
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Psychol., 23 February 2016
Sec. Perception Science
Volume 7 - 2016 | https://doi.org/10.3389/fpsyg.2016.00224
This article is part of the Research TopicUnderstanding the role of the time dimension in the brain information processingView all 13 articles
Neural oscillations are ubiquitous in the mammalian brain and they are typically classified according to their specific frequency responses (Buzsáki, 2006). Neural oscillations are hypothesized to organize communication within and between brain networks (e.g., Fries, 2015). Neural oscillations have increasingly been associated with various cognitive functions such as attention (Klimesch, 2012), working memory (Gulbinaite et al., 2014a; Haegens et al., 2014), and cognitive control (Cavanagh et al., 2009; Gulbinaite et al., 2014b) but also temporal expectation (Praamstra et al., 2006; Cravo et al., 2011; Rohenkohl and Nobre, 2011) and timing (Treisman, 1963; van Wassenhove, 2009; Kösem et al., 2014; Kononowicz and van Rijn, 2014). One quest in cognitive neuroscience is to explain how neural oscillations can subserve complex cognitive processes. Here, we mainly focus on the role of spontaneous rhythms in interval timing (also see van Wassenhove, in press); however, some hypotheses are supported by the literature on rhythmic entrainment.
One of the possible cognitive abilities neural oscillations may support is interval timing (Treisman et al., 1994), which is the ability to perceive, store, encode, and reproduce temporal intervals ranging from few 100 milliseconds to minutes. Over decades, experimental psychologists have proposed the existence of a cognitive mechanism akin to an internal clock. In search for the neural bases of the internal clock(s), it may be tempting to draw an analogy between ticking clocks and oscillating neuronal networks, as one of the reference papers in neurosciences states, “Clocks tick, bridges, and skyscrapers vibrate, neuronal networks oscillate” (Buzsáki and Draguhn, 2004, pp. 1926). Indeed, some interval timing theories have followed through this idea: Treisman (1963) suggested, that the internal clock could consist of a pacemaker which at the beginning of the to-be-timed interval would start sending pulses, that are then stored in the accumulator. The pulse count could serve as a subjective estimate of time. Furthermore, to implement this model into biologically plausible mechanisms, Treisman proposed that the pulse rate of the pacemaker would be driven by neural oscillations in the alpha range (8–12 Hz, see Figure 1A): faster alpha rhythms would thus result in longer estimates of time than slower alpha rhythms considering, that more pulses would accumulate during the same physical time interval (Treisman et al., 1994). As no simple relationships have been found between the rate of visual flicker, neural oscillations, and the subjective perception of duration when using oscillatory entrainment (Herbst et al., 2013, 2014; although see Johnston et al., 2006), it remains plausible that spontaneous fluctuations of alpha peaks could modulate perceived duration. For example, Haegens et al. (2014) have shown, that alpha peak frequency changed as a function of cognitive load in a N-back working memory (WM) task such that the larger the WM load, the higher the alpha peak frequency. These results indicate that subjectively longer durations could be associated with larger alpha peak if WM is implicated in the estimation of duration (Gu et al., 2015; van Wassenhove, in press).
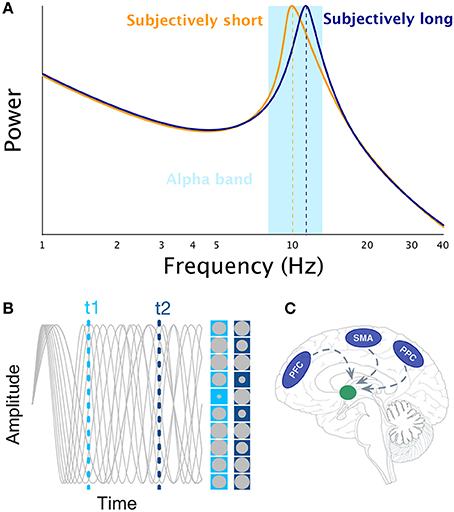
Figure 1. Illustration of the main interval timing theories of interval timing that rely on the notion of neural oscillations. Panel (A) illustrates the idea that faster alpha rhythms results in longer estimates of time as more pulses could be accumulated in a given physical time interval (Treisman, 1963). Panel (B) illustrates the SBF model. The gray sinusoids depict oscillators in an example trial. The amplitude of each oscillator is represented by the size of gray circle at t1 and t2 times, respectively. Panel (C) illustrates the main brain regions engaged in interval timing (PFC, SMA, PPC) and their presumed projections to the striatum as suggested by the SBF model.
Despite mechanistic attempts to link oscillatory processes with internal clock models, direct implementations of internal clock models still lack solid neural foundations whereas, more biologically grounded frameworks have been more plausible (Buhusi and Meck, 2005).
The most prominent neurobiologically plausible model of interval timing is the Striatal Beat Frequency (SBF) model (Matell and Meck, 2000, 2004; Buhusi and Meck, 2005) developed on the basis of the beat frequency model (Miall, 1989). One major assumption of SBF (Figure 1B) is the existence of cortical oscillators of various frequency responses most likely located in the Pre-Frontal Cortex (PFC) which is part of the mesocortical pathway. However, other cortical regions cannot be excluded [e.g., Supplementary Motor Area (SMA), Posterior Parietal Cortex (PPC), or sensory cortices, Figure 1C]. At the onset of an interval to be timed, the model posits, that cortical oscillators are phase-reset and, at the offset of the interval, the state of these cortical oscillators is read out by medium spiny neurons located in the striatum. Hence, the SBF model considers, that the phase of cortical oscillators gives rise to a unique activation pattern over time (Buhusi and Meck, 2005; Oprisan and Buhusi, 2011, 2014) and, that spiny neurons are coincidence detectors reading out the state of these cortical oscillators. Note that in SBF, the pattern of activation is identical whether one reads the phase or the amplitude of the oscillators. Although, cortical oscillators seem to be a key element of the SBF model, only little evidence currently supports the existence of a dedicated set of cortical oscillators for interval timing (e.g., Matell, 2014). It is also unclear whether cortical oscillators are really necessary for the SBF model, as any stable pattern of neural activation (Crowe et al., 2014; Merchant et al., 2014; Mello et al., 2015) within to-be-timed intervals but variable across to-be-timed intervals would be sufficient as input to insure reliable coincidence detection (also see Meck et al., 2013).
When considering oscillatory processes in the context of the SBF model, at least two important predictions regarding neural oscillators have to be taken into account. The first prediction is, that in order to provide a meaningful pattern, cortical oscillators have to be phase-reset, such that they always start from the same fixed state. For example, the results by Parker et al. (2014) suggest, that more precise phase reset of ongoing theta oscillations in the medial frontal cortex results in better timing accuracy (Kononowicz, 2015), something that would be in line with the SBF model. This hypothesis awaits future tests and more compelling evidence have to be provided.
The second prediction is linked to the idea, that the speed of internal clock can be modulated by the speed of cortical oscillators (Oprisan and Buhusi, 2014), which are modulated by tonic levels of dopamine (Oprisan and Buhusi, 2011). It is very often assumed, that the clock speed could be represented by the alpha band regime (Treisman et al., 1990, 1994) as it is the most prevalent spontaneous rhythm in the mammalian brain (Oprisan and Buhusi, 2014). However, as previously discussed, the relationship between alpha peak power and fluctuations in subjective timing has not been clearly established; direct attempts to test this hypothesis have not succeeded (Treisman et al., 1990, 1994). The power of alpha is a good marker of temporal expectation (Praamstra et al., 2006; Cravo et al., 2011; Rohenkohl and Nobre, 2011), which is in line with the hypothesized role of alpha as a selective coordinator implicated in the temporal prioritization of sensory events (Jensen et al., 2014). Hence, one possible departure from the early proposals could be that a single dominating frequency may not be necessary to represent the clock speed as neural oscillations outside of the alpha range have been implicated in interval timing (Busch et al., 2004; Kaiser et al., 2007; Sperduti et al., 2011), raising the possibility that other rhythms could serve as “pacemakers.” For instance, recent studies suggest a signifant role of beta oscillations in timing (Iversen et al., 2009; Fujioka et al., 2012, 2015; Bartolo et al., 2014; Teki, 2014; Kononowicz and van Rijn, 2014; Wiener and Kanai, 2016) and the phase characteristics of low-frequency oscillators can predict subjective timing (Cravo et al., 2011; Kösem et al., 2014), suggesting, that different neural oscillations have the potentiality to track time. Therefore, instead of focusing on one single neural oscillation, future studies should explore local trial-to-trial fluctuations across frequency bands and how subdominant frequencies vary as a function of subjectively perceived time intervals. Complementary to this, addressing the implications of such markers at different time scales and across sensory modalities may be desirable.
Interestingly, a recent review by Gu et al. (2015) proposes to unify interval timing and working memory models. Specifically, these authors proposed, that working memory and interval timing can originate from the same oscillatory processes such as gamma and theta oscillations, and phase-amplitude coupling between these frequency bands (Lisman, 2010). The proposed model largely focuses on oscillatory processes that could be shared between working memory and SBF. Nonetheless, the empirical ways to assess the principles of SBF model are still lacking. As the gist of the SBF lies in the notion of communication between cortical areas and the striatum, here we discuss the possibility of testing this hypothesis by investigating functional connectivity between the striatum and PFC.
Striatal neurons are ideal candidates for coincidence detection as they receive direct inputs from cortical neurons. Through coincidence detection of spiking activity from two or more cortical regions, the same striatal neuron will discharge within a given time window. For instance, Matell et al. (2003) showed, that neural activity in the striatum and the anterior cingulate cortex varied before 10 and 40 s when the reinforcement was presented at one of these two time points, suggesting, that neuronal populations respond to to particular time intervals. However, this pattern although predicted by SBF could largely be confounded by motor activity of lever pressing. Nevertheless, note, that Riehle et al. (1997) observed transient synchronization of neurons in motor cortex when stimuli were expected, but failed to appear. Although, this work is very important it only shows pattern of activity that fits into the SBF model under certain conditions. Given, that an important premise of the SBF model is the communication between striatal neuronal ensembles and cortical neurons, we propose, that investigating functional connectivity between subcortical and cortical structures can serve as an important step extending the results of Matell et al. (2003) and giving further support to the SBF model. For example, Antzoulatos and Miller (2014) found, that perceptual (non-temporal) category learning was accompanied by increased synchronization within beta band range (12–30 Hz) between the PFC and striatum, demonstrating the role of functional connectivity in learning. Specifically, synchronization was larger for correct trials. On the basis of the SBF model, a change of cortical-striatal synaptic weights through learning is predicted to reflect a memory mechanism such as the one implemented in the Scalar Expectancy Theory (Gibbon, 1977). Taken together, striatal neurons are predicted to become more sensitive to firing as a function of specific PFC neurons, and these learning effects should be visible during training of temporal discrimination as a change in inter-areal synchronization. Moreover, according to the SBF model and in line with the results of Antzoulatos and Miller (2014), inter-areal synchronization should be enhanced in “correct” trials (Kononowicz, 2015). Particularly, the striatal-PFC synchrony enhancement should emerge at the time of a standard interval, for example in the task where subjects compare a comparison interval, that could vary in length to a fixed standard interval. That is because striatal and PFC structures should become transiently synchronous due to previous learning enhancing sensitivity/tuning of striatum to the particular neural pattern exhibited at the time of standard interval.
The synchronization of neural oscillations has been associated with neuronal mechanisms such as coincidence detection, neural plasticity though long term potentiation/depression mechanism, and neuronal communication (Fell and Axmacher, 2011). These processes seem like a plausible candidate to coordinate striatum-PFC communication in recognition for specific patterns considered by SBF model. Specifically, the simplest scenario would predict an increase in coherence or spike-filed coherence for accurately timed trials. Coherence was proposed to reflect facilitated communication between brain regions (e.g., Fries, 2015). Effective communication should be linked to the successful timing performance if indeed communication between the striatum and PFC is a key component of timing system as proposed in the SBF model. This cortico-striatal spike-field coherence should be specifically enhanced at the time of standard interval if striatal neurons recognize cortical pattern (see Kononowicz and van Rijn, 2015). Furthermore, the role of cortico-cortical coherence has been shown in passive rhythmical stimulation paradigms, in which an increase in coherence coincided with the next tone occurrence (Fujioka et al., 2012). These results do support the hypothesis sketched in this paper and also suggest cortico-cortical analysis. Moreover, recent progress in neuroscientific methods allows to adress this questions in animals and humans using MEG/EEG modeling (David et al., 2011), but also deep brain recordings.
All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work has been supported by ERC-YSt-263584 to VW.
Antzoulatos, E. G., and Miller, E. K. (2014). Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron 83, 216–225.
Bartolo, R., Prado, L., and Merchant, H. (2014). Information processing in the primate basal ganglia during sensory-guided and internally driven rhythmic tapping. J. Neurosci. 34, 3910–3923. doi: 10.1523/JNEUROSCI.2679-13.2014
Buhusi, C. V., and Meck, W. H. (2005). What makes us tick? Functional and neural mechanisms of interval timing. Nat. Rev. Neurosci. 6, 755–765. doi: 10.1038/nrn1764
Busch, N. A., Debener, S., Kranczioch, C., Engel, A. K., and Herrmann, C. S. (2004). Size matters: effects of stimulus size, duration and eccentricity on the visual gamma-band response. Clin. Neurophysiol. 115, 1810–1820. doi: 10.1016/j.clinph.2004.03.015
Buzsáki, G., and Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929. doi: 10.1126/science.1099745
Cavanagh, J. F., Cohen, M. X., and Allen, J. J. (2009). Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 29, 98–105. doi: 10.1523/JNEUROSCI.4137-08.2009
Cravo, A. M., Rohenkohl, G., Wyart, V., and Nobre, A. C. (2011). Endogenous modulation of low frequency oscillations by temporal expectations. J. Neurophysiol. 106, 2964–2972. doi: 10.1152/jn.00157.2011
Crowe, D. A., Zarco, W., Bartolo, R., and Merchant, H. (2014). Dynamic representation of the temporal and sequential structure of rhythmic movements in the primate medial premotor cortex. J. Neurosci. 34, 11972–11983. doi: 10.1523/JNEUROSCI.2177-14.2014
David, O., Maess, B., Eckstein, K., and Friederici, A. D. (2011). Dynamic causal modeling of subcortical connectivity of language. J. Neurosci. 31, 2712–2717. doi: 10.1523/JNEUROSCI.3433-10.2011
Fell, J., and Axmacher, N. (2011). The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118. doi: 10.1038/nrn2979
Fries, P. (2015). Rhythms for cognition: communication through coherence. Neuron 88, 220–235. doi: 10.1016/j.neuron.2015.09.034
Fujioka, T., Ross, B., and Trainor, L. J. (2015). Beta-band oscillations represent auditory beat and its metrical hierarchy in perception and imagery. J. Neurosci. 35, 15187–15198. doi: 10.1523/JNEUROSCI.2397-15.2015
Fujioka, T., Trainer, L. J., Large, E. W., and Ross, B. (2012). Internalized timing of isochronous sounds is represented in neuromagnetic beta oscillations. J. Neurosci. 32, 1791–1802. doi: 10.1523/JNEUROSCI.4107-11.2012
Gibbon, J. (1977). Scalar expectancy theory and Weber's law in animal timing. Psychol. Rev. 84:279. doi: 10.1037/0033-295X.84.3.279
Gu, B. M., van Rijn, H., and Meck, W. H. (2015). Oscillatory multiplexing of neural population codes for interval timing and working memory. Neurosci. Biobehav. Rev. 48, 160–185. doi: 10.1016/j.neubiorev.2014.10.008
Gulbinaite, R., Johnson, A., de Jong, R., Morey, C. C., and van Rijn, H. (2014a). Dissociable mechanisms underlying individual differences in visual working memory capacity. Neuroimage 99, 197–206. doi: 10.1016/j.neuroimage.2014.05.060
Gulbinaite, R., van Rijn, H., and Cohen, M. X. (2014b). Fronto-parietal network oscillations reveal relationship between working memory capacity and cognitive control. Front. Hum. Neurosci. 8:761. doi: 10.3389/fnhum.2014.00761
Haegens, S., Cousijn, H., Wallis, G., Harrison, P. J., and Nobre, A. C. (2014). Inter-and intra-individual variability in alpha peak frequency. Neuroimage 92, 46–55. doi: 10.1016/j.neuroimage.2014.01.049
Herbst, S. K., Chaumon, M., Penney, T. B., and Busch, N. A. (2014). Flicker-induced time dilation does not modulate EEG correlates of temporal encoding. Brain Topogr. 1–11. doi: 10.1007/s10548-014-0389-z
Herbst, S. K., Javadi, A. H., van der Meer, E., and Busch, N. A. (2013). How long depends on how fast—perceived flicker dilates subjective duration. PLoS ONE 8:e76074. doi: 10.1371/journal.pone.0076074
Iversen, J. R., Repp, B. H., and Patel, A. D. (2009). Top-down control of rhythm perception modulates early auditory responses. Ann. N.Y. Acad. Sci. 1169, 58–73. doi: 10.1111/j.1749-6632.2009.04579.x
Jensen, O., Gips, B., Bergmann, T. O., and Bonnefond, M. (2014). Temporal coding organized by coupled alpha and gamma oscillations prioritize visual processing. Trends Neurosci. 37, 357–369. doi: 10.1016/j.tins.2014.04.001
Johnston, A., Arnold, D. H., and Nishida, S. (2006). Spatially localized distortions of event time. Curr. Biol. 16, 472–479. doi: 10.1016/j.cub.2006.01.032
Kaiser, J., Leiberg, S., Rust, H., and Lutzenberger, W. (2007). Prefrontal gamma-band activity distinguishes between sound durations. Brain Res. 1139, 153–162. doi: 10.1016/j.brainres.2006.12.085
Klimesch, W. (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617. doi: 10.1016/j.tics.2012.10.007
Kononowicz, T. W. (2015). Dopamine-dependent oscillations in frontal cortex index ‘start-gun’ signal in interval timing. Front. Hum. Neurosci. 9, 331. doi: 10.3389/fnhum.2015.00331
Kononowicz, T. W., and van Rijn, H. (2014). Decoupling interval timing and climbing neural activity: a dissociation between CNV and N1P2 amplitudes. J. Neurosci. 34, 2931–2939. doi: 10.1523/JNEUROSCI.2523-13.2014
Kononowicz, T. W., and van Rijn, H. (2015). Single trial beta oscillations index time estimation. Neuropsychologia 75, 381–389. doi: 10.1016/j.neuropsychologia.2015.06.014
Kösem, A., Gramfort, A., and van Wassenhove, V. (2014). Encoding of event timing in the phase of neural oscillations. Neuroimage 92, 274–284. doi: 10.1016/j.neuroimage.2014.02.010
Lisman, J. (2010). Working memory: the importance of theta and gamma oscillations. Curr. Biol. 20, 490–492. doi: 10.1016/j.cub.2010.04.011
Matell, M. S. (2014). “Searching for the Holy Grail: temporally informative firing patterns in the Rat,” in Neurobiology of Interval Timing, ed H. Merchant (New York, NY: Springer), 209–234.
Matell, M. S., and Meck, W. H. (2000). Neuropsychological mechanisms of interval timing behavior. Bioessays 22, 94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E
Matell, M. S., and Meck, W. H. (2004). Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn. Brain Res. 21, 139–170. doi: 10.1016/j.cogbrainres.2004.06.012
Matell, M. S., Meck, W. H., and Nicolelis, M. A. (2003). Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav. Neurosci. 117, 760–773. doi: 10.1037/0735-7044.117.4.760
Meck, W. H., Church, R. M., and Matell, M. S. (2013). Hippocampus, time, and memory—A retrospective analysis. Behav. Neurosci. 127, 642–654. doi: 10.1037/a0034201
Mello, G. B., Soares, S., and Paton, J. J. (2015). A scalable population code for time in the striatum. Curr. Biol. 25, 1113–1122. doi: 10.1016/j.cub.2015.02.036
Merchant, H., Bartolo, R., Pérez, O., Méndez, J. C., Mendoza, G., Gámez, J., et al. (2014). “Neurophysiology of timing in the hundreds of milliseconds: multiple layers of neuronal clocks in the medial premotor areas,” in Neurobiology of Interval Timing, ed V. de Lafuente (New York, NY: Springer), 143–154.
Miall, C. (1989). The storage of time intervals using oscillating neurons. Neural Comput. 1, 359–371. doi: 10.1162/neco.1989.1.3.359
Oprisan, S. A., and Buhusi, C. V. (2011). Modeling pharmacological clock and memory patterns of interval timing in a striatal beat-frequency model with realistic, noisy neurons. Front. Integr. Neurosci. 5:52. doi: 10.3389/fnint.2011.00052
Oprisan, S. A., and Buhusi, C. V. (2014). What is all the noise about in interval timing? Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20120459. doi: 10.1098/rstb.2012.0459
Parker, K. L., Chen, K. H., Kingyon, J. R., Cavanagh, J. F., and Narayanan, N. S. (2014). D1-dependent 4 Hz oscillations and ramping activity in rodent medial frontal cortex during interval timing. J. Neurosci. 34, 16774–16783. doi: 10.1523/JNEUROSCI.2772-14.2014
Praamstra, P., Kourtis, D., Kwok, H. F., and Oostenveld, R. (2006). Neurophysiology of implicit timing in serial choice reaction-time performance. J. Neurosci. 26, 5448–5455. doi: 10.1523/JNEUROSCI.0440-06.2006
Riehle, A., Grün, S., Diesmann, M., and Aertsen, A. (1997). Spike synchronization and rate modulation differentially involved in motor cortical function. Science 278, 1950–1953. doi: 10.1126/science.278.5345.1950
Rohenkohl, G., and Nobre, A. C. (2011). Alpha oscillations related to anticipatory attention follow temporal expectations. J. Neurosci. 31, 14076–14084. doi: 10.1523/JNEUROSCI.3387-11.2011
Sperduti, M., Tallon-Baudry, C., Hugueville, L., and Pouthas, V. (2011). Time is more than a sensory feature: attending to duration triggers specific anticipatory activity. Cogn. Neurosci. 2, 11–18. doi: 10.1080/17588928.2010.513433
Teki, S. (2014). Beta drives brain beats. Front. Syst. Neurosci. 8:155. doi: 10.3389/fnsys.2014.00155
Treisman, M. (1963). Temporal discrimination and the indifference interval: implications for a model of the “internal clock.” Psychol. Monogr. Gen. Appl. 77, 1–31. doi: 10.1037/h0093864
Treisman, M., Cook, N., Naish, P. L., and MacCrone, J. K. (1994). The internal clock: electroencephalographic evidence for oscillatory processes underlying time perception. Q. J. Exp. Psychol. 47, 241–289. doi: 10.1080/14640749408401112
Treisman, M., Faulkner, A., Naish, P. L., and Brogan, D. (1990). The internal clock: evidence for a temporal oscillator underlying time perception with some estimates of its characteristic frequency. Perception 19, 705–743. doi: 10.1068/p190705
van Wassenhove, V. (2009). Minding time in an amodal representational space. Philos. Trans. R. Soc. B Biol. Sci. 364, 1815–1830. doi: 10.1098/rstb.2009.0023
Keywords: time perception, interval timing, internal clock, oscillations, striatal beta frequency
Citation: Kononowicz TW and van Wassenhove V (2016) In Search of Oscillatory Traces of the Internal Clock. Front. Psychol. 7:224. doi: 10.3389/fpsyg.2016.00224
Received: 08 November 2015; Accepted: 03 February 2016;
Published: 23 February 2016.
Edited by:
Hugo Merchant, Universidad Nacional Autónoma de México, MexicoReviewed by:
Peter Keller, University of Western Sydney, AustraliaCopyright © 2016 Kononowicz and van Wassenhove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadeusz W. Kononowicz, dC53Lmtvbm9ub3dpY3pAaWNsb3VkLmNvbQ==;
Virginie van Wassenhove, dmlyZ2luaWUudmFuLndhc3NlbmhvdmVAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.