
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Psychol. , 11 June 2015
Sec. Movement Science
Volume 6 - 2015 | https://doi.org/10.3389/fpsyg.2015.00799
Since the discovery of the mirror neuron system in the 1980s, little, if any, research has been devoted to the study of interactive motor tasks (Goldman, 2012). Scientists interested in the neuropsychophysiological markers of joint motor action have relied on observation paradigms and passive tasks rather than dynamic paradigms and interactive tasks (Konvalinka and Roepstorff, 2012). Within this research scenario, we introduce a novel research paradigm that uses cooperative juggling as a platform to capture peripheral (e.g., skin conductance, breathing and heart rates, electromyographic signals) and central neuropsychophysiological (e.g., functional connectivity within and between brains) markers underlying the notion of team mental models (TMM). We discuss the epistemological and theoretical grounds of a cooperative juggling paradigm, and propose testable hypotheses on neuropsychophysiological markers underlying TMM. Furthermore, we present key methodological concerns that may influence peripheral responses as well as single and hyperbrain network configurations during joint motor action. Preliminary findings of the paradigm are highlighted. We conclude by delineating avenues for future research.
Across domains of human interest, science has always evolved through research paradigms (Kuhn, 1962). In sport science and performance psychology, neuropsychophysiological research has been primarily shaped by the expert-novice paradigm (Eklund and Tenenbaum, 2014). Scholars have aimed to identify the neuropsychophysiological markers (i.e., neural and physiological markers associated with psychological constructs) that distinguish high-performing individuals (“experts”) from their low-performing counterparts (“novices”), and optimal (e.g., “flow-feeling”) from poor (e.g., “choke”) performance states (Yarrow et al., 2009; Bertollo et al., 2013). Although various technological methodologies (e.g., fMRI, PET, NIRS, TMS) have been used to study motor tasks, most of what is known about the neuropsychophysiological markers of skilled performance is derived from electroencephalography (EEG) studies in precision sports, such as archery and pistol shooting (Hatfield and Kerick, 2007).
While the study of self-paced sports using EEG has evolved our knowledge of the neuropsychophysiological markers and networks of individuals’ skilled motor performance (Nakata et al., 2010), little is known about the neuropsychophysiological networks involved in successful interactive team actions (Reed et al., 2006; Tognoli, 2008). Since the discovery of the mirror neuron system in the early 1980s, little, if any, research has focused on interactive motor tasks (Goldman, 2012; Schilbach et al., 2013). Furthermore, social biology and social neuroscience researchers have primarily relied on passive (i.e., information flows unidirectionally from an active to a disengaged subject, such as avatars) rather than interactive paradigms (information flows multi-directionally between two or more active individuals; see Di Paolo and De Jaegher, 2012; Konvalinka and Roepstorff, 2012). Our perspective herein is to propose a novel paradigm, using cooperative juggling as a platform, to identify peripheral and central neuropsychophysiological mechanisms underlying the conceptual notion of team mental models (TMM).
We start by providing support for a juggling paradigm. The theoretical roots of TMM, illustrating how a juggling paradigm can advance research on TMM, are then presented. Next, we elaborate on a series of methodological considerations needed to advance our understanding of the neuropsychophysiological markers of TMM. We conclude by presenting preliminary findings and commenting on avenues of future research.
There is cross-disciplinary evidence that juggling offers a robust platform to advance knowledge in a variety of research domains, including motor behavior, brain sciences, and mathematics (Draganski et al., 2004; Dessing et al., 2012). Our proposal is aimed at identifying the peripheral and central physiological markers of team actions in general, and joint motor actions in particular. We propose that a juggling paradigm can greatly advance knowledge of “multi-brain” interactions during joint motor actions, akin to how research on self-paced sports was used to advance our knowledge of “single brains.” Cooperative juggling presents epistemological and methodological advantages that might help in the identification of neuropsychophysiological markers underlying TMM.
From an epistemological standpoint, cooperative juggling establishes that the locus of interest is on a given team, as two or more jugglers share the goal of “keeping the balls in the air.” To become a team a group of individuals should share a common goal (Carron et al., 2007). Without a shared goal, an assembly of individuals is a “group” rather than a “team.” Moreover, social loafing (i.e., a decrease in personal effort when individuals work in groups) is unlikely to occur in cooperative juggling given that individual mistakes and lack of effort are visible to the self and others. With a shared goal and clear performance expectations, the search for the neuropsychophysiological markers underlying TMM and other team-level phenomena (e.g., cohesion, collective-efficacy) is epistemologically valid within a juggling paradigm.
From a methodological standpoint, cooperative juggling represents an interactive task, in the sense that information flows multi-directionally between two or more jugglers. Most existing studies have been based on passive paradigms and cognitive task-analysis (e.g., card playing; music), thus limiting scholars’ ability to ask and respond to questions on joint motor interactions (Schilbach et al., 2013). For instance, in card playing one must play first so that another player has the opportunity to respond. Studies in music have considered musicians playing their own instrument rather than interacting through shared instruments (Lindenberger et al., 2009; Müller et al., 2013). Recent technological advancements, particularly mobile EEG synchronized with kinematic recording devices, allow for the reliable and multimodal monitoring of complex motor actions, including joint actions in cooperative juggling (Reis et al., 2014; Schack et al., 2014). Altogether, we posit that cooperative juggling offers an ideal epistemological and methodological platform to advance the theory of TMM.
Team expertise has been associated with the development of team-level cognitive schemas or TMM (Mathieu et al., 2000; Mohammed et al., 2010). To date, however, there is no reliable neuropsychophysiological evidence that team-level cognition exists. In this section, we provide an overview of the concept of TMM, while advancing several testable hypotheses to assess peripheral and central neuropsychophysiological markers of TMM (Figure 1).
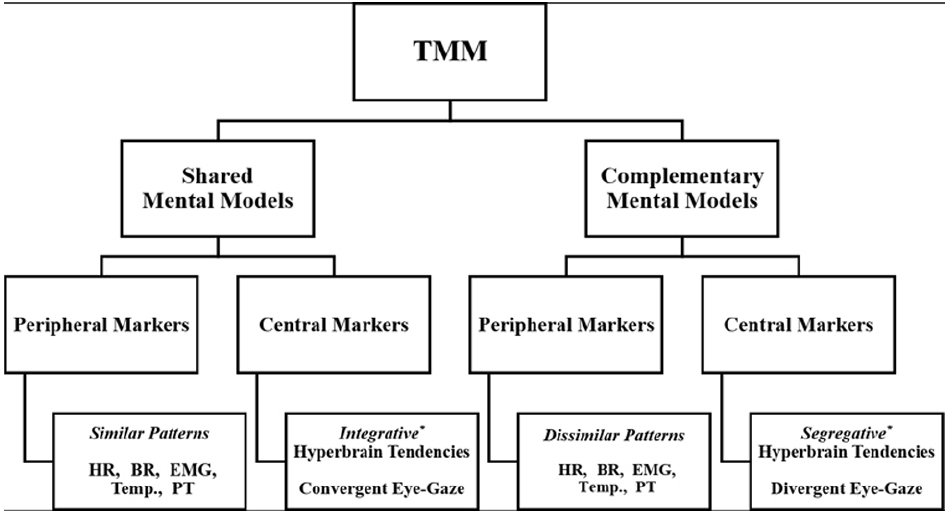
Figure 1. Proposed hypothetical neuropsychophysiological markers of TMM. HR, heart rate; BR, breathing rate; EMG, electromyography; Temp., temperature; PT, posture. *Prevalent Topological Configuration between two or more brains.
The term TMM has been defined as “task and team relevant knowledge that team members bring to a situation” (Cooke et al., 2000, p. 153). While a unified theory of TMM is not available to date (Filho et al., 2015), scholars concur that TMM represent two main forms of “task and team relevant knowledge,” namely shared and complementary mental models (Cannon-Bowers and Salas, 2001; Mohammed et al., 2010). Shared mental models refer to communal schemas about team tasks, strategies, and teammates’ characteristics. Complementary mental models pertain to idiosyncratic schemas held by teammates about team tasks, strategies, and teammates’ characteristics (Xinwen et al., 2006). To be successful, a team needs both shared and complementary mental models (Cannon-Bowers and Salas, 2001; Mohammed et al., 2010). Without shared knowledge, teammates cannot develop heuristic routes to facilitate team coordination and optimize decision-making under high-pressure situations (Bearman et al., 2010; Filho and Tenenbaum, 2012). Without complementary knowledge, teammates are unable to compensate for coordination breakdowns or generate creative solutions (Mohammed et al., 2010; Filho and Tenenbaum, 2012).
The existence of shared and complementary mental models has been established through the observation of coordination mechanisms (Cannon-Bowers et al., 1993; Mohammed et al., 2010). From a socio-cognitive standpoint, coordination refers to spatio-temporal synchronized action and effort among teammates and includes (a) explicit coordination, manifested through spoken verbal communication; and (b) implicit coordination, exhibited through non-verbal behavior (Filho and Tenenbaum, 2012). An abundance of research on explicit coordination exists (Mohammed et al., 2010). However, the neuropsychophysiological markers of implicit coordination remain understudied, especially in real-time interactive tasks (Reed et al., 2006; Schilbach et al., 2013). Accordingly, we focus on how the theoretical notions of shared and complementary mental models can be related to peripheral and central neuropsychophysiological variables.
To reliably identify neuropsychophysiological markers of TMM, a “control condition” must be defined. In juggling, a control condition would consist of a “solo juggling” (i.e., individual) task to be contrasted with an “interactive” condition (i.e., two or more jugglers established a cooperative interaction by juggling balls with each other) as illustrated in Figures 2A,B, respectively. The absence of a control condition prevents the researcher from being able to identify differences between individual and coupled peripheral and central neuropsychophysiological responses. This rationale is equivalent to current praxis in social neuroscience, where non-clinical individuals are used as controls in studies about social brain disorders (Harris, 2003). The solo and interactive conditions must be similar in terms of difficulty level, as established by the same number of degrees of freedom (i.e., the number of balls juggled minus the number of hands). Based on this rationale, if the interactive team-level task is defined as “dyadic juggling with five balls in the cascade style,” then the control condition would consist of “solo juggling with three balls in the cascade style.” Variations in the number of balls can occur as long as the solo and interactive conditions remain comparable. The difficulty level can also be established by quantitative (e.g., regression models), survey (e.g., Rates of Perceived Effort), and qualitative (e.g., interview with performers) methods.
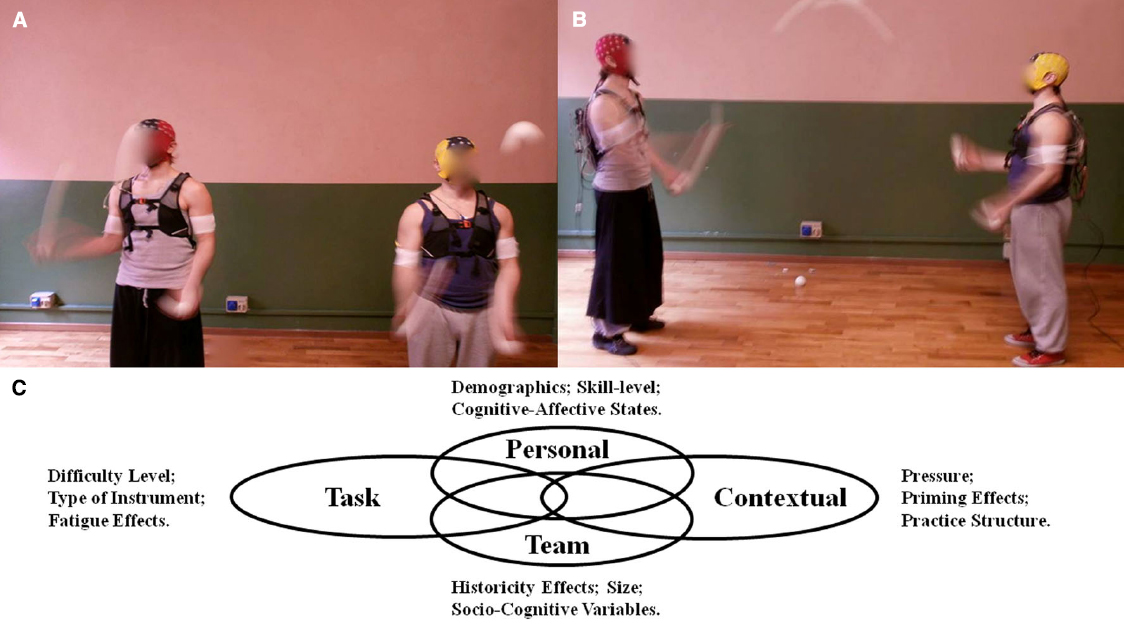
Figure 2. Illustration of EEG acquisitions in the individual condition of solo juggling (A) and using a cooperative juggling paradigm (B). Venn Diagram illustrating personal, contextual, task, and team-level factors to be experimentally manipulated and accounted for (C).
A reliable control condition allows for testing of hypotheses. The first hypothesis is that teammates’ neuropsychophysiological responses (Figure 1) should differ in interactive team-level tasks in comparison to individually performed tasks. This difference is due to the coordination effort needed for cooperative work in team settings (Eccles and Tenenbaum, 2004). Although conceptually appealing, this hypothesis has yet to be examined from a neuropsychophysiological standpoint. The second hypothesis is that similar patterns among peripheral neuropsychophysiological responses of teammates performing an interactive motor task might be indicative of shared mental models. This would be in line with the “mimicry coordination mechanism,” which is at the core of Theory of Mind and has greatly influenced research on team processes (Goldman, 2012). Conversely, dissimilar patterns of peripheral neuropsychophysiological responses might be indicative of compensatory activations aimed at reaching team coordination, similar to the notion of complementary mental models.
Conclusions about the relationship of shared and complementary inter-individual neuropsychophysiological patterns should be drawn with care, as these patterns might also be due to task characteristics or the reaching of a physiological plateau. Noteworthy, non-linear functions may also signal TMM, as teammates’ compensatory behaviors and bio-psycho-social states are not necessarily linear processes (Carron et al., 2007). For instance, teammates’ idiosyncratic rhythms, as indicated by the lack of linear or non-linear relationship among paired neuropsychophysiological responses, may signal either complementary mental models (e.g., teammates’ responses are related to task) or the absence of TMM (e.g., teammates’ responses are unrelated to task).
The interpretation of teammates’ coupled neuropsychophysiological patterns should be made in light of previous research on the mind-body connection. For instance, heart rate patterns have been linked to cognitive load and attentional control (Veltman and Gaillard, 1998; Bertollo et al., 2013), whereas breathing pattern is considered an indicator of motor coordination for skills of differing complexity levels (Martin-Harris, 2006; Seifert et al., 2010). Moreover, electromyography (EMG) and posture data can be used to inform research on TMM. Grounded on the notion of mirror neurons, jugglers exhibiting markedly similar EMG waves (forms, intensity, and frequency) in a given muscle group, while leaning toward the same location, may be relying on shared mental models. Conversely, jugglers displaying different EMG activations and distinct yet action-related compensatory posture may be relying on complementary mental models. Measures of hormones in blood plasma, temperature and skin conductance may also help to establish whether teammates share a similar emotional state. For instance, cortisol levels, low temperature on body extremities, and reduced skin conductance have been associated with stress responses (Eklund and Tenenbaum, 2014).
Electroencephalography is commonly considered the most reliable method for studying interactive brains during motor tasks (Konvalinka and Roepstorff, 2012). In this context, functional connectivity and efficiency measures are particularly suitable for the study of joint actions (Sänger et al., 2013). Functional connectivity maps can quantify the functional interdependencies related to shared or complementary mental models. Efficiency measures, such as those provided in Graph Theory, may help to reveal, through a hyperbrain approach, between-brains functional network topologies related to shared or complementary mental models.
The first hypothesis on central markers of TMM would test the notion that each individual possesses idiosyncratic neural functional patterns (complementary mental models) related to the interactive motor task. For instance, a highly skilled juggler may exhibit higher neural efficiency than a less skilled juggler. To this extent, eye-tracking technology could add information on the behavioral basis for the skill level of each juggler. Indeed, fixation and duration of eye-gaze have been linked to central mnemonic adaptations and associated with skill level (Eklund and Tenenbaum, 2014), with experts exhibiting context control (e.g., gaze at a central location) and novices showing target control (e.g., following “targets”) strategies.
The second hypothesis is that two brains engaged in a joint action should show unique systemic and communication characteristics, in comparison to the individual control condition. We would expect that integrative hyperbrain patterns (i.e., shared activity among brain cortices) reflect shared mental models, whereas segregative brain tendencies (i.e., low hyperbrain functional connectivity) indicate complementary mental models. Altogether, hyperbrain analysis performed through Graph Theory allows for the identification of functional flexibility or meta-stability (both integrative and segregative tendencies) of multi-brain networks (Tognoli, 2008), which in turn can serve as a neural index of individual preferences and team expertise. It has long been noted in Gestalt Psychology that a team is “greater than the sum of its parts” (TMM > Σ individuals’ mental models), or “only as strong as its weakest link” (TMM ≤ Σ individuals’ mental models). A hyperbrain approach applied to cooperative juggling can ultimately advance our knowledge of team expertise in interactive tasks by providing evidence of possible dynamic links between two interactive brains. Further manipulating the personal, task, contextual, and team-level factors may help to identify the ensemble of neuropsychophysiological markers of team expertise.
A methodological cornerstone pertains to the synchronization of two or more acquisition systems used to record neuropsychophysiological signals of joint motor action. Without precisely synchronized systems, it is impossible to reliably identify peripheral and central markers of joint motor action. As opposed to large-scale nomothetic studies, an idiographic approach through a series of well-controlled case studies might be the most appropriate design given that each cooperative team may have a unique “modus operandi.” Furthermore, case studies and small-n studies are appropriate when data acquisition is complex, costly and time intensive, and when potential participants are rare (Editorial Nature Neuroscience, 2004).
Other methodological aspects pertain to personal, task, contextual, and team-level factors (Figure 2C). The person-task-context notion has been the basis of studies in human action, as per the well-established Action Theory (Schack and Hackfort, 2007). Over the past 30 years, scholars have manipulated personal, task, and contextual variables in the search for answers about skilled movement action (Schmidt and Lee, 2011). Additionally, it is important to account for variance on team-level factors when conducting socio-cognitive research (Feltz et al., 2008). Therefore, we expand on the personal, task, contextual, and team-level factors that can be manipulated to advance knowledge of the neuropsychophysiological markers of TMM within a juggling paradigm.
Demographic variables (e.g., age, gender, hand dominance) should be accounted for as such variables influence performance in individual and team-level actions (Carron et al., 2007). Skill level is also likely to influence joint motor action (Eccles and Tenenbaum, 2004). For instance, during cooperative juggling, an expert may have to compensate for mistakes from a novice juggler. Furthermore, cognitive (e.g., self-efficacy; associative-dissociative focus) and affective states (e.g., arousal and pleasantness) have been associated with performance in motor tasks (Hanin, 2007). Single-item measures, the most ecologically valid approach for collecting data during motor task, can be used to assess the aforementioned factors (Kamata et al., 2002).
Task difficulty can be manipulated by increasing the number of elements to be juggled (easy, moderate, hard levels). Furthermore, changing the juggling instrument type (e.g., balls, clubs, diablo) should activate different neuropsychophysiological mechanisms (Beek and Lewbel, 1995). A juggling dyad can be proficient juggling with balls and only mediocre juggling with clubs. Finally, it is important to control for fatigue effects that can influence performance and the reliability of the assessment of neuropsychophysiological markers (Marcora and Staiano, 2010).
Manipulating pressure through different means (e.g., audience effects; panel of judges) can be used to explore neuropsychophysiological changes (Schilbach et al., 2013). One could explore whether hyperbrain networks change under pressure in comparison to a non-pressure condition. Further, priming effects influence a range of social actions (Kuzyakov et al., 2000). Priming positive and negative emotions about a context or unknown juggling partner may induce neuropsychophysiological changes that can affect the interactive motor action. Additionally, manipulating practice structure (e.g., blocked or random practice; see Schmidt and Lee, 2011) can advance knowledge on “team learning.” The assumption is that the quality and quantity of TMM can be influenced by practice structure.
Controlling for historicity effects is essential in social interaction studies. The existence, nature and extent of previous interactions influence team processes (Di Paolo and De Jaegher, 2012). Furthermore, the size of the team influences team dynamics and performance (Carron et al., 2007). Dyadic teams represent realistic target samples, with larger teams adding complexity to data collection and analysis. Socio-cognitive variables, such as cohesion and collective-efficacy, can be used as triangulation sources to help interpret neuropsychophysiological markers of TMM (Filho et al., 2015).
The juggling paradigm proposed herein has been implemented in two case studies using two different cooperative juggling dyads and two different experimental conditions: “individual” and “interactive” tasks. In study-1 we targeted peripheral markers (i.e., breathing and heart rate). In study-2 we targeted central markers of TMM by using two synchronized EEG systems. Results from study-1 revealed a strong correlation between the jugglers’ heart rate (r = 0.87, p < 0.01) and breathing rate (r = 0.77, p < 0.01) in the interactive condition. Results from study-2, based on a graph theoretical approach, suggested that the juggling dyad presented a hyperbrain pattern that varied with task difficulty, wherein higher values of “small-world-ness” were observed for an easier task in both theta (0.84) and alpha bands (0.82), as compared to a harder task (theta = 0.56; alpha = 0.52). That is, easier tasks fostered more integrative hyperbrain tendencies (shared models), whereas harder tasks elicited more segregative (complementary models) hyperbrain tendencies.
Future research should aim to answer three main questions. First, what are the neuropsychophysiological markers of TMM? Second, how do potential neuropsychophysiological markers of TMM vary in respect to personal, task, contextual, and team-level factors? Third, are changes (i.e., learning) in shared and complementary mental models observable through neuropsychophysiological longitudinal monitoring of cooperative dyads practicing together? The influence of co-regulation training on both functional (integrative and segregative brain tendencies) and structural (neuroplasticity; see Draganski et al., 2004) neurological adaptations should also be advanced. Beyond cooperative tasks, hyperbrain research in competitive tasks may advance knowledge on broader meta-cognitive concepts, including anticipation skills in sports, “strategic mindreading” (Game Theory), and “collective-consciousness.”
All authors have contributed with their specific expertise to designing the juggling paradigm presented herein.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Edson Filho started to work on this paradigm with support by the Association for Applied Sport Psychology (2013–2014) in a project titled “From Explicit to Implicit Coordination: Interactive Brains in Circus Acts.”
Bearman, C., Paletz, S. B. F., Orasanu, J., and Thomas, M. J. W. (2010). The breakdown of coordinated decision making in distributed systems. Hum. Factors 52, 173–188. doi: 10.1177/0018720810372104
Beek, P. J., and Lewbel, A. (1995). The science of juggling. Sci. Am. 273, 92–97. doi: 10.1038/scientificamerican1195-92
Bertollo, M., Bortoli, L., Gramaccioni, G., Hanin, Y., Comani, S., and Robazza, C. (2013). Behavioural and psychophysiological correlates of athletic performance: a test of the multi-action plan model. Appl. Psychophysiol. Biofeedback 38, 91–99. doi: 10.1007/s10484-013-9211-z
Cannon-Bowers, J. A., Salas, E., and Converse, S. A. (1993). “Shared mental models in expert decision making teams,” in Current Issues in Individual and Group Decision Making, ed. N. J. Jr. Castellan (Hillsdale, NJ: Erlbaum), 221–246.
Cannon-Bowers, J. A., and Salas, E. (2001). Reflections on shared cognition. J. Organ. Behav. 22, 195–202. doi: 10.1002/job.82
Carron, A. V., Eys, M. A., and Burke, S. M. (2007). “Team cohesion: nature, correlates, and development,” in Social Psychology in Sport, eds S. Jowette and D. Lavallee (Champaign, IL: Human Kinetics), 91–102.
Cooke, N. J., Salas, E., Cannon-Bowers, J. A., and Stout, R. (2000). Measuring team knowledge. Hum. Factors 42, 151–173. doi: 10.1518/001872000779656561
Dessing, J. C., Rey, F. P., and Beek, P. J. (2012). Gaze fixation improves the stability of expert juggling. Exp. Brain Res. 216, 635–644. doi: 10.1007/s00221-011-2967-6
Di Paolo, E., and De Jaegher, H. (2012). The interactive brain hypothesis. Front. Hum. Neurosci. 6:163. doi: 10.3389/fnhum.2012.00163
Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., and May, A. (2004). Neuroplasticity: changes in grey matter induced by training. Nature 427, 311–312. doi: 10.1038/427311a
Eccles, D. W., and Tenenbaum, G. (2004). Why an expert team is more than a team of experts: a social-cognitive conceptualization of team coordination and communication in sport. J. Sport Exerc. Psychol. 26, 542–560.
Editorial Nature Neuroscience. (2004). When once is enough. Nat. Neurosci. 7, 93. doi: 10.1038/nn0204-93
Eklund, R. C., and Tenenbaum, G. (2014). Encyclopedia of Sport and Exercise Psychology. Thousand Oaks, CA: Sage Publications.
Feltz, D. L., Short, S. E., and Sullivan, P. J. (2008). Self-efficacy in Sport. Champaign, IL: Human Kinetics.
Filho, E., and Tenenbaum, G. (2012). “Team mental models in sports: an overview,” in Athletic Insight’s Writings in Sport Psychology, ed. R. Schinke (Hauppauge, NY: Nova Science Publishers), 329–342.
Filho, E., Tenenbaum, G., and Yang, Y. (2015). Cohesion, team mental models and collective efficacy: towards an integrated model of team dynamics in sports. J. Sports Sci. 33, 641–653. doi: 10.1080/02640414.2014.957714
Goldman, A. I. (2012). “Theory of mind,” in Oxford Handbook of Philosophy and Cognitive Science, eds E. Margolis, R. Samuels, and S. Stich (Oxford: Oxford University Press), 402–424.
Hanin, Y. L. (2007). “Emotions in sport: current issues and perspectives,” in Handbook of Sport Psychology, 3rd Edn, eds G. Tenenbaum and R. C. Eklund (New York, NY: Wiley & Sons), 31–58.
Harris, J. C. (2003). Social neuroscience, empathy, brain integration, and neurodevelopmental disorders. Physiol. Behav. 79, 525–531. doi: 10.1016/S0031-9384(03)00158-6
Hatfield, B. D., and Kerick, S. E. (2007). “The psychology of superior sport performance: a cognitive and affective neuroscience perspective,” in Handbook of Sport Psychology, 3rd Edn, eds G. Tenenbaum and R. C. Eklund (Hoboken, NJ: Wiley), 84–109.
Kamata, A., Tenenbaum, G., and Hanin, Y. L. (2002). Individual zone of optimal functioning (IZOF): a probabilistic estimation. J. Sports Exerc. Psychol. 24, 189–208.
Konvalinka, I., and Roepstorff, A. (2012). The two-brain approach: how can mutually interacting brains teach us something about social interaction? Front. Hum. Neurosci. 6:215. doi: 10.3389/fnhum.2012.00215
Kuhn, T. S. (1962). The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press.
Kuzyakov, Y., Friedel, J. K., and Stahra, K. (2000). Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498. doi: 10.1016/S0038-0717(00)00084-5
Lindenberger, U., Li, S., Gruber, W., and Müller, V. (2009). Brains swinging in concert: cortical phase synchronization while playing guitar. BMC Neurosci. 10:22. doi: 10.1186/1471-2202-10-22
Marcora, S. M., and Staiano, W. (2010). The limit to exercise tolerance in humans: mind over muscle? Eur. J. Appl. Physiol. 109, 763–770. doi: 10.1007/s00421-010-1418-6
Mathieu, J. E., Heffner, T. S., Goodwin, G. F., Salas, E., and Cannon-Bowers, J. (2000). The influence of shared mental models on team process and performance. J. Appl. Psychol. 85, 273–283. doi: 10.1037/0021-9010.85.2.273
Mohammed, S., Ferzandi, L., and Hamilton, K. (2010). Metaphor no more: a 15-year review of the team mental model construct. J. Manage. 36, 876–910. doi: 10.1177/0149206309356804
Müller, V., Sänger, J., and Lindenberger, U. (2013). Intra- and inter-brain synchronization during musical improvisation on the guitar. PLoS ONE 8:e73852. doi: 10.1371/journal.pone.0073852
Nakata, H., Yoshie, M., Miura, A., and Kudo, K. (2010). Characteristics of the athletes’ brain: evidence from neurophysiology and neuroimaging. Brain Res. Rev. 62, 197–211. doi: 10.1016/j.brainresrev.2009.11.006
Reed, K. B., Peshkin, M., Hartmann, M. J., Patton, J., Vishton, P. M., and Grabowecky, M. (2006). “Haptic cooperation between people, and between people and machines.” in Proceedings of the 2006 IEEE/RSJ International Conference on Intelligent Robots and Systems October 9–15, 2006, Beijing, 2109–2114. doi: 10.1109/IROS.2006.282489
Reis, P. M., Hebenstreit, F., Gabsteiger, F., von Tscharner, V., and Lochmann, M. (2014). Methodological aspects of EEG and body dynamics measurements during motion. Front Hum. Neurosci. 8:156. doi: 10.3389/fnhum.2014.00156
Sänger, J., Müller, V., and Lindenberger, U. (2013). Directionality in hyperbrain networks discriminates between leaders and followers in guitar duets. Front. Hum. Neurosci. 7:234. doi: 10.3389/fnhum.2013.00234
Schack, T., Bertollo, M., Koester, D., Maycock, J., and Essig, K. (2014). “Technological advancements in sport psychology,” in Routledge Companion to Sport and Exercise Psychology, eds A. G. Papaioannou and D. Hackfort (London: Routledge), 953–965.
Schack, T., and Hackfort, D. (2007). “An action theory approach to applied sport psychology,” in Handbook of Sport Psychology, 3rd Edn, eds G. Tenenbaum and R. C. Eklund (New York, NY: John Wiley), 332–351.
Schilbach, L., Timmermans, B., Reddy, V., Costall, A., Bente, G., Schlicht, T., et al. (2013). Toward a second-person neuroscience. Behav. Brain Sci. 36, 393–414. doi: 10.1017/S0140525X12000660
Schmidt, R., and Lee, T. (2011). Motor Control and Learning: A Behavioral Emphasis, 5th Edn. Champaign, IL: Human Kinetics.
Seifert, L. L., Chollet, D. D., and Sanders, R. R. (2010). Does breathing disturb coordination in butterfly? Int. J. Sports Med. 31, 167–173. doi: 10.1055/s-0029-1243640
Tognoli, E. (2008). “EEG coordination dynamics: neuromarkers of social coordination,” in Coordination: Neural, Behavioral and Social Dynamics, eds A. Fuchs and V. K. Jirsa (Heidelberg: Springer), 309–323.
Veltman, J. A., and Gaillard, A. W. K. (1998). Physiological workload reactions to increasing levels of task difficulty. Ergonomics 41, 656–669. doi: 10.1080/001401398186829
Xinwen, B., Erping, W., Ying, Z., Dafei, M., and Jing, R. (2006). Developmental characteristics of two types of shared mental models. Acta Psychol. Sin. 38, 598–606.
Keywords: team mental models, shared mental models, complementary mental models, juggling, neuropsychophysiology, hyperbrains, social neuroscience
Citation: Filho E, Bertollo M, Robazza C and Comani S (2015) The juggling paradigm: a novel social neuroscience approach to identify neuropsychophysiological markers of team mental models. Front. Psychol. 6:799. doi: 10.3389/fpsyg.2015.00799
Received: 23 March 2015; Accepted: 27 May 2015;
Published: 11 June 2015.
Edited by:
Gordon Binsted, University of British Columbia, CanadaReviewed by:
Marco Taubert, Max Planck Institute for Human Cognitive and Brain Sciences, GermanyCopyright © 2015 Filho, Bertollo, Robazza and Comani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Comani, Behavioral Imaging and Neural Dynamics Center and Department of Neuroscience, Imaging and Clinical Sciences, University “G. d’Annunzio” of Chieti-Pescara, Via dei Vestini 33, 66013 Chieti, Italy,Y29tYW5pQHVuaWNoLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.