
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol., 18 May 2017
Sec. Psychopathology
Volume 8 - 2017 | https://doi.org/10.3389/fpsyg.2017.00797
Background: Although depression symptoms are often experienced by individuals who develop posttraumatic stress disorder (PTSD) following trauma exposure, little is know about the biological correlates associated with PTSD and depression co-morbidity vs. those associated with PTSD symptoms alone.
Methods: Here we examined salivary cortisol responses to trauma activation in a sample of 60 survivors of the World Trade Center attacks on September 11, 2001. Participants recalled the escape from the attacks 7 months post 9/11. Salivary cortisol levels were measured before and after their recollection of the trauma. PTSD, depression, and somatic symptoms were also assessed. From the behavioral assessment scales, the participants were grouped into three conditions: those with comorbid PTSD and depressive symptoms, PTSD alone symptoms, or no-pathology.
Results: Baseline and cortisol response levels differed between the comorbid, PTSD alone, and no-pathology groups. Individuals endorsing co-morbid symptoms had higher PTSD and somatic symptom severity and their cortisol response decreased following their trauma reminder while a trend of an elevated response to the trauma was found in the PTSD alone group. Our findings show distinct psychological and biological correlates related to the endorsement of PTSD with and without depression comorbidity.
Conclusions: The findings suggest that comorbidity symptoms manifestation entails a separate trauma induced condition from PTSD. Future research on biological correlates of comorbid PTSD and depression is warranted.
A significant number of individuals who encounter a traumatic event during the course of their lives experience temporary psychiatric symptoms, yet some go on and develop enduring and debilitating psychiatric conditions. Posttraumatic stress disorder (PTSD) is the most commonly associated disorder induced by trauma exposure (Breslau et al., 1997; Dekel et al., 2013b). Furthermore, many individuals endorsing PTSD report symptoms of depression as well (Kessler et al., 1995; O'Donnell et al., 2004), with comorbidity as high as 70% of the cases (Dekel et al., 2014). Considerable research has documented that the clinical expression of comorbid depression and PTSD differs from the presentation of PTSD alone (Sher, 2005). Comorbidity is associated with greater trauma exposure (Morina et al., 2013), PTSD symptom severity (Ikin et al., 2010), higher level of impairment (Mollica et al., 1999), sucidiality (Oquendo et al., 2003), and more hospitalizations (Sher, 2005). Depression may further mediate and intensify associated symptoms of PTSD such as somatization (Gupta, 2013) and contribute to an enduring sense of perceived threat (Lancaster et al., 2016). Although this clinical expression of the comorbidity condition suggests a more severe and enduring pathology, associated (unique) biological factors are relatively unknown. The present study explored potential differences in the biological stress response of individuals with PTSD and those endorsing PTSD as well as depressive symptoms.
The hypothalamic-pituitary adrenocortical (HPA) axis is an important part of the biological response to stress, promoting adaptation and accommodation to threatening and traumatic exposure (Gillespie et al., 2009). Stress exposure triggers a cascade of events in HPA activity with the end product being the release of the glucocorticoid hormone cortisol. Cortisol is generally elevated following trauma exposure (Kotozaki and Kawashima, 2012). In the short run cortisol facilitates the stress response and promotes the system's homeostasis and allostasis (Schulkin et al., 1994). However, prolonged activation of the HPA response can result in aggregated physiological and psychological wear and tear (McEwen, 2003). Cortisol abnormalities are associated with PTSD alone and depression alone (Morris et al., 2012b), but little is know about the cortisol pattern in comorbid PTSD and depression.
Neuroendocrine studies have consistently found cortisol variations in PTSD, which may in part account for the pathogenesis of the disorder. In general, cortisol (basal) abnormalities in PTSD have been largely manifested in hypoactivation of the system. Individuals with PTSD following a single traumatic event tend to have lower basal cortisol levels than healthy or trauma-exposed individuals without PTSD (Meewisse et al., 2007). The blunted cortisol response in PTSD is attributed to an enhanced HPA feedback function (Yehuda, 2002) leading to a progressive sensitization of the HPA-axis (Kendall-Tackett, 2000). Interestingly, depression is generally associated with hypercortisolemia (Holsboer, 2001), increased activity of the HPA axis and reduced inhibitory feedback (Kendall-Tackett, 2000). These findings raise the question whether individuals with PTSD + depression would differ from individuals with PTSD based on their HPA stress response.
Studies examined basal cortisol levels in individuals with co-morbid depression and PTSD reveal mixed findings. A few studies have documented that their (basal) cortisol levels are similar to those of healthy individuals, suggesting that the contrasting HPA patterns in depression and PTSD “counterbalance” one another when conditions co-occur (Halbreich et al., 1989). However, other recent studies have documented, lowered cortisol (basal) levels in the co-morbid group, as seen in pure PTSD, suggesting that comorbid depression does not effect the predominant PTSD cortisol pattern. For example, in a recent meta-analysis lower basal diurnal cortisol was evident in both PTSD as well as PTSD + depression groups relative to non-trauma exposed individuals (Morris et al., 2012a). Similarly, studies using pharmacological challenge tests [e.g., dexamethasone suppression test (DST)] for the most part conclude no effects of depression on HPA activity in PTSD (de Kloet et al., 2006). These results suggest that individuals with comorbid PTSD and depression resemble individuals with PTSD alone.
An important factor in understanding trauma-related disorders is the cortisol response to stressful stimulus and trauma-related reminders. Psychological distress and physiological reactivity upon exposure to trauma-related cues (DSM-5 B.4, B.5) are a salient character of PTSD. The failure of extinction of fear responsiveness to trauma-related cues is an underlying feature of the disorder. Responses to trauma stimulus provide a strong method for examining biological abnormalities in PTSD and co-morbid conditions. A consistent picture has emerged demonstrating greater peripheral nervous system reactivity including autonomic hyper-reactivity to trauma-related stimuli in individuals who develop PTSD (Dekel et al., 2016). Examining cortisol response in PTSD under symptom provocation may therefore offer a more promising assessment (Pitman et al., 2012) than measures of cortisol at rest.
In contrast to low basal levels, studies examining cortisol response in PTSD to stressful stimuli and trauma-reminders are few and for the most part point the direction of augmented cortisol responses (Liberzon et al., 1999; Bremner et al., 2003; Elzinga et al., 2003; Dekel et al., 2013a). Although these augmented cortisol responses are associated with PTSD, the contribution of comorbid depression symptoms remains unclear. Depression is associated with a sense of perceived threat (Lancaster et al., 2016) and may modify cortisol response to trauma reminder. Somatization symptoms are also frequently associated with depression and interact with the activity of the HPA-axis (Rief and Auer, 2000). To the best of our knowledge no study has compared the cortisol response to trauma-related reminders in comorbid depression + PTSD to that of PTSD alone. Thus, in the present study we examined cortisol response induced by trauma recollections in a sample of high-exposure 9/11 survivors. We initially examined differences in trauma exposure and symptom severity. We focused our analysis on the question whether individuals with comorbid PTSD and depressive symptoms differ from those who suffer from PTSD alone in their cortisol response.
The present analysis is part of a larger inquiry on psychological adaption to terrorism of Bonanno et al. (2005). Data at 7 months (Time 1: March, 2001, [T1]) and 18 months (Time 2: April, 2002, [T2]) post- 9/11 were obtained from individuals who had been in or within four blocks of the World Trade Center (WTC) towers at the time of the 9/11 attacks. Following approval from Columbia University's Institutional Review Board, we enlisted participants through their employers in the WTC, posted flyers in downtown Manhattan, and through public announcements. All participants signed written consent forms. The final prospective sample of individuals with data at the two assessment points included 60 participants (29 men and 31 women); the average age was 39.30 years (SD = 10.51), average annual income was $70,000, ethnically 77% were White (n = 48), and 44% resided in Manhattan (n = 28). There were no gender differences in demographics.
Threat exposure during the event was measured by six items pertaining to feelings of physical danger and being highly distressed (“perceived danger”), assessed on a 0 = no to 4 = very much; scale (α = 0.83), and four items pertaining to witnessing deaths and injuries (“objective exposure”), assessed on a 0 = none to 3 = three or more; scale (α between 0.70 and 0.80). Exploratory factor analysis supported 2-factor structures for the measure at T1.
PTSD symptoms at T1 and T2 were measured using the PTSD Symptom Scale-Self Report (PSS-SR, Foa et al., 1993), a 17-item scale reflecting frequency and severity of PTSD symptoms according to the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013) in the past month using a 0 (never/rarely) to 3 (almost always) scale. The PSS-SR has produced adequate internal consistency (.91) and concurrent validity (Foa et al., 1993). Internal consistency in the present study was 0.91. Previous research indicates that a PSS-SR total score of 14 serves as a reliable cutoff score for screening for PTSD (Coffey et al., 2006).
Depressive symptoms at T1 and T2 were measured using the Center for Epidemiologic Studies Depression Scale, Brief Version (CES-D, Kohout et al., 1993), consisting of nine-item scale and has evidenced reliability and validity statistics comparable to the full-scale version (Kohout et al., 1993). Respondents used a 1 (hardly ever) to 5 (almost always) scale to indicate how often they experienced depression symptoms in the past 2 weeks. Internal consistency for the nine-item scale in the current study was 0.79. A CES-D total score of 16 is the standard cutoff for low vs. high depression symptoms.
Somatic symptoms were assessed at T1 with an 18-item self-report checklist used in the Whitehall II study, a large scale study of morbidity among civil servants in London, England (Marmot et al., 1991; Stansfeld et al., 1993).
Trauma recollections were obtained at T1 in a 30-min open-ended standardized interview asking participants to freely recall their personal experiences escaping from the attacks prompted by the instruction: “I would like you to speak about what you went through on September 11; your experiences, thoughts and feelings on that day” (Dekel and Bonanno, 2013). Participants were instructed to report as openly as possible about virtually anything that came to mind.
Salivary cortisol measured at T1 was collected 2 min before and after the 30-min interview using Salivette collection devices (Sarstedt, Newton, NC) and stored at −20°C. Salivary cortisol was analyzed by a high-sensitivity cortisol enzyme immunoassay (Salimetrics, State College, PA) as described in Dozier et al. (2006). Intra-assay (2.20%) and interassay (5.70%) variability and pH of the assays were within the range of precision expected by Salimetrics. Cortisol concentration (μg/dl) was measured by optical density of duplicate samples using a Dynex spectrophotometer.
First, we categorized participants as having a high or low number of symptoms of depression and PTSD. Categorization of a high vs. low number of depression symptoms was based on the standard cutoff for the CES-D Scale of 16; categorization of a high vs. low number of PTSD symptoms was based on the standard cutoff for PSS-SR Scale of 14. Next, we categorized participants into one of three psychopathology groups: “comorbid group,” i.e., high levels of both PTSD and depressive symptoms (n = 18); “PTSD group,” i.e., high levels of PTSD symptoms alone (n = 15); and “no-pathology group,” i.e., low levels of PTSD and depressive symptoms (n = 27). Only 3 participants had elevated depressive symptoms alone (without PTSD) and their data were not included in the analysis. There were no gender differences between study groups.
To examine whether the study groups (comorbid, PTSD only, and no-pathology) differ in the extent of perceived danger and objective exposure to WTC attacks, we conducted two separate tests of one-way analysis of variance (ANOVA). Means, standard deviations, statistics and effect sizes are presented in Table 1.
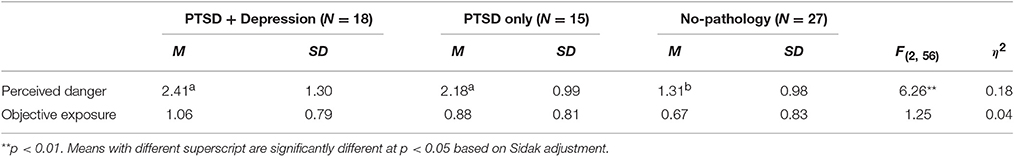
Table 1. Means, standard deviations, statistics, and effects sizes for the differences between psychopathology groups in perceived danger and objective exposure to 9/11 events.
The analyses indicated that the study groups significantly differed in the extent of perceived danger but not in the objective exposure to the 9/11 events. Sidak post-hoc analyses revealed that participants classified as comorbid PTSD + depressive symptoms or PTSD alone perceived the danger of the attacks to be higher than those of the no-pathology group (Šidák, 1967).
To examine whether the study groups (comorbid, PTSD only, and no-pathology) differ in the intensity of PTSD symptoms 7 and 18 months after the attacks and in somatic symptoms 7 months post the attack, we conducted multivariate analysis of variance (MANOVA). Means, standard deviations, statistics and effect sizes are presented in Table 2.
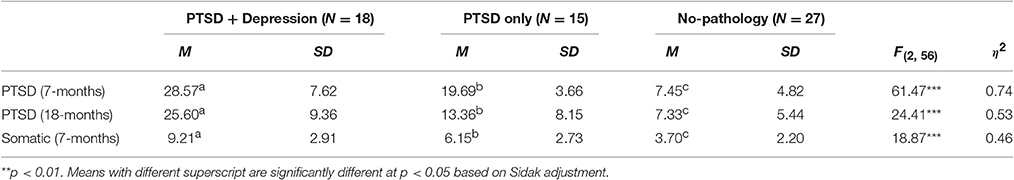
Table 2. Means, standard deviations, statistics, and effects sizes for the differences between psychopathology groups in PTSD and somatic-related symptoms.
The analysis indicated that the study groups significantly differed in the multivariate factor of symptom severity, Pillai's T = 0.86, F(6, 86) = 10.72, p < 0.001. Complementary one-way ANOVAs and discriminant analysis indicated that the comorbid group suffered from significantly higher intensity of PTSD symptoms (7 and 18 months) and somatic-related symptoms than the PTSD and no-pathology groups, with the largest differences in PTSD symptoms at 7 months (β = 0.94), followed by PTSD symptoms at 18 months (β = 0.58), and somatic-related symptoms at 7 months (β = 0.52). Both the comorbid and PTSD only groups had higher levels of PTSD symptom and somatic-related symptom severity than the no-pathology group.
First, we examined differences in baseline levels of cortisol between PTSD groups (PTSD + MDD, PTSD only, resilience), and we conducted one-way analysis of variance (ANOVA) with Brown-Forsythe adjustment to account for inequality of variance (a correction to the degrees of freedom). The analysis revealed significant results, F(2, 25.96) = 3.85, p = 0.034, η2 = 0.16. Although the effect size was small, planned contrasts with Bonferroni correction because of non-orthogonal comparisons indicated that the baseline cortisol level of the PTSD + depression group (M = 0.17, SD = 0.12) was significantly higher than that of the PTSD only group (M = 0.10, SD = 0.06, p = 0.048) and/or that of the resilience group (M = 0.10, SD = 0.07, p = 0.020).
Next, to examine whether the study groups significantly differed in their cortisol reactivity, we conducted a mixed-designed analysis of covariance, in which the between-subject independent variable was study group (comorbid, PTSD only, no-pathology), the within-subject independent variable was time (before, after interview), and the dependent variable was level of cortisol. Because the groups differed in the extent of perceived danger, we adjusted the analysis for its contribution. The μg/dl of salivary cortisol means and standard deviations are presented in Table 3.
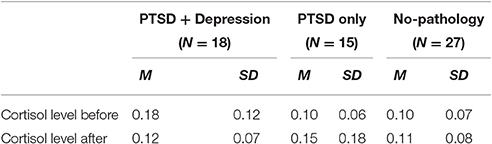
Table 3. Means and standard deviations for the differences between psychopathology groups in cortisol level.
While there were no main effects of group or time, the analysis revealed a significant interaction between study group and time, F(2, 43) = 4.22, p = 0.021, η2p = 0.16. Simple effects tests with Sidak adjustment revealed distinct differences in cortisol reactivity between the psychopathology groups (see Figure 1). Whereas, the cortisol significantly decreased among the comorbid group, it marginally increased in the PTSD group (and remained the same among the no-pathology group).
There are two competing theories concerning the tight relationship between PTSD and depression associated with trauma exposure (Flory and Yehuda, 2015). First, the comorbidity manifestation reflects merely overlapping symptoms of the two conditions, (Southwick et al., 1991; Franklin and Zimmerman, 2001). Second, comorbid PTSD and depression represents a distinct trauma-induced phenotype (O'Donnell et al., 2004; Dekel et al., 2014). We explored predictions from these theories by measuring cortisol levels among high-exposure survivors of the WTC attacks in response to recalling their personal experience escaping from the attack 7 months after the event.
Our study is the first to show that individuals with comorbid PTSD and depressive symptoms have decreased cortisol reactivity following trauma reminder while those with PTSD symptoms alone and healthy trauma-exposed do not. A trend of an elevated rather than decreased response to the trauma reminder was found in the PTSD alone group. The data also reveal that individuals endorsing comorbid symptoms have higher PTSD symptoms and somatization than individuals with PTSD alone.
The findings of comorbid PTSD and depression. Ourelevated symptoms associated with the comorbid condition indicate that phenotypically this condition is more severe than PTSD alone, as previously noted (Sher, 2005). However, the cortisol data demonstrating differences in baseline and activation cortisol levels between the comorbid and the PTSD only groups may suggest distinct conditions and not only one of degree. Our findings are in accord with the few biological studies comparing functional and molecular processes, such as lower amygdala activation (Kemp et al., 2007) and lower epigenetic methylation (Yehuda et al., 2015) in PTSD alone vs. comorbid PTSD and depression. Our findings, along with these studies, demonstrate that individuals with comorbid symptoms differ biologically from those with PTSD; possibly they all or some suffer from separate trauma-related psychopathologies. Furthermore, the differential cortisol responses to retelling the trauma highlight the importance of trauma-activation, and not only basal cortisol levels, in accounting for depression associated with PTSD and PTSD alone psychopathologies.
Baseline levels of cortisol agree fairly well with the general views of cortisol in major depressive disorder and PTSD (Yehuda, 2002). That is, baseline cortisol levels of those with depressive symptomology had higher baseline levels than the PTSD only group. With the trauma reminder, those with depressive symptomology had a blunted cortisol response to trauma-activation, whereas the PTSD only group had a strong trend toward a sensitized cortisol response to trauma-activation. While both groups had PTSD symptoms, cortisol levels moved in different directions after trauma-activation suggesting that when depressive symptoms are comorbid with PTSD, the depressive HPA and cortisol mechanisms predominate over the PTSD mechanisms. Blunt cortisol associated with depressive symptoms may relate to mechanisms of psychological disengagement with environmental stressors (Burke et al., 2005) and excessive shutting down of the hypothalamic-pituitary-adrenal cortical (HPA) axis due to impairment in negative feedback. An enhanced cortisol response in PTSD alone may be related with re-experiencing symptoms (Dekel et al., 2013a). On the other hand, the comorbidity may have very different pathological mechanisms than either PTSD or depression alone. These speculations should be followed up in future research.
Several limitations in this study should be noted. We obtained only a single post-interview measure of cortisol, and we did not include information on menstrual cycle, oral contraceptive use, medications, smoking, eating, and physical exercise just before the interview. Although we measured PTSD and depressive symptoms, whether a participant had clinically diagnosed PTSD or depression was not asked. The small sample size, although commonly used in studies like ours, raises the possibility of sample bias, and in the case of baseline cortisol levels, a significant but small effect size. The study assessed cortisol levels and responses 7 months post 9/11 and we might have overlooked variations in the cortisol response associated with symptom manifestation in the more immediate aftermath of trauma exposure or in the recovery 18 months after 9/11.
In summary, this study demonstrates higher baseline cortisol levels and lowered HPA-axis response correlates with PTSD + depressive symptoms and not with PTSD alone in individuals when confronted by trauma-reminders of the 9/11 WTC terrorist attacks. Importantly, trauma recollection, even 7 months post-trauma, might be an important tool to assess prolonged effects of trauma and their biological correlates. Biological markers of trauma-related psychopathology may offer an objective clinical assessment in addition to the existing DSM mental health classification based on subject description of symptoms. Further studies are needed to elucidate distinct biological abnormalities of various trauma-related conditions. A clearer understanding of unique biological targets may improve therapeutic interventions for trauma-exposed individuals endorsing comorbidity symptoms.
The study was approved by Teachers College, Columbia University Review Board. All subjects signed written consent.
SD collected the study data, initiated and generated the study paradigm, and prepared and wrote the manuscript. TE conducted the main statistical analysis and contributed to the writing of the manuscript. JR conducted the cortisol analysis and contributed to the writing of the manuscript. GB is the principle investigator of 9/11 study, which the sample of the current study is derived from. GB contributed to the manuscript editing.
This study was support by a Brain and Behavior Research Foundation (NARSAD) Young Investigator grant awarded to SD and her ECOR Claflin Distinguish Scholar award. GB received grants BCS-0202772 and BCS-0337643 from the National Science Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Kathleen Gordon for her technical assistance in performing the cortisol assays.
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association
Bonanno, G. A., Rennicke, C., and Dekel, S. (2005). Self-enhancement among high-exposure survivors of the september 11th terrorist attack: resilience or social maladjustment? J. Pers. Soc. Psychol. 88, 984–998. doi: 10.1037/0022-3514.88.6.984
Bremner, J. D., Vythilingam, M., Vermetten, E., Adil, J., Khan, S., Nazeer, A., et al. (2003). Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology 28, 733–750. doi: 10.1016/S0306-4530(02)00067-7
Breslau, N., Davis, G. C., Peterson, E. L., and Schultz, L. (1997). Psychiatric sequelae of posttraumatic stress disorder in women. Arch. Gen. Psychiatry 54, 81–87. doi: 10.1001/archpsyc.1997.01830130087016
Burke, H. M., Fernald, L. C., Gertler, P. J., and Adler, N. E. (2005). Depressive symptoms are associated with blunted cortisol stress responses in very low-income women. Psychosom. Med. 67, 211–216. doi: 10.1097/01.psy.0000156939.89050.28
Coffey, S. F., Gudmundsdottir, B., Beck, J. G., Palyo, S. A., and Miller, L. (2006). Screening for PTSD in motor vehicle accident survivors using the PSS-SR and IES. J. Trauma. Stress 19, 119–128. doi: 10.1002/jts.20106
Dekel, S., Ein-Dor, T., Gordon, K., Rosen, J., and Bonanno, G. (2013a). Cortisol and PTSD symptoms among male and female high-exposure 9/11 Survivors. J. Trauma. Stress 26, 621–625. doi: 10.1002/jts.21839
Dekel, S., Gilberston, M., Orr, S., Rauch, S., Nellie, W., and Pitman, R. (2016). “Trauma and posttraumatic stress disorder,” in Massachusetts General Hospital Comprehensive Clinical Psychiatry 2/e, eds T. A. Stern, M. Fava, T. Wilens, and J. F. Rosenbaum (Philadelphia, PA: Elsevier), 380–394.
Dekel, S., Solomon, Z., Horesh, D., and Ein-Dor, T. (2014). Posttraumatic stress disorder and depressive symptoms: joined or independent sequelae of trauma? J. Psychiatr. Res. 54, 64–69. doi: 10.1016/j.jpsychires.2014.03.003
Dekel, S., Solomon, Z., and Rozenstreich, E. (2013b). Secondary salutogenic effects in veterans whose parents were Holocaust survivors. J. Psychiatr. Res. 47, 266–271. doi: 10.1016/j.jpsychires.2012.10.013
Dekel, S., and Bonanno, G. A. (2013). Changes in trauma memory and patterns of posttraumatic stress. Psychol. Trauma 5, 26–34. doi: 10.1037/a0022750
de Kloet, C. S., Vermetten, E., Geuze, E., Kavelaars, A., Heijnen, C. J., and Westenberg, H. G. M. (2006). Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J. Psychiatr. Res. 40, 550–567. doi: 10.1016/j.jpsychires.2005.08.002
Dozier, M., Manni, M., Gordon, M. K., Peloso, E., Gunnar, M. R., Stovall-McClough, K. C., et al. (2006). Foster children's diurnal production of cortisol: an exploratory study. Child Maltreat. 11, 189–197. doi: 10.1177/1077559505285779
Elzinga, B. M., Schmahl, C. G., Vermetten, E., van Dyck, R., and Bremner, J. D. (2003). Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology 28, 1656–1665. doi: 10.1038/sj.npp.1300226
Flory, J. D., and Yehuda, R. (2015). Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin. Neurosci. 17, 141–150.
Foa, A., Riggs, D. S., Dancu, C. V., and Rotham, B. O. (1993). Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J. Trauma. Stress 6, 459–473. doi: 10.1002/jts.2490060405
Franklin, C. L., and Zimmerman, M. (2001). Posttraumatic stress disorder and major depressive disorder: investigating the role of overlapping symptoms in diagnostic comorbidity. J. Nerv. Ment. Dis. 189, 548–551. doi: 10.1097/00005053-200108000-00008
Gillespie, C. F., Phifer, J., Bradley, B., and Ressler, K. J. (2009). Risk and resilience: genetic and environmental influences on development of the stress response. Depress. Anxiety 26, 984–992. doi: 10.1002/da.20605
Gupta, M. A. (2013). Review of somatic symptoms in post-traumatic stress disorder. Int. Rev. Psychiatry 25, 86–99. doi: 10.3109/09540261.2012.736367
Halbreich, U., Olympia, J., Carson, S., Glogowski, J., Yeh, C. M., Axelrod, S., et al. (1989). Hypothalamo-pituitary-adrenal activity in endogenously depressed post-traumatic stress disorder patients. Psychoneuroendocrinology 14, 365–370. doi: 10.1016/0306-4530(89)90006-1
Holsboer, F. (2001). Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J. Affect. Disord. 62, 77–91. doi: 10.1016/S0165-0327(00)00352-9
Ikin, J. F., Creamer, M. C., Sim, M. R., and McKenzie, D. P. (2010). Comorbidity of PTSD and depression in Korean War veterans: prevalence, predictors, and impairment. J. Affect. Disord. 125, 279–286. doi: 10.1016/j.jad.2009.12.005
Kemp, A. H., Felmingham, K., Das, P., Hughes, G., Peduto, A. S., Bryant, R. A., et al. (2007). Influence of comorbid depression on fear in posttraumatic stress disorder: an fMRI study. Psychiatry Res. 155, 265–269. doi: 10.1016/j.pscychresns.2007.01.010
Kendall-Tackett, K. A. (2000). Physiological correlates of childhood abuse: chronic hyperarousal in PTSD, depression, and irritable bowel syndrome. Child Abuse Negl. 24, 799–810. doi: 10.1016/S0145-2134(00)00136-8
Kessler, R. C., Sonnega, A., Bromet, E., Hughes, M., and Nelson, C. B. (1995). Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry 52, 1048–1060. doi: 10.1001/archpsyc.1995.03950240066012
Kohout, F. J., Berkman, L. F., Evans, D. A., and Cornoni-Huntley, J. (1993). Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. J. Aging Health 5, 179–193. doi: 10.1177/089826439300500202
Kotozaki, Y., and Kawashima, R. (2012). Effects of the Higashi-Nihon earthquake: posttraumatic stress, psychological changes, and cortisol levels of survivors. PLoS ONE 7:e34612. doi: 10.1371/journal.pone.0034612
Lancaster, C. L., Cobb, A. R., Lee, H. J., and Telch, M. J. (2016). The role of perceived threat in the emergence of PTSD and depression symptoms during warzone deployment. Psyhol. Trauma 8, 528–534. doi: 10.1037/tra0000129
Liberzon, I., Abelson, J. L., Flagel, S. B., Raz, J., and Young, E. A. (1999). Neuroendocrine and psychophysiologic responses in PTSD: a symptom provocation study. Neuropsychopharmacology 21, 40–50. doi: 10.1016/S0893-133X(98)00128-6
Marmot, M. G., Smith, G. D., Stansfeld, S., Patel, C., North, F., Head, J., et al. (1991). Health inequalities among British civil servants: the Whitehall II study. Lancet 337, 1387–1393. doi: 10.1016/0140-6736(91)93068-K
McEwen, B. S. (2003). Mood disorders and allostatic load. Biol. Psychiatry 54, 200–207. doi: 10.1016/S0006-3223(03)00177-X
Meewisse, M. L., Reitsma, J. B., de Vries, G. J., Gersons, B. P., and Olff, M. (2007). Cortisol and post-traumatic stress disorder in adults: systematic review and meta-analysis. Br. J. Psychiatry 191, 387–392. doi: 10.1192/bjp.bp.106.024877
Mollica, R. F., McInnes, K., Sarajlic, N., Lavelle, J., Sarajlic, I., and Massagli, M. P. (1999). Disability associated with psychiatric comorbidity and health status in Bosnian refugees living in Croatia. JAMA 282, 433–439. doi: 10.1001/jama.282.5.433
Morina, N., Ajdukovic, D., Bogic, M., Franciskovic, T., Kucukalic, A., Lecic-Tosevski, D., et al. (2013). Co-occurrence of major depressive episode and posttraumatic stress disorder among survivors of war: how is it different from either condition alone? J. Clin. Psychiatry 74, 212–218. doi: 10.4088/JCP.12m07844
Morris, M. C., Compas, B. E., and Garber, J. (2012a). Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin. Psychol. Rev. 32, 301–315. doi: 10.1016/j.cpr.2012.02.002
Morris, M. C., Rao, U., and Garber, J. (2012b). Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J. Affect. Disord. 143, 223–230. doi: 10.1016/j.jad.2012.05.059
O'Donnell, M. L., Creamer, M., and Pattison, P. (2004). Posttraumatic stress disorder and depression following trauma: understanding comorbidity. Am. J. Psychiatry 161, 1390–1396. doi: 10.1176/appi.ajp.161.8.1390
Oquendo, M. A., Friend, J. M., Halberstam, B., Brodsky, B. S., Burke, A. K., Grunebaum, M. F., et al. (2003). Association of comorbid posttraumatic stress disorder and major depression with greater risk for suicidal behavior. Am. J. Psychiatry 160, 580–582. doi: 10.1176/appi.ajp.160.3.580
Pitman, R. K., Rasmusson, A. M., Koenen, K. C., Shin, L. M., Orr, S. P., Gilbertson, M. W., et al. (2012). Biological studies of post-traumatic stress disorder. Nat. Rev. Neurosci. 13, 769–787. doi: 10.1038/nrn3339
Rief, W., and Auer, C. (2000). Cortsiol and somatization. Biol. Psychol. 53, 13–23. doi: 10.1016/S0301-0511(00)00042-9
Schulkin, J., McEwen, B. S., and Gold, P. W. (1994). Allostasis, amygdala, and anticipatory angst. Neurosci. Biobehav. Rev. 18, 385–396. doi: 10.1016/0149-7634(94)90051-5
Sher, L. (2005). The concept of post-traumatic mood disorder. Med. Hypotheses 65, 205–210. doi: 10.1016/j.mehy.2005.03.014
Šidák, Z. (1967). Rectangular confidence regions for the means of multivariate normal distributions. J. Am. Stat. Assoc. 62, 626–633. doi: 10.1080/01621459.1967.10482935
Southwick, S. M., Yehuda, R., and Giller, E. L. Jr. (1991). Characterization of depression in war-related posttraumatic stress disorder. Am. J. Psychiatry 148, 179–183.
Stansfeld, S. A., Smith, G. D., and Marmot, M. (1993). Association between physical and psychological morbidity in the Whitehall II Study. J. Psychosom. Res. 37, 227–238. doi: 10.1016/0022-3999(93)90031-A
Yehuda, R. (2002). Current status of cortisol findings in post-traumatic stress disorder. Psychiatr. Clin. North Am. 25, 341–368, vii. doi: 10.1016/S0193-953X(02)00002-3
Keywords: PTSD symptoms, depressive symptom, September 11 terrorist attacks, cortisol, traumatic stress
Citation: Dekel S, Ein-Dor T, Rosen JB and Bonanno GA (2017) Differences in Cortisol Response to Trauma Activation in Individuals with and without Comorbid PTSD and Depression. Front. Psychol. 8:797. doi: 10.3389/fpsyg.2017.00797
Received: 14 January 2017; Accepted: 01 May 2017;
Published: 18 May 2017.
Edited by:
Alexandre Heeren, Harvard University, United StatesReviewed by:
Yael Lahav, University of Southern Denmark, DenmarkCopyright © 2017 Dekel, Ein-Dor, Rosen and Bonanno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharon Dekel, c2Rla2VsQG1naC5oYXJ2YXJkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.