
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychol. , 11 August 2011
Sec. Emotion Science
volume 2 - 2011 | https://doi.org/10.3389/fpsyg.2011.00192
Cognition and emotion interact in important ways to shape ongoing behaviors. In this study, we investigated the interaction between conflict-driven executive control adjustments and emotion during a face-word Stroop-like paradigm. Neutral and negative images were employed to manipulate emotion. We were particularly interested in contrasting two hypotheses of the impact of emotion on conflict adaptation effects. On the one hand, resource accounts of cognitive–emotional interactions predict that behavioral adjustments following incongruent trials would be decreased when participants also have to process a negative stimulus. On the other hand, affect regulation models predict that negative emotion should increase behavioral adjustments. We found that task-irrelevant negative stimuli significantly reduced conflict-driven control effects (i.e., conflict adaptation) compared to neutral images. We interpret the findings in terms of shared resources between proactive control mechanisms and emotional processing. Our findings demonstrate that emotion interacts with executive mechanisms responsible for dynamic behavioral adjustments that are tied to environmental demands, a central facet of flexible, goal-directed behavior.
Previous studies have investigated interactions between emotion and executive function. For instance, during a working memory task, task-irrelevant negative images shown during the maintenance period disrupted task performance to a larger extent than neutral images (Dolcos and McCarthy, 2006; Anticevic et al., 2010). Other studies have probed interactions between emotion and conflict processing (Blair et al., 2007; Hart et al., 2010; Hu et al., 2011; Kanske and Kotz, 2011) and response inhibition (Verbruggen and De Houwer, 2007; Sagaspe et al., 2011; Pessoa et al., 2011), among others. What is the impact of emotion on executive functions? Recently, we proposed a dual competition model, which attempts to explain the interaction between emotion and executive function in terms of shared processing resources (Pessoa, 2009). According to the model, task performance is typically impaired in the presence of a task-irrelevant emotional stimulus because resources needed for the primary task are utilized, at least in part, toward the processing of the emotion-laden stimulus. For instance, when a task-irrelevant negative image was presented just before an incongruent trial in a Stroop-like task, increased reaction time (RT) interference effects were reported (Hart et al., 2010). According to the dual competition model, a possible interpretation of this effect is that resources needed for conflict processing are shared or diverted by the processing of negative pictures – hence, the increased interference.
Previous studies that investigated the impact of emotion on response conflict focused on reactive control mechanisms. In these studies, interference effects are indexed by RT differences between incongruent (I) and congruent (C) trials, which can then be compared between emotional and neutral conditions. But in many real life situations, cognitive control has to be adjusted dynamically based on external demands. In such cases, proactive control mechanisms play an important role. For example, a person driving a car on a highway, when entering city limits, detects increased traffic and enhances the focus on driving when compared to driving outside the city. A frequently used method to investigate dynamic behavioral adjustments across trials in the laboratory involves so-called conflict adaptation (Gratton et al., 1992). To investigate conflict adaptation effects, a response conflict task is used but, instead of focusing on RT interference, differences in RT interference are compared as a function of the previous-trials (congruent or incongruent). The idea here is that when participants experience an incongruent trial, they up-regulate control so as to be in a better position to handle conflict in the subsequent trial – thus leading to reduced RT interference (relative to the situation in which the initial trial was congruent).
In conflict adaptation studies, the RT interference effect (I–C) on trials preceded by an incongruent trial is smaller than the RT interference effect (I–C) on trials preceded by a congruent trial. Thus, the overall RT pattern exhibits a two-way interaction. In the typical nomenclature used in these studies, incongruent trials following an incongruent one are termed “iI” trials, and incongruent trials following a congruent one are termed “cI” trials; likewise, incongruent trials following a congruent one are termed “cI,” and congruent trials following a congruent one are termed “cC.” Thus, the RT interaction pattern implies that (iI − iC) < (cI − cC). Several theoretical accounts have been proposed to explain adaptation effects, including conflict-monitoring (Botvinick et al., 2001), feature-integration mechanisms involving priming (Mayr et al., 2003; Hommel et al., 2004), and expectancy mechanisms (Gratton et al., 1992). Although there is disagreement in the field about the exact nature of conflict adaptation effects, cognitive control mechanisms appear to be important even when one controls for potentially confounding effects (Egner, 2007).
How does emotion interact with proactive control mechanisms such as those leading to conflict adaptation effects? In a recent study, emotional state was manipulated via a mood induction procedure to provoke negative (anxious, sad) or positive (happy, calm) states (van Steenbergen et al., 2010). Negative moods increased conflict adaptation effects relative to positive moods in a classic flanker task. Specifically, mood induction prior to the flanker task did not significantly change the overall conflict effect (I–C) between negative and positive moods although, intriguingly, conflict adaptation [(iI − iC) − (cI − cC)] increased during negative compared to positive mood. The latter result suggests that, under certain conditions, emotion can actually increase control processes. In a separate study, the same group reported that non-performance-contingent monetary reward reduced these conflict adaptation effects (van Steenbergen et al., 2009). Specifically, conflict adaptation [(iI − iC) − (cI − cC)] was significantly reduced during the reward compared to no-reward feedback condition, again with no significant differences in the basic conflict effect (I–C) between conditions. Based on the latter findings, van Steenbergen and colleagues proposed that conflict itself may engender a brief negative affective state (Botvinick, 2007) that is counteracted by the delivery of reward (thus reducing conflict adaptation). Another recent study, however, did not detect an effect of anxiety (manipulated via anticipating shock) on conflict adaptation effects (Robinson et al., 2011).
The goal of the present study was to further probe the processes by which emotion interacts with proactive control. Here, we investigated how phasic manipulations of task-irrelevant emotion interacted with conflict-driven control effects. Participants performed a face-word interference task where they identified the gender of the face while ignoring overlaid words (Egner et al., 2010). Immediately following the face-plus-word stimulus, we presented a neutral or negative task-irrelevant image and investigated how conflict adaptation was influenced by the processing of these images. Based on our dual competition framework, we hypothesized that processing negative compared to neutral images between trials would expend or divert resources needed for control implementation. Thus, conflict adaptation effects would be reduced given that control would be compromised – for instance, the up-regulation of control following an incongruent trial would be impaired. Alternatively, a phasic manipulation of emotion could be expected to have a similar effect as more sustained emotional manipulations, as described by Hommel and colleagues (van Steenbergen et al., 2010). In this case, conflict adaptation effects would be expected to increase, as in their results. The present study thus evaluated the two competing hypotheses to better understand the interactions between negative emotion and conflict-driven control adjustments.
Thirty-eight, native English participants (24 females, age range: 19–28 years) gave informed written consent and participated in the study, which was approved by the Institutional Review Board of Indiana University, Bloomington. All subjects were in good health, free of medication, and had no history of psychiatric or neurological disease.
After providing consent to participate in the study, subjects filled the state–trait anxiety inventory (Spielberger et al., 1970).
Each trial started with the presentation of a white fixation cross for 500 ms followed by a compound face-plus-word stimulus for 1 s (Figure 1). For the compound stimulus, images of male or female neutral faces were overlaid with words (“MALE”/“male” or “FEMALE”/“female”) to create congruent and incongruent trial types. After 200 ms from the offset of the compound stimulus, a neutral or negative image (see below) was shown for 500 ms. Each trial ended with a 500-ms inter-trial interval. Participants were instructed to press the “/” button on the keyboard with the right index finger if they saw a male face and the “z” button with the left index finger if they saw a female face, while ignoring the word (response button mapping was counterbalanced across participants). Participants were given 1200 ms from the stimulus onset to make a response. Participants were told to focus on the center of the monitor during the experimental blocks (see below) and they were informed that the presentation of neutral/negative image after each trial was task-irrelevant and did not depend on their performance.
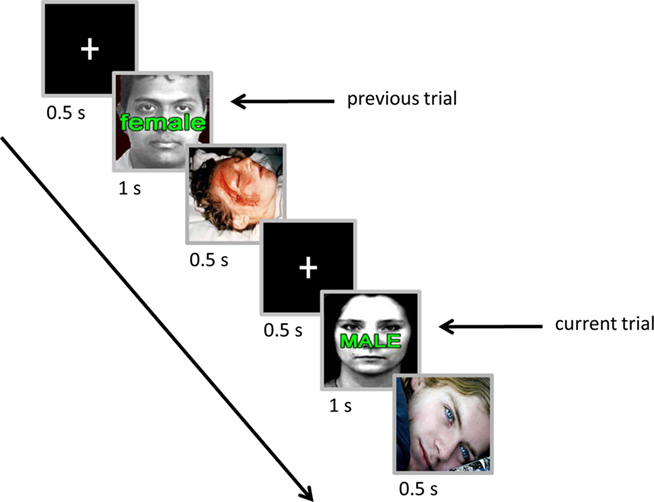
Figure 1. Experimental paradigm. Two consecutive trials are shown, illustrating an incongruent trial preceded by an incongruent trial with a negative emotional picture between them. The current-trial is followed by a neutral image. For simplicity, non-critical parts of the trials were omitted.
For the presentation of visual stimuli and recording of participant’s responses, Presentation software (Neurobehavioral Systems, Albany, CA, USA) was used. Before the start of the main experimental blocks, participants performed a practice block for 2 min, where explicit performance feedback was provided after each trial (a different set of stimuli was used). Following the brief practice, each participant performed six blocks of trials, each lasting about 2.5 min. Each block consisted of 48 trials, resulting in a total of 288 trials for the entire experimental session.
All experimental conditions and stimuli were intermixed randomly with the following constraints aimed at carefully balancing the order of experimental conditions, which is critical when studying conflict adaptation effects (Egner, 2007). The trial order of each block (except the first trial) was balanced in terms of previous- and current-trial congruency, specifically the following trial types: congruent trials preceded by a congruent trial (cC trials), incongruent trials preceded by a congruent trial (cI trials), congruent trials preceded by an incongruent trial (iC trials), and incongruent trials preceded by a incongruent trial (iI trials). Half of the trials from each of these four conditions were preceded by a neutral image, and the other half were preceded by a negative image. Trials were presented such that both the task-relevant feature (i.e., image of the face) and the task-irrelevant feature (i.e., overlaid word) of the compound stimulus were never physically the same across successive trials to minimize potential priming effects (Mayr et al., 2003). In particular, note that words were not alternated between the two gender categories in successive trials. Instead, both upper and lower case words were used and alternated between these two cases in successive trials (Egner et al., 2010). Finally, in each of the eight conditions of our study, half of the trials were response–repeat trials and half of them were response-switch trials. A total of 72 male and 72 female neutral face images were employed. Across the experiment, they were repeated only once; thus, they were shown once preceded by an incongruent trial and once preceded by a congruent trial. The identity of faces was counterbalanced across participants with respect to neutral and negative conditions.
To manipulate emotion, we employed images from the International Affective Picture System (IAPS) database (Lang et al., 1997). Seventy-two neutral (normative valence ratings: 5.16 ± 0.12; normative arousal ratings: 3.62 ± 0.93) and 72 negative (normative valence ratings: 1.84 ± 0.25; normative arousal ratings: 6.29 ± 0.64) images were employed, including strongly emotional images such as mutilated bodies. Images were chosen so as to attempt to carefully balance them for content and complexity. They were repeated once (once preceded by a congruent trial, once preceded by an incongruent trial).
Following most conflict adaptation studies, we focused on RT data, although additional analyses of error rate data were undertaken. For the RT analysis, the first trial of each block, error trials, trials immediately following an error, and trials with an RT exceeding 3 SD from the condition-specific mean (0.6% of the trials) were excluded in each participant. For the error rate data, the first trial of each run and trials that were preceded by an error were excluded. For each participant, mean RT and mean error rate data were determined as a function of previous-trial congruency (congruent, incongruent), current-trial congruency (congruent, incongruent) and image type between the previous- and current-trial (neutral, negative). Repeated-measures analyses of variance (ANOVAs) were conducted on the mean RT and mean error rate data, with those variables as within-subject factors. We used an alpha-level of 0.05 for all statistical tests.
Reaction time data (Table 1) were evaluated according to a 2 previous-trial congruency (congruent, incongruent) × 2 current-trial congruency (congruent, incongruent) × 2 emotion (neutral, negative) repeated-measures ANOVA. The main goal of this study was to investigate the effect of emotion on conflict adaptation. In other words, we were interested in evaluating a three-way interaction pattern, which was indeed borne out by the RT data [F(1,37) = 4.22, p = 0.047, 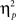 = 0.10; Figure 2A].
= 0.10; Figure 2A].
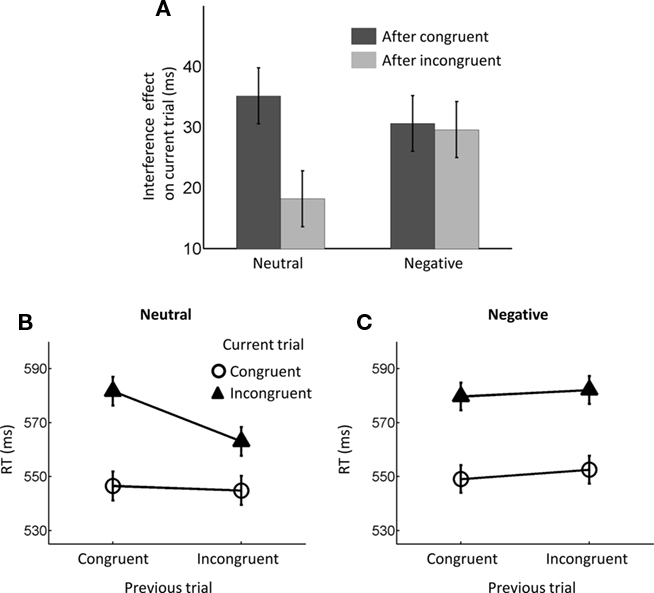
Figure 2. Reaction time (RT) conflict adaptation effects. (A) Bar plot of interference (incongruent–congruent) RT effects based on previous-trial congruency displayed for both neutral and negative conditions. Error bars represent standard within-subject standard error bars (Loftus and Masson, 1994) for the three-way interaction. (B) Mean RT data displayed as a function of previous- and current-trial congruency during trials preceded by neutral emotion and (C) negative emotion. Error bars in (B,C) represent standard within-subject standard error bars (Loftus and Masson, 1994) for the previous-trial congruency × current-trial congruency interaction.
As outlined in the Section “Introduction,” the two competing hypothesis tested in this study predict a three-way interaction but with contrasting patterns. To understand the nature of the three-way interaction, we ran two additional two-way analyses (previous- × current-trial congruency) separately for neutral and negative conditions. A significant conflict adaptation effect (i.e., previous- × current-trial congruency interaction) was observed during the neutral condition [F(1,37) = 7.13, p = 0.011, 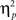 = 0.16; Figure 2B]. In the neutral condition, the interference effect was almost reduced by half when trials were preceded by incongruent trials (18 ms) compared to when they were preceded by congruent ones (35 ms). This is precisely the conflict adaptation pattern reported in the literature. In contrast, during the negative condition, a conflict adaptation effect (i.e., previous- × current-trial congruency interaction) was not observed [F(1,37) = 0.03, p = 0.867,
= 0.16; Figure 2B]. In the neutral condition, the interference effect was almost reduced by half when trials were preceded by incongruent trials (18 ms) compared to when they were preceded by congruent ones (35 ms). This is precisely the conflict adaptation pattern reported in the literature. In contrast, during the negative condition, a conflict adaptation effect (i.e., previous- × current-trial congruency interaction) was not observed [F(1,37) = 0.03, p = 0.867, 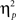 = 0.00; Figure 2C]. In fact, the interference effect on the trials preceded by incongruent trials (29 ms) was nearly identical to the one on the trials preceded by congruent trials (30 ms).
= 0.00; Figure 2C]. In fact, the interference effect on the trials preceded by incongruent trials (29 ms) was nearly identical to the one on the trials preceded by congruent trials (30 ms).
For completeness, all statistical results are provided in Table 2. Of note, the main effect of current-trial congruency was significant [F(1,37) = 75.33, p < 0.001, 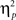 = 0.67], revealing the standard response conflict effect. As expected, mean RT was slower on incongruent (577 ms) compared to congruent (548 ms) condition. The main effect of emotion was significant [F(1,37) = 10.23, p = 0.003,
= 0.67], revealing the standard response conflict effect. As expected, mean RT was slower on incongruent (577 ms) compared to congruent (548 ms) condition. The main effect of emotion was significant [F(1,37) = 10.23, p = 0.003, 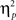 = 0.22], such that the mean RT of trials preceded by negative images was slower (566 ms) compared to those preceded by neutral images (559 ms).
= 0.22], such that the mean RT of trials preceded by negative images was slower (566 ms) compared to those preceded by neutral images (559 ms).
We also analyzed error rate data. A 2 previous-trial congruency (congruent, incongruent) × 2 current-trial congruency (congruent, incongruent) × 2 emotion (neutral, negative) repeated-measures ANOVA on error rate data (Table 1) did not reveal a three-way interaction [F(1,37) = 0.01, p = 0.918, 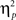 = 0.00]. Other results of interest related to main effects and interactions are as follows. The main effect of current-trial congruency was significant [F(1,37) = 57.33, p < 0.001,
= 0.00]. Other results of interest related to main effects and interactions are as follows. The main effect of current-trial congruency was significant [F(1,37) = 57.33, p < 0.001, 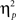 = 0.61], such that errors were more frequent during incongruent (11%) compared to congruent (4%) trials; other main effects were not significant (Fs < 1.5; ps > 0.2). The two-way interaction between previous- and current-trial congruency was statistically significant [F(1,37) = 6.01, p = 0.019,
= 0.61], such that errors were more frequent during incongruent (11%) compared to congruent (4%) trials; other main effects were not significant (Fs < 1.5; ps > 0.2). The two-way interaction between previous- and current-trial congruency was statistically significant [F(1,37) = 6.01, p = 0.019, 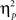 = 0.14], demonstrating the typical conflict adaptation effect, such that interference (incongruent vs. congruent) was lower after incongruent trials (6%) compared to after congruent trials (8%).
= 0.14], demonstrating the typical conflict adaptation effect, such that interference (incongruent vs. congruent) was lower after incongruent trials (6%) compared to after congruent trials (8%).
Next, we sought to confirm that differences in conflict adaptation effects observed in the RT data during neutral and negative conditions were not caused by a speed–accuracy tradeoff. To do so, we correlated the conflict adaptation effect [(cI − cC) − (iI − iC)] with the corresponding error rates during the neutral and, separately, negative conditions. We used robust regression for this analysis to safeguard against potential outliers that can greatly affect the standard Pearson correlation (Wilcox, 2005). No significant linear relationship was observed between the RT data and error rate data in either neutral (robust R2 = 0.02, p = 0.608) or negative (robust R2 = 0.05, p = 0.206) conditions.
Finally, we correlated the observed differences in RT conflict adaptation effects between neutral and negative conditions [(cI − cC) − (iI − iC)]NEGATIVE − [(cI − cC) − (iI − iC)NEUTRAL] with state and trait anxiety scores (in separate analyses) across participants. As in the above analysis, we used robust regression. No significant linear relationship was observed (state anxiety: robust R2 = 0.02, p = 0.600 and trait anxiety: robust R2 = 0.01, p = 0.830), but we report these negative findings here because of their theoretical relevance.
In this study, we investigated interactions between emotion and proactive control mechanisms. Our main finding was that conflict adaptation decreased when a negative, task-irrelevant picture was shown between the main conflict-inducing stimuli. Next, we discuss some of the implications of our findings.
Generally speaking, two classes of predictions could have been made concerning the influence of emotion on conflict adaptation. On the one hand, models that suggest that emotion and cognition share processing resources would predict that conflict adaptation would decrease in the conditions of our experiment (e.g., Wyble et al., 2008; Pessoa, 2009). This is because the processing of high-intensity negative images engages processing resources that are also needed by cognition. For instance, subjects may implicitly regulate their emotional reaction to the aversive stimulus. In this case, mechanisms needed for successful regulation may tap into those also needed to dynamically regulate control. In this manner, participants are less able to up-regulate control after an incongruent trial and, hence, a subsequent incongruent trial will exhibit substantial response interference.
According to the conflict-monitoring model (Botvinick et al., 2001), conflict-driven control adjustments happen because top-down attentional mechanisms are triggered after conflict is detected. These regulatory mechanisms would then exert top-down control during the subsequent trial, leading to decreased interference. Thus, a potential interpretation of our results is that the processing of a negative stimulus might consume/divert resources needed for top-down control engagement, leading to poorer control on the subsequent trial.
Recently, Mansouri et al. (2009) proposed a model for conflict-driven control adjustments based on mnemonic processes. Their model proposes that, after the initial detection of conflict on an incongruent trial, the information about the experienced conflict is stored in short-term memory. This information is then used to exert top-down control during the subsequent trial. At the same time, there are studies that reported disruption of working memory performance by task-irrelevant, negative pictures shown during the maintenance period (Dolcos and McCarthy, 2006; Anticevic et al., 2010). Taken together, an alternative interpretation of our results would be then that the processing of negative pictures disrupted the working memory representation of the experienced conflict, leading to reduced behavioral adjustments during the subsequent trial.
The findings of studies by Hommel and colleagues (van Steenbergen et al., 2009, 2010) suggest that negative emotion might increase conflict adaptation. In particular, van Steenbergen et al. (2010) suggested that conflict adaptation is sensitive to the modulation of pleasure level. The present findings run counter this suggestion, at least when the manipulation involves a phasic stimulus – van Steenbergen et al. (2010) investigated a longer-lasting mood manipulation. It is also worth noting that it has been suggested that conflict signals are aversive in nature and might lead to the selection of strategies that reduce conflict in future occurrences (Botvinick, 2007). Based on this notion, van Steenbergen et al. (2009) suggested that non-performance-contingent reward acted as a positive event that canceled out the negative experience of a conflict signal, thus leading to a reduction in control adjustments – given that conflict was less aversive in such cases. According to this interpretation, one would expect that viewing a negative image, such as the mutilated-body stimuli employed here, after the conflict signal would increase the overall aversive level. And to reduce task aversiveness, participants would increase subsequent behavioral control. Again, our results run counter this prediction. Although, in principle, it could be argued that our phasic manipulation of emotion is qualitatively different from the mood induction employed by van Steenbergen et al. (2010) and that negative signals linked to experiencing conflict are distinct from those obtained when viewing highly negative images, the present findings indicate that models of conflict processing need to be extended so as to incorporate mechanisms of cognitive–emotional interactions.
In conclusion, we investigated the effects of negative emotion on conflict-driven control mechanisms by using a face-word Stroop-like task. We found that conflict adaptation effects were significantly reduced during the negative emotional condition. We interpret the current findings in terms of shared resources between the processing of negative emotional stimuli and proactive control mechanisms. Our findings demonstrate that emotion interacts with executive mechanisms responsible for dynamic behavioral adjustments that are tied to environmental demands, a central facet of flexible, goal-directed behavior.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Support for this work was provided in part by the National Institute of Mental Health (MH071589). We would like to thank Andrea Kenzer for assistance with data collection, and Jong Moon Choi for discussions.
Anticevic, A., Repovs, G., and Barch, D. M. (2010). Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn. Affect. Behav. Neurosci. 10, 159–173.
Blair, K. S., Smith, B. W., Mitchell, D. G., Morton, J., Vythilingam, M., Pessoa, L., Fridberg, D., Zametkin, A., Sturman, D., Nelson, E. E., Drevets, W. C., Pine, D. S., Martin, A., and Blair, R. J. (2007). Modulation of emotion by cognition and cognition by emotion. Neuroimage 35, 430–440.
Botvinick, M. (2007). Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 7, 356–366.
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., and Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652.
Dolcos, F., and McCarthy, G. (2006). Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 26, 2072–2079.
Egner, T. (2007). Congruency sequence effects and cognitive control. Cogn. Affect. Behav. Neurosci. 7, 380–390.
Egner, T., Ely, S., and Grinband, J. (2010). Going, going, gone: characterizing the time-course of congruency sequence effects. Front. Psychol. 1:154. doi: 10.3389/fpsyg.2010.00154
Gratton, G., Coles, M. G., and Donchin, E. (1992). Optimizing the use of information: strategic control of activation of responses. J. Exp. Psychol. Gen. 121, 480–506.
Hart, S. J., Green, S. R., Casp, M., and Belger, A. (2010). Emotional priming effects during Stroop task performance. Neuroimage 49, 2662–2670.
Hommel, B., Proctor, R. W., and Vu, K. P. (2004). A feature-integration account of sequential effects in the Simon task. Psychol. Res. 68, 1–17.
Hu, K., Bauer, A., Padmala, S., and Pessoa, L. (2011). Threat of bodily harm has opposing effects on cognition. Emotion. doi: 10.1037/a0024345. [Epub ahead of print].
Kanske, P., and Kotz, S. A. (2011). Emotion speeds up conflict resolution: a new role for the ventral anterior cingulate cortex? Cereb. Cortex 21, 911–919.
Lang, P. J., Bradley, M. M., and Cuthbert, B. N. (2008). “International Affective Picture System (IAPS)”: Affective Ratings of Pictures and Instruction Manual. Technical Report, A–8. Gainesville, FL: University of Florida.
Loftus, G. R., and Masson, M. E. (1994). Using confidence intervals in within-subject designs. Psychon. Bull. Rev. 1, 476–490.
Mansouri, F. A., Tanaka, K., and Buckley, M. J. (2009). Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat. Rev. Neurosci. 10, 141–152.
Mayr, U., Awh, E., and Laurey, P. (2003). Conflict adaptation effects in the absence of executive control. Nat. Neurosci. 6, 450–452.
Pessoa, L. (2009). How do emotion and motivation direct executive function? Trends Cogn. Sci. 13, 160–166.
Pessoa, L., Padmala, S., Kenzer, A., and Bauer, A. (2011). Interactions between cognition and emotion during response inhibition. Emotion. doi: 10.1037/a0024109. [Epub ahead of print].
Robinson, O. J., Letkiewicz, A. M., Overstreet, C., Ernst, M., and Grillon, C. (2011). The effect of induced anxiety on cognition: threat of shock enhances aversive processing in healthy individuals. Cogn. Affect. Behav. Neurosci. 12, 217–227.
Sagaspe, P., Schwartz, S., and Vuilleumier, P. (2011). Fear and stop: a role for the amygdala in motor inhibition by emotional signals. Neuroimage 55, 1825–1835.
Spielberger, C. D., Gorsuch, R. L., and Lushene, R. E. (1970). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press.
van Steenbergen, H., Band, G. P., and Hommel, B. (2009). Reward counteracts conflict adaptation. Evidence for a role of affect in executive control. Psychol. Sci. 20, 1473–1477.
van Steenbergen, H., Band, G. P., and Hommel, B. (2010). In the mood for adaptation: how affect regulates conflict-driven control. Psychol. Sci. 21, 1629–1634.
Verbruggen, F., and De Houwer, J. (2007). Do emotional stimuli interfere with response inhibition? Evidence from the stop signal paradigm. Cogn. Emot. 21, 391–403.
Wilcox, R. R. (2005). Introduction to Robust Estimation and Hypothesis Testing. Burlington, MA: Elsevier Academic Press.
Keywords: emotion, conflict adaptation, cognition, shared resources
Citation: Padmala S, Bauer A and Pessoa L (2011) Negative emotion impairs conflict-driven executive control. Front. Psychology 2:192. doi: 10.3389/fpsyg.2011.00192
Received: 25 May 2011;
Accepted: 27 July 2011;
Published online: 11 August 2011.
Edited by:
Marco Tamietto, Tilburg University, NetherlandsReviewed by:
Jack Van Honk, Utrecht University, NetherlandsCopyright: © 2011 Padmala, Bauer and Pessoa. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Luiz Pessoa, Department of Psychology, University of Maryland, College Park, MD 20742, USA. e-mail:cGVzc29hQHVtZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.