
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Psychol. , 07 July 2020
Sec. Psychology for Clinical Settings
Volume 11 - 2020 | https://doi.org/10.3389/fpsyg.2020.01353
 Christian Franceschini1*
Christian Franceschini1* Chiara Fante2
Chiara Fante2 Marco Filardi3,4
Marco Filardi3,4 Maria Claudia Folli1
Maria Claudia Folli1 Francesca Brazzi1
Francesca Brazzi1 Fabio Pizza3,4
Fabio Pizza3,4 Anita D’Anselmo3,4
Anita D’Anselmo3,4 Francesca Ingravallo5
Francesca Ingravallo5 Elena Antelmi6
Elena Antelmi6 Giuseppe Plazzi3,4
Giuseppe Plazzi3,4Introduction: Narcolepsy type 1 (NT1) is a chronic and rare sleep disorder typically arising during adolescence and young adulthood. The main symptoms are excessive daytime sleepiness and cataplexy, a prototypical fall down elicited by huge emotions. Social relationships, school, work, and general health perception are frequently impaired in patients, who often show lower quality-of-life scores. We report which management strategies a young patient (DMG) adopted to cope with NT1 during his growth, avoiding exhibiting serious impairments to his global functioning.
Methods: A clinical psychologist explores the history of the patient’s disease and the self-acquired strategies used to cope with the symptoms. The patient’s global adaptation to the disease, stress-related managing skills, and overall well-being are assessed by standardized scales [Illness Behavior Questionnaire (IBQ); Coping Orientations to Problems Experienced (COPE); and Psychological General Well-Being Index (PGWBI)]. We conducted a qualitative analysis of the patient’s narration of his illness according to the procedure of the Grounded Theory. The MAXQDA software program was used to code the verbatim transcript.
Results: From the qualitative analysis of the interview, three thematic cores emerged: 1) the disease history; 2) the patient’s friendship with AD, a friend of his age diagnosed with NT1 since childhood; 3) the strategies used to deal with his symptoms before the diagnosis of NT1 and the related treatment. From the psychometric tests, the patient presents good coping strategies in dealing with stressful problems and events based mainly on acceptance and positive reinterpretation of the stressful situation.
Conclusion: This case shows that comparing peers of the same age and suffering from the same illness improve the patient’s self-management ability to cope and live well with NT1.
Narcolepsy type 1 (NT1) is a rare and chronic central nervous system hypersomnia characterized by excessive daytime sleepiness (EDS), sudden loss of muscle tone elicited by intense emotions, usually laughing and joking (cataplexy), sleep paralysis, hypnagogic hallucinations, and disrupted nocturnal sleep [American Academy of Sleep Medicine (AASM), 2014]. The diagnosis relies on polysomnography (PSG) and multiple sleep latency test (MSLT) [American Academy of Sleep Medicine (AASM), 2014], documenting the neurophysiological hallmarks of narcolepsy: shortened sleep latency and sleep-onset rapid eye movement (REM) periods (SOREMPs). Low cerebrospinal fluid hypocretin-1 (CSF hcrt-1) levels (below 110 pg/ml) and EDS complaints are also sufficient to establish this diagnosis.
The disease is caused by hypocretin neuronal loss, most likely on autoimmune basis (Latorre et al., 2018).
NT1 onset displays a bimodal incidence peak around 15 and around 36 years of age (Plazzi et al., 2011; Thorpy and Krieger, 2014), with a prevalence of 0.02–0.06% in the American and European population (Mignot, 1998).
In NT1 patients, high comorbidity for other medical conditions is usually reported: weight gain up to obesity (Ponziani et al., 2016), precocious puberty (Postiglione et al., 2018), and psychiatric disorders, in particular depression (37.9%) (Dauvilliers et al., 2009; Fortuyn et al., 2011; Ruoff et al., 2017) and anxiety disorders (53%) (Fortuyn et al., 2010). An association with schizophrenia-like psychosis can also be noticed (Plazzi et al., 2015). Besides, from a psychological point of view, illness-related problems at school/work and in maintaining stable romantic relationships can seriously impair the patient’s quality of life (Ingravallo et al., 2012; Plazzi et al., 2018; Raggi et al., 2019).
Available treatments for NT1 are only symptomatic, primarily focused on EDS and cataplexy. The former is generally treated with stimulants, while antidepressants are suggested for cataplexy. Sodium oxybate (gamma-hydroxybutyric acid B-subtype receptor agonist - GABAb) and pitolisant (H3 receptor antagonist/inverse agonist) can be administered for both symptoms (Franceschini et al., 2020).
As we mentioned before, NT1 implies psychological issues that, if managed, can facilitate better adherence to pharmacological therapy and improve the quality of life. In this regard, as suggested by clinical international guidelines (Britton et al., 2002; Billiard et al., 2006), scheduling naps, work planning, psychosocial support, and behavioral therapy may reduce the negative consequences of this disease.
Quality of life in patients suffering from chronic disease is related to a good management of the disease. Accordingly, identifying and proposing novel management strategies to patients may significantly improve their overall well-being: psychological counseling successfully helps both the patient and his/her parents in developing management skills, and educational programs can improve the patient’s knowledge and confidence related to his/her disorder (Shilling et al., 2013).
Other techniques concentrate on recognizing symptoms and taking appropriate actions by developing strategies to deal with the illness and interact with the healthcare system over time (Schulman-Green et al., 2012). Among these, self-management is “the ability of the individual, in conjunction with family, community, and healthcare professionals, to manage symptoms, treatments, lifestyle changes, and psychosocial, cultural, and spiritual consequences of health conditions” (Richard and Shea, 2011) and stands as a dynamic and interactive process that directs individuals to manage a chronic illness (Lorig and Holman, 2003).
We present a case of a young NT1 patient who was able to develop the self-management process on his symptoms and perform academic activities without serious impairments. For this purpose, we conducted a qualitative analysis of the patient’s narration of his illness and correlated it to a psychological evaluation (carried out through standardized tests) to describe the management strategy/process that the patient adopted to cope with the symptoms.
The report has been conducted according to the CARE guidelines for case reports (Riley et al., 2017).
At the age of 21, the patient (DMG are his fake initials) was referred to the center for narcolepsy of the University of Bologna for complaints of EDS and cataplexy.
During the clinical interview, the patient claimed to be already aware of having narcolepsy, as one of his classmates (AD are his fake initials) has been afflicted with the pathology since childhood. DMG recollected the onset of daytime sleepiness at the age of 6, especially in the early afternoon, with multiple sleep episodes when he was mentally exhausted or bored. The sleep need was described as unavoidable, and nap duration was generally short (i.e., 5 min) and associated with feelings of fatigue and a sensation of warmth upon awakening. The patient reported cataplexy episodes involving mainly the facial district and elicited by intense laugh (1–2 times a week). He never experienced sleep paralysis or hypnagogic/hypnopompic hallucinations.
At diagnostic hospitalization, the patient presented with severe sleepiness [Epworth Sleepiness Scale (ESS) = 18] (Johns, 1991; Vignatelli et al., 2003) and was markedly overweight [body mass index (BMI) = 29] (Ponziani et al., 2016).
The 24-hour continuous video-polysomnography (v-PSG) documented several daytime naps with SOREMPs. Nocturnal sleep was disturbed by frequent awakenings and described as non-refreshing (Figure 1A). The MSLT confirmed pathological sleep propensity (mean sleep latency = 2.30 min) with 5/5 SOREMPs (Figure 1B).
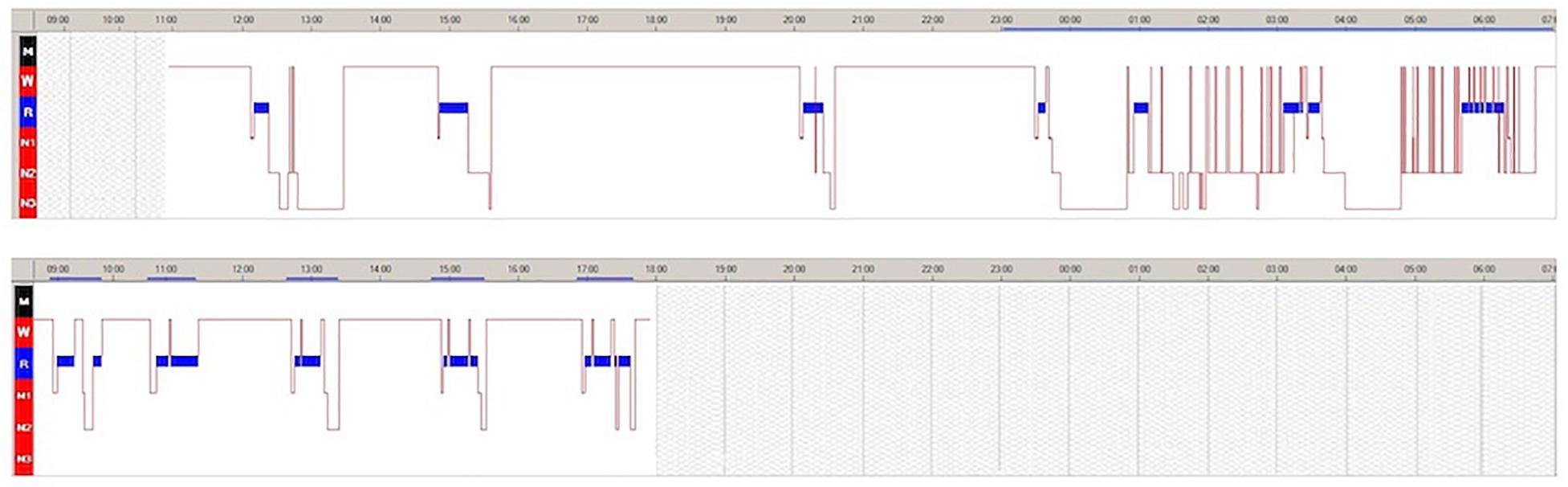
Figure 1. Twenty-four-hour video-polysomnography (v-PSG) (A) and multiple sleep latency test (MSLT) (B) during the hospitalizations. Blue bar indicates rapid eye movement (REM) sleep. The solid blue line indicates the period between when the light was switched off and when it was switched on.
The patient had undetectable CSF hcrt-1. Accordingly, NT1 was diagnosed, and a treatment with sodium oxybate up to 9 g was started with a remarkable improvement on outpatient follow-up (after 12 months) on both hypersomnolence (ESS = 5) and cataplexy (1/2 times a month to the face); moreover, his weight returned within the normal range (BMI 25 kg/m2).
Although narcolepsy-related symptoms emerged in his clinical history since childhood, with an escalation during adolescence, by the time of the diagnosis, DMG showed quite a good adaptation. Indeed, as reported, the patient, already aware of the pathology’s symptoms for some considerable time, managed to deal with both sleepiness symptoms and cataplexy episodes, without falling into scholastic failures and/or social isolation.
An interview that lasted about an hour and a half with a clinical psychologist (ChrF) was carried out to collect the patient’s disease history and information on the self-acquired strategies adopted to cope with the symptoms. Moreover, the patient completed standardized scales assessing global adaptation to the disease, stress-related managing skills, and overall well-being. More in detail, the Coping Orientations to Problems Experienced (COPE) (Carver et al., 1989; Sica et al., 1997) was used to assess coping skills defined as the abilities and strategies used to deal with problematic or stress-related situations. The COPE consists of 15 scales (four items each), namely, Activity, Planning, Suppression of competitive activities, Containment, Information research, Search for understanding, Emotional outburst, Positive reinterpretation and growth, Acceptance, Religious devotion, Humor, Mental detachment, Behavioral detachment, Denial, and Alcohol and drug use. The Illness Behavior Questionnaire (IBQ) (Pilowsky and Spence, 1975; Fava et al., 1982) is used to evaluate modalities in which individuals react to aspects of their physical functionality and consists of 62 items that explore emotions and inclinations regarding the disease, perception of significant people’s reaction (including the physician), and vision of his psychosocial situation. The IBQ is formed by the following scales: Global hypochondria, Belief to suffer from the illness, Psychological/somatic perception, Emotional inhibition, Denial, and Irritability.
Finally, health-related quality of life was evaluated through the Psychological General Well-Being Index (PGWBI) (Dupuy, 1984; Grossi et al., 2002). The questionnaire consists of 22 items that explore six different dimensions: Anxiety, Depression, Positivity and wellness, Self-control, All- around health, and Vitality.
The Grounded Theory (GT) is a method inspired by an interpretative model that aims to deeply analyze the meanings connected to the studied subject (Arcidiacono and Gregorio, 2008). While a quantitative method aims to validate prior theories by referring to a linear paradigm, GT systematically gathers and analyzes research data that cannot be manipulated by the researcher but are instead coherent with the real collection situations. In this way, it is possible to generate theories truly based on data (Glaser and Strauss, 2017).
Accordingly, the open-codification procedure of the GT was followed in order to analyze the interview (Strauss and Corbin, 1990).
The recording of the interview was transcribed verbatim. The transcript was coded using the MAXQDA software program (VERBI GmbH, Marburg, Germany), applying open and axial coding (Boeije, 2010).
We started with an explorative analysis of the general content, following a bottom-up approach where no categories are defined before looking at the material. To reduce errors in this process and identify all possible categories, two different researchers analyzed the material independently (ChrF; CF). As the study concerns a single case, we preferred to consider every single theme that emerged, without considering potential long passages.
Each of the researchers was asked to identify in vivo a series of emerging themes and possible subthemes (“codes”). A codebook was created based on the preliminary results achieved by the two encoders. Later, the two researchers (separately) analyzed the interview by assigning codes (identified according to their content) to specific portions of the text. This way, they obtained a satisfactory intersubjective agreement grade (kappa = 0.82).
Unrefined scores of each scale were calculated for every qualitative instrument that was administered to the patient and then converted into corresponding Z-scores.
Results of standardized tests are reported in Table 1. Regarding coping strategies developed to deal with stressful situations, the patient shows significant marks in relation to the dimensions “Activity” and “Positive attitude.” These scales are considered adaptive in problem-focused coping: undertaking an action to eliminate or reduce stress effects (Activity scale, z = 1.5); thinking, planning and elaborating strategies to solve the problem (Planning scale, z = 1.5), and putting aside every other activity, avoiding distractions to better face situations (Suppression of competitive activities scale, z = 1.8) are modalities frequently used by the patient. Other significant strategies are critical elaboration (positive/growth) of his experience (Positive reinterpretation scale, z = 1.8) and acceptance of the situation and/or his inability to face it (Acceptance scale, z = 1.9). There is no evidence of an excessive resort to unsuitable modalities shaped on avoidance or denial (his marks do not deviate from the cross-section’s ones).
The patient does not show any higher score on IBQ scales, suggesting the absence of clinically significant behaviors or attitudes related to the pathology (i.e., hypochondria, denial, or emotional inhibition). General perceived well-being, assessed with the PGWBI, was slightly lower than the reference value for the male population (70 vs. 88).
Three thematic cores emerged from the qualitative analysis of the interview: (1) history of the disease; (2) story of DMG’s friendship with AD; (3) strategies (partially self-acquired) adopted to deal with the symptoms before diagnosis of NT1 and related treatment.
A series of codes were identified to describe each thematic core. The identified codes, their definition, and the number of text’s portions they codify are shown in Table 2.

Table 2. Identified codes for each thematic core, their definition and number of text’s sections codified.
Within the “History of the disease” thematic core, we collect the patient’s early memories originated from what his mother told him about his childhood (code: “mother’s memories of symptoms during childhood”), together with DMG’s own memories about EDS (code: “early memories of sleepiness”) and cataplexy episodes (code: “early memories of cataplexy-related symptoms”) during his adolescence.
With regard to the “Relationship with his peer afflicted with narcolepsy,” in the patient’s report, we notice the importance of his relationship with his friend of the same age afflicted by NT1. The comparison between his situation and the one of his peer allowed the patient to better recognize NT1 symptoms (code: “knowledge of the disease thanks to his friendship with AD”) but also raise his awareness about it (code: “awareness of the disease thanks to his friendship with AD”).
Finally, in the thematic core, “Strategies to cope with the disease,” some strategies used by the patient to manage his condition, implemented before having a confirmed diagnosis, emerged. They vary from management of sleepiness symptoms (code: “management through naps” and “management through good sleep”) to specific learning strategies to fulfill academic requests and reduce limitations (code: “study planning through learning strategies”) and global adaptive stress management strategies (code: “problem-focused coping,” “stress management,” and “observation of peers”). Moreover, DMG reported a regular practice of physical activity as a means to maintain his well-being (code: “management through physical activity”).
Example sentences for each thematic core and codes from the patient’s interview are reported in Table 3.
This is the first qualitative study focused on symptomatology self-management in a patient with NT1, a potential approach to facilitate well-being and coping strategies in patients affected by this sleep disease.
Interestingly, before the diagnosis, the patient, although untreated and without any medical advice, showed an optimal adaptation to narcolepsy symptoms. Standardized tests did not highlight any particular critical areas, except for moderate levels of general distress, confirming proper psychological and social functioning. Regarding his disease history, no unusual and suffering-orientated behaviors have been observed. Moreover, DMG adopted good coping strategies, based on acceptance and positive reinterpretation of situations, to face problems and stressful events.
Qualitative analysis of the patient seems to confirm the collection of these heterogeneous symptoms in the diagnosis of NT1. DMG is aware of the disease, thanks to his relationship with a peer diagnosed with NT1 during childhood. This friendship allowed DMG to become familiar with his symptoms and recognize them in his global functioning (code: “knowledge of the disease thanks to his friendship with AD”): the fact that DMG attributed his first episodes of muscular weakness and sudden sleep attacks to a medical condition (code: “awareness of the disease thanks to his friendship with AD”) stands as a striking example of such process.
The opportunity to compare what he was undergoing with the experience of his friend provided him an explanation that has reasonably reduced his feelings of insecurity, confusion, or fear, which are typical of NT1 patients before getting a diagnosis (Thorpy and Krieger, 2014). Besides, the patient was able to acquire functional strategies to manage NT1 symptoms, especially sleepiness (taking several short naps). Accordingly, the patient’s statements confirm the hypothesis that peers’ support and mutual learning are key factors to improve management skills and self-trust and therefore reduce the sense of diversity and contribute to a more positive self-image (Johnston et al., 2012; Jormfeldt et al., 2012).
The currently available literature proposes a distinction in three macro-categories when referring to self-management processes related to chronic illnesses (Schulman-Green et al., 2012): (a) focusing on illness needs (i.e., all tasks and skills needed to the individual to manage daily needs); (b) activating resources (i.e., all individuals and social resources); and (c) living with a chronic illness (i.e., all tasks and skills related to coping with the illness and growing as a person). By analyzing the patient’s interview, we can see how he developed adaptive strategies for each of these categories; for instance, as for “Focusing on illness needs (a),” the patient reports how he learned to reduce sleepiness symptoms by adequate sleep hygiene, planned naps throughout the day, and physical activity. DMG seemed to have globally activated psychological and social resources, and therefore, he managed his most stressful moments by sharing with peers and learning through observation (b). Finally, organizing specific strategies for studying and a problem-oriented coping style allowed him to live well with NT1 (c). COPE results coupled with the evaluation of the qualitative interview analysis show how the patient tends to activate confident strategies like thinking, planning, and elaborating ways to overcome problems when faced with stressful situations, which are re-elaborated in a positive connotation.
Moreover, literature has often reported that among all symptoms, EDS has a major impact on the quality of life, relationships, and school/work conditions of an NT1 patient: being in public may be extremely troubling because of the fear of a sudden sleep attack or falling to the ground as a consequence of cataplexy (Daniels et al., 2001; Vignatelli et al., 2004, 2011; Avis et al., 2015; Raggi et al., 2019). This constant preoccupation with others’ opinions often results in a stigmatization process that affects these patients’ social life (Rovere et al., 2008; Kapella et al., 2015; Franceschini et al., 2020). It has been clear for years (Alaia, 1992; Rovere et al., 2008; Franceschini et al., 2020) that narcolepsy treatments cannot be limited to pharmacological therapy but needs to be implemented with social support and help from a peer who is aware of the challenges brought by this disease.
Finally, this clinical observation of DMG’s story supports the hypothesis that patients with chronic illnesses can feel better and generally more hopeful about their condition when they can compare themselves to someone else who, suffering from the same disease, seems to manage his/her symptoms efficiently (upward social comparison) (Stenberg et al., 2016). One of the key strengths of this manuscript is that it offers the opportunity to develop a discussion around innovative approaches in increasing NT1 patients’ management skills and adaptation. Providing settings where patients can freely share their everyday problems and experiences may ease the process of dealing with symptoms-related emotions, as well as discovering new self-management strategies and finally gain a more fulfilling quality of life. On the other hand, a possible limitation of this report may be the fact that it reflects only the psychological experience and outcomes of a single individual. Nevertheless, this study certainly underlines the importance of creating a setting where NT1 patients can get a real feeling of understanding when explaining controversial emotions, such as peer support-oriented groups and psycho-educational interventions to promote adaptation and management skills. Finally, future clinical research studies should be centered on understanding the role played by patients’ coping strategies and by familial environmental factors to better help the well-being of NT patients.
To date, this is the first original study adopting a qualitative approach to explore the efficacy of peer support in self-management processes specific for NT1. The clinical history of DMG and his management strategies, strongly influenced by the observation of his friend afflicted with the same illness, suggest the importance for patients to share their own experiences. Creating settings where NT1 patients can interact with each other and talking about symptoms may be strongly beneficial, together with providing support for those who are experiencing NT1 symptoms for the first time.
The opportunity to learn new perspectives about perceiving and managing the disease represents a crucial element of an effective self-management promoting team for patients with NT1.
Ethical approval was not provided for this study on human participants because the study has been conducted according to the principles set forth by the Declaration of Helsinki (59th WMA General Assembly, Seoul, October 2008) and in accordance with the Medical Research Involving Human Subjects Act (WMO). Written informed consent was obtained from DMG both for the purposes of research participation as well as for the publication of the case report, including indirectly identifiable data. The patients or participants provided their written informed consent to participate in this study.
The case report study was based on a concept developed by CFr who wrote the manuscript and took part in the review and critique processes as PI. CFr conducted the clinical interview to the patient. CFa organized the study, performed the neuropsychological assessment (organization and execution), and participated in the review and critique processes. MF participated in the interpretation of all the psychological results and participated in the review and critique processes. MCF participated in the review and critique process of manuscripts. FB, FP, FI, and EA critically reviewed the manuscripts and gave their approval of this version of the manuscript to be submitted. GP had followed the clinical developmental of the case report and the critical review process of manuscripts. All authors contributed to the article and approved the submitted version.
GP participated in the advisory board of UCB Pharma, Jazz Pharmaceuticals, and BioProject.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We gently thank Cecilia Baroncini, Graziella Magistrali, and Maria Gina Torreggiani for their great help in editing the English text.
EDS, excessive daytime slepiness; NT1, narcolepsy type 1.
Alaia, S. L. (1992). Life effects of narcolepsy. Loss Grief Care 5, 1–22. doi: 10.1300/J132v05n03_01
American Academy of Sleep Medicine (AASM) (2014). International Classification Of Sleep Disorders, 3rd Edn, Darien, IL: American Academy of Sleep Medicine.
Arcidiacono, F., and Gregorio, E. D. (2008). Methodological thinking in psychology: starting from mixed methods. Intern. J. Mult. Res. Approach. 2, 118–126. doi: 10.5172/mra.455.2.1.118
Avis, K. T., Shen, J., Weaver, P., and Schwebel, D. C. (2015). Psychosocial characteristics of children with central disorders of hypersomnolence versus matched healthy children. J. Clin. Sleep. Med. 11, 1281–1288. doi: 10.5664/jcsm.5186
Billiard, M., Bassetti, C., Dauvilliers, Y., Dolenc-Groselj, L., Lammers, G. J., Mayer, G., et al. (2006). EFNS guidelines on management of narcolepsy. Eur. J. Neurol. 13, 1035–1048. doi: 10.1111/j.1468-1331.2006.01473.x
Britton, T., Hansen, A., Hicks, J., Howard, R., and Meredith, A. (2002). Guidelines on the Diagnosis And Management Of Narcolepsy In Adults And Children: Evidence-Based Guidelines for the UK With Graded Recommendations. Ashtead: Taylor Patten Communications Ltd.
Carver, C. S., Scheier, M. F., and Weintraub, J. K. (1989). Assessing coping strategies: a theoretically based approach. J. Pers. Soc. Psychol. 56, 267–283. doi: 10.1037//0022-3514.56.2.267
Daniels, E., King, M. A., Smith, I. E., and Shneerson, J. M. (2001). Health-related quality of life in narcolepsy. J. Sleep Res. 10, 75–81. doi: 10.1046/j.1365-2869.2001.00234.x
Dauvilliers, Y., Paquereau, J., Bastuji, H., Drouot, X., Weil, J.-S., and Viot-Blanc, V. (2009). Psychological health in central hypersomnias: the french harmony study. J. Neurol. Neurosurg. Psychiatr. 80, 636–641. doi: 10.1136/jnnp.2008.161588
Dupuy, H. J. (1984). “The psychological general well-being (PGWB) index,” in Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapie, eds N. K. Wenger, M. E. Mattson, C. D. Furburg, and J. Elinson (New York, NY: Le Jacq Publishing).
Fava, G. A., Bernardi, M., Pilowsky, I., and Spence, N. D. (1982). “Versione italiana dell’ “Illness behavior questionnaire” (IBQ) di pilowsky e spence,” in Nuovi metodi in Psicometria, ed. R. Canestrari (Florence: Organizzazioni Speciali), 65–73.
Fortuyn, H. A. D., Lappenschaar, M. A., Furer, J. W., Hodiamont, P. P., Rijnders, C. A., Renier, W. O., et al. (2010). Anxiety and mood disorders in narcolepsy: a case-control study. Gen. Hosp. Psychiatry 32, 49–56. doi: 10.1016/j.genhosppsych.2009.08.007
Fortuyn, H. A. D., Mulders, P. C., Renier, W. O., Buitelaar, J. K., and Overeem, S. (2011). Narcolepsy and psychiatry: an evolving association of increasing interest. Sleep Med. 12, 714–719. doi: 10.1016/j.sleep.2011.01.013
Franceschini, C., Fante, C., Folli, M. C., Filosa, M., Pizza, F., Antelmi, E., et al. (2020). Giving a voice to cataplectic experience: recollections from patients with narcolepsy type 1. J. Clin. Sleep Med. 16, 597–603. doi: 10.5664/jcsm.8286
Franceschini, C., Pizza, F., Antelmi, E., Folli, M. C., and Plazzi, G. (2020). Narcolepsy treatment: pharmacological and behavioral strategies in adults and children. Sleep Breath 24, 615–627. doi: 10.1007/s11325-019-01894-1894
Glaser, B. G., and Strauss, A. L. (2017). Discovery of Grounded Theory: Strategies For Qualitative Research. New York, NY: Routledge.
Grossi, E., Mosconi, P., Groth, N., Niero, M., and Apolone, G. (2002). Questionario Psychological General Well-Being Index: Versione Italiana. Milano: Istituto Farmacologico Mario Negri.
Ingravallo, F., Gnucci, V., Pizza, F., Vignatelli, L., Govi, A., Dormi, A., et al. (2012). The burden of narcolepsy with cataplexy: how disease history and clinical features influence socio-economic outcomes. Sleep Med. 13, 1293–1300. doi: 10.1016/j.sleep.2012.08.002
Johns, M. W. (1991). A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep 14, 540–545. doi: 10.1093/sleep/14.6.540
Johnston, S., Irving, H., Mill, K., Rowan, M. S., and Liddy, C. (2012). The patient’s voice: an exploratory study of the impact of a group self-management support program. BMC Fam. Pract. 13:65. doi: 10.1186/1471-2296-13-65
Jormfeldt, H., Rask, M., Brunt, D., Bengtsson, A., and Svedberg, P. (2012). Experiences of a person-centred health education group intervention–a qualitative study among people with a persistent mental illness. Issues Ment. Health Nurs. 33, 209–216. doi: 10.3109/01612840.2011.653041
Kapella, M. C., Berger, B. E., Vern, B. A., Vispute, S., Prasad, B., and Carley, D. W. (2015). Health-related stigma as a determinant of functioning in young adults with narcolepsy. PLoS One 10:e0122478. doi: 10.1371/journal.pone.0122478
Latorre, D., Kallweit, U., Armentani, E., Foglierini, M., Mele, F., Cassotta, A., et al. (2018). T cells in patients with narcolepsy target self-antigens of hypocretin neurons. Nature 562, 63–68. doi: 10.1038/s41586-018-0540-541
Lorig, K. R., and Holman, H. (2003). Self-management education: history, definition, outcomes, and mechanisms. Ann. Behav. Med. 26, 1–7. doi: 10.1207/S15324796ABM2601_01
Mignot, E. (1998). Genetic and familial aspects of narcolepsy. Neurology 50, S16–S22. doi: 10.1212/wnl.50.2_suppl_1.s16
Pilowsky, I., and Spence, N. D. (1975). Patterns of illness behaviour in patients with intractable pain. J. Psychosom. Res. 19, 279–287. doi: 10.1016/0022-3999(75)90026-90024
Plazzi, G., Clawges, H. M., and Owens, J. A. (2018). Clinical characteristics and burden of illness in pediatric patients with narcolepsy. Pediatr. Neurol. 85, 21–32. doi: 10.1016/j.pediatrneurol.2018.06.008
Plazzi, G., Fabbri, C., Pizza, F., and Serretti, A. (2015). Schizophrenia-like symptoms in narcolepsy type 1: shared and distinctive clinical characteristics. Neuropsychobiology 71, 218–224. doi: 10.1159/000432400
Plazzi, G., Pizza, F., Palaia, V., Franceschini, C., Poli, F., Moghadam, K. K., et al. (2011). Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain 134, 3477–3489. doi: 10.1093/brain/awr244
Ponziani, V., Gennari, M., Pizza, F., Balsamo, A., Bernardi, F., and Plazzi, G. (2016). Growing Up with Type 1 narcolepsy: its anthropometric and endocrine features. J. Clin. Sleep Med. 12, 1649–1657. doi: 10.5664/jcsm.6352
Postiglione, E., Antelmi, E., Pizza, F., Lecendreux, M., Dauvilliers, Y., and Plazzi, G. (2018). The clinical spectrum of childhood narcolepsy. Sleep Med. Rev. 38, 70–85. doi: 10.1016/j.smrv.2017.04.003
Raggi, A., Plazzi, G., and Ferri, R. (2019). Health-related quality of life in patients with narcolepsy: a review of the literature. J. Nerv. Ment. Dis. 207, 84–99. doi: 10.1097/NMD.0000000000000918
Richard, A. A., and Shea, K. (2011). Delineation of self-care and associated concepts. J. Nurs. Scholarsh. 43, 255–264. doi: 10.1111/j.1547-5069.2011.01404.x
Riley, D. S., Barber, M. S., Kienle, G. S., Aronson, J. K., von Schoen-Angerer, T., Tugwell, P., et al. (2017). CARE guidelines for case reports: explanation and elaboration document. J. Clin. Epidemiol. 89, 218–235. doi: 10.1016/j.jclinepi.2017.04.026
Rovere, H., Rossini, S., and Reimão, R. (2008). Quality of life in patients with narcolepsy: a WHOQOL-bref study. Arq. Neuropsiquiatr. 66, 163–167. doi: 10.1590/s0004-282x2008000200004
Ruoff, C. M., Reaven, N. L., Funk, S. E., McGaughey, K. J., Ohayon, M. M., Guilleminault, C., et al. (2017). High rates of psychiatric comorbidity in narcolepsy: findings from the burden of narcolepsy disease (BOND) study of 9,312 patients in the United States. J. Clin. Psychiatry 78, 171–176. doi: 10.4088/JCP.15m10262
Schulman-Green, D., Jaser, S., Martin, F., Alonzo, A., Grey, M., McCorkle, R., et al. (2012). Processes of self-management in chronic illness. J. Nurs. Scholarsh. 44, 136–144. doi: 10.1111/j.1547-5069.2012.01444.x
Shilling, V., Morris, C., Thompson-Coon, J., Ukoumunne, O., Rogers, M., and Logan, S. (2013). Peer support for parents of children with chronic disabling conditions: a systematic review of quantitative and qualitative studies. Dev. Med. Child. Neurol. 55, 602–609. doi: 10.1111/dmcn.12091
Sica, C., Novara, C., Dorz, S., and Sanavio, E. (1997). Coping strategies: evidence of cross-cultural differences? a preliminary study with the Italian version of coping orientations to problems experienced (COPE). Pers. Indiv. Differ. 23, 1025–1029.
Stenberg, U., Haaland-Øverby, M., Fredriksen, K., Westermann, K. F., and Kvisvik, T. (2016). A scoping review of the literature on benefits and challenges of participating in patient education programs aimed at promoting self-management for people living with chronic illness. Pat. Educ. Couns. 99, 1759–1771. doi: 10.1016/j.pec.2016.07.027
Strauss, A., and Corbin, J. (1990). Basics of Qualitative Research: Grounded Theory Procedures And Techniques. Newbury Park, CA: Sage.
Thorpy, M. J., and Krieger, A. C. (2014). Delayed diagnosis of narcolepsy: characterization and impact. Sleep Med. 15, 502–507. doi: 10.1016/j.sleep.2014.01.015
Vignatelli, L., D’Alessandro, R., Mosconi, P., Ferini-Strambi, L., Guidolin, L., De Vincentiis, A., et al. (2004). Health-related quality of life in Italian patients with narcolepsy: the SF-36 health survey. Sleep Med. 5, 467–475. doi: 10.1016/j.sleep.2004.04.003
Vignatelli, L., Plazzi, G., Barbato, A., Ferini-Strambi, L., Manni, R., Pompei, F., et al. (2003). Italian version of the epworth sleepiness scale: external validity. Neurol. Sci. 23, 295–300. doi: 10.1007/s100720300004
Keywords: narcolepsy, case report, clinical psychology, peer support, self-management, coping strategies, narrative medicine
Citation: Franceschini C, Fante C, Filardi M, Folli MC, Brazzi F, Pizza F, D’Anselmo A, Ingravallo F, Antelmi E and Plazzi G (2020) Can a Peer Support the Process of Self-Management in Narcolepsy? A Qualitative Narrative Analysis of a Narcoleptic Patient. Front. Psychol. 11:1353. doi: 10.3389/fpsyg.2020.01353
Received: 11 March 2020; Accepted: 22 May 2020;
Published: 07 July 2020.
Edited by:
Gianluca Castelnuovo, Catholic University of the Sacred Heart, ItalyReviewed by:
Chiara Baglioni, Università degli Studi Guglielmo Marconi, ItalyCopyright © 2020 Franceschini, Fante, Filardi, Folli, Brazzi, Pizza, D’Anselmo, Ingravallo, Antelmi and Plazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Franceschini, Y2hyaXN0aWFuLmZyYW5jZXNjaGluaUB1bmlwci5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.