
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 28 October 2016
Sec. Psychology for Clinical Settings
Volume 7 - 2016 | https://doi.org/10.3389/fpsyg.2016.01689
This article is part of the Research Topic Pain management in clinical and health psychology View all 13 articles
Objectives: Attributions about how comorbid symptoms worsen or improve each other are central cognitive components of chronic pain that are shown to facilitate or impede the recovery process. Still, these attributions have been poorly illuminated in chronic pain patients. The present study explored perceptions of how sleep, pain, and mood influence each other in patients awaiting total hip arthroplasty (THA).
Design and Methods: In this cross-sectional study, 291 patients (mean age 67.8, 65.3% female) rated 12 statements about how much a given symptom (pain, sleep, mood) changed when another symptom (pain, sleep, mood) worsened or improved on a response scale ranging from much worse (-2) via no change (0) to much better (2). Sleep (Bergen Insomnia Scale), pain (McGill Pain Questionnaire), anxiety and depression (Hospital Anxiety and Depression Scale) were assessed as background variables.
Results: Of the patients in the study, 56% reported symptoms indicating insomnia. Anxiety and depression were indicated in 16 and 10%, respectively. Over 80% rated their pain as horrible/unbearable and reported that pain occurred always/daily. When experiencing increased pain, a majority perceived that sleep (90%) and mood (70%) worsened, whilst experiencing reduced pain improved sleep and mood in 50%. Poor sleep increased pain and worsened mood in 45 and 60% of the patients, respectively. Better sleep was perceived to reduce pain and improve mood in 50%. Worsened mood increased pain (46%) and worsened sleep (52%). Improved mood decreased pain and improved sleep in 25 and 35%, respectively.
Discussion: In this study, a novel approach was used to investigate perceptions of reciprocal relationships between symptoms. We found that THA patients perceived interrelationships between pain, sleep and mood. These perceived interrelations were stronger when symptoms worsened than when symptoms improved. They also held stronger beliefs about the effect of pain on sleep and mood, than the effect of sleep and mood on pain. Attributions are central in illness perception and ultimately affect illness behavior. For patients who perceive symptoms to interrelate, the door has already been opened to utilize these attributions in treatments aiming to disrupt vicious cycles, hence supporting the use of multimodal treatments.
Pain in patients eligible for total hip arthroplasty (THA) is normally caused by arthritis (Hamel et al., 2008). The experience and expression of such pain is commonly modulated by the presence of comorbid conditions like sleep and mood disturbances (Chiu et al., 2005; Lautenbacher et al., 2006; Roehrs et al., 2006; Haack et al., 2007; Smith et al., 2007; O’Brien et al., 2011; Blagestad et al., 2012) as well as expectancies and appraisals about these conditions (Tracey, 2010; Bjorkedal and Flaten, 2012). Chronic pain patients often attribute specific causal relationships in terms of how these conditions influence each other (Morin et al., 1998; Hawker et al., 2008; Tang et al., 2009; Theadom and Cropley, 2010). Shown to shape symptom expression, such attributions also influence a person’s overall perceived symptom load (Petrie et al., 2007). Attributions typically enable a person to predict and influence future events, and are, accordingly, found to predict thoughts and behavior aimed at getting well, or motivation to perform preventive health behavior (Michela and Wood, 1986). In chronic pain specifically, such attributions are found to be central cognitive facilitators or impediments to the recovery process (Dean, 1986; Michela and Wood, 1986; DeGood and Kiernan, 1996; Roesch and Weiner, 2001).
Sleep and mood disturbances are frequently experienced as a consequence of pain in chronic pain patients (Brennan and Lieberman, 2009), and often interact to worsen pain (Chiu et al., 2005; Zautra et al., 2005; Vitiello et al., 2009; Ong et al., 2010; Theadom and Cropley, 2010; Sivertsen et al., 2015). Conversely, there is also recent research highlighting the amplifying effect of improvements of sleep and mood involved in the recovery from chronic pain (Zautra et al., 2005; Davies et al., 2008; Ashworth et al., 2010; Ong et al., 2010). Sleep and mood are therefore central components both in expression of illness, and as part of the multimodality treatment of chronic pain patients. There is emerging evidence that chronic pain patients with comorbid sleep problems are aware of the bidirectional relationship between the constructs (Tang et al., 2009; Ramlee et al., 2016). Hence, there is great potential in assessing and utilizing attributions to aid accurate understanding and treatment of chronic pain and its comorbid conditions.
Attributions about the perceived relationship between pain, sleep and mood have been poorly illuminated empirically. A few studies have explored the perceived effect of pain on sleep and mood and found, first, that good sleep and emotional well-being are rated as very important for chronic pain patients (Turk et al., 2008). Furthermore, many pain patients are convinced that their sleep problems result from their pain (Morin et al., 1998; Hawker et al., 2008), and consequently when they experience severe pain, it is difficult for them to sleep (Edwards et al., 2011; Tang et al., 2012a). In line with this, chronic pain patients often believe that their sleep problem will disappear when their pain is gone (Morin et al., 1998). Of the studies to date, only one has explored this reciprocal relationship from the perspective of sleep, finding that fibromyalgia patients directly associate poor sleep with feelings of pain and fatigue, in addition to reduced coping abilities (Theadom and Cropley, 2010). Knowledge of attributions about the perceived mutual influence of mood, pain and sleep is lacking in chronic pain patients. Also missing are studies exploring attributions about how improvements, and not only worsening, of symptoms, are perceived to influence other symptoms. Finally, in order to investigate whether bidirectional relationships exist in how patients attribute reciprocal symptom influence, these multidirectional attributions need to be explored within the same individuals.
To improve our understanding of attributions of symptoms in chronic pain patients, we developed an instrument to explore how patients waiting to undergo THA perceived pain, sleep and mood to influence each other. The questionnaire contained 12 statements assessing two main aspects of symptom influence: (1) how levels of pain influence sleep and mood, but also, conversely, the influence of sleep and mood on pain, and (2) the perceived effect on pain, sleep and mood both when symptoms are worse than usual and when symptoms are better than usual. Based on the responses to these statements, bidirectional relationships between pain, sleep and mood were investigated.
This questionnaire-based study was part of a prospective, multi-center study that evaluated pain, sleep, anxiety, depression and symptom attribution in patients 6–0 weeks before THA. These results are reported elsewhere.
Participants were recruited from four different orthopedic departments in hospitals across Norway (Haukeland University Hospital, Diakonhjemmet Hospital, Coastal Hospital Hagevik and Sørlandet Hospital Arendal) between May 2014 and November 2015. A total of 643 patients who entered the waiting lists for THA were invited to participate and 314 patients accepted. The response rate differed between the hospitals, with response rates of 75.2, 72.0, 58.7, and 23.2%, respectively. Due to the low response rate in the last hospital, sensitivity analyses were performed whereby results with all hospitals included were compared to results from all hospitals without the hospital with the lowest response rate. In all cases, the results did not significantly differ, with differences in effect (measured by Cohen’s d effect size) of less than 0.1. Hence, including data from the hospital with low response rate had negligible effects on the results. Eighteen participants were excluded from the analysis due to missing signed consent form pre-operatively, and five because their THA was canceled. Thus, the final sample consisted of 291 participants.
The participants were recruited consecutively from the waiting lists for THA. When sending the notice of the date for their operation, an administrative staff member at the respective hospital enclosed information about the study, provided a questionnaire consisting of several validated scales as well as an informed consent form. Patients willing to participate were asked to complete the questionnaire at home and return the questionnaire and signed consent form when arriving at the pre-operative consultation. At one hospital, the patients were asked to return the questionnaire in a prepaid return envelope. Date of surgery was extracted from the Norwegian Arthroplasty Register via the participant’s unique identifying code provided in the questionnaire. The participant’s address was provided by the respective hospitals.
The study was approved by The Regional Committee for Medical and Health Research Ethics in Western Norway (2014/63/REK Vest) and was also approved at each of the hospitals involved.
The questionnaire contained a selection of measures that registered the participant’s name, identifying code and data on the participant’s demographics (age, sex, ethnicity, education level, employment, income, marital status, and number of children) and self-reported health. The following clinical background variables were assessed; pain intensity and frequency [from the McGill Pain Questionnaire (Melzack, 1975), in addition to reporting additional pain in the hip being replaced], sleep [Bergen Insomnia Scale (BIS; Pallesen et al., 2008)], symptoms of anxiety and depression [Hospital Anxiety and Depression Scale (Zigmond and Snaith, 1983)], and specific hip-related outcomes [Hip Osteoarthritis Outcome Scale (Nilsdotter et al., 2003)]. In addition, the participants completed a questionnaire assessing attribution of symptoms specifically designed for this study. These questionnaires are briefly described in the following paragraphs.
General pain was assessed using two verbal descriptor scales from the McGill Pain Questionnaire (Melzack, 1975), validated in Norwegian (Kim et al., 1995). The magnitude of pain was assessed by the phrase: “place a cross in the box fitting your pain,” with the response alternatives “no pain,” “weak,” “unpleasant,” “bothersome,” “terrible” or “unbearable.” The frequency of pain was assessed by the phrase: “How often do you have pain?” The response alternatives were “constantly,” “daily,” “several times a week,” “about once a week,” “several times a month,” “about once a month,” “less than once a month” and “never.” Patients were also asked whether the pain was chronic (>3 months), if they had additional pain to the hip being replaced and whether they felt that analgesics relieved their pain.
Sleep was assessed using the BIS which measures self-reported symptoms of insomnia corresponding to the criteria for insomnia in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR (American Psychiatric Association, 2000). The scale includes six items that are scored on an eight-point scale indicating the number of days per week for which a specific symptom is experienced (0–7 days, total scores ranging from 0 to 42). The BIS is validated using subjective as well as polysomnographic data and is found to possess good psychometric properties (Pallesen et al., 2008). Participants were categorized as insomniacs if scoring 3 or more on at least one of items 1–4, and 3 or more on at least one of items 5 and 6. The scale provided a Cronbach’s alpha of 0.91 in the present study.
The Hospital Anxiety and Depression Scale (HADS) was used to assess the presence of anxiety and depression. The HADS contains 14 items describing non-vegetative symptoms of anxiety and depression (scoring range 0–21 for both anxiety and depression subscales) (Zigmond and Snaith, 1983). Higher scores indicate greater symptom severity. A score of 8 or higher on the HADS subscales of anxiety and depression respectively is considered a clinical cut-off. A validated Norwegian version of the HADS was used in the present study (Bjelland et al., 2002), for which the Cronbach’s alpha for each subscale was 0.86.
The Hip Osteoarthritis Outcome Scale (HOOS) evaluated hip related outcomes through 5 subscales [pain, symptoms, functioning in activities of daily living (ADL), functioning in sport and recreation, and hip-related quality of life]. Standardized response alternatives are provided on a 5-point Likert scale (0–4). Then, a normalized score from 0 to 100 is calculated for each subscale (100 indicating no symptoms, and 0 indicating extreme symptoms) (Nilsdotter et al., 2003). The Cronbach’s alpha coefficient was 0.96 in the present study.
The main outcome variable was symptom attribution. In order to assess how participants perceived symptoms of pain, sleep and mood to influence each other, a questionnaire was developed containing 12 statements about how much a given symptom (pain, sleep, mood) changed when another symptom (pain, sleep, mood) worsened or improved. Six statements explored the effect on the other two symptoms when a given symptom worsened, and six statements explored the effect on the other two symptoms when a given symptom improved. The participants were asked to provide responses on a 5-point scale (from 1 to 5) for each statement. Table 1 presents the 12 statements together with the response alternatives.
Analyses were performed using SPSS, version 21. For the symptom attribution questionnaire, the rating scale was recoded in order to display the positive or negative properties of the perceived influence. Much worse was recoded to -2, a bit worse was recoded to -1, as usual was recoded to 0 (indicating no change), a bit better was recoded to 1 and much better was recoded as 2. Descriptive statistics were used to characterize symptom attributions and the difference of the mean from 0 (no change) was measured through one-sample t-tests. A paired sample t-test was used to compare items in bidirectional relationships in order to assess the directionality of symptom attribution. All statements are listed in Table 1. For example, whether pain influences sleep more than sleep influences pain was assessed by comparing statements 1a and 2a for the worsening relationships between symptoms, and statements 1c and 2c for the improving relationships between symptoms. For the pain-mood relationship, statements 1b and 3a and statements 1d and 3c were compared for the worsening and improving effect of symptoms, respectively. For the sleep-mood relationship, statements 2b and 3b and statements 2d and 3d were compared for the worsening and improving effect of symptoms, respectively. Pairs with one or more missing values were removed from analyses (excluded pairwise). To measure the magnitude of the effect, effect sizes (Cohen’s d) were estimated using DSTAT (Johnson, 1995). An effect size of 0.2 is regarded as a small, 0.5 a medium, and effect sizes of 0.8 or higher are regarded as large (Cohen, 1988). A Bonferroni-correction was applied due to multiple comparisons, setting the new critical p-value to 0.002.
Table 2 presents the participants’ characteristics. The mean age was 67.9 years and 65.3% were female. The majority were retired, married/cohabiting, and had 2 or 3 children. The majority had an income between 100 000 and 399 999 NOK (equivalent to approximately 12 000–50 000 USD). Clinical background variables are presented in Table 3. On the PPI, most participants rated their pain to be horrible (66.3%) or unbearable (16.2%), and over 90% rated their pain to occur daily or be present constantly. Over 70% also reported additional pain in the hip being replaced. In total, 54.0% reported symptoms indicating insomnia (the average BIS score was 16.4, SD = 12.0). Symptoms indicating caseness of anxiety or depression were reported by 16.2 and 10.3%, respectively. According to the hip-specific outcome measure (HOOS), the self-reported hip-related pain, function, quality of life, ADL and sports and recreation were poor (between 40 and 24 on a scale of 100–0 where 100 indicates no symptoms, and 0 indicates extreme symptoms).
A substantial portion of patients perceived that worsening of symptoms influenced their pain, sleep and mood (Table 4, Figure 1). Ninety per cent of the patients reported that sleep worsened in the presence of increased pain and 70% reported mood to worsen with increased pain. Close to 45% perceived their pain to worsen with poorer sleep, and almost 60% perceived mood to worsen with poorer sleep. Worse mood was perceived to have the least influence on pain (64.3% perceived there to be no change), but 51.9% reported mood to influence sleep. As displayed in Table 5, the mean on all subscales differed significantly from 0 (all t-values significant on the 0.002-level) with effect sizes ranging from 0.6 to 2.3 (medium to very large effect size).
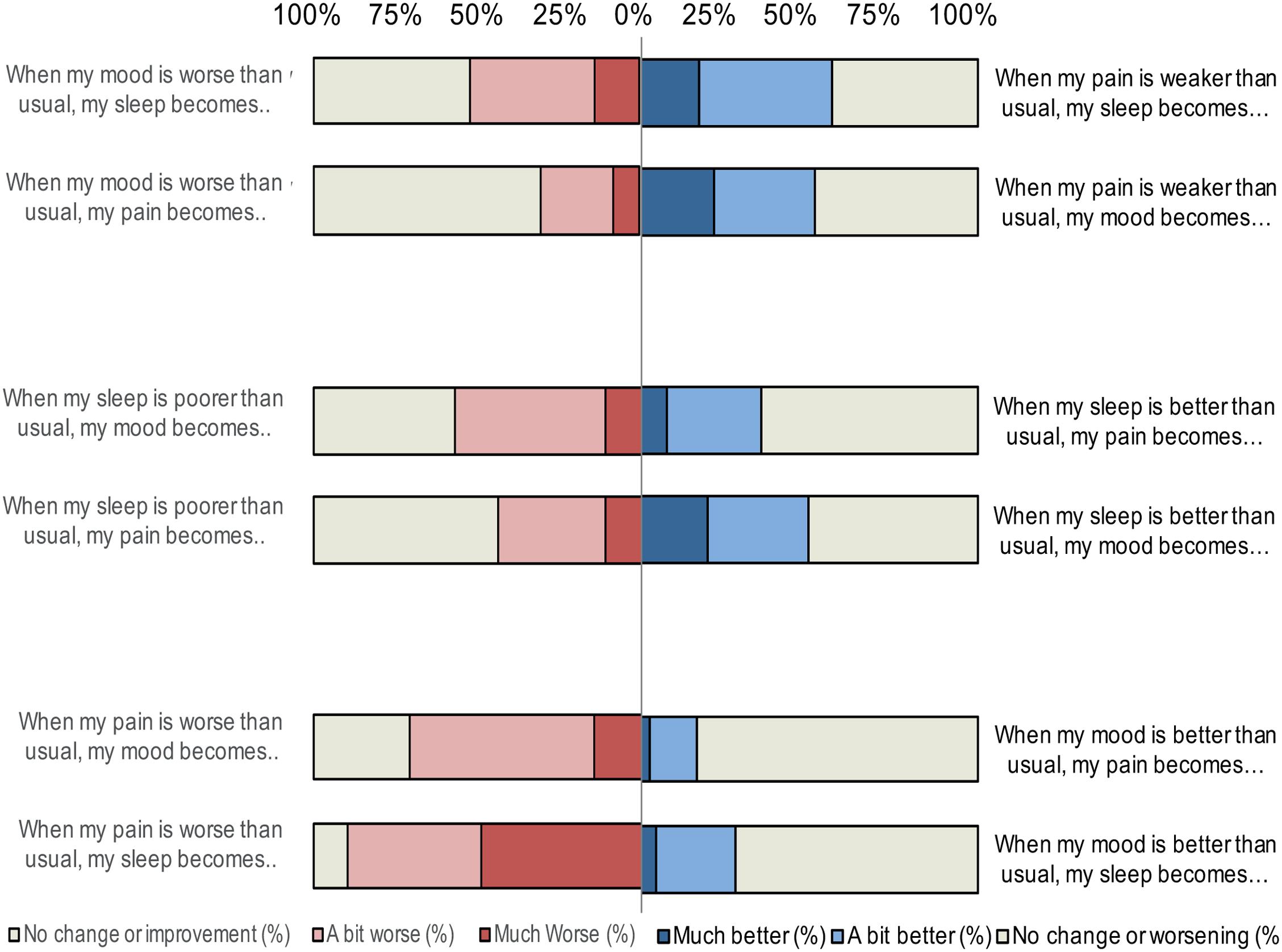
FIGURE 1. Percentages of patients attributing changes in their pain, sleep, and mood when symptoms worsen or improve.
Patients reported improvement of one symptom to influence the other symptoms to a smaller degree than did worsening of it (Table 4, Figure 1). Still, reduced pain was perceived to improve sleep and mood in 56.7 and 51.8% of the patients, respectively. Improved sleep was also perceived to improve pain in 35.4% of the patients. Improved sleep had a strong influence on improvements of mood and was reported by 47.2% of the patients. Again, mood was perceived to have the least influence on pain and sleep; improved mood was perceived not to have an effect in 74.9% for pain and 63.2% for sleep. Regardless, all variables differed significantly from 0 (all t-values significant on a 0.002-level). Table 5 displays the effect sizes (ranging from 0.3 = small effect size to 0.9 = large effect size).
When symptoms worsened, pain was significantly perceived to influence sleep more than sleep influenced pain (t = -19.2, df = 279). The effect size was large (d = 1.1) (Table 6, Figure 2). Increased pain was also perceived to influence mood significantly more than worsened mood influenced pain (t = -10.5, df = 269). This effect size was medium (d = 0.6). There was no significant difference to which degree the participants perceived sleep and mood to influence each other.
Reduced pain significantly influenced sleep more than improved sleep influenced pain (Table 6, Figure 3, t = 5.7, df = 272). The effect size was small to medium (d = 0.4). Reduced pain also influenced mood more than improved mood influenced pain (t = 10.3, df = 268) with a medium effect size (d = 0.6). Lastly, improved sleep was perceived to influence mood more than improved mood influenced sleep (t = 8.0, df = 269) with a medium effect size (d = 0.5).
In contrast to the number of studies that aim to disentangle the relationship between chronic pain, sleep and mood, limited effort has been devoted to investigating how patients themselves perceive how these symptoms influence each other. The present study explored perceived bi-directional influences of pain, sleep and mood when symptoms worsened or improved in patients awaiting THA. We found that a large majority perceived sleep and mood to worsen when experiencing worse pain than usual and less intense pain than usual was perceived to improve sleep and mood. A significant proportion of the patients perceived pain to worsen with poorer sleep, and better sleep was perceived to reduce pain. Overall, pain stood out as the symptom with the largest perceived influence on the other symptoms, while mood was the symptom perceived by the fewest patients as influencing the other symptoms.
We found that almost all of the patients in the present study perceived increased pain to lead to poorer sleep, corroborating the impact of pain on sleep in previous qualitative and quantitative studies (Smith et al., 2000; Breivik et al., 2006; Hawker et al., 2008; Ashworth et al., 2010; Theadom and Cropley, 2010; Henderson et al., 2013; Thomazeau et al., 2014). For example, many chronic pain patients firmly believe that when they are in pain, it is simply impossible for them to get comfortable and go to sleep (Edwards et al., 2011; Tang et al., 2012a). The rate of patients perceiving pain to negatively impact sleep was higher in the present study than found in chronic pain patients in general (90% vs. 65%) (Breivik et al., 2006); also, the intensity and frequency of pain was higher in the present sample. The present study highlights the importance of effective treatments for chronic pain.
One third of our patient’s perceived pain and mood to worsen with poorer sleep, mirroring one qualitative study where a poor night’s sleep was found to be directly associated with increased pain (Theadom and Cropley, 2010). Our results are also in line with increasing numbers of observational and experimental studies establishing an effect of sleep on pain. However, more than half of the patients in our study did not perceive poorer sleep to increase pain. One of the most distressing features of chronic pain is the unpredictable fluctuation in its type and intensity (Hawker et al., 2008), and it may thus be difficult for patients to perceive how these symptoms are influenced by sleep and mood. This is supported by a recent daily process study that reported the pain-relieving effect of good sleep to be short-lived. Although sleep quality showed an inverse relationship with pain upon waking and during the first half of the day, no association was found during the second half of the day (Tang et al., 2012c). The authors suggest that for some patients, reduced pain might actually lead to over-extending activity. This would cause even more pain during the night, consequently masking the positive effect of good sleep on pain. Hence, perceived improvement of pain as a result of good sleep might be masked by the fluctuations or other sources of increasing pain. In addition, many clinicians do not regularly assess, diagnose or treat comorbid sleep problems in pain patients, since they are under the false impression that treatment of the underlying organic/psychiatric condition will resolve any residual sleep complaints (Ozminkowski et al., 2007). This lack of focus might contribute to these patients’ perception of illness.
Although depression, anxiety and negative mood are closely related to chronic pain (Lin et al., 2003; Argoff, 2007; Montin et al., 2007; O’Brien et al., 2010; Wylde et al., 2011; Hoogeboom et al., 2012), worse mood than usual was perceived by the fewest patients to impact pain and sleep in our study. In a study of middle-aged women with chronic pain, an increase in negative affect during the previous week predicted greater pain during subsequent weeks (Zautra et al., 2005). One could assume that patients would perceive this same effect to a larger degree than what we found. Our results might indicate, as suggested by Lavigne (2005), that negative mood affects sleep and pain in a more indirect way. Alternatively, if the perception about reciprocal relationships between symptoms depend on the presence of the symptom in question, our results might simply reflect lower rates of anxiety and depression compared to pain and sleep complaints in the present study. Future investigations of symptom attributions in chronic pain patients with larger samples of comorbid anxiety and depression would clarify this matter.
The present study is to the authors’ knowledge the first to explore how improvement in one symptom (pain, sleep, or mood) is perceived to influence other symptoms. We found that a majority of our patients perceive reduction of pain to improve sleep and mood. Although chronic pain is intractable by definition, this underlines the importance of optimal pain management, whereby reducing pain may also improve comorbid symptoms (Turk and Cohen, 2010). More noteworthy is the finding that one third perceived better sleep than usual to improve pain. The role of sound sleep is key in chronic pain patients. Firstly, restorative sleep is shown to be involved in the resolution of chronic pain (Davies et al., 2008), and chronic pain patients that are “good sleepers” report less pain at night, less negative consequences from their pain and less depression or pain-related anxiety (Ashworth et al., 2010). Accordingly, the concurrent treatment of pain-related sleep problems is found either to reduce pain itself, or to reduce pain interference, which might be an important aspect of pain in chronic pain patients (Edinger et al., 2005; Vitiello et al., 2009; Jungquist et al., 2010; Tang et al., 2012b). Furthermore, positive emotions are seen as resilience factors decreasing the negative impact of chronic pain conditions (Zautra et al., 2005; Ong et al., 2010). In a study investigating positive and negative affect in women with chronic pain, people who tend to have higher levels of positive affect also had less pain over time (Zautra et al., 2005). Hence, adequate sleep and positive mood seems to be a buffer involved not only in the biological foundation of pain perception (Davies et al., 2008), but also in the ability to cope with daily pain (Theadom and Cropley, 2010). Positive emotions and good sleep may therefore play an important role in fostering recovery after episodes of severe pain (Zautra et al., 2001).
Taken together, the present findings have implications for the assessment and treatment of chronic pain and pain-related sleep and mood disturbances. That symptoms interact to worsen and improve each other forms the basis of multimodality treatments. This emphasizes the benefit of interventions aiming at disrupting vicious circles between symptoms (Argoff, 2007; Smith et al., 2009). The results of the present study support the use of interventions that target sleep and mood in addition to pain. Furthermore, attributions are found to be central cognitive facilitators or impediments to the recovery process (Dean, 1986; DeGood and Kiernan, 1996; Roesch and Weiner, 2001). According to attribution theory, individuals with chronic illness who make internal, unstable and controllable attributions also believe they can do something to minimize the impact of their illness. This leads directly to certain motivated coping cognitions and behavior, and ultimately to more positive psychological adjustment (Weiner, 1985). For chronic pain patients who perceive symptoms to interrelate, the door has already been opened to utilize these attributions in the treatment of chronic pain and its comorbid conditions. For our patients awaiting THA specifically, these attributions might aid a positive reinforcing cycle of symptom improvement when pain is reduced after surgery.
The limitations of the study should be noted. Firstly, due to the lack of previous studies that include the key attribution elements aimed at in the present study, a questionnaire was constructed for this purpose. It is therefore not previously validated. The questions used for assessing reciprocal relationships between pain, sleep and mood should be validated in other types of samples (e.g., normal subjects as well as in patients suffering from sleep and mood disorders). In the process of developing the questionnaire, mood was intentionally chosen as a general symptom-effector instead of specifying anxiety and depression, for several reasons. By broadening the term into “mood,” we are convinced that aspects of disturbed mood such as “helplessness” or “frustrations” often experienced by these patients would be included in addition to aspects of anxiety and depression. Furthermore, there is no equivalent positive category to diagnoses such as anxiety and depression, and we also wanted to capture eventual positive attributions of improved mood, beyond the absence of negative symptoms. Another limitation is that since the patients completed the questionnaires without assistance from the researchers we had no way to ensure that participants understood the intention of the attribution questionnaire. Third, it is important to note that one of the hospitals included in the study had a very low response rate (22%), due to unknown factors. In order to ensure representativeness of our data, sensitivity analyses were performed and showed no major changes in results when the respective hospital was removed from analyses.
Despite the limitations, there are several strengths of this novel study. It places itself in a line of studies focusing on obtaining wider knowledge about the sleep-pain domain from the patient’s perspective (Hawker et al., 2008; Turk et al., 2008), but it extends the scope to also explore attributions about sleep and mood, and to illuminate both the attributions related to worsening as well as improvement of symptoms. The natural path forward is to extend this newly acquired perspective into different chronic pain populations or populations where pain is a frequently experienced comorbid symptom.
The present study found that patients awaiting THA perceive pain, sleep and mood to influence each other when symptoms worsen or improve. Pain was perceived to have a stronger influence on sleep and mood, than sleep and mood had on pain. Attributions of symptom dynamics as investigated in the present study may play a key role in overall pain experience and illness behavior.
TB designed the study, developed the main questionnaire, recruited the hospitals participating in the study and collected the data. She also analyzed the data and wrote the manuscript. SP supervised the project, including participating in the design of the study and developing the main questionnaire. He also took part in deciding the choice of analyses, and critically reviewed the manuscript. JG also supervised the project, including participating in the design of the study and developing the main questionnaire. she also took part in deciding the choice of analyses, and critically reviewed the manuscript. NT took part in deciding the choice of analyses, the interpretation of the results and critically reviewed the manuscript. IN was the main supervisor of the project and participated in the design of the study and the development of the main questionnaire. She also took part in deciding the choice of analyses, and critically reviewed the manuscript. All authors have approved of the final version of the manuscript to be published.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This research was conducted as the doctoral dissertation of the first author (TB) at the Department of Clinical Psychology, University of Bergen, Norway. In addition, this research was supported by a grant from Kavli Trust (grant number 808014). This grant did not influence any aspect of the project and the content is solely the responsibility of the authors. The authors have no other competing interest to report. The authors are grateful to all involved staff at Haukeland University Hospital, Sørlandet Hospital Arendal, Coastal Hospital Hagevik and Diakonhjemmet Hospital, and to the patients participating in this study.
American Psychiatric Association (2000). Diagnostic and Statistical Manual of Mental Disorders-IV- TR. Washington DC: American Psychiatric Association.
Argoff, C. E. (2007). The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin. J. Pain 23, 15–22. doi: 10.1097/01.ajp.0000210945.27052.b300002508-200701000-00003
Ashworth, P. C., Davidson, K. M., and Espie, C. A. (2010). Cognitive-behavioral factors associated with sleep quality in chronic pain patients. Behav. Sleep Med. 8, 28–39. doi: 10.1080/15402000903425587
Bjelland, I., Dahl, A. A., Haug, T. T., and Neckelmann, D. (2002). The validity of the hospital anxiety and depression Scale. An updated literature review. J. Psychosom. Res. 52, 69–77. doi: 10.1016/S0022-3999(01)00296-3
Bjorkedal, E., and Flaten, M. A. (2012). Expectations of increased and decreased pain explain the effect of conditioned pain modulation in females. J. Pain Res. 5, 289–300. doi: 10.2147/JPR.S33559
Blagestad, T., Pallesen, S., Lunde, L. H., Sivertsen, B., Nordhus, I. H., and Gronli, J. (2012). Sleep in older chronic pain patients: a comparative polysomnographic study. Clin. J. Pain 28, 277–283. doi: 10.1097/AJP.0b013e3182313899
Breivik, H., Collett, B., Ventafridda, V., Cohen, R., and Gallacher, D. (2006). Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain 10, 287–333. doi: 10.1016/j.ejpain.2005.06.009
Brennan, M. J., and Lieberman, J. A. III. (2009). Sleep disturbances in patients with chronic pain: effectively managing opioid analgesia to improve outcomes. Curr. Med. Res. Opin. 25, 1045–1055. doi: 10.1185/03007990902797790
Chiu, Y., Silman, A., Macfarlane, G., Ray, D., Gupta, A., Dickens, C., et al. (2005). Poor sleep and depression are independently associated with a reduced pain threshold. Results of a population based study. Pain 115, 316–321. doi: 10.1016/j.pain.2005.03.009
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences, 2nd Edn. Hillsdale, NJ: Lawrence Erlbaum Associates.
Davies, K. A., Macfarlane, G. J., Nicholl, B. I., Dickens, C., Morriss, R., Ray, D., et al. (2008). Restorative sleep predicts the resolution of chronic widespread pain: results from the EPIFUND study. Rheumatology (Oxford) 47, 1809–1813. doi: 10.1093/rheumatology/ken389
DeGood, D. E., and Kiernan, B. (1996). Perception of fault in patients with chronic pain. Pain 64, 153–159. doi: 10.1016/0304-3959(95)00090-9
Edinger, J. D., Wohlgemuth, W. K., Krystal, A. D., and Rice, J. R. (2005). Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Arch. Intern. Med. 165, 2527–2535. doi: 10.1001/archinte.165.21.2527
Edwards, M. J., Tang, N. K., Wright, A. M., Salkovskis, P. M., and Timberlake, C. M. (2011). Thinking about thinking about pain: a qualitative investigation of rumination in chronic pain. Pain Manag. 1, 311–323. doi: 10.2217/pmt.11.29
Haack, M., Sanchez, E., and Mullington, J. M. (2007). Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 30, 1145–1152.
Hamel, M. B., Toth, M., Legedza, A., and Rosen, M. P. (2008). Joint replacement surgery in elderly patients with severe osteoarthritis of the hip or knee: decision making, postoperative recovery, and clinical outcomes. Arch. Intern. Med. 168, 1430–1440. doi: 10.1001/archinte.168.13.1430
Hawker, G. A., Stewart, L., French, M. R., Cibere, J., Jordan, J. M., March, L., et al. (2008). Understanding the pain experience in hip and knee osteoarthritis–an OARSI/OMERACT initiative. Osteoarthritis Cartilage 16, 415–422. doi: 10.1016/j.joca.2007.12.017
Henderson, J. V., Harrison, C. M., Britt, H. C., Bayram, C. F., and Miller, G. C. (2013). Prevalence, causes, severity, impact, and management of chronic pain in Australian general practice patients. Pain. Med. 14, 13461361. doi: 10.1111/pme.12195
Hoogeboom, T. J., den Broeder, A. A., Swierstra, B. A., de Bie, R. A., and van den Ende, C. H. (2012). Joint-pain comorbidity, health status, and medication use in hip and knee osteoarthritis: a cross-sectional study. Arthritis Care Res. (Hoboken) 64, 54–58. doi: 10.1002/acr.20647
Johnson, B. T. (1995). DSTAT: Software for the Meta-Analytic Review of Research Literature (Version 1.11). Hillsdale, NJ: Erlbaum.
Jungquist, C. R., O’Brien, C., Matteson-Rusby, S., Smith, M. T., Pigeon, W. R., Xia, Y., et al. (2010). The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 11, 302–309. doi: 10.1016/j.sleep.2009.05.018
Kim, H. S., Schwartz-Barcott, D., Holter, I. M., and Lorensen, M. (1995). Developing a translation of the McGill pain questionnaire for cross-cultural comparison: an example from Norway. J. Adv. Nurs. 21, 421–426. doi: 10.1111/j.1365-2648.1995.tb02722.x
Lautenbacher, S., Kundermann, B., and Krieg, J. C. (2006). Sleep deprivation and pain perception. Sleep Med. Rev. 10, 357–369. doi: 10.1016/j.smrv.2005.08.001
Lin, E. H., Katon, W., Von Korff, M., Tang, L., Williams, JW Jr, Kroenke, K., et al. (2003). Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA 290, 2428–2429. doi: 10.1001/jama.290.18.2428290/18/2428
Melzack, R. (1975). The McGill pain questionnaire: major properties and scoring methods. Pain 1, 277–299. doi: 10.1016/0304-3959(75)90044-5
Michela, J., and Wood, J. (1986). “Causal attributions in health and illness,” in Advances in Cognitive-Behavioral Research and Therapy, ed. P. C. Kendal (New York, NY: Academic Press), 179–235.
Montin, L., Leino-Kilpi, H., Katajisto, J., Lepisto, J., Kettunen, J., and Suominen, T. (2007). Anxiety and health-related quality of life of patients undergoing total hip arthroplasty for osteoarthritis. Chronic Illn. 3, 219–227. doi: 10.1177/1742395307084405
Morin, C. M., Gibson, D., and Wade, J. (1998). Self-reported sleep and mood disturbance in chronic pain patients. Clin. J. Pain 14, 311–314. doi: 10.1097/00002508-199812000-00007
Nilsdotter, A. K., Lohmander, L. S., Klassbo, M., and Roos, E. M. (2003). Hip disability and osteoarthritis outcome score (HOOS)–validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 4:10. doi: 10.1186/1471-2474-4-10
O’Brien, E. M., Waxenberg, L. B., Atchison, J. W., Gremillion, H. A., Staud, R. M., McCrae, C. S., et al. (2010). Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin. J. Pain 26, 310–319. doi: 10.1097/AJP.0b013e3181c328e9
O’Brien, E. M., Waxenberg, L. B., Atchison, J. W., Gremillion, H. A., Staud, R. M., McCrae, C. S., et al. (2011). Intraindividual variability in daily sleep and pain ratings among chronic pain patients: bidirectional association and the role of negative mood. Clin. J. Pain 27, 425–433. doi: 10.1097/AJP.0b013e318208c8e4
Ong, A. D., Zautra, A. J., and Reid, M. C. (2010). Psychological resilience predicts decreases in pain catastrophizing through positive emotions. Psychol. Aging 25, 516–523. doi: 10.1037/a0019384
Ozminkowski, R. J., Wang, S., and Walsh, J. K. (2007). The direct and indirect costs of untreated insomnia in adults in the United States. Sleep 30, 263–273.
Pallesen, S., Bjorvatn, B., Nordhus, I. H., Sivertsen, B., Hjornevik, M., and Morin, C. M. (2008). A new scale for measuring insomnia: the Bergen Insomnia Scale. Percept. Mot. Skills 107, 691–706. doi: 10.2466/PMS.107.7.691-706
Petrie, K. J., Jago, L. A., and Devcich, D. A. (2007). The role of illness perceptions in patients with medical conditions. Curr. Opin. Psychiatry 20, 163–167. doi: 10.1097/YCO.0b013e328014a871
Ramlee, F., Afolalu, E., and Tang, N. (2016). Do people with chronic pain judge their sleep differently? A qualitative study. Behav. Sleep Med. 1–16. doi: 10.1080/15402002.2016.1188393 [Epub ahead of print].
Roehrs, T., Hyde, M., Blaisdell, B., Greenwald, M., and Roth, T. (2006). Sleep loss and REM sleep loss are hyperalgesic. Sleep 29, 145–151.
Roesch, S. C., and Weiner, B. (2001). A meta-analytic review of coping with illness: do causal attributions matter? J. Psychosom. Res. 50, 205–219. doi: 10.1016/S0022-3999(01)00188-X
Sivertsen, B., Lallukka, T., Petrie, K. J., Steingrimsdottir, O. A., Stubhaug, A., and Nielsen, C. S. (2015). Sleep and pain sensitivity in adults. Pain 156, 1433–1439. doi: 10.1097/j.pain.0000000000000131
Smith, M. T., Edwards, R. R., McCann, U. D., and Haythornthwaite, J. A. (2007). The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep 30, 494–505.
Smith, M. T., Perlis, M. L., Smith, M. S., Giles, D. E., and Carmody, T. P. (2000). Sleep quality and presleep arousal in chronic pain. J. Behav. Med. 23, 1–13. doi: 10.1023/A:1005444719169
Smith, M. T., Quartana, P. J., Okonkwo, R. M., and Nasir, A. (2009). Mechanisms by which sleep disturbance contributes to osteoarthritis pain: a conceptual model. Curr. Pain Headache Rep. 13, 447–454. doi: 10.1007/s11916-009-0073-2
Tang, N. K., Goodchild, C. E., Hester, J., and Salkovskis, P. M. (2012a). Pain-related insomnia versus primary insomnia: a comparison study of sleep pattern, psychological characteristics, and cognitive-behavioral processes. Clin. J. Pain 28, 428–436. doi: 10.1097/AJP.0b013e31823711bc
Tang, N. K., Goodchild, C. E., and Salkovskis, P. M. (2012b). Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: a pilot randomised controlled trial. Behav. Res. Ther. 50, 814–821. doi: 10.1016/j.brat.2012.08.006
Tang, N. K., Goodchild, C. E., Sanborn, A. N., Howard, J., and Salkovskis, P. M. (2012c). Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep 35, 675A–687A. doi: 10.5665/sleep.1830
Tang, N. K., Salkovskis, P. M., Hodges, A., Soong, E., Hanna, M. H., and Hester, J. (2009). Chronic pain syndrome associated with health anxiety: a qualitative thematic comparison between pain patients with high and low health anxiety. Br. J. Clin. Psychol. 48(Pt 1), 1–20. doi: 10.1348/014466508X336167
Theadom, A., and Cropley, M. (2010). ‘This constant being woken up is the worst thing’–experiences of sleep in fibromyalgia syndrome. Disabil. Rehabil. 32, 1939–1947. doi: 10.3109/09638281003797331
Thomazeau, J., Perin, J., Nizard, R., Bouhassira, D., Collin, E., Nguyen, E., et al. (2014). Pain management and pain characteristics in obese and normal weight patients before joint replacement. J. Eval. Clin. Pract. 20, 611–616. doi: 10.1111/jep.12176
Tracey, I. (2010). Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med. 16, 1277–1283. doi: 10.1038/nm.2229
Turk, D. C., and Cohen, M. J. (2010). Sleep as a marker in the effective management of chronic osteoarthritis pain with opioid analgesics. Semin. Arthritis Rheum. 39, 477–490. doi: 10.1016/j.semarthrit.2008.10.006
Turk, D. C., Dworkin, R. H., Revicki, D., Harding, G., Burke, L. B., Cella, D., et al. (2008). Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 137, 276–285. doi: 10.1016/j.pain.2007.09.002
Vitiello, M. V., Rybarczyk, B., Von Korff, M., and Stepanski, E. J. (2009). Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J. Clin Sleep Med 5, 355–362.
Weiner, B. (1985). An attributional theory of achievement motivation and emotion. Psychol. Rev. 92, 548–573. doi: 10.1037/0033-295X.92.4.548
Wylde, V., Hewlett, S., Learmonth, I. D., and Dieppe, P. (2011). Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain 152, 566–572. doi: 10.1016/j.pain.2010.11.023
Zautra, A., Smith, B., Affleck, G., and Tennen, H. (2001). Examinations of chronic pain and affect relationships: applications of a dynamic model of affect. J. Consult. Clin. Psychol. 69, 786–795. doi: 10.1037/0022-006X.69.5.786
Zautra, A. J., Johnson, L. M., and Davis, M. C. (2005). Positive affect as a source of resilience for women in chronic pain. J. Consult. Clin. Psychol. 73, 212–220. doi: 10.1037/0022-006X.73.2.212
Keywords: chronic pain, sleep, mood, attribution, reciprocal relationships between symptoms
Citation: Blågestad T, Pallesen S, Grønli J, Tang NKY and Nordhus IH (2016) How Perceived Pain Influence Sleep and Mood More Than The Reverse: A Novel, Exploratory Study with Patients Awaiting Total Hip Arthroplasty. Front. Psychol. 7:1689. doi: 10.3389/fpsyg.2016.01689
Received: 04 July 2016; Accepted: 13 October 2016;
Published: 28 October 2016.
Edited by:
Gianluca Castelnuovo, Catholic University of the Sacred Heart, ItalyReviewed by:
Michelle Dow Keawphalouk, Harvard University and Massachusetts Institute of Technology, USACopyright © 2016 Blågestad, Pallesen, Grønli, Tang and Nordhus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tone Blågestad, dG9uZS5ibGFnZXN0YWRAdWliLm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.