- 1Department of Psychological and Brain Sciences, University of Massachusetts, Amherst, MA, United States
- 2Neuroscience and Behavior, University of Massachusetts, Amherst, MA, United States
- 3Rady School of Management, University of California, San Diego, San Diego, CA, United States
When offered a choice of $40 today or $50 later, many would choose the immediate reward over the greater delayed reward. Such behavior is a result of future gains being discounted such that their value is rendered less than that of the immediate gain. Extreme discounting behaviors are associated with impulsivity and addiction. Given recent evidence of sleep’s role in decision making, we tested the hypothesis that sleep would reduce delayed discounting behavior. Twenty young adults (M = 20.19 years, SD = 0.98 years; 6 males) performed a hypothetical delay discounting task, making a series of choices between an immediate reward (from $0 to $50) or a larger reward ($50) available at a delay of 2, 4, 8, 14, or 22 weeks. Participants performed the task before and after a mid-day nap, and before and after an equivalent interval of wake (within subject, order counterbalanced, wake, and sleep conditions separated by 1 week). As expected, indifference points decreased with longer delays both prior to and following the nap/wake interval. However, the impact of a nap interval on discounting did not differ from the impact of a wake interval. Thus, while sleep has been shown to play an active role in some financial decision-making tasks, a nap is not sufficient to change delay discounting behavior.
Introduction
The “marshmallow experiment" of Mischel et al. (1989) is known for demonstrating the inability of children to wait for two marshmallows, with them opting instead for a single, immediate marshmallow. While we find humor in these behaviors in children, adults show similar deficits on tasks typically involving monetary rewards. For instance, a person who accepts $50 now over $100 in a year is greatly devaluing the delayed reward relative to the present amount, to the point where the future value is subjectively less than the present value (Mitchell and Wilson, 2012). Delay discounting – choosing an immediate smaller reward over a delayed larger reward – can be economically disadvantageous (Mazur, 1987). Excessive discounting behavior may have its roots in impulsivity (Glimcher et al., 2007), and may underlie other maladaptive behaviors (Bickel et al., 1999; MacKillop et al., 2011; Odum, 2011).
Decision-making behavior can become more impulsive following sleep deprivation. Reynolds and Schiffbauer (2004) observed steeper discounting rates in sleep-deprived individuals compared to those that were allowed to sleep. Sleep deprivation likewise impairs performance on the Iowa Gambling Task (IGT), an affectively guided decision-making task (Harrison and Horne, 2000; Killgore et al., 2006). These deficits from a lack of sleep may reflect reduced function and connectivity of the prefrontal cortex and other brain areas (Drummond et al., 2000; Verweij et al., 2014), causing more impulsive behavior. However, impairments are not consistently observed following sleep deprivation (Acheson et al., 2007; Libedinsky et al., 2013; Demos et al., 2016; Chan, 2017).
While sleep deprivation can impair decisions, sleep itself may have positive effects on economic choices with subjective components. For example, people have more positive perceptions toward available choice options following sleep relative to wake (Karmarkar et al., 2017), and affect-guided IGT decisions improve following sleep (Pace-Schott et al., 2012). In addition, amygdala reactivation during REM sleep is thought to contribute to emotion processing, including emotional aspects of decision-making (moral judgements; Cellini et al., 2017).
This raises the question of whether sleep can actively reduce impulsivity during financial choices. To test this, we measured individual discounting rates before (which served as baseline) and after a nap and an equivalent period of wake. A nap paradigm allowed us to assess the active role of sleep compared to a wake control without the confound of sleep depriving subjects. We hypothesized that people would show less discounting after sleep.
Materials and Methods
Participants
Participants were 20 young adults 18–30 years (M = 20.19 years, SD = 0.98 years; 6 males). All participants were screened against high or low sleep quantity (<5 h or >11 h per night), the use of sleep-affecting medications, and the presence of sleep, neurological, or psychiatric disorders.
Procedure
The protocol was approved by the Institutional Review Board at the University of Massachusetts. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Accordingly, written informed consent was obtained prior to testing. All participants completed a nap and wake condition. In both conditions, participants arrived at the laboratory at 12:30 PM and were instructed to wear an actigraph throughout the day. Participants completed the delay discounting task in the lab and then returned home to either (1) attempt a 3-h nap by turning off the lights and keeping their eyes closed (Nap-First condition) or (2) stay awake (Wake-First condition). They returned to the lab at 5:30 PM to perform the discounting task again (Figure 1A). This procedure was repeated exactly 1 week later when participants completed the opposite condition.
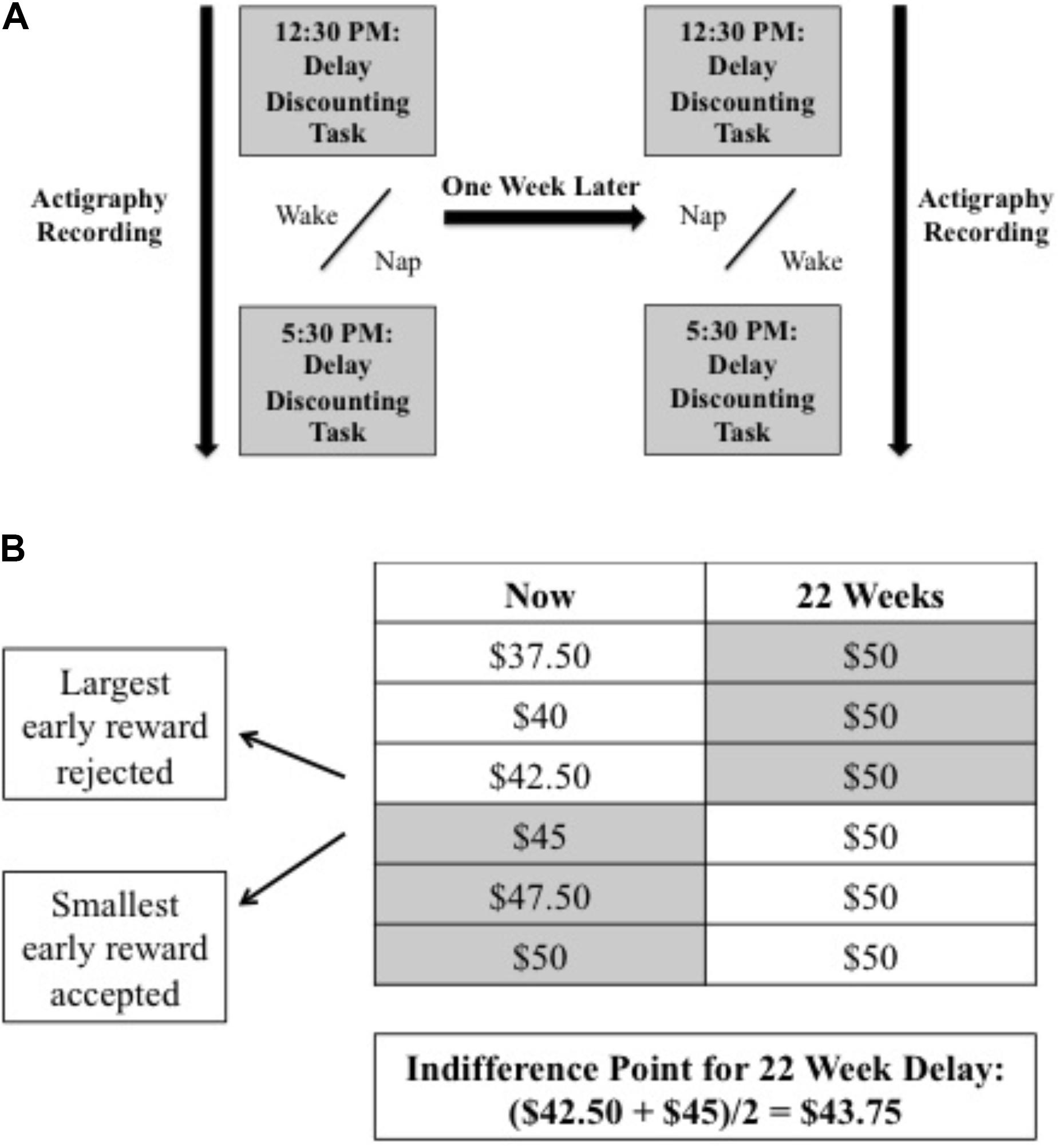
FIGURE 1. Experimental design indicating (A) study procedures (gray bars indicate time points when the delay discounting task was performed) and (B) a sample choice (22-week delay) and respective indifference point calculation. Shaded cells represent participant’s choice between the now and later option.
Delay Discounting Task
The task was adapted from Mitchell and Wilson (2012). Monetary choices between a smaller, more immediate reward, or a larger, delayed reward, were presented one pair at a time on a computer screen. Each pair represented a unique combination of two factors: reward magnitude of the immediate option and/or delay of delivery of the larger, later reward. The immediate outcome was always offered at no delay, but with a payout that varied from $0 to $50, increasing in increments of $2.50 (e.g., $0, $2.50). The reward magnitude of the larger, delayed reward was held constant at $50, while its payout delay varied between 2, 4, 8, 14, or 22 weeks. Parametrically varying over these options resulted in a total of 105 monetary choices. All participants were presented with all 105 choices, and their responses were not restricted by a time limit.
Questionnaires
Habitual sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). During each session, current affect (Positive and Negative Affect Scale, PANAS; Watson et al., 1988) and sleepiness (Stanford Sleepiness Scale; Hoddes et al., 1973) were also recorded.
Actigraphy
The Actiwatch Spectrum was worn on the non-dominant wrist. The actigraph, which uses a triaxial accelerometer to estimate sleep, is deemed valid relative to polysomnography (Mantua et al., 2016). Participants were instructed to press an event marker at “lights off” and at “lights on” for the nap. Data from the watch was scored in 15-s epochs and analyzed using the Actiwatch software. Nap duration and nap efficiency, the amount of time spent asleep relative to time in bed, were calculated (Spencer et al., 2016).
Analyses
The primary outcome measures were the individual Indifference Points (Mitchell and Wilson, 2012). These were defined as the midway point between the largest immediate reward rejected and the smallest immediate reward accepted for each time delay (2, 4, 8, 14, and 22 weeks; Figure 1B). In addition, Discounting Functions (k) were estimated per individual across the six delay points1 using the hyperbolic model: V = A/(1+kD), where “V” is the Indifference Point, “A” the delayed reward (set at $50), “D” the length of the delay, and “k” the free parameter that represents the steepness of the discounting (Johnson and Bickel, 2002). Fits were estimated before and after nap and wake using this model with the Matlab fit function.
To examine changes in Indifference Points we conducted mixed ANOVAs with within-subjects variables Condition (Wake, Nap), Time Point (before or after nap/wake), and Delay (5 delays), as well as between-subjects factor Order (Wake-First, Nap-First). A similar analysis, (without the Delay factor), was used on the estimated discounting steepness (k). Post hoc paired samples t-tests were conducted where main effects and interactions were significant.
Results
All participants stayed awake in the wake condition and successfully napped in the nap condition. Average nap duration was 110.1 min (SD = 56.2), with all participants napping for at least 30 min. Naps were efficient, with an average sleep efficiency of 92.05% (SD = 5.85). None of the participants reported poor subjective sleep quality (PSQI scores <7; M = 3.90, SD = 1.74). Negative mood, as assessed with the PANAS, did not differ before [t(19) = -0.514, p = 0.613] or after [t(19) = 0.664, p = 0.515] the nap/wake interval. Positive mood was greater before the wake compared to the nap interval [t(19) = -2.776, p = 0.012], with no difference after the nap/wake intervals [t(19) = -0.365, p = 0.715].
Indifference Points
A mixed ANOVA revealed a significant main effect of Delay [F(4,72) = 17.651, p < 0.001, = 0.495], such that Indifference Points were lower for longer delays, consistent with prior studies (e.g., Laibson, 1997). There was a weak main effect of Time Point [F(1,18) = 4.003, p = 0.061, = 0.182] qualified by a significant Time Point x Delay interaction [F(4,72) = 2.619, p = 0.042, = 0.127], such that Indifference Points were higher for long delays following time spent away from the decision task, regardless of sleep.
The Condition × Delay interaction was significant [F(4,72) = 3.029, p = 0.023, = 0.144]. Post hoc paired samples t-tests revealed higher Indifference Points – reflective of less discounting – in the Nap condition compared to the Wake condition for the 4-week delay only [t(19) = -2.202, p = 0.040, Cohen’s d = 0.492; Figure 2). However, given that this analysis collapses across Time Point (before/after interval), we presume this to reflect baseline differences in the conditions. Indeed, the Condition × Time Point [F(1,18) = 0.607, p = 0.446] and Condition × Time Point × Delay [F(4,72) = 0.695, p = 0.598] interactions were not significant.
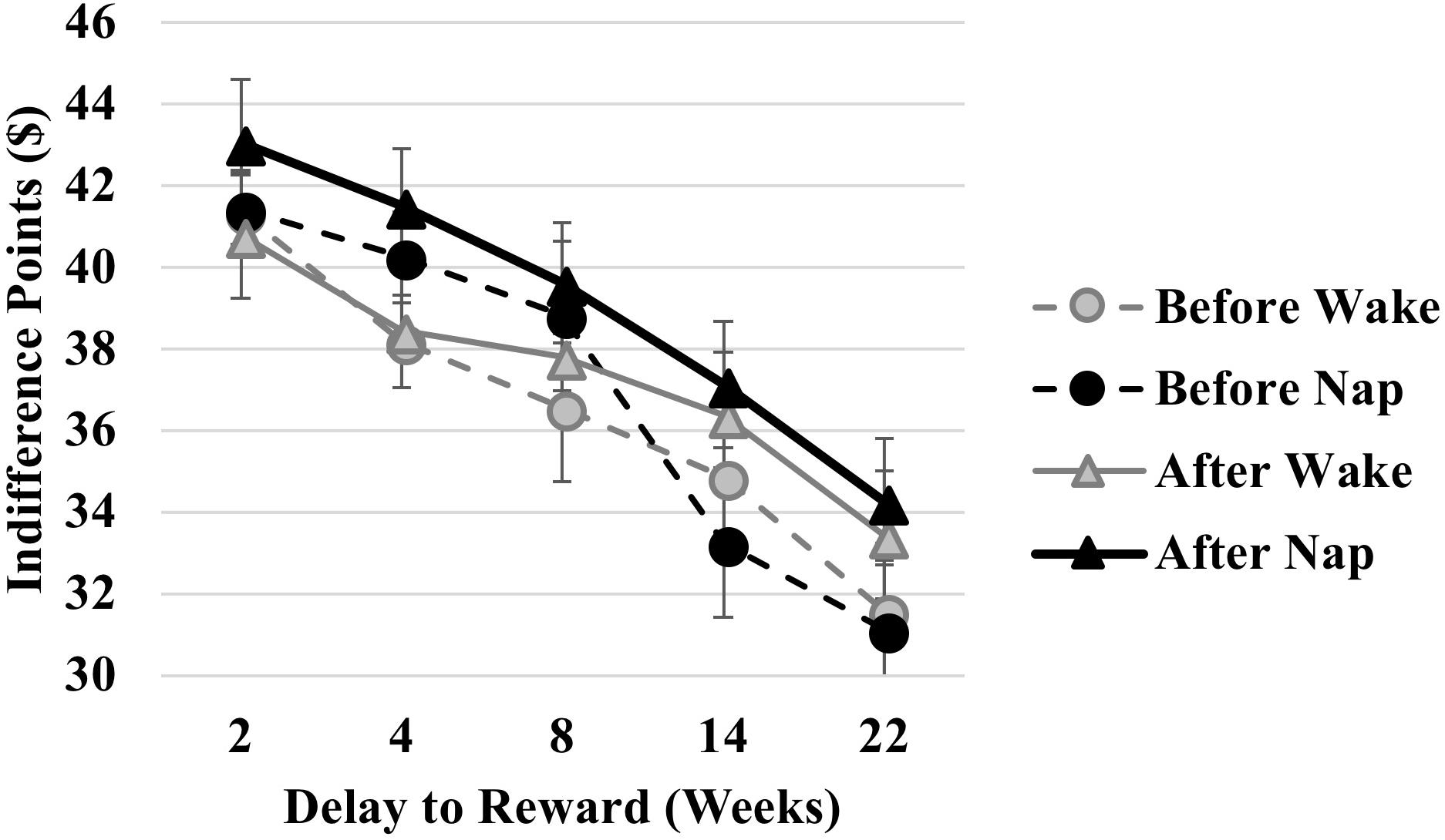
FIGURE 2. Comparison of indifference points across time delays (2, 4, 8, 14, and 22 weeks) before (circles) and after (triangles) an afternoon nap (black symbols/lines) and equivalent interval awake (gray symbols/lines).
While the Condition × Order interaction was significant [F(1,18) = 4.599, p = 0.046, = 0.203], such that Indifference Points were higher for the Nap Condition in the Wake-first group compared to the Nap-first group, the 3-way interaction of Condition × Order × Time Point was not [F(4,18) = 0.315, p = 0.582, = 0.017], ruling out the possibility that any sleep related changes in Indifference Points were related to whether the individuals had completed the task before. No other main effects or interactions were significant (p > 0.2).
Discounting Functions
We examined the differences in participants’ “k” discount parameters before and after periods of nap and wake using a repeated-measures ANOVA. The Order (e.g., nap/wake session order) covariate was not significant (p = 0.742). Consistent with the Indifference Points analyses, there was a marginally significant difference between the steepness of the discount rates overall in the nap and wake tasks within subjects [F(1,18) = 4.375, p = 0.051, = 0.196], and similarly a marginally significant decrease in steepness when comparing k before and after a nap or wake interval [F(1,18) = 3.677, p = 0.071, = 0.170]. However, the interaction between these factors did not reach significance [F(1,18) = 1.99, p = 0.175, = 0.100], indicating that the magnitude of the changes was not different with sleep.
Discussion
In this study, we find that sleep, operationalized as a mid-day nap, does not modulate delay discounting behavior. As expected, indifference points were lower for longer delays. Although there was a significant Delay × Condition interaction for the Indifference Points, the Delay × Condition × Time Point interaction was not significant. Because we randomized order, used a within-subject design, and found the Delay × Condition effect only at the 4 weeks delay, we assume the Delay × Condition interaction to be a false positive.
A similar lack of differences between the effects of nap and wake intervals was observed when fitting a hyperbolic discounting model to the indifference points across the range of delays. This analysis is useful as it offers a summary of the general steepness (k) of an individual’s overall discounting function. However, it should be noted that fitting these functions to a relatively small number of delays (6) is sensitive to variation (and noise) in the data.
These results run counter to our central hypothesis, arising from prior evidence that a bout of sleep can influence some forms of consumer and/or economic decisions (e.g., Pace-Schott et al., 2012; Karmarkar et al., 2017). That earlier research employed an overnight sleep interval, which raises the possibility that a longer sleep bout is necessary to impact delay discounting behavior. It has been suggested that delay discounting may be less sensitive than other tasks of inhibitory control and decision making (Acheson et al., 2007). In our task, the mean nap length was greater than 90 min, suggesting many subjects likely reached REM sleep (see Milner and Cote, 2009), but it may be that late-night REM is needed to see sleep-relevant changes. Others have suggested that a longer interval of sleep deprivation (as opposed to our mid-day napless condition) may be necessary to see such effects (Libedinsky et al., 2013; see also Killgore et al., 2006). Alternatively, this study, combined with other recent findings could point to delay discounting behavior as independent of sleep (e.g., Acheson et al., 2007; Libedinsky et al., 2013; Demos et al., 2016; Chan, 2017). Our results motivate future studies employing longer periods of sleep (one or more overnight sleep bouts) to distinguish between these possibilities.
There are several limitations to consider. First, we report a null result, though we believe it is important to document to avoid publication bias and to move the field forward (Matosin et al., 2014). Second, as noted, our design would have benefited from the use of polysomnography in the nap interval. An in-lab study would also have allowed us to carefully control the nap and wake behaviors. Finally, this study used hypothetical rewards which may not replicate fully incentivized behavior (Paloyelis et al., 2010).
Overall, understanding sleep’s role in these types of behaviors is important in supporting healthy decisions in impulsive populations as well as other meaningful groups. This paper expands the research on sleep and decision-making, and finds that delay discounting is not sensitive to short periods of sleep, compared to non-deprived wake.
Author Contributions
RS contributed to the study conceptualization. SO, AS, and RS contributed to the study design. SO contributed to the data collection. SO and AS did the data analysis. SO, AS, UK, and RS contributed to the data interpretation and manuscript writing.
Funding
This work was funded by an Honors Research Grant to SO from Commonwealth Honors College at the University of Massachusetts, Amherst.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
- ^Given the sparse number of data points and the standard delayed reward, the canonical data point of zero delay and $50 indifference was explicitly included in the fits.
References
Acheson, A., Richards, J. B., and de Wit, H. (2007). Effects of sleep deprivation on impulsive behaviors in men and women. Physiol. Behav. 91, 579–587. doi: 10.1016/j.physbeh.2007.03.020
Bickel, W. K., Odum, A. L., and Madden, G. J. (1999). Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology 146, 447–454. doi: 10.1007/PL00005490
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., and Kupfer, D. J. (1989). The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213. doi: 10.1016/0165-1781(89)90047-4
Cellini, N., Lotto, L., Pletti, C., and Sarlo, M. (2017). Daytime REM sleep affects emotional experience but not decision choices in moral dilemmas. Sci. Rep. 7:11059. doi: 10.1038/s41598-017-11530-4
Chan, W. S. (2017). Delay discounting and response disinhibition moderate associations between actigraphically measured sleep parameters and body mass index. J. Sleep Res. 26, 21–29. doi: 10.1111/jsr.12437
Demos, K. E., Hart, C. N., Sweet, L. H., Mailloux, K. A., Trautvetter, J., Williams, S. E., et al. (2016). Partial sleep deprivation impacts impulsive action but not impulsive decision-making. Physiol. Behav. 164, 214–219. doi: 10.1016/j.physbeh.2016.06.003
Drummond, S. P. A., Brown, G. G., Gillin, J. C., Stricker, J. L., Wong, E. C., and Buxton, R. B. (2000). Altered brain response to verbal learning following sleep deprivation. Nature 403, 655–657. doi: 10.1038/35001068
Glimcher, P. W., Kable, J., and Louie, K. (2007). Neuroeconomic studies of impulsivity: now or just as soon as possible? Am. Econ. Rev. 97, 142–147. doi: 10.1016/j.forsciint.2015.06.016
Harrison, Y., and Horne, J. A. (2000). The impact of sleep deprivation on decision making: a review. J. Exp. Psychol. Appl. 6, 236–249. doi: 10.1037/1076-898X.6.3.236
Hoddes, E., Zarcone, V., Smythe, H., Phillips, R., and Dement, W. C. (1973). Quantification of sleepiness: A new approach. Psychophysiology 10, 431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x
Johnson, M. W., and Bickel, W. K. (2002). Within-subject comparison of real and hypothetical money rewards in delay discounting. J. Exp. Anal. Behav. 77, 129F–146F. doi: 10.1901/jeab.2002.77-129
Karmarkar, U. R., Shiv, B., and Spencer, R. (2017). Should you sleep on it? The effects of overnight sleep on subjective preference-based choice. J. Behav. Decis. Mak. 30, 70–79. doi: 10.1002/bdm.1921
Killgore, W. D. S., Balkin, T. J., and Wesensten, N. J. (2006). Impaired decision making following 49 h of sleep deprivation. J. Sleep Res. 15, 7–13. doi: 10.1111/j.1365-2869.2006.00487.x
Laibson, D. (1997). Golden eggs and hyperbolic discounting. J. Econ. 112, 443–478. doi: 10.1162/003355397555253
Libedinsky, C., Massar, S. A. A., Ling, A., Chee, W. Y., Huettel, S. A., and Chee, M. W. L. (2013). Sleep deprivation alters effort discounting but not delay discounting of monetary rewards. Sleep 36, 899–904. doi: 10.5665/sleep.2720
MacKillop, J., Amlung, M. T., Few, L. R., Ray, L. A., Sweet, L. H., and Munafo, M. R. (2011). Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology 216, 305–321. doi: 10.1007/s00213-011-2229-0
Mantua, J., Gravel, N., and Spencer, R. M. C. (2016). Reliability of sleep measures from four personal health monitoring devices compared to research-based actigraphy and polysomnography. Sensors 16:e636. doi: 10.3390/s16050646
Matosin, N., Frank, E., Engel, M., Lum, J. S., and Newell, K. A. (2014). Negativity towards negative results: a discussion of the disconnect between scientific worth and scientific culture. Dis. Model Mech. 7, 171–173. doi: 10.1242/dmm.015123
Mazur, J. E. (1987). “An adjusting procedure for studying delayed reinforcement,” in Quantitative Analysis of Behavior: The Effect of Delay and of Intervening Events on Reinforcement Value, Vol. 5, eds M. L. Commons, J. E. Mazur, J. A. Nevin, and H. Rachlin (Hillsdale, NJ: Erlbaum), 55–73.
Milner, C. E., and Cote, K. A. (2009). Benefits of napping in healthy adults: impact of nap length, time of day, age, and experience with napping. J. Sleep Res. 18, 272–281. doi: 10.1111/j.1365-2869.2008.00718.x
Mischel, W., Shoda, Y., and Rodriguez, M. L. (1989). Delay of gratification in children. Science 244, 933–938. doi: 10.1126/science.2658056
Mitchell, S. H., and Wilson, V. B. (2012). Differences in delay discounting between smokers and nonsmokers remain when both rewards are delayed. Psychopharmacology 219, 549–562. doi: 10.1007/s00213-011-2521-z
Odum, A. L. (2011). Delay discounting: Trait variable? Behav. Process. 87, 1–9. doi: 10.1016/j.beproc.2011.02.007
Pace-Schott, E., Nave, G., Morgan, A., and Spencer, R. (2012). Sleep-dependent modulation of affectively guided decision making. J. Sleep Res. 21, 30–39. doi: 10.1111/j.1365-2869.2011.00921.x
Paloyelis, Y., Asherson, P., Mehta, M. A., Faraone, S. V., and Kuntsi, J. (2010). DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology 35, 2414–2426. doi: 10.1038/npp.2010.124
Reynolds, B., and Schiffbauer, R. (2004). Measuring state changes in human delay discounting: an experiential discounting task. Behav. Proc. 67, 343–356. doi: 10.1016/S0376-6357(04)00140-8
Spencer, R. M., Campanella, C., de Jong, D. M., Desrochers, P., Root, H., Cremone, A., et al. (2016). Sleep and behavior of preschool children under typical and nap-promoted conditions. Sleep Health 2, 35–41. doi: 10.1016/j.sleh.2015.12.009
Verweij, I. M., Romeijn, N., Smith, D. J. A., Piantoni, G., Van Someren, E. J. W., and van der Werf, Y. D. (2014). Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci. 15:88. doi: 10.1186/1471-2202-15-88
Keywords: discounting, sleep, impulsivity, decision making, naps, economics
Citation: O’Connor S, Sonni A, Karmarkar U and Spencer RMC (2018) Naps Do Not Change Delay Discounting Behavior in Young Adults. Front. Psychol. 9:921. doi: 10.3389/fpsyg.2018.00921
Received: 21 February 2018; Accepted: 18 May 2018;
Published: 22 June 2018.
Edited by:
Thomas Kleinsorge, Leibniz Research Centre for Working Environment and Human Factors (LG), GermanyReviewed by:
Stijn A. A. Massar, National University of Singapore, SingaporePoppy Watson, University of New South Wales, Australia
Copyright © 2018 O’Connor, Sonni, Karmarkar and Spencer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca M. C. Spencer, cnNwZW5jZXJAdW1hc3MuZWR1
†These authors have contributed equally to this work.
 Sean O’Connor
Sean O’Connor Akshata Sonni
Akshata Sonni Uma Karmarkar
Uma Karmarkar Rebecca M. C. Spencer
Rebecca M. C. Spencer