- 1Department of Psychiatry and Forensic Medicine, School of Medicine, Universitat Autònoma de Barcelona, Barcelona, Spain
- 2Department of Mental Health, Parc Taulí Hospital Universitari, Institut d’Investigació i Innovació Parc Taulí I3PT, Universitat Autònoma de Barcelona, Sabadell, Spain
- 3Specialized Department in Mental Health and Intellectual Disability, Institut Assistència Sanitària (IAS), Institut d’Investigació Biomèdica de Girona, Parc Hospitalari Martí i Julià, Salt, Spain
- 4Institute of Neuropsychiatry and Addictions, Parc de Salut Mar, Barcelona, Spain
- 5Anxiety Unit, Institute of Neuropsychiatry and Addictions, Hospital del Mar, CIBERSAM, Barcelona, Spain
- 6IMIM, Hospital del Mar Medical Research Institute, Barcelona, Spain
- 7University Research Institute on Health Sciencies (IUNICS), Universitat de les Illes Balears, Mallorca, Spain
- 8PROMOSAM Red de Investigación en Procesos, Mecanismos y Tratamientos Psicológicos para la Promoción de la Salud Mental, Mallorca, Spain
Attentional control (AC) and fear extinction learning are known to be involved in pathological anxiety. In this study we explored whether individual differences in non-emotional AC were associated with individual differences in the magnitude and gradient of fear extinction (learning and recall). In 50 individuals with fear of spiders, we collected measures of non-emotional AC by means of self-report and by assessing the functioning of the major attention networks (executive control, orienting, and alerting). The participants then underwent a paradigm assessing fear extinction learning and extinction recall. The two components of the orienting network functioning (costs and benefits) were significantly associated with fear extinction gradient over and above the effects of trait anxiety. Specifically, participants with enhanced orienting costs (i.e., difficulties in disengaging attention from cues not relevant for the task) showed faster extinction learning, while those with enhanced orienting benefits (i.e., attention facilitated by valid cues) exhibited faster extinction recall as measured by fear-potentiated startle and Unconditioned Stimulus expectancies, respectively. Our findings suggest that, in non-emotional conditions, the orienting component of attention may be predictive of fear extinction. They also show that the use of fear extinction gradients and the exploration of individual differences in non-emotional AC (using performance-based measures of attentional network functioning) can provide a better understanding of individual differences in fear learning. Our findings also may help to understand differences in exposure therapy outcomes.
Introduction
Several attentional and learning processes have been found to play a major role in pathological anxiety (i.e., anxiety disorders). Recent research suggests that attentional control (AC) and fear extinction learning feature prominently among such processes (e.g., Bar-Haim et al., 2007; Cisler and Koster, 2010; Eysenck and Derakshan, 2011; Milad and Quirk, 2012; Heeren et al., 2013; VanElzakker et al., 2014; Duits et al., 2015; Hadwin et al., 2016). In this study, we explore the possible association between these two processes.
Attentional control is a construct that defines our ability to regulate attention allocation, including our ability to maintain sustained attention, ignore distractors, and shift attention between tasks (Derryberry and Reed, 2002). Deficient AC has been found to characterize both clinical (Olatunji et al., 2011) and subclinical anxiety (e.g., Fajkowska and Derryberry, 2010; Sportel et al., 2013). Such deficits may also account for the attention bias to threat commonly observed in anxious individuals (Hadwin et al., 2016). Moreover, such deficits are associated with reduced ability to regulate emotion (Fajkowska and Derryberry, 2010; Armstrong et al., 2011; Tortella-Feliu et al., 2014; Hsu et al., 2015; Morillas-Romero et al., 2015; O’Bryan et al., 2017). AC can be assessed under emotional conditions (emotional AC, e.g., Barry et al., 2013) or neutral conditions (non-emotional AC, e.g., Derryberry and Reed, 2002; Pacheco-Unguetti et al., 2011; Richey et al., 2016).
Fear extinction learning refers to the decrease in fear following non-reinforced exposure to a feared conditioned stimulus (CS) and is typically investigated in humans within a differential fear learning paradigm preceded by a conditioning (i.e., acquisition) phase. The test of how fear extinction learning is retrieved after re-exposure to the extinguished CS is usually called extinction recall. Deficient fear extinction learning (Duits et al., 2015) or extinction recall (Graham and Milad, 2011; Milad and Quirk, 2012) could be a marker of anxiety disorders (e.g., Graham and Milad, 2011). Importantly, fear extinction is a form of emotion regulation (Hartley and Phelps, 2010) and there is evidence that similar neurobiological mechanisms (i.e., hipoactivity of the prefrontal cortex) may be involved in fear extinction, reduced emotion regulation capabilities, and low AC in non-emotional conditions (Bishop, 2007, 2008, 2009; Hartley and Phelps, 2010; Milad and Quirk, 2012; Ochsner et al., 2012; Shiba et al., 2016; Ball et al., 2017). Therefore, individual differences in AC under non-emotional conditions may be associated with individual differences in fear extinction, although this has not been investigated so far, as far as we are aware.
Moreover, fear extinction learning procedures are considered experimental models for exposure therapy (Craske et al., 2014: Pittig et al., 2016) and both fear extinction learning (e.g., Waters and Pine, 2016; Ball et al., 2017; Forcadell et al., 2017) and attentional functioning (e.g., Barry et al., 2015a) may be associated with the outcomes of exposure therapy, and constitute putative targets for improving such outcomes (Bar-Haim, 2010; Craske et al., 2012, 2014; Barry et al., 2015b; Mogg and Bradley, 2016; Pittig et al., 2016). A better understanding of the association between AC and fear extinction may therefore have important therapeutic implications.
The role of attention in fear learning has been a topic of research for years (e.g., Mackintosh, 1975; Wagner, 1981; Le Pelley et al., 2016). Most studies have focused on how attention allocation changes during or after acquisition (e.g., Beaver et al., 2005; Koster et al., 2005) or extinction (e.g., Robbins, 1990; Van Damme et al., 2006; Barry et al., 2016b) affect the magnitude of learning. Moreover, attentional biases to threat in anxiety could reflect a much broader dysregulation of AC (Bishop, 2009; Moriya and Tanno, 2009; Pacheco-Unguetti et al., 2011). The use of non-emotional stimuli allows to isolate potential general attention deficits beyond those observed when individuals face emotional materials (see further below). A few recent studies have focused on how baseline individual differences in attention predict the magnitude or gradient (“speed”) of fear extinction learning (Waters and Kershaw, 2015; Barry et al., 2016a, 2017).
The study by Waters and Kershaw (2015) belongs to the research tradition that focuses on analyzing how attentional bias to threat-related information is associated with increased anxiety (valence-specific models) (for a review see Heeren et al., 2013; Hadwin et al., 2016). Waters and Kershaw (2015) found that clinically anxious children who showed attention to threat in a visual probe task exhibited greater fear extinction learning than those who avoided threat.
The studies by Barry and colleagues (Barry et al., 2016a, 2017) represent a second research tradition that has explored how deficient AC is associated with anxiety vulnerability, and more specifically with cognitive and inhibitory control impairments observed in anxious individuals. In two separate studies in healthy participants, these authors investigated how emotional AC, as measured by self-report (Barry et al., 2013), was associated with fear extinction learning (Barry et al., 2016a, 2017). In the first study, participants were confronted with a perceptually similar stimulus presented after extinction of the original CS, and it was observed that higher emotional AC was associated with faster extinction learning and greater return of fear (Barry et al., 2016a). In the second study, during extinction learning participants were confronted with a similar stimulus as during acquisition, and were instructed to attend toward the common features between the acquisition and extinction stimuli, or toward the unique features of the extinction stimulus. For participants who, during extinction, were instructed to attend toward the unique features of the extinction stimulus, lower emotional AC tended to be associated with a greater return of fear (Barry et al., 2017). The authors suggested that those with low emotional AC may have been unable to shift attention to other features of the extinction stimulus, which may have facilitated the return of fear when confronted with a perceptually similar stimulus.
In the studies mentioned above, individual differences in attention functioning were investigated using emotional conditions. We share the view of Heeren et al. (2015b, p. 136) that the focus on emotional materials has “neglected the empirical exploration of basic attentional deficits from non-emotional material,” and precludes the assessment of general attentional abilities that may be relevant to several clinical phenomena (see also Snyder et al., 2015). Furthermore, in the two studies investigating the association between AC and extinction learning, AC was assessed by self-report (Barry et al., 2016a, 2017). The use of performance-based tasks (see below) may provide important information on the role of different attentional networks beyond general AC (see Heeren et al., 2015a; Heeren and McNally, 2016).
According to the attention system model (Posner and Petersen, 1990; Posner and Rothbart, 2007), attention consists of three major networks, which can be assessed separately: executive control, orienting, and alerting (see Posner et al., 2007 for a review). The executive control network is specialized in conflict resolution and voluntary control of attention while resisting distraction by other competing stimuli. While the executive control network has traditionally been equated to AC, some authors have recently expanded the definition of AC to include the orienting and alerting networks (e.g., Heeren and McNally, 2016). The orienting network is involved in attention engagement to new stimuli and attention disengagement from the current focus. Finally, the alerting network is devoted to maintaining adequate sensitivity to perceive and process stimuli. The functioning of these three attentional networks when facing non-emotional cues has been related to anxiety and emotion regulation. For example, reduced efficiency of the executive control and orienting networks has been associated with high trait and clinical anxiety (Moriya and Tanno, 2009; Pacheco-Unguetti et al., 2010; Pacheco-Unguetti et al., 2011; Heeren et al., 2015b; Heeren and McNally, 2016), and faster spontaneous emotion regulation (Morillas-Romero et al., 2015). Finally, increased efficiency of the alerting network has been associated with state anxiety (Pacheco-Unguetti et al., 2010).
In this present study, we explore whether individual differences in non-emotional AC (defined as a multifaceted construct including executive control, orienting, and alerting attentional networks), are associated with individual differences in fear extinction (learning and recall) in a sample of subclinical phobic participants (individuals with moderate to strong fear of spiders). The use of subclinical samples is a valid strategy for studying anxiety-related processes, can be generalized to individuals with an anxiety diagnosis (Stopa and Clark, 2001; Abramowitz et al., 2014) and also has some advantages (e.g., avoid comorbidity, medications or the impact from previous treatments) compared to clinical samples. Furthermore, previous studies exploring the association between attentional bias to threat and fear extinction (Waters and Kershaw, 2015) and between fear learning and treatment outcome (Waters and Pine, 2016) included children with specific phobias, but to the best of our knowledge, ours is the first study exploring the role of non-emotional attention and its association with fear extinction in (subclinical) adult phobic individuals using “truly” non-emotional stimuli.
We used self-report and performance-based measures of AC under non-emotional conditions. Fear extinction was assessed using three different measures: Unconditioned Stimulus (US) expectancies, Skin Conductance Response (SCR), and Fear-Potentiated Startle (FPS). Given the well-established association between trait anxiety and AC (e.g., Pacheco-Unguetti et al., 2010, 2011; Sportel et al., 2011), and between trait anxiety and fear extinction (Sehlmeyer et al., 2011; Gazendam et al., 2013; Haaker et al., 2015), we tested the magnitude of these associations after controlling for trait anxiety.
Materials and Methods
Participants
We selected individuals with moderate to strong fear of spiders, as assessed by a dimensional instrument. Participants were recruited by advertisement to participate in a study on “physiological responses to anxiety” (see participants flow chart in Figure 1). Initially, 1504 individuals were screened with the validated Spanish version (Forcadell et al., 2014) of the Fear of Spiders Questionnaire (FSQ; Szymanski and O’Donohue, 1995) via a secure web system. In the online stage we used online forms with encryption technology that guaranteed the privacy of the participants. The information could only be processed by a person with access to the matrix and passwords. Participants who scored in the top quartile of the study distribution (FSQ ≥ 33; n = 386) were invited to participate. Of those, 92 agreed to be interviewed by a doctoral-level clinical psychologist using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998).
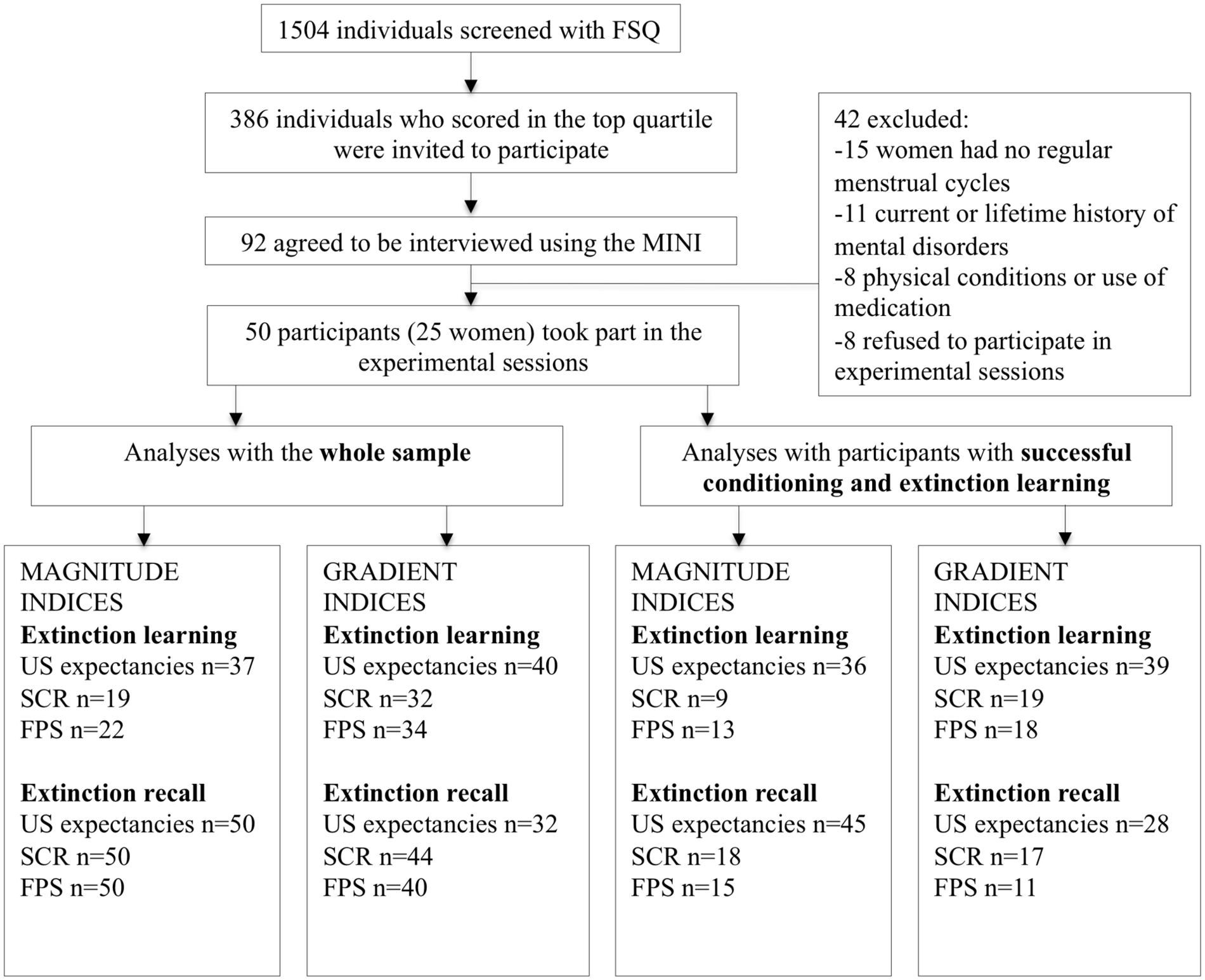
FIGURE 1. Diagram of the sample selection procedure and participants included in each analysis. FSQ, Fear of Spiders Questionnaire; MINI, Mini International Neuropsychiatric Interview; US, Unconditioned stimulus; SCR, Skin Conductance Response; FPS, Fear-Potentiated startle.
Exclusion criteria were: (a) current or lifetime history of mental disorders other than specific phobia (animal type, spiders), as determined by the MINI, supplemented with the specific phobia section of the Structured Clinical Interview for DSM (SCID; First et al., 2002); (b) use of medication/illicit drugs or medical problems that could interfere with study performance or interpretation; (c) alcohol abuse; (d) pregnancy; (e) not being Spanish-speaker. Female participants had regular menstrual cycles (as per self-report), had not used oral contraceptives or hormone replacement therapy during the previous 3 months (as per self-report), and participated in the study during their early follicular phase (days 3–8 of a 28–30-day cycle) to avoid possible confounding by sex hormones in fear extinction (Milad et al., 2006; Merz et al., 2012; Pineles et al., 2016). All participants were tested between 5 and 8 PM.
The final sample consisted of 50 participants with moderate to strong fear of spiders (MFSQ = 58.98, SD = 17.94; Mage = 21.50 years, SD = 2.93; 25 women). The number of participants included in each analysis is reported in Figure 1. Participants gave written informed consent to take part in the study, which was approved by the corresponding institution’s Clinical Research Ethics Committee. Participants were paid €25.
Procedure Overview
Participants took part in two experimental sessions on 2 consecutive days. On day 1, they completed (a) a self-report measure of AC (Attentional Control Scale; ACS; Derryberry and Reed, 2002), (b) the trait version of the State-Trait Anxiety Inventory (STAI-T; Spielberger et al., 1970), (c) a task assessing attentional network functioning (Attentional Network Test-Interactions task, ANT-I, Callejas et al., 2004) (see below and Figure 2A), and they underwent the first part of the fear learning paradigm (conditioning and extinction learning) (see below and Figure 2B). On day 2, they participated in the second part of the fear learning paradigm (extinction recall) (Figure 2C). Psychophysiological responses were recorded continually during the fear learning paradigm (see below).
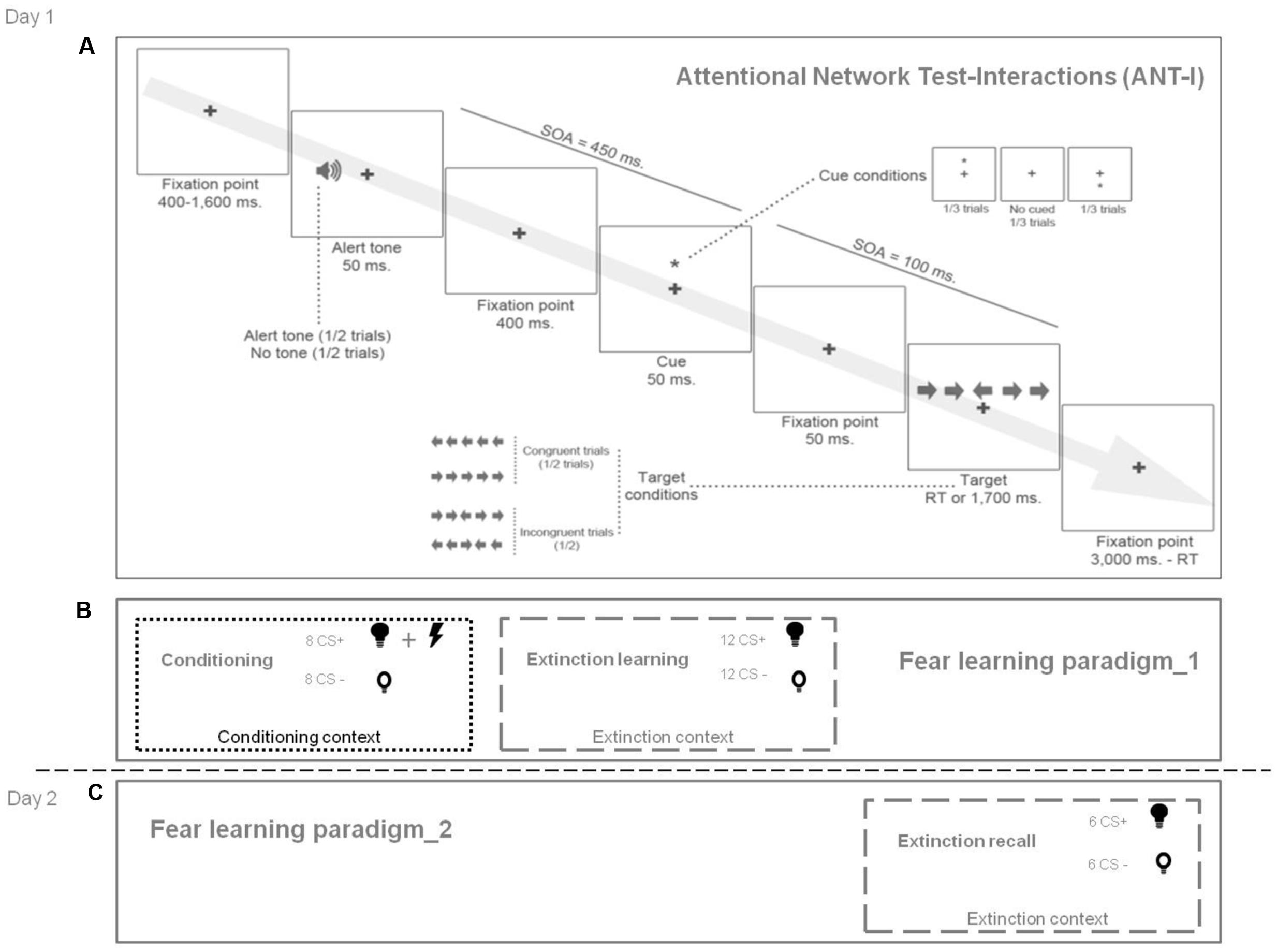
FIGURE 2. Summary of experimental design. (A) Attentional Network Test-Interactions (ANT-I): Trials began with a fixation point (400–1600 ms). Then, in half of the trials, an alerting tone is presented (50 ms). In two-thirds of the trials, this is followed after 400 ms by an asterisk as an orienting signal (50 ms) either above or below the fixation point (cued trials). No asterisk is presented in the remaining third of the trials (uncued condition). Then, the asterisk disappears leaving only the fixation point. After 50 ms, an arrow flanked by four distractor arrows (two on each side) is presented. The distractors point either in the same direction as the arrow target (i.e., congruent trials) or in the opposite direction (i.e., incongruent trials), and in the same position as the orienting cue (i.e., valid trials) or at the opposite location (i.e., invalid trials). Participants have to indicate the direction in which the target was pointing (right or left). After the response, the fixation point is presented for up to 3000 ms. (B) Fear learning paradigm, day 1 (conditioning and extinction learning). A conditioning phase was followed by an extinction learning phase. The visual contexts were photographs of two different rooms (i.e., conditioning context and extinction context). A lamp switched on to one of two different colors (blue or yellow), which were the CS+ and the CS–. Participants were not instructed about the CS-US contingency. During conditioning, the CS+ co-terminated with an electric shock (US). During extinction learning (immediately after conditioning), the CS+ was not followed by the US. The CS– was never followed by the US. (C) Fear learning paradigm, day 2 (extinction recall). This phase was identical to extinction learning on day 1. The extinguished CS+ and the CS- were presented again in the extinction context. SOA, stimulus onset asynchrony; RT, reaction time; CS+, conditioned stimulus associated with the unconditioned stimulus during the conditioning phase; CS–, conditioned stimulus never associated with the unconditioned stimulus; US, unconditioned stimulus.
Self-report Measures
Attentional Control
We used the Spanish version (Pacheco-Unguetti et al., 2011) of the Attentional Control Scale (ACS; Derryberry and Reed, 2002) to measure individual differences in non-emotional AC (e.g., “My concentration is good even if there is music in the room around me,” “It is easy for me to read or write while I am also talking on the phone”). The scale consists of 20 4-point items (1 = Almost never; 4 = Always), with higher scores indicating higher AC. The scale is divided into two subscales: focusing (i.e., ability to intentionally hold attentional focus) and shifting (i.e., ability to intentionally shift attentional focus). In line with Ólafsson et al. (2011), item 9 was excluded when calculating the total score. Cronbach’s alpha for the ACS was 0.70.
Trait Anxiety
We used the Spanish version (Spielberger et al., 1982) of the STAI-trait (STAI-T; Spielberger et al., 1970) to measure trait anxiety (e.g., “I worry too much over something that really does not matter,” “I am content”). It consists of 20 4-point items (1 = Almost never; 4 = Almost always). Scores range from 0 to 60 in the Spanish version of the STAI-T, with higher scores indicating higher trait anxiety. Cronbach’s alpha for the STAI-T was 0.87.
Performance-Based Measures
Attentional Network Functioning
We used the Attentional Network Test for Interactions (ANT-I; Callejas et al., 2004), a modified version of the Attentional Network Test (Fan et al., 2002), to assess the functioning of the three major attentional networks (executive control, orienting and alerting), and the two components of orienting, namely costs (i.e., disengagement of attention from invalid cues), and benefits (i.e., facilitated orientation from valid cues). The task consisted of four blocks of 48 trials. On each trial, a non-emotional cue (an alerting tone and/or an asterisk) preceded an arrow flanked by other distractor arrows (see Figure 2A). Participants had to indicate the direction of the target arrow by pressing one of two keys as quickly and accurately as possible (we measured reaction time and error rate; see below). Following previous research (Callejas et al., 2004; Pacheco-Unguetti et al., 2010, 2011) we computed an efficiency index for each attentional network, and for the two components of orienting (see Supplementary Methods), where higher scores indicated enhanced functioning of the network (except for executive control and orienting costs, where higher scores indicated diminished functioning).
Fear Learning Paradigm
We adapted the paradigm developed by Milad et al. (2005) which assesses conditioning, extinction learning, and extinction recall separately. The original task used Skin Conductance Response (SCR) as the only measure of conditioned fear, and we added two other measures (Unconditioned Stimulus [US] expectancies and Fear-Potentiated Startle [FPS]). Briefly, the visual contexts were photographs of two different rooms (conditioning context, CX+; extinction context, CX-) containing a lamp that switched on to one of two different colors (blue or yellow), which were the CS+ and the CS- (CS not paired with the US). Contexts and CSs were displayed on a computer monitor in front of the participant. On day 1, a conditioning phase (in CX+) was followed by an extinction learning phase (in CX-). During conditioning, the CS+ co-terminated with an electric shock (US). The US was individually adjusted before the experiment (day 1), presenting shocks of gradually increasing intensity until a ‘definitely annoying but not painful’ shock was selected [Mshocklevel = 4.9 milliamperes (mA), SD = 3.3]. Participants were not instructed about the CS-US contingency. During extinction learning (immediately after conditioning), the CS+ was not followed by the US. The CS– was never followed by the US. The extinction learning phase was divided in two equal parts by a 1-min pause (early and late extinction learning). Day 2 consisted of an extinction recall phase (in CX-). During day 2, the CS+ and the CS– were never followed by the US. The US was not recalibrated during day 2.
Each trial of the experiment started with presentation of the context for 10, 12, or 14 s. Then the CS was presented (i.e., the lamp switched on) for 8 s, and a startle probe (50 ms duration, 100 dB) was delivered 7 s after CS onset. Between trials, a fixation cross was shown for 1 s. In one third of the trials (noise-alone trials, NA), no CS was presented, and instead the context was present for 8 more seconds; the startle probe was presented at second 7 of this extra time. The inter-probe interval varied between 18, 20, and 22 s. Eight trials of each type (CS+, CS–, and NA) were presented during conditioning, and six trials of each type were presented during each of the remaining phases (early and late extinction learning and extinction recall). Trial order was randomized across participants in blocks of nine trials (three of each type), with the restriction that no more than two trials of the same type occurred consecutively. Assignment of the photographs of the rooms to the conditioning and the extinction contexts, and of the CS+ and the CS–, was counterbalanced across participants.
SCR, FPS, and US expectancy ratings were calculated for each trial type. The SCR signal was sampled at a rate of 125 Hz. SCR magnitudes were computed in microsiemens (μS) as the difference between the maximum SCR value and the value at response onset, occurring 1–7 s after CS onset. Trials in which no response was detected or with a response magnitude of <0.02 μS were considered non-response trials (see Dunsmoor et al., 2009), and trials showing interference or excessive baseline activity (1.3%) were rejected after visual inspection. To normalize the distribution of the SCR data, we applied a square root transformation.
Startle amplitudes were computed in microvolts (μV) as the difference between the EMG value at response peak and the average EMG during the 50 ms preceding the probe. If no response was detected in a given trial, the amplitude was scored as 0 μV. To be considered a valid response, elevations in the EMG recording had to start between 20 and 100 ms, and their peak had to occur between 20 and 150 ms after the probe. After visual inspection, trials with excessive noise (3.2%) were rejected. Raw data were transformed into T-scores to control for differences in reactivity. Scorers of SCR and FPS data were blinded to the stimuli presented.
Regarding US expectancy ratings, for each trial participants were told to try to predict whether the shock would occur in the following seconds each time the lamps in the rooms turned blue or yellow. They had to answer as quickly as possible by clicking on the scale from 0 (no shock) to 10 (shock) displayed at the bottom of the screen (see Supplementary Materials-Methods for further information).
Fear Extinction Indices
For each participant, we calculated an index based on the magnitude (amount of learning) and an index based on the gradient (slope of change, i.e., “speed”) for both extinction learning and extinction recall.
Magnitude-Based Indices
Based on previous research (Rabinak et al., 2013, 2014; Garfinkel et al., 2014; Pineles et al., 2016), for each participant and each measure (US expectancies, SCR and FPS) we calculated an index expressing the “amount of learning,” reflecting differences between CS+ and CS- during extinction learning and extinction recall.
The extinction learning index was calculated according to Pineles et al. (2016): Extinction learning = 100 – ([Mean [CS+]-[CS-] during the last two trials of early extinction/Mean [CS+]-[CS-] during the first two trials of early extinction × 100]). Since the extinction learning index was calculated as a percentage, higher scores indicated enhanced extinction learning (i.e., less discrimination between CS+ and CS-).
The extinction recall index was calculated, based on Rabinak et al. (2013), as the mean of (CS+)-(CS-) in the first two extinction recall trials. Lower scores indicated enhanced extinction recall.
Gradient-Based Indices
In line with Barry et al. (2016a, 2017), we computed the slope of change (i.e., gradient) across extinction learning and extinction recall trials using the area under the curve with respect to the decrease in US expectancies, SCR, and FPS scores. For each participant we calculated the percentage change in the difference between CS+ and CS- in each trial during extinction learning and recall relative to the first extinction trial. We used the percentage change between the first and last extinction trials as the baseline to account for individual differences in the intercept of the extinction curves. Lower scores in the gradient-based indices indicated faster fear extinction learning and extinction recall.
Statistical Analyses
In manipulation checks, we examined main effects and interactions between networks and reaction time in the ANT-I using a factorial mixed ANOVA with executive control (congruent and incongruent), orienting (valid, invalid, uncued), and alerting (no alerting, alerting tone) as within-subject factors, and reaction time as the dependent variable. We also studied the association between trait anxiety and AC variables using Pearson bivariate correlations. We also studied the performance of the sample during the fear learning paradigm using repeated-measures ANOVA for each phase (conditioning, extinction learning and extinction recall) and for each measure (US expectancies, SCR and FPS), with CS type (CS+ vs. CS-) and Block as within-subject factors. We averaged SCR and FPS responses over two consecutive trials of the same type (blocks), and applied Greenhouse–Geisser corrections for main effects and interactions involving repeated measures. For indices calculated as a percentage, extreme values (>150% or <-150%) were excluded.
We used Pearson bivariate correlation analyses to test for association between AC with fear extinction indices. Following recent guidelines for the analyses of fear learning data in humans (Lonsdorf et al., 2017), we performed those analyses with the whole sample. Since extinction learning can theoretically only occur if there is conditioning, and extinction recall can occur only if there is extinction learning, we repeated our extinction learning analyses using only those participants showing successful conditioning, and our extinction recall analyses using only those participants showing successful extinction learning. The criteria for establishing successful fear conditioning/extinction learning were based on Schiller et al. (2012): the differential SCR to the CS+ and CS- by the end of the conditioning phase (mean of second half of the conditioning trials) had to be in the right direction (i.e., CS+ > CS-) and >0.1 μs for SCR, >1 μV for FPS, or >1 point for US expectancies. Similarly, the criteria for establishing successful extinction learning were: differential SCR to the CS+ and CS- by the end of the late extinction learning phase (last trial) had to be ≤0.1 μs for SCR, ≤1 μV for FPS, or ≤1 for US expectancies.
For associations that were significant at p < 0.10, we conducted hierarchical regression analyses using AC as the independent variables and fear extinction indices as the dependent variables. For these analyses, trait anxiety was entered in Step 1, and the AC variables were entered independently in Step 2.
Finally, we conducted additional analyses using an alternative method to calculate indices based on fear extinction gradients (see Barry et al., 2016a,b).
Results
Manipulation Checks
Our manipulation checks on the ANT-I confirmed (see Supplementary Table S1 in Supplementary Results) the typically observed pattern for this task (i.e., reaction times were significantly shorter in: (i) trials including an alerting tone than in trials without this tone, (ii) trials including an orienting signal, and (iii) trials where distractors pointed in the same direction as the arrow target) (e.g., Callejas et al., 2004; Pacheco-Unguetti et al., 2010, 2011). Also consistent with previous literature, trait anxiety was significantly and negatively correlated with AC (see Supplementary Results). Regarding the associations between performance-based and self-reported AC, the overall AC scale was only significantly correlated with performance-based executive control (r = -0.438; p = 0.001), with a higher self-reported AC indicating a lower interference (i.e., greater executive control). The focusing AC subscale was positively associated with interference (i.e., lower executive control) (r = 0.323; p = 0.022). No significant correlations were found between self-reported AC and the orienting and alerting networks functioning. All the correlations are depicted in Supplementary Materials, Supplementary Table S2.
For all measures (US expectancies, SCR, FPS), we found evidence of successful conditioning (i.e., higher response to the CS+ than the CS- in the last block of conditioning), which allowed us to investigate fear extinction learning. We also found evidence of extinction learning (i.e., similar response to the CS+ and CS- in the last block of extinction) for all measures (US expectancies, SCR, FPS), which allowed us to investigate extinction recall. For further details, see Supplementary Results.
Correlational Analyses
Relationship between AC and Fear Extinction Magnitude-Based Indices
None of the AC variables investigated was significantly correlated with fear extinction learning or recall, as measured by the magnitude-based indices (Table 1).
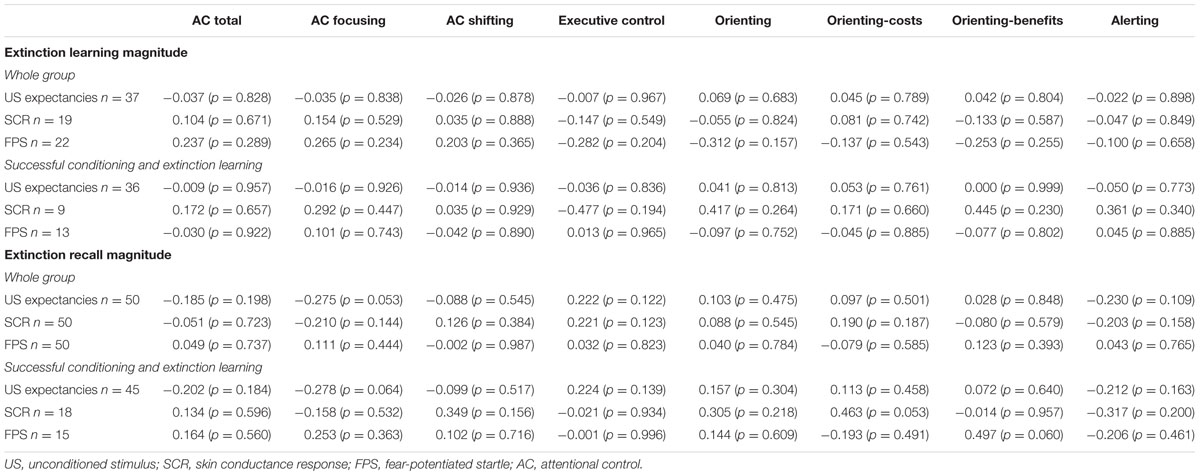
TABLE 1. Bivariate correlation between attentional control and fear extinction magnitude-based indices (results for the whole group and for participants with successful fear conditioning and extinction learning).
Relationship between AC and Fear Extinction Gradient-Based Indices
The two orienting network components (costs and benefits) showed a significant negative association with fear extinction (see Table 2). For the whole sample, orienting benefits (i.e., facilitated orientation) were inversely correlated with our gradient-based index of extinction recall, as measured with US expectancies (r = -0.358; p = 0.044), indicating that enhanced facilitated orientation was associated with faster extinction recall (see Table 2). When only those participants with successful conditioning and extinction learning were included in the analyses, orienting costs were inversely correlated with our gradient-based index of extinction learning using FPS (r = -0.493; p = 0.038), indicating that greater difficulty in disengaging attention from invalid cues was associated with faster extinction learning. Moreover, orienting benefits were inversely correlated again with our gradient-based index of extinction recall, as measured by US expectancies (r = -0.424; p = 0.025). We found no other significant associations between AC and fear extinction learning or recall.
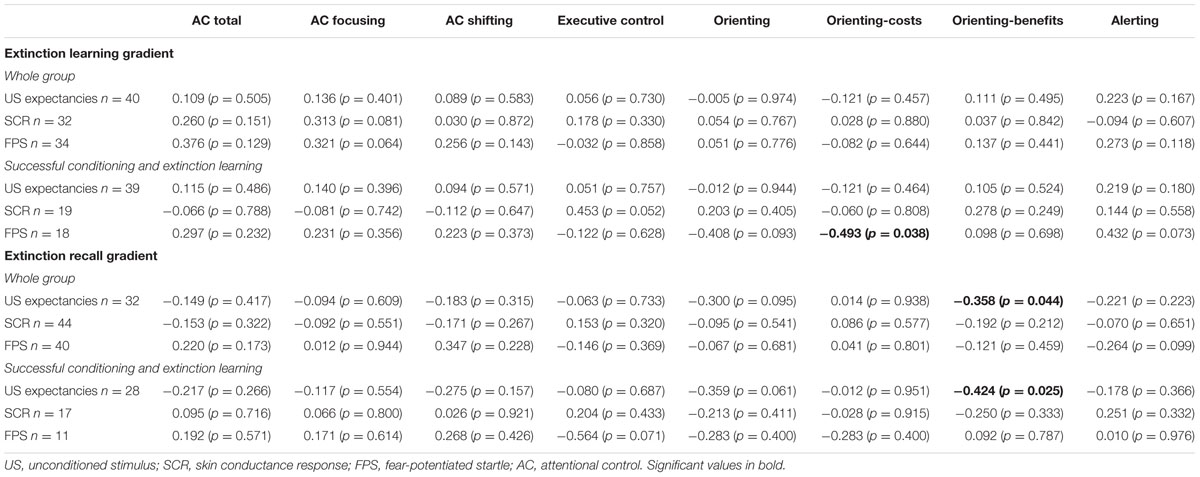
TABLE 2. Bivariate correlation between attentional control and fear extinction gradient-based indices (results for the whole group and for participants with successful fear conditioning and extinction learning).
Scatter plots for the main findings can be found in the Supplementary Materials (Supplementary Figure S1).
Predictive Power of AC on Magnitude and Gradient of Fear Extinction
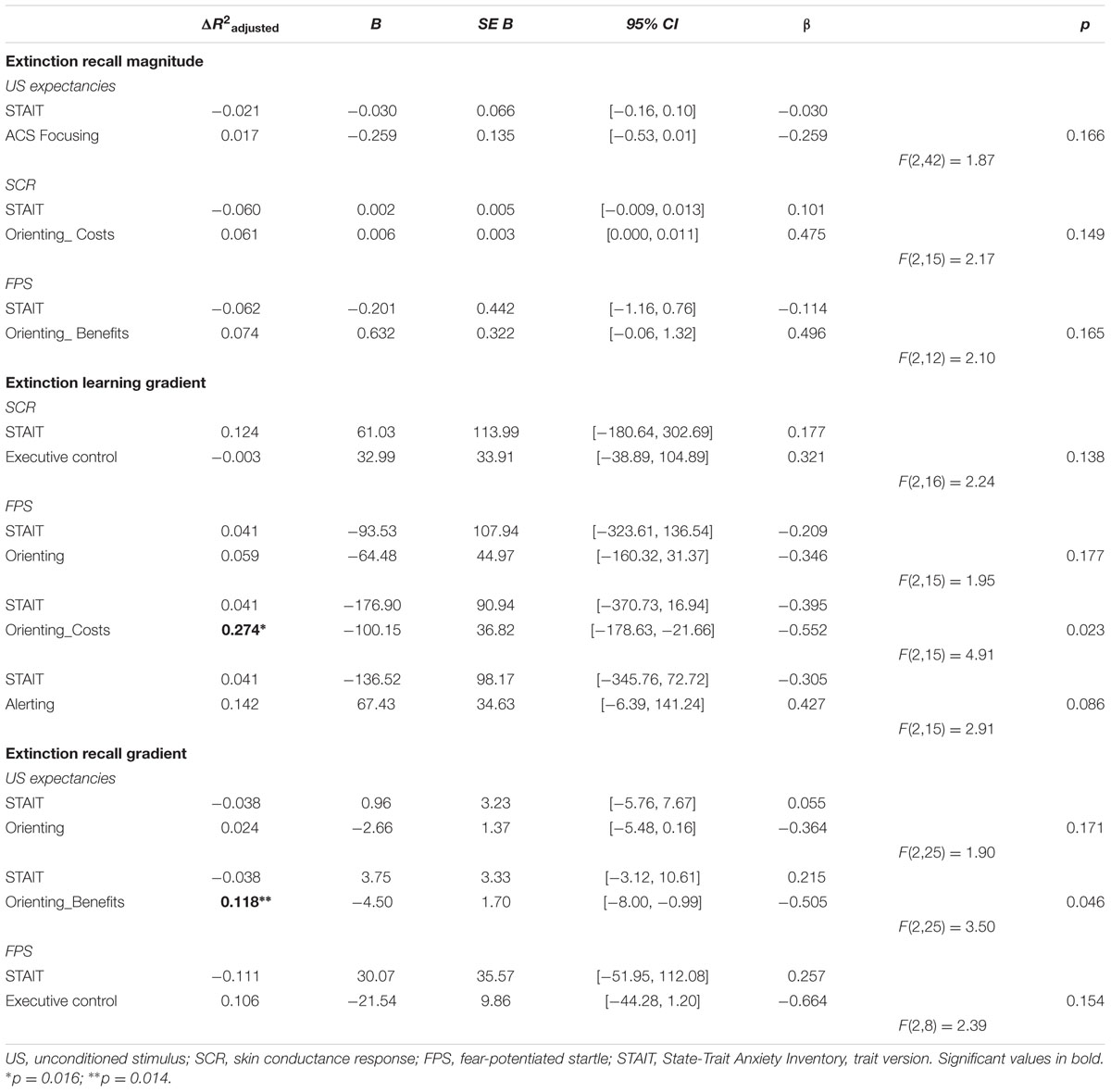
Results from the hierarchical regression analyses (Table 3) showed that, after controlling for trait anxiety, orienting costs were a significant predictor of extinction learning gradient (as measured by FPS), accounting for 27.4% of its variance (F[2,15] = 4.91, p = 0.023, R2Adjusted = 0.27). In other words, greater difficulty in disengaging attention from invalid neutral cues predicted faster extinction learning beyond what is attributable to trait anxiety.
After controlling for trait anxiety, orienting benefits were also a significant predictor of extinction recall gradient (as measured by US expectancies), accounting for 12% of its variance (F[2,25] = 3.50, p = 0.046, R2Adjusted = 0.12). Thus, facilitated orientation by valid neutral cues predicted faster extinction recall beyond what is attributable to trait anxiety.
Additional Analyses
When we calculated the gradient of fear extinction considering only the response to the CS+ (instead of the difference between CS+ and CS-) (Barry et al., 2017), we found that enhanced alerting was associated with faster extinction recall (as measured by FPS) (r = -0.695; p = 0.008) (see Supplementary Table S3).
Discussion
In this paper, we found that individual differences in self-reported non-emotional AC are not significantly associated with fear extinction learning and recall. However, we did find that the two components of orienting network functioning are significantly negatively associated with fear extinction learning and recall beyond that accounted for by trait anxiety. Specifically, participants with enhanced orienting costs (i.e., difficulties in disengaging attention from cues not relevant for the task) showed faster extinction learning, while those with enhanced orienting benefits (i.e., facilitated orientation by valid cues) exhibited faster extinction recall as measured by FPS and US expectancies, respectively.
The lack of a significant association between self-reported non-emotional AC and fear extinction is at odds with the findings of Barry et al. (2016a), who reported that higher self-reported emotional AC was associated with faster fear extinction learning. In our calculation of the extinction gradients, we considered the difference between CS+ and CS- in each trial; however, when we used the same criteria as Barry and colleagues (Barry et al., 2016a,b) (i.e., considering only the response to the CS+) the results did not change (see Supplementary Table S3). The main difference between Barry’s studies and ours is that we measured AC in non-emotional conditions whereas Barry et al. (2016a, 2017) measured emotional AC. This underlines the importance of the distinction between emotional and non-emotional AC.
Our results on orienting network functioning are not easily comparable to previous research because, to our knowledge, this is first study to analyze how individual differences in the functioning of attentional networks under non-emotional conditions are associated with individual differences in fear extinction learning and recall. Our results on orienting costs resemble those reported of Waters and Kershaw (2015) using threat-related stimuli. While Waters and Kershaw (2015) found that higher allocation to threat was a predictor of higher extinction learning, we found that lower capacity for disengaging attention from irrelevant cues was a predictor of faster extinction learning. It may be that both processes tap into a similar construct that becomes apparent using both threat-related and non-emotional conditions. Consistent with this, Heeren et al. (2015b) stated that specific impairment of the orienting network, such as difficulty in disengaging attention from task-irrelevant distractors, may be a mechanism underlying attentional bias. Then, during fear extinction learning we could assume that people with greater orienting costs with non-emotional stimuli also display higher attentional allocation to the CS+ that speeds up the extinction learning process.
However, this interpretation conflicts with data showing that higher self-reported emotional AC is positively associated with faster extinction learning, as shown by Barry et al. (2016a). Nevertheless, in the study by Barry et al. (2017), a different stimulus was presented during extinction learning and during conditioning. The authors interpreted that participants with high AC were better able to shift attention from the common, threatening features of the original CS+ to the distinct features of a different but similar CS+, therefore “speeding up” the extinction learning process.
The fact that orienting costs and orienting benefits showed a different association with extinction learning and recall also suggests that distinct attentional capabilities may have different relationships with various fear learning processes. This is also consistent with previous studies showing that extinction learning and extinction recall are independent processes (Phelps et al., 2004; Milad et al., 2007; Quirk and Mueller, 2008).
In a previous study, Morillas-Romero et al. (2015) reported that, under non-emotional conditions, orienting network functioning was not related to spontaneous emotion regulation, but with explicit emotion regulation styles. To our knowledge, ours is the first report showing that individual differences in orienting network functioning under non-emotional conditions are related to a form of spontaneous emotion regulation (i.e., fear extinction). Our results underline the potential prominence of the orienting component of attention in anxiety and related processes, as shown recently by Heeren et al. (2015b), Heeren and McNally (2016) in adults with social anxiety disorder.
We also explored the contribution of the alerting network to fear extinction, and found that enhanced alerting was associated with faster fear extinction recall. Previous studies (Tortella-Feliu et al., 2014) have shown that the alerting network is related to self-reported emotion regulation strategies (i.e., enhanced alerting predicts a higher probability of suppressing distressing cognitions). Therefore, there seems to be a positive relationship between alertness and emotional regulation, and future research will need to investigate this relationship further.
Our results could have several methodological and clinical implications. From a methodological perspective, our data indicate that fear extinction gradients can provide a better understanding of individual differences in fear learning (and, more generally, a broader view of fear learning processes) than those offered by “static” fear indices, as previously observed by Barry et al. (2016a, 2017). They support that the empirical exploration of individual differences in non-emotional AC can be relevant for several clinical phenomena, as recently emphasized by Heeren et al. (2015b), and shown here for extinction learning and recall. Furthermore, our results highlight the utility of performance-based measures of attentional network functioning beyond general and self-reported AC.
Taken together with previous studies on the association between both fear extinction (e.g., Waters and Pine, 2016; Ball et al., 2017) and attentional functioning (e.g., Barry et al., 2015a) and exposure-based interventions, our results suggest that differences in the orienting network functioning may explain differences in exposure therapy outcomes, a question that deserves to be explored in future studies.
Our result may also have implications for other forms of psychological interventions, especially attention training. Attention training is a generic term that refers to repetitive “practice in conflict-related tasks, working memory tasks or others tasks involving executive control mechanisms” (Tang and Posner, 2009, p. 222) that requires directed effortful attention control. Importantly, most of these interventions train attention using non-emotional materials, as is the case of the attention training technique for anxiety disorders (Fergus and Bardeen, 2016; Knowles et al., 2016). However, in the field of anxiety disorders the most common attention intervention is attention bias modification (ABM) training (Bar-Haim, 2010). Positive results on the use of ABM, mainly as an add-on to cognitive behavior therapy, have been reported in several anxiety disorders (Linetzky et al., 2015). However, results on the efficacy of these interventions are inconsistent (Mogoaşe et al., 2014; Kuckertz and Amir, 2015), and some authors have called for ABM procedures to be improved (Mogg and Bradley, 2016). Notably, although these procedures are intended to train participants to disengage attention from threatening information, several studies have found that any active attentional training procedure will reduce anxiety symptoms, including procedures that use only neutral stimuli (Klumpp and Amir, 2010; Heeren et al., 2015c). Heeren et al. (2015c) also reported that several attention training procedures improve the executive control and alerting components of AC, but not the orienting one, in socially anxious patients. Therefore we propose that attentional training with neutral stimuli should be further explored as a way to improve AC, especially by directly manipulating the orienting network, as proposed by Heeren et al. (2015b). This in turn could be related to increased effectiveness of exposure.
Our study has several strengths. It is the first to explore the association between non-emotional AC and fear learning. Moreover, we measured fear learning using three different fear measures, controlled for some variables that are known to affect fear learning (menstrual cycle, time of the day), and used both magnitude and gradient-based measures. Also, our AC variables included self-reported and performance-based measures. We included analyses and results for the whole sample and only for those participants with successful conditioning and extinction learning. Finally, while previous studies focused on extinction learning, we also studied extinction recall.
We also note some limitations. Participants in our study were individuals with moderate to strong fear of spiders, which also involves disgust, not only fear (Cisler et al., 2009), and this may affect some of the processes investigated. Second, in the paradigm employed here conditioning and extinction learning occurred consecutively with no time for longer consolidation of fear conditioning. Third, some of our analyses including only participants with successful conditioning and extinction learning (those involving SCR and FPS measures and magnitude-based indices) were based on relatively small samples (less than a half of the whole sample for some analyses). However, the main findings from these analyses are fully consistent with those obtained using the whole sample (n = 50), which is a relatively large one for fear learning psychophysiological experiments using a 2-day procedure, compared to many previous studies (e.g., Milad et al., 2005; Rabinak et al., 2013, 2014; Garfinkel et al., 2014; Pineles et al., 2016). We computed post-hoc power (two-tailed) for our significant bivariate correlations using sample size, the effect size, and the alpha error (0.05). We had 53–63% (moderate) power to detect a significant correlation between the orienting network functioning and extinction learning and recall. This indicates that Type II error probability was still relatively high in our study, and therefore a replication with larger samples is warranted to ensure the generalizability of our findings. Finally, most significant findings could not be replicated across different fear measures, although this is consistent with many previous fear-learning studies (e.g., Sevenster et al., 2014).
Despite these limitations, we consider that these results contribute to a better understanding of how non-emotional AC is related to fear extinction learning and recall. The most important theoretical contribution is that attentional biases to threat in anxiety could reflect a much broader dysregulation of AC observed in face of non-emotional material.
In summary, in this exploratory study we showed that orienting network functioning is related to fear extinction learning and recall over and above trait anxiety. To the best of our knowledge, this is the first study that links non-emotional AC to fear extinction. An important avenue for future research is to explore the association between AC and anxiety treatments (i.e., exposure therapy).
Ethics Statement
This study was carried out in accordance with the recommendations of Clinical Research Ethics Committee of Institut Hospital del Mar d’Investigacions Mèdiques, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Clinical Research Ethics Committee.
Author Contributions
EF, DT-R, MF, and MT-F contributed to the development of the study hypothesis, and to the contribution of the study and experiment designs. EF, DT-R, and DT prepared the stimuli, and prepared data and software for analysis. EF, DT-R, and MT-F performed the data analysis. All authors drafted the manuscript, discussed the results, implications, and literature, and approved the final version of the manuscript for submission.
Funding
This work was supported by ANPIR under Grant to EF (BA-3/2016); PROMOSAM Investigación en procesos, mecanismos y tratamientos psicológicos para la promoción de la salud mental under Grant to MT-F (PSI2014-56303- RED); and Carlos III Health Institute/FEDER under Grant to MF (PI16/00144).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpsyg.2017.01654/full#supplementary-material
References
Abramowitz, J. S., Fabricant, L. E., Taylor, S., Deacon, B. J., McKay, D., and Storch, E. A. (2014). The relevance of analogue studies for understanding obsessions and compulsions. Clin. Psychol. Rev. 34, 206–217. doi: 10.1016/j.cpr.2014.01.004
Armstrong, T., Zald, D. H., and Olatunji, B. O. (2011). Attentional control in OCD and GAD: specificity and associations with core cognitive symptoms. Behav. Res. Ther. 49, 756–762. doi: 10.1016/j.brat.2011.08.003
Ball, T. M., Knapp, S. E., Paulus, M. P., and Stein, M. B. (2017). Brain activation during fear extinction predicts exposure success. Depress. Anxiety 34, 257–266. doi: 10.1002/da.22583
Bar-Haim, Y. (2010). Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. J. Child Psychol. Psychiatry 51, 859–870. doi: 10.1111/j.1469-7610.2010.02251.x
Bar-Haim, Y., Lamy, D., Pergamin, L., Bakermans-Kranenburg, M. J., and van IJzendoorn, M. H. (2007). Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 133, 1–24. doi: 10.1037/0033-2909.133.1.1
Barry, T. J., Griffith, J. W., Vervliet, B., and Hermans, D. (2016a). The role of stimulus specificity and attention in the generalization of extinction. J. Exp. Psychopathol. 7, 143–152. doi: 10.5127/jep.048615
Barry, T. J., Hermans, D., Lenaert, B., Debeer, E., and Griffith, J. W. (2013). The eACS: attentional control in the presence of emotion. Pers. Individ. Dif. 55, 777–782. doi: 10.1016/j.paid.2013.06.014
Barry, T. J., Sewart, A. R., Arch, J. J., and Craske, M. G. (2015a). Deficits in disengaging attention from threat predict improved response to cognitive behavioral therapy for anxiety. Depress. Anxiety 32, 892–899. doi: 10.1002/da.22421
Barry, T. J., Vervliet, B., and Hermans, D. (2015b). An integrative review of attention biases and their contribution to treatment for anxiety disorders. Front. Psychol. 6:968. doi: 10.3389/fpsyg.2015.00968
Barry, T. J., Vervliet, B., and Hermans, D. (2016b). Threat-related gaze fixation and its relationship with the speed and generalisability of extinction learning. Austr. J. Psychol. 68, 200–208. doi: 10.1111/ajpy.12124
Barry, T. J., Vervliet, B., and Hermans, D. (2017). Feature specific attention and the return of fear after extinction. J. Exp. Psychopathol. 8, 76–87. doi: 10.5127/jep.051115
Beaver, J. D., Mogg, K., and Bradley, B. P. (2005). Emotional conditioning to masked stimuli and modulation of visuospatial attention. Emotion 5, 67–79. doi: 10.1037/1528-3542.5.1.67
Bishop, S. J. (2007). Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn. Sci. 11, 307–316. doi: 10.1016/j.tics.2007.05.008
Bishop, S. J. (2008). Neural mechanisms underlying selective attention to threat. Ann. N. Y. Acad. Sci. 129, 141–152. doi: 10.1196/annals.1417.016
Bishop, S. J. (2009). Trait anxiety and impoverished prefrontal control of attention. Nat. Neurosci. 12, 92–98. doi: 10.1038/nn.2242
Callejas, A., Lupiáñez, J., and Tudela, P. (2004). The three attentional networks: on their independence and interactions. Brain Cogn. 54, 225–227. doi: 10.1016/j.bandc.2004.02.012
Cisler, J. M., and Koster, E. H. W. (2010). Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clin. Psychol. Rev. 30, 203–216. doi: 10.1016/j.cpr.2009.11.003
Cisler, J. M., Olatunji, B. O., and Lohr, J. M. (2009). Disgust, fear, and the anxiety disorders: a critical review. Clin. Psychol. Rev. 29, 34–46. doi: 10.1016/j.cpr.2008.09.007
Craske, M. G., Liao, B., Brown, L., and Verliet, B. (2012). Role of inhibition in exposure therapy. J. Exp. Psychopathol. 3, 322–345. doi: 10.5127/jep.026511
Craske, M. G., Treanor, M., Conway, C. C., Zbozinek, T., and Vervliet, B. (2014). Maximizing exposure therapy: An inhibitory learning approach. Behav. Res. Ther. 58, 10–23. doi: 10.1016/j.brat.2014.04.006
Derryberry, D., and Reed, M. A. (2002). Anxiety-related attentional biases and their regulation by attentional control. J. Abnorm. Psychol. 111, 225–236. doi: 10.1037/0021-843X.111.2.225
Duits, P., Cath, D. C., Lissek, S., Hox, J. J., Hamm, A. O., Engelhard, I. M., et al. (2015). Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety 32, 239–253. doi: 10.1002/da.22353
Dunsmoor, J. E., Mitroff, S. R., and LaBar, K. S. (2009). Generalization of conditioned fear along a dimension of increasing fear intensity. Learning & Memory, 16(7), 460–9. doi: 10.1101/lm.1431609
Eysenck, M. W., and Derakshan, N. (2011). New perspectives in attentional control theory. Pers. Individ. Dif. 50, 955–960. doi: 10.1016/j.paid.2010.08.019
Fajkowska, M., and Derryberry, D. (2010). Psychometric properties of attentional control scale: the preliminary study on a polish sample. Polish Psychol. Bull. 41, 1–7.
Fan, J., McCandliss, B. D., Sommer, T., Raz, A., and Posner, M. I. (2002). Testing the efficiency and independence of attentional networks. J. Cogn. Neurosci. 14, 340–347. doi: 10.1162/089892902317361886
Fergus, T. A., and Bardeen, J. R. (2016). The attention training technique: a review of a neurobehavioral therapy for emotional disorders. Cogn. Behav. Pract. 23, 502–516. doi: 10.1016/j.cbpra.2015.11.001
First, M. B., Spitzer, R. L., Gibbon, M., and Williams, J. B. W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York, NY: New York State Psychiatric Institute.
Forcadell, E., Torrents-Rodas, D., Martinez, E., Torrubia, R., and Fullana, M. A. (2014). “Reliability and validity of the Spanish version of the fear of spiders questionnaire,” in Poster Presented at the Meeting of VII Congreso Internacional y XII Nacional de Psicologia clínica, Seville.
Forcadell, E., Torrents-Rodas, D., Vervliet, B., Leiva, D., Tortella-Feliu, M., and Fullana, M. A. (2017). Does fear extinction in the laboratory predict outcomes of exposure therapy? A treatment analog study. Int. J. Psychophysiol. doi: 10.1016/j.ijpsycho.2017.09.001 [Epub ahead of print].
Garfinkel, S. N., Abelson, J. L., King, A. P., Sripada, R. K., Wang, X., Gaines, L. M., et al. (2014). Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J. Neurosci. 34, 13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014
Gazendam, F. J., Kamphuis, J. H., and Kindt, M. (2013). Deficient safety learning characterizes high trait anxious individuals. Biol. Psychol. 92, 342–352. doi: 10.1016/j.biopsycho.2012.11.006
Graham, B. M., and Milad, M. R. (2011). The study of fear extinction: Implications for anxiety disorders. Am. J. Psychiatry 168, 1255–1265. doi: 10.1176/appi.ajp.2011.11040557
Haaker, J., Lonsdorf, T. B., Schümann, D., Menz, M., Brassen, S., Bunzeck, N., et al. (2015). Deficient inhibitory processing in trait anxiety: evidence from context-dependent fear learning, extinction recall and renewal. Biol. Psychol. 111, 65–72. doi: 10.1016/j.biopsycho.2015.07.010
Hadwin, J. A., Visu-Petra, L., Muris, P., Derakshan, N., and Macleod, C. (2016). Introduction to the special issue: Understanding the role of attentional control in the development of anxiety in childhood, adolescence, and across lifespan. J. Exp. Psychopathol. 7, 277–295.
Hartley, C. A., and Phelps, E. A. (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35, 136–146. doi: 10.1038/npp.2009.121
Heeren, A., Billieux, J., Philippot, P., and Maurage, P. (2015a). Looking under the hood of executive function impairments in psychopathology: a commentary on “Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches”. Front. Psychol. 6:1170. doi: 10.3389/fpsyg.2015.01170
Heeren, A., Maurage, P., and Philippot, P. (2015b). Revistiong attentional processing of non-emotional cues in social anxiety: a specific impairment for the orienting network of attention. Psychiatry Res. 228, 136–142. doi: 10.1016/j.psychres.2015.04.030
Heeren, A., Mogoaşe, C., McNally, R. J., Schmitz, A., and Philippot, P. (2015c). Does attention bias modification improve attentional control? A double-blind randomized experiment with individuals with social anxiety disorder. J. Anxiety Disord. 29, 35–42. doi: 10.1016/j.janxdis.2014.10.007
Heeren, A., De Raedt, R., Koster, E. H. W., and Philippot, P. (2013). The (neuro)cognitive mechanisms behind attention bias modification in anxiety: proposals based on theoretical accounts of attentional bias. Front. Hum. Neurosci. 7:119. doi: 10.3389/fnhum.2013.00119
Heeren, A., and McNally, R. J. (2016). An integrative network approach to social anxiety disorders: The complex dynamic interplay among attentional bias for threat, attentional control, and symptoms. J. Anxiety Disord. 42, 95–104. doi: 10.1016/j.janxdis.2016.06.009
Hsu, K. J., Beard, C., Rifkin, L., Dillon, D. G., Pizzagalli, D. A., and Björgvinsson, T. (2015). Transdiagnostic mechanisms in depression and anxiety: the role of rumination and attentional control. J. Affect. Disord. 188, 22–27. doi: 10.1016/j.jad.2015.08.008
Klumpp, H., and Amir, N. (2010). Preliminary study of attention training to threat and neutral faces on anxious reactivity to a social stressor in social anxiety. Cogn. Ther. Res. 34, 263–271. doi: 10.1007/s10608-009-9251-0
Knowles, M. M., Foden, P., El-Deredy, W., and Wells, A. (2016). A systematic review of efficacy of the attention training technique in clinical and nonclinical samples. J. Clin. Psychol. 72, 999–1025. doi: 10.1002/jclp.22312
Koster, E., Crombez, G., Van Damme, S., Verschuere, B., and De Houwer, J. (2005). Signals for threat modulate attentional capture and holding: fear-conditioning and extinction during the exogenous cueing task. Cogn. Emot. 19, 771–780. doi: 10.1080/02699930441000418
Kuckertz, J. M., and Amir, N. (2015). Attention bias modification for anxiety and phobias: current status and future directions. Curr. Psychiatry Rep. 17:9. doi: 10.1007/s11920-014-0545-x
Le Pelley, M. E., Mitchell, C. J., Beesley, T., George, D. N., and Wills, A. J. (2016). Attention and associative learning in humans: an integrative review. Psychol. Bull. 142, 1111–1140. doi: 10.1037/bul0000064
Linetzky, M., Pergamin-Hight, L., Pine, D. S., and Bar-Haim, Y. (2015). Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depress. Anxiety 32, 383–391. doi: 10.1002/da.22344
Lonsdorf, T., Menz, M., Andreatta, M., Fullana, M., Golkar, A., Haaker, J., et al. (2017). Don’t fear ‘fear conditioning’: methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci. Biobehav. Rev. 77, 247–285. doi: 10.1016/j.neubiorev.2017.02.026
Mackintosh, N. J. (1975). A theory of attention: variations in the associability of stimuli with reinforcement. Psychol. Rev. 82, 276–298. doi: 10.1037/h0076778
Merz, C. J., Tabbert, K., Schweckendiek, J., Klucken, T., Vaitl, D., Stark, R., et al. (2012). Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm. Behav. 62, 531–538. doi: 10.1016/j.yhbeh.2012.09.001
Milad, M. R., Goldstein, J. M., Orr, S. P., Wedig, M. M., Klibanski, A., Pitman, R. K., et al. (2006). Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav. Neurosci. 120, 1196–1203. doi: 10.1037/0735-7044.120.5.1196
Milad, M. R., Orr, S. P., Pitman, R. K., and Rauch, S. L. (2005). Context modulation of memory for fear extinction in humans. Psychophysiology 42, 456–464. doi: 10.1111/j.1469-8986.2005.00302.x
Milad, M. R., and Quirk, G. J. (2012). Fear extinction as a model for translational neuroscience: ten years of progress. Annu. Rev. Psychol. 63, 129–151. doi: 10.1146/annurev.psych.121208.131631
Milad, M. R., Wright, C. I., Orr, S. P., Pitman, R. K., Quirk, G. J., and Rauch, S. L. (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry 62, 446–454. doi: 10.1016/j.biopsych.2006.10.011
Mogg, K., and Bradley, B. P. (2016). Anxiety and attention to threat: cognitive mechanisms and treatment with attention bias modification. Behav. Res. Ther. 87, 76–108. doi: 10.1016/j.brat.2016.08.001
Mogoaşe, C., David, D., and Koster, E. H. W. (2014). Clinical efficacy of attentional bias modification procedures: An updated meta-analysis. J. Clin. Psychol. 70, 1133–1157. doi: 10.1002/jclp.22081
Morillas-Romero, A., Tortella-Feliu, M., Balle, M., and Bornas, X. (2015). Spontaneous emotion regulation and attentional control. Emotion 15, 162–175. doi: 10.1037/emo0000016
Moriya, J., and Tanno, Y. (2009). Competition between endogenous and exogenous attention to nonemotional stimuli in social anxiety. Emotion 9, 739–743. doi: 10.1037/a0016817
O’Bryan, E. M., Kraemer, K. M., Johnson, A. L., McLeish, A. C., and McLaughlin, L. E. (2017). Examining the role of attentional control in terms of specific emotion regulation difficulties. Pers. Individ. Dif. 108, 158–163. doi: 10.1016/j.paid.2016.12.015
Ochsner, K. N., Silvers, J. A., and Buhle, J. T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 1251, E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x
Ólafsson, R. P., Smári, J., Guðmundsdóttir, F., Ólafsdóttir, G., Harðardóttir, H. L., and Einarsson, S. M. (2011). Self reported attentional control with the Attentional Control Scale: factor structure and relationship with symptoms of anxiety and depression. J. Anxiety Disord. 25, 777–782. doi: 10.1016/j.janxdis.2011.03.013
Olatunji, B. O., Ciesielski, B. G., Armstrong, T., Zhao, M., and Zald, D. H. (2011). Making something out of nothing: neutral content modulates attention in generalized anxiety disorder. Depress. Anxiety 28, 427–434. doi: 10.1002/da.20806
Pacheco-Unguetti, A. P., Acosta, A., Callejas, A., and Lupiáñez, J. (2010). Attention and anxiety: different attentional functioning under state and trait anxiety. Psychol. Sci. 21, 298–304. doi: 10.1177/0956797609359624
Pacheco-Unguetti, A. P., Acosta, A., Marqués, E., and Lupiáñez, J. (2011). Alterations of the attentional networks in patients with anxiety disorders. J. Anxiety Disord. 25, 888–895. doi: 10.1016/j.janxdis.2011.04.010
Phelps, E. A., Delgado, M. R., Nearing, K. I., and Ledoux, J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905. doi: 10.1016/j.neuron.2004.08.042
Pineles, S. L., Nillni, Y. I., King, M. W., Patton, S. C., Bauer, M. R., Mostoufi, S. M., et al. (2016). Extinction retention and the menstrual cycle: different associations for women with posttraumatic stress disorder. J. Abnorm. Psychol. 125, 349–355. doi: 10.1037/abn0000138
Pittig, A., Van Den Berg, L., and Vervliet, B. (2016). The key role of extinction learning in anxiety disorders: behavioral strategies to enhance exposure-based treatments. Curr. Opin. Psychiatry 29, 39–47. doi: 10.1097/YCO.0000000000000220
Posner, M. I., and Petersen, S. E. (1990). The attention system of the human brain. Annu. Rev. Neurosci. 13, 25–42. doi: 10.1146/annurev.ne.13.030190.000325
Posner, M. I., and Rothbart, M. K. (2007). Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 58, 1–23. doi: 10.1146/annurev.psych.58.110405.085516
Posner, M. I., Rueda, M. R., and Kanske, P. (2007). “Probing the mechanisms of attention,” in Handbook of Psychophysiology, eds J. T. Cacioppo, J. G. Tassinary, and G. G. Berntson (Cambridge: Cambridge University Press), 410–432.
Quirk, G. J., and Mueller, D. (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33, 56–72. doi: 10.1038/sj.npp.1301555
Rabinak, C. A., Angstadt, M., Lyons, M., Mori, S., Milad, M. R., Liberzon, I., et al. (2014). Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol. Learn. Mem. 113, 125–134. doi: 10.1016/j.nlm.2013.09.009
Rabinak, C. A., Angstadt, M., Sripada, C. S., Abelson, J. L., Liberzon, I., Milad, M. R., et al. (2013). Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology 64, 396–402. doi: 10.1016/j.neuropharm.2012.06.063
Richey, J. A., White, B. A., Valdespino, A., Ghane, M., and Schmidt, N. B. (2016). Attentional control mediates fearful responding to an ecologically valid stressor. Anxiety Stress Coping 29, 60–79. doi: 10.1080/10615806.2015.1015424
Robbins, S. (1990). Mechanisms underlying spontaneous recovery in autoshaping. J. Exp. Psychol. 16, 235–249. doi: 10.1037/0097-7403.16.3.235
Schiller, D., Raio, C. M., and Phelps, E. A. (2012). Extinction training during the reconsolidation window prevents recovery of fear. J. Vis. Exp. 66:e3893. doi: 10.3791/3893
Sehlmeyer, C., Dannlowski, U., Schöning, S., Kugel, H., Pyka, M., Pfleiderer, B., et al. (2011). Neural correlates of trait anxiety in fear extinction. Psychol. Med. 41, 789–798. doi: 10.1017/S0033291710001248
Sevenster, D., Beckers, T., and Kindt, M. (2014). Fear conditioning of SCR but not the startle reflex requires conscious discrimination of threat and safety. Front. Behav. Neurosci. 8:32. doi: 10.3389/fnbeh.2014.00032
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., et al. (1998). The mini-international neuropsychiatric interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33. doi: 10.1016/S0924-9338(99)80239-9
Shiba, Y., Santangelo, A. M., and Roberts, A. C. (2016). Beyond the medial regions of prefrontal cortex in the regulation of fear and anxiety. Front. Syst. Neurosci. 10:12. doi: 10.3389/fnsys.2016.00012
Snyder, H. R., Miyake, A., and Hankin, B. L. (2015). Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front. Psychol. 6:328. doi: 10.3389/fpsyg.2015.00328
Spielberger, C., Gorsuch, R., and Lushene, R. (1970). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press.
Spielberger, C., Gorsuch, R., and Lushene, R. (1982). STAI, Cuestionario de Ansiedad Estado/Rasgo [STAI: Manual for the State-Trait Anxiety Inventory]. Madrid: TEA Ediciones, S.A.
Sportel, B. E., Nauta, M. H., de Hullu, E., and de Jong, P. J. (2013). Predicting internalizing symptoms over a two year period by BIS, FFFS and attentional control. Pers. Individ. Dif. 54, 236–240. doi: 10.1016/j.paid.2012.08.043
Sportel, B. E., Nauta, M. H., de Hullu, E., de Jong, P. J., and Hartman, C. A. (2011). Behavioral inhibition and attentional control in adolescents: robust relationships with anxiety and depression. J. Child Fam. Stud. 20, 149–156. doi: 10.1007/s10826-010-9435-y
Stopa, L., and Clark, D. M. (2001). Social phobia: comments on the viability and validity of an analogue research strategy and British norms for the fear of negative evaluation questionnaire. Behav. Cogn. Psychother. 29, 423–430. doi: 10.1017/S1352465801004039
Szymanski, J., and O’Donohue, W. (1995). Fear of spiders questionnaire. J. Behav. Ther. Exp. Psychiatry 26, 31–34. doi: 10.1016/0005-7916(94)00072-T
Tang, Y.-Y., and Posner, M. I. (2009). Attention training and attention state training. Trends Cogn. Sci. 13, 222–227. doi: 10.1016/j.tics.2009.01.009
Tortella-Feliu, M., Morillas-Romero, A., Balle, M., Bornas, X., Llabrés, J., and Pacheco-Unguetti, A. P. (2014). Attentional control, attentional network functioning, and emotion regulation styles. Cogn. Emot. 28, 769–780. doi: 10.1080/02699931.2013.860889
Van Damme, S., Crombez, G., Hermans, D., Koster, E. H. W., and Eccleston, C. (2006). The role of extinction and reinstatement in attentional bias to threat: a conditioning approach. Behav. Res. Ther. 44, 1555–1563. doi: 10.1016/j.brat.2005.11.008
VanElzakker, M. B., Kathryn Dahlgren, M., Caroline Davis, F., Dubois, S., and Shin, L. M. (2014). From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 113, 3–18. doi: 10.1016/j.nlm.2013.11.014
Wagner, A. R. (1981). “SOP: a model of automatic memory processing in animal behavior,” in Information Processing in Animals: Memory, eds N. Spear and R. Miller (Hillsdale, NJ: Erlbaum), 5–47.
Waters, A. M., and Kershaw, R. (2015). Direction of attention bias to threat relates to differences in fear acquisition and extinction in anxious children. Behav. Res. Ther. 64, 56–65. doi: 10.1016/j.brat.2014.11.010
Keywords: attentional control, attentional network functioning, fear extinction, extinction learning, extinction recall, anxiety disorders
Citation: Forcadell E, Torrents-Rodas D, Treen D, Fullana MA and Tortella-Feliu M (2017) Attentional Control and Fear Extinction in Subclinical Fear: An Exploratory Study. Front. Psychol. 8:1654. doi: 10.3389/fpsyg.2017.01654
Received: 17 July 2017; Accepted: 08 September 2017;
Published: 26 September 2017.
Edited by:
Alexandre Heeren, Harvard University, United StatesReviewed by:
Bruno Kluwe Schiavon, University of Zürich - Psychiatrische Universitätsklinik Zürich, SwitzerlandKatie Moraes de Almondes, Federal University of Rio Grande do Norte, Brazil
Copyright © 2017 Forcadell, Torrents-Rodas, Treen, Fullana and Tortella-Feliu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miquel A. Fullana, bWlndWVsYW5nZWxmdWxsYW5hQGdtYWlsLmNvbQ==
 Eduard Forcadell
Eduard Forcadell David Torrents-Rodas
David Torrents-Rodas Devi Treen
Devi Treen Miquel A. Fullana
Miquel A. Fullana Miquel Tortella-Feliu
Miquel Tortella-Feliu