) and c) were implemented. If there was no such clear differentiation, the actigraphy protocol was excluded. In the main part of the DEP.PAIN.DEM study, 162 patients were enrolled (Erdal et al., 2018). However, 7 patients had Parkinson’s disease, 11 did not consent to wear an actigraph, 8 patients removed the actigraph and 30 were excluded for other reasons (missing data, malfunctioning actigraphs, etc.) and were thus excluded in the actigraph sub-project. As a result, 106 were included in the actigraph subproject in the DEP.PAIN.DEM -study. 95 of these patients had valid MMSE measurements and were thus included in the present study. In the COSMOS study the actigraphy subproject included 107 patients, 24 of whom were excluded due to actigraph malfunction or because of missing data. Of these 46 had dementia according to the MMSE measurements and were thus included in the final sample. The following parameters were derived from actigraphy measurements: total sleep time (TST, min), time in bed (TiB, hrs:min), sleep efficiency (SE,% [TST/TiB]∗100), sleep onset latency (SOL, min), wake after sleep onset (WASO, min), early morning awakening (EMA, min) and day time total sleep time (DTS, min). DTS is a measure of sleep during daytime (from light on in the morning to lights off at night) and TST is a measure of sleep during nighttime (from light off in the night to lights on in the morning).
We used the validated CSDD to measure depression (Barca et al., 2010). The CSDD consists of 19 items assessing five domains of depression (mood, behavioral disturbances, physical signs, cyclic functions and ideational disturbances). A cut-off point of 8/9 has demonstrated the best accuracy for diagnosing depression, ≥11 corresponds with severe depression according to the ICD-10 criteria (Barca et al., 2010). We used the following cut-offs in classifying the categorical variables: no depression = CSDD ≤ 6, moderate depression = 7 ≤ CSDD ≤ 10, severe depression = CSDD ≥ 11 (Barca et al., 2010).
Cognitive function was assessed using the validated MMSE (Perneczky et al., 2006). The MMSE is a cognitive screening test with a 30-point scale consisting of 20 tasks, which was developed to differentiate potential dementia from normal functioning. In the present study, we used the following cut-offs: a score from 0 to 10 represents severe dementia, a score from 11 to 20 represents moderate dementia, a score from 21 to 25 represents mild cognitive impairment, and a score from 26 to 30 represents no dementia (Perneczky et al., 2006).
To measure pain, we used the Mobilization-Observation-Behavior-Intensity-Dementia-2 Pain Scale (MOBID-2), a validated staff-administered instrument (Husebo et al., 2010). The instrument provides a total score based on observations ranging from 0 to 10, in which 10 represents the worst possible pain. In line with previous research, a cut-off of ≥3 was used to indicate clinically relevant pain (Husebo et al., 2007, 2014).
Use of the following medications was registered based on daily prescriptions registered in the patient health records: N02A (opioids), N02B (other analgesics), N05C (hypnotics and sedatives) and N06A (antidepressants). Patients were registered categorically as users or non-users within each ATC group.
Descriptive statistics (means, standard deviations and percentages) were calculated for the full sample (Table 1) and for the DEP.PAIN.DEM dataset and the COSMOS dataset separately (Table 2). In addition, we conducted independent samples t-tests to investigate if there were any statistically significant differences in relevant characteristics between the respondents in the two studies. Sleep characteristics were calculated for the full sample, for patients with and without pain as measured with MOBID-2, for patients with different degrees of depression according to the CSDD, and with different degrees of cognitive function according to the MMSE. We also conducted independent samples t-tests to investigate if there were any statistically differences between any of the different sleep parameters regarding these categories (pain/no pain, moderate dementia/severe dementia and no depression/moderate depression versus severe depression, Table 3).
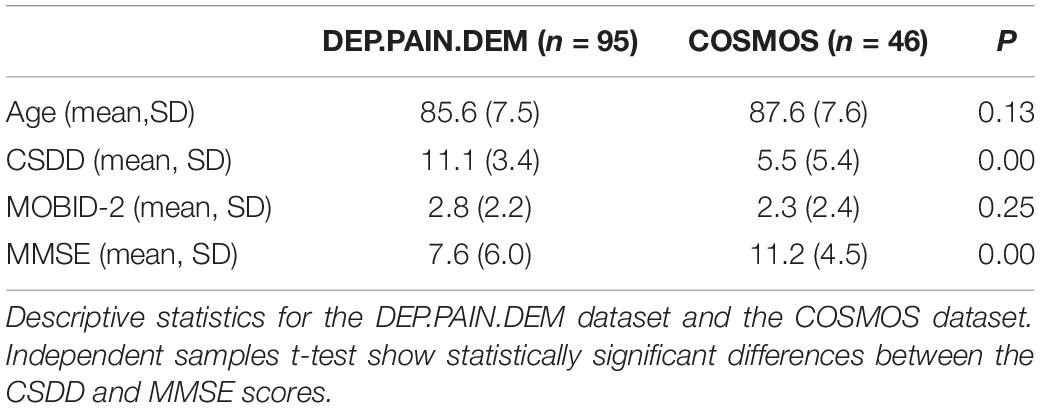
Table 2. Descriptive statistics for age and CSDD, MOBID-2, and MMSE scores, comparing data from the DEP.PAIN.DEM and COSMOS studies.
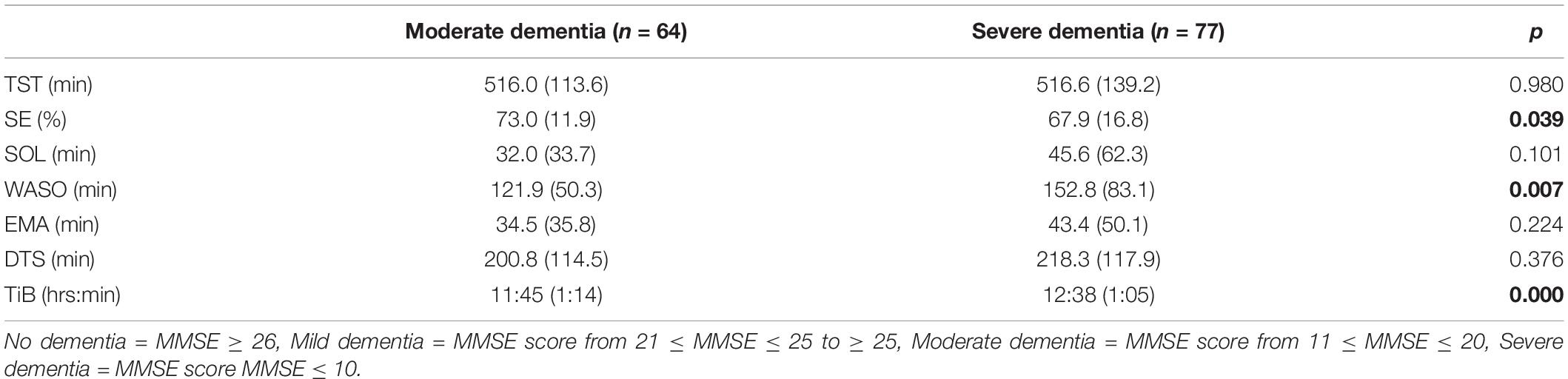
Table 3. Panel D: Sleep outcomes for patients with different levels of dementia, n = 141 (mean, SD).
Two-step hierarchical multiple regression were conducted to investigate how the different sleep parameters (total sleep time, sleep efficiency, sleep onset latency, wake after sleep onset, early morning awakening, time in bed and daytime total sleep time) correlated with varying levels of pain, depression and dementia in NH patients (see Table 4). Multicollinearity tests using variance inflation factor showed no severe correlation between independent variables that warrant corrective measures These variables were entered in the first step of the hierarchical regressions. In the second step, we adjusted for the use of analgesics, antidepressants, sedative-hypnotics and opioids. These variables were implemented in the regression models to investigate their potential effect on the associations between the main variables of interest. We used categorical variables with the cut-off points described above for pain, depression and dementia. In addition, we include age and gender in both steps. We regarded p < 0.05 as statistically significant in all analyses.
Table 4. hierarchical regression models for various sleep parameters, with and without adjusting for medication use.
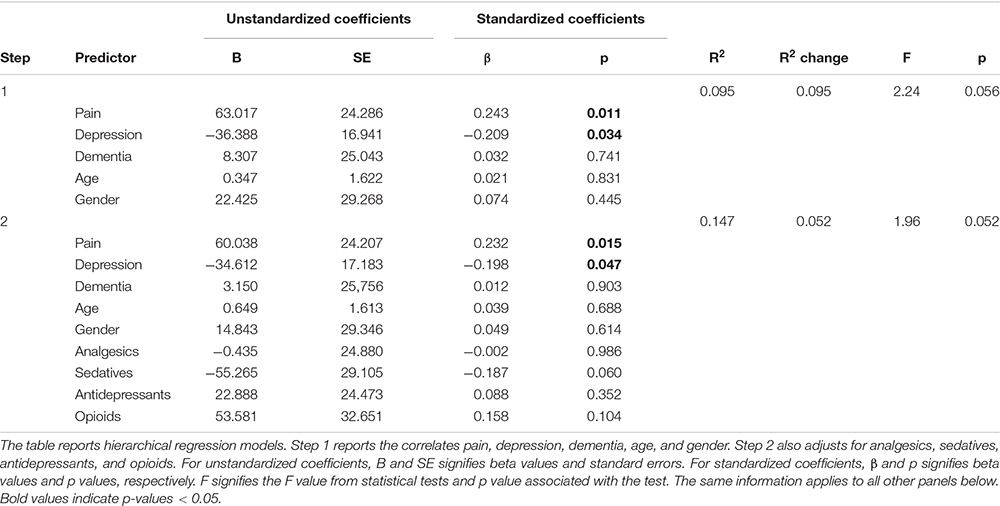
Table 4. Panel A: Hierarchical regression models predicting total sleep time (TST) with and without adjusting for medication use.
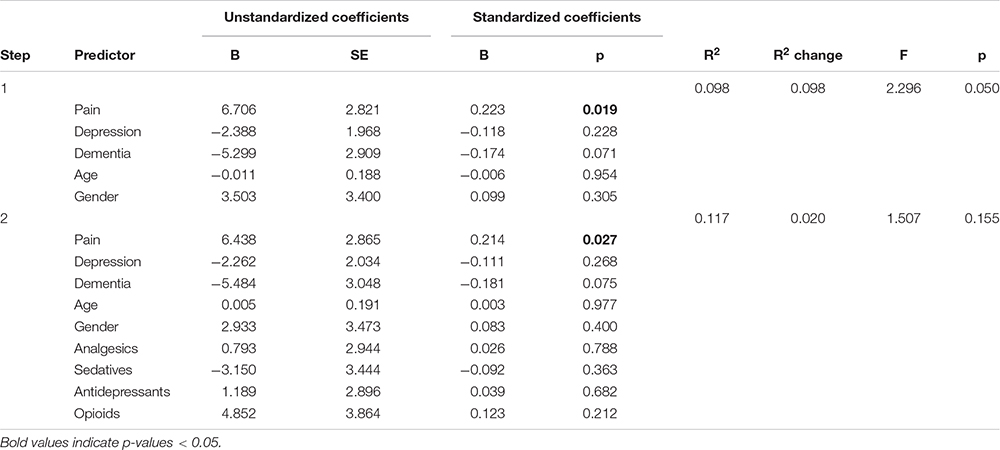
Table 4. Panel B: Hierarchical regression models predicting sleep efficiency (SE) with and without adjusting for medication use.
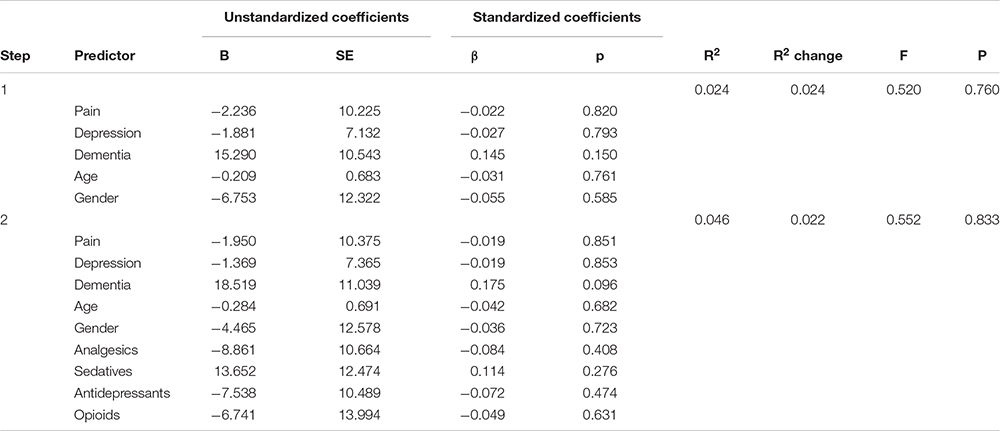
Table 4. Panel C: Hierarchical regression models predicting sleep onset latency (SOL) with and without adjusting for medication use.
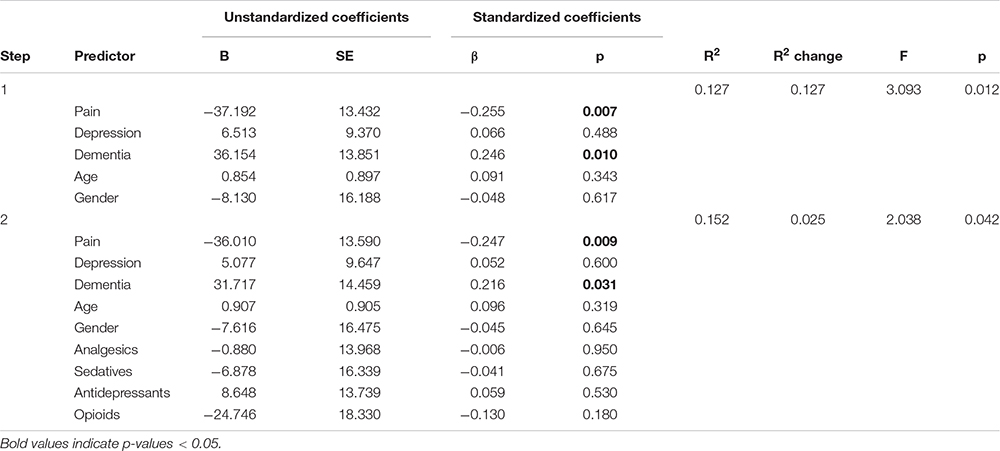
Table 4. Panel D: Hierarchical regression models predicting wake after sleep onset (WASO) with and without adjusting for medication use.
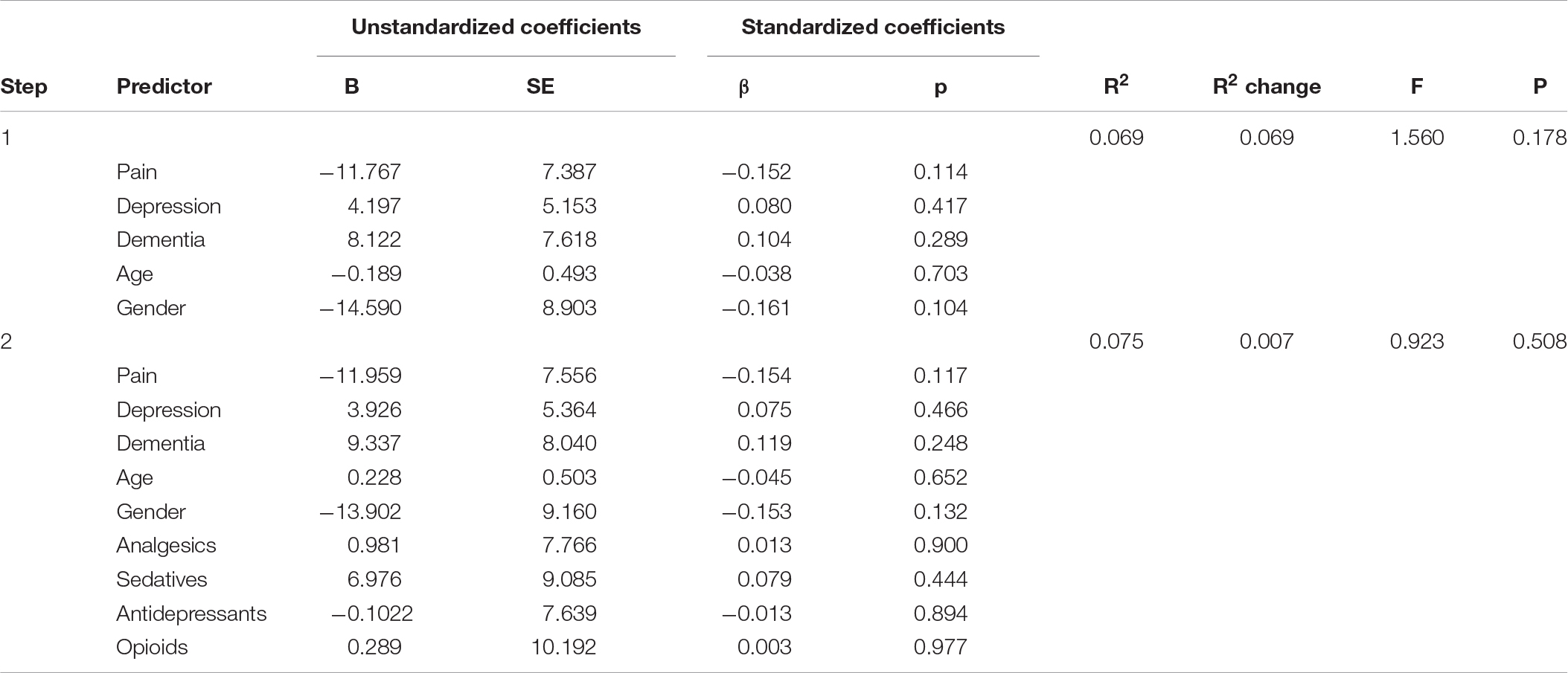
Table 4. Panel E: Hierarchical regression models predicting early morning awakening (EMA) with and without adjusting for medication use.
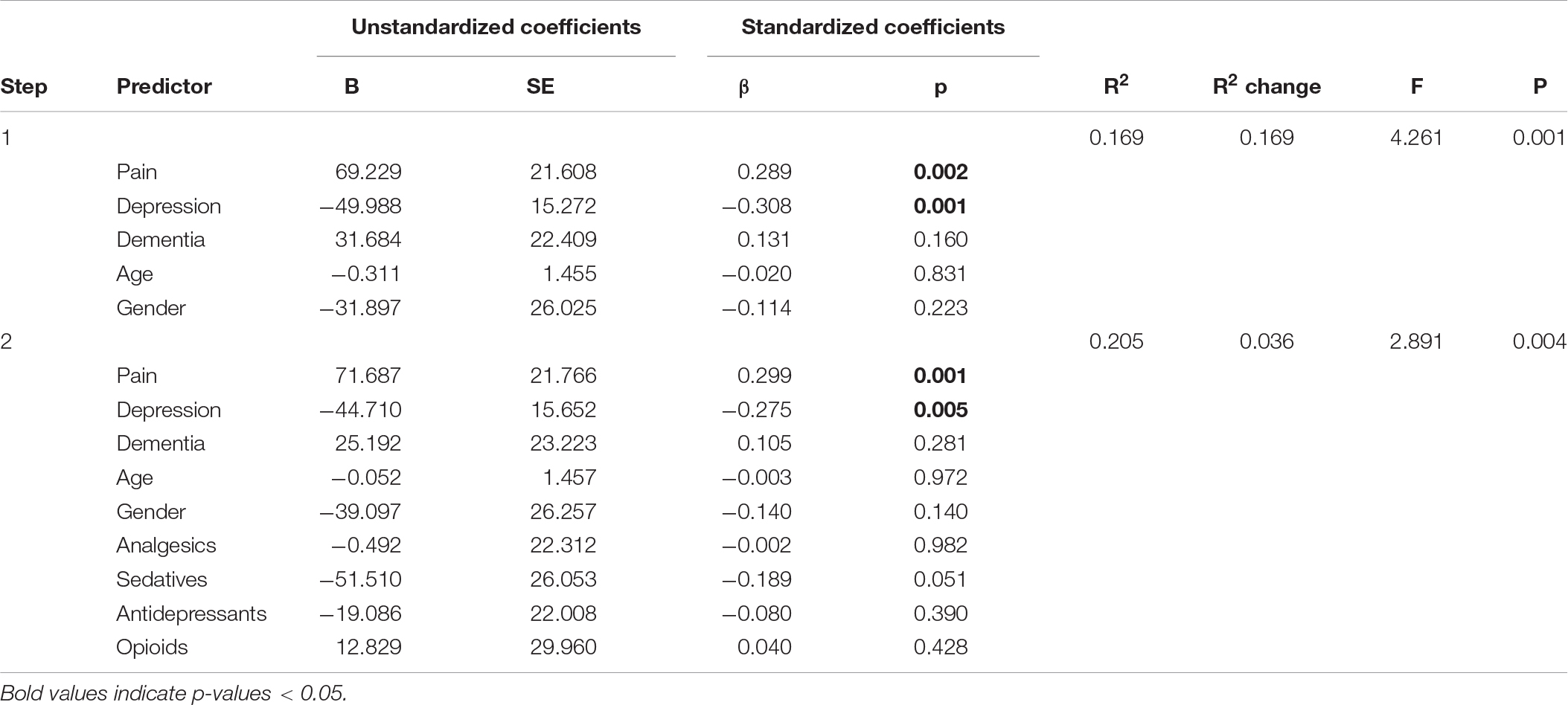
Table 4. Panel F: Hierarchical regression models predicting daytime total sleep time (DTS) with and without adjusting for medication use.
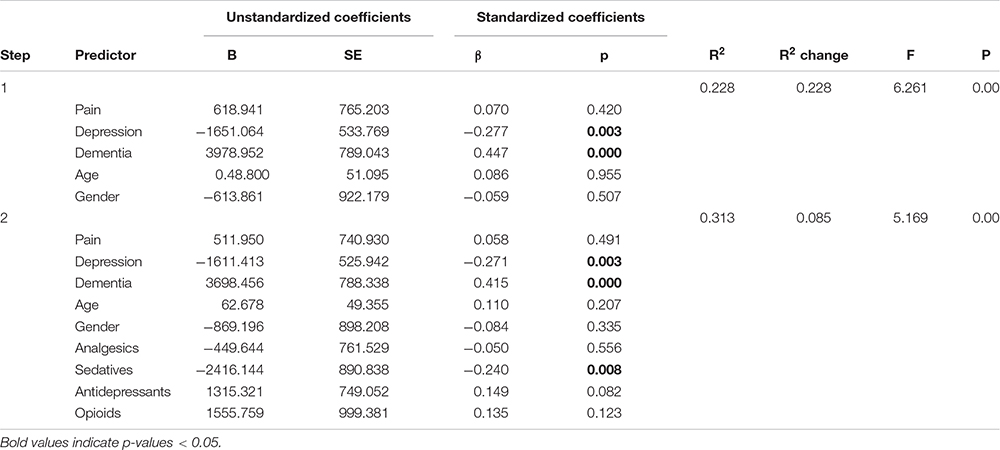
Table 4. Panel G: Hierarchical regression models with and without predicting time in bed (TiB) adjusting for medication use.
Mean age in the total sample was 86.2 years and 76.3% were female (see Table 1 for the number of observations for the different variables). The mean CSDD, MOBID-2, and MMSE scores were 9.5 (SD 4.7), 2.6 (SD 2.3), and 8.8 (SD 5.8), respectively. The patients from the DEP.PAIN.DEM trial had significantly higher depression and lower MMSE mean scores than the patients in the COSMOS trial (Table 2). In the total sample, 46.8% of the patients were prescribed non-opioid analgesics, 27.7% were prescribed sedative-hypnotics, 47.5% were prescribed antidepressants and 17.7% were prescribed opioids (see Table 5). Table 4 reports sleep characteristics for the full sample (Panel A), for those with and without pain (Panel B), for different levels of depression (Panel C), and for various levels of cognitive impairment (Panel D).
We comment on significant associations between the predictors and the dependent variables for all models that are reported in Table 4, but note that some of the regression models are not statistically significant. The hierarchical regression predicting TST showed that being in pain was associated with more TST, while more severe depression was associated with less TST (Table 4, Panel A). The regression predicting SE showed that being in pain was associated with higher SE (Table 4, Panel B). There were no significant results in the regression predicting SOL (Table 4, Panel C). The linear regression predicting WASO (Table 4, Panel D) showed that more severe dementia and pain were both associated with more WASO. The regression predicting EMA showed no significant results (Table 4, Panel E). The regression predicting DTS showed that being in pain was associated with more DTS and that higher depression scores were associated with less DTS. The final regression predicting TiB showed that more severe dementia was associated with more TiB. More severe depression was associated with less TiB (Table 4, Panel G).
In the second step of the hierarchical regressions, we adjusted for the use of analgesics, antidepressants, sedative-hypnotics and opioids. In all the regressions, the results reported above held when adjusting for the use of these medications. In addition, we noted that in the regression predicting DTS, the negative association between the use of sedatives and DTS was only marginally insignificant (p = 0.051) (Table 4, Panel F). Furthermore, in the regression predicting TiB, the use of sedatives was associated with less TiB (Table 4, Panel G).
In order to correct for multiple comparisons, we conducted False Discovery Rate corrections. Overall, the results largely held when taking these corrections into account. The exception was that the significant association between depression and TST became insignificant (p = 0.034 with a corrected threshold of p = 0.021).
Contrary to our hypotheses, NH patients with more severe pain and dementia had longer total sleep time and slept more during daytime, which indicates that NH patients with dementia and pain sleep more. Furthermore, their sleep efficiency was higher, indicating that the patients had more sleep within the time they spent in bed. In line with our hypotheses, our results showed that more severe dementia was associated with more time spent awake at night. Furthermore, people with more severe dementia spent more time in bed and people with more severe depression slept less at daytime and had less total sleep time. In addition, when adjusting for the use of medications, we found that patients who were prescribed sedative-hypnotic medications spent less time in bed.
To the best of our knowledge, this study is the first to explore how different levels of pain and depression correlate with a wide set of actigraphy-measured sleep parameters in NH patients with dementia (see Table 4). The results are of key importance since they provide new and valuable insight on the relationship between NH patients’ sleep, including sleep during daytime, and its associations with different levels of pain and depression. In light of previous research that has demonstrated that pain conditions are commonly associated with worse sleep in people without dementia (e.g., Andersen et al., 2018), the results from the present study are particularly interesting.
The results from the present study were unexpected. A potential explanation for these results could be that patients in pain receive more analgesics, antidepressants, sedative-hypnotics or opioids and thereby experience more sedation as an intended or adverse treatment effect. In turn, sedation and lack of movement could be recorded as sleep using actigraphy. However, we controlled for the use of different medications, rendering this explanation unlikely.
Another potential explanation of these somewhat surprising results may be that many older adults who experience chronic pain may develop behavior in which painful movements are avoided. This is known as the pain-avoidance effect (Vlaeyen and Linton, 2012; Achterberg et al., 2013). If this interpretation is valid, it would imply that the patients may in fact not be sleeping but are lying still in order to avoid pain, and by extension this highlight that pain assessment is fundamental. When a patient is lying still, it may be interpreted as sleep when measured with actigraphy. The fact that DTS was also higher in NH patients with pain and dementia may indicate that painful movements/activity were avoided at daytime as well. At the same time, it is noteworthy that previous research shows more agitated behavior among patients with mild to moderate pain (Husebo et al., 2014). In Blytt et al. (2018a), which is based on sleep-related data from the DEP.PAIN.DEM trial, we found that in the full sample, pain treatment improved sleep as measured with actigraphy compared to placebo after one week with pain treatment (paracetamol and buprenorphine). The results from the DEP.PAIN.DEM trial do not allow differentiation between sedation as a side-effect of the administered treatment and actual sleep. Importantly, we found no long-term effect of pain treatment on sleep (Blytt et al., 2018b). In the present study, we registered the use of different medications that can lead to sedation as a side-effect. However, this did not affect the results, and the only finding related to medication use was that the use of sedatives was associated with less time spent in bed.
Based on the present findings, we argue that there is a need for studies examining more thoroughly and systematically the association between pain, depression and sleep. It is furthermore important to acknowledge that it is very difficult to ascertain pain in people with dementia. The use of observation-based pain instruments applied by a proxy-rater who is familiar with the patients is a common method to assess pain in this population. Since a patient’s behavior is typically changed in reaction to experiencing pain, it is essential to examine pain expressions in relation to the person’s usual behavior (Hadjistavropoulos et al., 2014). Most tools used for classifying pain in NH patients are developed against this backdrop (Corbett et al., 2012; Flo et al., 2014; Lichtner et al., 2014). The MOBID-2 instrument has good validity, interrater and test-retest reliability, and responsiveness to change has been shown (Husebo et al., 2014). Yet, pain is a complex phenomenon, and we cannot be sure that the patient is experiencing pain, and in what intensity, location and duration.
Previous research indicates that increased wakefulness and fragmented sleep at night are common sleep problems in patients with dementia (McCleery et al., 2016). Our results showed that the patients with more severe dementia spent more time awake during the night. Sleep problems in patients with dementia may be a result of neural loss in the suprachiasmatic nuclei and/or other neuropathological changes to sleep-wake circuits (Swaab et al., 1985; Van Someren, 2000). The results of the present study indicate that as dementia progresses, sleep problems may be exacerbated. While more severe dementia was also associated with spending more time in bed, we cannot tell whether this is a result of the patient’s own wishes, or NH routines.
Previous studies investigating sleep and depression have found that 60 to 80% of depressed adults and older adults report having difficulty falling or staying asleep or being tired the next day (Almeida and Pfaff, 2005; Ancoli-Israel, 2009). Furthermore, depression in people with dementia is associated with lack of initiative and reduced memory, and there is thus overlap between the core symptoms of dementia and depression (Brailean et al., 2016). The present study indicates that the patients with more severe depression was somewhat more active as we found that they slept less during daytime, had lower total sleep time and spent less time in bed. A potential explanation for this could be agitation – a symptom often seen in people with dementia and depression (Husebo et al., 2014).
As pointed out by McCleery et al. (2016), there is a lack of evidence regarding drug treatment of sleep problems in people with dementia, and this is thus highly needed. However, the present study was not designed to look at the effect of sedatives on sleep in this population. Therefore, we cannot be sure if the patients receiving sedatives (27.7% of the total sample) were systematically different in other ways that can explain these results.
This study has several limitations. Due to the cross-sectional design of the study, we cannot draw any causal conclusions. Furthermore, the COSMOS and the DEP.PAIN.DEM trials have somewhat different inclusion and exclusion criteria. Importantly, the DEP.PAIN.DEM study, from which most of the data is derived, had depression and dementia as inclusion criteria (CSDD ≥ 8, MMSE ≤ 20). As the results from the independent samples t-tests showed, the patients from the DEP.PAIN.DEM trial had significantly higher depression scores and lower MMSE scores. This implies that the patients from the two trials may not be comparable, which may affect generalizability. The results from the present study may arguably be generalized to NH patients with dementia and probable symptoms of depression.
To measure sleep in the NH setting is challenging due to patients’ reduced ability to give valid self-report (Blytt et al., 2017). Therefore, objective measurement using actigraphy or use of proxy-rater assessment tools (where health personnel or relatives answer on behalf of the patient) are the current alternatives. Both have several limitations. When using actigraphy, immobility is recorded as sleep, which makes it difficult to interpret the results. Furthermore, previous research has shown that SOL has been particularly difficult to ascertain (Marino et al., 2013). When proxy-rater assessment tools are evaluated, they are often compared with actigraphy as the gold standard (Blytt et al., 2017; Hjetland et al., 2020). This indicates that actigraphy is considered a better method for measuring sleep in this population. When sleep is evaluated using proxy-rater assessment tools like the Neuropsychiatric Inventory and the Cornell Scale for Depression in Dementia, raters report significantly fewer sleep problems compared to actigraphy (Blytt et al., 2017). This highlights that measuring sleep in this population is difficult. However, individual sleep diaries – addressed by the patient’s primary nurse – would have strengthened the study.
It is furthermore important to note that we have just conducted a coarse mapping of the medications; N02A (opioids), N02B (other analgesics), N05C (hypnotics and sedatives), and N06A (antidepressants). However, effects such as sedation may differ within the different drug groups and according to the prescribed dose. Additionally, we did not assess whether the daily prescriptions were in fact administered to the patient. Only daily prescribed treatments were recorded we did not control for the use of as-needed treatment.
Another limitation in the present study was that we did not have complete datasets for all the different variables (for an overview of missing data, see the number of observations in Table 1). We cannot be sure whether the results would be different if we had complete data for all the relevant parameters investigated.
Together, the results of the present study revealed new and interesting insights on the relationship between sleep, pain and depression in a fragile patient group. Importantly, the results indicated that being in pain was associated with more sleep as measured with actigraphy, also when controlling for use of potentially sleep-enhancing medications like sedative-hypnotics, analgesics, opioids and antidepressants. Future research should focus on further investigating the mechanisms underlying these results.
The datasets generated for this study will not be made publicly available in order to maintain confidentiality of the study participants. Requests to access the datasets should be directed to the corresponding author.
The studies involving human participants were reviewed and approved by The Regional Ethics Committee REC-West 2013/1474 and REC-West 2013/1765. The patients/participants provided their written informed consent to participate in this study.
BH is the primary investigator of the COSMOS and the DEP.PAIN.DEM trial from which the data originates. KB designed the study, analyzed the data, and wrote the manuscript. BB, AE, EF-G and BH designed the study, helped with the analysis of the data, and wrote the manuscript. All authors have read and approved the manuscript prior to publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank the patients, their relatives, and the nursing home staff for their willingness and motivation, which made this work possible. BH would like to thank the GC Rieber Foundation and the Norwegian Directorate of Health for supporting our work at the Centre for Elderly and Nursing Home Medicine, University of Bergen, Norway. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. A special thanks to Andrea Rørvik Marti for scoring the actigraphy data. KB had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analyses. A conference paper based on the same data material has been previously published as a poster at the Nordic Sleep Conference in 2019, KB, EF-G, AE, BB, and BH: “Pain, depression and their association with sleep in nursing home patients with advanced dementia – a cross-sectional study.”
Achterberg, W. P., Gambassi, G., Finne-Soveri, H., Liperoti, R., Noro, A., Frijters, D. H., et al. (2010). Pain in European long-term care facilities: cross-national study in Finland, Italy and The Netherlands. Pain 148, 70–74. doi: 10.1016/j.pain.2009.10.008
Achterberg, W. P., Pieper, M. J., van Dalen-Kok, A. H., de Waal, M. W., Husebo, B. S., Lautenbacher, S., et al. (2013). Pain management in patients with dementia. Clin. Interv. Aging 8, 1471–1482.
Almeida, O. P., and Pfaff, J. J. (2005). Sleep complaints among older general practice patients: association with depression. Br. J. Gen. Pract. 55, 864–866.
Andersen, M. L., Araujo, P., Frange, C., and Tufik, S. (2018). Sleep disturbance and pain: a tale of two common problems. Chest 154, 1249–1259.
Bair, M. J., Robinson, R. L., Katon, W., and Kroenke, K. (2003). Depression and pain comorbidity: a literature review. Arch. Intern. Med. 163, 2433–2445. doi: 10.1001/archinte.163.20.2433
Ballard, C., Corbett, A., Chitramohan, R., and Aarsland, D. (2009). Management of agitation and aggression associated with Alzheimer’s disease: controversies and possible solutions. Curr. Opin. Psychiatry 22, 532–540. doi: 10.1097/yco.0b013e32833111f9
Barca, M. L., Engedal, K., and Selbæk, G. (2010). A reliability and validity study of the cornell scale among elderly inpatients, using various clinical criteria. Dement. Geriatr. Cogn. Disord. 29, 438–447. doi: 10.1159/000313533
Blytt, K. M. (2019). Sleep in Nursing Home Patients: Clinical Assessment and the Effects of Pain Treatment on Sleep. Ph.D. thesis, University of Bergen, Bergen. doi: 10.1159/000313533
Blytt, K. M., Bjorvatn, B., Husebo, B., and Flo, E. (2017). Clinically significant discrepancies between sleep problems assessed by standard clinical tools and actigraphy. BMC Geriatr. 17:253. doi: 10.1186/s12877-017-0653-7
Blytt, K. M., Bjorvatn, B., Husebo, B., and Flo, E. (2018a). Effects of pain treatment on sleep in nursing home patients with dementia and depression: a multicenter placebo-controlled randomized clinical trial. Int. J. Geriatr. Psychiatry 33, 663–670. doi: 10.1002/gps.4839
Blytt, K. M., Husebo, B., Flo, E., and Bjorvatn, B. (2018b). Long-term pain treatment did not improve sleep in nursing home patients with comorbid dementia and depression: a 13-week randomized placebo-controlled trial. Front. Psychol. 9:134. doi: 10.3389/fpsyg.2018.00134
Brailean, A., Comijs, H. C., Aartsen, M. J., Prince, M., Prina, A. M., Beekman, A., et al. (2016). Late-life depression symptom dimensions and cognitive functioning in the Longitudinal Aging Study Amsterdam (LASA). J. Affect. Disord. 201, 171–178. doi: 10.1016/j.jad.2016.05.027
Chopra, K., and Arora, V. (2014). An intricate relationship between pain and depression: clinical correlates, coactivation factors and therapeutic targets. Expert. Opin. Ther. Targets 18, 159–176. doi: 10.1517/14728222.2014.855720
Corbett, A., Husebo, B., Malcangio, M., Staniland, A., Cohen-Mansfield, J., Aarsland, D., et al. (2012). Assessment and treatment of pain in people with dementia. Nat. Rev. Neurol. 8:264. doi: 10.1038/nrneurol.2012.53
Enache, D., Winblad, B., and Aarsland, D. (2011). Depression in dementia: epidemiology, mechanisms, and treatment. Curr. Opin. Psychiatry 24, 461–472. doi: 10.1097/yco.0b013e32834bb9d4
Erdal, A., Flo, E., Aarsland, D., Ballard, C., Slettebo, D. D., and Husebo, B. S. (2018). Efficacy and safety of analgesic treatment for depression in people with advanced dementia: randomised, multicentre, double-blind, placebo-controlled trial (DEP. PAIN. DEM). Drugs Aging 35, 545–558. doi: 10.1007/s40266-018-0546-2
Erdal, A., Flo, E., Selbaek, G., Aarsland, D., Bergh, S., Slettebo, D. D., et al. (2017). Associations between pain and depression in nursing home patients at different stages of dementia. J. Affect. Disord. 218, 8–14. doi: 10.1016/j.jad.2017.04.038
Flo, E., Bjorvatn, B., Corbett, A., Pallesen, S., and Husebo, B. S. (2017). Joint occurrence of pain and sleep disturbances in people with dementia. a systematic review. Curr Alzheimer Res. 14, 538–545. doi: 10.2174/1567205013666160602234932
Flo, E., Gulla, C., and Husebo, B. S. (2014). Effective pain management in patients with dementia: benefits beyond pain? Drugs Aging 31:863. doi: 10.1007/s40266-014-0222-0
Goldenberg, D. L. (2010). Pain/depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am. J. Med. 123, 675–682. doi: 10.1016/j.amjmed.2010.01.014
Hadjistavropoulos, T., Herr, K., Prkachin, K. M., Craig, K. D., Gibson, S. J., Lukas, A., et al. (2014). Pain assessment in elderly adults with dementia. Lancet Neurol. 13, 1216–1227. doi: 10.1016/s1474-4422(14)70103-6
Hemmingsson, E. S., Gustafsson, M., Isaksson, U., Karlsson, S., Gustafson, Y., Sandman, P. O., et al. (2018). Prevalence of pain and pharmacological pain treatment among old people in nursing homes in 2007 and 2013. Eur. J. Clin. Pharmacol. 74, 483–488. doi: 10.1007/s00228-017-2384-2
Hjetland, G. J., Nordhus, I. H., Pallesen, S., Cummings, J., Tractenberg, R. E., Thun, E., et al. (2020). An actigraphy-based validation study of the sleep disorder inventory in the nursing home. Front. Psychiatry 11:173.
Husebø, B. S., Ballard, C., Aarsland, D., Selbaek, G., Slettebo, D. D., Gulla, C., et al. (2019). The effect of a multicomponent intervention on quality of life in residents of nursing homes: a randomized controlled trial (COSMOS). J. Am. Med. Dir. Assoc. 20, 330–339. doi: 10.1016/j.jamda.2018.11.006
Husebo, B. S., Flo, E., Aarsland, D., Selbaek, G., Testad, I., Gulla, C., et al. (2015). COSMOS—improving the quality of life in nursing home patients: protocol for an effectiveness-implementation cluster randomized clinical hybrid trial. Implement. Sci. 10:131.
Husebo, B. S., Ostelo, R., and Strand, L. I. (2014). The MOBID-2 pain scale: reliability and responsiveness to pain in patients with dementia. Eur. J. Pain 18, 1419–1430. doi: 10.1002/ejp.507
Husebo, B. S., Strand, L. I., Moe-Nilssen, R., Husebo, S. B., and Ljunggren, A. E. (2010). Pain in older persons with severe dementia. Psychometric properties of MOBID-2 Pain Scale in a clinical setting. Scand. J. Caring Sci. 24, 380–391. doi: 10.1111/j.1471-6712.2009.00710.x
Husebo, B. S., Strand, L. I., Moe-Nilssen, R., Husebo, S. B., Snow, A. L., and Ljunggren, A. E. (2007). Mobilization-observation-behavior-intensity-dementia pain scale (MOBID): development and validation of a nurse-administered pain assessment tool for use in dementia. J. Pain Symptom Manage 34, 67–80. doi: 10.1016/j.jpainsymman.2006.10.016
Jean-Louis, G., Von Gizycki, H., Zizi, F., Hauri, P., Spielman, A., and Taub, H. (1997). The actigraph data analysis software: I. A novel approach to scoring and interpreting sleep-wake activity. Percept. Mot. Skills 85, 207–216. doi: 10.2466/pms.1997.85.1.207
Kroenke, K., Wu, J., Bair, M. J., Krebs, E. E., Damush, T. M., and Tu, W. (2011). Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J. Pain 12, 964–973. doi: 10.1016/j.jpain.2011.03.003
Kyle, S. D., Espie, C. A., and Morgan, K. (2010). “…Not just a minor thing, it is something major, which stops you from functioning daily”: quality of life and daytime functioning in insomnia. Behav. Sleep Med. 8, 123–140. doi: 10.1080/15402002.2010.487450
Lichtner, V., Dowding, D., Esterhuizen, P., Closs, S. J., Long, A. F., Corbett, A., et al. (2014). Pain assessment for people with dementia: a systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 14:138.
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734.
Marino, M., Li, Y., Rueschman, M. N., Winkelman, J. W., Ellenbogen, J. M., Solet, J. M., et al. (2013). Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep 36, 1747–1755. doi: 10.5665/sleep.3142
McCleery, J., Cohen, D. A., and Sharpley, A. L. (2016). Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst. Rev. 11:CD009178.
McCrae, C. S., Dzierzewski, J. M., McNamara, J. P., Vatthauer, K. E., Roth, A. J., and Rowe, M. A. (2016). Changes in sleep predict changes in affect in older caregivers of individuals with alzheimer’s dementia: a multilevel model approach. J. Gerontol. B Psychol. Sci. Soc. Sci. 71, 458–462. doi: 10.1093/geronb/gbu162
Nicassio, P. M., Ormseth, S. R., Kay, M., Custodio, M., Irwin, M. R., Olmstead, R., et al. (2012). The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain 153, 107–112. doi: 10.1016/j.pain.2011.09.024
Perneczky, R., Wagenpfeil, S., Komossa, K., Grimmer, T., Diehl, J., and Kurz, A. (2006). Mapping scores onto stages: mini-mental state examination and clinical dementia rating. Am. J. Geriatr. Psychiatry 14, 139–144. doi: 10.1097/01.jgp.0000192478.82189.a8
Ravyts, S. G., Dzierzewski, J. M., Grah, S. C., Buman, M. P., Aiken-Morgan, A. T., and Giacobb, P. R. Jr., et al. (2018). Pain inconsistency and sleep in mid to late-life: the role of depression. Aging Ment. Health 23, 1174–1179. doi: 10.1080/13607863.2018.1481929
Sadeh, A., Sharkey, K. M., and Carskadon, M. A. (1994). Activity-based sleep—wake identification: an empirical test of methodological issues. Sleep 17, 201–207. doi: 10.1093/sleep/17.3.201
Sivertsen, B., Lallukka, T., Petrie, K. J., Steingrímsdóttir, ÓA., Stubhaug, A., and Nielsen, C. S. (2015). Sleep and pain sensitivity in adults. Pain 156, 1433–1439. doi: 10.1097/j.pain.0000000000000131
Sivertsen, B., Omvik, S., Havik, O. E., Pallesen, S., Bjorvatn, B., Nielsen, G. H., et al. (2006). A comparison of actigraphy and polysomnography in older adults treated for chronic primary insomnia. Sleep 29, 1353–1358. doi: 10.1093/sleep/29.10.1353
Swaab, D. F., Fliers, E., and Partiman, T. S. (1985). The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 342, 37–44. doi: 10.1016/0006-8993(85)91350-2
Van Someren, E. J. W. (2000). Circadian and sleep disturbances in the elderly. Exp. Gerontol. 35, 1229–1237. doi: 10.1016/s0531-5565(00)00191-1
Vlaeyen, J. W., and Linton, S. J. (2012). Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 153, 1144–1147. doi: 10.1016/j.pain.2011.12.009
Wagatsuma, S., Yamaguchi, T., Berge, L. I., Husebo, B., Habiger, T. F., Nouchi, R., et al. (2020). How, why and where it hurts—breaking down pain syndrome among nursing home patients with dementia: a cross-sectional analysis of the COSMOS trial. Pain Manag. Nurs. doi: 10.1016/j.pmn.2020.11.014 Online ahead of print
Webster, L., Powell, K., Costafreda, S. G., and Livingston, G. (2020a). The impact of sleep disturbances on care home residents with dementia: the SIESTA qualitative study. Int. Psychogeriatr. 32, 839–847. doi: 10.1017/s1041610220000642
Webster, L., Costafreda Gonzalez, S., Stringer, A., Lineham, A., Budgett, J., Kyle, S., et al. (2020b). Measuring the prevalence of sleep disturbances in people with dementia living in care homes: a systematic review and meta-analysis. Sleep 43:zsz251.
World Health Organization [WHO] (2019). Dementia Fact Sheet No. 362. Available online at: http://www.who.int/mediacentre/factsheets/fs362/en/. [Accessed August 02, 2020]
Keywords: sleep, pain, depression, nursing home patients, actigraphy, dementia
Citation: Blytt KM, Flo-Groeneboom E, Erdal A, Bjorvatn B and Husebo BS (2021) Sleep and its Association With Pain and Depression in Nursing Home Patients With Advanced Dementia – a Cross-Sectional Study. Front. Psychol. 12:633959. doi: 10.3389/fpsyg.2021.633959
Received: 26 November 2020; Accepted: 26 March 2021;
Published: 20 April 2021.
Edited by:
Christian Franceschini, University of Parma, ItalyReviewed by:
Luigi De Gennaro, Sapienza University of Rome, ItalyCopyright © 2021 Blytt, Flo-Groeneboom, Erdal, Bjorvatn and Husebo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kjersti Marie Blytt, kjersti.blytt@hvl.no
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.