- 1Guangdong Key Laboratory of Mental Health and Cognitive Science, School of Psychology, Center for Studies of Psychological Application, South China Normal University, Guangzhou, China
- 2Center for Mental Health Education, Hainan University, Haikou, China
Previous studies have found that individuals exhibit empathic responses when others are treated unfairly. However, there remains a lack of clarity over the extent to which self-interest regulates these empathic responses, and in identifying which component of empathy is more likely to be affected. To investigate these issues, an experiment was designed based on a money distribution task with two conditions [observation condition (OC) vs. participation condition (PC)], and carried out using scalp-recorded event-related potentials (ERPs). Behavioral data showed that the participants’ empathic responses were consistent with their coplayers’ emotional expressions in the OC, whereas they were inconsistent with the coplayers’ expressions in the PC. The electrophysiological data showed that the neural encoding of facial expressions (reflected in the N170) was not affected by self-interest. However, the late stage of empathic responses (LPP) showed a decline when participants’ self-interest was involved. Disadvantageous inequality and relatively fair distribution to others elicited a more pronounced feedback-related negativity (FRN) than advantageous inequality distribution in both the OC and PC. As the late stage of empathic responses is also indexed by the LPP amplitude, these results indicate that the participants were more concerned for their own outcomes than for others’ benefits when self-interest was involved, which reduced their empathy toward their coplayers at the late stage of empathic responses.
Introduction
Empathy is an important contributor to successful social interaction and a key motivator for altruistic behavior, allowing us to predict and understand others’ behavior and to react accordingly. As social creatures, we are able not only to infer the intentions of others’ behaviors, but also to share the mental and emotional states of others due to our capability for empathy (Decety and Lamm, 2006; de Vignemont and Singer, 2006; Singer, 2006; Frith and Singer, 2008; Hein and Singer, 2008; Singer and Lamm, 2009; Kirman and Teschl, 2010; Cuff et al., 2016). Surprisingly, however, most studies to date have focused on empathy for physical pain rather than empathy for negative social experiences, such as unfair treatment, even though observing the latter is likely to be a more frequent occurrence. Numerous studies on the neural bases of empathy have shown the anterior insula (AI) and mid-anterior cingulate cortex (mACC) to be implicated in first-hand and vicarious experiences of pain (Singer et al., 2004; Farrell et al., 2005; Salimi-Khorshidi et al., 2009; Corradi-Dell’Acqua et al., 2011; Lamm et al., 2011); disgust (Murphy et al., 2003; Wicker et al., 2003; Jabbi et al., 2007; Vytal and Hamann, 2010); and unfairness (Sanfey et al., 2003; Civai et al., 2012; Corradi-Dell’Acqua et al., 2013; Gabay et al., 2014; Feng et al., 2015). The findings of these studies suggest that we feel unpleasant when unfair treatment is meted out to ourselves or to others. These results have provided support for the inequity aversion and fairness preference in Ultimatum Games (UG). The findings from the UG task indicated that the participants respond to a proposer’s unfair offers by lowering both their own and others’ payoffs (Charness and Rabin, 2002; Sanfey et al., 2003). That is, our empathy for others may promote altruistic behavior, a preference for fairness, and disgust toward unfair transactions or distributions.
However, in real life, we usually tend to help others generously when our own interests are not involved. It is difficult for most people to make every effort to sacrifice their own interests to help others. This may be because, when self-interest is involved, we are more concerned about our own interest, resulting in reduced empathic responses toward others. Previous studies have confirmed empathic responses are not adaptive to all social interactions, being modulated by contextual appraisal (Gu and Han, 2007; Hein and Singer, 2008; Singer and Lamm, 2009; Yamada et al., 2011). For example, Yamada et al. (2011) showed that congruence (in the cooperative setting) between the co-player’s affective expression and the participant’s outcome (win or loss) enhanced initial empathic responses, whereas incongruence (in the competitive setting) led to counter-empathic responses. These results showed that empathic responses can be reduced by a competitive relationship.
Even if there is no direct competition, we are also likely to exhibit incongruent emotions toward others once self-interest is involved. In an electrophysiological study, observers evaluated the outcomes for their friends versus strangers in a gambling task (Ma et al., 2011). The discrepancy of the FRN loss–win difference wave (d-FRN) between the outcomes for friends and strangers could only be observed when the observer did not participate in the gamble. These results indicate that the participants experienced more motivational relevance toward their friends than to strangers, but also that the participants’ empathic responses toward friends were only salient when they were not directly involved in the gamble. In other words, we are more concerned about our own outcomes compared with others’ benefits (Fukushima and Hiraki, 2006; Itagaki and Katayama, 2008). We are used to evaluating others’ outcomes from an egocentric perspective. The larger the extent to which self-benefit is involved, the more likely it is that egoism will influence one’s affective response toward others’ monetary outcomes (Wang et al., 2014a). The stronger empathy for a friend compared to a stranger may also be because the friend is self-related, in that the participants may have selfish incentives for increasing the chance of future cooperative interactions.
In reviewing previous empathy research, it is evident that there are still some issues that are unclear. How does the involvement of self-interest regulate empathic responses toward others who suffer unfair treatment? It has been suggested that empathy involves both an early automatic component characterized by emotional sharing (bottom-up processing) and a late controlled component characterized by cognitive evaluation (top-down processing) (Decety and Lamm, 2006; Shamay-Tsoory et al., 2007, 2009; Xiang et al., 2018). This raises the question, ‘Which component is more likely to be affected by self-interest?’ Addressing this issue could help to resolve some of the gaps and apparent anomalies in the current literature on empathy.
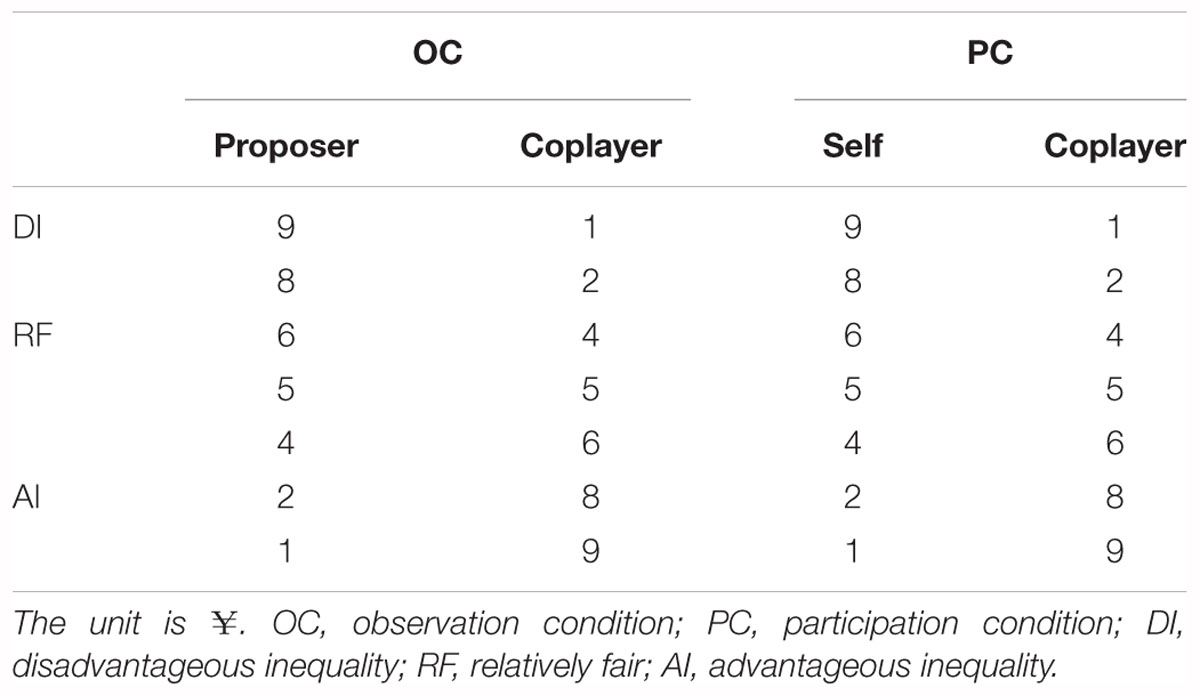
In an attempt to resolve these issues, the current study used scalp-recorded event-related potentials (ERPs) which provide a continuous measure of processing between a stimulus and a response, making it possible to determine which stage(s) of empathy are being affected by our experimental manipulation. We designed a money distribution task with two conditions [observation condition (OC) vs. participation condition (PC)], adapted from the classic Dictator Game (Engel, 2011). In the OC, the participants took part in the game as observers and observed a proposer allocating money to a coplayer. In the PC, the proposer allocated money to the participant and the coplayer. The EEGs from the participants were recorded simultaneously. There were three kinds of money distribution to the coplayer, as shown in Table 1: disadvantageous inequality (DI, in which the coplayer receives less money than the proposer or the participant), relatively fair (RF; the coplayer’s share is basically equal to that of the others); and advantageous inequality (AI, the coplayer receives more money than the proposer or the participant).
Our analyses focused on three distinct ERP components. The first component was N170, a face-sensitive ERP component (Caharel et al., 2009) characterized by a negative deflection in wave amplitude that occurs around 150–200 ms over occipito-temporal sites (Bentin et al., 1996; Maurer et al., 2005). A study has demonstrated a relationship between the magnitude of N170 and the occurrence of spontaneous facial mimicry (Achaibou et al., 2008). Mimicry is an ability to adopt the emotional state of another person, which can occur automatically and has been designated as the most basic form of empathy (Christov-Moore et al., 2014). This means that N170 is associated with the early stage of empathy (the bottom-up process of emotional sharing). As a targeted component in the current study, N170 was used to investigate whether self-interest influences the early stage of empathy. The second component of ERPs, called the late positive potential (LPP), is a centro-parietal positive deflection that usually appears between 300 and 700 ms, reflecting a long latency empathic response (Fan and Han, 2008). Through the use of LPP, we can examine whether self-interest affects the later stages of empathy. The third component of ERPs is called feedback-related negativity (FRN). FRN is an ERP component that peaks at about 250 ms after the onset of the outcome feedback, and is more prominent for stimuli with unfavorable outcomes as opposed to favorable outcomes (Gehring and Willoughby, 2002; Holroyd and Coles, 2002; Nieuwenhuis et al., 2005; Holroyd et al., 2006; Masaki et al., 2006; Hajcak et al., 2007; Yu et al., 2007; Walsh and Anderson, 2012). Through the FRN, we can identify with greater accuracy the point in time when self-interest affects empathy.
Based on previous evidence, we predict that involvement of self-interest could weaken empathic responses toward those who suffer unfair treatment. If there are differences in the amplitude of N170 between the OC and PC, this indicates that self-interest has affected the neural encoding of facial expressions. This could interfere with the emotional resonance with others; that is to say, self-interest could affect the early stage of empathic responses to economic inequality (i.e., emotional sharing). If there are no significant differences, this indicates on the contrary that self-interest has not affected the recognition of facial expressions, and therefore has not weakened emotional resonance. If there are differences in the LPP amplitude between the OC and PC, this indicates that self-interest has affected the late stage of empathy; the absence of significant differences indicates that self-interest has not affected the late stage of empathy. If the FRN amplitude in the disadvantageous inequality situation is higher than in the advantageous inequality situation under both the OC and PC, this indicates that the participants viewed the outcome of money distribution from the coplayer’s perspective (i.e., an empathic response) at the beginning of empathic responses. If the FRN amplitude shows the opposite trend under both conditions, this indicates that the participants viewed the outcome from their own perspective (a counter-empathic response) at the beginning of empathic responses.
At the behavioral level, we predicted that the participants would exhibit opposite emotional responses to the others’ facial expressions in the OC compared to the PC. More specifically, the participants’ emotional responses should be emotionally congruent with the coplayer in the OC, and emotionally incongruent with the coplayer in the PC.
Materials and Methods
Participants
Twenty-six participants were recruited to this study (13 women, 13 men; mean age: 20.46 years ± 2.14 SD). All participants were college students, right handed, had normal or corrected to normal vision, and did not have any history of neurological or mental disorders. This study was carried out in accordance with the recommendations of Ethics Committee of South China Normal University. All participants provided informed written consent before taking part in the experiment and were paid for their participation. The procedure of the experiment was consistent with the principles of international research involving human subjects as stated in the Declaration of Helsinki (World Medical Organization, 1999). The study was approved by the Ethics Committee of South China Normal University.
Visual Stimuli
Participants were comfortably seated in a dimly lit, sound-attenuated, and electrically shielded room. The stimuli were presented at the center of a 17 inch color monitor with a white background. Each stimulus (inclusion of text information) was a 10.5 cm × 14 cm (width × height) picture, subtending a visual angle of 6.02° × 8.02° at a viewing distance of 100 cm. As an observer, the participant saw a proposer giving an initial endowment of 10 yuan to a coplayer of the game. As a participant, he or she saw the proposer allocating 10 yuan to himself or herself and the coplayer. The facial expressions of the male or female proposer (gender-matched) serving during the experiment were neutral and selected from an ID photo. Feedback consisted of three kinds of facial expressions (frown, neutral, and smile) of the male or female coplayer (gender-matched), selected from the CAS-PEAL Face Database (Wen et al., 2008). Three still shots were used, clearly expressing a discernible frown, neutral expression, and smile, in response to a disadvantageous inequality (DI) distribution, relatively fair (RF) distribution, and advantageous inequality (AI) distribution to the coplayer, respectively. Brightness, size, contrast, and color settings of these pictures were unified with photo editing software.
Experimental Procedure
Before recording, participants read the instructions and the rules of the experimental task. We used a payoff distribution task preceded by an Ultimatum Game (UG; Sanfey et al., 2003). The participants were asked to perform a classical UG task for 1 min. The aim of the task was to encourage the participants to empathize with others, thereby enhancing the ecological validity of the formal experiment. In each trial of the UG task, the participants faced an unknown proposer who made an offer on how to divide an initial endowment of 10 yuan. The participants could accept the offer or reject it. Participants were instructed to use the keypad to make their choices. Data with respect to the UG were not relevant to the current study and thus not recorded. At the end of this session, participants were asked two questions, as follows. The first was, “As an observer, what are your feelings if your coplayer receives less money?” (1 = “unpleasant”, 2 = “pleasant”, 3 = “no feeling”); and the second was, “As an observer, what are your feelings if your coplayer receives more money?” (1 = “unpleasant,” 2 = “pleasant,” 3 = “no feeling”). All of the participants’ answers to the questions were “unpleasant” and “pleasant,” respectively. This suggests that the participants responded empathically toward the coplayer in relation to the money distribution outcome. Following the UG task, we then conducted the formal experiment, including the OC and PC.
In the OC, the participants were convinced that the proposer was empowered to distribute 10 yuan to the coplayer. As observers, the participants did not have the chance to receive a payment of 10 yuan. Table 1 shows the offers that were defined a priori by the experimenters. These ranged from extreme DI (distribution ratio of 1:9 or 2:8, where the proposer offered 1 or 2 yuan out of 10 yuan to the coplayer, leaving the rest to himself/herself), to RF (distribution ratio of 5:5, 6:4, or 4:6), and AI (distribution ratio of 9:1 or 8:2). Each observer was asked to pay attention to the facial expression of the coplayer and the outcome of the distribution. The three levels of fairness were randomly presented.
At the beginning of each trial, a fixation mark was displayed at the center of the screen for a duration of 400–600 ms. After the fixation marker, the participants were presented with the proposer’s photograph for 800 ms, followed by a 500–800 ms blank screen prior to the onset of the coplayer’s facial expression (frown, neutral, and smile, in response to DI, RF, and AI distribution to the coplayer, respectively). The facial expression remained on screen for 1000 ms. Subsequently, the financial outcome was presented for 1000 ms (see Figure 1). Each level of fairness was presented for 60 trials, making a total of 180 trials. To encourage the participants to concentrate on the task, a question mark was presented instead of the outcome presentation every 30 trials. The participants were asked to judge whether the money offered to the coplayer was AI, RF, or DI at this time. The keyboard numbers 1, 2, and 3 represented AI, RF, and DI, respectively. At the end of the OC task, the participants completed a rating scale to measure the degree of unpleasantness experienced for each level of fairness, using a 5-point scale ranging from 0 (“not at all”) to 4 (“very much”). Each distribution ratio (1:9, 2:8, 4:6, 5:5, 6:4, 8:2, and 9:1) was presented once.
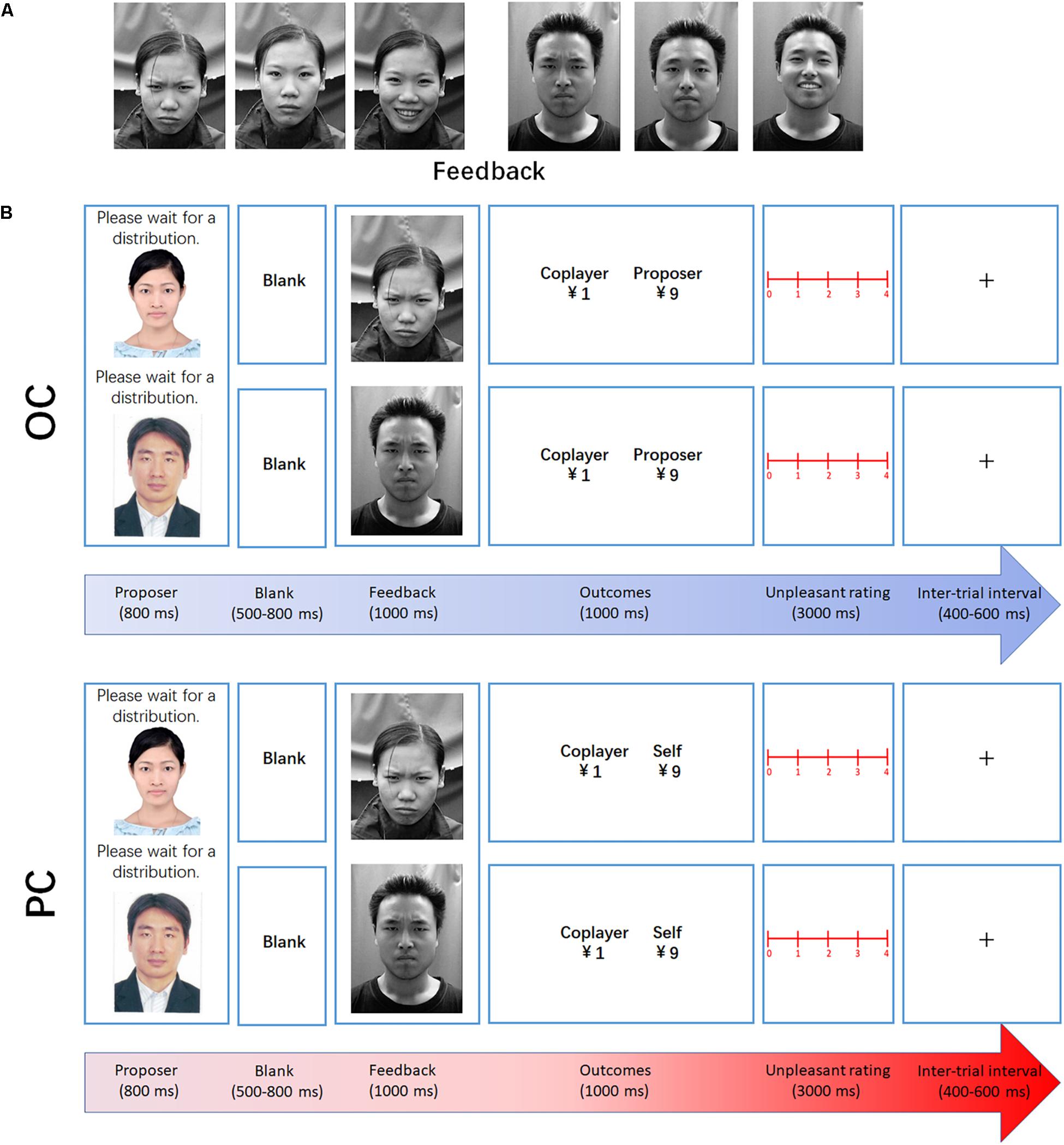
Figure 1. Experimental design. (A) Examples of the facial expressions used in the experiment (feedback: frown, neutral, and smile, in response to DI, RF, and AI distribution to the coplayer, respectively). (B) Examples of DI trials in the observation and participation conditions. At the beginning of each trial, a fixation mark was displayed at the center of the screen for a duration of 400–600 ms. After the fixation marker, the participants were presented with the proposer’s photograph for 800 ms, followed by a 500–800 ms blank screen prior to the onset of the coplayer’s facial expression. Subsequently, the financial outcome was presented for 1000 ms.
In the PC, the proposers were empowered to distribute 10 yuan to the participants and the coplayer. As shown in Table 1, the offers were defined a priori by the experimenters, and were identical to those in the OC. Each level of fairness was presented for 60 trials, making a total of 180 trials. The participants were told that one trial from the pool of money distribution would be drawn at random as their reward.
In summary, there were 400 trials in the whole experiment, including 14 practice trials, 12 judgment trials, and 14 rating trials. Note that only 360 trials of the formal experiment were analyzed; the judgment and assessment data were not included. The participants took part in the game firstly as observers and subsequently participated in the money distribution task.
EEG Recording and Analysis
The electroencephalogram (EEG) was recorded continuously from 62 scalp electrodes and the addition of two mastoid electrodes with Brain Amp DC amplifiers (Brain Products, Germany). All electrodes were re-referenced offline to an averaged mastoid reference, with a forehead ground being employed. Eye blinks and vertical eye movements were monitored with electrodes located above the right eye. The horizontal EOG (HEOG) recording electrodes were positioned at the outer canthi of both eyes. EEG and EOG activity was amplified by applying a bandpass filter from 0.01 to 100 Hz and was continuously sampled at 500 Hz/channel. All electrode impedances were maintained at <5 kΩ. The EEGs averaged for the trials under each condition were computed separately offline using BrainVision Analyzer 2.0 software (Brain Products, Germany; Fritsch and Kuchinke, 2013). Each epoch continued for 1,200 ms, with 200 ms before the facial expression (feedback) onset for the baseline correction. In addition, we used independent component analysis (ICA) to remove artifacts. Trials contaminated by eye blinks, eye movements, or muscle potentials exceeding ±100 μV at any electrode were excluded from averaging. The total rejection rate of the trials was 2.49%.
Our analyses focused on three distinct ERP components: N170, LPP, and FRN. As explained in the introduction, the reasons for choosing these three components can be summarized as follows: N170 is associated with the neural encoding of facial expressions; LPP is sensitive to changes in perceived emotional intensity; and the FRN component is well-established to distinguish between one’s own positive and negative outcomes (San Martin, 2012). Based on previous studies and visual observation, we analyzed the mean amplitude from 140 to 180 ms after the onset of the facial expression for N170; from 330 to 550 ms after the onset of the facial expression for LPP; and from 250 to 350 ms after the onset of the facial expression for FRN. For statistical analysis, we selected two electrodes (P7 and P8) in the occipito-temporal area for N170; nine electrodes (C3, C4, P3, P4, Cz, Pz, CPz, CP3, and CP4) in the central-parietal area for LPP; and nine electrodes (F3, F4, C3, C4, Fz, Cz, FCz, FC3, and FC4) in the fronto-central area for FRN.
The within-subject repeated measures ANOVA was performed with three factors: condition (OC and PC), level of fairness (DI, RF, and AI), and electrodes for N170, LPP, and FRN. The Greenhouse–Geisser correction was applied for the violation of the sphericity assumption in ANOVA where appropriate, and the Bonferroni correction was used for multiple comparisons.
Results
Behavioral Data
Figure 2 shows the average unpleasantness rating in each condition (OC-DI, OC-RF, OC-AI, PC-DI, PC-RF, and PC-AI). Repeated-measures analysis of variance (ANOVA) was conducted for the behavioral data, with condition (OC/PC) and fairness (DI/RF/AI) as two independent factors. The main effects for condition [F(1,25) = 0.030, p > 0.05, = 0.001] and fairness [F(2,50) = 2.426, p > 0.05, = 0.088] were not significant. However, the interaction effect between condition and fairness was significant [F(2,50) = 89.107, p < 0.001, = 0.781].
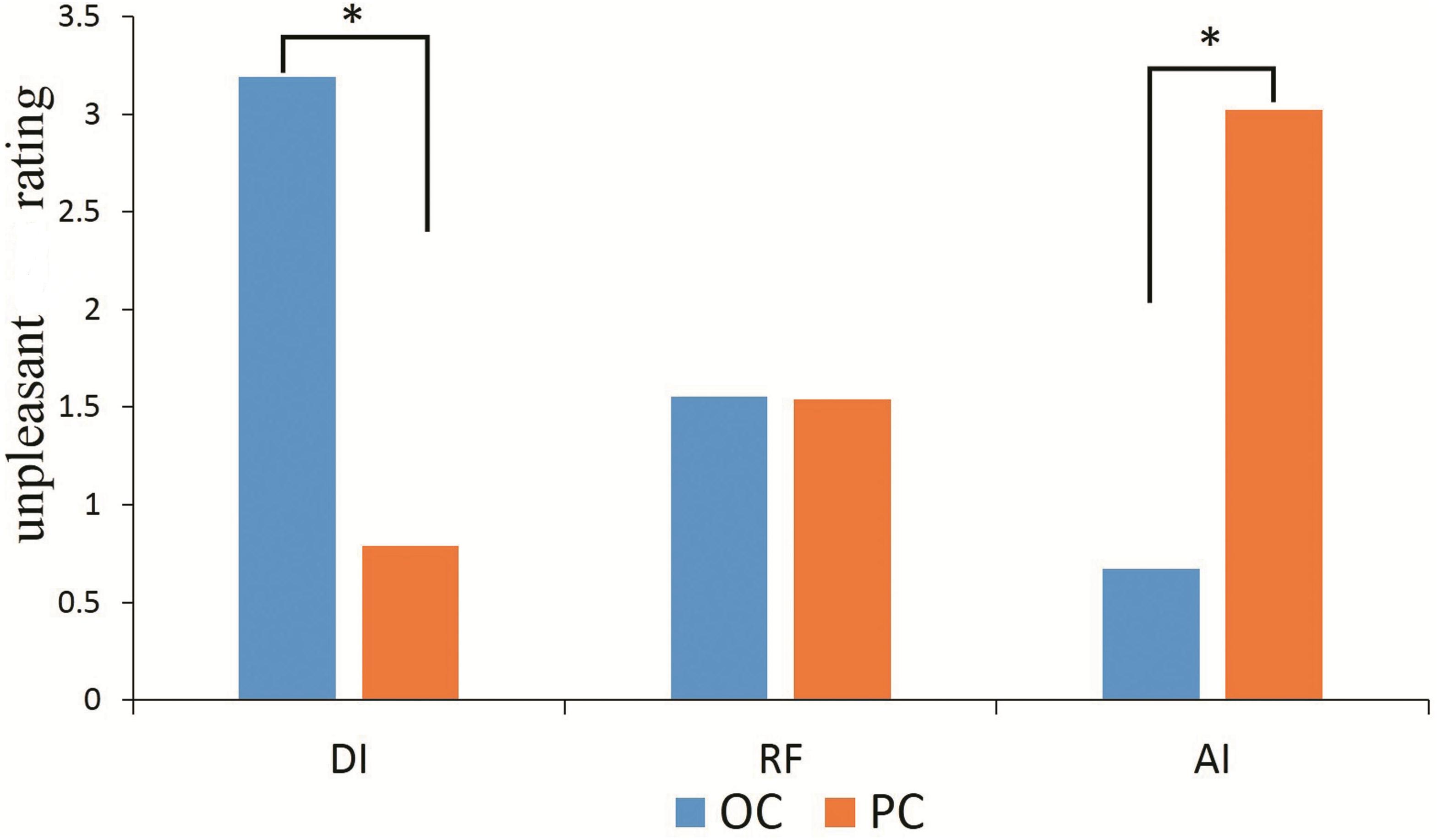
Figure 2. The self-reported unpleasant ratting in each condition (OC-DI, OC-RF, OC-AI, PC-DI, PC-RF, and PC-AI). ∗P < 0.05.
Further simple effect analysis revealed that in the OC, participants rated themselves as feeling more unpleasant in the DI situation (3.192 ± 0.176) than in the AI [0.673 ± 0.149; t(25) = 10.455, p < 0.001], and RF situations [1.551 ± 0.162; t(25) = 7.285, p < 0.001]. They also felt more unpleasant in the RF than in the AI situation [t(25) = 3.343, p < 0.01]. In the PC, however, the participants rated themselves as feeling more unpleasant in the AI situation (3.019 ± 0.219) than in the DI [0.788 ± 0.222; t(25) = 6.519, p < 0.001] and RF situations [1.538 ± 0.165; t(25) = 5.454, p < 0.001]. Additionally, they felt more unpleasant in the RF than in the DI situation [t(25) = 2.705, p < 0.05].
The participants rated themselves in the DI situation as feeling more unpleasant in the OC than in the PC [t(25) = 8.012, p < 0.001]. In contrast, in the AI situation they felt more unpleasant in the PC than in the OC [t(25) = 8.469, p < 0.001]. There was no significant difference between the OC and PC in the assessment of unpleasantness in the RF situation (p > 0.05).
ERP Data
N170
Supplementary Figures S1, S2 show the grand-averaged ERPs and topographic maps for the N170 amplitudes in different situations. The statistical results for grand-averaged ERPs of N170 with three factors (condition, fairness, and electrode) revealed no significant differences. The main effect for condition [F(1,25) = 1.057, p > 0.05, = 0.041], fairness [F(2,50) = 2.736, p > 0.05, = 0.099] and electrode [F(1,25) = 0.838, p > 0.05, = 0.032] were not significant. The three-way interaction of condition, fairness and electrode was not significant [F(2,50) = 0.144, p > 0.05, = 0.006]. The interaction effect between condition and fairness [F(2,50) = 0.521, p > 0.05, = 0.020], condition and electrode [F(1,25) = 0.070, p > 0.05, = 0.003], fairness and electrode [F(2,50) = 0.020, p > 0.05, = 0.001] were not significant. Table 2 summarizes the ANOVA results for the N170 amplitudes (140–180 ms) with the condition (OC and PC), level of fairness (DI, RF, and AI), and electrode distribution as the within-subject factors. These results indicated that the neural encoding of facial expressions was not affected by self-interest. Therefore, self-interest could not have interfered with the emotional resonance with others. In particular, self-interest did not affect the early stage of empathic responses to economic inequality (i.e., emotional sharing).
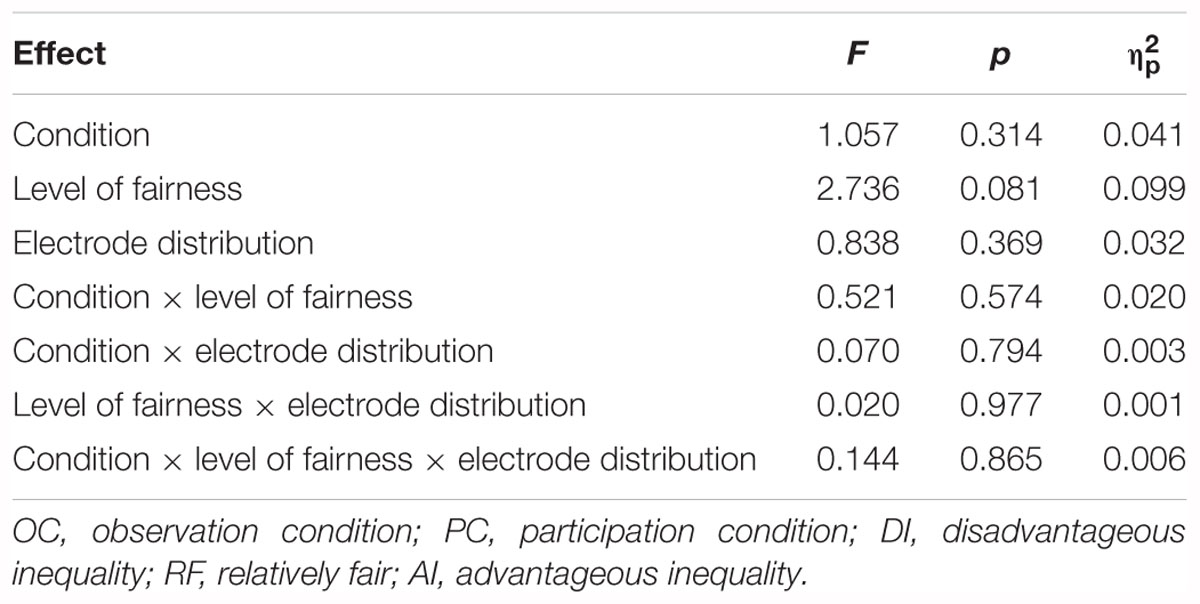
Table 2. Summary of ANOVA results for the N170 amplitudes (140–180 ms) with the condition (OC and PC), level of fairness (DI, RF, and AI), and electrode distribution as the within-subject factors.
Late Positive Potential (LPP)
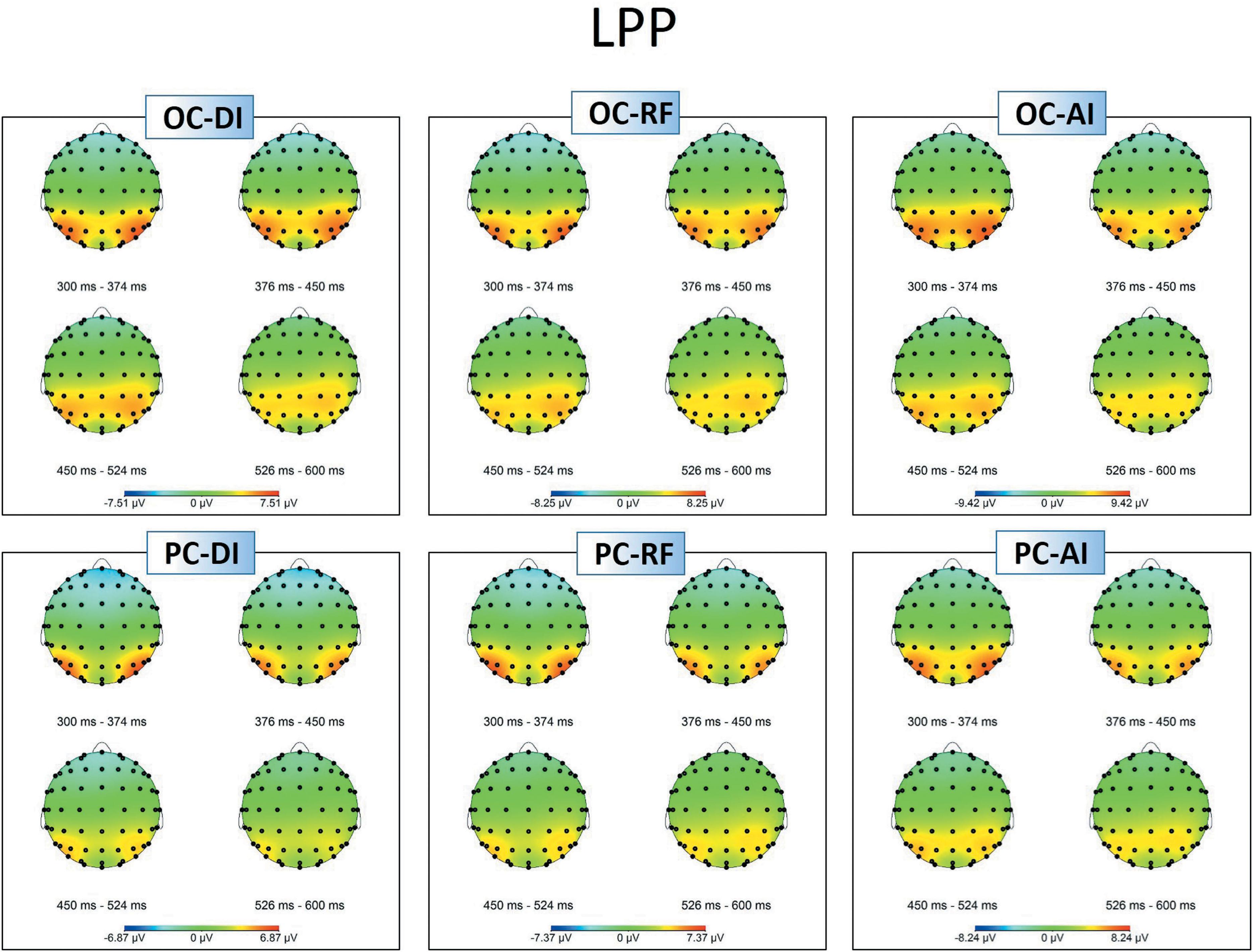
Figures 3A, 4 show grand-averaged ERPs and topographic maps for LPP amplitudes in different situations. The statistical results for grand-averaged ERPs of LPP with three factors (condition, fairness, and electrode) revealed main effects for condition [F(1,25) = 11.936, p < 0.01, = 0.323], with fairness and electrode being significant [fairness: F(2,50) = 6.341, p < 0.01, = 0.202; electrode: F(8,200) = 10.552, p < 0.001, = 0.824]. Specifically, the OC (MOC-DI = 3.632, SEOC-DI = 0.563; MOC-RF = 3.788, SEOC-RF = 0.601; MOC-AI = 4.403, SEOC-AI = 0.639) elicited significantly enhanced LPP amplitude compared to PC (MPC-DI = 2.247, SEPC-DI = 0.474; MPC-RF = 2.729, SEPC-RF = 0.528; MPC-AI = 3.240, SEPC-AI = 0.557) in all levels of fairness (see Figure 3B). The statistically significant differences were, respectively, as follows: tDI(25) = 4.414, pDI < 0.001; tRF(25) = 2.144, pRF < 0.05; and tAI(25) = 2.951, pAI < 0.01. In sum, the results showed that the grand-averaged ERP of the LPP was larger for the OC than for the PC, regardless of the level of fairness. Post hoc pairwise comparisons with Bonferroni correction showed the comparisons of AI (MAI = 3.822, SEAI = 0.566) with DI (MDI = 2.940, SEDI = 0.596) and RF (MRF = 3.258, SERF = 0.509) were significant [tAI,DI(25) = 3.336, pAI,DI < 0.01; tAI,RF(25) = 2.364, pAI,RF < 0.05], whereas the comparison between RF and DI was not.
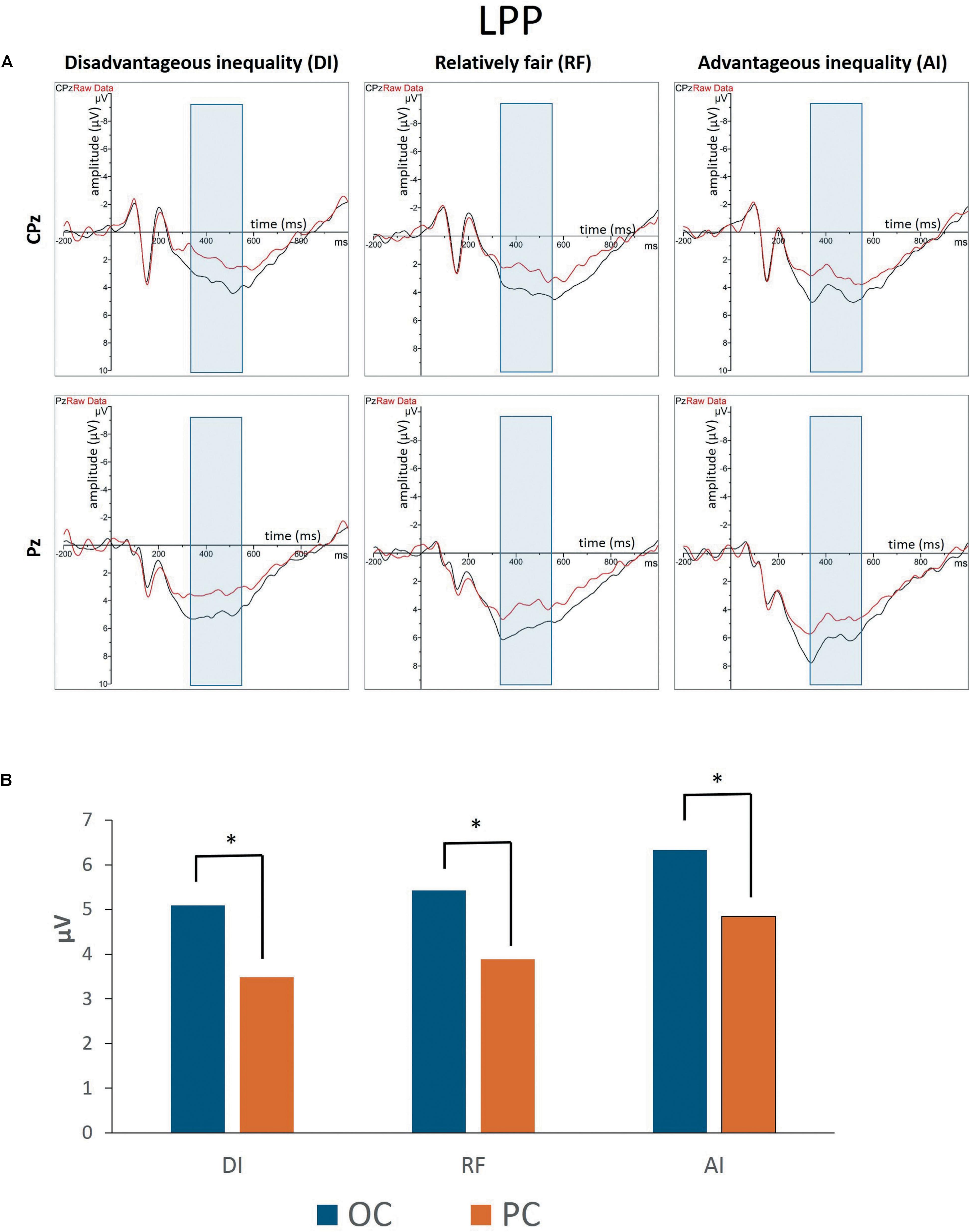
Figure 3. (A) Grand-averaged event-related brain potentials (ERPs) for LPP amplitudes in different situations from the CPz and Pz regions. (B) The bar graphs show the mean value of the LPP amplitude in each condition at Pz. ∗P < 0.05.
In addition, compared with the electrodes of Cz (M = 1.146, SE = 0.712), C3 (M = 1.139, SE = 0.620), C4 (M = 1.854, SE = 0.625), CPz (M = 3.270, SE = 0.633), CP3 (M = 3.120, SE = 0.524), and CP4 (M = 3.633, SE = 0.550), more pronounced LPPs were observed at Pz (M = 4.845, SE = 0.530), P3 (M = 5.507, SE = 0.515), and P4 (M = 5.545, SE = 0.498). The interaction effect between condition and electrode was marginal significant [F(8,200) = 2.541, p = 0.048, = 0.530]. Specifically, the P4 amplitudes in the OC (M = 6.088, SE = 0.521) were higher than those of P3 (M = 5.979, SE = 0.591), t(25) = 0.286, p > 0.05. However, the P4 amplitudes in the PC (M = 5.003, SE = 0.526) were lower than those of P3 (M = 5.036, SE = 0.507), t(25) = -0.093, p > 0.05. The Cz amplitudes in the OC (M = 1.815, SE = 0.795) were higher than those of C3 (M = 1.636, SE = 0.672), t(25) = 0.686, p > 0.05. In the PC, however, the Cz amplitudes (M = 0.477, SE = 0.687) were lower than those of C3 (M = 0.642, SE = 0.604), t(25) = -0.702, p > 0.05. The CPz amplitudes in the OC (M = 4.035, SE = 0.716) were higher than those of CP3 (M = 3.614, SE = 0.603), t(25) = 1.618, p > 0.05, whereas the CPz amplitudes in the PC (M = 2.506, SE = 0.617) were lower than those of CP3 (M = 2.626, SE = 0.494), t(25) = -0.478, p > 0.05. The interaction effects of other variables were not significant.
Feedback-Related Negativity (FRN)
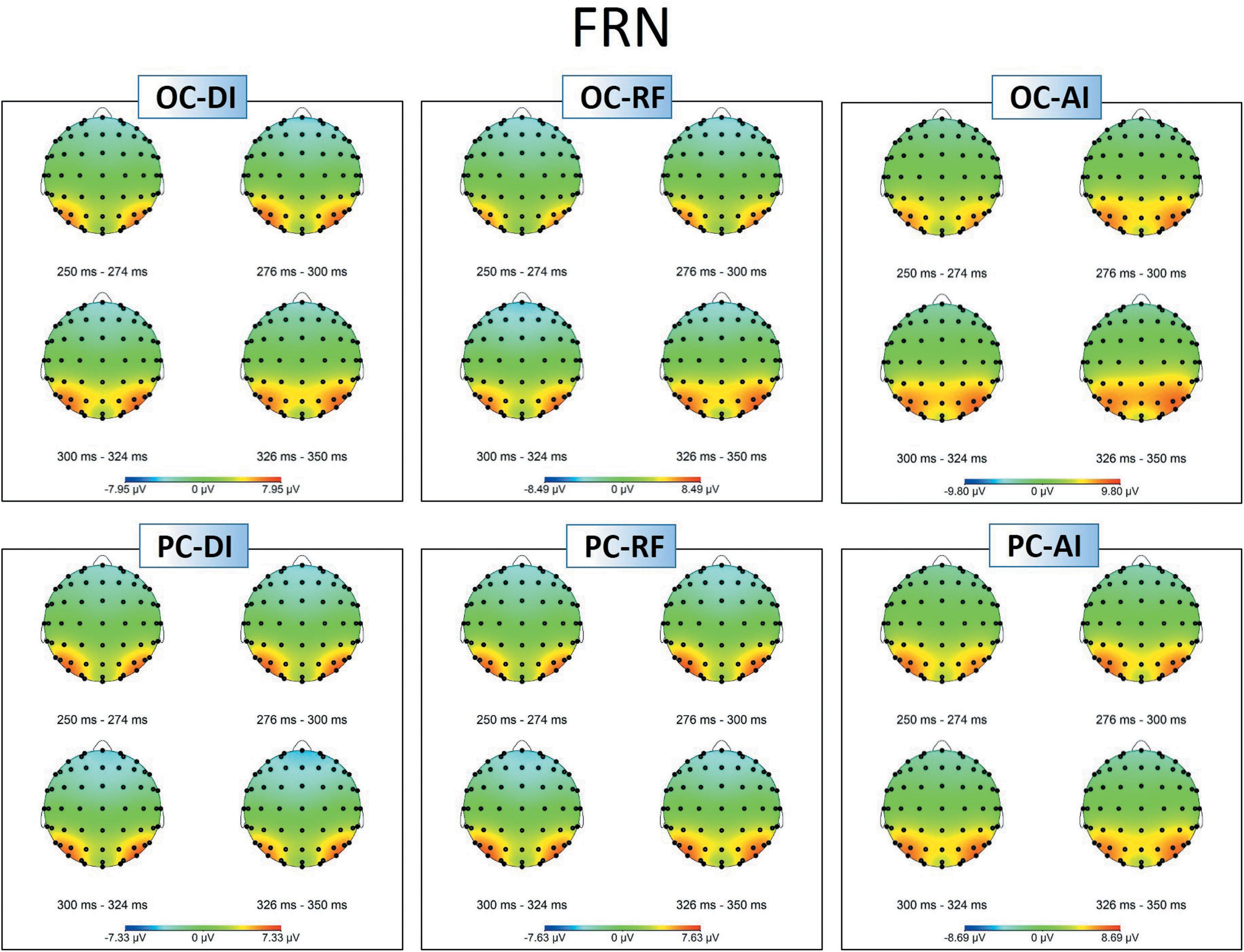
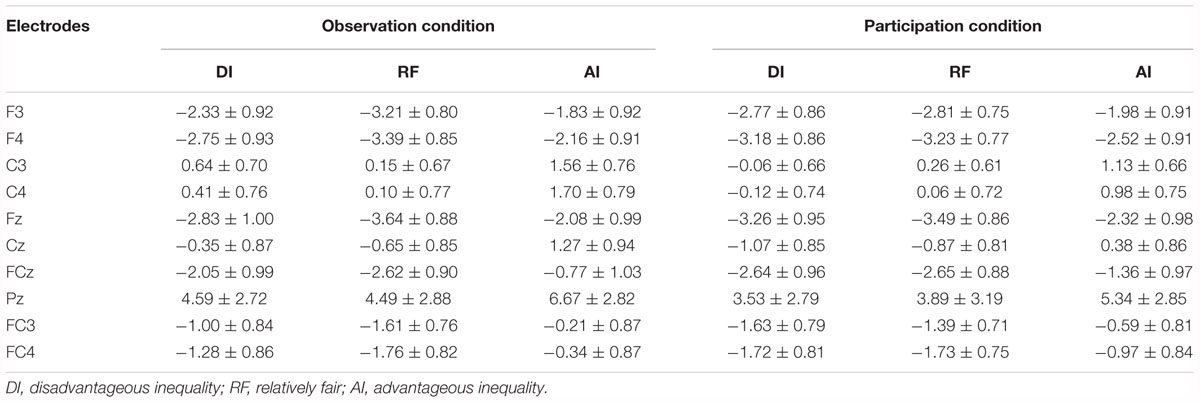
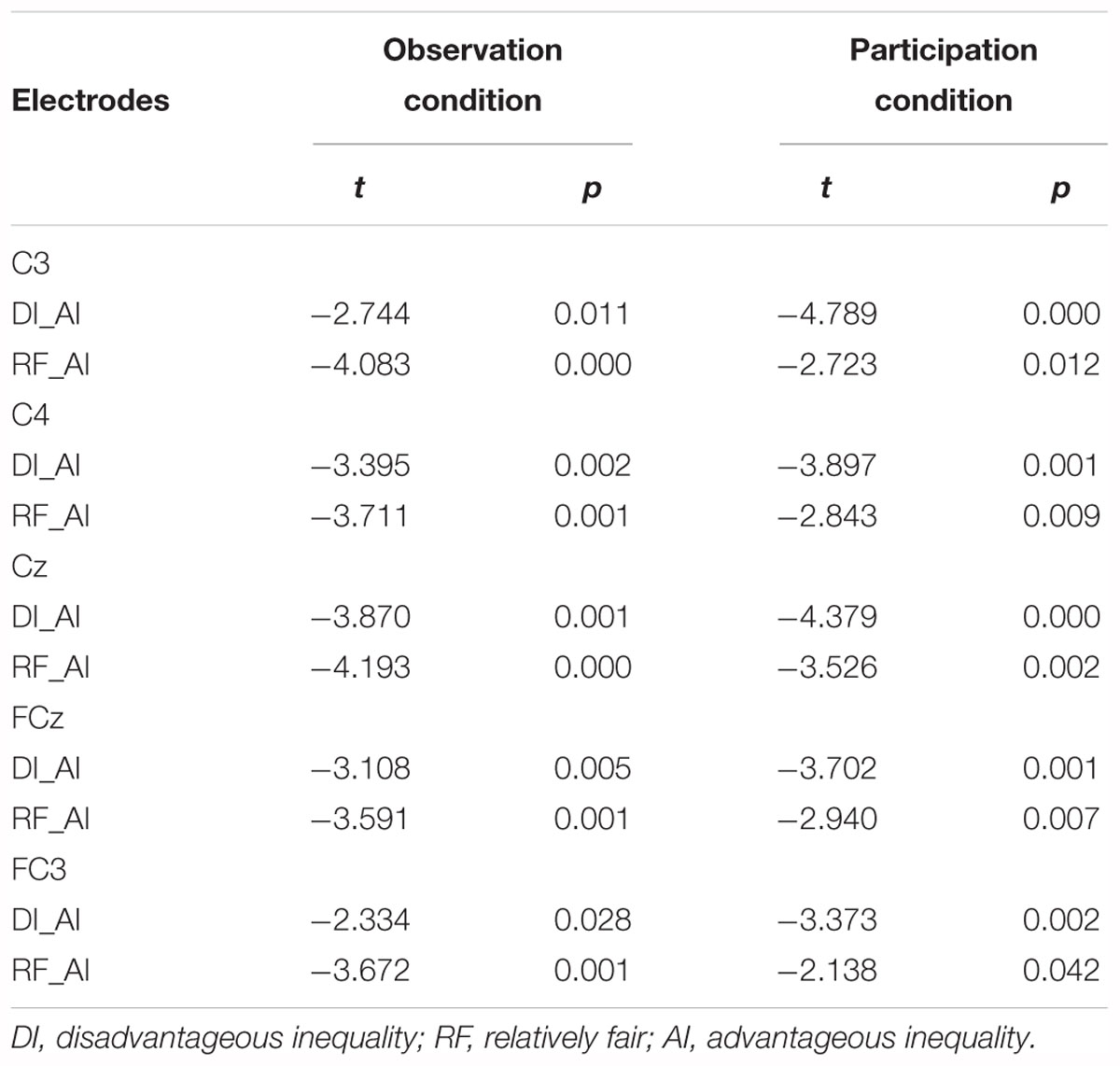
Figures 5A, 6 show grand-averaged ERPs and topographic maps for FRN amplitudes in different situations. Similar statistical analyses were conducted for the grand-averaged ERP of FRN. The results revealed main effects for fairness [F(2,50) = 9.935, p < 0.001, = 0.284] and electrode [F(8,200) = 16.824, p < 0.001, = 0.882]. The main effect for condition was not significant [F(1,25) = 0.913, p > 0.05, = 0.035]. Post hoc pairwise comparisons with Bonferroni correction showed the comparisons of AI (MAI = -0.562, SEAI = 0.826) with RF (MRF = -1.804, SERF = 0.740) and with DI (MDI = -1.555, SEDI = 0.809) were both significant [tAI,RF(25) = 3.819, pAI,RF < 0.01; tAI,DI(25) = 3.561, pAI,DI < 0.01], whereas the comparison between RF and DI was not (see Figure 5B). The results indicate that the grand-averaged ERPs of FRN were larger for the DI and RF than for the AI distribution, regardless of condition. In addition, compared with the electrodes of Cz (M = -0.215, SE = 0.811), C3 (M = 0.613, SE = 0.638), C4 (M = 0.521, SE = 0.711), FCz (M = -2.012, SE = 0.902), FC3 (M = -1.071, SE = 0.759), and FC4 (M = -1.300, SE = 0.780), more pronounced FRNs were observed at Fz (M = -2.936, SE = 0.892), F3 (M = -2.489, SE = 0.819), and F4 (M = -2.873, SE = 0.819). Table 3 shows the mean amplitudes and standard error in each condition at FRN. The interaction effect between fairness and electrodes was also significant [F(16,400) = 4.663, p < 0.01, = 0.882). DI and RF distribution to the coplayers elicited a more pronounced FRN than AI distribution at electrodes C3, C4, Cz, FCz, and FC3. Table 4 shows the significant t-test results for these electrodes in each condition at FRN. There was a similar tendency at other electrodes, but these were not statistically significant. The interaction effects of other variables were also not significant.
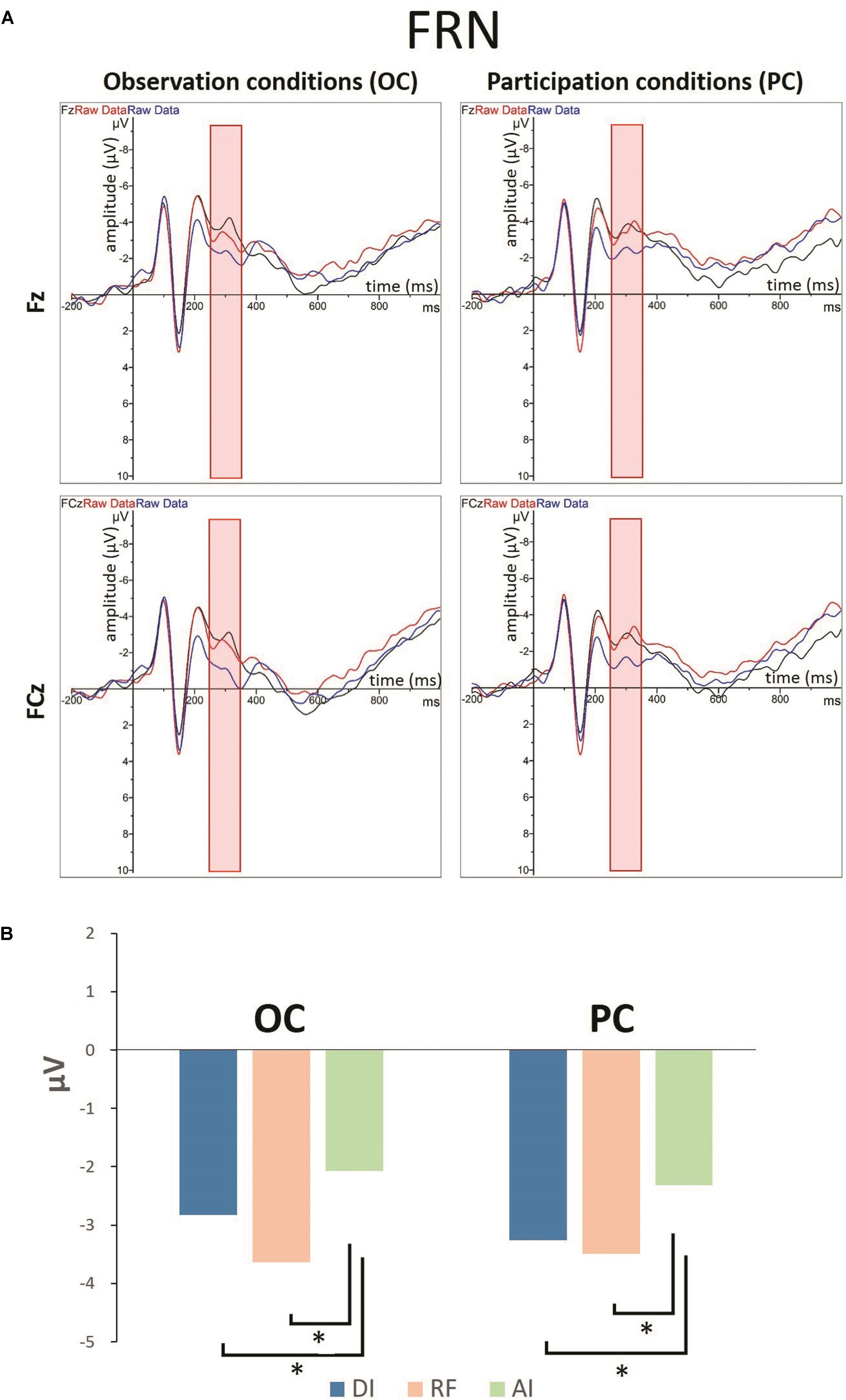
Figure 5. (A) Grand-averaged ERPs for LPP amplitudes in different situations from the Fz and FCz regions. (B) The bar graphs show the mean value of the FRN amplitude for each condition in Fz region. ∗P < 0.05.
Discussion
The main aim of this study was to investigate how the involvement of self-interest regulates empathic responses toward those who suffer unfair treatment. A related aim was to identify which component of empathy is more likely to be affected by self-interest. The electrophysiological data showed a decreased amplitude of the LPP when self-interest was involved. However, self-interest did not significantly affect the amplitude of N170. Disadvantageous inequality and relatively fair distribution to the coplayers elicited a more pronounced FRN than advantageous inequality distribution in both the OC and PC. Moreover, the behavioral data showed that the participants’ empathic responses were congruent with the coplayers’ emotional expressions in the OC, whereas in the PC their emotional responses were inconsistent with the coplayers’ expressions. These findings show that the participants’ empathic responses were aroused spontaneously when they observed the facial expressions of the coplayers at the early stage of emotional responses. However, self-interest modulated their empathy toward others at the late stage of responses.
At the temporal dynamics level, the neural encoding of facial expressions (reflected in the N170) was not affected by self-interest. Thus self-interest did not interfere with the emotional resonance with others. In other words, the results indicate that the early stage of empathy to economic inequality (i.e., emotional sharing) was not weakened by self-interest. However, the late stage of empathic responses (reflected in the LPP) showed differences between the OC and PC.
According to previous studies, there are three reasons for assuming that empathic responses toward others declines when self-interest is involved. Firstly, previous research has confirmed that LPP amplitude is associated with empathic responses, with statistical results showing a positive correlation. Participants displaying the greater LPP ‘pain effect’ said that they were more likely to experience empathic concern and to imaginatively transpose themselves into fictional social situations (Ikezawa et al., 2014). Our results indicate that the participants produced more empathic responses toward others in the OC.
Secondly, a series of studies found that LPP is associated with emotional regulation (Olofsson et al., 2008; Hajcak et al., 2010). A study found that when participants were asked to decrease the intensity of their emotional responses to unpleasant images, LPP amplitude was reduced (Moser et al., 2006). Foti and Hajcak’s (2008) finding further suggested that changing the meaning is indeed capable of modifying LPP responses to affective images (Foti and Hajcak, 2008). Based on previous studies, we consider that the amplitude of LPP can reflect the strength of empathy. If the participants have a stronger empathic response to the coplayers, they need greater emotional regulation which results in greater LPP amplitude. Similarly, weaker empathic responses could result in a smaller LPP amplitude. In our study, the amplitude of LPP increased in the OC and decreased in the PC. This suggests that the participants exhibited stronger empathic responses to the coplayers in the OC. In the PC, however, the participants’ concern about their own benefits might have weakened the empathic responses toward the coplayers, producing a lower LPP than in the OC.
Thirdly, previous studies have found that empathic brain responses are modulated by top–down control processes such as attention. Empathic responses can be reduced when attention is distracted away from others’ pain (Gu and Han, 2007). In the current study, the participants who were actively involved in the distribution of money were more concerned for their own interests; thus their attention shifted away from the coplayers’ outcomes while their empathic responses were reduced, reflected in the reduction in LPP amplitude.
Similarly to the LPP results, the behavioral results showed that participants in the OC rated themselves as feeling more unpleasant in the disadvantageous inequality situation than in the advantageous inequality and relatively fair situations. Meanwhile, they felt more unpleasant in the relatively fair situation than in the advantageous inequality situation. However, participants in the PC rated themselves as feeling more unpleasant in the advantageous inequality situation than in the disadvantageous inequality and relatively fair situations. Moreover, they felt more unpleasant in the relatively fair situation than in the disadvantageous inequality situation.
Additionally, participants in the disadvantageous inequality situation rated themselves as feeling more unpleasant in the OC than in the PC. In contrast, participants in the advantageous inequality situation felt more unpleasant in the PC than in the OC. There was no significant difference in the assessment of unpleasantness in the relatively fair situation between the OC and PC. These results suggest that fairness preferences and empathic responses depend on condition. The participants empathetically respond to others’ unfair payoffs only when their own interests are not involved.
These results are consistent with some previous studies, which revealed that individuals empathetically respond to others’ financial rewards. For instance, a handful of functional magnetic resonance imaging (fMRI) studies have demonstrated that the participants had greater reward region activation when they observed their friends or other people who were socially similar to themselves win at gambling (Mobbs et al., 2009; Molenberghs et al., 2014; Varnum et al., 2014). In contrast, when their self-interest was involved (i.e., when their low payoffs resulted from others’ high payoffs), the participants gave a higher unpleasantness rating to others’ high payoffs than to others’ low payoffs, suggesting that the advantageous inequality enjoyed by the coplayer was perceived as unfavorable by the participants, and vice versa. Thus the participants’ emotional responses were incongruent with the coplayers.
Evidence from ERP studies has shown that the temporal dynamics of empathy consists of an early affective arousal component followed by a late cognitive reappraisal and regulation component (Gu and Han, 2007; Decety et al., 2017). Our results suggest that self-interest reduces empathic responses toward others at the late stage of empathy. This implies that empathic brain responses are modulated by top–down processes. We speculate that the modification of empathic responses through self-involvement might be due to attention being drawn away from others’ emotional responses and toward the concern for self-interest. Research has confirmed that shifting attention away from emotional material results in reduced LPP amplitudes (Dunning and Hajcak, 2009; MacNamara and Hajcak, 2009; Thiruchselvam et al., 2011, 2012; Wangelin et al., 2011; Wiens et al., 2011; Nordstrom and Wiens, 2012; Paul et al., 2013; Wiens and Syrjanen, 2013). In addition, a series of studies showed that distraction yielded an attenuation of LPP magnitude in response to negative pictures and positive pictures (Thiruchselvam et al., 2011; Wangelin et al., 2011; Van Dillen and Derks, 2012; Paul et al., 2013; Schönfelder et al., 2014). Thus, as regards the current study, we speculate that participants’ attention could have shifted away from others’ outcomes to their own interests, resulting in reduced empathic responses that were reflected in the reduced LPP amplitude; i.e., distraction could have regulated their empathic responses. Although the studies mentioned above applied different experimental paradigms compared to the current study, the internal mental processing may be similar. Future research is needed to verify this speculation.
The ERP results also indicate that the disadvantageous inequality situation elicited a more pronounced FRN than advantageous inequality in both the OC and PC. Previous studies have shown that FRN is more prominent for stimuli with unfavorable as opposed to favorable outcomes (Gehring and Willoughby, 2002; Holroyd and Coles, 2002; Nieuwenhuis et al., 2005; Holroyd et al., 2006; Masaki et al., 2006; Hajcak et al., 2007; Yu et al., 2007; Walsh and Anderson, 2012). From this point of view, our study suggests that the participants perceived others’ frowns as unfavorable results at the early stage of empathy, regardless of whether they participated personally in the money distribution task. These results indicate that the participants’ initial emotional responses were congruent with the coplayers’, and therefore produced empathic responses. This is supported by the N170 results, which indicate that facial expression recognition was not affected by self-interest; thus emotions were aroused automatically. According to the two components of empathy theory, the empathic responses that take place at the early stage of empathy are elicited automatically by the perception of others’ emotional states. After the spontaneous empathic response toward others, their attention begins to drift away from others’ emotional states to their own interests, resulting in an attenuation of empathic response. These results further confirm that empathic brain responses are modulated by top–down processes such as attention. Concern about self-interest shifted attention away from others’ emotional responses and reduced empathic responses toward others at the late stage of empathy.
In summary, our results indicate that the participants exhibited empathic responses when others were treated unfairly. In the money distribution task, the participants’ empathic responses were aroused spontaneously when they saw the coplayer’s facial expression, regardless of whether their personal interest was involved, and especially at the beginning of their emotional responses. In the late stage of empathy, however, when self-interest was involved, the participants were more concerned about their own outcomes compared with others’ benefits, which reduced their empathic responses toward the coplayers. These results confirm that empathic responses are indeed regulated by the involvement of self-interest at the late stage of empathy. The participants showed a strong preference for self-gain. They preferred advantageous inequity for themselves, although the outcome might have been disadvantageous inequity for the coplayer. These findings are partially similar to the results of previous studies that have examined competitive situations; those results indicate that when individuals participate in a competitive task, they are most affected by their own outcomes. In comparison to strangers, participants’ empathic responses toward close friends were only salient when they did not directly participate in a gambling game (Leng and Zhou, 2010; Ma et al., 2011; Wang et al., 2014b). Research also suggests that the larger the extent of self-benefit involvement, the more likely it is that egoism will influence one’s affective response toward others’ monetary outcomes (Wang et al., 2014a). Our findings complement those studies; that is, in demonstrating on the contrary that people are not wholly concerned about personal interests. They can also be affected by other people’s emotional states, producing empathic responses at an initial stage.
Furthermore, empathic responses toward the coplayers were reduced when attention was diverted away from the coplayers’ outcomes. Therefore, we speculate that distraction cannot only regulate spontaneous emotions but also regulate vicarious emotions such as empathy. Empathy helps us to understand other people’s emotional states, motivates prosocial behavior, and facilitates social communication (Acevedo et al., 2012). However, professional workers such as counselors and doctors should not confuse their own experience with that of their clients (Luo et al., 2013); otherwise, it would lead to personal distress and detrimentally affect their well-being. In addition, high levels of empathic accuracy have been observed in individuals with psychopathic disorders, borderline personality disorders, and high levels of narcissism and anxiety, suggesting that a heightened awareness of, or sensitivity to, others’ internal states has the potential for negative outcomes (Simpson et al., 1999). An implication of our findings is that distraction is an effective means of regulating vicarious emotion, such as empathy. This has profound significance and important practical value for research in psychological counseling and clinical psychology.
Limitations and Future Directions
Note that there are still some limitations to our current study. First, we only examined some healthy college students as participants in this research. The empathic responses of people with affective defects should be considered, and the differences between the temporal dynamics of empathy regulation by self-interest involved in healthy individuals and those with psychopathology should also be detected. We infer that the weakening of empathic responses toward others through concern about self-interest may be due to distraction. It is possible that regulatory processes in certain affective disorders may be characterized by distinct temporal dynamics. For instance, relative to low anxious individuals, those high in anxiety show a slowed disengagement of attention from threatening stimuli (Fox et al., 2001; Yiend and Mathews, 2001). Comparing healthy and psychopathological samples could illuminate the mechanisms behind the empathy regulatory impairments that underlie specific psychiatric disorders.
A second limitation is that the participants completed two types of money distribution task with the same gender-matched people. In daily life, we are in contact with people not only of the same sex but also of the opposite sex. Takahashi and his colleagues found that when the target person’s possession was superior and self-relevant (including the same sex), stronger envy and stronger anterior cingulate cortex (ACC) activation were induced (Takahashi et al., 2009). This implies that we may produce varying degrees of empathic responses to people of a different gender. Future studies should examine whether our findings are applicable to the opposite sex when they suffer from unfair money distribution.
Author Contributions
JJ designed the experiments, analyzed the data, and wrote the manuscript. PL designed the experiments and wrote the manuscript. MZ, BL, YP, and JL collected the data. XZ designed the experiments and worked on the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31771218) and the Major Program of the National Social Science Foundation of China (Grant No. 14ZDB259). Research Fund for Higher Education of Hainan, China (Grant No. Hnky2016-2). Educational Science Project during the Twelfth Five-year Plan Period of Hainan, China (Grant No. QJY1251505).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2019.00372/full#supplementary-material
FIGURE S1 | Grand-averaged event-related brain potentials (ERPs) for the N170 amplitudes in different situations from the P7 and P8 regions. OC, observation condition; PC, participation condition.
FIGURE S2 | Topographic maps for the N170 amplitudes in different situations. OC, observation condition; DI, disadvantageous inequality; RF, relatively fair; AI, advantageous inequality; PC, participation condition.
References
Acevedo, B., Aron, A., Fisher, H., and Brown, L. (2012). Neural correlates of marital satisfaction and well-being: reward, empathy, and affect. Clin. Neuropsychiatry 9, 20–31.
Achaibou, A., Poutois, G., Schwartz, S., and Vuilleumier, P. (2008). Simultaneous recording of EEG and facial muscle reactions during spontaneous emotional mimicry. Neuropsychologia 46, 1104–1113. doi: 10.1016/j.neuropsychologia.2007.10.019
Bentin, S., Allison, T., Puce, A., Perez, E., and McCarthy, G. (1996). Electrophysiological studies of face perception in humans. J. Cogn. Neurosci. 8, 551–565. doi: 10.1162/jocn.1996.8.6.551
Caharel, S., d’Arripe, O., Ramon, M., Jacques, C., and Rossion, B. (2009). Early adaptation to repeated unfamiliar faces across viewpoint changes in the right hemisphere: evidence from the N170 ERP component. Neuropsychologia 47, 639–643. doi: 10.1016/j.neuropsychologia.2008.11.016
Charness, G., and Rabin, M. (2002). Understanding social preference with simple tests. Q. J. Econ. 117, 817–869. doi: 10.1111/j.1748-6653.2012.02038.x
Christov-Moore, L., Simpson, E. A., Coudé, G., Grigaityte, K., Iacoboni, M., and Ferrari, P. F. (2014). Empathy: gender effects in brain and behavior. Neurosci. Biobehav. Rev. 46, 604–627. doi: 10.1016/j.neubiorev.2014.09.001
Civai, C., Crescentini, C., Rustichini, A., and Rumiati, R. I. (2012). Equality versus self-interest in the brain: differential roles of anterior insula and medial prefrontal cortex. Neuroimage 62, 102–112. doi: 10.1016/j.neuroimage.2012.04.037
Corradi-Dell’Acqua, C., Civai, C., Rumiati, R. I., and Fink, G. R. (2013). Disentangling self- and fairness-related neural mechanisms involved in the ultimatum game: an fMRI study. Soc. Cogn. Affect. Neurosci. 8, 424–431. doi: 10.1093/scan/nss014
Corradi-Dell’Acqua, C., Hofstetter, C., and Vuilleumier, P. (2011). Felt and seen pain evoke the same local patterns of cortical activity in insular and cingulate cortex. J. Neurosci. 31, 17996–18006. doi: 10.1523/JNEUROSCI.2686-11.2011
Cuff, B. M. P., Brown, S. J., Taylor, L., and Howat, D. J. (2016). Empathy: a review of the concept. Emot. Rev. 8, 144–153. doi: 10.1177/1754073914558466
de Vignemont, F., and Singer, T. (2006). The empathic brain: how, when and why? Trends Cogn. Sci. 10, 435–441. doi: 10.1016/j.tics.2006.08.008
Decety, J., and Lamm, C. (2006). Human empathy through the lens of social neuroscience. Sci. World J. 6, 1146–1163. doi: 10.1100/tsw.2006.221
Decety, J., Meidenbauer, K. L., and Cowell, J. M. (2017). The development of cognitive empathy and concern in preschool children: a behavioral neuroscience investigation. Dev. Sci. 21:e12570. doi: 10.1111/desc.12570
Dunning, J. P., and Hajcak, G. (2009). See no evil: directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology 46, 28–33. doi: 10.1111/j.1469-8986.2008.00723.x
Engel, C. (2011). Dictator games: a meta study. Exp. Econ. 14, 583–610. doi: 10.1007/s10683-011-9283-7
Fan, Y., and Han, S. (2008). Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia 46, 160–173. doi: 10.1016/j.neuropsychologia.2007.07.023
Farrell, M. J., Laird, A. R., and Egan, G. F. (2005). Brain activity associated with painfully hot stimuli applied to the upper limb: a meta-analysis. Hum. Brain Mapp. 25, 129–139. doi: 10.1002/hbm.20125
Feng, C., Luo, Y. J., and Krueger, F. (2015). Neural signatures of fairness-related normative decision making in the ultimatum game: a coordinate-based meta-analysis. Hum. Brain Mapp. 36, 591–602. doi: 10.1002/hbm.22649
Foti, D., and Hajcak, G. (2008). Deconstructing reappraisal: descriptions preceding arousing pictures modulate the subsequent neural response. J. Cogn. Neurosci. 20, 977–988. doi: 10.1162/jocn.2008.20066
Fox, E., Russo, R., Bowles, R., and Dutton, K. (2001). Do threatening stimuli draw or hold visual attention in subclinical anxiety? J. Exp. Psychol. Gen. 130, 681–700. doi: 10.1037/0096-3445.130.4.681
Frith, C. D., and Singer, T. (2008). The role of social cognition in decision making. Philos. Trans. R. Soc. B 363, 3875–3886. doi: 10.1098/rstb.2008.0156
Fritsch, N., and Kuchinke, L. (2013). Acquired affective associations induce emotion effects in word recognition: an ERP study. Brain Lang. 124, 75–83. doi: 10.1016/j.bandl.2012.12.001
Fukushima, H., and Hiraki, K. (2006). Perceiving an opponent’s loss: gender-related differences in the medial-frontal negativity. Soc. Cogn. Affect. Neurosci. 1, 149–157. doi: 10.1093/scan/nsl020
Gabay, A. S., Radua, J., Kempton, M. J., and Mehta, M. A. (2014). The Ultimatum Game and the brain: a meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 47, 549–558. doi: 10.1016/j.neubiorev.2014.10.014
Gehring, W. J., and Willoughby, A. R. (2002). The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295, 2279–2282. doi: 10.1126/science.1066893
Gu, X., and Han, S. (2007). Attention and reality constraints on the neural processes of empathy for pain. Neuroimage 36, 256–267. doi: 10.1016/j.neuroimage.2007.02.025
Hajcak, G., MacNamara, A., and Olvet, D. M. (2010). Event-related potentials, emotion, and emotion regulation: an integrative review. Dev. Neuropsychol. 35, 129–155. doi: 10.1080/87565640903526504
Hajcak, G., Moser, J. S., Holroyd, C. B., and Simons, R. F. (2007). It’s worse than you thought: the feedback negativity and violations of reward prediction in gambling tasks. Psychophysiology 44, 905–912. doi: 10.1111/j.1469-8986.2007.00567.x
Hein, G., and Singer, T. (2008). I feel how you feel but not always: the empathic brain and its modulation. Curr. Opin. Neurobiol. 18, 153–158. doi: 10.1016/j.conb.2008.07.012
Holroyd, C. B., and Coles, M. G. (2002). The neural basis of human error processing reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 109, 679–709. doi: 10.1037/0033-295X.109.4.679
Holroyd, C. B., Hajcak, G., and Larsen, J. T. (2006). The good, the bad and the neutral: electrophysiological responses to feedback stimuli. Brain Res. 1105, 93–101. doi: 10.1016/j.brainres.2005.12.015
Ikezawa, S., Corbera, S., and Wexler, B. E. (2014). Emotion self-regulation and empathy depend upon longer stimulus exposure. Soc. Cogn. Affect. Neurosci. 9, 1561–1568. doi: 10.1093/scan/nst148
Itagaki, S., and Katayama, J. (2008). Self-relevant criteria determine the evaluation of outcomes induced by others. Neuroreport 19, 383–387. doi: 10.1097/WNR.0b013e3282f556e8
Jabbi, M., Swart, M., and Keysers, C. (2007). Empathy for positive and negative emotions in the gustatory cortex. Neuroimage 34, 1744–1753. doi: 10.1016/j.neuroimage.2006.10.032
Kirman, A., and Teschl, M. (2010). Selfish or selfless? The role of empathy in economics. Philos. Trans. R. Soc. B 365, 303–317. doi: 10.1098/rstb.2009.0192
Lamm, C., Decety, J., and Singer, T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502. doi: 10.1016/j.neuroimage.2010.10.014
Leng, Y., and Zhou, X. (2010). Modulation of the brain activity in outcome evaluation by interpersonal relationship: an ERP study. Neuropsychologia 48, 448–455. doi: 10.1016/j.neuropsychologia.2009.10.002
Luo, P., Qu, C., Chen, X., Zheng, X., Jiang, Y., and Zheng, X. (2013). A comparison of counselors and matched controls in maintaining different brain responses to the same stimuli under the self-perspective and the other-perspective. Brain Imaging Behav. 7, 188–195. doi: 10.1007/s11682-012-9214-z
Ma, Q., Shen, Q., Xu, Q., Li, D., Shu, L., and Weber, B. (2011). Empathic responses to others’ gains and losses: an electrophysiological investigation. Neuroimage 54, 2472–2480. doi: 10.1016/j.neuroimage.2010.10.045
MacNamara, A., and Hajcak, G. (2009). Anxiety and spatial attention moderate the electrocortical response to aversive pictures. Neuropsychologia 47, 2975–2980. doi: 10.1016/j.neuropsychologia.2009.06.026
Masaki, H., Takeuchi, S., Gehring, W. J., Takasawa, N., and Yamazaki, K. (2006). Affective-motivational influences on feedback-related ERPs in a gambling task. Brain Res. 1105, 110–121. doi: 10.1016/j.brainres.2006.01.022
Maurer, U., Brem, S., Bucher, K., and Brandeis, D. (2005). Emerging neurophysiological specialization for letter strings. J. Cogn. Neurosci. 17, 1532–1552. doi: 10.1162/089892905774597218
Mobbs, D., Yu, R., Meyer, M., Passamonti, L., Seymour, B., Calder, A. J., et al. (2009). A key role for similarity in vicarious reward. Science 324:900. doi: 10.1126/science.1170539
Molenberghs, P., Bosworth, R., Nott, Z., Louis, W. R., Smith, J. R., Amiot, C. E., et al. (2014). The influence of group membership and individual differences in psychopathy and perspective taking on neural responses when punishing and rewarding others. Hum. Brain Mapp. 35, 4989–4999. doi: 10.1002/hbm.22527
Moser, J. S., Hajcak, G., Bukay, E., and Simons, R. F. (2006). Intentional modulation of emotional responding to unpleasant pictures: an ERP study. Psychophysiology 43, 292–296. doi: 10.1111/j.1469-8986.2006.00402.x
Murphy, F. C., Nimmosmith, I., and Lawrence, A. D. (2003). Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 3, 207–233. doi: 10.3758/CABN.3.3.207
Nieuwenhuis, S., Aston-Jones, G., and Cohen, J. D. (2005). Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol. Bull. 131, 510–532. doi: 10.1037/0033-2909.131.4.510
Nordstrom, H., and Wiens, S. (2012). Emotional event-related potentials are larger to figures than scenes but are similarly reduced by inattention. BMC Neurosci. 13:49. doi: 10.1186/1471-2202-13-49
Olofsson, J., Nordin, S., Sequeira, H., and Polich, J. (2008). Affective picture processing: an integrative review of ERP findings. Biol. Psychol. 77, 247–265. doi: 10.1016/j.biopsycho.2007.11.006
Paul, S., Simon, D., Kniesche, R., Kathmann, N., and Endrass, T. (2013). Timing effects of antecedent- and response-focused emotion regulation strategies. Biol. Psychol. 94, 136–142. doi: 10.1016/j.biopsycho.2013.05.019
Salimi-Khorshidi, G., Smith, S. M., Keltner, J. R., Wager, T. D., and Nichols, T. E. (2009). Meta-analysis of neuroimaging data: a comparison of image-based and coordinate-based pooling of studies. Neuroimage 45, 810–823. doi: 10.1016/j.neuroimage.2008.12.039
San Martin, R. (2012). Event-related potential studies of outcome processing and feedback-guided learning. Front. Hum. Neurosci. 6:304. doi: 10.3389/fnhum.2012.00304
Sanfey, A. G., Rilling, J. K., Aronson, J. A., Nystrom, L. E., and Cohen, J. D. (2003). The neural basis of economic decision-making in the ultimatum game. Science 300, 1755–1758. doi: 10.1126/science.1082976
Schönfelder, S., Kanske, P., Heissler, J., and Wessa, M. (2014). Time course of emotion-related responding during distraction and reappraisal. Soc. Cogn. Affect. Neurosci. 9, 1310–1319. doi: 10.1093/scan/nst116
Shamay-Tsoory, S. G., Aharon-Peretz, J., and Perry, D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 132(Pt 3), 617–627. doi: 10.1093/brain/awn279
Shamay-Tsoory, S. G., Shur, S., Barcai-Goodman, L., Medlovich, S., Harari, H., and Levkovitz, Y. (2007). Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res. Neuroimaging 149, 11–23. doi: 10.1016/j.psychres.2005.10.018
Simpson, J. A., Ickes, W., and Grich, J. (1999). When accuracy hurts: reactions of anxious ambivalent dating partners to a relationship-threatening situation. J. Pers. Soc. Psychol. 76, 754–769. doi: 10.1037/0022-3514.76.5.754
Singer, T. (2006). The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci. Biobehav. Rev. 30, 855–863. doi: 10.1016/j.neubiorev.2006.06.011
Singer, T., and Lamm, C. (2009). The social neuroscience of empathy. Ann. N. Y. Acad. Sci. 1156, 81–96. doi: 10.1111/j.1749-6632.2009.04418.x
Singer, T., Seymour, B., Odoherty, J. P., Kaube, H., Dolan, R. J., and Frith, C. (2004). Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162. doi: 10.1126/science.1093535
Takahashi, H., Kato, M., Matsuura, M., Mobbs, D., Suhara, T., and Okubo, Y. (2009). When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science 323, 937–939. doi: 10.1126/science.1165604
Thiruchselvam, R., Blechert, J., Sheppes, G., Rydstrom, A., and Gross, J. J. (2011). The temporal dynamics of emotion regulation: an EEG study of distraction and reappraisal. Biol. Psychol. 87, 84–92. doi: 10.1016/j.biopsycho.2011.02.009
Thiruchselvam, R., Hajcak, G., and Gross, J. J. (2012). Looking inward: shifting attention within working memory representations alters emotional responses. Psychol. Sci. 23, 1461–1466. doi: 10.1177/0956797612449838
Van Dillen, L. F., and Derks, B. (2012). Working memory load reduces facilitated processing of threatening faces: an ERP study. Emotion 12, 1340–1349. doi: 10.1037/a0028624
Varnum, M. E., Shi, Z., Chen, A., Qiu, J., and Han, S. (2014). When ”Your” reward is the same as ”My” reward: self-construal priming shifts neural responses to own vs. friends’ rewards. Neuroimage 87, 164–169. doi: 10.1016/j.neuroimage.2013.10.042
Vytal, K., and Hamann, S. (2010). Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J. Cogn. Neurosci. 22, 2864–2885. doi: 10.1162/jocn.2009.21366
Walsh, M. M., and Anderson, J. R. (2012). Learning from experience: event-related potential correlates of reward processing, neural adaptation, and behavioral choice. Neurosci. Biobehav. Rev. 36, 1870–1884. doi: 10.1016/j.neubiorev.2012.05.008
Wang, Y., Qu, C., Luo, Q. L., Qu, L. L., and Li, X. (2014a). Like or dislike? Affective preference modulates neural response to others’ gains and losses. PLoS One 9:e105694. doi: 10.1371/journal.pone.0105694
Wang, Y., Yuan, B., Roberts, K., Wang, Y., Lin, C., and Simons, R. F. (2014b). How friendly is a little friendly competition? Evidence of self-interest and empathy during outcome evaluation. Int. J. Psychophysiol. 91, 155–162. doi: 10.1016/j.ijpsycho.2013.10.009
Wangelin, B. C., Low, A., McTeague, L. M., Bradley, M. M., and Lang, P. J. (2011). Aversive picture processing: effects of a concurrent task on sustained defensive system engagement. Psychophysiology 48, 112–116. doi: 10.1111/j.1469-8986.2010.01041.x
Wen, G., Bo, C., Shiguang, S., Xilin, C., Delong, Z., Xiaohua, Z., et al. (2008). The CAS-PEAL large-scale Chinese face database and baseline evaluations. IEEE Trans. Syst. Man Cybern. A Syst. Hum. 38, 149–161. doi: 10.1109/tsmca.2007.909557
Wicker, B., Keysers, C., Plailly, J., Royet, J.-P., Gallese, V., and Rizzolatti, G. (2003). Both of Us disgusted in my insula. Neuron 40, 655–664. doi: 10.1016/s0896-6273(03)00679-2
Wiens, S., Sand, A., Norberg, J., and Andersson, P. (2011). Emotional event-related potentials are reduced if negative pictures presented at fixation are unattended. Neurosci. Lett. 495, 178–182. doi: 10.1016/j.neulet.2011.03.042
Wiens, S., and Syrjanen, E. (2013). Directed attention reduces processing of emotional distracters irrespective of valence and arousal level. Biol. Psychol. 94, 44–54. doi: 10.1016/j.biopsycho.2013.05.001
Xiang, Y., Wang, Y., Gao, S., Zhang, X., and Cui, R. (2018). Neural mechanisms with respect to different paradigms and relevant regulatory factors in empathy for pain. Front. Neurosci. 12:507. doi: 10.3389/fnins.2018.00507
Yamada, M., Lamm, C., and Decety, J. (2011). Pleasing frowns, disappointing smiles: an ERP investigation of counterempathy. Emotion 11, 1336–1345. doi: 10.1037/a0023854
Yiend, J., and Mathews, A. (2001). Anxiety and attention to threatening pictures. Q. J. Exp. Psychol. 54, 665–681. doi: 10.1080/713755991
Keywords: empathy, fairness preference, event-related potential (ERP), N170, late positive potential (LPP), feedback-related negativity (FRN)
Citation: Jie J, Luo P, Zhuang M, Li B, Pang Y, Li J and Zheng X (2019) Self-Interest Induces Counter- Empathy at the Late Stage of Empathic Responses to Others’ Economic Payoffs. Front. Psychol. 10:372. doi: 10.3389/fpsyg.2019.00372
Received: 24 October 2018; Accepted: 06 February 2019;
Published: 25 February 2019.
Edited by:
Qinghua He, Southwest University, ChinaCopyright © 2019 Jie, Luo, Zhuang, Li, Pang, Li and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xifu Zheng, emhlbmd4aWZ1QG0uc2NudS5lZHUuY24=
†These authors have contributed equally to this work
 Jing Jie
Jing Jie Pinchao Luo1†
Pinchao Luo1† Xifu Zheng
Xifu Zheng