
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Psychol. , 05 November 2020
Sec. Decision Neuroscience
Volume 11 - 2020 | https://doi.org/10.3389/fpsyg.2020.550204
This article is part of the Research Topic Consumer Neuroscience - Foundation, Validation, and Relevance View all 19 articles
This article reviews a wide range of functional magnetic resonance imaging (fMRI) studies conducted in the field of consumer neuroscience to (1) highlight common interpretative approaches of neuroimaging data (i.e., forward inference and reverse inference), (2) discuss potential interpretative issues associated with these approaches, and (3) provide a framework that employs a multi-method approach aimed to possibly raise the explanatory power and, thus, the validity of functional neuroimaging research in consumer neuroscience. Based on this framework, we argue that the validity of fMRI studies can be improved by the triangulation of (1) careful design of neuroimaging studies and analyses of data, (2) meta-analyses, and (3) the integration of psychometric and behavioral data with neuroimaging data. Guidelines on when and how to employ triangulation methods on neuroimaging data are included. Moreover, we also included discussions on practices and research directions that validate fMRI studies in consumer neuroscience beyond data triangulation.
Since the dawn of consumer neuroscience, researchers have employed neuroimaging techniques, especially functional magnetic resonance imaging (fMRI), to explore latent mental processes underlying consumer behavior (e.g., Yoon et al., 2006; Hedgcock and Rao, 2009; Reimann et al., 2010; Meade and Craig, 2012; Plassmann and Weber, 2015). Thanks to their collective effort through more than a decade of research, consumer neuroscientists have accumulated a wealth of insights that sheds light upon many aspects of consumer behavior. However, an unresolved challenge exists: that of reverse inference. Defined as the use of neuroimaging data to infer the presence of specific cognitive and affective processes (Poldrack, 2008), reverse inference may be tricky when interpretating neuroimaging results (Reimann et al., 2011, 2016; Plassmann et al., 2012, 2015).
In this article, we attempt to summarize potential issues associated with reverse inference, and we argue that proper data triangulation can help address these issues. Herein, we define data triangulation as the practice of utilizing multiple methods and data sources to study the same phenomenon (Patton, 1999; Carter et al., 2014). In neuroimaging research, triangulation would thus entail integrating neuroimaging data with data from meta-analyses, psychometric assessments, and consumer behavior.
Because the neuroimaging techniques employed in consumer neuroscience often consist of fMRI, we focus exclusively on fMRI research in our review of research papers, examinations of research methods, and recommendations to consumer researchers (hereinafter, we use the terms “neuroimaging” and “fMRI” synonymously). However, many of our analyses and recommendations could possibly be applied to other imaging techniques as well.
This paper is organized as follows. First, we review more than 50 empirical articles employing fMRI techniques published in consumer and marketing research journals as well as neuroscience journals, and we use this review to discusses fMRI’s contributions to consumer research. Second, we discuss two major interpretative approaches of neuroimaging research—forward inference and reverse inference—and explain how these approaches contribute to understanding consumer behavior. Third, we discuss potential issues and challenges associated with reverse inference. Fourth, we argue that the utilization of data triangulation of fMRI data with meta-analytic data as well as psychometric and behavioral data can enhance the validity of fMRI studies. Fifth, we provide and discuss several recommendations for future consumer neuroscience research.
Over the past one and a half decades, the field of consumer neuroscience has significantly contributed to consumer research. In Table 1, we have reviewed and attempted to summarize more than 50 empirical articles utilizing fMRI techniques. The works summarized here have been published in consumer research journals such as the Journal of the Academy of Marketing Science, the Journal of Consumer Research, the Journal of Marketing Research, the Journal of Consumer Psychology, and the Journal of the Association for Consumer Research, as well as journals in fields outside consumer research, including psychology, neuroscience, and the general science. For journals outside consumer research, we have focused our review on Cortex, Journal of Neuroscience, Journal of Neuroscience, Psychology, and Economics, NeuroImage, Neuron, Psychological Science, the Proceedings of the National Academy of Sciences, and Social Cognitive and Affective Neuroscience, in which some of the earlier work on consumer neuroscience appeared and more recent work has continued to appear. For consumer research journals, the list of included works is based on research results for methodological keywords such as “neuroimaging” and “fMRI.” For journals outside consumer research, the list of included works is focused on fMRI studies generated from searching keywords such as “consumer neuroscience,” “neuromarketing,” “market,” “marketing,” “consumer,” and “purchase.” Given our focus on specific journals and keywords, we acknowledge that some works related to consumer neuroscience may not be included in this review. Additionally, since our article’s focus is on fMRI, we would like to note that important work has been done using other imaging techniques such as electroencephalography (EEG). Nevertheless, the works included in Table 1 cover a broad range of topics relevant to consumer researchers, including theoretical domains such as information processing (Craig et al., 2012; Esch et al., 2012), judgment and decision making (Hedgcock and Rao, 2009; Karmarkar et al., 2015), and goals and motivation (Hedgcock et al., 2012; Wiggin et al., 2019), as well as substantive domains such as advertising (Craig et al., 2012; Venkatraman et al., 2012; Falk et al., 2016), pricing (Plassmann et al., 2008; Plassmann and Weber, 2015), branding (Reimann et al., 2012, 2018; Chan et al., 2018), and packaging design (Reimann et al., 2010). The table also examines the papers’ usage of meta-analyses as well as psychometrics/behavioral data, two major data source that we recommend triangulating with the fMRI data. Taken together, Table 1 provides a summary of the status of the consumer neuroscience literature and the type of data being used, showing that over the past one and a half decades researchers have started to integrate different data forms in their investigations.
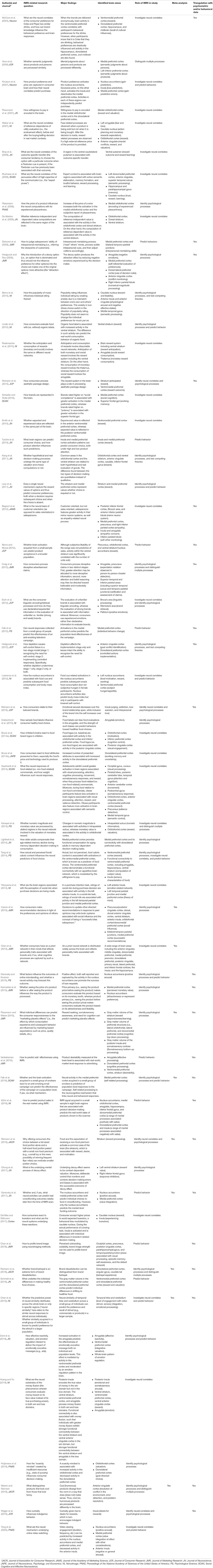
Table 1. Consumer neuroscience research in marketing, consumer research, and neuroscience journals (chronological order).
According to Wixted and Mickes (2013), neuroimaging studies take at least two major interpretative approaches. The first approach examines the neuroanatomical localization of behaviors and mental tasks, a process often referred to as forward inference (Henson, 2005; Poldrack, 2011). Forward inference has been adopted in consumer neuroscience to investigate the neurophysiological substrates of a wide range of consumer research concepts including, but not limited to, valuation (De Martino et al., 2009), social influence of buying decisions (Yokoyama et al., 2014), perception of money (Huang and Yu, 2019), and consumer curiosity (Wiggin et al., 2019). A typical forward inference question is this: While the concept of consumers’ willingness to pay (WTP) for a product is not unfamiliar to consumer researchers, where is WTP encoded in the brain? Plassmann et al. (2007) attempted to answer this interesting question by having hungry participants bid for the right to eat food. The authors pinpointed the medial prefrontal cortex and the dorsolateral prefrontal cortex as encoding locations of WTP. While the forward inference approach is laudable, it does not come without limitations. One such limitation is that while forward inference may highlight that a particular consumer research concept is correlated with activation of a certain brain area, forward inference often leaves open the psychological interpretation of possible underlying processes at play. This limitation may be less problematic in research that is largely interested in brain mapping but becomes more challenging in research that also cares about underlying psychological processes.
The second major approach of neuroimaging research involves using brain activation patterns to infer psychological processes. Specifically, by building on previously established functions and/or connectivity of specific brain regions, consumer researchers can make educated guesses as to which cognitive and emotional processes may be taking place as consumers process information or make decisions. This process is known as reverse inference (Poldrack, 2006, 2011). For instance, if a researcher posits that visual processing is taking place in an fMRI study because of increased activation in the occipital lobe, the researcher is reverse inferencing.
Reverse inference is important for consumer research (and for neuroscience in general) because it allows consumer researchers to access implicit or latent physiological processes (Reimann et al., 2011; Karmarkar and Plassmann, 2019). Specifically, the reverse inference approach can contribute to consumer research in at least three ways, as discussed below.
The first and most direct application of this research approach is that it allows consumer researchers to allude to underlying implicit psychological processes by observing activities of specific brain regions. This application requires a previously established link between brain regions and their functions. For instance, in studying how consumers process aesthetic package design, Reimann et al. (2010) recorded participants’ brain activity as they viewed aesthetic (vs. standardized) product package designs using fMRI. The authors observed that when processing aesthetic (vs. standardized) package designs, participants’ brains featured greater activation in the striatum, a brain area that has been associated with reward evaluations and processing (Delgado et al., 2003; Balleine et al., 2007; Kable and Glimcher, 2007; Báez-Mendoza and Schultz, 2013), among other activations. Following a similar interpretative logic, Plassmann et al. (2008) found that increasing the price of wine increases both reports of pleasantness as well as activity of the medial orbitofrontal cortex, a brain area that has widely been thought to encode pleasantness.
Second, neuroimaging can aid consumer researchers when competing theories exist to explain an observed phenomenon. Specifically, researchers have relied on established theories of brain regions’ functions to determine which theory is (or theories are) supported by actual brain activation. One such paper is Hedgcock et al. (2012) seminal examination of self-control depletion. Building on a two-stage model of self-control (i.e., recognizing the need for self-control → implementing self-control), the authors examined three completing models of depletion: (1) depletion only impairs the ability to recognize the need for self-control, (2) depletion only impairs the ability to implement self-control, and (3) depletion impairs both abilities. An fMRI study demonstrated that under depletion, a brain region associated with the implementation of controlled action (i.e., the right middle frontal gyrus located in the dorsolateral prefrontal cortex) features reduced activation, but such patterns were not found in the brain region associated with the detection of goal conflict (i.e., the anterior cingulate cortex). Therefore, the authors were able to conclude that only the implementation stage was likely to be affected by depletion (Hedgcock et al., 2012).
Third, the reverse inference approach is also useful in distinguishing different processes, networks, or stages of processing (i.e., neurophysiological dissociation). Such studies usually involve the observation of activations in different brain functional regions and/or networks (and occasionally also at different times) when participants undertake different cognitive tasks. For instance, Yoon et al. (2006) investigated whether semantic judgments of brands and humans are processed similarly. It has long been observed that consumers, marketers, and researchers alike tend to use similar if not identical words to describe both brands and people (Fournier, 1998; Fournier and Alvarez, 2012; MacInnis and Folkes, 2017). For instance, a brand can be “reliable” like a person and can form relationships with humans as other humans do. This tendency naturally raises the question of whether brands and humans are processed identically or at least similarly in the human brain. Yoon et al. (2006) work, however, showed that this may not be the case: Processing humans was associated with the medial prefrontal cortex, whereas processing brands was associated with the left inferior prefrontal cortex, which has been found to be involved in object processing.
In summary, neuroimaging studies often take two major interpretative approaches: Using both behaviors and mental tasks to explore neurophysiological correlates and to map the brain (i.e., forward inference) and using brain activations to infer certain psychological processes (i.e., reverse inference). With reverse inference, we have further identified three ways in which reverse inference contributes to consumer research: (1) determining underlying, implicit, and unknown psychological processes; (2) determining the plausibility of enhancing and/or completing theories; and (3) distinguishing multiple processes, networks, or stages of processing. Both approaches are of great value and are widely employed in consumer neuroscience and neuroscience in general. In the present paper, we focus on reverse inference due to its theoretical and methodological intricacies and highlight its potential issues when employed.
Although reverse inference is by no means an incorrect research approach per se (Hutzler, 2014), neuroscientists have noticed interpretational issues associated with it (Poldrack, 2006; Del Pinal and Nathan, 2017). More specifically, when activation in a brain region of interest is not particularly selective for a specific cognitive process (i.e., when multiple cognitive processes can activate the same brain region), the validity of reverse inference can be undermined (Poldrack, 2006, 2011).
For instance, it has long been known that the anterior cingulate cortex is heavily involved in the processing of conflicting information from the environment (Botvinick et al., 2001; Van Veen et al., 2001; Carter and van Veen, 2007), so it is tempting to assume that the consumer brain is processing conflicting information when activation in this brain region is observed. However, prior literature shows that the anterior cingulate cortex is also sensitive to the gain and loss of rewards (e.g., Taylor et al., 2006; Seo and Lee, 2007). As a result, researchers cannot, without committing a logical fallacy, argue for the involvement of conflicting information based on the activation observed in the anterior cingulate cortex alone.1
Unfortunately, multi-functional brain regions are quite common (e.g., Miller and Cohen, 2001; Postuma and Dagher, 2006). As a result, issues associated with reverse inference are neither foreign to consumer neuroscience nor foreign to neuroscience in general. Indeed, in the recent consumer neuroscience literature, the medial prefrontal cortex has been associated with reward processing (Reimann et al., 2010; Karmarkar et al., 2015), pleasantness (Plassmann et al., 2008), self-referential evaluation of preferences (Hedgcock and Rao, 2009), and social processes (Dietvorst et al., 2009; Cascio et al., 2015). While the exact three-dimensional locations of the medial prefrontal cortex may slightly vary across these investigations, they generally belong to the same anatomical brain area. Similarly, activation in the insula has been claimed to indicate negative affect status (Berns et al., 2010), social influence (Cascio et al., 2015), and desire for rewards (Wiggin et al., 2019).
What, then, can and should consumer researchers do to address this issue? Below, we will focus on three major strategies that can mitigate potential issues inherent in reverse inference: (1) reducing false alarms via refined study design and analysis, (2) employing neuroimaging meta-analyses to estimate the extent of reverse inference, and (3) integrating neuroimaging data with psychometric assessments and data from behavioral studies to provide additional confidence in the findings. We also posit that data triangulation employing these methods may greatly enhance the validity of neuroimaging research.
To address the potential issues associated with reverse inference, we first explain its logic. Let COG be the cognitive process of interest, and let ACT be the activation in the brain region purportedly associated with this process. The validity of reverse inference is thus given by the probability that COG takes place given that ACT is present, or, formally, P(COG|ACT). According to Bayes’ theorem:
It should be noted that the term COG here is always conditioned on the specific task used in the studies. In other words, the prior probability of P(COG) in this equation should in fact be P(COG|TASK), where TASK stands for the task setting and design. We have omitted this term in the equation for the sake of succinctness, but we will revisit this task-relevant nature of reverse inference in later discussions.
The equation above translates the question of reverse inference into “When the activation of specific brain regions is observed, how likely is it that the cognitive process of interest indeed took place?” To be able to make any meaningful argument based on such reverse inference, consumer researchers need to estimate and, if possible, systematically increase the value of P(COG|ACT). This can be done in multiple ways, as summarized below.
Activations in a brain region that (1) is purportedly associated with a cognitive process of interest but (2) does not actually reflect the proposed cognitive are called false alarms, which is a major culprit of reverse inference’s validity issues (Hutzler, 2014). Mathematically, the denominator in the above equation, P(ACT), is the prior probability that indicates the tendency of this brain region to become activated by default. If a brain region can easily become activated (i.e., if the false alarm rate is high) – because, for instance, it is involved in many different cognitive processes – then P(COG|ACT)will be relevantly small because P(ACT) is large, rendering reverse inference invalid.
Although how easily a brain region can become active and in how many different functions a brain region can get involved are questions largely beyond consumer researchers’ control, methods exist to control the level of P(ACT).
First, consumer researchers could design studies in such a way that the chances of false alarms are reduced. To illustrate, the term P(ACT) in the equation of Bayes’ Theorem can be further broken down to P(ACT|COG)P(COG) + P(ACT|¬COG)P(¬COG); thus,
where ¬COG indicates that the cognitive process of interest is not present.
According to this equation, if a brain region can be activated by a mental process different from the one of interest (i.e., the false alarm, the probability of which is captured by P[ACT|¬COG]), then the validity of reverse inference P(COG|ACT) will decrease as a result (all else being equal). Conversely, if, based on the systematic review of prior literature and study design, consumer researchers can lessen the possibility that such alternative cognitive processes take place, then the term P(ACT|¬COG)P(¬COG)] should shrink in value, and, as a result, the validity of reverse inference will increase. For instance, in a thought experiment, Hutzler (2014) discusses an experiment that infers the recognition of visual words from activation of the fusiform gyrus. Because the imagery study only involves the presentation of words, consumer researchers can reasonably rule out the competing cognitive theory of facial recognition. In this way, although the fusiform gyrus is multifunctional, the study design creates functional specificity by eliminating an alternative theory and, thus, controlling the baseline level of P(ACT), which is in fact P(ACT|TASK). Needless to say, ruling out alternative cognitive processes via design requires a good understanding of the literature on the brain region(s) of interest and depends heavily upon how much is known about the functionality of the brain region (cf. Agis and Hillis, 2017).
Another way to reduce false alarms is to use finer (i.e., smaller and/or better-defined) structures for reverse inference. Smaller ROIs are more selective and, thus, more predictive of cognitive processes than larger regions (Poldrack, 2006). In addition, instead of relying on the activation of individual brain regions, consumer researchers could use patterns of such activations to decode underlying cognitive processes, a method called multi-voxel pattern analysis (MVPA). This method utilizes pattern recognition to identify connections between cognitive processes and neuroimaging activation patterns. Such connections can then be used to reduce false alarms, as holistic activation patterns are much more selective for specific cognitive processes than individual brain regions are (Norman et al., 2006; Poldrack, 2008, 2011; Mahmoudi et al., 2012; Del Pinal and Nathan, 2017).
Why can meta-analyses help validate reverse inference? To answer this question, we must first discuss two important notions that are pertinent to reverse inference: consistency and specificity (Wager et al., 2009). Consistency is the extent to which brain activations replicate across studies, scanners, and labs, when certain cognitive processes are engaged. For instance, when participants see fear-inducing stimuli in a scanner, do their amygdalae tend to become activated regardless of where and in which labs they are scanned, who is scanning them, and how fear is induced in these scans? If the answer is “yes,” then the amygdala can be assumed to be consistently associated with fear. In the equation above, consistency is captured by P(ACT|COG), which is in proportion to P(COG|ACT). In other words, if consistency is inflated, then the evaluation of reverse inference will also be inflated, causing a validity issue. Unfortunately, in neuroimaging studies, P(ACT|COG) does tend to be inflated, because the usually small sample sizes and large numbers of tests give rise to underpowered studies and high false positive rates (Wager et al., 2009; Yarkoni et al., 2011). As a result, to evaluate the validity of reverse inference, consistency must be accounted for. On the other hand, specificity captures whether a brain region is selective for a mental process, which is directly associated with the validity of reverse inference as discussed in section “Reducing False Alarms in Reverse Inference via Careful Design and Analysis.”
Therefore, to estimate P(COG|ACT) and assess the validity of reverse inference, it is crucial to estimate consistency and specificity. However, neither feature can be acquired from a single study. Rather, one must consult a substantial number of studies to make a good estimation of consistency and specificity and, in turn, the validity of reverse inference.
Neuroimaging databases, such as neurosynth.org (Yarkoni et al., 2011), attempt to provide a means to account for consistency and specificity based on prior neuroimaging literature and meta-analyses generated using the database. Such databases thus attempt to quantify the extent of reverse inference by estimating the posterior probability P(COG|ACT). Neurosynth.org claims to be an open-source, large-scale, automated synthesis of functional neuroimaging data, utilizing text-mining and meta-analytic techniques to synthesize more than 14,000 published research papers (as of June 2020) and providing probabilistic mappings between brain regions and terms. These terms describe cognitive states (e.g., “pain,” “working memory,” or “fear”) and are used in the abstract of a paper, so they serve as a proxy of the mental process of interest. Based on this database and the meta-analysis provided therein, consumer researchers are able to tell whether there tends to be a non-zero association between the usages of certain terms (e.g., “emotion”) and the activation of a given brain region (e.g., the amygdala) in the extant literature. More importantly, the database also provides the posterior probability of a term, if the activation of a specific brain region is found (i.e., an estimated P(COG|ACT) based on extant literature).
To illustrate the usefulness and usage of meta-analysis, imagine that a consumer researcher is interested in how product evaluation can be influenced by the physical attractiveness of photos of human models that appear on product packages. Let us further assume after a literature review, the researcher hypothesizes that highly attractive models are more rewarding than average-looking models and thus lead to more favorable product evaluation. Since the reward valuation of physical attractiveness is associated with activity in the ventromedial prefrontal cortex (O’Doherty et al., 2003; Pegors et al., 2015), the researcher chooses the ventromedial prefrontal cortex as the ROI that reflects reward processing in this hypothesis. [Note, however, that in this scenario we focus on a single brain region only to make the example as simple as possible. In reality, it is always advisable to expect more than one ROI to be activated and, as a result, to (1) always include a whole brain analyses under the proper family-wise error rate (FWER)/false discovery rate (FDR) threshold (i.e., techniques to control false positives in fMRI data analysis, see Bennett et al., 2009) and/or (2) investigate brain regions in their activation patterns rather than in isolation, for instance employing MVPA as discussed in Section “Reducing False Alarms in Reverse Inference via Careful Design and Analysis”].
The researcher then sets out to test the hypothesis with fMRI, in which participants are shown a battery of product packages with images of either highly attractive models or average-looking models on them. Consistent with the hypothesis, when viewing packages with highly attractive (vs. average-looking) models, participants exhibit a brain activation in the ventromedial prefrontal cortex, in a region of interest with the center being the MNI coordinates x = −4, y = 38, z = −16. Also consistent with the hypothesis, the BOLD signal in the ventromedial prefrontal cortex mediates the liking of the product reported by in-scanner button pressing.
Based on these results, it would seem that the hypothesis is supported by the fMRI result. However, is this truly the case? Can the researcher reasonably infer reward processing from this brain activation, understanding the risks of reverse inference, especially regarding such notoriously multifunctional regions as the ventromedial prefrontal cortex?
To answer this question, the researcher needs to estimate P(RewardProcessing|vmPFCactivationinandaround[−4,38,−16]), which indicates how much confidence the researcher can put in this reverse inference. Neurosynth.org is designed precisely to quantify such reverse inferences. After inputting the coordinates of the peak voxel activation in neurosynth.org, it generates a table under the “Associations” tab (see Figure 1) based on its meta-analytical data corpus. Clearly, some terms in the table are brain regions (e.g., “ventromedial,” “vmpfc”), while others are cognitive processes (e.g., “terms,” “decision,” “choice”).
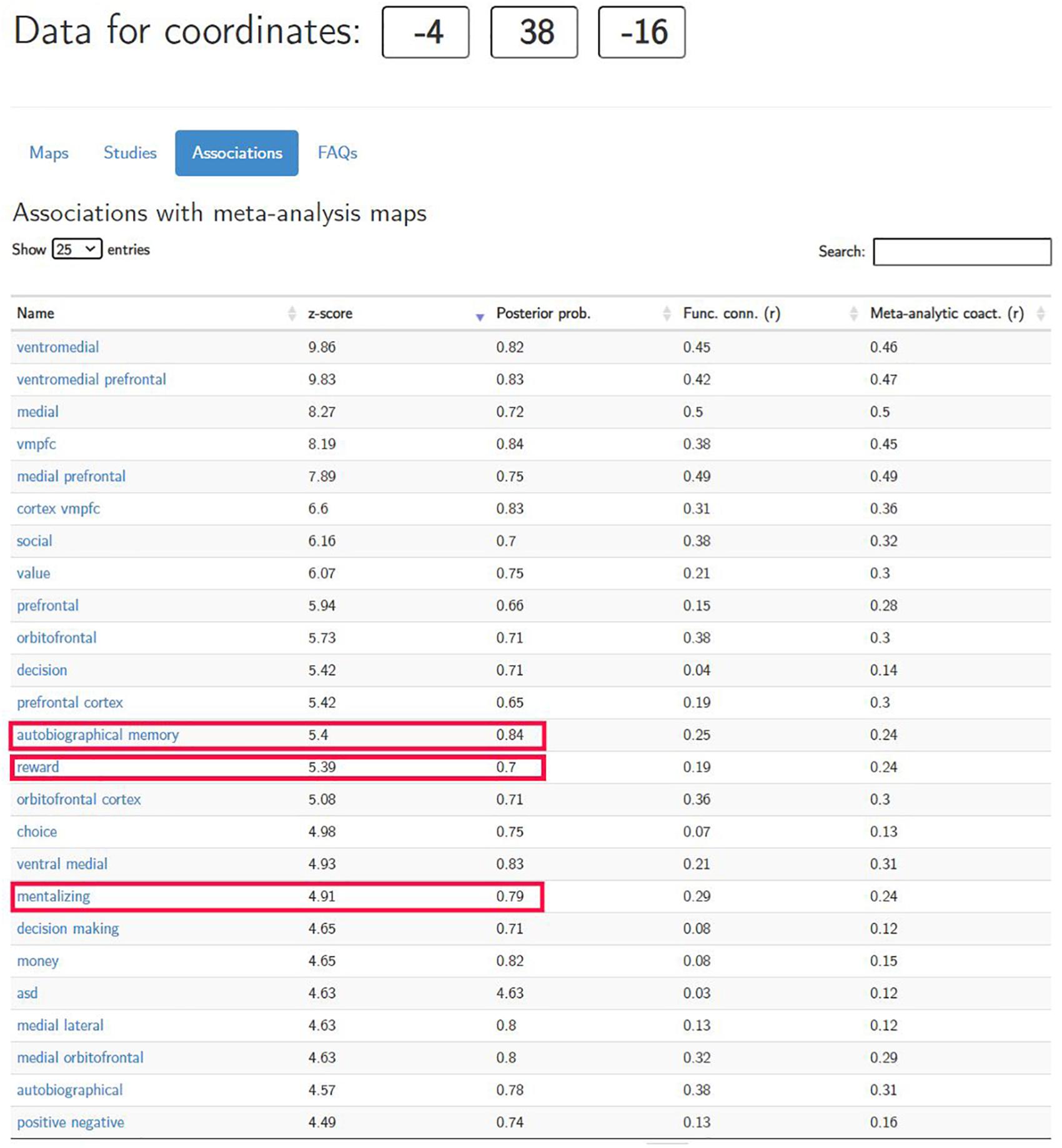
Figure 1. Meta-analysis results generated by neurosynth.org (retrieved on June 26, 2020).
Not surprisingly, the term “reward” (highlighted in Figure 1) appears in the association table. For this term of interest, the meta-analysis first returns a z-score of 5.39. This z-score shows whether there exists a non-zero relationship between the term (“reward” in this case) and the location (i.e., x = −4, y = 38, z = −16). The larger the z-score, the more confidence the researcher can have in claiming that this brain location is indeed associated with reward in the extant literature. Note that this z-score, however, cannot be used to determine the validity of the reverse inference being employed, nor can it be used to infer the strength of the association between the mental process (i.e., the term) and the location of the activation in the brain (Yarkoni, 2015b).
More informative is the number under the “posterior prob.” column. This number is the estimation of P(COG|ACT) and, thus, of the validity of the reverse inference. In our case, the posterior probability is 0.7 (see Figure 1). However, what does this number mean? Note that Bayes’ Theorem dictates that posterior probabilities must be calculated based on prior probabilities. In the case of neurosynth.org, its creators arbitrarily impose a uniform 50% prior probability on all terms [P(COG) = 0.5foranyCOG]. In other words, neurosynth.org assumes that if we take a random paper in the database, the prior probability of that paper using any term is exactly 50%. This is, of course, not true in reality; however, by making this assumption, neurosynth.org provides a threshold that applies to all terms, such that if the posterior probability is greater than 0.5, then the researcher can reasonably assume that the reverse inference he or she is examining has some merit at an above-chance level (Yarkoni et al., 2011; Yarkoni, 2015b). In addition, this uniform, arbitrary 0.5 prior probability allows researchers to make quantitative comparisons across all terms, such that if a location has a greater posterior probability for a term, then this region is more preferentially associated with that term than are the terms with lower posterior probabilities. It should be noted, however, that if the arbitrary prior probability is changed to other values from 0.5, the posterior probability will also vary as a result. Therefore, the absolute value of the posterior probability does not carry much meaning per se, and interpretations thereof should be made only in relation to other probabilities, such as the default 0.5, or the posterior probability for a different term associated with the same voxel.
Therefore, in our case, the 0.7 posterior probability means that if there is a 50–50 chance that a study uses the term “reward” a priori, then the estimated posterior probability for the term “reward” to be used increases to 70% after we observe the activation of the ventromedial prefrontal cortex in and around MNI coordinates x = −4, y = 38, z = −16. Thus, it seems that there is an above-chance likelihood that reward process is present in this hypothetical study. Please note again that one can only interpret change (i.e., 0.7 vs. the baseline 0.5) and not the absolute value.
However, we should take care here to avoid jumping to conclusions. It is very important to note that the fact that there is a decent chance of reward processing having taken place given the observed activation does not necessarily mean that this region is selective for reward. Rather, other terms may also have comparable or even higher levels of posterior probability in this reported region. Indeed, if we pay close attention to other terms in Figure 1, terms such as “autobiographical memory” and “mentalizing” (both highlighted) will arise, each having a posterior probability even greater than that of “reward” (0.84 and 0.79, respectively). Admittedly, many terms with the highest posterior probabilities in this table are reward-related (e.g., “reward,” “value,” “money”). However, even after taking all these terms into consideration, the conclusion the researcher can draw from this meta-analysis is still inconclusive; that is, the ventromedial prefrontal cortex in and around (x = −4, y = 38, z = −16) is preferentially (but not selectively) associated with reward. In other words, at this point in the analysis, we are still not sure if reward processing is truly happening.
Thus far, we have discussed what the neurosynth.org database tells us and, equally importantly, what it does not tell us (also cf. Yarkoni, 2015a,b for a discussion). The most important question, however, still remains. The researcher in our hypothetical example theorizes that reward is underlying the processing of product packages with images of highly attractive (vs. average-looking) human models on them. The fMRI result seems to support this argument, and the meta-analysis suggests that there is a good chance that the researcher is on the right track. However, the researcher still cannot draw decisive conclusions, due to the existence of alternative, equally viable (at least judging from the meta-analysis) mental processes (in this case, autobiographical memory and mentalizing). So, what should the researcher do now?
This question can be partially addressed by the meta-analysis that brings up this very question. Indeed, a valuable function of meta-analysis is to identify a (limited) set of competing theories so the proposed hypothesis can be supported by eliminating other (at least major) alternatives. Needless to say, the easiest alternatives to exclude are those that are unlikely to occur under the particular study design being employed. For instance, in the design of the hypothetical study described above, autobiographical memory is very unlikely to have been involved (since there is not a memory-related task involved) and thus can be reasonably excluded.
However, mentalizing – the process by which individuals make sense of the mental state of others or oneself – may well survive this “exclusion by study design.” After all, when consumers see other human beings (i.e., human models used in our hypothetical fMRI experiment), especially if they show emotions, consumers may well engage in mentalizing and, in turn, feature the well-documented activation in the ventromedial prefrontal cortex (Lombardo et al., 2010; Atique et al., 2011; Schnell et al., 2011). At this point, the researcher needs to think above and beyond the meta-analysis. One possible solution, for example, is to acquire the activation pattern of mentalizing; if this pattern is inconsistent with the actual results, then it is very likely that mentalizing may not have taken place. In this case, mentalizing involves not only the activation of the ventromedial prefrontal cortex but also activity in the precuneus and/or the temporoparietal junction, which often accompany ventromedial activations. Thus, if the researcher fails to find meaningful activations in these additional regions, mentalizing may not have been engaged in the study. Needless to say, the researcher should turn to extant literature to identify such patterns; however, another newly launched meta-analysis database, neuroquery.org, may also greatly assist in this process, in that it helps researchers predict which brain regions are likely to become activated given designated cognitive processes. Moreover, pattern-based activation can prove useful even if mentalizing does take place. For instance, if reward processing indeed occurs, it may not only involve the ventromedial prefrontal cortex but also trigger the orbitofrontal cortex and the ventral striatum. If the researcher can find activity in these regions that are also involved in reward processing, it becomes more evident that reward is taking place, even in the presence of mentalizing.
Although meta-analyses can shed important light upon the validity of reverse inference, it is not without limitations. Other than the information meta-analysis cannot yield, which we already discussed in Section “Integrating Information from Neuroimaging Meta-Analyses,” there exist other factors that limit the usefulness of meta-analyses.
First, when the term related to the cognitive process of interest has been rarely examined in the literature, the database will provide little information to the researcher. For instance, consumer researchers interested in personal control may recognize the scantiness of neuroimaging studies investigating perceived control and loss thereof, despite the richness of non-imaging work, which has been conducted mostly on non-human animals (e.g., Maier and Watkins, 2005; Amat et al., 2010, 2014).
Second, when a term is used inconsistently in the literature, the usefulness of meta-analysis is also limited. For instance, in the literature, the term “control” not only is used to refer to “personal control” but also is found in works involving self-control, motor control, executive control, and even the control condition in experimental studies. These terms are not distinguishable in the database due to the limitation of text-recognition tools, thus rendering meta-analyses on such terms virtually uninformative.
Third, meta-analysis databases like neurosynth.org cannot capture nuances across studies and designs. Such situations may introduce noise to meta-analyses and thus undermine the usefulness of their results, except in the case of well-defined and consistently-used cognitive processes and experimental procedures (Dockès et al., 2020).
For instance, meta-analysis databases may not be able to distinguish between “perceptual curiosity” and “epistemic curiosity,” as the terms tend to be aggregated together, despite their conceptual and neurophysiological distinctions in the literature on curiosity (Loewenstein, 1994; Jepma et al., 2012; Kidd and Hayden, 2015; Wiggin et al., 2019). To make matters worse, there are too few neuroimaging studies on curiosity to provide sufficient statistical power for the meta-analysis, if such terms exist in the database at all. Moreover, the inconsistent study procedures introduce noise to the database. Thus, if a researcher hypothesizes perceptual (but not epistemic) curiosity as the cognitive process underlying a certain consumer behavior, meta-analysis might not be able to provide much useful insight. In the following section, we argue that behavioral data can prove useful in addressing these concerns.
In addition, another database, NeuroQuery.org (Dockès et al., 2020), has recently been made publicly available.2 This new database complements the previously discussed databases in important ways, which we discuss below. An issue with the traditional databases is that the results they generate are largely based on decontextualized, aggregated data and arbitrary thresholding. Specifically, such databases rely on automated text-analysis algorithms and scripts to analyze and document the neuroscience concepts (in the form of “terms”) included in a sizable corpus of literature with considerable statistic power. However, to investigate these neuroscience concepts, the original research papers included in the corpus may take different approaches, employ different designs, and use different terms. Due to current limitations of text-mining techniques, traditional databases are unable to capture such task-related information, nuances across studies and designs, and idiosyncratic usages of the same terms. This situation can prove problematic, because the output of such analysis can suffer from a low signal-to-noise ratio and thus can be difficult to control and interpret, except in the case of well-defined concepts and/or highly standardized procedures (Dockès et al., 2020). Neuroquery.org, on the other hand, attempts to employ a different approach. Specifically, NeuroQuery.org begins with keywords (e.g., psychological processes, neuropsychological conditions, and anatomy) and uses these terms to predict what brain regions are likely to become activated if proposed psychological processes are engaged. The model this database utilizes can produce accurate and usable predictions of ROIs even for less common terms, terms that are named inconsistently in neuroscience vocabulary, or sets of terms that have not been studied together previously.
Although many researchers turn to neuroimaging techniques to discover mental processes precisely because of behavioral studies’ limitations, psychometric and behavioral data are useful in neuroimaging studies in many ways. To begin with, in relation to our discussions on meta-analyses, psychometric and behavioral data can prove helpful in compensating the shortcomings of meta-analyses, as they can provide a better-calibrated, task-relevant environment to investigate psychological processes of interest. In the aforementioned case of curiosity, for instance, extant literature provides rich behavioral manipulations to introduce perceptual vs. epistemic curiosity (e.g., Loewenstein, 1994), and the researcher can thus conduct behavioral experiments to observe whether perceptual (but not epistemic) curiosity produces the consumer behavior in question. If so, such studies can provide more evidence to complement the fMRI data.
Even when meta-analyses can be employed without the issues discussed in the previous section, psychometric and behavioral data can still prove helpful to reverse inference studies by provide additional evidence that the proposed cognitive process is indeed implicated in the phenomenon being studied. Mathematically, doing so increases P(COG) and in turn P(COG|ACT). For instance, Plassmann and Weber (2015) investigate the consumer responsiveness to the “marketing placebo effects” (MPE; the effect by which consumption experience and subsequent behavior are influenced by marketing-based expectancy such price, quality beliefs, etc.). The authors provide a comprehensive account of the cognitive processes that can influence MPE: reward responsiveness, cognitive top-down processing, and somatosensory bottom-up processing. In an MRI study, the gray matter volume of brain regions associated with these cognitive processes is found to be predictive of the MPE effect. To provide further support for this theoretical account, the authors further conducted a study demonstrating that personality traits related to these cognitive processes are also predictive of MPE, as are gray matter volume measures, thus fostering extra confidence in the proposed processes.
Note that for triangulation purposes, the behavioral data do not need to be “purely behavioral” (i.e., collected from separate studies that do not feature neuroimaging elements at all). Rather, the collection of psychometric and behavioral data can either be part of the original fMRI study and/or collected in separate studies.
For instance, imagine a study investigating how food print ads drive consumers’ willingness to purchase. To explore the cognitive process underlying food ads, suppose the researcher shows hungry participants multiple food ad images in the scanner as they report how much they want to purchase the food advertised. Let us further assume that the researcher finds that more successful ads are associated with (1) a neural network associated with reward and impulse (which might include, say, the nucleus accumbens, orbital prefrontal cortex, ventromedial prefrontal cortex, anterior insula, etc.) and (2) importantly, reduced activation in the medial prefrontal gyrus, a region associated with inhibition behavior (e.g., Batterink et al., 2010). Based on this pattern, our hypothetical researcher speculates that more successful food ads might be associated with greater inhibition/control failure and, in turn, higher willingness to purchase. (Here again, we caution our readers that the theorization and selection of ROIs herein are overly simplified for the sake of clarity and succinctness).
This theorizing is clearly an example of potentially problematic reverse inferences. How, then, can behavioral data help the researcher with this proposed process? Of course, the aforementioned “purely behavioral” studies can be conducted, wherein the researcher shows that depleted participants exhibit greater willingness to purchase to the same food ad than non-depleted participants do, thus demonstrating the involvement of control process. Alternatively, the researcher could show that people’s acceptance of the same food ad is a function of their trait self-control. However, the researchers can also collect behavioral data and combine them with neuroimaging data collected in either the existing or a new study to provide support for the proposed hypothesis.
For instance, if inhibition/control indeed plays a role in the acceptance of food ads, the aggregated activation in related brain regions acquired from a group as small as the participants scanned may be predictive of the effectiveness of the ads in the real world on a market or population level (i.e., neuroforecasting, Genevsky et al., 2017). Such “related brain regions” may be the medial prefrontal gyrus found in the existing hypothetical study or may involve a more extensive inhibitory network established by the literature. Similarly, the researcher could also run a structural scan to see whether the cortical thickness of these brain regions is predictive of individual participants’ acceptance of the ads (e.g., rating, choice, etc.). Furthermore, the researcher can even use transcranial magnetic stimulation (TMS) to temporarily disrupt the function in these brain regions (if they are relative shallow) or recruit participants with lesions in these areas and obverse how these participants react to the ads in comparison to control participants. In all these approaches, behavioral data are combined with brain data to shed light upon the underlying cognitive processes – in this case, inhibition and control.
As shown above, psychometric and behavioral data can be triangulated with brain data in a very flexible manner and can provide interesting and useful insights to researchers in many ways. Based on the specific issues they are investigating, the nature of the existing design and data, and the technology and resources available to them, researchers can select approaches to behavioral data to fit their needs.
In summary, in consumer neuroscience, researchers can address the validity issue of reverse inference by (1) refining the neuroimaging study design and analysis to reduce false alarms, (2) utilizing neuroimaging databases and the meta-analysis data therein to quantify the extent of reverse inference, and (3) using psychometric and behavioral data to provide additional process evidence.
In this paper, we advocate not the isolated employment but the triangulation of these methods. Such triangulation can enable each method to compensate for the others’ shortcomings and disadvantages, thus providing synergy toward improved overall validity. As discussed earlier, notwithstanding their unique advantages, each of these methods suffers from certain shortcomings: fMRI studies can investigate hidden mental processes but are potentially vulnerable to reverse inference issues; neuroimaging meta-analysis can assist researchers in quantifying reverse inference, but its usability can be limited and its analysis may not necessarily be conclusive; and behavioral data are flexible and can be well calibrated to the stimuli and procedures but may not be able to address the mental process of interest in a direct manner (the very reason researchers turn to neuroimaging in the first place).
Understanding the pros and cons of each method is important, as the cons serve as a starting point to identify the potential validity issues with one’s data, while the pros help researchers understand what data triangulations should be employed to address these issues. Indeed, this is precisely what the literature on Multitrait-Multimethod Matrices (MTMM, Campbell and Fiske, 1959; Bagozzi and Yi, 1993) suggests: that is, a single method may not be enough to distinguish the cognitive process of interest from unsubstantial, random-method variance and noise and, instead, multiple methods taking different angles can possibly redress single-method concern effectively.
Therefore, despite the increased time, effort, and expertise needed to adopt a multi-method approach, we still believe that the triangulation approach we have proposed herein should be considered by consumer neuroscience researchers. Specifically, for neuroimaging studies in consumer neuroscience, we believe the researcher should always (1) carefully refine the design of the fMRI study and (2) utilize meta-analyses (of course, sometimes the database is not usable for certain keywords and designs, but this is not known to the researcher until they have utilized the database). On the other hand, we believe that the researcher should base their decision of whether to include (3) psychometric and behavioral data, as well as what to include, on the specific issue they are facing. If, for example, the researcher makes a claim about certain mental cognitive processes based on brain activation, but the subsequent study design and meta-analysis cannot rule out competing theories and provide conclusive judgments, we would then recommend that psychometric and behavioral data be included. However, if it is already well-established in the behavioral literature that treatment X (e.g., seeing a picture of a snake) will cause mental activity M (e.g., fear) in the population of interest, and if, with due caution and prudence, the researcher can reasonably believe there is no good reason why this causal link would disappear in the scanner, then perhaps in such a case the researcher does not need to reinvent the wheel. Therefore, much as we applaud the merit of psychometric and behavioral data in general and believe they are beneficial most of the time, as a general principle we encourage researchers to include meaningful psychometric and behavioral data that address existing issues stemming from their particular research question and design, instead of triangulating for the sake of triangulating.
In addition to the hypothetical examples we used in previous sections, real-world examples of triangulation can also be found in the extant literature in consumer neuroscience. Regarding the works we summarized in Table 1, in Section “Integrating Psychometric and Behavioral Data,” we already discussed how Plassmann and Weber (2015) triangulated anatomical neuroimaging data with psychometric measures to explore the marketing placebo effect, and we would like to note that in this work the authors also utilize the NeuroSynth meta-analytical tool in support of their conclusions. Similarly, in Reimann et al. (2018), an fMRI study demonstrates that brand betrayal and brand satisfaction involve different brain areas. This result is triangulated with both psychometric measures and the NeuroSynth database. The authors were able to pinpoint a number of aspects on which brand dissatisfaction is differentiated from brand betrayal, including prior relationship with the brand, anger, and rumination. Furthermore, by combining fMRI, individual-level data and marketing-level behavioral data, Genevsky et al. (2017) found that nucleus accumbens activity not only predicts individual choices to fund in crowdfunding but also market-level funding outcome. The authors also examined the role sensory processes play in the identified pattern, and NeuroSynth was employed to predefine the volumes of interest (VOIs).
In addition to data triangulation, other practices can also improve the validity of individual studies and consumer neuroscience. Although a thorough discussion of these practices would be outside the scope of this paper, we would still like to take this opportunity to briefly advocate some of these practices for future studies in the field.
As discussed in Section “Summary: The Importance of Triangulation,” the notion of data triangulation proposed in this paper is consistent with that of the Multitrait-Multimethod Matrix (MTMM, Campbell and Fiske, 1959). The MTMM provides useful information that can benefit fMRI studies in three ways. First, the MTMM provides estimations of reliability of each measure, which could potentially help address the issue that fMRI measures tend to suffer from poor overall reliability (Elliott et al., 2020). Second, the MTMM provides assessment of convergent validity: if a concept (e.g., a mental process) is measured through multiple methods, the measures should be strongly correlated. Third, the MTMM also helps researchers to establish discriminant (or divergent) of measures, such that measures for different constructs should not be correlated (Campbell and Fiske, 1959).
Therefore, the MTMM would be a good tool to evaluate how well the triangulation improves the validity. The challenge is, however, how to directly compare the vastly different methods (e.g., behavioral data of distinct nature and neuroimaging data) on the MTMM. Although the analysis above already lays out the means in which triangulation can help improve the validity of fMRI studies, quantifying such improvement would provide the proposed triangulation with even more solid footing. Thus, we advocate the quantification of the proposed triangulation as a direction for future research.
Recent years have witnessed an increase in the important discussion regarding how to promote openness and transparency of research in the fields of psychology and neuroscience (e.g., Aarts et al., 2015; Gorgolewski and Poldrack, 2016; Kidwell et al., 2016; Nichols et al., 2016; Gilmore et al., 2017; Klein et al., 2018; Shrout and Rodgers, 2018). In solidarity with the fields’ joint effort toward a more precise and interpretable discipline, we also take the opportunity to advocate these practices in consumer neuroscience. For example, we suggest that consumer researchers could report information regarding experimental design, image acquisition, preprocessing, statistical modeling and statistical inferences, as well as result tables, which could be provided in a web appendix alongside the main paper. Some guidelines and checklists are provided, for instance, by Nichols et al. (2016) and in the appendix to Reimann et al. (2018). These practices may be helpful to make research reports more interpretable.
Another laudable practice is data sharing. This practice is more than just academic integrity, but also improves the validity of data analysis. In a recent paper published in Nature (Botvinik-Nezer et al., 2020), 70 independent teams of researchers were asked to analyze the same dataset. Since no two teams employed the same workflow of analysis, this flexibility in data analysis creates a considerable variation in the results of hypotheses testing. When the results from teams are aggregated, however, they show a significant convergence on activated regions. Thus, for better cross-validation, consumer neuroscientists may want to consider sharing data, which can be done with dedicated online repositories such as OpenfMRI.org (Poldrack et al., 2013), NeuroVault.org (Gorgolewski et al., 2015), and OpenVoxel.org. Furthermore, we encourage consumer researchers to share the workflow by which the data were analyzed, and report (if applicable) the code they used to analyze scan data on online platforms such as GitHub.com to further promote transparency, openness, and cross-validation.
Consumer neuroscience may hold valuable insights for the advancement of consumer research, with perhaps the most intriguing of these being its potential to (1) reveal hidden cognitive and emotional processes that have been inaccessible to traditional research methods and (2) to confirm physiological and psychological processes underlying consumer behavior. Indeed, over the years, consumer researchers have been employing fMRI, along with other neuroimaging techniques, in an attempt to open the black boxes of consumer experience, motivation, decision-making, and so on. Needless to say, fMRI has shed new light upon our understanding of consumer behavior, and we fully acknowledge the important contributions made by the field. That being said, as we pointed out earlier, many studies’ reliance on reverse inference may render them vulnerable to validity issues. To promote the validity of individual neuroimaging studies as well as consumer neuroscience as a field, in this paper we advocate for the triangulation of neuroimaging data with meta-analyses as well as psychometric and behavioral data. We believe these research practices may substantially increase the conclusions we can draw from fMRI data. Therefore, we encourage the employment of data triangulation in consumer neuroscience research. Lastly, we take this opportunity to offer our special acknowledgment to the intensive methodological discussions and disputes taking place in our parent disciplines, such as cognitive neuroscience and functional neuroimaging, as this paper draws largely upon their lessons and wisdom.
The original contributions generated for this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
CC conducted the review on extant literature and the analysis on reverse inference in the frontend with the consent of MR. Both CC and MR drafted the frontend. Both authors contributed to the article and approved the submitted version.
This work has benefited from financial support of Lingnan University, Hong Kong Special Administrative Region, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank the editors, TR, Hilke Plassmann, Carolyn Yoon, and Joseph T. Devlin, for their thoughtful remarks and their guidance through the review process and the reviewers for helpful remarks on earlier versions of this article.
Aarts, A. A., Anderson, J. E., Anderson, C. J., Attridge, P. R., Attwood, A., Axt, J., et al. (2015). Estimating the reproducibility of psychological science. Science 349, aac4716. doi: 10.1126/science.aac4716
Agis, D., and Hillis, A. E. (2017). The cart before the horse: when cognitive neuroscience precedes cognitive neuropsychology. Cogn. Neuropsychol. 34, 420–429. doi: 10.1080/02643294.2017.1314264
Amat, J., Aleksejev, R. M., Paul, E., Watkins, L. R., and Maier, S. F. (2010). Behavioral control over shock blocks behavioral and neurochemical effects of later social defeat. Neuroscience 165, 1031–1038. doi: 10.1016/j.neuroscience.2009.11.005
Amat, J., Christianson, J. P., Aleksejev, R. M., Kim, J., Richeson, K. R., Watkins, L. R., et al. (2014). Control over a stressor involves the posterior dorsal striatum and the act/outcome circuit. Eur. J. Neurosci. 40, 2352–2358. doi: 10.1111/ejn.12609
Atique, B., Erb, M., Gharabaghi, A., Grodd, W., and Anders, S. (2011). Task-specific activity and connectivity within the mentalizing network during emotion and intention mentalizing. Neuroimage 55, 1899–1911. doi: 10.1016/j.neuroimage.2010.12.036
Báez-Mendoza, R., and Schultz, W. (2013). The role of the striatum in social behavior. Front. Neurosci 7:233. doi: 10.3389/fnins.2013.00233
Bagozzi, R. P., Verbeke, W. J. M. I., van den Berg, W. E., Rietdijk, W. J. R., Dietvorst, R. C., and Worm, L. (2012). Genetic and neurological foundations of customer orientation: field and experimental evidence. J. Acad. Mark. Sci. 40, 639–658. doi: 10.1007/s11747-011-0271-4
Bagozzi, R. P., and Yi, Y. (1993). Multitrait–multimethod matrices in consumer research: critique and new developments. J. Consum. Psychol. 2, 143–170. doi: 10.1016/S1057-7408(08)80022-8
Balleine, B. W., Delgado, M. R., and Hikosaka, O. (2007). The role of the dorsal striatum in reward and decision-making. J. Neurosci. 27, 8161–8165.
Batterink, L., Yokum, S., and Stice, E. (2010). Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage 52, 1696–1703. doi: 10.1016/j.neuroimage.2010.05.059
Bennett, C. M., Wolford, G. L., and Miller, M. B. (2009). The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 4, 417–422. doi: 10.1093/scan/nsp053
Berns, G. S., Capra, C. M., Moore, S., and Noussair, C. (2010). Neural mechanisms of the influence of popularity on adolescent ratings of music. Neuroimage 49, 2687–2696. doi: 10.1016/j.neuroimage.2009.10.070
Berns, G. S., and Moore, S. E. (2012). A neural predictor of cultural popularity. J. Consum. Psychol. 22, 154–160. doi: 10.1016/j.j.2011.05.001
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., and Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychol. Rev. 108:624.
Botvinik-Nezer, R., Holzmeister, F., Camerer, C. F., Dreber, A., Huber, J., Johannesson, M., et al. (2020). Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88. doi: 10.1038/s41586-020-2314-9
Bray, S., Rangel, A., Shimojo, S., Balleine, B., and O’Doherty, J. P. (2008). The neural mechanisms underlying the influence of pavlovian cues on human decision making. J. Neurosci. 28, 5861–5866. doi: 10.1523/JNEUROSCI.0897-08.2008
Bruce, A. S., Bruce, J. M., Black, W. R., Lepping, R. J., Henry, J. M., Cherry, J. B. C., et al. (2014a). Branding and a child’s brain: an fMRI study of neural responses to logos. Soc. Cogn. Affect. Neurosci. 9, 118–122. doi: 10.1093/scan/nss109
Bruce, A. S., Lusk, J. L., Crespi, J. M., Cherry, J. B. C., Bruce, J. M., McFadden, B. R., et al. (2014b). Consumers’ neural and behavioral responses to food technologies and price. J. Neurosci. Psychol. Econ. 7, 164–173. doi: 10.1037/npe0000023
Campbell, D. T., and Fiske, D. W. (1959). Convergent and discriminant validation by the multitrait-multimethod matrix. Psychol. Bull. 56, 81–105. doi: 10.1037/h0046016
Carter, C. S., and van Veen, V. (2007). Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn. Affect. Behav. Neurosci. 7, 367–379. doi: 10.3758/CABN.7.4.367
Carter, N., Bryant-Lukosius, D., Dicenso, A., Blythe, J., and Neville, A. J. (2014). The use of triangulation in qualitative research. Oncol. Nurs. Forum 41, 545–547. doi: 10.1188/14.ONF.545-547
Cascio, C. N., O’Donnell, M. B., Bayer, J., Tinney, F. J., and Falk, E. B. (2015). Neural correlates of susceptibility to group opinions in online word-of-mouth recommendations. J. Mark. Res. 52, 559–575. doi: 10.1509/jmr.13.0611
Chan, H. Y., Boksem, M., and Smidts, A. (2018). Neural profiling of brands: mapping brand image in consumers’ brains with visual templates. J. Mark. Res. 55, 600–615. doi: 10.1509/jmr.17.0019
Chan, H. Y., Smidts, A., Schoots, V. C., Dietvorst, R. C., and Boksem, M. A. S. (2019). Neural similarity at temporal lobe and cerebellum predicts out-of-sample preference and recall for video stimuli. Neuroimage 197, 391–401. doi: 10.1016/j.neuroimage.2019.04.076
Chen, Y. P., Nelson, L. D., and Hsu, M. (2015). From “Where” to “What”: distributed representations of brand associations in the human brain. J. Mark. Res. 52, 453–466. doi: 10.1509/jmr.14.0606
Chung, H. K., Sjöström, T., Lee, H. J., Lu, Y. T., Tsuo, F. Y., Chen, T. S., et al. (2017). Why do irrelevant alternatives matter? An fMRI-TMS study of context-dependent preferences. J. Neurosci. 37, 11647–11661. doi: 10.1523/JNEUROSCI.2307-16.2017
Craig, A. W., Loureiro, Y. K., Wood, S., and Vendemia, J. M. C. (2012). Suspicious minds: exploring neural processes during exposure to deceptive advertising. J. Mark. Res. 49, 361–372. doi: 10.1509/jmr.09.0007
Dal Mas, D. E., and Wittmann, B. C. (2017). Avoiding boredom: caudate and insula activity reflects boredom-elicited purchase bias. Cortex 92, 57–69. doi: 10.1016/j.cortex.2017.03.008
De Martino, B., Kumaran, D., Holt, B., and Dolan, R. J. (2009). The neurobiology of reference-dependent value computation. J. Neurosci. 29, 3833–3842. doi: 10.1523/JNEUROSCI.4832-08.2009
Del Pinal, G., and Nathan, M. J. (2017). “Two kinds of reverse inference in cognitive neuroscience,” in The Human Sciences after the Decade of the Brain, eds J. Leefmann and E. Hildt (Cambridge, MA: Academic Press), 121–139. doi: 10.1016/B978-0-12-804205-2.00008-2
Delgado, M. R., Locke, H. M., Stenger, V. A., and Fiez, J. A. (2003). Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn. Affect. Behav. Neurosci. 3, 27–38.
Dietvorst, R. C., Verbeke, W. J. M. I., Bagozzi, R. P., Yoon, C., Smits, M., and Van Der, L. (2009). A sales force-specific theory-of-mind scale: tests of its validity by classical methods and functional magnetic resonance imaging. J. Mark. Res. 46, 653–668. doi: 10.1509/jmkr.46.5.653
Dockès, J., Poldrack, R. A., Primet, R., Gözükan, H., Yarkoni, T., Suchanek, F., et al. (2020). Neuroquery, comprehensive meta-analysis of human brain mapping. eLife 9, 1–36. doi: 10.7554/eLife.53385
Doré, B. P., Tompson, S. H., O’Donnell, M. B., An, L. C., Strecher, V., and Falk, E. B. (2019). Neural mechanisms of emotion regulation moderate the predictive value of affective and value-related brain responses to persuasive messages. J. Neurosci. 39, 1293–1300. doi: 10.1523/JNEUROSCI.1651-18.2018
Elliott, M. L., Knodt, A. R., Ireland, D., Morris, M. L., Poulton, R., Ramrakha, S., et al. (2020). What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol. Sci. 31, 792–806. doi: 10.1177/0956797620916786
Esch, F. R., Möll, T., Schmitt, B., Elger, C. E., Neuhaus, C., and Weber, B. (2012). Brands on the brain: do consumers use declarative information or experienced emotions to evaluate brands? J. Consum. Psychol. 22, 75–85. doi: 10.1016/j.jcps.2010.08.004
Falk, E. B., Berkman, E. T., and Lieberman, M. D. (2012). From neural responses to population behavior neural focus group predicts population-level media effects. Psychol. Sci 23, 439–445.
Falk, E. B., O’Donnell, M. B., Tompson, S., Gonzalez, R., Dal Cin, S. D., Strecher, V., et al. (2016). Functional brain imaging predicts public health campaign success. Soc. Cogn. Affect. Neurosci. 11, 204–214. doi: 10.1093/scan/nsv108
Fournier, S. (1998). Consumers and their brands: developing relationship theory in consumer research. J. Consum. Res. 24, 343–353. doi: 10.1086/209515
Fournier, S., and Alvarez, C. (2012). Brands as relationship partners: warmth, competence, and in-between. J. Consum. Psychol. 22, 177–185. doi: 10.1016/j.jcps.2011.10.003
Gearhardt, A. N., Yokum, S., Stice, E., Harris, J. L., and Brownell, K. D. (2014). Relation of obesity to neural activation in response to food commercials. Soc. Cogn. Affect. Neurosci. 9, 932–938. doi: 10.1093/scan/nst059
Genevsky, A., and Knutson, B. (2015). Neural affective mechanisms predict market-level microlending. Psychol. Sci. 26, 1411–1422. doi: 10.1177/0956797615588467
Genevsky, A., Yoon, C., and Knutson, B. (2017). When brain beats behavior: neuroforecasting crowdfunding outcomes. J. Neurosci. 37, 8625–8634. doi: 10.1523/JNEUROSCI.1633-16.2017
Gilmore, R. O., Diaz, M. T., Wyble, B. A., and Yarkoni, T. (2017). Progress toward openness, transparency, and reproducibility in cognitive neuroscience. Ann. N.Y. Acad. Sci. 1396, 1–14. doi: 10.1111/nyas.13325
Gorgolewski, K. J., and Poldrack, R. A. (2016). A practical guide for improving transparency and reproducibility in neuroimaging research. PLoS Biol. 14:e1002506. doi: 10.1371/journal.pbio.1002506
Gorgolewski, K. J., Varoquaux, G., Rivera, G., Schwarz, Y., Ghosh, S. S., Maumet, C., et al. (2015). NeuroVault.Org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform. 9:8. doi: 10.3389/fninf.2015.00008
Grabenhorst, F., Schulte, F. P., Maderwald, S., and Brand, M. (2013). Food labels promote healthy choices by a decision bias in the amygdala. Neuroimage 74, 152–163. doi: 10.1016/j.neuroimage.2013.02.012
Hedgcock, W. M., and Rao, A. R. (2009). Trade-Off aversion as an explanation for the attraction effect: a functional magnetic resonance imaging study. J. Mark. Res. 46, 1–13. doi: 10.1509/jmkr.46.1.1
Hedgcock, W. M., Vohs, K. D., and Rao, A. R. (2012). Reducing self-control depletion effects through enhanced sensitivity to implementation: evidence from fMRI and behavioral studies. J. Consum. Psychol. 22, 486–495. doi: 10.1016/J.JCPS.2012.05.008
Henson, R. (2005). What can functional neuroimaging tell the experimental psychologist? Q. J. Exp. Psychol. Sect. A Hum. Exp. Psychol. 58, 193–233. doi: 10.1080/02724980443000502
Huang, Y., and Yu, R. (2019). Common and distinct neural substrates of the money illusion in win and loss domains. Neuroimage 184, 109–118. doi: 10.1016/j.neuroimage.2018.09.032
Huijsmans, I., Ma, I., Micheli, L., Civai, C., Stallen, M., and Sanfey, A. G. (2019). A scarcity mindset alters neural processing underlying consumer decision making. Proc. Natl. Acad. Sci. U.S.A. 116, 11699–11704. doi: 10.1073/pnas.1818572116
Hutzler, F. (2014). Reverse inference is not a fallacy per se: cognitive processes can be inferred from functional imaging data. Neuroimage 84, 1061–1069. doi: 10.1016/j.neuroimage.2012.12.075
Jepma, M., Verdonschot, R. G., van Steenbergen, H., Rombouts, S. A. R. B., and Nieuwenhuis, S. (2012). Neural mechanisms underlying the induction and relief of perceptual curiosity. Front. Behav. Neurosci. 6:5. doi: 10.3389/fnbeh.2012.00005
Kable, J. W., and Glimcher, P. W. (2007). The neural correlates of subjective value during intertemporal choice. Nat. Neurosci. 10, 1625–1633. doi: 10.1038/nn2007
Kanayet, F. J., Opfer, J. E., and Cunningham, W. A. (2014). The value of numbers in economic rewards. Psychol. Sci. 25, 1534–1545. doi: 10.1177/0956797614533969
Kang, M. J., Rangel, A., Camus, M., and Camerer, C. F. (2011). Hypothetical and real choice differentially activate common valuation areas. J. Neurosci. 31, 461–468. doi: 10.1523/JNEUROSCI.1583-10.2011
Karmarkar, U. R., and Plassmann, H. (2019). Consumer neuroscience: past. present, and future. Organ. Res. Methods 22, 174–195. doi: 10.1177/1094428117730598
Karmarkar, U. R., Shiv, B., and Knutson, B. (2015). Cost conscious? The neural and behavioral impact of price primacy on decision making. J. Mark. Res. 52, 467–481. doi: 10.1509/jmr.13.0488
Kidd, C., and Hayden, B. Y. (2015). The psychology and neuroscience of curiosity. Neuron 88, 449–460. doi: 10.1016/j.neuron.2015.09.010
Kidwell, M. C., Lazareviæ, L. B., Baranski, E., Hardwicke, T. E., Piechowski, S., Falkenberg, L. S., et al. (2016). Badges to acknowledge open practices: a simple, low-cost, effective method for increasing transparency. PLoS Biol. 14:e1002456. doi: 10.1371/journal.pbio.1002456
Klein, O., Hardwicke, T. E., Aust, F., Breuer, J., Danielsson, H., Mohr, A. H., et al. (2018). A practical guide for transparency in psychological science. Collabra Psychol. 4, 1–15. doi: 10.1525/collabra.158
Klucharev, V., Smidts, A., and Fernández, G. (2008). Brain mechanisms of persuasion: how “expert power” modulates memory and attitudes. Soc. Cogn. Affect. Neurosci. 3, 353–366. doi: 10.1093/scan/nsn022
Knutson, B., and Genevsky, A. (2018). Neuroforecasting aggregate choice. Curr. Dir. Psychol. Sci. 27, 110–115. doi: 10.1177/0963721417737877
Knutson, B., Rick, S., Wimmer, G. E., Prelec, D., and Loewenstein, G. (2007). Neural predictors of purchases. Neuron 53, 147–156. doi: 10.1016/j.neuron.2006.11.010
Kühn, S., Strelow, E., and Gallinat, J. (2016). Multiple “buy buttons” in the brain: forecasting chocolate sales at point-of-sale based on functional brain activation using fMRI. Neuroimage 136, 122–128. doi: 10.1016/j.neuroimage.2016.05.021
Lawrence, N. S., Hinton, E. C., Parkinson, J. A., and Lawrence, A. D. (2012). Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage 63, 415–422. doi: 10.1016/j.neuroimage.2012.06.070
Levy, I., Lazzaro, S. C., Rutledge, R. B., and Glimcher, P. W. (2011). Choice from non-choice: predicting consumer preferences from blood oxygenation level-dependent signals obtained during passive viewing. J. Neurosci. 31, 118–125. doi: 10.1523/JNEUROSCI.3214-10.2011
Lighthall, N. R., Huettel, S. A., and Cabeza, R. (2014). Functional compensation in the ventromedial prefrontal cortex improves memory-dependent decisions in older adults. J. Neurosci. 34, 15648–15657. doi: 10.1523/JNEUROSCI.2888-14.2014
Linder, N. S., Uhl, G., Fliessbach, K., Trautner, P., Elger, C. E., and Weber, B. (2010). Organic labeling influences food valuation and choice. Neuroimage 53, 215–220. doi: 10.1016/j.neuroimage.2010.05.077
Loewenstein, G. (1994). The psychology of curiosity: a review and reinterpretation. Psychol. Bull. 116, 75–98. doi: 10.1037/0033-2909.116.1.75
Lombardo, M. V., Chakrabarti, B., Bullmore, E. T., Wheelwright, S. J., Sadek, S. A., Suckling, J., et al. (2010). Shared neural circuits for mentalizing about the self and others. J. Cogn. Neurosci. 22, 1623–1635. doi: 10.1162/jocn.2009.21287
MacInnis, D. J., and Folkes, V. S. (2017). Humanizing brands: when brands seem to be like me, part of me, and in a relationship with me. J. Consum. Psychol. 27, 355–374. doi: 10.1016/j.jcps.2016.12.003
Mahmoudi, A., Takerkart, S., Regragui, F., Boussaoud, D., and Brovelli, A. (2012). Multivoxel pattern analysis for fMRI data: a review. Comput. Math. Methods Med. 2012:961257. doi: 10.1155/2012/961257
Maier, S. F., and Watkins, L. R. (2005). Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci. Biobehav. Rev. 29, 829–841. doi: 10.1016/j.neubiorev.2005.03.021
McClure, S. M., Li, J., Tomlin, D., Cypert, K. S., Montague, L. M., and Montague, P. R. (2004). Neural correlates of behavioral preference for culturally familiar drinks. Neuron 44, 379–387. doi: 10.1016/j.neuron.2004.09.019
Meade, A. W., and Craig, S. B. (2012). Identifying careless responses in survey data. Psychol. Methods 17, 437–455. doi: 10.1037/a0028085
Miller, E. K., and Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 24, 167–202. doi: 10.1146/annurev.neuro.24.1.167
Nichols, T., Das, S., Eickhoff, S., Evans, A., Glatard, T., Hanke, M., et al. (2016). Best practices in data analysis and sharing in neuroimaging using MRI. bioRxiv [Preprint]. doi: 10.1101/054262
Norman, K. A., Polyn, S. M., Detre, G. J., and Haxby, J. V. (2006). Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci. 10, 424–430. doi: 10.1016/j.tics.2006.07.005
O’Doherty, J., Winston, J., Critchley, H., Perrett, D., Burt, D. M., and Dolan, R. J. (2003). Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 41, 147–155. doi: 10.1016/S0028-3932(02)00145-8
Patton, M. Q. (1999). Enhancing the quality and credibility of qualitative analysis. Health Serv. Res. 34, 1189–1208.
Pegors, T. K., Kable, J. W., Chatterjee, A., and Epstein, R. A. (2015). Common and unique representations in pFC for face and place attractiveness. J. Cogn. Neurosci. 27, 959–973. doi: 10.1162/jocn_a_00777
Plassmann, H., O’Doherty, J., and Rangel, A. (2007). Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J. Neurosci. 27, 9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007
Plassmann, H., O’Doherty, J., Shiv, B., and Rangel, A. (2008). Marketing actions can modulate neural representations of experienced pleasantness. Proc. Natl. Acad. Sci. U.S.A. 105, 1050–1054. doi: 10.1073/pnas.0706929105
Plassmann, H., Ramsøy, T. Z., and Milosavljevic, M. (2012). Branding the brain: a critical review and outlook. J. Consum. Psychol. 22, 18–36. doi: 10.1016/j.jcps.2011.11.010
Plassmann, H., Venkatraman, V., Huettel, S., and Yoon, C. (2015). Consumer neuroscience: applications, challenges, and possible solutions. J. Mark. Res. 52, 427–435. doi: 10.1509/jmr.14.0048
Plassmann, H., and Weber, B. (2015). Individual differences in marketing placebo effects: evidence from brain imaging and behavioral experiments. J. Mark. Res. 52, 493–510. doi: 10.1509/jmr.13.0613
Poldrack, R. A. (2006). Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 10, 59–63. doi: 10.1016/j.tics.2005.12.004
Poldrack, R. A. (2008). The role of fMRI in cognitive neuroscience: where do we stand? Curr. Opin. Neurobiol. 18, 223–227.
Poldrack, R. A. (2011). Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron 72, 692–697. doi: 10.1016/j.neuron.2011.11.001
Poldrack, R. A., Barch, D. M., Mitchell, J. P., Wager, T. D., Wagner, A. D., Devlin, J. T., et al. (2013). Towards open sharing of task-based fMRI data: the OpenfMRI project. Front. Neuroinform. 7:12. doi: 10.3389/fninf.2013.00012
Postuma, R. B., and Dagher, A. (2006). Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex 16, 1508–1521. doi: 10.1093/cercor/bhj088
Rademacher, L., Krach, S., Kohls, G., Irmak, A., Gründer, G., and Spreckelmeyer, K. N. (2010). Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage 49, 3276–3285. doi: 10.1016/j.neuroimage.2009.10.089
Reimann, M., Castaño, R., Zaichkowsky, J., and Bechara, A. (2012). How we relate to brands: psychological and neurophysiological insights into consumer-brand relationships. J. Consum. Psychol. 22, 128–142. doi: 10.1016/j.jcps.2011.11.003
Reimann, M., MacInnis, D., and Bechara, A. (2016). Can smaller meals make you happy? Behavioral, neurophysiological, and psychological insights into motivating smaller portion choice. J. Assoc. Consum. Res. 1, 71–91. doi: 10.1086/684285
Reimann, M., MacInnis, D. J., Folkes, V. S., Uhalde, A., and Pol, G. (2018). Insights into the experience of brand Betrayal: from what people say and what the brain reveals. J. Assoc. Consum. Res. 3, 240–254. doi: 10.1086/697077
Reimann, M., Schilke, O., Weber, B., Neuhaus, C., and Zaichkowsky, J. (2011). Functional magnetic resonance imaging in consumer research: a review and application. Psychol. Mark. 28, 608–637. doi: 10.1002/mar.20403
Reimann, M., Zaichkowsky, J., Neuhaus, C., Bender, T., and Weber, B. (2010). Aesthetic package design: a behavioral, neural, and psychological investigation. J. Consum. Psychol. 20, 431–441. doi: 10.1016/j.jcps.2010.06.009
Schaefer, M., and Rotte, M. (2010). Combining a semantic differential with fMRI to investigate brands as cultural symbols. Soc. Cogn. Affect. Neurosci. 5, 274–281. doi: 10.1093/scan/nsp055
Schmidt, L., Tusche, A., Manoharan, N., Hutcherson, C., Hare, T., and Plassmann, H. (2018). Neuroanatomy of the vmPFC and dlPFC predicts individual differences in cognitive regulation during dietary self-control across regulation strategies. J. Neurosci. 38, 5799–5806. doi: 10.1523/JNEUROSCI.3402-17.2018
Schnell, K., Bluschke, S., Konradt, B., and Walter, H. (2011). Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. Neuroimage 54, 1743–1754. doi: 10.1016/j.neuroimage.2010.08.024
Seo, H., and Lee, D. (2007). Temporal filtering of reward signals in the dorsal anterior cingulate cortex during a mixed-strategy game. J. Neurosci. 27, 8366–8377. doi: 10.1523/JNEUROSCI.2369-07.2007
Shrout, P. E., and Rodgers, J. L. (2018). Psychology, science, and knowledge construction: broadening perspectives from the replication crisis. Annu. Rev. Psychol. 69, 487–510. doi: 10.1146/annurev-psych-122216-011845
Smith, D. V., Hayden, B. Y., Truong, T. K., Song, A. W., Platt, M. L., and Huettel, S. A. (2010). Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J. Neurosci. 30, 2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010
Tang, D. W., Fellows, L. K., and Dagher, A. (2014). Behavioral and neural valuation of foods is driven by implicit knowledge of caloric content. Psychol. Sci. 25, 2168–2176. doi: 10.1177/0956797614552081
Taylor, S. F., Martis, B., Fitzgerald, K. D., Welsh, R. C., Abelson, J. L., Liberzon, I., et al. (2006). Medial frontal cortex activity and loss-related responses to errors. J. Neurosci. 26, 4063–4070. doi: 10.1523/JNEUROSCI.4709-05.2006
Tong, L. C., Yavuz Acikalin, M., Genevsky, A., Shiv, B., and Knutson, B. (2020). Brain activity forecasts video engagement in an internet attention market. Proc. Natl. Acad. Sci. U.S.A. 117, 6936–6941. doi: 10.1073/pnas.1905178117
Tusche, A., Bode, S., and Haynes, J. D. (2010). Neural responses to unattended products predict later consumer choices. J. Neurosci. 30, 8024–8031. doi: 10.1523/JNEUROSCI.0064-10.2010
Van Veen, V., Cohen, J. D., Botvinick, M. M., Stenger, V. A., and Carter, C. S. (2001). Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14, 1302–1308. doi: 10.1006/nimg.2001.0923
Venkatraman, V., Clithero, J. A., Fitzsimons, G. J., and Huettel, S. A. (2012). New scanner data for brand marketers: how neuroscience can help better understand differences in brand preferences. J. Consum. Psychol. 22, 143–153. doi: 10.1016/j.jcps.2011.11.008
Venkatraman, V., Dimoka, A., Pavlou, P. A., Vo, K., Hampton, W., Bollinger, B., et al. (2015). Predicting advertising success beyond traditional measures: new insights from neurophysiological methods and market response modeling. J. Mark. Res. 52, 436–452. doi: 10.1509/jmr.13.0593
Wager, T. D., Lindquist, M. A., Nichols, T. E., Kober, H., and Van Snellenberg, J. X. (2009). Evaluating the consistency and specificity of neuroimaging data using meta-analysis. Neuroimage 45, S210–S221. doi: 10.1016/j.neuroimage.2008.10.061
Warren, C., and Reimann, M. (2019). Crazy-funny-cool theory: divergent reactions to unusual product designs. J. Assoc. Consum. Res. 4, 409–421. doi: 10.1086/705036
Weber, B., Aholt, A., Neuhaus, C., Trautner, P., Elger, C. E., and Teichert, T. (2007). Neural evidence for reference-dependence in real-market-transactions. Neuroimage 35, 441–447. doi: 10.1016/j.neuroimage.2006.11.034
Wiggin, K. L., Reimann, M., and Jain, S. P. (2019). Curiosity tempts indulgence. J. Consum. Res. 45, 1194–1212. doi: 10.1093/jcr/ucy055
Wixted, J. T., and Mickes, L. (2013). On the relationship between fMRI and theories of cognition: the arrow points in both directions. Perspect. Psychol. Sci. 8, 104–107. doi: 10.1177/1745691612469022
Yarkoni, T. (2015a). No, the Dorsal Anterior cingulate is Not Selective for Pain: Comment on Lieberman and Eisenberger (2015). Available online at: https://www.talyarkoni.org/blog/2015/12/05/no-the-dorsal-anterior-cingulate-is-not-selective-for-pain-comment-on-lieberman-and-eisenberger-2015/ (Accessed June 25, 2020).
Yarkoni, T. (2015b). Still Not Selective: Comment on Comment on Comment on Lieberman & Eisenberger (2015). Available online at: https://www.talyarkoni.org/blog/2015/12/14/still-not-selective-comment-on-comment-on-comment-on-lieberman-eisenberger-2015/ (accessed June 25, 2020).
Yarkoni, T., Poldrack, R. A., Nichols, T. E., Van Essen, D. C., and Wager, T. D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 8, 665–670. doi: 10.1038/nmeth.1635
Yokoyama, R., Nozawa, T., Sugiura, M., Yomogida, Y., Takeuchi, H., Akimoto, Y., et al. (2014). The neural bases underlying social risk perception in purchase decisions. Neuroimage 91, 120–128. doi: 10.1016/j.neuroimage.2014.01.036
Keywords: consumer neuroscience, fMRI, data triangulation, neuroimaging database, validity
Citation: Cao CC and Reimann M (2020) Data Triangulation in Consumer Neuroscience: Integrating Functional Neuroimaging With Meta-Analyses, Psychometrics, and Behavioral Data. Front. Psychol. 11:550204. doi: 10.3389/fpsyg.2020.550204
Received: 09 April 2020; Accepted: 08 October 2020;
Published: 05 November 2020.
Edited by:
Thomas Zoëga Ramsøy, Neurons Inc., DenmarkReviewed by:
Ale Smidts, Erasmus University Rotterdam, NetherlandsCopyright © 2020 Cao and Reimann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Clark Cao, Y2xhcmtjYW9AbG4uZWR1Lmhr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.