- 1Department of Clinical Psychiatry, University of Bourgogne Franche-Comté, University Hospital, Besançon, France
- 2E.A. 481, Laboratory of Neurosciences, University of Franche-Comté, Besançon, France
- 3E.A. 3188, Laboratory of Psychology, University of Franche-Comté, Besançon, France
- 4UMSR 3124/FED 4209 MSHE Ledoux, Centre National de la Recherche Scientifique/Université de Franche-Comté, Besançon, France
- 5Fondation FondaMental, Albert Chenevier Hospital, Créteil, France
- 6CIC-IT 808 Inserm, Besançon University Hospital, Besançon, France
Background: The analysis of eye movements (EM) by eye-tracking has been carried out for several decades to investigate mood regulation, emotional information processing, and psychomotor disturbances in depressive disorders.
Method: A systematic review of all English language PubMed articles using the terms “saccadic eye movements” OR “eye-tracking” AND “depression” OR “bipolar disorders” was conducted using PRISMA guidelines. The aim of this review was to characterize the specific alterations of EM in unipolar and bipolar depression.
Results: Findings regarding psychomotor disturbance showed an increase in reaction time in prosaccade and antisaccade tasks in both unipolar and bipolar disorders. In both disorders, patients have been reported to have an attraction for negative emotions, especially for negative pictures in unipolar and threatening images in bipolar disorder. However, the pattern could change with aging, elderly unipolar patients disengaging key features of sad and neutral stimuli. Methodological limitations generally include small sample sizes with mixed unipolar and bipolar depressed patients.
Conclusion: Eye movement analysis can be used to discriminate patients with depressive disorders from controls, as well as patients with bipolar disorder from patients with unipolar depression. General knowledge concerning psychomotor alterations and affective regulation strategies associated with each disorder can also be gained thanks to the analysis. Future directions for research on eye movement and depression are proposed in this review.
Introduction
The study of eye movements (EM) in psychiatry and psychopathology began in 1908 based on the pioneer research of Allen Ross Diefendorf and Raymond Dodge. These authors were the first to study the ocular reaction in depression, mania, hebephrenic disease, epilepsy, and imbecile populations (Diefendorf and Dodge, 1908). The interest in EM stems from the information we can gain concerning brain functioning and the earliest stages of motor organization (Leigh and Zee, 2006) as well as psychopathology (Helmchen, 1989). Recently, the development of sophisticated eye tracking technologies such as the infra-red limbus or pupil detection method and the camera using the corneal reflection to measure eye movement (Young and Sheena, 1975) facilitated the study of EM in mental disorders. This technique enables to clarify some diagnoses as well as to assess the effect of drugs during the course of a disease and the recoveries or adaptations during treatment. For example, in schizophrenic populations, EM studies have revealed cognitive impairments of inhibition as well as a link between the genetics of physiological traits and smooth pursuit eye movements (SPEM; Matthysse et al., 2004; Gooding and Basso, 2008). In the case of affective disorders, EM studies may help specify the extent of psychomotor symptoms—which are usually reported across the spectrum of depressive disorders (Bennabi et al., 2013)–, and improve the understanding of mood regulation and emotional information processing as well as the prediction of outcome after treatment initiation.
Depression criteria and the use of categorical DSM definitions may lead to difficulties differentiating bipolar from unipolar depression. Some subtle symptoms help distinguish between unipolar and bipolar depression. Among these symptoms, higher rates of psychomotor retardation, greater difficulty to think, more early morning awakening, more morning worsening of mood, and more frequent psychotic symptoms are mainly related to bipolar depression (Mitchell et al., 2008). In spite of these differences in symptoms, a clear-cut distinction between the two pathologies remains currently difficult (Goodwin et al., 2008). Indeed, a patient can experience depressive episodes for several years without experiencing mania or hypomania (Smith and Craddock, 2011). That's why many patients with bipolar depression are often misdiagnosed and thus treated as having unipolar depression, leading to insufficient treatment and poor outcomes (Bowden, 2001, 2010).
It is then of importance to distinguish between unipolar and bipolar depression at an early stage so as to improve care management. A recent pilot study using fMRI and pattern classification to discriminate unipolar and bipolar depression (Grotegerd et al., 2013) during facial emotional picture presentation has given a diagnosis with up to 90% correct classifications. However, this study was conducted in a small sample of subjects and the use of fMRI is not appropriate in routine clinical practice. Depression has also been characterized by a reduction in positive expression recognition coupled with an increase in the recognition of negative emotions when the stimulus is ambiguous (Surguladze et al., 2004). Impairments in emotional information processing have been associated with social dysfunction (Tse and Bond, 2004) and may be implicated in the maintenance of the disease (Fossati et al., 2004). At the physiopathological level, these deficits have been related to structural and functional anomalies, in particular to the prefrontal cortex (Rogers et al., 2004).
On the other hand, modern eye-tracking techniques have been used to characterize both informational processing by establishing the point of gaze, and more basic characteristics of reflexive and voluntary psychomotor activity that can be altered with mood disorders. EM and fixations provide information concerning cortical mechanisms underlying cognitive functions (Hutton, 2008; Henderson et al., 2013); may help to improve the understanding of the pathological mechanisms underlying mood disorders (Leigh and Zee, 2006); and could be a promising behavioral tool to differentiate unipolar and bipolar depression.
Our aim in the following review was to summarize the literature regarding the study of saccadic EM in adult depressive (unipolar and bipolar) population. Firstly, we describe the main eye-tracking paradigms, their characteristics and usefulness. Then, we present studies addressing unipolar and bipolar depression through the use of eye-tracking paradigms. Finally, we suggest perspectives for future research.
Methods
A search of the literature was conducted in accordance with Preferred Reporting Items for Systematic reviews and Meta Analyses (PRISMA; Moher et al., 2010). Relevant manuscripts were identified in PubMed database in April 2014 using the following keywords: “saccadic eye movements” OR “eye-tracking” AND “depression” OR “bipolar disorders.” The reference lists of the selected manuscripts were scrutinized for additional studies. Searches were limited to human studies reported in English and were eligible for inclusion if they investigated oculomotor performances or emotional information processing with infrared video-oculography or electrooculography in unipolar or bipolar disorders. Articles were included if they contained primary data derived from clinical trials, meta-analysis or case reports. Excluded studies were those addressing mood disorders due to specific disease processes (e.g., Parkinson's disease or dementia), conducted in children or adolescent psychiatric patients or with no abstract available. We initially applied the above eligibility criteria to the citations and abstracts generated by the search. Based on this information, we excluded the publications that did not meet the inclusion criteria.
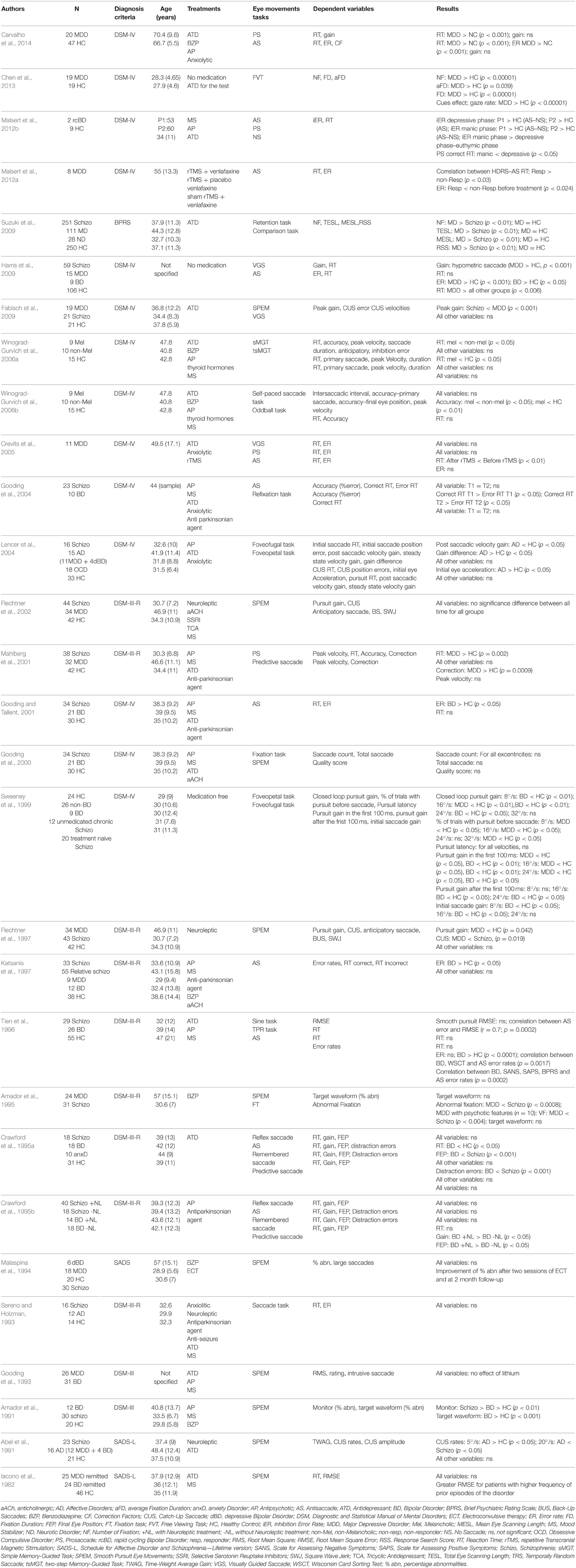
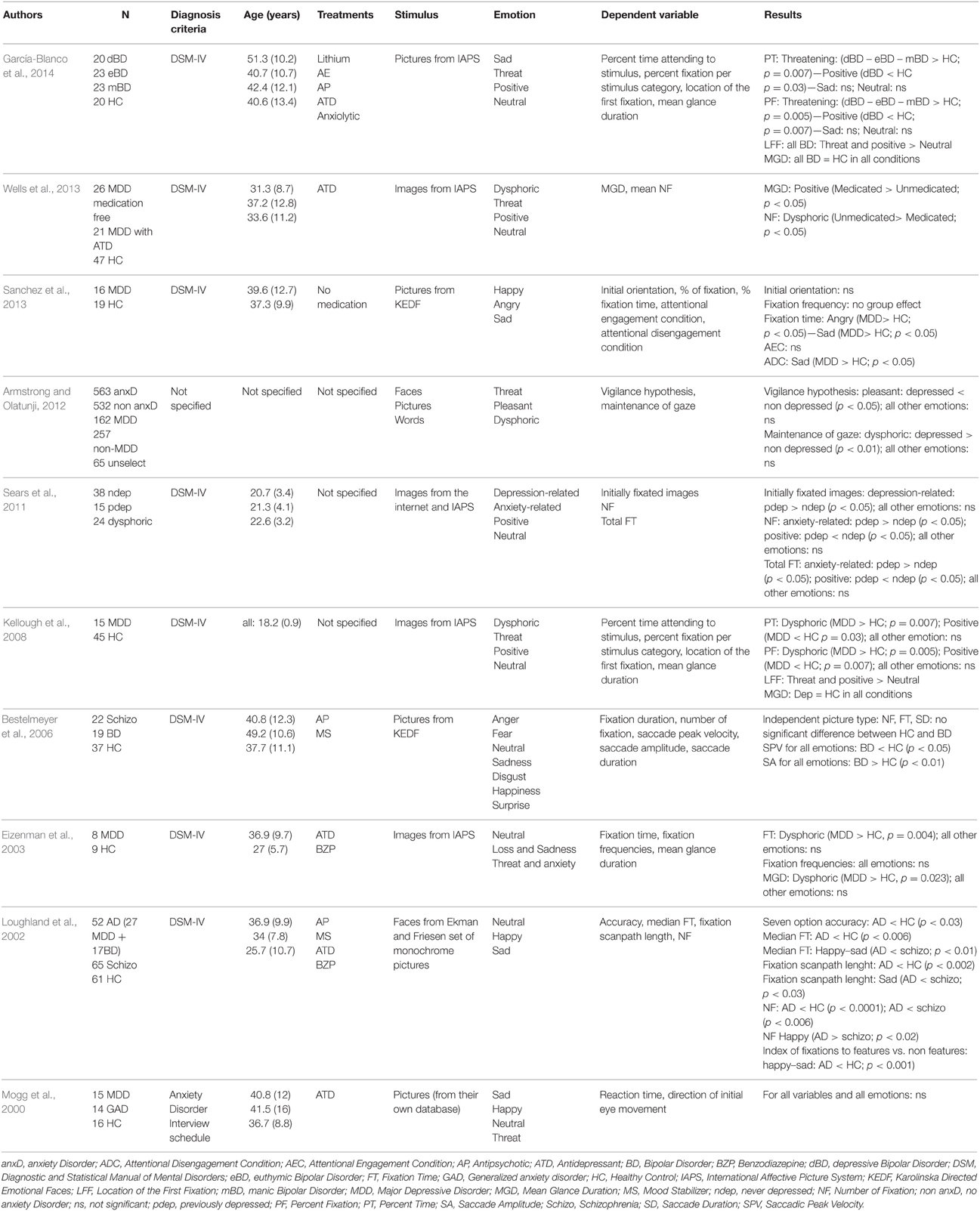
When an article met the inclusion criteria, or when there was not sufficient information to definitely exclude it, we retrieved the full text. We then reviewed these potentially relevant articles to determine whether the inclusion criteria were really met. Out of the 71 papers with full-text reviewed, a total of 30 articles that did not meet eligibility criteria were excluded. Thus, data were obtained from 41 papers that met our eligibility criteria (Figure 1). The reviewed studies were listed in Tables 1, 2 according to sample, measure and results.
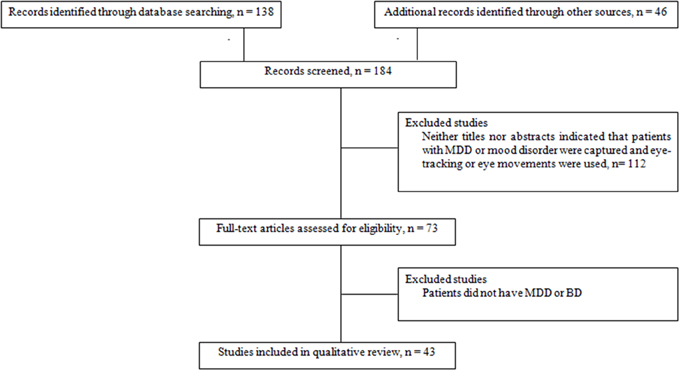
Figure 1. Flow chart of information through the different study phases according to the Preferred Reporting Item for System reviews and Meta-analyses (PRISMA). MDD, Major Depressive Disorder; BD, Bipolar Disorder.
Main Eye-tracking Paradigms
Several eye-tracking paradigms have been developed over the years to probe the behavioral—and underlying brain—processes associated with the various psychopathologies. We reviewed below some of the most commonly used EM tasks in research. Depending on the authors, and due to the lack of standardization, some tasks may involve similar instructions and receive different terminologies (Holmqvist et al., 2011). This is the reason why we sometimes provide the reader with several terminologies.
Prosaccades (PS), Visually Guided Saccades (VGS), and Refixation Tasks
In PS, VGS, or refixation tasks, a fixation stimulus appears at the center of the screen and a visual target is presented at the peripheral location. In the first version of the PS task, known as the step PS, the central fixation stimulus disappears at the same time as the peripheral target appears. This task assesses the integrity of saccade-generating circuitry and their involvement in the initiation of reflexive saccade. In a second version of PS task, known as the gap PS, a gap-time is added between the disappearance of the central fixation stimulus and the appearance of the peripheral target (Elderkin-Thompson et al., 2009). The gap task is namely used to study the express saccades, which are saccades characterized by short latencies (80–130 ms). In a third version, known as the overlap PS, the central fixation stimulus remains on the screen when the peripheral target appears. The overlap task is used to examine the ability to flexibly disengage and reorient cognitive resources through eye movement.
In these three experimental conditions, subjects are instructed to fix the target at the peripheral location as soon as the latter appears (Figure 2). Typical measures in PS are the latencies, error rates and amplitudes. This task measures the basic EM characteristics of the subject. The cortical structures linked to the PS performances are the superior colliculus (SC), the frontal eye field (FEF), the cerebellum and the parietal cortex (PC; Ettinger et al., 2005; Leigh and Zee, 2006).
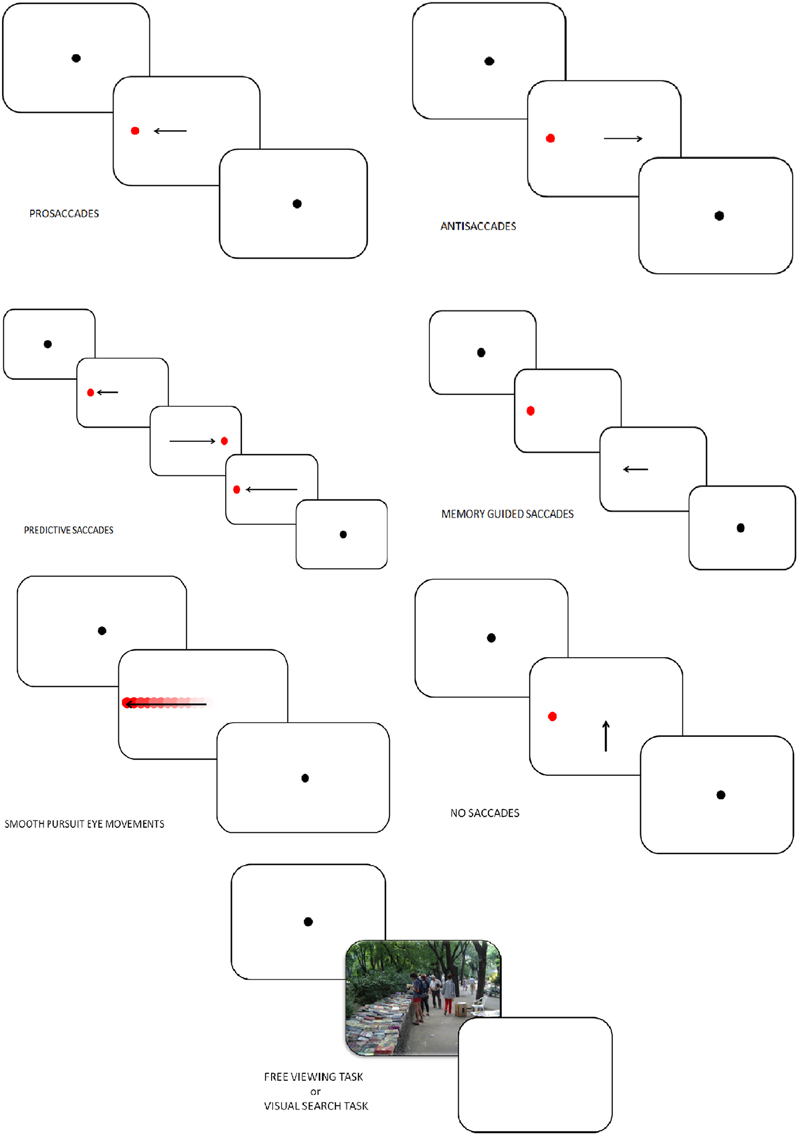
Figure 2. Tasks in which eye-tracking is commonly used (oculomotor, free-viewing, and visual search tasks).
Antisaccades (AS)
In AS, the subject looks at a fixation point and a visual target is presented. Subjects are instructed to make a saccade away from the target (Everling and Fischer, 1998; Figure 2). A correct AS involves two mechanisms depending on automatic processes (Theeuwes et al., 1998): the inhibition of reflexive saccade to the onset location and the execution of a voluntary EM to the mirror location of the onset. Consequently, longer latencies and more erroneous saccades characterize AS performances rather than PS. Typical measures in AS are the error rates (reflecting the inhibition failure), and saccadic reaction time. These two measures are linked to cognitive abilities and may help quantify an inhibition deficit (Currie and Ramsden, 1991). The dorsolateral prefrontal cortex (DLPFC; Crevits et al., 2005), the FEF (Gaymard et al., 1998) and the supplementary eye field (SEF; Everling and Munoz, 2000) are more active during AS. Inhibition deficits are generally linked to frontal area dysfunctions.
Predictive Saccades, “Oddball Task,” Self-paced Saccade
In predictive saccade, “oddball,” and self-paced saccade tasks, subjects are required to keep their eyes fixed on a target that moves regularly back and forth between two known locations (left and right to the center, remaining in each position for some period of time; Figure 2). This task is used to assess participant's ability to adjust their oculomotor response to predictably moving visual stimulus (negative RT, anticipation) and erroneous anticipatory saccades (Bronstein and Kennard, 1987). Moreover, the oddball task also captures the ability to inhibit an expected motor program and reprogram a new saccade to correspond to an “oddball” trajectory. Predictive saccade may help to quantify inhibition of reflexive saccades, performed by the DLPFC.
Memory-Guided Saccade (MGS)
Participants are instructed to look at a central fixation point. During this fixation period a target appears in the periphery and the participant is not allowed to make a saccade toward the target. The peripheral target disappears, and, after a variable delay period, the subject is required to make a saccade toward the memorized location (Figure 2). Accuracy, number of anticipatory errors and saccade latency are typical measures in MGS. These saccades are used to assess damages of the basal ganglia and regions of the frontal lobe, involved in processes of working memory (Herrera-Guzmán et al., 2009) and seriously affected in depression (Austin et al., 2001). Performances in MGS are also associated to cortical areas such as DLPFC and SEF (Ettinger et al., 2005).
Smooth Pursuit Eye Movements (SPEM), Foveofugal, and Foveopetal Tasks
Participants are instructed to track a target moving between two fixation points with a sinusoidal or constant velocity (Figure 2). Typical measures in SPEM include the pursuit gain (ratio of eye velocity to target velocity), the catch-up saccade (CUS), employed when the gaze position lags behind the target it follows, and therefore temporarily needs to increase velocity to catch up with the target, anticipatory saccades and square wave jerks (SWJ, small pairs of saccade in opposite directions and separated by an intersaccadic interval of 200–450 ms). Analysis of SPEM performances is used to assess the quality of the pursuit system, could therefore indicate cerebellar and basal ganglia disorders, and can be influenced by age, level of attention, and pharmacological treatments (Leigh and Zee, 2006). Two cortical areas play a major role in SPEM: the medial superior temporal area (MST) and the FEF (Nuding et al., 2008).
No Saccade (NS) and Fixation Tasks
Participants have to keep their gaze fixed on a target placed at the center of the screen (Figure 2). After 2 s and only if the participant stared at the fixation dot, a target appears simultaneously in periphery of the screen as distractor. A typical measure in the NS task is the inhibition error, which consists, in this condition, in the movement of the eye. A key cortex area involved in this task is the DLPFC (Pierrot-Deseilligny et al., 2003).
Free Viewing Tasks (FVT) and Visual Search Tasks (VST)
In FVT, participants are instructed to look at series of images freely, for instance “as if they were watching television.” In VST, subjects are required to explore a complex visual scene to identify specific stimulus features or to compare multiple scenes (Holmqvist et al., 2011; Figure 2). Various parameters can be studied in those tasks: fixation time, fixation number, location of the first fixation, mean glance duration, saccadic amplitude, saccadic duration and peak saccade velocity. Those kinds of task have usually been used to assess attentional biases, and emotion, face, and scene processing.
Results
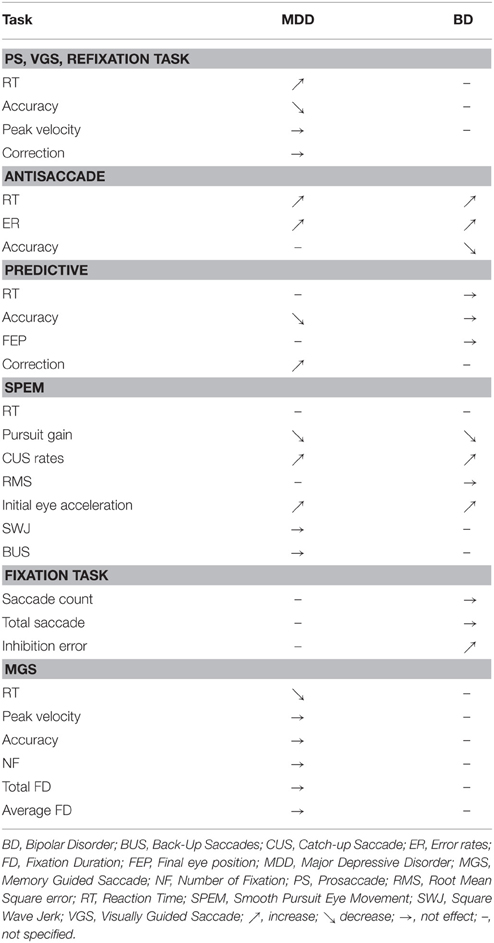
A summary of eye movement characteristics in the two groups (i.e., patients with unipolar depression and patients with bipolar depression), in each task, is listed below (Table 3).
Prosaccades (PS), Visually Guided Saccades (VGS), and Refixation Tasks
In 1993, Sereno et al. investigated saccadic performance in affective disorder groups composed by depressed and bipolar patients. They found no difference between affective disorder, schizophrenic and control participants for RT and ER in gap and no-gap conditions. Harris et al. (2009) obtained divergent results with an electro-oculogram (EOG), saccades being more hypometric in unipolar depressed patients than in controls but not between bipolar patient and controls. Mahlberg et al. (2001) explored saccadic EM of 32 patients with MDD using high-resolution infrared oculography. Patients with MDD exhibited longer RT and needed more corrective saccades (saccade to correct the remaining error relative to the target position and the eye position after the main saccade) to reach the target than healthy controls (HC). Crevits et al. (2005) observed no impact of 10 sessions of fast rTMS at a frequency of 10 Hz (50 impulses) on RT and ER. As for antisaccade performances, Gooding et al. (2004) failed to find temporal stability for prosaccade characteristics. Winograd-Gurvich et al. (2006a) explored differences between melancholic, non-melancholic depressed patients and controls in VGS. Melancholic patients were characterized by longer latencies than non-melancholic and controls, and non-melancholic performed the task slower than controls. Malsert et al. (2012b) found very short reaction times in manic phases in patients with rapid cycling BD. Finally, Carvalho et al. (2014) found an increase of RT in patients with MDD compared to controls but the same accuracy in PS.
Antisaccades
In unipolar and bipolar disorders, Harris et al. (2009) found an increase in RT and ER in comparison to HC. In their report on antisaccades performances in bipolar disorder, Crawford et al. (1995a) found an increase in spatial amplitude errors in AS with hypometric saccades compared to controls. In a subsequent report, these authors found no differences between bipolar and schizophrenic patients in terms of latency, gain (corresponding to the ratio of the saccade amplitude divided by the target step amplitude) and distraction errors. Tien et al. (1996) have investigated AS performances in bipolar, schizophrenic and HC, focusing on reaction time and error rates. They found no differences between the three groups concerning RT. However, they observed significantly higher ER in BD than in HC. Based on the same paradigm, other authors obtained concordant results in BD, with no effects of psychotropic medications on performances (Katsanis et al., 1997; Gooding et al., 2000). In 2004, Gooding and collaborators explored test-retest reliabilities on EM performances in bipolar patients, and reported that ER previously described were not temporally stable. More recently, Malsert et al. (2012b) have observed a link between antisaccade performances and clinical scores, suggesting that error rates could be a predictor of treatment response. Moreover, these authors compared antisaccade performances in two patients suffering from rapid cycling bipolar disorder, and found a higher ER in patients during depressive and manic phases, in comparison to HC. Additionally, lower ER characterized depressive and euthymic rather than manic states. In 2014, Carvalho et al. found an increase of RT, ER for MDD patients. However, MDD patients presented a similar correction factor to that observed in controls. This indicated that unipolar patients had not altered abilities for error detection and correction. Crevits et al. (2005) investigated the impact of 10 sessions of rTMS at 10 Hz frequency applied in DLPFC in a cohort of 11 depressed patients with each sessions consisting of 50 train of 5 s duration separated by 30 s pauses, and found a decrease in latency and no effect on ER.
Predictive Saccades
In their first study in 1995, Crawford et al. found no differences between BD, schizophrenic patients and HC in predictive saccade performances. In a second study, they investigated the impact of antipsychotic treatments on predictive saccadic performances in BD patients. Patients with BD treated with antipsychotics had more accurate saccades than non-treated BD when the target was visible or temporarily withdraw (Crawford et al., 1995b). In a predictive task, Mahlberg et al. (2001) showed that depressed patients with major depression (as schizophrenic patients) needed more corrections than HC to reach the target. In 2006, Winograd-Gruvich et al. investigated performances of melancholic and non-melancholic depressed patients in an “oddball” task. There were no differences in RT between the two groups of depressed patients, but the melancholic depressed were less accurate than non-melancholic and HC. Melancholia had no effects on performance in self-paced saccade tasks.
Smooth Pursuit Eye Movements (SPEM)
In 1991, Abel et al. studied smooth pursuit gain and CUS in affective disorders and schizophrenia. When the constant stimulus velocity was 5°/s, MDD and BD patients had higher CUS rates than HC, whereas for 20°/s velocity, MDD and BD subjects had only fewer CUS errors than schizophrenic patients but not than HC. Amador et al. (1991) observed that manic patients had abnormalities in SPEM in comparison with controls leading to a failure to engage the smooth pursuit system. Another study by Tien et al. (1996) found no difference between BD and control in RMS and RT in a pursuit task. Flechtner et al. (2002) explored SPEM in 34 MDD patients. Patients exhibited lower pursuit gain than HC and lower CUS errors than schizophrenic patients. Sweeney et al. (1999) assessed pursuit EM in foveopetal and foveofugal task in BD, MDD, schizophrenic, and control groups. In tasks based on foveopetal motion, depressed and bipolar patients demonstrated reduced pursuit gain compared to HC. Moreover, MDD patients had more difficulty initiating pursuit before their first CUS than HC. In tasks based on foveofugal motion, MDD, and bipolar patients also exhibited lower open loop (i.e., early period of pursuit) pursuit gain and lower closed loop (i.e., late period of pursuit) pursuit gain than controls. Depressed patients had fewer abnormal visual fixations (SWJ) than schizophrenic patients regardless of psychotic features (Amador et al., 1995). Neuroleptic medication and variation of clinical state in MDD had no impact on SPEM performances (Flechtner et al., 2002). Lencer et al. (2004) found in a pursuit task that MDD and BD patients had higher initial eye acceleration and lower post-saccadic velocity gain (i.e., ratio of eye to target velocity) than HC. These characteristics of pursuit performances led to a higher gain difference in MDD and BD patients than in HC. Fabisch et al. (2009) showed that unipolar depressed patients had also higher peak gain than schizophrenic patients. Iacono et al. (1982) observed that lithium induced a greater number of errors during SPEM in unipolar and bipolar patients. However, Gooding et al. (1993) found no effect of lithium treatment on pursuit performance from the time of initial testing to the time of retest. Furthermore, electroconvulsive therapy (ECT) “transiently disrupted” SPEM but improved pursuit performances after two sessions of ECT and at 2 months follow-up (Malaspina et al., 1994). The authors proposed that SPEM could be a “state marker in severe major depression.”
No Saccade and Fixation Task
In 2000, Gooding et al. found no differences in saccade count regardless of the amplitude of EM, and no differences in the total number of saccades between BD patients, HC and schizophrenic patients, whatever the eccentricities. Studying rapid cycling BD, Malsert et al. (2012b) observed that patients in manic phase had a higher number of NS inhibition errors than those in depressive or euthymic phase. Furthermore, for all phases of BD, patients had a higher percentage of NS inhibition errors than HC.
Memory-guided Saccade Task
In 2006a, Winograd-Gurvich et al. compared melancholic and non-melancholic depressions in a memory-guided saccade task. A higher RT, a decrease in peak velocity, and an increase in the number of hypometric saccades characterized melancholic patients. Non-melancholic patients only had an increase in peak velocity. Suzuki et al. (2009) explored EM dysfunction using retention and comparison tasks in mood disorders, schizophrenia, neurotic disorders, and in the control population. Mood disorders groups, mainly composed of MDD patients, had a higher number of fixations, total eye scanning length, mean saccade length, and responsive search score (corresponding to the data of EM that occurred for the 5-s period immediately following the question: “Are there any other differences?”) than schizophrenic patients. However, MDD did not differ from healthy subjects. Chen et al. (2013) investigated memory impairment in MDD. These authors found higher fixation number, total and average fixation duration, in MDD than in non-MDD participants. This corresponds to a difficulty in shifting attention and extracting information.
Emotion and Oculomotor Behaviors
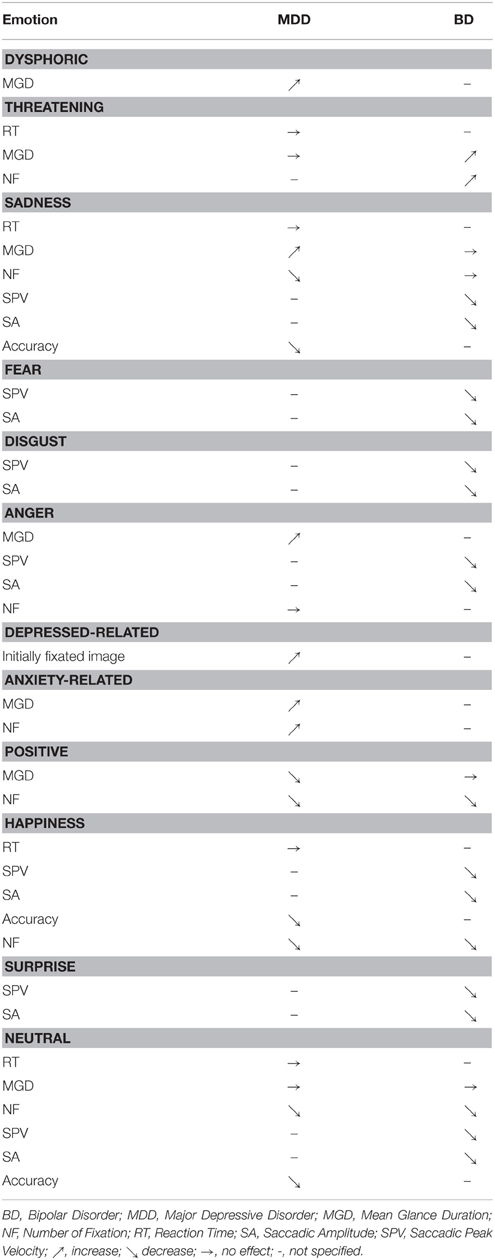
Beyond the analysis of the general dynamics of the oculomotor function, eye-tracking can be employed in order to characterize emotional processing. A detailed table listing emotional exploration characteristics in the two groups is presented in Table 4.
In 2000, Mogg et al. investigated emotional biases in generalized anxiety disorder (GAD) and MDD, by using EM recording. Examining four different types of emotions (sad, happy, neutral, threatening), depressed patients did not differ from GAD patients or HC for RT and the direction of initial EM. However, Eizenman et al. (2003) found an increase in fixation time and average glance duration on dysphoric images for MDD when compared to HC but not for other emotions (i.e., threat and anxiety, interpersonal attachment and social contact). In BD population, Bestelmeyer et al. (2006) observed an impact of picture type (i.e., landscapes, fractals, faces, noise) rather than social content of the picture on EM. Patients with BD were characterized by lower saccadic peak velocity and lower saccade amplitude compared to HC for all picture types. Using the same paradigms as Eizenman et al. (2003), Kellough et al. (2008) obtained concordant results in MDD. Depressed patients made more and longer fixations on dysphoric content compared to HC, and the opposite was true for positive pictures. However, depressed patients fixed first positive and threat pictures rather than dysphoric or neutral. A study by Sears et al. (2011) explored the effect of history of depression on emotion attention biases. Depressed patients with history of depression had higher number of fixations and higher total fixation time for anxiety related pictures compared to patients with no history of depression. Opposite results were found for positive images. In comparison with patients without depression history, previously depressed patients fixed more often depression-related pictures in comparison with non-previously depressed patients. A recent meta-analysis carried out by Armstrong and Olatunji (2012) summarized the attentional biases in affective disorders. Depressed individuals were characterized by a reduction of initial orientation to pleasant stimuli, compared to non-depressed. However, this meta-analysis did not show any increase in vigilance for threatening stimuli. Regarding the maintenance of gaze, depressed subjects had an increase in attention for dysphoric pictures and a decrease for positive picture. A more recent work of Sanchez et al. (2013), which studied stress in depression, found no effect of emotional valence of picture on initial orientation and fixation frequency. However, depressed patients had longer total fixation time on angry and sad emotional faces than HC. Wells et al. (2013) explored the effect of antidepressant (ATD) medication on emotion perception in MDD. The consumption of ATD led to higher mean gaze duration on positive pictures and fewer fixations on dysphoric images in medicated depressed patients. García-Blanco et al. (2014) investigated visual behaviors at different phases of BD, as well as in HC. In their task, four pictures—three “emotional” (i.e., happy, sad, threatening) and one “neutral”—were simultaneously presented. Compared to HC, only bipolar disorder patients who were in a depressive episode (dBD) had fewer fixations on happy images. BD patients, whatever their episodes, had a higher number of fixations on threatening pictures than HC. Similar results were found for the percentage of fixations. Moreover, all participants had a higher number of first fixations on the happy pictures than on the other ones.
Discussion
Psychomotor Disturbance in Unipolar and Bipolar Depression
Eye movement tasks are a useful tool to investigate cognitive and motor functioning, through exploration of both low (i.e., automatic) and high (i.e., resource-demanding) levels of motor control. Varieties of processes such as automatic relocation of visual search, spatial working memory, prediction, and response suppression can be evaluated through eye tracking, and related to mood disorders.
Unipolar depressed patients present psychomotor retardation expressed by an increase in RT in both prosaccade and antisaccade tasks (Mahlberg et al., 2001; Carvalho et al., 2014). These impairments of motor and cognitive features involved in movement production were previously observed in other tasks such as fine motor tasks (Sabbe et al., 1996; Pier et al., 2004), gait analysis (Hausdorff et al., 2004) or while measuring ideational retardation (Smith et al., 1994; Brébion et al., 1995). The alterations of movement production were more pronounced in melancholic depressed patients compared to non-melancholic (Parker et al., 2000; Winograd-Gurvich et al., 2006a) with a decrease of saccade accuracy in melancholic depressed patients. Several studies hypothesized specific alterations of motricity in melancholic depression (Parker et al., 2000; Winograd-Gurvich et al., 2006a).
Patients with BD have also been characterized by an increase in reaction time in prosaccade and antisaccade tasks. These deficits were higher in the depressive phase than in the manic phase. Moreover, dBD and mBD could present an inhibition deficit leading to an increase in the antisaccade error rates (Malsert et al., 2012b) and inhibition errors in the NS task are numerous in mBD (Malsert et al., 2012b). These characteristics may be related to some clinical dimensions of BD such as the production of impulsive processes (Swann, 2010). Indeed, the behavioral disinhibition could represent a core dimension of the manic phase (Swann et al., 2003; Larson et al., 2005) causing inability to shift from a given behavior over time.
Psychopathology of Depression and EM: Potential Neurophysiological Foundations
The psychopathology of depression has been associated with an alteration in prefrontal and orbitofrontal cortices (Figure 3). As regards EM control, depressed patients have demonstrated a reduction of performance in visual pursuit tasks. Indeed, they would retain a good perception of visual information, but would have alterations in sensorimotor integration processes that could be responsible for precision alteration found in this task (Van der Linden and Hupet, 1994; Fabisch et al., 2009). A deficit in PS performance could be related to functional alterations affecting cortical structures such as the FEF and the superior colliculus (Schall, 2004). The impairment of deep FEF regions could also account for deficits in the visual pursuit system (Rosano et al., 2002).
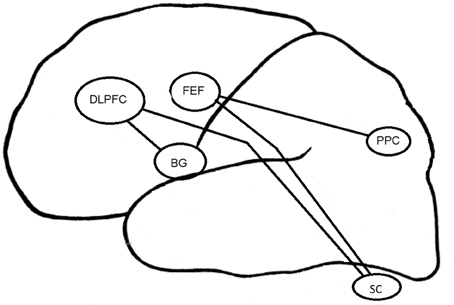
Figure 3. Cortical structures associated to EM in depression. DLPFC, DorsoLateral Prefrontal Cortex; FEF, Frontal Eye Field; BG, Basal Ganglia; PPC, Parietal Posterior Cortex; SC, Superior Colliculus.
The increase in RT in MGS task for the melancholic population could result from a change in the FEF and the posterior PC (Winograd-Gurvich et al., 2006a). Non-melancholic depressed patients were not characterized by a psychomotor retardation but rather by an increase in saccadic peak velocity. Brain structures specifically involved in MGS performances are mainly the cerebellum and basal ganglia (Ivry and Keele, 1989; Dreher and Grafman, 2002). The cerebellum is involved in the timing of movement within short time intervals (Clarke et al., 1996), whereas the basal ganglia are involved within longer time intervals (Meck, 1996). All these structures interact with other regions such as the DLPFC (Pierrot-Deseilligny and Burke, 2005).
In the antisaccade task, the mBD patients have more severe inhibition deficits than dBD or euthymic BD patients. The DLPFC seems to be involved in the inhibition of saccades generated by the superior colliculus (Condy et al., 2004; Kaufman et al., 2010). The EM inhibition deficit observed in BD could be linked to previously reported activation deficits in (ventrolateral) prefrontal cortex and impulsivity in BD patients (Jeanningros et al., 2008).
Negative Emotion
A basic analysis carried out with eye-tracking in depressed patients concerns the visual processing of emotional information. The majority of the studies included in our review found an attraction for negative emotion and a reduced orientation to positive emotion in depression (Armstrong and Olatunji, 2012). The capacity of emotional information processing would depend on perceptive, attentional, and memory resources (Brosch et al., 2013).
Depression is associated with a greater perceptual focus on negative pictures. Depressed patients tend to maintain their attention on negative images. This process contributes to keep the disorder by rumination of negative information (Joormann and Gotlib, 2007, 2008; Levens and Gotlib, 2010; Horn and Leigh, 2011) and by the inability to inhibit the negative emotional processing (Goeleven et al., 2006; Joormann and Gotlib, 2010). Some studies have highlighted a lack of initial orientation on negative information (Mogg et al., 2006; Kellough et al., 2008; Wisco, 2009; De Raedt and Koster, 2010). According to these studies, depression is characterized by a negative bias in memory encoding processes rather than by a change in early attentional processes (Williams, 1997).
The early attentional bias has been found to be specific to anxiety (vigilance hypothesis) and the attentional maintenance bias, specific to depression (Weierich and Treat, 2008; Peckham et al., 2010). The presence of a negativity bias was also confirmed by studies using emotional picture presentation with a presentation time superior to 10 s (Armstrong and Olatunji, 2012). However, some factors, such as aging, may well moderate those effects. In our very recent study (Noiret et al., 2015), we found specific characteristics of visual fixations and scanning strategies in elderly MDD patients. Older adults with depression have been characterized by a disengagement of their visual fixations from key features of sad and neutral faces (i.e., lower total fixation duration and fewer fixations on emotional regions [eyes and mouth] compared to HC). In this case, positivity effects accompanying emotional processing in aging could account for interactions between aging and depression. In any event, a reversal seems to occur in comparison to what is usually reported in younger depressive patients.
Patients with BD focus more on threatening pictures regardless of the disease phase, but more frequently during the euthymic phase. BD patients who are in a depressive episode, as MDD patients, exhibit a decrease in fixation time on positive pictures and an increase on negative images. García-Blanco et al. (2014) have shown that the bipolar phase effect (depressive–euthymic–manic) had an impact on attention. Patients with dBD were unable to maintain eye contact on a positive picture. This cognitive bias likely alters emotional self-regulation processes and plays a role in the maintenance of the disease. This relative lack of interest in the positive image could be related to an “anhedonic bias.” Similar kinds of cognitive effects were found in MDD and would also be involved in the maintenance of the disease in that population (Fritzsche et al., 2010). In contrast, these effects were not found in mBD. It could mean that, in this situation, an evaluation conflict between negative and positive emotions occurs (Mansell et al., 2007). The presence of a bias toward threatening pictures in the eBD could be associated with an increase in emotional reactivity and with the onset or exacerbation of affective episodes. The bias toward threatening images therefore seems to be a marker of heightened sensitivity to emotional content and possibly a marker of vulnerability to depressive episodes.
Effect of Drugs on the Basic Dynamics of EM
The effects of drugs on EM have been studied for a long time (Wise, 1984). One reason for this is they inform us about drug effects on the central nervous system (Park et al., 2015). Psychotropic drugs can alter the basic EM but also have an impact on oculomotor performances in emotional information processing (Sweeney et al., 1994; Reilly et al., 2008). Most studies evaluating the effect of drugs were conducted in the animal or in healthy human adults. These studies revealed that benzodiazepines cause a reduction in saccade velocity (Ball et al., 1991), increases saccadic RT (Fafrowicz et al., 1995) and ER of AS (Green and King, 1998). Antidepressants have an effect on RT of both PS and AS, as well as on ER of AS (Green et al., 2000). Morrens et al. (2007) have shown an increase in saccadic peak velocity in healthy subjects treated with paroxetine.
In depressed patients, contradictory results have been found. Some studies have shown an effect of antidepressants on oculomotor performances (Green et al., 2000) while other studies have reported no effect of this treatment on RT and ER in depression (Katsanis et al., 1997; Flechtner et al., 2002). Benzodiazepine could cause a decrease in saccadic peak velocity (Green et al., 2000), an increase in saccadic RT in both gap and overlap conditions (Fafrowicz et al., 1995), and antisaccade ER (Green and King, 1998) as well as an alteration of visual pursuit (Van Nechel, 2007). Antipsychotic drugs in depression (Flechtner et al., 2002) would not influence antisaccade velocity, RT and ER. Sweeney et al. (1997) highlighted an increase in saccadic RT and a decrease in saccadic velocity in schizophrenic patients treated by antipsychotics.
Other studies have shown that antidepressants reduce the recognition of negative emotions and increase the recognition of positive emotions in depression (Fu et al., 2004; Harmer et al., 2009; Wells et al., 2013) whereas others did not show any difference (García-Blanco et al., 2014). Difficulties have been encountered while assessing the impact of these drugs, because patients often take multiple treatments. Although the analysis is complex, it seems necessary to develop new studies evaluating the effects of specific drugs on the basic characteristics of EM and emotional processing in depression.
Limitations of the Reviewed Research
As regards the overall limitation of the research field, the main critical observations are that (i) most sample sizes were relatively small, (ii) there is a great variability in the eye movement parameters studied, (iii) most of the studies included patients treated with psychotropic medication and rigorous control of the medication effects over EM was generally lacking.
Conclusion
EM have been used to identify the characteristics of motor and cognitive alteration in MDD and BD. The psychomotor retardation specificity associated with each disorder helps us distinguish these two populations (Parker et al., 2000). Depressed and bipolar patients have been characterized by an increase in RT. However, melancholic depressed patient have a more important increase in RT than non-melancholic patients. Among BD patients, only those who are in their depressive phase have longer latency. Eye movement studies have also been used to differentiate melancholic from non-melancholic depressed patients (Winograd-Gurvich et al., 2006a). RT in prosaccade and memory-guided saccade task, accuracy in predictive saccade task and peak velocity in memory-guided saccade tasks could be used to discriminate the two populations. The association between the analysis technique of EM and other exploratory methods of motricity (clinical ERD, fine motor tasks, gait analysis, cognitive measure) could also contribute to improve the comprehension of these mechanisms. The analysis of bipolar patients' inhibition capacities through AS performance could have a diagnostic interest to identify the different phases of the disorder at an early stage (depressive–euthymic–manic).
Future eye tracking studies should also further improve the comprehension of physiopathological mechanisms in depression by focusing on the involvement of specific cortical regions (especially, DLPFC and FEF) (Funahashi, 2001). Visual information processing, dependent on genetic factors and brain physiology could constitute a sensitive analysis vector of pathophysiological processes of depression (Arolt et al., 1996; Matthysse et al., 2004). At the therapeutic level, the study of RT changes could be a predictive factor of treatment response as suggested by the studies of Malsert et al. (2012a) and Crevits et al. (2005).
Depression is a mood disorder linked to an alteration of emotional perception that may lead to a reduction of social interaction skills. The analysis of emotional information processing based on eye-tracking technologies could be used to further identify negative biases that may be associated with the reduction of attentional allocation to positive stimuli as a function of episode severity (Sears et al., 2011). Even if the alteration is dependent on the severity of the disease, this impairment seems to be relatively stable over time and is sometimes also present in healthy subjects with increased risk of depression, which suggests endophenotypic characteristics (Bediou et al., 2009). Similarly several studies have reported the presence of altered EM in remitted patients (Joormann and Gotlib, 2010; Malsert et al., 2012b).
To conclude, the results reported in this review highlight the need for additional research efforts in order to account for complex interactions discussed in the preceding sections (e.g., interactions between age and depressive disorders), and to systematically account for medication effects and their potential combinations with the core perceptual-motor effects of each disorder. Moreover, the current state of the literature on EM and depression has permitted us to establish that (i) unipolar depressed patients have been characterized by psychomotor retardation and negative emotional bias, and (ii) bipolar depressed patients present psychomotor retardation, inhibition deficit and are attracted by negative emotions such as threat. All these data are clinically useful for (i) understanding the link between emotion regulation, cognition and mood disorders, (ii) differentiating unipolar and bipolar disorders, and (iii) evaluating therapeutic response.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Richard Medeiros, Medical Editor of Medical Editing International, for editing the manuscript. This study was supported by a grant from the French Ministry of Health (Programme Hospitalier de Recherche Clinique: PHRC n°2009-A00942-55). The sponsor has no role in the study.
References
Abel, L. A., Friedman, L., Jesberger, J., Malki, A., and Meltzer, H. Y. (1991). Quantitative assessment of smooth pursuit gain and catch-up saccades in schizophrenia and affective disorders. Biol. Psychiatry 29, 1063–1072. doi: 10.1016/0006-3223(91)90248-K
Amador, X. F., Malaspina, D., Sackeim, H. A., Coleman, E. A., Kaufmann, C. A., Hasan, A., et al. (1995). Visual fixation and smooth pursuit eye movement abnormalities in patients with schizophrenia and their relatives. J. Neuropsychiatry Clin. Neurosci. 7, 197–206. doi: 10.1176/jnp.7.2.197
Amador, X. F., Sackeim, H. A., Mukherjee, S., Halperin, R., Neeley, P., Maclin, E., et al. (1991). Specificity of smooth pursuit eye movement and visual fixation abnormalities in schizophrenia. Comparison to mania and normal controls. Schizophr. Res. 5, 135–144. doi: 10.1016/0920-9964(91)90040-x
Armstrong, T., and Olatunji, B. O. (2012). Eye tracking of attention in the affective disorders: a meta-analytic review and synthesis. Clin. Psychol. Rev. 32, 704–723. doi: 10.1016/j.cpr.2012.09.004
Arolt, V., Lencer, R., Nolte, A., Müller-Myhsok, B., Purmann, S., Schürmann, M., et al. (1996). Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am. J. Med. Genet. 67, 564–579.
Austin, M. P., Mitchell, P., and Goodwin, G. M. (2001). Cognitive deficits in depression: possible implications for functional neuropathology. Br. J. Psychiatry 178, 200–206. doi: 10.1192/bjp.178.3.200
Ball, D. M., Glue, P., Wilson, S., and Nutt, D. J. (1991). Pharmacology of saccadic eye movements in man. 1. Effects of the benzodiazepine receptor ligands midazolam and flumazenil. Psychopharmacology 105, 361–367. doi: 10.1007/BF02244431
Bediou, B., Saoud, M., Harmer, C., and Krolak-Salmon, P. (2009). L'analyse des visages dans la dépression. L'Évolution Psychiatrique 74, 79–91. doi: 10.1016/j.evopsy.2008.12.015
Bennabi, D., Vandel, P., Papaxanthis, C., Pozzo, T., and Haffen, E. (2013). Psychomotor retardation in depression: a systematic review of diagnostic, pathophysiologic, and therapeutic implications. Biomed Res. Int. 2013:158746. doi: 10.1155/2013/158746
Bestelmeyer, P. E., Tatler, B. W., Phillips, L. H., Fraser, G., Benson, P. J., and St Clair, D. (2006). Global visual scanning abnormalities in schizophrenia and bipolar disorder. Schizophr. Res. 87, 212–222. doi: 10.1016/j.schres.2006.06.015
Bowden, C. L. (2001). Strategies to reduce misdiagnosis of bipolar depression. Psychiatr. Serv. 52, 51–55. doi: 10.1176/appi.ps.52.1.51
Bowden, C. L. (2010). Diagnosis, treatment, and recovery maintenance in bipolar depression. J. Clin. Psychiatry 71:e01. doi: 10.4088/jcp.8125cc5c
Brébion, G., Smith, M. J., and Allilaire, J. F. (1995). Psychometric characteristics of ideational retardation in depressives. Br. J. Clin. Psychol. 34(Pt 3), 371–381. doi: 10.1111/j.2044-8260.1995.tb01472.x
Bronstein, A. M., and Kennard, C. (1987). Predictive eye saccades are different from visually triggered saccades. Vision Res. 27, 517–520. doi: 10.1016/0042-6989(87)90037-X
Brosch, T., Scherer, K. R., Grandjean, D., and Sander, D. (2013). The impact of emotion on perception, attention, memory, and decision-making. Swiss Med. Wkly. 143, w13786. doi: 10.4414/smw.2013.13786
Carvalho, N., Noiret, N., Vandel, P., Monnin, J., Chopard, G., and Laurent, E. (2014). Saccadic eye movements in depressed elderly patients. PLoS ONE 9:e105355. doi: 10.1371/journal.pone.0105355
Chen, S., Zhou, R., Cui, H., and Chen, X. (2013). Deficits in cue detection underlie event-based prospective memory impairment in major depression: an eye tracking study. Psychiatry Res. 209, 453–458. doi: 10.1016/j.psychres.2013.01.015
Clarke, S., Ivry, R., Grinband, J., Roberts, S., and Shimizu, N. (1996). “Exploring the domain of the cerebellar timing system,” in Advances in Psychology, eds A. P. María and A. Julio (Amsterdam: North-Holland), 257–280. doi: 10.1016/s0166-4115(96)80063-x
Condy, C., Rivaud-Péchoux, S., Ostendorf, F., Ploner, C. J., and Gaymard, B. (2004). Neural substrate of antisaccades: role of subcortical structures. Neurology 63, 1571–1578. doi: 10.1212/01.WNL.0000142990.44979.5A
Crawford, T. J., Haeger, B., Kennard, C., Reveley, M. A., and Henderson, L. (1995a). Saccadic abnormalities in psychotic patients. I. Neuroleptic-free psychotic patients. Psychol. Med. 25, 461–471. doi: 10.1017/S0033291700033389
Crawford, T. J., Haeger, B., Kennard, C., Reveley, M. A., and Henderson, L. (1995b). Saccadic abnormalities in psychotic patients. II. The role of neuroleptic treatment. Psychol. Med. 25, 473–483. doi: 10.1017/S0033291700033390
Crevits, L., Van den Abbeele, D., Audenaert, K., Goethals, M., and Dierick, M. (2005). Effect of repetitive transcranial magnetic stimulation on saccades in depression: a pilot study. Psychiatry Res. 135, 113–119. doi: 10.1016/j.psychres.2003.10.008
Currie, J., and Ramsden, B. (1991). Validation of a clinical antisaccadic eye movements test in the assessment of dementia. Arch. Neurol. 48, 949. doi: 10.1001/archneur.1991.00530180102024
De Raedt, R., and Koster, E. H. (2010). Understanding vulnerability for depression from a cognitive neuroscience perspective: a reappraisal of attentional factors and a new conceptual framework. Cogn. Affect. Behav. Neurosci. 10, 50–70. doi: 10.3758/CABN.10.1.50
Diefendorf, A. R., and Dodge, R. (1908). An experimental study of the ocular reactions of the insane from photographic records. Brain 31, 451–489. doi: 10.1093/brain/31.3.451
Dreher, J. C., and Grafman, J. (2002). The roles of the cerebellum and basal ganglia in timing and error prediction. Eur. J. Neurosci. 16, 1609–1619. doi: 10.1046/j.1460-9568.2002.02212.x
Eizenman, M., Yu, L. H., Grupp, L., Eizenman, E., Ellenbogen, M., Gemar, M., et al. (2003). A naturalistic visual scanning approach to assess selective attention in major depressive disorder. Psychiatry Res. 118, 117–128. doi: 10.1016/S0165-1781(03)00068-4
Elderkin-Thompson, V., Hellemann, G., Pham, D., and Kumar, A. (2009). Prefrontal brain morphology and executive function in healthy and depressed elderly. Int. J. Geriatr. Psychiatry 24, 459–468. doi: 10.1002/gps.2137
Ettinger, U., Antonova, E., Crawford, T. J., Mitterschiffthaler, M. T., Goswani, S., Sharma, T., et al. (2005). Structural neural correlates of prosaccade and antisaccade eye movements in healthy humans. Neuroimage 24, 487–494. doi: 10.1016/j.neuroimage.2004.08.019
Everling, S., and Fischer, B. (1998). The antisaccade: a review of basic research and clinical studies. Neuropsychologia 36, 885–899. doi: 10.1016/S0028-3932(98)00020-7
Everling, S., and Munoz, D. P. (2000). Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J. Neurosci. 20, 387–400.
Fabisch, K., Fitz, W., Fabisch, H., Haas-Krammer, A., Klug, G., Zapotoczky, S., et al. (2009). Sinusoidal smooth pursuit eye tracking at different stimulus frequencies: position error and velocity error before catch-up saccades in schizophrenia and in major depressive disorder. Aust. N.Z. J. Psychiatry 43, 855–865. doi: 10.1080/00048670903107542
Fafrowicz, M., Unrug, A., Marek, T., van Luijtelaar, G., Noworol, C., and Coenen, A. (1995). Effects of diazepam and buspirone on reaction time of saccadic eye movements. Neuropsychobiology 32, 156–160. doi: 10.1159/000119316
Flechtner, K. M., Steinacher, B., Sauer, R., and Mackert, A. (1997). Smooth pursuit eye movements in schizophrenia and affective disorder. Psychol. Med. 27, 1411–1419. doi: 10.1017/S0033291797005709
Flechtner, K. M., Steinacher, B., Sauer, R., and Mackert, A. (2002). Smooth pursuit eye movements of patients with schizophrenia and affective disorder during clinical treatment. Eur. Arch. Psychiatry Clin. Neurosci. 252, 49–53. doi: 10.1007/s004060200011
Fossati, P., Harvey, P. O., Le Bastard, G., Ergis, A. M., Jouvent, R., and Allilaire, J. F. (2004). Verbal memory performance of patients with a first depressive episode and patients with unipolar and bipolar recurrent depression. J. Psychiatr. Res. 38, 137–144. doi: 10.1016/j.jpsychires.2003.08.002
Fritzsche, A., Dahme, B., Gotlib, I. H., Joormann, J., Magnussen, H., Watz, H., et al. (2010). Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychol. Med. 40, 815–826. doi: 10.1017/S0033291709990948
Fu, C. H., Williams, S. C., Cleare, A. J., Brammer, M. J., Walsh, N. D., Kim, J., et al. (2004). Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch. Gen. Psychiatry 61, 877–889. doi: 10.1001/archpsyc.61.9.877
Funahashi, S. (2001). Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci. Res. 39, 147–165. doi: 10.1016/S0168-0102(00)00224-8
García-Blanco, A., Salmerón, L., Perea, M., and Livianos, L. (2014). Attentional biases toward emotional images in the different episodes of bipolar disorder: an eye-tracking study. Psychiatry Res. 215, 628–633. doi: 10.1016/j.psychres.2013.12.039
Gaymard, B., Ploner, C. J., Rivaud, S., Vermersch, A. I., and Pierrot-Deseilligny, C. (1998). Cortical control of saccades. Exp. Brain Res. 123, 159–163. doi: 10.1007/s002210050557
Goeleven, E., De Raedt, R., Baert, S., and Koster, E. H. (2006). Deficient inhibition of emotional information in depression. J. Affect. Disord. 93, 149–157. doi: 10.1016/j.jad.2006.03.007
Gooding, D. C., and Basso, M. A. (2008). The tell-tale tasks: a review of saccadic research in psychiatric patient populations. Brain Cogn. 68, 371–390. doi: 10.1016/j.bandc.2008.08.024
Gooding, D. C., and Tallent, K. A. (2001). The association between antisaccade task and working memory task performance in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis. 189, 8–16. doi: 10.1097/00005053-200101000-00003
Gooding, D. C., Grabowski, J. A., and Hendershot, C. S. (2000). Fixation stability in schizophrenia, bipolar, and control subjects. Psychiatry Res. 97, 119–128. doi: 10.1016/S0165-1781(00)00226-2
Gooding, D. C., Iacono, W. G., Katsanis, J., Beiser, M., and Grove, W. M. (1993). The association between lithium carbonate and smooth pursuit eye tracking among first-episode patients with psychotic affective disorders. Psychophysiology 30, 3–9. doi: 10.1111/j.1469-8986.1993.tb03199.x
Gooding, D. C., Mohapatra, L., and Shea, H. B. (2004). Temporal stability of saccadic task performance in schizophrenia and bipolar patients. Psychol. Med. 34, 921–932. doi: 10.1017/S003329170300165X
Goodwin, G. M., Anderson, I., Arango, C., Bowden, C. L., Henry, C., Mitchell, P. B., et al. (2008). ECNP consensus meeting. Bipolar depression. Nice, March 2007. Eur. Neuropsychopharmacol. 18, 535–549. doi: 10.1016/j.euroneuro.2008.03.003
Green, J. F., and King, D. J. (1998). The effects of chlorpromazine and lorazepam on abnormal antisaccade and no-saccade distractibility. Biol. Psychiatry 44, 709–715. doi: 10.1016/S0006-3223(97)00452-6
Green, J. F., King, D. J., and Trimble, K. M. (2000). Antisaccade and smooth pursuit eye movements in healthy subjects receiving sertraline and lorazepam. J. Psychopharmacol. 14, 30–36. doi: 10.1177/026988110001400103
Grotegerd, D., Suslow, T., Bauer, J., Ohrmann, P., Arolt, V., Stuhrmann, A., et al. (2013). Discriminating unipolar and bipolar depression by means of fMRI and pattern classification: a pilot study. Eur. Arch. Psychiatry Clin. Neurosci. 263, 119–131. doi: 10.1007/s00406-012-0329-4
Harmer, C. J., O'sullivan, U., Favaron, E., Massey-Chase, R., Ayres, R., Reinecke, A., et al. (2009). Effect of acute antidepressant administration on negative affective bias in depressed patients. Am. J. Psychiatry 166, 1178–1184. doi: 10.1176/appi.ajp.2009.09020149
Harris, M. S., Reilly, J. L., Thase, M. E., Keshavan, M. S., and Sweeney, J. A. (2009). Response suppression deficits in treatment-naive first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res. 170, 150–156. doi: 10.1016/j.psychres.2008.10.031
Hausdorff, J. M., Peng, C. K., Goldberger, A. L., and Stoll, A. L. (2004). Gait unsteadiness and fall risk in two affective disorders: a preliminary study. BMC Psychiatry 4:39. doi: 10.1186/1471-244X-4-39
Helmchen, H. (1989). Eye movements and psychopathology. Eur. Arch. Psychiatry Neurol. Sci. 239, 1–2. doi: 10.1007/BF01739735
Henderson, J. M., Shinkareva, S. V., Wang, J., Luke, S. G., and Olejarczyk, J. (2013). Predicting cognitive state from eye movements. PLoS ONE 8:e64937. doi: 10.1371/journal.pone.0064937
Herrera-Guzmán, I., Gudayol-Ferré, E., Herrera-Guzmán, D., Guardia-Olmos, J., Hinojosa-Calvo, E., and Herrera-Abarca, J. E. (2009). Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. J. Psychiatr. Res. 43, 855–863. doi: 10.1016/j.jpsychires.2008.10.015
Holmqvist, K., Nyström, M., Andersson, R., Dewhurst, R., Jarodzka, H., and van de Weijer, J. (2011). Eye Tracking: A Comprehensive Guide to Methods and Measures. Oxford: OUP.
Horn, A. K., and Leigh, R. J. (2011). The anatomy and physiology of the ocular motor system. Handb. Clin. Neurol. 102, 21–69. doi: 10.1016/B978-0-444-52903-9.00008-X
Hutton, S. B. (2008). Cognitive control of saccadic eye movements. Brain Cogn. 68, 327–340. doi: 10.1016/j.bandc.2008.08.021
Iacono, W. G., Peloquin, L. J., Lumry, A. E., Valentine, R. H., and Tuason, V. B. (1982). Eye tracking in patients with unipolar and bipolar affective disorders in remission. J. Abnorm. Psychol. 91, 35–44. doi: 10.1037/0021-843X.91.1.35
Ivry, R. B., and Keele, S. W. (1989). Timing functions of the cerebellum. J. Cogn. Neurosci. 1, 136–152. doi: 10.1162/jocn.1989.1.2.136
Jeanningros, R., Mazzola-Pomietto, P., and Kaladjian, A. (2008). [Neuroanatomical correlates of impulse control disorders in manic states]. L'Information Psychiatrique 84, 121–128. doi: 10.3917/inpsy.8402.0121
Joormann, J., and Gotlib, I. H. (2007). Selective attention to emotional faces following recovery from depression. J. Abnorm. Psychol. 116, 80–85. doi: 10.1037/0021-843X.116.1.80
Joormann, J., and Gotlib, I. H. (2008). Updating the contents of working memory in depression: interference from irrelevant negative material. J. Abnorm. Psychol. 117, 182–192. doi: 10.1037/0021-843X.117.1.182
Joormann, J., and Gotlib, I. H. (2010). Emotion regulation in depression: relation to cognitive inhibition. Cogn. Emot. 24, 281–298. doi: 10.1080/02699930903407948
Katsanis, J., Kortenkamp, S., Iacono, W. G., and Grove, W. M. (1997). Antisaccade performance in patients with schizophrenia and affective disorder. J. Abnorm. Psychol. 106, 468–472. doi: 10.1037/0021-843X.106.3.468
Kaufman, L. D., Pratt, J., Levine, B., and Black, S. E. (2010). Antisaccades: a probe into the dorsolateral prefrontal cortex in Alzheimer's disease. A critical review. J. Alzheimers Dis. 19, 781–793. doi: 10.3233/JAD-2010-1275
Kellough, J. L., Beevers, C. G., Ellis, A. J., and Wells, T. T. (2008). Time course of selective attention in clinically depressed young adults: an eye tracking study. Behav. Res. Ther. 46, 1238–1243. doi: 10.1016/j.brat.2008.07.004
Larson, E. R., Shear, P. K., Krikorian, R., Welge, J., and Strakowski, S. M. (2005). Working memory and inhibitory control among manic and euthymic patients with bipolar disorder. J. Int. Neuropsychol. Soc. 11, 163–172. doi: 10.1017/s1355617705050228
Leigh, R. J., and Zee, D. S. (2006). The Neurology of Eye Movements. New York, NY: Oxford University Press.
Lencer, R., Trillenberg, P., Trillenberg-Krecker, K., Junghanns, K., Kordon, A., Broocks, A., et al. (2004). Smooth pursuit deficits in schizophrenia, affective disorder and obsessive-compulsive disorder. Psychol. Med. 34, 451–460. doi: 10.1017/S0033291703001314
Levens, S. M., and Gotlib, I. H. (2010). Updating positive and negative stimuli in working memory in depression. J. Exp. Psychol. Gen. 139, 654–664. doi: 10.1037/a0020283
Loughland, C. M., Williams, L. M., and Gordon, E. (2002). Schizophrenia and affective disorder show different visual scanning behavior for faces: a trait versus state-based distinction? Biol. Psychiatry 52, 338–348. doi: 10.1016/S0006-3223(02)01356-2
Mahlberg, R., Steinacher, B., Mackert, A., and Flechtner, K. M. (2001). Basic parameters of saccadic eye movements–differences between unmedicated schizophrenia and affective disorder patients. Eur. Arch. Psychiatry Clin. Neurosci. 251, 205–210. doi: 10.1007/s004060170028
Malaspina, D., Amador, X. F., Coleman, E. A., Mayr, T. L., Friedman, J. H., and Sackeim, H. A. (1994). Smooth pursuit eye movement abnormality in severe major depression: effects of ECT and clinical recovery. J. Neuropsychiatry Clin. Neurosci. 6, 36–42. doi: 10.1176/jnp.6.1.36
Malsert, J., Guyader, N., Chauvin, A., Polosan, M., Poulet, E., Szekely, D., et al. (2012a). Antisaccades as a follow-up tool in major depressive disorder therapies: a pilot study. Psychiatry Res. 200, 1051–1053. doi: 10.1016/j.psychres.2012.05.007
Malsert, J., Guyader, N., Chauvin, A., Polosan, M., Szekely, D., Bougerol, T., et al. (2012b). Saccadic performance and cortical excitability as trait-markers and state-markers in rapid cycling bipolar disorder: a two-case follow-up study. Front. Psychiatry 3:112. doi: 10.3389/fpsyt.2012.00112
Mansell, W., Morrison, A. P., Reid, G., Lowens, I., and Tai, S. (2007). The interpretation of, and responses to, changes in internal states: an integrative cognitive model of mood swings and bipolar disorders. Behav. Cogn. Psychother. 35, 515–539. doi: 10.1017/s1352465807003827
Matthysse, S., Holzman, P. S., Gusella, J. F., Levy, D. L., Harte, C. B., Jørgensen, A., et al. (2004). Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: additional evidence. Am. J. Med. Genet. B, Neuropsychiatr. Genet. 128B, 30–36. doi: 10.1002/ajmg.b.30030
Meck, W. H. (1996). Neuropharmacology of timing and time perception. Brain Res. Cogn. Brain Res. 3, 227–242. doi: 10.1016/0926-6410(96)00009-2
Mitchell, P. B., Goodwin, G. M., Johnson, G. F., and Hirschfeld, R. M. (2008). Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord. 10(1 Pt 2),144–152. doi: 10.1111/j.1399-5618.2007.00559.x
Mogg, K., Bradbury, K. E., and Bradley, B. P. (2006). Interpretation of ambiguous information in clinical depression. Behav. Res. Ther. 44, 1411–1419. doi: 10.1016/j.brat.2005.10.008
Mogg, K., Millar, N., and Bradley, B. P. (2000). Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. J. Abnorm. Psychol. 109, 695–704. doi: 10.1037/0021-843X.109.4.695
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2010). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int. J. Surg. 8, 336–341. doi: 10.1016/j.ijsu.2010.02.007
Morrens, M., Wezenberg, E., Verkes, R. J., Hulstijn, W., Ruigt, G. S., and Sabbe, B. G. (2007). Psychomotor and memory effects of haloperidol, olanzapine, and paroxetine in healthy subjects after short-term administration. J. Clin. Psychopharmacol. 27, 15–21. doi: 10.1097/jcp.0b013e31802dfff0
Noiret, N., Carvalho, N., Laurent, É., Vulliez, L., Bennabi, D., Chopard, G., et al. (2015). Visual scanning behavior during processing of emotional faces in older adults with major depression. Aging Ment. Health 19, 264–273. doi: 10.1080/13607863.2014.926473
Nuding, U., Ono, S., Mustari, M. J., Büttner, U., and Glasauer, S. (2008). A theory of the dual pathways for smooth pursuit based on dynamic gain control. J. Neurophysiol. 99, 2798–2808. doi: 10.1152/jn.90237.2008
Park, K. M., Shin, K. J., Ha, S. Y., Park, J., Kim, S. E., Kim, H. C., et al. (2015). Can the adverse effects of antiepileptic drugs be detected in saccadic eye movements? Seizure 25, 33–36. doi: 10.1016/j.seizure.2014.12.003
Parker, G., Roy, K., Wilhelm, K., Mitchell, P., and Hadzi-Pavlovic, D. (2000). The nature of bipolar depression: implications for the definition of melancholia. J. Affect. Disord. 59, 217–224. doi: 10.1016/S0165-0327(99)00144-5
Peckham, A. D., McHugh, R. K., and Otto, M. W. (2010). A meta-analysis of the magnitude of biased attention in depression. Depress. Anxiety 27, 1135–1142. doi: 10.1002/da.20755
Pier, M. P., Hulstijn, W., and Sabbe, B. G. (2004). Differential patterns of psychomotor functioning in unmedicated melancholic and nonmelancholic depressed patients. J. Psychiatr. Res. 38, 425–435. doi: 10.1016/j.jpsychires.2003.11.008
Pierrot-Deseilligny, C., Müri, R. M., Ploner, C. J., Gaymard, B., Demeret, S., and Rivaud-Pechoux, S. (2003). Decisional role of the dorsolateral prefrontal cortex in ocular motor behaviour. Brain 126, 1460–1473. doi: 10.1093/brain/awg148
Pierrot-Deseilligny, E., and Burke, D. C. (2005). The Circuitry of the Human Spinal Cord: Its Role in Motor Control and Movement Disorders. New York, NY: Cambridge University Press. doi: 10.1017/cbo9780511545047
Reilly, J. L., Lencer, R., Bishop, J. R., Keedy, S., and Sweeney, J. A. (2008). Pharmacological treatment effects on eye movement control. Brain Cogn. 68, 415–435. doi: 10.1016/j.bandc.2008.08.026
Rogers, M. A., Kasai, K., Koji, M., Fukuda, R., Iwanami, A., Nakagome, K., et al. (2004). Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci. Res. 50, 1–11. doi: 10.1016/j.neures.2004.05.003
Rosano, C., Krisky, C. M., Welling, J. S., Eddy, W. F., Luna, B., Thulborn, K. R., et al. (2002). Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cereb. Cortex 12, 107–115. doi: 10.1093/cercor/12.2.107
Sabbe, B., Hulstijn, W., Van Hoof, J., and Zitman, F. (1996). Fine motor retardation and depression. J. Psychiatr. Res. 30, 295–306. doi: 10.1016/0022-3956(96)00014-3
Sanchez, A., Vazquez, C., Marker, C., LeMoult, J., and Joormann, J. (2013). Attentional disengagement predicts stress recovery in depression: an eye-tracking study. J. Abnorm. Psychol. 122, 303–313. doi: 10.1037/a0031529
Schall, J. D. (2004). On the role of frontal eye field in guiding attention and saccades. Vision Res. 44, 1453–1467. doi: 10.1016/j.visres.2003.10.025
Sears, C., Newman, K., Ference, J., and Thomas, C. (2011). Attention to emotional images in previously depressed individuals: an eye-tracking study. Cognit. Ther. Res. 35, 517–528. doi: 10.1007/s10608-011-9396-5
Sereno, A. B., and Holzman, P. S. (1993). Express saccades and smooth pursuit eye movement function in schizophrenic, affective disorder, and normal subjects. J. Cogn. Neurosci. 5, 303–316. doi: 10.1162/jocn.1993.5.3.303
Smith, D. J., and Craddock, N. (2011). Unipolar and bipolar depression: different of the same? Br. J. Psychiatry 199, 272–274. doi: 10.1192/bjp.bp.111.092726
Smith, M. J., Brébion, G., Banquet, J. P., and Allilaire, J. F. (1994). Experimental evidence for two dimensions of cognitive disorders in depressives. J. Psychiatr. Res. 28, 401–411. doi: 10.1016/0022-3956(94)90021-3
Surguladze, S. A., Young, A. W., Senior, C., Brébion, G., Travis, M. J., and Phillips, M. L. (2004). Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology 18, 212–218. doi: 10.1037/0894-4105.18.2.212
Suzuki, M., Takahashi, S., Matsushima, E., Tsunoda, M., Kurachi, M., Okada, T., et al. (2009). Exploratory eye movement dysfunction as a discriminator for schizophrenia: a large sample study using a newly developed digital computerized system. Eur. Arch. Psychiatry Clin. Neurosci. 259, 186–194. doi: 10.1007/s00406-008-0850-7
Swann, A. C. (2010). Mechanisms of impulsivity in bipolar disorder and related illness. Epidemiol. Psichiatr. Soc. 19, 120–130. doi: 10.1017/S1121189X00000828
Swann, A. C., Pazzaglia, P., Nicholls, A., Dougherty, D. M., and Moeller, F. G. (2003). Impulsivity and phase of illness in bipolar disorder. J. Affect. Disord. 73, 105–111. doi: 10.1016/S0165-0327(02)00328-2
Sweeney, J. A., Bauer, K. S., Keshavan, M. S., Haas, G. L., Schooler, N. R., and Kroboth, P. D. (1997). Adverse effects of risperidone on eye movement activity: a comparison of risperidone and haloperidol in antipsychotic-naive schizophrenic patients. Neuropsychopharmacology 16, 217–228. doi: 10.1016/S0893-133X(96)00195-9
Sweeney, J. A., Clementz, B. A., Haas, G. L., Escobar, M. D., Drake, K., and Frances, A. J. (1994). Eye tracking dysfunction in schizophrenia: characterization of component eye movement abnormalities, diagnostic specificity, and the role of attention. J. Abnorm. Psychol. 103, 222–230.
Sweeney, J. A., Luna, B., Haas, G. L., Keshavan, M. S., Mann, J. J., and Thase, M. E. (1999). Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol. Psycchiatry 46, 671–680.
Theeuwes, J., Kramer, A. F., Hahn, S., and Irwin, D. E. (1998). Our eyes do not always go where we want them to go: capture of the eyes by new objects. Psychol. Sci. 9, 379–385. doi: 10.1111/1467-9280.00071
Tien, A. Y., Ross, D. E., Pearlson, G., and Strauss, M. E. (1996). Eye movements and psychopathology in schizophrenia and bipolar disorder. J. Nerv. Ment. Dis. 184, 331–338. doi: 10.1097/00005053-199606000-00001
Tse, W. S., and Bond, A. J. (2004). The impact of depression on social skills. J. Nerv. Ment. Dis. 192, 260–268. doi: 10.1097/01.nmd.0000120884.60002.2b
Van der Linden, M., and Hupet, M. (1994). Le Vieillissement Cognitif. Paris: Presses Universitaires de France - PUF.
Van Nechel, C. (2007). Les anomalies oculomotrices dues aux médicaments. Bull. Soc. Ophtalmol. 304, 179–184.
Weierich, M. R., and Treat, T. A. (2008). Theories and measurement of visual attentional processing in anxiety. Cogn. Emot. 22, 985–1018. doi: 10.1080/02699930701597601
Wells, T. T., Clerkin, E. M., Ellis, A. J., and Beevers, C. G. (2013). Effect of antidepressant medication use on emotional information processing in major depression. Am. J. Psychiatry. 171, 195–200. doi: 10.1176/appi.ajp.2013.12091243
Williams, J. M. G. (1997). Cognitive Psychology and Emotional Disorders. Chichester: John Wiley and Sons.
Winograd-Gurvich, C., Georgiou-Karistianis, N., Fitzgerald, P. B., Millist, L., and White, O. B. (2006a). Ocular motor differences between melancholic and non-melancholic depression. J. Affect. Disord. 93, 193–203. doi: 10.1016/j.jad.2006.03.018
Winograd-Gurvich, C., Georgiou-Karistianis, N., Fitzgerald, P. B., Millist, L., and White, O. B. (2006b). Self-paced and reprogrammed saccades: differences between melancholic and non-melancholic depression. Neurosci. Res. 56, 253–260. doi: 10.1016/j.neures.2006.07.003
Wisco, B. E. (2009). Depressive cognition: self-reference and depth of processing. Clin. Psychol. Rev. 29, 382–392. doi: 10.1016/j.cpr.2009.03.003
Wise, S. P. (1984). Saccadic eye movements in response to drug action in the midbrain. Trends Neurosci. 7, 357–358. doi: 10.1016/S0166-2236(84)80049-1
Keywords: unipolar depression, bipolar depression, eye movement, saccade, emotion
Citation: Carvalho N, Laurent E, Noiret N, Chopard G, Haffen E, Bennabi D and Vandel P (2015) Eye Movement in Unipolar and Bipolar Depression: A Systematic Review of the Literature. Front. Psychol. 6:1809. doi: 10.3389/fpsyg.2015.01809
Received: 02 May 2015; Accepted: 09 November 2015;
Published: 15 December 2015.
Edited by:
John Monterosso, University of Southern California, USAReviewed by:
Philip B. Mitchell, University of New South Wales, AustraliaBreno Satler Diniz, Federal University of Minas Gerais, Brazil
Copyright © 2015 Carvalho, Laurent, Noiret, Chopard, Haffen, Bennabi and Vandel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicolas Carvalho, bmljLmNhcnZhbGhAZ21haWwuY29t;
Eric Laurent, ZXJpYy5sYXVyZW50QHVuaXYtZmNvbXRlLmZy
 Nicolas Carvalho
Nicolas Carvalho Eric Laurent
Eric Laurent Nicolas Noiret1,3
Nicolas Noiret1,3 Djamila Bennabi
Djamila Bennabi Pierre Vandel
Pierre Vandel