
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychol., 06 January 2016
Sec. Cognition
Volume 6 - 2015 | https://doi.org/10.3389/fpsyg.2015.01986
This article is part of the Research TopicMechanisms of well-adjusted and disordered self-soothing: From Oxytocin and Thermo-Regulation to Addiction and Emotional CopingView all 13 articles
Inter-individual touch can be a desirable reward that can both relieve negative affect and evoke strong feelings of pleasure. However, if other sensory cues indicate it is undesirable to interact with the toucher, the affective experience of the same touch may be flipped to disgust. While a broad literature has addressed, on one hand the neurophysiological basis of ascending touch pathways, and on the other hand the central neurochemistry involved in touch behaviors, investigations of how external context and internal state shapes the hedonic value of touch have only recently emerged. Here, we review the psychological and neurobiological mechanisms responsible for the integration of tactile “bottom–up” stimuli and “top–down” information into affective touch experiences. We highlight the reciprocal influences between gentle touch and contextual information, and consider how, and at which levels of neural processing, top-down influences may modulate ascending touch signals. Finally, we discuss the central neurochemistry, specifically the μ-opioids and oxytocin systems, involved in affective touch processing, and how the functions of these neurotransmitters largely depend on the context and motivational state of the individual.
Inter-individual touch is frequently used to communicate positive messages, like reassurance, comfort, sympathy, and support (Hertenstein et al., 2006b). For the recipient, touch from another person can be soothing (Feldman et al., 2010b; Fairhurst et al., 2014), give rise to pleasurable feelings (Löken et al., 2009; Morrison et al., 2010), and potentially suppress pain and negative emotion (Coan et al., 2006; Liljencrantz et al., 2012; Mancini et al., 2014, 2015). On the other hand the hedonic experience of touch can be flipped from pleasure to displeasure if the perceived intentions or the identity of the toucher does not match the preferences of the recipient of touch (Gazzola et al., 2012).
The hedonic value of touch, the pleasantness or unpleasantness, is intrinsically related to the physical characteristics of tactile stimuli, like softness (Rolls et al., 2003), temperature (Schepers and Ringkamp, 2009; Ackerley et al., 2014), force and velocity (Löken et al., 2009). However, as in other sensory modalities, the signals from the peripheral receptors are processed and modulated by several “top–down” mechanisms before the subjective experience of touch arises in the brain (Kveraga et al., 2007; Ellingsen, 2014). First, sensory information enters subjective awareness through the gate of attention (Johansen-Berg et al., 2000) – presuming you are sitting down right now, you might not be aware of the physical pressure of the chair pressing against your skin until this very moment when your attention is directed toward this stimulus (Schubert et al., 2008). Second, when sensory signals do gain access to awareness, the resulting sensation is influenced by the brain’s pre-existing models, or predictions, of what these sensory signals mean, which are shaped by learning (Knill and Richard, 1996; Kersten et al., 2004; Schmack et al., 2013). Third, other available cues carrying information about the importance, relevance and affective valence of this sensation, weigh in. For a given affective touch stimulus, contextual information such as visual or auditory cues about the toucher (Macaluso and Driver, 2001; Taylor-Clarke et al., 2002), and internal motivational state or mood (Kalaska, 1994; Montoya and Sitges, 2006; Triscoli et al., 2014; Løseth et al., in press), is essential for deciding how important this particular touch is (how much attention should be paid to it), how desirable it is (positive or negative), and how to respond behaviorally.
Most of the research on affiliative touch has been done from the vantage point of the touch stimulus itself, e.g. the neurobiology of mechanoreceptive skin receptors and the ascending touch pathways (Vallbo et al., 1993; Wessberg et al., 2003; Vrontou et al., 2013), observational studies of animals’ engagement in specific touch behaviors (Harlow and Zimmermann, 1959; Dunbar, 1991; Alberts, 2007), or human psychophysical (Loken et al., 2011; Ackerley et al., 2014; Fairhurst et al., 2014) and neuroimaging (Rolls et al., 2003; Olausson et al., 2008; McGlone et al., 2012; Bjornsdotter et al., 2014; Kaiser et al., 2015) studies assessing the sensations and brain activity in respect to different touch stimuli. Much less is known about the neurobiological processes whereby top-down factors – cross-sensory, cognitive, and affective information – shape touch signals.
Here, we review the neural circuitry and neurochemistry underpinning top-down modulation of affective touch, and suggest how the brain integrates sensory and prior information into affective touch sensations. First, we discuss how context modulates the meaning and in turn the hedonic value of touch, and how this shapes both the affective experience and the behavioral consequences. We will then review the central neurochemistry, primarily μ-opioids and oxytocin, underpinning the seemingly opposite stimulatory and soothing effects of touch, and propose how these outcomes are highly dependent on the individual’s affective-motivational state.
Much of human behavior is geared toward seeking pleasant experiences, while avoiding unnecessary painful, or aversive experiences. Hedonic valuation of sensation guides decisions about which behaviors to engage in and which to avoid, rendering hedonic processing essential to survival (For review, see Berridge and Kringelbach, 2015). In order to be useful, however, these systems need to take into account the individual’s short-term and long-term needs. While high-calorie food is usually thought of as a desirable reward, it loses its utility and ceases to be pleasurable upon satiety (Small et al., 2001). Similarly, the utility and consequently the hedonic experience of interpersonal touch largely depends on the context and internal needs and motivational goals.
When being touched by another individual, inferences about the identity, physical characteristics, and the intentions of the toucher, conveyed through visual and auditory stimuli, gives useful information about the importance of the touch and how preferable it is (Suvilehto et al., 2015). This can dramatically shape both the hedonic experience and the behavioral response (e.g., approach or withdraw).
In two similar experimental studies of interpersonal touch, the recipient’s beliefs about the toucher affected the pleasantness of gentle sensual caresses (Figure 1; Gazzola et al., 2012; Scheele et al., 2014). The study participants, who were all heterosexual men, rated experimentally applied sensual caresses as pleasant when they were lead to believe, via a visual cue, that they were being caressed by a female experimenter, but unpleasant when the cue indicated a male experimenter. In reality the same female experimenter, who was blinded to the cues, did all the caresses. For the study participants, the visual information about the sex and appearance of the believed toucher changed the meaning and desirability of the touch, which in turn impacted the hedonic touch experience – touch by an attractive female felt better.
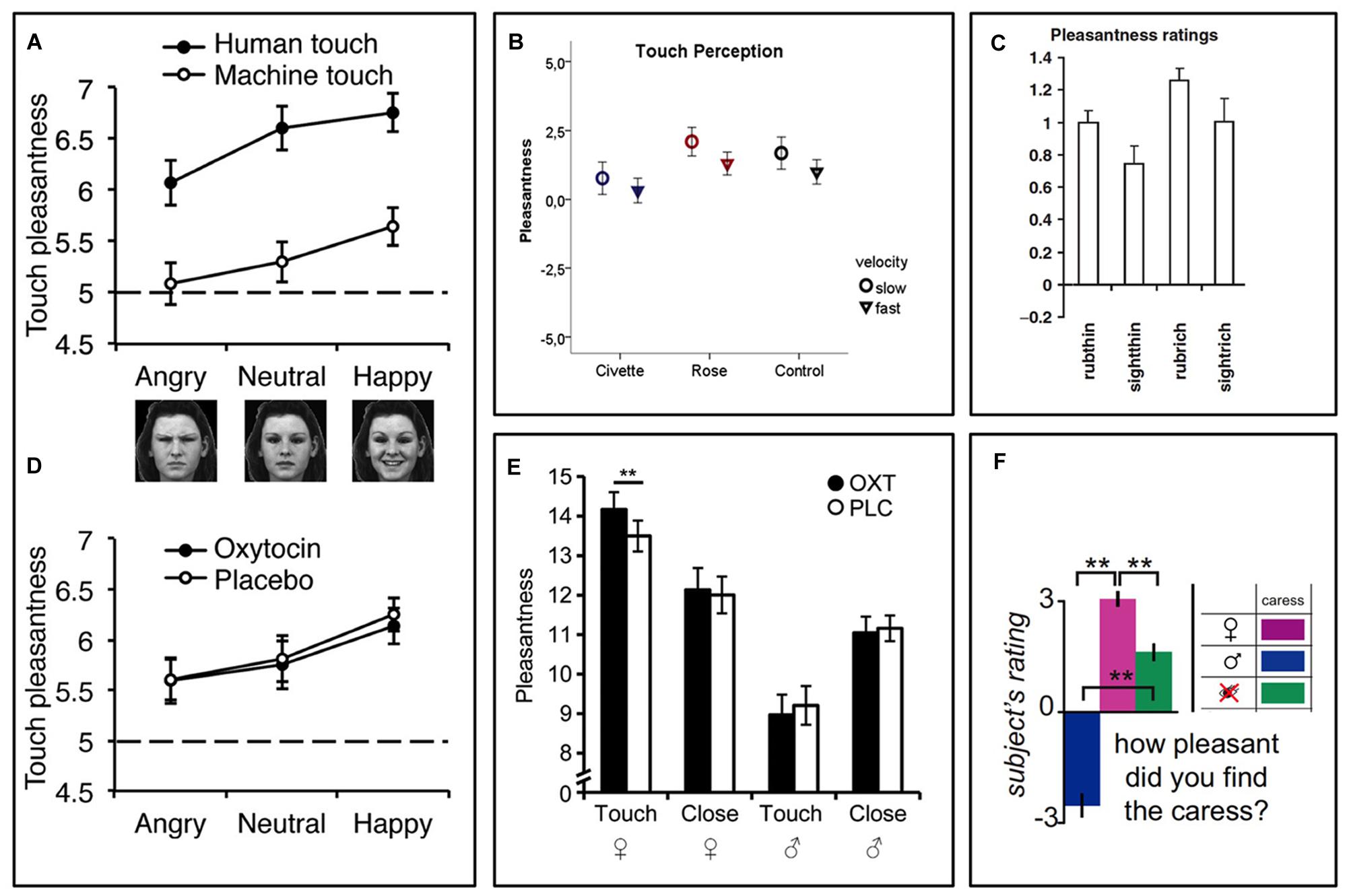
FIGURE 1. Contextual modulation of touch pleasantness during identical tactile stimuli. (A) Touch pleasantness of both gentle stroking (human) touch and equally intense vibratory (machine) touch is highest during concomitant presentation of smiling faces and lowest during presentation of frowning faces (Ellingsen et al., 2014). (B) In a similar fashion, touch pleasantness is highest during concomitant presentation of pleasant (rose) odors and lowest during presentation of disgusting (civette) odors (Croy et al., 2014). (C) People find the gently rubbing of a skin cream more pleasant when being told it is a “rich moisturizing cream” (rubrich) relative to a “basic cream” (rubthin) (McCabe et al., 2008). (D) While one study found no effect of intranasal oxytocin on touch pleasantness (Ellingsen et al., 2014), another study (E) found that oxytocin increased touch pleasantness in heterosexual men when they believed they were being sensually caressed by a woman, but not when they believed the caresser was a man (Scheele et al., 2014). (E–F) Caresses are more pleasant when the caresser is believed to be a woman relative to a man (Gazzola et al., 2012; Scheele et al., 2014). Figure adapted from (McCabe et al., 2008; Gazzola et al., 2012; Ellingsen et al., 2014; Croy et al., 2014; Scheele et al., 2014). ∗∗p < 0.01.
Using a different design, we recently showed that the visual presentation of faces with emotional expressions affected the pleasantness of concomitant touch stimuli. Study participants rated touch as most pleasant when combined with a photograph of a smiling face and least pleasant when combined with a frowning face (Figure 1A, Ellingsen et al., 2014). Interestingly, this effect was seen even though participants were fully aware that the person they saw in front of them was not the person touching them. This suggests that affective cross-sensory stimuli that are time-locked with the touch, but seemingly non-informative (i.e., does not provide any specific information about the value of the touch stimulus), can still influence the hedonic impact of this touch. Along the same lines, a recent experiment showed that disgusting odors presented simultaneously with a gentle stroking touch reduced the pleasantness of this touch (Figure 1B). Again, the participants were fully aware that the stimuli originated from independent sources (Croy et al., 2014). Instead of carrying information about the value of the touch itself, these effects may appear as a result of a shift in affective or motivational state, which in turn change the hedonic impact of touch, perhaps similar to affective priming (Winkielman et al., 2005; Schwarz, 2012). This bears similarity to the tendency of unpleasant and pleasant sensory events to exert immediate reciprocal inhibitory effects. For example, pleasant images, odors, music, and food can reduce pain (For review, see Leknes and Tracey, 2008). On the other hand, pain and negative affect can reduce the capacity for pleasure, as demonstrated by the strong comorbidity between chronic pain, depression, and anhedonia (i.e., a lack of capacity for pleasure; Pizzagalli et al., 2008; Elvemo et al., 2015; Romer Thomsen et al., 2015). However, there are also cases where pain can enhance the pleasure of reward (Carstens et al., 2002; Rozin et al., 2013; Bastian et al., 2014; Leknes and Bastian, 2014), highlighting that the relationship between pleasure and displeasure is not one of a simple, universal mutual inhibition, but rather involves a complex integrative process weighing the importance of contextual cues.
A widespread notion is that sensations in general are shaped by inferences about the relative importance, or utility, of sensory signals (Cabanac, 1971; Tindell et al., 2006) – how useful or relevant these are in relation to the organisms’ goals, which are often ultimately related to survival, well-being, and procreation. The motivation-decision model of pain was put forward to explain the often-dramatic variability in pain experience due to the individual’s internal motivational state (Fields, 2006, 2007). This framework describes brain mechanisms that reduce or increase the hedonic impact of nociceptive events based on their relative importance at the given time. The model was initially developed to explain modulation of pain, but the basic idea is applicable to affective touch, as well as other sensory events that fall within a reward-punishment continuum. The model postulates that, as a result of an unconscious decision-making process, any concurrent or impending event deemed more important to the individual than a pain stimulus should suppress the hedonic impact of this pain. The event of superior importance may be a greater threat or a potential reward. Likewise, anything judged as more important than an impending reward – for example a threat or a bigger reward – should suppress the hedonic impact and motivation for this reward (Fields, 2011).
Like pain, touch stimuli usually happen in a multisensory context. As a facet of this, the occurrence of touch can affect the experience of non-touch stimuli – just as other sensory stimuli co-occurring with touch can affect touch experience (Taylor-Clarke et al., 2002; Calvert and Thesen, 2004).
Being touched by another human being can evoke powerful emotions. People are remarkably accurate in detecting a wide range of emotional messages, even when these are communicated exclusively through touch (Hertenstein et al., 2006a). A series of observational studies has showed that brief, casual touch from strangers can have positive behavioral effects in people, and even make them more generous. Restaurant diners tip more if the waitress casually touches them when returning their change (Crusco and Wetzel, 1984), and people are more satisfied with a library visit if the librarian casually touches their hand (Fisher et al., 1976). Similar studies report that when casually touched, people are more likely to return money left in a public phone (Kleinke, 1977), spend money in a supermarket (Hornik, 1992), rate salespeople at car showrooms more positively (Erceau and Guguen, 2007), or give away cigarettes (Joule and Gueguen, 2007). There are also studies suggesting positive health effects of touch in therapeutic relationships (Whitcher and Fisher, 1979; Eaton et al., 1986; Monroe, 2009), and within romantic couples (Grewen et al., 2003; Ditzen et al., 2007).
In most such studies, however, touch formed part of an affectively congruent situation. Less is known about the effects of, and appraisal of, touch in contexts where other available information is affectively incongruent, such as being casually touched by someone expressing anger. On the interplay between touch and concomitant non-touch signals, touch has been proposed to intensify the emotional display of other senses (Knapp and Hall, 1997; Hertenstein et al., 2006a). Touch ultimately means that someone – or something – is making physical contact, for better or for worse, which often calls for immediate action. A potential intensifying effect of touch on other sensory signals might therefore facilitate a rapid decision on whether the toucher is a friend or a foe, which is essential when this person is close.
We recently found that gentle touch from another human shaped social impressions of visually presented faces differentially depending on the emotional expression of the face (Ellingsen et al., 2014). Whereas concomitant human touch made innocuous neutral and smiling faces seem more attractive and friendly, it made angry faces seem less attractive and friendly, relative to equally intense touch from a device. This effect was potentiated by intranasal administration of an oxytocin receptor agonist (Ellingsen et al., 2014) (See below for more on oxytocin).
To understand how affective touch experiences are created in the brain, it is useful to examine first, how touch stimuli are transmitted from the periphery to the brain, and second, how these signals are modified by and integrated with top-down information.
The processing of touch starts with the activation of mechanoreceptive afferents in the skin, such as fast-conducting, myelinated A-beta or slow-conducting, unmyelinated C-tactile (CT) afferents (Olausson et al., 2010; McGlone et al., 2014). While A-beta afferents respond to a wide variety of touch stimuli, CT afferents may be more specifically tuned to respond to stimuli slowly moving over the skin, like a caress, and their firing rates in the peripheral afferent correspond closely to touch pleasantness (Löken et al., 2009). Moreover, CTs activate most vigorously in response to touch stimuli that are close to skin-temperature, but less to colder or warmer stimuli, which again corresponds closely to pleasantness ratings (Ackerley et al., 2014).
Less is known about the relationship between CT signaling and positive affect during different contexts or motivational-affective states (but see Croy et al., 2014). Notably, however, recent studies suggest that CT afferents may play a role in the tactile “hedonic flip” following injury or inflammation of the skin (such as tactile hypersensitivity and allodynia), whereby light gentle touch becomes less pleasant or even painful (Liljencrantz and Olausson, 2014). Recent animal studies have found that inflammation-induced hypersensitivity is reduced in mice whose transmission of C-low-threshold mechanoreceptive afferents (equivalent to CTs in humans) has been genetically knocked out (Seal et al., 2009; Lou et al., 2013). In humans, experimentally induced allodynia-like pain, provoked by light touch to the skin overlaying an aching muscle, persists after functional compression blockage of myelinated skin afferents (Nagi et al., 2011). Other studies have found that, using the experimental heat/capcaicin model of allodynia, light CT-optimal touch (3 cm/s velocity) to the skin adjacent to the sensitized area was more unpleasant than CT-suboptimal touch (30 cm/s), reversing the relationship between velocity and pleasantness seen under healthy conditions (Liljencrantz et al., 2014). This is consistent with the view that a potential antinociceptive role of CT is disrupted, or that CT afferents may even signal negative affect, during injury or inflammation of the skin (Liljencrantz and Olausson, 2014). Thus, it is possible that during such physiological “threat conditions” (Fields, 2006; Porges, 2007), a state-induced shift in the function of CT afferents may contribute to the motivation to protect and care for a wounded limb.
Given the strong contextual influences on touch pleasantness, it is unknown whether there are qualities of certain touch stimuli that inherently carry a positive hedonic value (i.e., are pleasant or give rise to positive affect), or whether the hedonic value of touch is always dependent on other contextual or internal factors (Ellingsen et al., 2015).
A-beta afferents from the upper and lower extremities terminate in the cuneate and gracile nuclei of the dorsal column (Perl et al., 1962; Petit and Burgess, 1968), where they synapse onto neurons that transmit to the ventral posteriolateral nuclei of the thalamus. C-tactile afferents likely take a different route to the brain, through the spinothalamic tract (Andrew, 2010). From the thalamus, touch signals are relayed to cortical sensory processing areas such as the insular (Olausson et al., 2002; Bjornsdotter et al., 2009) and primary and secondary somatosensory areas (McGlone et al., 2002; Gazzola et al., 2012) as well as to other higher-order areas such as the prefrontal, orbitofrontal, anterior cingulate cortices, and the superior temporal sulcus (Francis et al., 1999; Lindgren et al., 2012; McGlone et al., 2012; Gordon et al., 2013; Scheele et al., 2014). There is also evidence that subcortical areas such as ventral striatum and amygdala, which are key structures in the processing of affect and motivation in general, are implicated in the processing of affective touch (Ellingsen et al., 2013; Perini et al., 2015).
Although the subjective experience of pleasant touch is thought to arise from cortical activation, it is not clear how other information, such as visual contextual information or memory, modulates the sensory signals. Does such information target the neural systems that generate pleasure or displeasure, e.g., hedonic hot and cold spots (Pecina and Berridge, 2005; Ho and Berridge, 2013; Castro and Berridge, 2014), or does it also modulate ascending sensory signaling? If so, at what levels does this modulation take place? Evidence from different fields indicates that top-down influences can modulate sensory signals at early stages of sensory processing. Focused auditory attention in humans can modulate signaling in the auditory sensory cortex as early as 20 ms post stimulus (Woldorff et al., 1993). Moreover, visual spatial attention can modulate pre-cortical signals in the lateral geniculate nucleus of the thalamus, the first relay between the retina and the cortex (McAlonan et al., 2008). It is well documented that ascending nociceptive neurons in the spinal dorsal horn are modulated by signaling descending from the brain (Wall, 1967; Woolf, 2011). The periaqueductal gray (PAG) in the midbrain controls incoming nociceptive signals indirectly through the rostroventral medulla (RVM;Millan, 2002; Fields, 2004). Neurons in the RVM project to the spinal dorsal horn, with inhibitory or excitatory effects on nociceptive transmission (Urban and Gebhart, 1999; Neubert et al., 2004). The PAG receives direct input from the limbic structures amygdala and ventral striatum, and from the prefrontal cortex, constituting a descending pathway by which affective or cognitive information can influence ascending sensory information already at the spinal dorsal horn (Fields, 2004).
Modulation of innocuous touch is less studied, especially in humans. A few studies on somatosensory evoked potentials (SEPs) have shed light on what levels of sensory processing may be modulated by top-down influences. One study found SEP differences as early as 50 ms post-stimulus when study participants were attending to, relative to not attending to, tactile stimulation of the index finger (Schubert et al., 2008). Another study found SEP differences when people were led to expect a more intense tactile stimulus, relative to an expected low-intensity tactile stimulus (Fiorio et al., 2012). These studies, in which the stimuli were identical regardless of attention or expectation, suggest that somatosensory processing can be modified by top-down processing at least as early as the primary somatosensory area.
In rodents, there is electrophysiological evidence that corticofugal projections, originating from the primary somatosensory area (SI), modulate innocuous touch signals in the cuneate and gracile nuclei of the dorsal column – the earliest relay stages for many low-threshold mechanoreceptive afferents (Nunez and Malmierca, 2007). Further, branches of low-threshold mechanoreceptors synapse at the segmental level in the spinal dorsal horn, but it is not known if central cognitive or affective information can alter touch processing at this level (Abraira and Ginty, 2013).
Modulation of hedonic sensations by context, expectations, attention, and mood, can sometimes alter widespread sensory processing in the brain (Small et al., 2001; Wager et al., 2004; de Araujo et al., 2005; Petrovic et al., 2005; Nitschke et al., 2006; Tracey and Mantyh, 2007; Berna et al., 2010; Knudsen et al., 2011; Woods et al., 2011; Amanzio et al., 2013). Such modulations have been widely studied in paradigms evoking placebo effects, i.e., beneficial effects from clinical treatment due to patients’ or study participants’ positive expectations or appraised contextual meaning, rather than the active ingredient of the treatment itself (Schedlowski et al., 2015; Wager and Atlas, 2015). In these experiments, contextual cues are often manipulated to alter the subjects’ expectations of the effects of the treatment, which can be an inactive substance or procedure. Thus, one can study how an unpleasant sensation or symptom, such as pain, changes across different contexts. A series of functional neuroimaging studies indicate that placebo improvement is often underpinned by modulation of neural circuitry that traditionally are considered pathways for bottom-up, ascending sensory signals (Buchel et al., 2014). For example, placebo-induced reduction of pain is often associated with widespread reductions of somatosensory processing in thalamus, insula, primary and secondary somatosensory areas, and dorsal anterior cingulate cortex (ACC; Price et al., 2007; Eippert et al., 2009a; Lu et al., 2010; Amanzio et al., 2013). Moreover, some studies suggest that nociceptive processing in the spinal cord can be modified by expectations of pain relief (Eippert et al., 2009b) or pain worsening (Geuter and Buchel, 2013). Increased activity in a set of brain regions collectively involved in cognition, valuation, and affective processing, consisting of ventromedial (vmPFC) and dorsolateral (dlPFC) prefrontal cortex, orbitofrontal cortex (OFC), anterior insula, ventral striatum, amygdala and the midbrain, is often observed in placebo studies (Petrovic et al., 2002; Wager et al., 2004; Zubieta et al., 2005; Scott et al., 2007, 2008; Watson et al., 2009; Geuter et al., 2013; Pecina et al., 2013; Bingel and Placebo Competence Team, 2014; Hashmi et al., 2014; Kessner et al., 2014; Wrobel et al., 2014; Sevel et al., 2015), and is thought to be responsible for the suppression of pain processing. The functional architecture of modulatory networks for placebo responsiveness has yet to be disentangled, but these regions play central roles in hedonic valuation more generally, in the monitoring and updating of expectation, and the integration of available relevant information (Craig, 2009; McDannald et al., 2011; Schoenbaum et al., 2011; Roy et al., 2012; Lebreton et al., 2015; Lindquist et al., 2015).
There are relatively few investigations of the brain mechanisms underpinning contextual modulation of affective touch. A handful of studies have used functional Magnetic Resonance Imaging (fMRI) to investigate brain activity responses to the same touch gentle stimulus during different contexts. We recently investigated whether placebo improvement of touch pleasantness (hyperhedonia) involves a modulation in somatosensory processing circuitry and whether this is related to activation of a prefrontal–subcortical modulatory neural circuit, similarly to that observed in placebo analgesia (Ellingsen et al., 2013). We suggested to a group of healthy volunteers that a nasal spray would increase both the pleasantness of gentle touch and reduce the unpleasantness of pain. After self-administration of a placebo nasal spray, which the participants were lead to believe would improve the affective aspects of both gentle touch and pain sensations, they found touch more pleasant and pain less unpleasant. While fMRI recordings during pain stimuli indicated decreased somatosensory processing, recordings during gentle touch stimuli showed instead increased activity in somatosensory areas (SI, SII, and the posterior insula). Those participants who showed the strongest placebo hyperhedonia and analgesia, also had the strongest placebo-induced activity increase in vmPFC, Nucleus Accumbens, amygdala, and brainstem regions. Furthermore, the magnitude of this activity increase was related to the modulation of somatosensory circuitry. Specifically, those with the strongest placebo increase in functional coupling between vmPFC and PAG also had the strongest hyperhedonic increases and analgesic decreases in somatosensory areas (Figure 2A), consistent with previous findings for placebo analgesia (Wager et al., 2007; Eippert et al., 2009a). Another fMRI study investigated the modulation of touch pleasantness during the application of a skin cream, by the visual presentation of labels saying either “rich moisturizing cream” or “basic cream” (Figure 1C, McCabe et al., 2008). Although it was always the same cream, participants reported the application of the rich cream as richer and more pleasant. This improvement in hedonics was associated with increased activations in the ventral striatum, pregenual ACC (pgACC), SI/SII, and the parietal area 7. One study found that when manipulating study participants’ beliefs about the gender identity of the toucher, touch pleasantness of “female caresses” increased, along with activation increases in SI and the OFC (Figures 1E and 2B; Gazzola et al., 2012). Using a similar design (Figure 1D; Scheele et al., 2014) partly replicated these results, showing increased activation in the SI, as well as the caudate, when participants believed the caresser was female. Moreover, the intranasal administration of an oxytocin receptor agonist further increased the touch pleasantness of the “female caresses”, which was underpinned by activation increases in the anterior insula, pgACC, and precuneus.
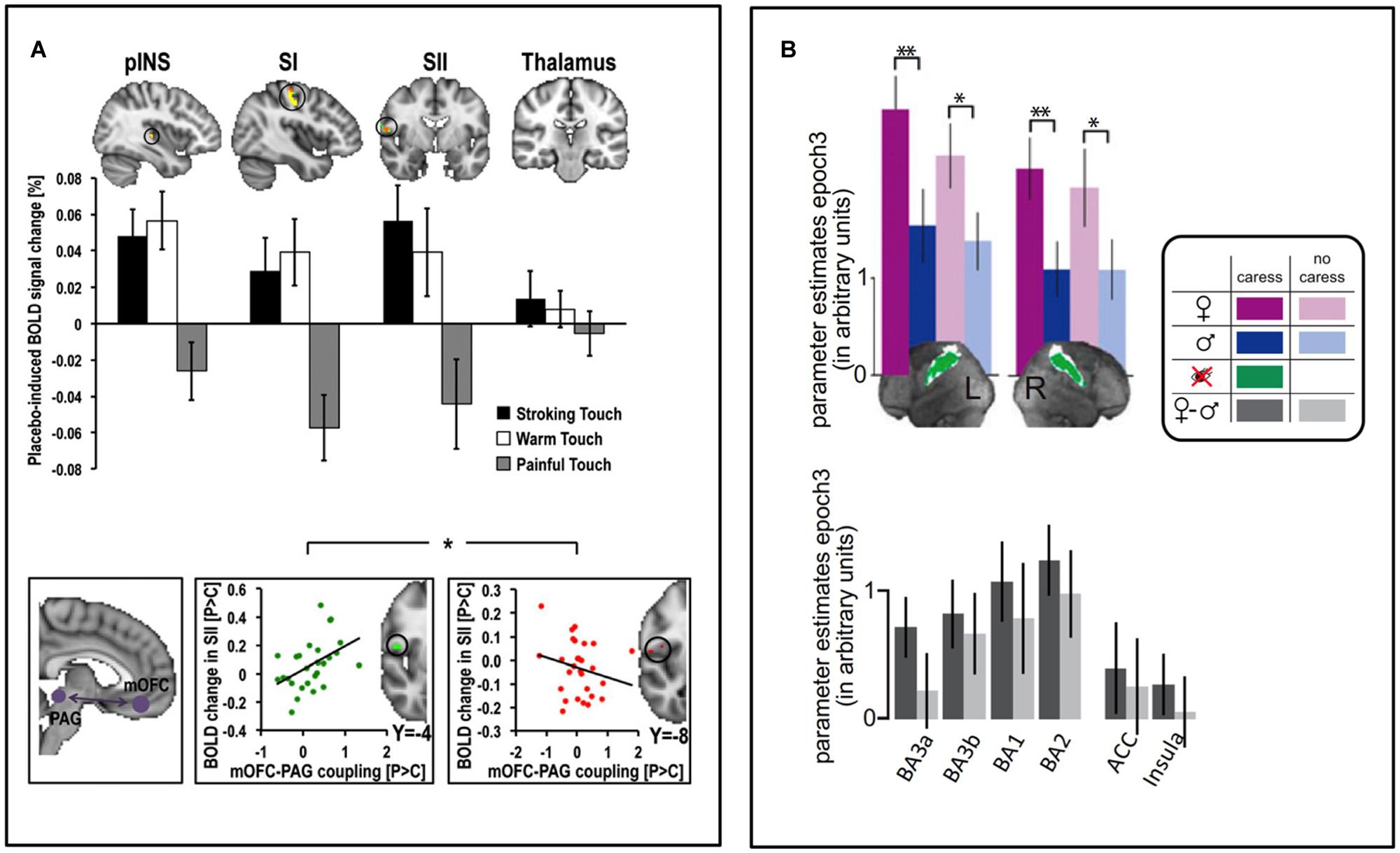
FIGURE 2. Contextual modulation of brain to affective touch. (A) After self-administrating a (placebo) nasal spray believed to have beneficial effects on gentle touch and pain perception, placebo-induced increases in touch pleasantness and reductions in pain unpleasantness were underpinned by respective increases and decreases in somatosensory processing of pleasant touch and pain (top). The individual magnitude of this somatosensory modulation was associated with the degree to which placebo treatment increased the functional connectivity between the medial OFC (mOFC) and PAG (bottom, left), an important pathway for pain modulation – those with the strongest increase in mOFC-PAG connectivity had the strongest somatosensory increases to pleasant touch (bottom, middle) and the strongest somatosensory decreases to pain stimuli (bottom, right) (Ellingsen et al., 2013). (B) In heterosexual men, SI activity during gentle caresses was larger when they believed a woman, relative to a man, performed the caress. The same pattern was seen across different sub-regions of SI, as well as (non-significantly) in the ACC and insula (bottom) (Gazzola et al., 2012). Figure adapted from (Gazzola et al., 2012; Ellingsen et al., 2013). ∗p < 0.05, ∗∗p < 0.01.
It has not yet been demonstrated whether the modulation of pleasurable touch, like pain, involves descending modulation of cutaneous afferents in the spinal cord, perhaps via the RVM. Nevertheless, these findings suggest that, like negative hedonic feelings such as pain, psychological modulation of pleasant sensations may involve a more comprehensive modulation of the underlying sensory processing, and not only within higher-level valuation circuitry. One caveat, which is shared with research on other sensory modalities, is that although thinking in terms of one ascending “sensory” system and one descending “modulatory” system is useful, e.g., for forming testable research questions, it may be too simplistic. It has been suggested that, instead of functioning as separate systems where one can influence the other, it may be more accurate to consider this as one recurring sensory processing system with several integrative components, such as feed-forward signaling, feedback loops, influences from the “early” sensory processing of other modalities, and influences from more abstract cognitive and affective information (Kinchla and Wolfe, 1979; Finkel and Edelman, 1989; Ullman, 1995; Siegel et al., 2000; O’Reilly et al., 2013).
In affiliative interactions such as rough-and-tumble play, where touch has stimulatory or arousing behavioral or physiological effects (Feldman et al., 2010a; Gordon et al., 2010), the touch behaviors involved are typically different from interactions where the intent is soothing, consolidation or relaxation (Holt-Lunstad et al., 2008). These touch activities are likely driven by different motivational modes, depending on the individual’s underlying needs. There is evidence that primates and rodents frequently engage in soothing and soft touch activities, like social grooming and huddling, but rarely in rough-and-tumble play, when they are distressed or in homeostatic imbalance (For review, see Løseth et al., 2014). However, while stimulatory and soothing touch may generally be differentiated by their stimulus characteristics, the actual arousing or relaxing effects of a given kind of touch depends on the appraised meaning (Ellingsen, 2015). For example, although a gentle caress can be soothing in one context, it can be sexually arousing in a different context. Furthermore, a caress can arouse negative affect and withdrawal if coming from an unwanted person (Major, 1981).
The μμ-opioid receptor (MOR) system has a multi-faceted role in reward, both social (Machin and Dunbar, 2011; Chelnokova et al., 2014) and non-social (Drewnowski et al., 1995; Yeomans and Gray, 1997). As is well known, MOR activation promotes relief of negative affect (Hsu et al., 2013) – e.g., drugs that activate MOR in humans have potent analgesic effects (Zubieta et al., 2001). Moreover, opioids have an inhibitory effect on Hypothalamic–Pituitary–Adrenal (HPA) axis responses to environmental stress (Kreek, 1996; Wand et al., 2002). Furthermore, MOR promotes motivation for (Mahler and Berridge, 2012) and enjoyment of (Pecina and Berridge, 2005) appetitive reward. A wealth of studies using pharmacological manipulation of the MOR system in a variety of mammalian taxa have demonstrated a key role of the MOR system for affiliative touch behaviors, such as social grooming (Keverne et al., 1989; Machin and Dunbar, 2011), social play (Panksepp and Bishop, 1981; Vanderschuren et al., 1995), and huddling (Shapiro et al., 1989; Dunbar, 2010).
Notably, the directionality of MOR agonism and antagonism effects on affiliative touch behaviors has been diverging into two opposing “camps” – 1) studies of primates and infant rodents indicating that enhanced MOR signaling reduces affiliative touch behaviors, and 2) studies of adolescent and adult rodents indicating that enhanced MOR signaling increases affiliative touch behaviors. We recently proposed the State-dependent μ-Opioid Modulation of Social Motivation (SOMSoM) model as a resolution to this apparent paradox, in that, instead of reflecting a fundamental species-related difference in MOR function per se, these differences may instead be due to consistent differences in the animals’ motivational state during the experimental tasks (Løseth et al., 2014). Most of these studies made use of some variant of a “social relief paradigm”, where the animal was separated for a certain amount of time before reunited with its peers. Since primates and infant rodents rely on close social bonds with others for survival and protection, they are often very distressed by social separation (Panksepp et al., 1978). Adolescent and adult rodents on the other hand, are not as reliant on social support for survival or coping with stress, and typically form more transient bonds for mating and parenting. Thus, they are considerably less distressed by the social separation that is inherent in the majority of these studies (Nelson and Panksepp, 1998; van den Berg et al., 1999). Consequently, while socially isolated primates and infant rodents may be distressed and thus highly motivated for seeking relief and safety through social contact, adult and adolescent rodents may have less need for relief and thus more motivation for social exploration. The SOMSoM model proposes that during negative affective states, animals seek out affiliative touch interactions primarily for comfort and relief of negative emotion. By providing relief from distress, MOR activation by social contact or pharmacological stimulation therefore reduces contact seeking, while disruption of MOR signaling intensifies contact seeking. However, when the animal is in emotional equilibrium, social interactions are instead sought out for exploration, joy, and mating, which is also promoted by MOR (Løseth et al., 2014). During this motivational state, pharmacological stimulation of MOR signaling increases, while disruption of MOR signaling reduces, contact seeking and behaviors such as play.
Touch plays a central role in these interactions, and a body of behavioral research indicates a specific role for affiliative touch in health and wellbeing (for review, see Walker and McGlone, 2013). However, the social interactions in these studies are always happening in a rich multisensory context. An important challenge for future studies is therefore to disentangle the role of the MOR system in touch specifically.
The neuropeptide oxytocin also plays a central role in social affiliation and attachment in mammals (Tops et al., 2007; Feldman, 2012). Differences in oxytocin receptor distribution in limbic brain areas across rodent species reflect differences in social organization and bond formation (Young et al., 2011). The monogamous prairie vole has higher densities of oxytocin and vasopressin receptors in the ventral striatum than the closely related, but promiscuous, montane and meadow voles (Ross et al., 2009). Furthermore, the blockade of mesolimbic oxytocin signaling in prairie voles prevents both maternal behavior (Cho et al., 1999; Olazabal and Young, 2006) and the formation of long-term pair bonds (Insel and Hulihan, 1995; Cho et al., 1999; Ferguson et al., 2000). Oxytocin is also involved in a range of social and emotional processing in humans (Bartz et al., 2011; Leknes et al., 2013; Ellingsen et al., 2014), and has anxiolytic effects (Heinrichs et al., 2003; Kirsch et al., 2005), enhance parasympathetic responses (Gamer and Buchel, 2012), and increase heart rate variability – indicating increased vagal control (Kemp et al., 2012). Similar to the MOR system, oxytocin is associated with promoting social approach both for appetitive social reward (Panksepp et al., 1997; Nakajima et al., 2014), and relief of negative affect (Campbell, 2008; Bosch, 2011). Specific affiliative behavior such as social grooming (Drago et al., 1986; Pedersen et al., 1988; Witt et al., 1992; Francis et al., 2000; Champagne et al., 2001; Champagne, 2008) and maternal nurturing (Pedersen and Prange, 1979; Pedersen et al., 1982; Bosch, 2011) has been associated with oxytocin.
Several studies indicate that oxytocin suppresses the activity of the stress-induced HPA axis. In humans, oxytocin reduces the release of adrenocorticotropic hormone (ACTH; Chiodera and Coiro, 1987) and cortisol (Legros et al., 1988) in response to stressful stimuli. In rats, central blockade of oxytocin increases basal and stress-induced release of ACTH and corticosterone (Neumann et al., 2000b). However, a study using local injection of an oxytocin antagonist indicates differential effects on stress responses depending on the brain site. Local blockade in the Paraventricular Nuclei (PVN) led to increased basal ACTH, but reduced stress-induced release of ACTH, perhaps because of the increased baseline. On the other hand, injections in the amygdala and the medio-lateral septum, which projects directly and indirectly to the PVN, did not alter basal ACTH levels, but reduced stress-induced ACTH (Neumann et al., 2000a). Because of the effect of oxytocin on both stress regulation and social bonding, it has been suggested that the soothing and anxiolytic effects of stroking touch in mammals is mediated by oxytocin (Uvnas-Moberg et al., 2014).
In contrast to in rodent literature, relatively few studies have employed pharmacological modulation of oxytocin in primates. A recent study investigated pair-bonding in marmoset monkeys, and found that huddling behavior was increased by the administration of an oxytocin receptor agonist, but reduced by an oxytocin antagonist (Smith et al., 2010). Another study found that, in squirrel monkeys, intranasal oxytocin dampened the increases of blood plasma ACTH in response to (stressful) social isolation. However, plasma levels of cortisol were not affected (Parker et al., 2005), and, since behavioral changes were not assessed, it is difficult to directly relate this finding to affiliative touch behavior.
Primate studies investigating peripheral levels of oxytocin during social interactions provide indirect evidence for an involvement of oxytocin in affiliative touch behavior (although whether peripheral OT levels give an indication of central OT levels is as yet unclear – see Jokinen et al., 2012; Neumann and Landgraf, 2012; Kagerbauer et al., 2013). In rhesus monkeys, engagement in social grooming activities correlates positively with plasma (Maestripieri et al., 2009) and cerebrospinal fluid (Winslow et al., 2003) levels of oxytocin. In wild chimpanzees, increased urinary levels of oxytocin has been reported to follow grooming events, which is mediated by bond strength between the grooming partners, specifically grooming interactions between animals with closer social bonds showed larger increases in urinary oxytocin (Crockford et al., 2013). Another study found no relationship between plasma oxytocin and social behavior in free-ranging macaques (Schwandt et al., 2007). A recent study on pair bonding in cotton-top tamarins reported that inter-individual levels of urinary oxytocin co-varied closely with grooming and mutual contact in females and with sexual behavior in males (Snowdon et al., 2010). Moreover, one study reported higher urinary levels of oxytocin during social contact than during social isolation (Seltzer and Ziegler, 2007). Together, these studies are in line with a notion that oxytocin release is associated with the relief of negative states induced by social isolation or rejection, and that low levels of oxytocin may promote seeking of social support (Panksepp et al., 1997; Tops et al., 2007). It has been proposed that oxytocin release in social interaction may involve two “phases” – first, a social-salience related release during motivation for approach, and second – if leading to physical affiliative contact – an anti-stress related release (Uvnas-Moberg et al., 2014). This model is derived from the reports of oxytocin release in dogs and dog-owners, first in response to auditory and visual cues that the other “individual” is nearby, and then again when the owner strokes and caresses the dog, together with reductions in plasma cortisol (Miller et al., 2009; Handlin et al., 2011; Beetz et al., 2012; Rehn et al., 2014).
Similar to the effects of MOR system manipulations, the behavioral effects of oxytocin administration seem to vary across contexts and affective states (Bartz et al., 2011). In rodents, oxytocin is associated with both protective behavior toward pups and aggression against intruders (Campbell, 2008). Intranasal oxytocin in humans increases the recognition of both positive (Unkelbach et al., 2008; Marsh et al., 2010) and negative emotions (Bartz et al., 2010; Fischer-Shofty et al., 2010; Leknes et al., 2013), and increases empathizing and cooperation with in-group members, but may increase aggression toward threatening out-group members (De Dreu and Kret, 2015). We recently found that oxytocin promotes a social-touch induced “sharpening” of social impressions of others, relative to non-social touch (Ellingsen et al., 2014). Nevertheless, a single dose (40IU) of oxytocin did not affect the pleasantness or intensity of the actual touch experience. In contrast, a recent study found that, in a group of heterosexual men, intranasal oxytocin increased the pleasantness of sensual caresses specifically when they believed that a woman was touching (Scheele et al., 2014). However, oxytocin had no effect on touch pleasantness when the participants believed the caresser was a man, further highlighting the importance of multisensory context in oxytocin functioning. A popular hypothesis about central oxytocin functioning is that oxytocin may promote social approach behavior (in both positive and negative contexts), and inhibit social avoidance (Kemp and Guastella, 2010, 2011; Clark et al., 2013). However, while many of the studies using intranasal oxytocin in humans involve an experimental manipulation of context, they rarely assess – or manipulate – more profound changes in motivational or homeostatic state. Thus, it is not well known to what degree oxytocinergic modulation of social approach and avoidance in humans depends on the individual’s initial state. Interestingly, it has recently been suggested that oxytocin might promote approach behavior more strongly in novel contexts compared to familiar contexts (Tops et al., 2014). One study found that participants’ salivary oxytocin, during anticipation of a cognitive task, was positively correlated with state trust at the initial session, but negatively correlated with trust in the subsequent session, when they were familiar with the task (Tops et al., 2013). Similarly, another study reported that intranasal oxytocin increased the expression of affiliation in a clinical interview for depression, during an initial visit but not during a follow-up visit (Brune et al., 2015).
A series of studies assessing endogenous peripheral levels of oxytocin suggest a role of oxytocin in human affiliative touch (Lupoli et al., 2001; Matthiesen et al., 2001; Uvnas-Moberg, 2004; Light et al., 2005), although the exact mechanisms are unclear (Feldman, 2012). One study found that plasma oxytocin levels in mothers during pregnancy and the early postpartum period predicted maternal bonding behaviors such as eye gaze, high-pitched vocalizations and affectionate touch directed at the infant (Feldman et al., 2007). Another study reported that higher plasma levels of oxytocin correlated with more frequent infant-directed stimulatory touch by first-time fathers, but with more frequent affectionate touch (e.g., hugging, kissing, and stroking) by first-time mothers (Gordon et al., 2010). Furthermore, one study found that couples who were instructed to perform 30 minutes of reciprocal “warm, sensual” touch on their partner’s neck, shoulders, and hands, three days/week for 4 weeks, had increased post-intervention levels of salivary oxytocin, as well as reductions in stress-responsive markers such as blood pressure, plasma cortisol, and alpha amylase, compared to a control group (Holt-Lunstad et al., 2008). Unfortunately, this literature commonly quantified oxytocin in plasma using methods in which the validity has been questioned, and results may reflect non-oxytocin substances (McCullough et al., 2013; Christensen et al., 2014). Perhaps for this reason, along with potentially fine-grained variations in context and motivational state across studies, overall findings of touch-induced release of peripheral oxytocin in humans are inconsistent. While some studies have found peripheral oxytocin release in response to touch (Light et al., 2000; Odendaal and Meintjes, 2003; Light et al., 2005; Holt-Lunstad et al., 2008), others have found no effect (Turner et al., 1999; Heinrichs et al., 2001; Wikstrom et al., 2003; Grewen et al., 2005; Ditzen et al., 2007). Moreover, methodological limitations like the lack of useful oxytocin antagonists for human testing, as well as the current inability to assess oxytocin release in the human brain, limits the understanding of the functional neurobiology of oxytocin in humans. Finally, it is important to note that, like the MOR system, many of the functions of central oxytocin are not restricted to the social domain, but instead may reflect more fundamental mechanisms involved in generalized processing of salience, motivation, anxiety, and stress regulation (Churchland and Winkielman, 2012; Harari-Dahan and Bernstein, 2014).
In addition to MOR and oxytocin, neurotransmitters such as vasopressin (Winslow et al., 1993; Panksepp et al., 1997), serotonin (Insel and Winslow, 1998; ?), cannabinoids (Trezza and Vanderschuren, 2008a,b; Trezza et al., 2012) and dopamine (Champagne et al., 2004) modulate social touch behaviors in mammals. For example, tickling – an activity primarily associated with social play – increases NAc dopamine signaling in rats (Maruyama et al., 2012; Hori et al., 2013). In humans, massage therapy increases urinary dopamine and serotonin, and reduces urinary and salivary cortisol (as reviewed by Field et al., 2005). It is, however, unknown whether such peripheral assessment reflects concentrations of these neurotransmitters in the brain.
These neurotransmitter systems likely interact with MOR and oxytocin processing in key brain regions involved in social and emotional processing (Hagelberg et al., 2002; Liu and Wang, 2003; Depue and Morrone-Strupinsky, 2005a; Lintas et al., 2011; Colasanti et al., 2012; Tops et al., 2014). Understanding the nature of these interactions is an important challenge for future studies (Weisman and Feldman, 2013). Furthermore, in the periphery, oxytocin interacts with other hormones to affect behavior. For example, it has been reported that intranasal oxytocin increases human fathers’ expression of eye gaze and affectionate touch toward their infants, but only in those whose plasma testosterone levels also increase after the oxytocin administration (Weisman et al., 2014).
Although touch can be a source of safety, comfort, relief, and pleasure, this effect is likely confined to instances where contextual cues are affectively congruent with affiliative touch, e.g., when the other individual is friendly, has good intentions, and the touch is socioculturally appropriate. When touch occurs in combination with contextual cues indicating that the touch is undesirable, or is associated with danger, the same touch stimulus may instead be appraised as unpleasant or disgusting and promote avoidance. It is not yet known whether there are aspects of touch that are inherently positive, or if the hedonic value of all kinds of touch is dependent on context or internal state. Future research is needed to determine the flexibility and boundaries of bottom-up versus top-down influences on touch. Although relatively few, the existing studies on the neurobiological underpinnings of top-down modulation of affective touch indicate involvement of modulatory prefrontal and subcortical circuitry key to valuation, cross-sensory integration, and the construction of meaning (Ellingsen et al., 2013; Ellingsen et al., 2015). These studies also suggest that somatosensory processing in circuitry traditionally considered part of a “bottom-up” pathway can be modulated by expectations and contextual cues informative of the hedonic value of touch. This mechanism bears similarity to that involved in placebo improvement of negative hedonic experiences, such as pain (Scott et al., 2007; Eippert et al., 2009a; Benedetti, 2014). μ-opioids and oxytocin are two of the neurotransmitters that have been most extensively studied in relation to affiliative touch. Pharmacological manipulation of μ-opioid processing can dramatically influence touch behavior in mammals, but the directions of the effects seems to depend on motivational state. Whereas MOR antagonism increases social contact seeking when the animal is distressed, it tends to decrease contact seeking when the animal is in a non-stressed state, especially in animals that rely on social relationships for emotion regulation, such as primates and infant rodents (Panksepp et al., 1978). This may reflect a bimodal role of opioids in both comfort seeking and exploration for social reward, mirroring the dual effects of MOR in pain relief and pleasure (Løseth et al., 2014). The role of oxytocin in affiliative touch, and in social interactions in general, is similarly dependent on context. One line of research indicates that oxytocin may either increase the salience of socially relevant cues, or promote approach behavior in general (Shamay-Tsoory et al., 2009; Kemp and Guastella, 2010, 2011). Another line of research indicates an anxiolytic and stress-reducing effect of oxytocin, and it has been hypothesized to account for the relaxing and soothing effects of touch (Churchland and Winkielman, 2012). Unfortunately, most of the studies investigating the effects of pharmacological manipulation of μ-opioids and oxytocin systems on social touch behaviors do not give information about the contribution of touch relative to other sensory modalities. The same issue applies to investigations of the effects of interpersonal touch in naturalistic settings, where touch is part of a complex multisensory interaction. A future challenge is thus to disentangle the specific role of touch in relation to other sensory modalities, and how touch is integrated with other sensory signals. Isolating the specific role of touch in social interactions, while still keeping a certain level of ecological validity is particularly challenging, and poses an important task for future research.
DE prepared the manuscript. DE, SL, GL, JW, and HO revised the manuscript into its finalized form.
D-ME is supported by a postdoctoral scholarship from the Norwegian Research Council (FRIPRO) and the Marie Sklodowska-Curie Actions, under the COFUND program (240553/F20).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abraira, V. E., and Ginty, D. D. (2013). The sensory neurons of touch. Neuron 79, 618–639. doi: 10.1016/j.neuron.2013.07.051
Ackerley, R., Backlund Wasling, H., Liljencrantz, J., Olausson, H., Johnson, R. D., and Wessberg, J. (2014). Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J. Neurosci. 34, 2879–2883. doi: 10.1523/JNEUROSCI.2847-13.2014
Alberts, J. R. (2007). Huddling by rat pups: ontogeny of individual and group behavior. Dev. Psychobiol. 49, 22–32. doi: 10.1002/dev.20190
Amanzio, M., Benedetti, F., Porro, C. A., Palermo, S., and Cauda, F. (2013). Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum. Brain Mapp. 34, 738–752. doi: 10.1002/hbm.21471
Andrew, D. (2010). Quantitative characterization of low-threshold mechanoreceptor inputs to lamina I spinoparabrachial neurons in the rat. J. Physiol. 588, 117–124. doi: 10.1113/jphysiol.2009.181511
Bartz, J. A., Zaki, J., Bolger, N., Hollander, E., Ludwig, N. N., Kolevzon, A., et al. (2010). Oxytocin selectively improves empathic accuracy. Psychol. Sci. 21, 1426–1428. doi: 10.1177/0956797610383439
Bartz, J. A., Zaki, J., Bolger, N., and Ochsner, K. N. (2011). Social effects of oxytocin in humans: context and person matter. Trends Cogn. Sci. 15, 301–309. doi: 10.1016/j.tics.2011.05.002
Bastian, B., Jetten, J., and Hornsey, M. J. (2014). Gustatory pleasure and pain. The offset of acute physical pain enhances responsiveness to taste. Appetite 72, 150–155. doi: 10.1016/j.appet.2013.10.011
Beetz, A., Uvnas-Moberg, K., Julius, H., and Kotrschal, K. (2012). Psychosocial and psychophysiological effects of human-animal interactions: the possible role of oxytocin. Front. Psychol. 3:234. doi: 10.3389/fpsyg.2012.00234
Benedetti, F. (2014). Placebo effects: from the neurobiological paradigm to translational implications. Neuron 84, 623–637. doi: 10.1016/j.neuron.2014.10.023
Berna, C., Leknes, S., Holmes, E. A., Edwards, R. R., Goodwin, G. M., and Tracey, I. (2010). Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol. Psychiatry 67, 1083–1090. doi: 10.1016/j.biopsych.2010.01.014
Berridge, K. C., and Kringelbach, M. L. (2015). Pleasure systems in the brain. Neuron 86, 646–664. doi: 10.1016/j.neuron.2015.02.018
Bingel, U., and Placebo Competence Team (2014). Avoiding nocebo effects to optimize treatment outcome. JAMA 312, 693–694. doi: 10.1001/jama.2014.8342
Bjornsdotter, M., Gordon, I., Pelphrey, K. A., Olausson, H., and Kaiser, M. D. (2014). Development of brain mechanisms for processing affective touch. Front. Behav. Neurosci. 8:24. doi: 10.3389/fnbeh.2014.00024
Bjornsdotter, M., Loken, L., Olausson, H., Vallbo, A., and Wessberg, J. (2009). Somatotopic organization of gentle touch processing in the posterior insular cortex. J. Neurosci. 29, 9314–9320. doi: 10.1523/JNEUROSCI.0400-09.2009
Bosch, O. J. (2011). Maternal nurturing is dependent on her innate anxiety: the behavioral roles of brain oxytocin and vasopressin. Horm. Behav. 59, 202–212. doi: 10.1016/j.yhbeh.2010.11.012
Brune, M., Kolb, M., Ebert, A., Roser, P., and Edel, M. A. (2015). Nonverbal communication of patients with borderline personality disorder during clinical interviews a double-blind placebo-controlled study using intranasal oxytocin. J. Nerv. Ment. Dis. 203, 107–111. doi: 10.1097/NMD.0000000000000240
Buchel, C., Geuter, S., Sprenger, C., and Eippert, F. (2014). Placebo analgesia: a predictive coding perspective. Neuron 81, 1223–1239. doi: 10.1016/j.neuron.2014.02.042
Cabanac, M. (1971). Physiological role of pleasure. Science 173, 1103–1107. doi: 10.1126/science.173.4002.1103
Calvert, G. A., and Thesen, T. (2004). Multisensory integration: methodological approaches and emerging principles in the human brain. J. Physiol. Paris 98, 191–205. doi: 10.1016/j.jphysparis.2004.03.018
Campbell, A. (2008). Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol. Psychol. 77, 1–10. doi: 10.1016/j.biopsycho.2007.09.001
Carstens, E., Carstens, M. I., Dessirier, J. M., O’Mahony, M., Simons, C. T., Sudo, M., et al. (2002). It hurts so good: oral irritation by spices and carbonated drinks and the underlying neural mechanisms. Food Qual. Prefer. 13, 431–443. doi: 10.1016/S0950-3293(01)00067-2
Castro, D. C., and Berridge, K. C. (2014). Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J. Neurosci. 34, 4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014
Champagne, F. A. (2008). Epigenetic mechanisms and the transgenerational effects of maternal care. Front. Neuroendocrinol. 29:3. doi: 10.1016/j.yfrne.2008.03.003
Champagne, F. A., Chretien, P., Stevenson, C. W., Zhang, T. Y., Gratton, A., and Meaney, M. J. (2004). Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J. Neurosci. 24, 4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004
Champagne, F., Diorio, J., Sharma, S., and Meaney, M. J. (2001). Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. U.S.A. 98, 12736–12741. doi: 10.1073/pnas.221224598
Chelnokova, O., Laeng, B., Eikemo, M., Riegels, J., Løseth, G., Maurud, H., et al. (2014). Rewards of beauty: the opioid system mediates social motivation in humans. Mol. Psychiatry 19, 746–747. doi: 10.1038/mp.2014.1
Chiodera, P., and Coiro, V. (1987). Oxytocin reduces metyrapone-induced ACTH secretion in human subjects. Brain Res. 420, 178–181. doi: 10.1016/0006-8993(87)90257-5
Cho, M. M., DeVries, A. C., Williams, J. R., and Carter, C. S. (1999). The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci. 113, 1071–1079. doi: 10.1037/0735-7044.113.5.1071
Christensen, J. C., Shiyanov, P. A., Estepp, J. R., and Schlager, J. J. (2014). Lack of association between human plasma oxytocin and interpersonal trust in a prisoner’s dilemma paradigm. PLoS ONE 9:e116172. doi: 10.1371/journal.pone.0116172
Churchland, P. S., and Winkielman, P. (2012). Modulating social behavior with oxytocin: how does it work? What does it mean? Horm. Behav. 61, 392–399. doi: 10.1016/j.yhbeh.2011.12.003
Clark, C. L., St John, N., Pasca, A. M., Hyde, S. A., Hornbeak, K., Abramova, M., et al. (2013). Neonatal CSF oxytocin levels are associated with parent report of infant soothability and sociability. Psychoneuroendocrinology 38, 1208–1212. doi: 10.1016/j.psyneuen.2012.10.017
Coan, J. A., Schaefer, H. S., and Davidson, R. J. (2006). Lending a hand: social regulation of the neural response to threat. Psychol. Sci. 17, 1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x
Colasanti, A., Searle, G. E., Long, C. J., Hill, S. P., Reiley, R. R., Quelch, D., et al. (2012). Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol. Psychiatry 72, 371–377. doi: 10.1016/j.biopsych.2012.01.027
Craig, A. D. (2009). How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70. doi: 10.1038/nrn2555
Crockford, C., Wittig, R. M., Langergraber, K., Ziegler, T. E., Zuberbuhler, K., and Deschner, T. (2013). Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. Biol. Sci. R. Soc. 280:20122765. doi: 10.1098/rspb.2012.2765
Croy, I., Angelo, S. D., and Olausson, H. (2014). Reduced pleasant touch appraisal in the presence of a disgusting odor. PLoS ONE 9:e92975. doi: 10.1371/journal.pone.0092975
Crusco, A. H., and Wetzel, C. G. (1984). The midas touch: the effects of interpersonal touch on restaurant tipping. Pers. Soc. Psychol. Bull. 10, 512–517. doi: 10.1177/0146167284104003
de Araujo, I. E., Rolls, E. T., Velazco, M. I., Margot, C., and Cayeux, I. (2005). Cognitive modulation of olfactory processing. Neuron 46, 671–679. doi: 10.1016/j.neuron.2005.04.021
De Dreu, C. K., and Kret, M. E. (2015). Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biol. Psychiatry doi: 10.1016/j.biopsych.2015.03.020 [Epub ahead of print].
Depue, R. A., and Morrone-Strupinsky, J. V. (2005a). A neurobehavioral model of affiliative bonding: Implications for conceptualizing a human trait of affiliation. Behav. Brain Sci. 28, 313–350. doi: 10.1017/S0140525X05000063
Ditzen, B., Neumann, I. D., Bodenmann, G., von Dawans, B., Turner, R. A., Ehlert, U., et al. (2007). Effects of different kinds of couple interaction on cortisol and heart rate responses to stress in women. Psychoneuroendocrinology 32, 565–574. doi: 10.1016/j.psyneuen.2007.03.011
Drago, F., Pedersen, C. A., Caldwell, J. D., and Prange, A. J. Jr. (1986). Oxytocin potently enhances novelty-induced grooming behavior in the rat. Brain Res. 368, 287–295. doi: 10.1016/0006-8993(86)90573-1
Drewnowski, A., Krahn, D. D., Demitrack, M. A., Nairn, K., and Gosnell, B. A. (1995). Naloxone, an opiate blocker, reduces the consumption of sweet high-fat foods in obese and lean female binge eaters. Am. J. Clin. Nutr. 61, 1206–1212.
Dunbar, R. I. (2010). The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci. Biobehav. Rev. 34, 260–268. doi: 10.1016/j.neubiorev.2008.07.001
Dunbar, R. I. M. (1991). Functional-significance of social grooming in primates. Folia Primatol. 57, 121–131. doi: 10.1159/000156574
Eaton, M., Mitchell-Bonair, I. L., and Friedmann, E. (1986). The effect of touch on nutritional intake of chronic organic brain syndrome patients. J. Gerontol. 41, 611–616. doi: 10.1093/geronj/41.5.611
Eippert, F., Bingel, U., Schoell, E. D., Yacubian, J., Klinger, R., Lorenz, J., et al. (2009a). Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543. doi: 10.1016/j.neuron.2009.07.014
Eippert, F., Finsterbusch, J., Bingel, U., and Buchel, C. (2009b). Direct evidence for spinal cord involvement in placebo analgesia. Science 326:404. doi: 10.1126/science.1180142
Ellingsen, D. M. (2014) Central Modulation of Affective Touch, Pain, and Emotion in Humans, Ph.D. thesis, Institute of Neuroscience and Physiology, University of Gothenburg, Gothenburg.
Ellingsen, D. M. (2015). How Do C-tactile skin afferents contribute to erotic affect? J. Sex. Med. 12:1656. doi: 10.1111/jsm.12929
Ellingsen, D. M., Leknes, S., and Kringelbach, M. L. (2015). “Hedonic value,” in Handbook of Value: Perspectives from Economics, Neuroscience, Philosophy, Psychology and Sociology, eds T. Brosch and D. Sander (Oxford: Oxford University Press), 265–286.
Ellingsen, D. M., Wessberg, J., Chelnokova, O., Olausson, H., Laeng, B., and Leknes, S. (2014). In touch with your emotions: oxytocin and touch change social impressions while others’ facial expressions can alter touch. Psychoneuroendocrinology 39, 11–20. doi: 10.1016/j.psyneuen.2013.09.017
Ellingsen, D. M., Wessberg, J., Eikemo, M., Liljencrantz, J., Endestad, T., Olausson, H., et al. (2013). Placebo improves pleasure and pain through opposite modulation of sensory processing. Proc. Natl. Acad. Sci. U.S.A. 110, 17993–17998. doi: 10.1073/pnas.1305050110
Elvemo, N. A., Landro, N. I., Borchgrevink, P. C., and Haberg, A. K. (2015). Reward responsiveness in patients with chronic pain. Eur. J. Pain 19, 1537–1543. doi: 10.1002/ejp.687
Erceau, D., and Guguen, N. (2007). Tactile contact and evaluation of the toucher. J. Soc. Psychol. 147, 441–444. doi: 10.3200/SOCP.147.4.441-444
Fairhurst, M. T., Loken, L., and Grossmann, T. (2014). Physiological and behavioral responses reveal 9-month-old infants’ sensitivity to pleasant touch. Psychol. Sci. 25, 1124–1131. doi: 10.1177/0956797614527114
Feldman, R. (2012). Oxytocin and social affiliation in humans. Horm. Behav. 61, 380–391. doi: 10.1016/j.yhbeh.2012.01.008
Feldman, R., Gordon, I., and Zagoory-Sharon, O. (2010a). The cross-generation transmission of oxytocin in humans. Horm. Behav. 58, 669–676. doi: 10.1016/j.yhbeh.2010.06.005
Feldman, R., Singer, M., and Zagoory, O. (2010b). Touch attenuates infants’ physiological reactivity to stress. Dev. Sci. 13, 271–278. doi: 10.1111/j.1467-7687.2009.00890.x
Feldman, R., Weller, A., Zagoory-Sharon, O., and Levine, A. (2007). Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol. Sci. 18, 965–970. doi: 10.1111/j.1467-9280.2007.02010.x
Ferguson, J. N., Young, L. J., Hearn, E. F., Matzuk, M. M., Insel, T. R., and Winslow, J. T. (2000). Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288. doi: 10.1038/77040
Field, T., Hernandez-Reif, M., Diego, M., Schanberg, S., and Kuhn, C. (2005). Cortisol decreases and serotonin and dopamine increase following massage therapy. Int. J. Neurosci. 115, 1397–1413. doi: 10.1080/00207450590956459
Fields, H. (2004). State-dependent opioid control of pain. Nat. Rev. Neurosci. 5, 565–575. doi: 10.1038/nrn1431
Fields, H. L. (2006). “A motivation-decision model of pain: the role of opioids,” in Proceedings of the 11th World Congress on Pain, eds H. Flor, et al. (Seattle: IASP Press), 449–459.
Fields, H. L. (2007). Understanding how opioids contribute to reward and analgesia. Region. Anesth. Pain Med. 32, 242–246. doi: 10.1097/00115550-200705000-00012
Fields, H. L. (2011). “Mu opioid receptor mediated analgesia and reward,” in The Opiate Receptors, Vol. 23, ed. G. W. Pasternak (New York, NY: Humana Press), 239–264.
Finkel, L. H., and Edelman, G. M. (1989). Integration of distributed cortical systems by reentry: a computer simulation of interactive functionally segregated visual areas. J. Neurosci. 9, 3188–3208.
Fiorio, M., Recchia, S., Corra, F., Simonetto, S., Garcia-Larrea, L., and Tinazzi, M. (2012). Enhancing non-noxious perception: behavioural and neurophysiological correlates of a placebo-like manipulation. Neuroscience 217, 96–104. doi: 10.1016/j.neuroscience.2012.04.066
Fischer-Shofty, M., Shamay-Tsoory, S. G., Harari, H., and Levkovitz, Y. (2010). The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia 48, 179–184. doi: 10.1016/j.neuropsychologia.2009.09.003
Fisher, J. D., Rytting, M., and Heslin, R. (1976). Hands touching hands: affective and evaluative effects of an interpersonal touch. Sociometry 39, 416–421. doi: 10.2307/3033506
Francis, D. D., Champagne, F. C., and Meaney, M. J. (2000). Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 12, 1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x
Francis, S., Rolls, E. T., Bowtell, R., McGlone, F., O’Doherty, J., Browning, A., et al. (1999). The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport 10, 453–459. doi: 10.1097/00001756-199902250-00003
Gamer, M., and Buchel, C. (2012). Oxytocin specifically enhances valence-dependent parasympathetic responses. Psychoneuroendocrinology 37, 87–93. doi: 10.1016/j.psyneuen.2011.05.007
Gazzola, V., Spezio, M. L., Etzel, J. A., Castelli, F., Adolphs, R., and Keysers, C. (2012). Primary somatosensory cortex discriminates affective significance in social touch. Proc. Natl. Acad. Sci. U.S.A. 109, E1657–E1666. doi: 10.1073/pnas.1113211109
Geuter, S., and Buchel, C. (2013). Facilitation of pain in the human spinal cord by nocebo treatment. J. Neurosci. 33, 13784–13790. doi: 10.1523/JNEUROSCI.2191-13.2013
Geuter, S., Eippert, F., Hindi Attar, C., and Buchel, C. (2013). Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage 67, 227–236. doi: 10.1016/j.neuroimage.2012.11.029
Gordon, I., Voos, A. C., Bennett, R. H., Bolling, D. Z., Pelphrey, K. A., and Kaiser, M. D. (2013). Brain mechanisms for processing affective touch. Hum. Brain Mapp. 34, 914–922. doi: 10.1002/hbm.21480
Gordon, I., Zagoory-Sharon, O., Leckman, J. F., and Feldman, R. (2010). Oxytocin and the development of parenting in humans. Biol. Psychiatry 68, 377–382. doi: 10.1016/j.biopsych.2010.02.005
Grewen, K. M., Anderson, B. J., Girdler, S. S., and Light, K. C. (2003). Warm partner contact is related to lower cardiovascular reactivity. Behav. Med. 29, 123–130. doi: 10.1080/08964280309596065
Grewen, K. M., Girdler, S. S., Amico, J., and Light, K. C. (2005). Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom. Med. 67, 531–538. doi: 10.1097/01.psy.0000170341.88395.47
Hagelberg, N., Kajander, J. K., Någren, K., Hinkka, S., Hietala, J., and Scheinin, H. (2002). Mu-Receptor agonism with alfentanil increases striatal dopamine D2 receptor binding in man. Synapse 45, 25–30. doi: 10.1002/syn.10078
Handlin, L., Hydbring-Sandberg, E., Nilsson, A., Ejdeback, M., Jansson, A., and Uvnas-Moberg, K. (2011). Short-term interaction between dogs and their owners: effects on oxytocin, cortisol, insulin and heart Rate-an exploratory study. Anthrozoos 24, 301–315. doi: 10.2752/175303711X13045914865385
Harari-Dahan, O., and Bernstein, A. (2014). A general approach - avoidance hypothesis of Oxytocin: accounting for social and non-social effects of oxytocin. Neurosci. Biobehav. Rev. 47C, 506–519. doi: 10.1016/j.neubiorev.2014.10.007
Harlow, H. F., and Zimmermann, R. R. (1959). Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science 130, 421–432. doi: 10.1126/science.130.3373.421
Hashmi, J. A., Kong, J., Spaeth, R., Khan, S., Kaptchuk, T. J., and Gollub, R. L. (2014). Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J. Neurosci. 34, 3924–3936. doi: 10.1523/JNEUROSCI.3155-13.2014
Heinrichs, M., Baumgartner, T., Kirschbaum, C., and Ehlert, U. (2003). Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry 54, 1389–1398. doi: 10.1016/S0006-3223(03)00465-7
Heinrichs, M., Meinlschmidt, G., Neumann, I., Wagner, S., Kirschbaum, C., Ehlert, U., et al. (2001). Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J. Clin. Endocrinol. Metab. 86, 4798–4804. doi: 10.1210/jcem.86.10.7919
Hertenstein, M. J., Keltner, D., App, B., Bulleit, B. A., and Jaskolka, A. R. (2006a). Touch communicates distinct emotions. Emotion 6, 528–533. doi: 10.1037/1528-3542.6.3.528
Hertenstein, M. J., Verkamp, J. M., Kerestes, A. M., and Holmes, R. M. (2006b). The communicative functions of touch in humans, nonhuman primates, and rats: a review and synthesis of the empirical research. Genet. Soc. Gen. Psychol. Monogr. 132, 5–94. doi: 10.3200/MONO.132.1.5-94
Ho, C. Y., and Berridge, K. C. (2013). An orexin hotspot in ventral pallidum amplifies hedonic ‘liking’ for sweetness. Neuropsychopharmacology 38, 1655–1664. doi: 10.1038/npp.2013.62
Holt-Lunstad, J., Birmingham, W. A., and Light, K. C. (2008). Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom. Med. 70, 976–985. doi: 10.1097/PSY.0b013e318187aef7
Hori, M., Shimoju, R., Tokunaga, R., Ohkubo, M., Miyabe, S., Ohnishi, J., et al. (2013). Tickling increases dopamine release in the nucleus accumbens and 50 kHz ultrasonic vocalizations in adolescent rats. Neuroreport 24, 241–245. doi: 10.1097/WNR.0b013e32835edbfa
Hornik, J. (1992). Tactile stimulation and consumer response. J. Consum. Res. 19, 449–458. doi: 10.1086/209314
Hsu, D. T., Sanford, B. J., Meyers, K. K., Love, T. M., Hazlett, K. E., Wang, H., et al. (2013). Response of the mu-opioid system to social rejection and acceptance. Mol. Psychiatry 18, 1211–1217. doi: 10.1038/mp.2013.96
Insel, T. R., and Hulihan, T. J. (1995). A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 109, 782–789. doi: 10.1037/0735-7044.109.4.782
Insel, T. R., and Winslow, J. T. (1998). Serotonin and neuropeptides in affiliative behaviors. Biol. Psychiatry 44, 207–219. doi: 10.1016/S0006-3223(98)00094-8
Johansen-Berg, H., Christensen, V., Woolrich, M., and Matthews, P. M. (2000). Attention to touch modulates activity in both primary and secondary somatosensory areas. Neuroreport 11, 1237–1241. doi: 10.1097/00001756-200004270-00019
Jokinen, J., Chatzittofis, A., Hellstrom, C., Nordstrom, P., Uvnas-Moberg, K., and Asberg, M. (2012). Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology 37, 482–490. doi: 10.1016/j.psyneuen.2011.07.016
Joule, R. V., and Gueguen, N. (2007). Touch, compliance, and awareness of tactile contact. Percept. Mot. Skills 104, 581–588. doi: 10.2466/pms.104.2.581-588
Kagerbauer, S. M., Martin, J., Schuster, T., Blobner, M., Kochs, E. F., and Landgraf, R. (2013). Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J. Neuroendocrinol. 25, 668–673. doi: 10.1111/jne.12038
Kaiser, M. D., Yang, D. Y., Voos, A. C., Bennett, R. H., Gordon, I., Pretzsch, C., et al. (2015). Brain mechanisms for processing affective (and Nonaffective) touch are atypical in Autism. Cereb Cortex doi: 10.1093/cercor/bhv125 [Epub ahead of print].
Kalaska, J. F. (1994). Central neural mechanisms of touch and proprioception. Can. J. Physiol. Pharmacol. 72, 542–545. doi: 10.1139/y94-078
Kemp, A. H., and Guastella, A. J. (2010). Oxytocin: prosocial behavior, social salience, or approach-related behavior? Biol. Psychiatry 67, e33–e34. doi: 10.1016/j.biopsych.2009.11.019
Kemp, A. H., and Guastella, A. J. (2011). The role of oxytocin in human affect: a novel hypothesis. Curr. Dir. Psychol. Sci. 20, 222–231. doi: 10.1177/0963721411417547
Kemp, A. H., Quintana, D. S., Kuhnert, R. L., Griffiths, K., Hickie, I. B., and Guastella, A. J. (2012). Oxytocin increases heart rate variability in humans at rest: implications for social approach-related motivation and capacity for social engagement. PLoS ONE 7:e44014. doi: 10.1371/journal.pone.0044014
Kersten, D., Mamassian, P., and Yuille, A. (2004). Object perception as Bayesian inference. Annu. Rev. Psychol. 55, 271–304. doi: 10.1146/annurev.psych.55.090902.142005
Kessner, S., Forkmann, K., Ritter, C., Wiech, K., Ploner, M., and Bingel, U. (2014). The effect of treatment history on therapeutic outcome: psychological and neurobiological underpinnings. PLoS ONE 9:e109014. doi: 10.1371/journal.pone.0109014
Keverne, E. B., Martensz, N. D., and Tuite, B. (1989). Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology 14, 155–161. doi: 10.1016/0306-4530(89)90065-6
Kinchla, R. A., and Wolfe, J. M. (1979). Order of visual processing - top-down. Bottom up, or Middle-Out. Percept. Psychophys. 25, 225–231. doi: 10.3758/BF03202991
Kirsch, P., Esslinger, C., Chen, Q., Mier, D., Lis, S., Siddhanti, S., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005
Kleinke, C. L. (1977). Complience to requests made by gazing and touching experimenters in field settings. J. Exp. Soc. Psychol. 13, 218–223. doi: 10.1016/0022-1031(77)90044-0
Knapp, M. L., and Hall, J. A. (1997). Nonverbal Communication in Human Interaction, 4th Edn. Fort Worth, TX: Harcourt Brace College.
Knill, D. C., and Richard, W. (1996). Perception as Bayesian Inference. Cambridge: Cambridge University Press.
Knudsen, L., Petersen, G. L., Nørskov, K. N., Vase, L., Finnerup, N., Jensen, T. S., et al. (2011). Review of neuroimaging studies related to pain modulation. Scand. J. Pain 2, 108–120. doi: 10.1016/j.sjpain.2011.05.005
Kreek, M. J. (1996). Opioid receptors: some perspectives from early studies of their role in normal physiology, stress responsivity, and in specific addictive diseases. Neurochem. Res. 21, 1469–1488. doi: 10.1007/BF02532387
Kveraga, K., Ghuman, A. S., and Bar, M. (2007). Top-down predictions in the cognitive brain. Brain Cogn. 65, 145–168. doi: 10.1016/j.bandc.2007.06.007
Lebreton, M., Abitbol, R., Daunizeau, J., and Pessiglione, M. (2015). Automatic integration of confidence in the brain valuation signal. Nat. Neurosci. 18, 1159–1167. doi: 10.1038/nn.4064
Legros, J. J., Chiodera, P., and Geenen, V. (1988). Inhibitory action of exogenous oxytocin on plasma cortisol in normal human subjects: evidence of action at the adrenal level. Neuroendocrinology 48, 204–206. doi: 10.1159/000125009
Leknes, S., and Bastian, B. (2014). The Benefits of Pain. Rev. Philos. Psychol. 5, 57–70. doi: 10.1007/s13164-014-0178-3
Leknes, S., and Tracey, I. (2008). A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 9, 314–320. doi: 10.1038/nrn2333
Leknes, S., Wessberg, J., Ellingsen, D. M., Chelnokova, O., Olausson, H., and Laeng, B. (2013). Oxytocin enhances pupil dilation and sensitivity to ’hidden’ emotional expressions. Soc. Cogn. Affect. Neurosci. 8, 741–749. doi: 10.1093/scan/nss062
Light, K. C., Grewen, K. M., and Amico, J. A. (2005). More frequent partner hugs and higher oxytocin levels are linked to lower blood pressure and heart rate in premenopausal women. Biol. Psychol. 69, 5–21. doi: 10.1016/j.biopsycho.2004.11.002
Light, K. C., Smith, T. E., Johns, J. M., Brownley, K. A., Hofheimer, J. A., and Amico, J. A. (2000). Oxytocin responsivity in mothers of infants: a preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Health Psychol. 19, 560–567. doi: 10.1037/0278-6133.19.6.560
Liljencrantz, J., Ellingsen, D. M., Leknes, S., Kramer, H., Spadoni, A. D., Kosheleva, E., et al. (2012). “C-tactile afferent stimulation modulate pain perception,” in Society for Neuroscience, New Orleans, LA.
Liljencrantz, J., Marshall, A., Ackerley, R., and Olausson, H. (2014). Discriminative and affective touch in human experimental tactile allodynia. Neurosci. Lett. 563, 75–79. doi: 10.1016/j.neulet.2014.01.041
Liljencrantz, J., and Olausson, H. (2014). Tactile C fibers and their contributions to pleasant sensations and to tactile allodynia. Front. Behav. Neurosci. 8:37. doi: 10.3389/fnbeh.2014.00037
Lindgren, L., Westling, G., Brulin, C., Lehtipalo, S., Andersson, M., and Nyberg, L. (2012). Pleasant human touch is represented in pregenual anterior cingulate cortex. Neuroimage 59, 3427–3432. doi: 10.1016/j.neuroimage.2011.11.013
Lindquist, K. A., Satpute, A. B., Wager, T. D., Weber, J., and Barrett, L. F. (2015). the brain basis of positive and negative affect: evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex doi: 10.1093/cercor/bhv001 [Epub ahead of print].
Lintas, A., Chi, N., Lauzon, N. M., Bishop, S. F., Gholizadeh, S., Sun, N., et al. (2011). Identification of a dopamine receptor-mediated opiate reward memory switch in the basolateral amygdala-nucleus accumbens circuit. J. Neurosci. 31, 11172–11183. doi: 10.1523/JNEUROSCI.1781-11.2011
Liu, Y., and Wang, Z. X. (2003). Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121, 537–544. doi: 10.1016/S0306-4522(03)00555-4
Loken, L. S., Evert, M., and Wessberg, J. (2011). Pleasantness of touch in human glabrous and hairy skin: order effects on affective ratings. Brain Res. 1417, 9–15. doi: 10.1016/j.brainres.2011.08.011
Löken, L. S., Wessberg, J., Morrison, I., McGlone, F., and Olausson, H. (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nat. Neurosci. 12, 547–548. doi: 10.1038/nn.2312
Løseth, G. E., Ellingsen, D. M., and Leknes, S. (2014). State-dependent mu-opioid modulation of social motivation. Front. Behav. Neurosci. 8:430. doi: 10.3389/fnbeh.2014.00430
Løseth, G., Leknes, S., and Ellingsen, D. M. (in press). “The neurochemical basis of motivation for affiliative touch,” in Affective Touch and the Neurophysiology of CT Afferents, eds H. Olausson, et al. (Berlin: Springer).
Lou, S., Duan, B., Vong, L., Lowell, B. B., and Ma, Q. (2013). Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J. Neurosci. 33, 870–882. doi: 10.1523/JNEUROSCI.3942-12.2013
Lu, H. C., Hsieh, J. C., Lu, C. L., Niddam, D. M., Wu, Y. T., Yeh, T. C., et al. (2010). Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: a 3T-fMRI study. Pain 148, 75–83. doi: 10.1016/j.pain.2009.10.012
Lupoli, B., Johansson, B., Uvnas-Moberg, K., and Svennersten-Sjaunja, K. (2001). Effect of suckling on the release of oxytocin, prolactin, cortisol, gastrin, cholecystokinin, somatostatin and insulin in dairy cows and their calves. J. Dairy Res. 68, 175–187. doi: 10.1017/S0022029901004721
Macaluso, E., and Driver, J. (2001). Spatial attention and crossmodal interactions between vision and touch. Neuropsychologia 39, 1304–1316. doi: 10.1016/S0028-3932(01)00119-1
Machin, A. J., and Dunbar, R. I. M. (2011). The brain opioid theory of social attachment: a review of the evidence. Behaviour 148, 985–1025. doi: 10.1163/000579511X596624
Maestripieri, D., Hoffman, C. L., Anderson, G. M., Carter, C. S., and Higley, J. D. (2009). Mother-infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol. Behav. 96, 613–619. doi: 10.1016/j.physbeh.2008.12.016
Mahler, S. V., and Berridge, K. C. (2012). What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology 221, 407–426. doi: 10.1007/s00213-011-2588-6
Major, B. (1981). “Gender patterns in touching behavior,” in Gender and nonverbal behavior, eds C. Mayo and N. M. Henley (New York, NY: Springer), 15–37.
Mancini, F., Beaumont, A. L., Hu, L., Haggard, P., and Iannetti, G. D. (2015). Touch inhibits subcortical and cortical nociceptive responses. Pain 156, 1936–1944. doi: 10.1097/j.pain.0000000000000253
Mancini, F., Nash, T., Iannetti, G. D., and Haggard, P. (2014). Pain relief by touch: a quantitative approach. Pain 155, 635–642. doi: 10.1016/j.pain.2013.12.024
Marsh, A. A., Yu, H. H., Pine, D. S., and Blair, R. J. (2010). Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology 209, 225–232. doi: 10.1007/s00213-010-1780-4
Maruyama, K., Shimoju, R., Ohkubo, M., Maruyama, H., and Kurosawa, M. (2012). Tactile skin stimulation increases dopamine release in the nucleus accumbens in rats. J. Physiol. Sci. 62, 259–266. doi: 10.1007/s12576-012-0205-z
Matthiesen, A. S., Ransjo-Arvidson, A. B., Nissen, E., and Uvnas-Moberg, K. (2001). Postpartum maternal oxytocin release by newborns: effects of infant hand massage and sucking. Birth 28, 13–19. doi: 10.1046/j.1523-536x.2001.00013.x
McAlonan, K., Cavanaugh, J., and Wurtz, R. H. (2008). Guarding the gateway to cortex with attention in visual thalamus. Nature 456, 391–394. doi: 10.1038/nature07382
McCabe, C., Rolls, E. T., Bilderbeck, A., and McGlone, F. (2008). Cognitive influences on the affective representation of touch and the sight of touch in the human brain. Soc. Cogn. Affect. Neurosci. 3, 97–108. doi: 10.1093/scan/nsn005
McCullough, M. E., Churchland, P. S., and Mendez, A. J. (2013). Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci. Biobehav. Rev. 37, 1485–1492. doi: 10.1016/j.neubiorev.2013.04.018
McDannald, M. A., Lucantonio, F., Burke, K. A., Niv, Y., and Schoenbaum, G. (2011). Ventral striatum and orbitofrontal cortex are both required for model-based, but not model-free, reinforcement learning. J. Neurosci. 31, 2700–2705. doi: 10.1523/JNEUROSCI.5499-10.2011
McGlone, F., Kelly, E. F., Trulsson, M., Francis, S. T., Westling, G., and Bowtell, R. (2002). Functional neuroimaging studies of human somatosensory cortex. Behav. Brain Res. 135, 147–158. doi: 10.1016/S0166-4328(02)00144-4
McGlone, F., Olausson, H., Boyle, J. A., Jones-Gotman, M., Dancer, C., Guest, S., et al. (2012). Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. Eur. J. Neurosci. 35, 1782–1788. doi: 10.1111/j.1460-9568.2012.08092.x
McGlone, F., Wessberg, J., and Olausson, H. (2014). Discriminative and affective touch: sensing and feeling. Neuron 82, 737–755. doi: 10.1016/j.neuron.2014.05.001
Millan, M. J. (2002). Descending control of pain. Progr. Neurobiol. 66, 355–474. doi: 10.1016/S0301-0082(02)00009-6
Miller, S. C., Kennedy, C., Devoe, D., Hickey, M., Nelson, T., and Kogan, L. (2009). An Examination of changes in oxytocin levels in men and women before and after interaction with a bonded dog. Anthrozoos 22, 31–42. doi: 10.2752/175303708X390455
Monroe, C. M. (2009). The Effects of Therapeutic Touch on Pain. J. Holistic Nurs. 27, 85–92. doi: 10.1177/0898010108327213
Montoya, P., and Sitges, C. (2006). Affective modulation of somatosensory-evoked potentials elicited by tactile stimulation. Brain Res. 1068, 205–212. doi: 10.1016/j.brainres.2005.11.019
Morrison, I., Loken, L. S., and Olausson, H. (2010). The skin as a social organ. Exp. Brain Res. Exp. Hirnforsch. Exp. Cereb. 204, 305–314. doi: 10.1007/s00221-009-2007-y
Nagi, S. S., Rubin, T. K., Chelvanayagam, D. K., Macefield, V. G., and Mahns, D. A. (2011). Allodynia mediated by C-tactile afferents in human hairy skin. J. Physiol. 589, 4065–4075. doi: 10.1113/jphysiol.2011.211326
Nakajima, M., Gorlich, A., and Heintz, N. (2014). Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159, 295–305. doi: 10.1016/j.cell.2014.09.020
Nelson, E. E., and Panksepp, J. (1998). Brain substrates of infant-mother attachment: Contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev. 22, 437–452. doi: 10.1016/S0149-7634(97)00052-3
Neubert, M. J., Kincaid, W., and Heinricher, M. M. (2004). Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain 110, 158–165. doi: 10.1016/j.pain.2004.03.017
Neumann, I. D., Kromer, S. A., Toschi, N., and Ebner, K. (2000a). Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul. Pept. 96, 31–38. doi: 10.1016/S0167-0115(00)00197-X
Neumann, I. D., Wigger, A., Torner, L., Holsboer, F., and Landgraf, R. (2000b). Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J. Neuroendocrinol. 12, 235–243. doi: 10.1046/j.1365-2826.2000.00442.x
Neumann, I. D., and Landgraf, R. (2012). Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649–659. doi: 10.1016/j.tins.2012.08.004
Nitschke, J. B., Dixon, G. E., Sarinopoulos, I., Short, S. J., Cohen, J. D., Smith, E. E., et al. (2006). Altering expectancy dampens neural response to aversive taste in primary taste cortex. Nat. Neurosci. 9, 435–442. doi: 10.1038/nn1645
Nunez, A., and Malmierca, E. (2007). Corticofugal modulation of sensory information. Adv. Anat. Embryol. Cell Biol. 187, 1–74.
Odendaal, J. S., and Meintjes, R. A. (2003). Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J. 165, 296–301. doi: 10.1016/S1090-0233(02)00237-X
Olausson, H. W., Cole, J., Vallbo, A., McGlone, F., Elam, M., Kramer, H. H., et al. (2008). Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci. Lett. 436, 128–132. doi: 10.1016/j.neulet.2008.03.015
Olausson, H., Lamarre, Y., Backlund, H., Morin, C., Wallin, B. G., Starck, G., et al. (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nat. Neurosci. 5, 900–904. doi: 10.1038/nn896
Olausson, H., Wessberg, J., Morrison, I., McGlone, F., and Vallbo, A. (2010). The neurophysiology of unmyelinated tactile afferents. Neurosci. Biobehav. Rev. 34, 185–191. doi: 10.1016/j.neubiorev.2008.09.011
Olazabal, D. E., and Young, L. J. (2006). Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience 141, 559–568. doi: 10.1016/j.neuroscience.2006.04.017
O’Reilly, J. X., Jbabdi, S., Rushworth, M. F., and Behrens, T. E. (2013). Brain systems for probabilistic and dynamic prediction: computational specificity and integration. PLoS Biol. 11:e1001662. doi: 10.1371/journal.pbio.1001662
Panksepp, J., and Bishop, P. (1981). An autoradiographic map of (3H)diprenorphine binding in rat brain: effects of social interaction. Brain Res. Bull. 7, 405–410. doi: 10.1016/0361-9230(81)90038-1
Panksepp, J., Herman, B., Conner, R., Bishop, P., and Scott, J. P. (1978). The biology of social attachments: opiates alleviate separation distress. Biol. Psychiatry 13, 607–618.
Panksepp, J., Nelson, E., and Bekkedal, M. (1997). Brain systems for the mediation of social separation-distress and social-reward. Evolutionary antecedents and neuropeptide intermediaries. Ann. N. Y. Acad. Sci. 807, 78–100. doi: 10.1111/j.1749-6632.1997.tb51914.x
Parker, K. J., Buckmaster, C. L., Schatzberg, A. F., and Lyons, D. M. (2005). Intranasal oxytocin administration attenuates the ACTH stress response in monkeys. Psychoneuroendocrinology 30, 924–929. doi: 10.1016/j.psyneuen.2005.04.002
Pecina, M., Azhar, H., Love, T. M., Lu, T., Fredrickson, B. L., Stohler, C. S., et al. (2013). Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology 38, 639–646. doi: 10.1038/npp.2012.227
Pecina, S., and Berridge, K. C. (2005). Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci. 25, 11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005
Pedersen, C. A., Ascher, J. A., Monroe, Y. L., and Prange, A. J. Jr. (1982). Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650. doi: 10.1126/science.7071605
Pedersen, C. A., Caldwell, J. D., Drago, F., Noonan, L. R., Peterson, G., Hood, L. E., et al. (1988). Grooming behavioral effects of oxytocin. Pharmacology, ontogeny, and comparisons with other nonapeptides. Ann. N. Y. Acad. Sci. 525, 245–256. doi: 10.1111/j.1749-6632.1988.tb38610.x
Pedersen, C. A., and Prange, A. J. Jr. (1979). Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. U.S.A. 76, 6661–6665. doi: 10.1073/pnas.76.12.6661
Perini, I., Olausson, H., and Morrison, I. (2015). Seeking pleasant touch: neural correlates of behavioral preferences for skin stroking. Front. Behav. Neurosci. 9:8. doi: 10.3389/fnbeh.2015.00008
Perl, E. R., Whitlock, D. G., and Gentry, J. R. (1962). Cutaneous projection to second-order neurons of the dorsal column system. J. Neurophysiol. 25, 337–358.
Petit, D., and Burgess, P. R. (1968). Dorsal column projection of receptors in cat hairy skin supplied by myelinated fibers. J. Neurophysiol. 31, 849–855.
Petrovic, P., Dietrich, T., Fransson, P., Andersson, J., Carlsson, K., and Ingvar, M. (2005). Placebo in emotional processing–induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46, 957–969. doi: 10.1016/j.neuron.2005.05.023
Petrovic, P., Kalso, E., Petersson, K. M., and Ingvar, M. (2002). Placebo and opioid analgesia– imaging a shared neuronal network. Science 295, 1737–1740. doi: 10.1126/science.1067176
Pizzagalli, D. A., Iosifescu, D., Hallett, L. A., Ratner, K. G., and Fava, M. (2008). Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res. 43, 76–87. doi: 10.1016/j.jpsychires.2008.03.001
Porges, S. W. (2007). The polyvagal perspective. Biol. Psychol. 74, 116–143. doi: 10.1016/j.biopsycho.2006.06.009
Price, D. D., Craggs, J., Verne, G. N., Perlstein, W. M., and Robinson, M. E. (2007). Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain 127, 63–72. doi: 10.1016/j.pain.2006.08.001
Rehn, T., Handlin, L., Uvnas-Moberg, K., and Keeling, L. J. (2014). Dogs’ endocrine and behavioural responses at reunion are affected by how the human initiates contact. Physiol. Behav. 124, 45–53. doi: 10.1016/j.physbeh.2013.10.009
Rolls, E. T., O’Doherty, J., Kringelbach, M. L., Francis, S., Bowtell, R., and McGlone, F. (2003). Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. Cortex 13, 308–317. doi: 10.1093/cercor/13.3.308
Romer Thomsen, K., Whybrow, P. C., and Kringelbach, M. L. (2015). Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front. Behav. Neurosci. 9:49. doi: 10.3389/fnbeh.2015.00049
Ross, H. E., Freeman, S. M., Spiegel, L. L., Ren, X., Terwilliger, E. F., and Young, L. J. (2009). Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009
Roy, M., Shohamy, D., and Wager, T. D. (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci. 16, 147–156. doi: 10.1016/j.tics.2012.01.005
Rozin, P., Guillot, L., Fincher, K., Rozin, A., and Tsukayama, E. (2013). Glad to be sad, and other examples of benign masochism. Judgm Decis Mak 8, 439–447.
Schedlowski, M., Enck, P., Rief, W., and Bingel, U. (2015). Neuro-bio-behavioral mechanisms of placebo and nocebo responses: implications for clinical trials and clinical practice. Pharmacol. Rev. 67, 697–730. doi: 10.1124/pr.114.009423
Scheele, D., Kendrick, K. M., Khouri, C., Kretzer, E., Schlapfer, T. E., Stoffel-Wagner, B., et al. (2014). An oxytocin-induced facilitation of neural and emotional responses to social touch correlates inversely with autism traits. Neuropsychopharmacology 39, 2078–2085. doi: 10.1038/npp.2014.78
Schepers, R. J., and Ringkamp, M. (2009). Thermoreceptors and thermosensitive afferents. Neurosci. Biobehav. Rev. 33, 205–212. doi: 10.1016/j.neubiorev.2008.07.009
Schmack, K., de Castro, A., Rothkirch, M., Sekutowicz, M., Rossler, H., Haynes, J. D., et al. (2013). Delusions and the role of beliefs in perceptual inference. J. Neurosci. 33, 13701–13712. doi: 10.1523/JNEUROSCI.1778-13.2013
Schoenbaum, G., Roesch, M. R., Stalnaker, T. A., and Takahashi, Y. K. (2011). “Orbitofrontal cortex and outcome expectancies: optimizing behavior and sensory perception,” in Neurobiology of Sensation and Reward, ed. J. A. Gottfried (Boca Raton, FL: CRC Press).
Schubert, R., Ritter, P., Wustenberg, T., Preuschhof, C., Curio, G., Sommer, W., et al. (2008). Spatial attention related SEP amplitude modulations covary with BOLD signal in S1–a simultaneous EEG–fMRI study. Cereb. Cortex 18, 2686–2700. doi: 10.1093/cercor/bhn029
Schwandt, M. L., Howell, S., Bales, K., Jaffe, B. D., Westergaard, G. C., and Higley, J. D. (2007). Associations between the neuropeptides oxytocin and vasopressin and the behavior of free-ranging female rhesus macaques (Macaca mulatta). Am. J. Phys. Anthropol. 27, 210–211.
Schwarz, N. (2012). “Feelings-as-information theory,” in The Handbook of Theories of Social Psychology, eds P. A. M. Van Lange, et al. (Thousand Oaks, CA: Sage Publications Ltd.), 289–308.
Scott, D. J., Stohler, C. S., Egnatuk, C. M., Wang, H., Koeppe, R. A., and Zubieta, J. K. (2007). Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55, 325–336. doi: 10.1016/j.neuron.2007.06.028
Scott, D. J., Stohler, C. S., Egnatuk, C. M., Wang, H., Koeppe, R. A., and Zubieta, J. K. (2008). Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry 65, 220–231. doi: 10.1001/archgenpsychiatry.2007.34
Seal, R. P., Wang, X., Guan, Y., Raja, S. N., Woodbury, C. J., Basbaum, A. I., et al. (2009). Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature 462, 651–655. doi: 10.1038/nature08505
Seltzer, L. J., and Ziegler, T. E. (2007). Non-invasive measurement of small peptides in the common marmoset (Callithrix jacchus): a radiolabeled clearance study and endogenous excretion under varying social conditions. Horm. Behav. 51, 436–442. doi: 10.1016/j.yhbeh.2006.12.012
Sevel, L. S., O’Shea, A. M., Letzen, J. E., Craggs, J. G., Price, D. D., and Robinson, M. E. (2015). Effective connectivity predicts future placebo analgesic response: a dynamic causal modeling study of pain processing in healthy controls. Neuroimage 110C, 87–94. doi: 10.1016/j.neuroimage.2015.01.056
Shamay-Tsoory, S. G., Fischer, M., Dvash, J., Harari, H., Perach-Bloom, N., and Levkovitz, Y. (2009). Intranasal administration of oxytocin increases envy and schadenfreude (gloating). Biol. Psychiatry 66, 864–870. doi: 10.1016/j.biopsych.2009.06.009
Shapiro, L. E., Meyer, M. E., and Dewsbury, D. A. (1989). Affiliative behavior in voles: effects of morphine, naloxone, and cross-fostering. Physiol. Behav. 46, 719–723. doi: 10.1016/0031-9384(89)90357-0
Siegel, M., Kording, K. P., and Konig, P. (2000). Integrating top-down and bottom-up sensory processing by somato-dendritic interactions. J. Comput. Neurosci. 8, 161–173. doi: 10.1023/A:1008973215925
Small, D. M., Zatorre, R. J., Dagher, A., Evans, A. C., and Jones-Gotman, M. (2001). Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain 124, 1720–1733. doi: 10.1093/brain/124.9.1720
Smith, A. S., Agmo, A., Birnie, A. K., and French, J. A. (2010). Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 57, 255–262. doi: 10.1016/j.yhbeh.2009.12.004
Snowdon, C. T., Pieper, B. A., Boe, C. Y., Cronin, K. A., Kurian, A. V., and Ziegler, T. E. (2010). Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm. Behav. 58, 614–618. doi: 10.1016/j.yhbeh.2010.06.014
Suvilehto, J. T., Glerean, E., Dunbar, R. I., Hari, R., and Nummenmaa, L. (2015). Topography of social touching depends on emotional bonds between humans. Proc. Natl. Acad. Sci. U.S.A. 112, 13811–13816. doi: 10.1073/pnas.1519231112
Taylor-Clarke, M., Kennett, S., and Haggard, P. (2002). Vision modulates somatosensory cortical processing. Curr. Biol. 12, 233–236. doi: 10.1016/S0960-9822(01)00681-9
Tindell, A. J., Smith, K. S., Pecina, S., Berridge, K. C., and Aldridge, J. W. (2006). Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J. Neurophysiol. 96, 2399–2409. doi: 10.1152/jn.00576.2006
Tops, M., Huffmeijer, R., Linting, M., Grewen, K. M., Light, K. C., Koole, S. L., et al. (2013). The role of oxytocin in familiarization-habituation responses to social novelty. Front. Psychol. 4:761. doi: 10.3389/fpsyg.2013.00761
Tops, M., Koole, S. L., IJzerman, H., and Buisman-Pijlman, F. T. A. (2014). Why social attachment and oxytocin protect against addiction and stress: Insights from the dynamics between ventral and dorsal corticostriatal systems. Pharmacol. Biochem. 119, 39–48. doi: 10.1016/j.pbb.2013.07.015
Tops, M., van Peer, J. M., Korf, J., Wijers, A. A., and Tucker, D. M. (2007). Anxiety, cortisol, and attachment predict plasma oxytocin. Psychophysiology 44, 444–449. doi: 10.1111/j.1469-8986.2007.00510.x
Tracey, I., and Mantyh, P. W. (2007). The cerebral signature for pain perception and its modulation. Neuron 55, 377–391. doi: 10.1016/j.neuron.2007.07.012
Trezza, V., Damsteegt, R., Manduca, A., Petrosino, S., Van Kerkhof, L. W., Pasterkamp, R. J., et al. (2012). Endocannabinoids in amygdala and nucleus accumbens mediate social play reward in adolescent rats. J. Neurosci. 32, 14899–14908. doi: 10.1523/JNEUROSCI.0114-12.2012
Trezza, V., and Vanderschuren, L. J. M. J. (2008a). Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. Eur. Neuropsychopharmacol. 18, 519–530. doi: 10.1016/j.euroneuro.2008.03.001
Trezza, V., and Vanderschuren, L. J. M. J. (2008b). Bidirectional cannabinoid modulation of social behavior in adolescent rats. Psychopharmacology 197, 217–227. doi: 10.1007/s00213-007-1025-3
Triscoli, C., Ackerley, R., and Sailer, U. (2014). Touch satiety: differential effects of stroking velocity on liking and wanting touch over repetitions. PLoS ONE 9:e113425. doi: 10.1371/journal.pone.0113425
Turner, R. A., Altemus, M., Enos, T., Cooper, B., and McGuinness, T. (1999). Preliminary research on plasma oxytocin in normal cycling women: investigating emotion and interpersonal distress. Psychiatry 62, 97–113.
Ullman, S. (1995). Sequence seeking and counter streams: a computational model for bidirectional information flow in the visual cortex. Cereb. Cortex 5, 1–11. doi: 10.1093/cercor/5.1.1
Unkelbach, C., Guastella, A. J., and Forgas, J. P. (2008). Oxytocin selectively facilitates recognition of positive sex and relationship words. Psychol. Sci. 19, 1092–1094. doi: 10.1111/j.1467-9280.2008.02206.x
Urban, M. O., and Gebhart, G. F. (1999). Supraspinal contributions to hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 96, 7687–7692. doi: 10.1073/pnas.96.14.7687
Uvnas-Moberg, K. (2004). “Massage relaxation and well-being: A possible role for oxytocin as an integrative principle,” in Touch and Massage in Early Child Development, ed. T. Field (Calverton, NY: Johnson & Johnson Pediatric Institute), 191–208.
Uvnas-Moberg, K., Handlin, L., and Petersson, M. (2014). Self-soothing behaviors with particular reference to oxytocin release induced by non-noxious sensory stimulation. Front. Psychol. 5:1529. doi: 10.3389/fpsyg.2014.01529
Vallbo, A., Olausson, H., Wessberg, J., and Norrsell, U. (1993). A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 628, 301–304. doi: 10.1016/0006-8993(93)90968-S
van den Berg, C. L., Hol, T., Van Ree, J. M., Spruijt, B. M., Everts, H., and Koolhaas, J. M. (1999). Play is indispensable for an adequate development of coping with social challenges in the rat. Dev. Psychobiol. 34, 129–138. doi: 10.1002/(SICI)1098-2302(199903)34:2<129::AID-DEV6>3.0.CO;2-L
Vanderschuren, L. J., Niesink, R. J., Spruijt, B. M., and Van Ree, J. M. (1995). Effects of morphine on different aspects of social play in juvenile rats. Psychopharmacology 117, 225–231. doi: 10.1007/BF02245191
Vrontou, S., Wong, A. M., Rau, K. K., Koerber, H. R., and Anderson, D. J. (2013). Genetic identification of C fibres that detect massage-like stroking of hairy skin in vivo. Nature 493, 669–673. doi: 10.1038/nature11810
Wager, T. D., and Atlas, L. Y. (2015). The neuroscience of placebo effects: connecting context, learning and health. Nat. Rev. Neurosci. 16, 403–418. doi: 10.1038/nrn3976
Wager, T. D., Rilling, J. K., Smith, E. E., Sokolik, A., Casey, K. L., Davidson, R. J., et al. (2004). Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303, 1162–1167. doi: 10.1126/science.1093065
Wager, T. D., Scott, D. J., and Zubieta, J. K. (2007). Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. U.S.A. 104, 11056–11061. doi: 10.1073/pnas.0702413104
Walker, S. C., and McGlone, F. P. (2013). The social brain: neurobiological basis of affiliative behaviours and psychological well-being. Neuropeptides 47, 379–393. doi: 10.1016/j.npep.2013.10.008
Wall, P. D. (1967). The laminar organization of dorsal horn and effects of descending impulses. J. Physiol. 188, 403–423. doi: 10.1113/jphysiol.1967.sp008146
Wand, G. S., McCaul, M., Yang, X., Reynolds, J., Gotjen, D., Lee, S., et al. (2002). The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology 26, 106–114. doi: 10.1016/S0893-133X(01)00294-9
Watson, A., El-Deredy, W., Iannetti, G. D., Lloyd, D., Tracey, I., Vogt, B. A., et al. (2009). Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145, 24–30. doi: 10.1016/j.pain.2009.04.003
Weisman, O., and Feldman, R. (2013). Oxytocin administration affects the production of multiple hormones. Psychoneuroendocrinology 38, 626–627. doi: 10.1016/j.psyneuen.2013.03.004
Weisman, O., Zagoory-Sharon, O., and Feldman, R. (2014). Oxytocin administration, salivary testosterone, and father-infant social behavior. Progr. Neuro Psychopharmacol. Biol. Psychiatry 49, 47–52. doi: 10.1016/j.pnpbp.2013.11.006
Wessberg, J., Olausson, H., Fernstrom, K. W., and Vallbo, A. B. (2003). Receptive field properties of unmyelinated tactile afferents in the human skin. J. Neurophysiol. 89, 1567–1575. doi: 10.1152/jn.00256.2002
Whitcher, S. J., and Fisher, J. D. (1979). Multidimensional reaction to therapeutic touch in a hospital setting. J. Pers. Soc. Psychol. 37, 87–96. doi: 10.1037/0022-3514.37.1.87
Wikstrom, S., Gunnarsson, T., and Nordin, C. (2003). Tactile stimulus and neurohormonal response: a pilot study. Int. J. Neurosci. 113, 787–793. doi: 10.1080/00207450390200954
Winkielman, P., Berridge, K. C., and Wilbarger, J. L. (2005). Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers. Soc. Psychol. Bull. 31, 121–135. doi: 10.1177/0146167204271309
Winslow, J. T., Hastings, N., Carter, C. S., Harbaugh, C. R., and Insel, T. R. (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. doi: 10.1038/365545a0
Winslow, J. T., Noble, P. L., Lyons, C. K., Sterk, S. M., and Insel, T. R. (2003). Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology 28, 910–918.
Witt, D. M., Winslow, J. T., and Insel, T. R. (1992). Enhanced social interactions in rats following chronic, centrally infused oxytocin. Pharmacol. Biochem. Behav. 43, 855–861. doi: 10.1016/0091-3057(92)90418-F
Woldorff, M. G., Gallen, C. C., Hampson, S. A., Hillyard, S. A., Pantev, C., Sobel, D., et al. (1993). Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proc. Natl. Acad. Sci. U.S.A. 90, 8722–8726. doi: 10.1073/pnas.90.18.8722
Woods, A. T., Lloyd, D. M., Kuenzel, J., Poliakoff, E., Dijksterhuis, G. B., and Thomas, A. (2011). Expected taste intensity affects response to sweet drinks in primary taste cortex. Neuroreport 22, 365–369. doi: 10.1097/WNR.0b013e3283469581
Woolf, C. J. (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain 152, S2–S15. doi: 10.1016/j.pain.2010.09.030
Wrobel, N., Wiech, K., Forkmann, K., Ritter, C., and Bingel, U. (2014). Haloperidol blocks dorsal striatum activity but not analgesia in a placebo paradigm. Cortex 57, 60–73. doi: 10.1016/j.cortex.2014.02.023
Yeomans, M. R., and Gray, R. W. (1997). Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol. Behav. 62, 15–21. doi: 10.1016/S0031-9384(97)00101-7
Young, K. A., Gobrogge, K. L., Liu, Y., and Wang, Z. (2011). The neurobiology of pair bonding: insights from a socially monogamous rodent. Front. Neuroendocrinol. 32:6. doi: 10.1016/j.yfrne.2010.07.006
Zubieta, J. K., Bueller, J. A., Jackson, L. R., Scott, D. J., Xu, Y., Koeppe, R. A., et al. (2005). Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 25, 7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005
Keywords: touch, top–down modulation, hedonics, oxytocin, opioids, social processing, placebo effect
Citation: Ellingsen D-M, Leknes S, Løseth G, Wessberg J and Olausson H (2016) The Neurobiology Shaping Affective Touch: Expectation, Motivation, and Meaning in the Multisensory Context. Front. Psychol. 6:1986. doi: 10.3389/fpsyg.2015.01986
Received: 05 October 2015; Accepted: 12 December 2015;
Published: 06 January 2016.
Edited by:
Mattie Tops, VU University Amsterdam, NetherlandsReviewed by:
Susannah Claire Walker, Liverpool John Moores University, UKCopyright © 2016 Ellingsen, Leknes, Løseth, Wessberg and Olausson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan-Mikael Ellingsen, ZC5tLmVsbGluZ3NlbkBwc3lrb2xvZ2kudWlvLm5v
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.