
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Psychiatry , 25 February 2025
Sec. Psychopathology
Volume 16 - 2025 | https://doi.org/10.3389/fpsyt.2025.1502217
This article is part of the Research Topic Psychological Factors as Determinants of Medical Conditions, Volume III View all 8 articles
 Francesca Borghesi1*
Francesca Borghesi1* Gloria Simoncini1
Gloria Simoncini1 Riccardo Cremascoli2
Riccardo Cremascoli2 Laura Bianchi2
Laura Bianchi2 Leonardo Mendolicchio3
Leonardo Mendolicchio3 Simone Cappelli4
Simone Cappelli4 Federico Brusa3
Federico Brusa3 Stefania Cattaldo5
Stefania Cattaldo5 Elisa Prina6,7
Elisa Prina6,7 Alice Chirico8
Alice Chirico8 Alessandro Mauro2,9
Alessandro Mauro2,9 Pietro Cipresso1
Pietro Cipresso1Background: Affect dynamics, or variations in emotional experiences over time, are linked to psychological health and well-being, with moderate emotional variations indicating good psychophysical health. Given the impact of emotional state on cardiac variability, our objective was to develop a quantitative method to measure affect dynamics for better understanding emotion temporal management in Anorexia Nervosa (AN).
Methods: The study proposed an experimental and methodological approach to evaluate physiological affect dynamics in clinical settings. It tested affective transitions and temporal changes using emotional images from the International Affective Picture System (IAPS), examining physiological characteristics of a patient with AN. The methodology involved calculating a heart rate variability index, e.g., RMSSD, and using it in a Discrete Time and Discrete Space Markov chain to define, quantify, and predict emotional fluctuations over time.
Results: The patient with Anorexia Nervosa showed a high likelihood of transitioning from positive to negative emotional states, particularly at lower arousal levels. The steady state matrix indicated a tendency to remain in highly activated pleasant states, reflecting difficulties in maintaining emotional balance.
Conclusions: Employing Markov chains provided a quantitative and insightful approach for examining affect dynamics in a patient with AN. This methodology accurately measures emotional transitions and provides a clear and interpretable framework for clinicians and patients. By leveraging Markovian indexes, mental health professionals may gain a comprehensive understanding of emotional fluctuations’ patterns. Moreover, graphical representations of emotional transitions may enhance the clinician-patient dialogue, facilitating a clearer emotional and physiological profile for the implementation of personalized treatment procedures.
Increasing empirical evidence supports the notion that affect dynamics - as the temporal fluctuations of our emotional experiences – which is intricately linked with well-being and psychological health (1–3). This burgeoning field of research indicates that not all emotional experiences are created equal, and the way our emotions change and flow over time can provide critical insights into our overall mental health (4–7). In general, medium variability in affective dynamism is associated with good psychophysical health; the opposite effect is observed for extreme states of too high variability or extreme rigidity (e.g., inability to switch from one affective state to another) (8–11). The extreme in affective dynamism is opposite on a descriptive level but equal from a psycho-physiological point of view and have been deeply investigated in the realm of psychiatric or neurological disorders (8, 12). Specifically, affect dynamics have been studied to gain a better understanding of how individuals with conditions such as bipolar disorder, depression, anxiety and eating disorders, experience and feel emotion in time (13–16). In eating disorders, most of all in AN, contemporary theories acknowledge the importance of disordered emotional functioning in the disorder’s development and maintenance [e.g., (17, 18)]. AN is characterized by symptoms of extreme mental and emotional rigidity, making it a compelling case for studying issues in emotion regulation and affect dynamics. Individuals with AN often exhibit a pronounced inability to adaptively manage and transition between emotional states, reflecting significant challenges in emotional flexibility. This rigidity is manifested in both cognitive and emotional domains, leading to a limited range of emotional experiences and heightened difficulty in coping with negative affect (10, 19, 20). Most recent studies have focused on affective variability- pattern of frequent and large mood shifts over time- or on its opposite affective inertia- emotions that are resistant to change or shifts over time (18, 21, 22). The study of Vansteelandt and colleagues (17) investigated affect variability using Experience Sampling Method protocol in patient with AN restricted and Bulimia Nervosa (BN). Results of this exploratory study suggest that the diagnostic groups have the same mean levels of affect, but the AN restricted group showed a less variability in the quality of their affect. Recently, also Williams-Kerver et al. (23) demonstrated, using Experience sampling Method, that participants in the AN and BN groups experienced significantly greater Negative Affect (NA) intensity and better emotion differentiation- the ability to discriminate distinct emotional states- than participants in the Binge Eating Disorders (BED) group. Alternatively, the BN group demonstrated significantly greater NA variability than the AN group and greater NA inertia than the BED group.
All studies described relied most on the Russell’s circumplex model (24), and the predominant experimental design methodology was Ecological Momentary Assessment (EMA) (25). This method allowed collecting real-time data in naturalistic settings, providing a valuable window into individuals’ emotional lives (26–28). EMA involves prompting patients to report on their feelings, behaviors, and environmental context at random times throughout the day, thereby offering a rich, detailed account of affect dynamics (29).
Although EMA is an ecological and longitudinally accurate instrument, it necessitates high participant compliance, that is, the person must be able (and willing) to respond to most of the responses over time. Furthermore, EMA can only delimit and describe the long-term time series of affective states reported by the participant’s responses, assessing mostly the mood; it cannot provide information about short time affective transitions that the person can make during the day or week. Finally, to the best of our knowledge, no one study assesses the physiological characteristic of affect dynamics in eating disorders using EMA, may be due to the compliance and the difficulty of the continuous physiological signals (30–32). Incorporating Heart Rate Variability (HRV), particularly RMSSD, as a physiological signal for affect dynamics is supported by extensive research linking it to autonomic nervous system function and emotional regulation (33). Studies by Thayer et al. (34) and Schmalbach et al. (35) demonstrate that HRV is a robust indicator of emotional regulation and stress resilience, underscoring its relevance for psychological and neurological assessments. Particularly, research by Appelhans and Luecken (36) highlights RMSSD’s specificity in reflecting parasympathetic activity, making it a common measure for estimating the variations in heart rate that are mediated by the vagus nerve and therefore it represents an ideal measure for studying affective dynamics. Recent research underscores the validity of HRV, particularly RMSSD, as a physiological index in psychiatric conditions, linking it to emotional regulation and neurophysiological changes across disorders such as depression and anxiety. HRV reflects the body’s capacity for emotional and physiological regulation, with lower HRV associated with higher symptom severity in psychiatric disorders (12, 35). These findings support the use of HRV in monitoring emotional regulation, offering a rationale for its selection as a physiological index in our study on affect dynamics. Adding RMSSD as a key physiological measure in our study is further justified by its mathematical significance in quantifying short-term variations in heart rate. RMSSD stands for the Root Mean Square of Successive Differences between normal heartbeats. It’s calculated using the formula:
where (RRi) represents the duration of one heartbeat to the next (in milliseconds), and (N) is the total number of heartbeats analyzed. This formula captures the variability in heart rate over short periods, making it an excellent index for assessing autonomic nervous system function, particularly the parasympathetic branch. RMSSD’s focus on short-term variability provides a sensitive measure of vagal tone, a key aspect of emotional and physiological regulation.
For this, reason we developed and implemented a new experimental and methodological technique to study AN’s affect dynamics with physiological signals (e.g. RMSSD) in the clinical setting. The goal is to create a simple and effective assessment of affect dynamics, investigating all possible affective transitions and to measure it with Discrete-Time and Discrete Space Markov processes. While previous techniques have focused on group-level data analysis using panel data methodologies, thus, losing sight of the individuality and uniqueness of individual emotional experiences, here, our methodology allows for a personalized, patient-centered perspective. The experimental design involves the emotive images’ visualization of the International Affective Picture System (IAPS), (37) to stimulate all possible affective transitions, based on Russell’s Circumplex model (4). HRV is measured during all the experimental session, and the RMSSD is used into this novel methodological approach of analysis based on Discrete Time and Discrete Space Markovian stochastic processes. Markov chains are mathematical systems that describe a sequence of possible events, where the probability of each event depends only on the state attained in the previous event (38). In the context of affect dynamics, Markov chains can be employed to analyze and predict emotional state transitions over time (39), utilizing physiological measures like RMSSD to understand the probabilistic nature of emotional fluctuations. This approach can effectively capture the dynamic and stochastic nature of emotional fluctuations over time, offering insights into how emotional states evolve (4, 8, 40, 41).
This method stands out for its potential to provide individualized and detailed insights into the emotional patterns of individuals affected by Anorexia Nervosa, offering new perspectives on the relationship between emotions and physiology in these disorders. With this approach, we aspire to develop more effective experimental and methodological tools for monitoring and intervention, enhancing the understanding and treatment of these complex conditions. Through the simple and quantitatively idiographic use of the grapho describing probability transitions, a comprehensive profile can be drawn up that is easy for the patient and the clinician to interpret.
A female patient with Anorexia Nervosa with restricts behavior was selected as study’s participant. She is hospitalized at the IRCCS Istituto Auxologico Piancavallo for two weeks.
The patient affected by Anorexia Nervosa (AN) was a 35-year-old woman. She was admitted at the Unit of Eating Disorders of Istituo Auxologico Italiano in Piancavallo (Italy) in December 2023 because of severe malnutrition (Body mass index 9.7 kg/m2). She has suffered from AN since she was 19 years old. The neuropsychological evaluation showed a good mood stability with the ongoing therapy: Aripiprazole 5 mg/day, Delorazepam 1 mg/ml 5gtt+10gtt+25gtt/day, Sertraline 50 mg/day, Trazodone 60mg/ml 15gtt/day. During the 4 weeks of hospitalization the patient underwent a rehabilitation program based on refeeding in a medical setting. The refeeding program is implemented by a multidisciplinary team of doctors, dietitians, educators, and psychologists, with daily individual meetings.
The patient also went through a standardized rehabilitation program directed by a physiotherapist through exercises to improve her walking ability and endurance, and balance control an hour a day, from Monday through Saturday.
She was not given the planned pharmacological therapy close to the administration of the emotional stimuli and detection of physiological parameters.
The study was conducted in accordance with the Declaration of Helsinki, having been approved by the Ethics Committee of IRCCS Istituto Auxologico Italiano (Prot. 2022_10_25_05).
The participant gave written informed consent before participating in the study. In particular, to be included in the study, participant criteria were:
- Normative performance on three neuropsychological tests, respectively phonemic (Corrected Score = 33,16) and semantic fluency (Corrected score = 50,97, (42), Trail Making Test (Part A corrected score = 39; Part B corrected score = 71; Part B-A corrected score = 32), (43) and Tower of London Test (Time Score = 28; Accuracy score = 33), (44).
- Absence of additional concomitant pathological conditions, thus, achieving results within the expected range on neuropsychological tests investigating general cognitive functioning (MMSE: score = 25 with a cut-off = 21, (45), short-term and long-term mnestic abilities (Digit Span Forward: corrected score = 5,50, (46); Rey Auditory Verbal Learning Test: Immediate recall corrected score = 32; Deferred recall corrected score = 5,40, (47) attentional capacities (TMT Test: part A corrected score = 67; (43) and executive functions (Clock Drawing Test corrected score = 10,50, (48); Frontal Assessment Battery corrected score = 14,49, (49); Coloured Progressive Matrixes corrected score = 23,50, (50).
The psychometric tools were administered to confirm normative cognitive functioning and exclude conditions that could confound the study’s focus on affect dynamics. These assessments ensured the observed patterns were specific to Anorexia Nervosa.
The study followed a within-subject design to examine affect dynamics using affective induction through IAPS (37). In accordance with Russel’s Circumplex model, a total of twelve transitions were established between distinct blocks of arousal-valence (51). These transitions encompass horizontal, vertical, and oblique movements (see Figure 1). The visual stimuli were presented via Desktop monitor on a 42-inch screen while sitting comfortably in a chair with armrests and headrests to minimize head and arm movements, which inject noise into the data captured by the sensors. We positioned the chair about one meter from the monitor. The participant was exposed to 156 images from the IAPS dataset, arranged into 13 blocks of 2 minutes, mimic 12 transitions between arousal and valence states, as outlined by Russel’s Circumplex model (26 total minutes equivalent to 1560 seconds). Each block contains 12 images, displayed for 10 seconds, with images selected based on high (>6) or low (<4) arousal/valence Likert scores from the Self-Assessment Manikin (SAM) (52). The participant had a unique random images sequence, to avoid temporal or ceiling effect in emotional response. This design ensured a comprehensive exploration of emotional transitions, with the sequence of image presentation randomized to avoid order effects.
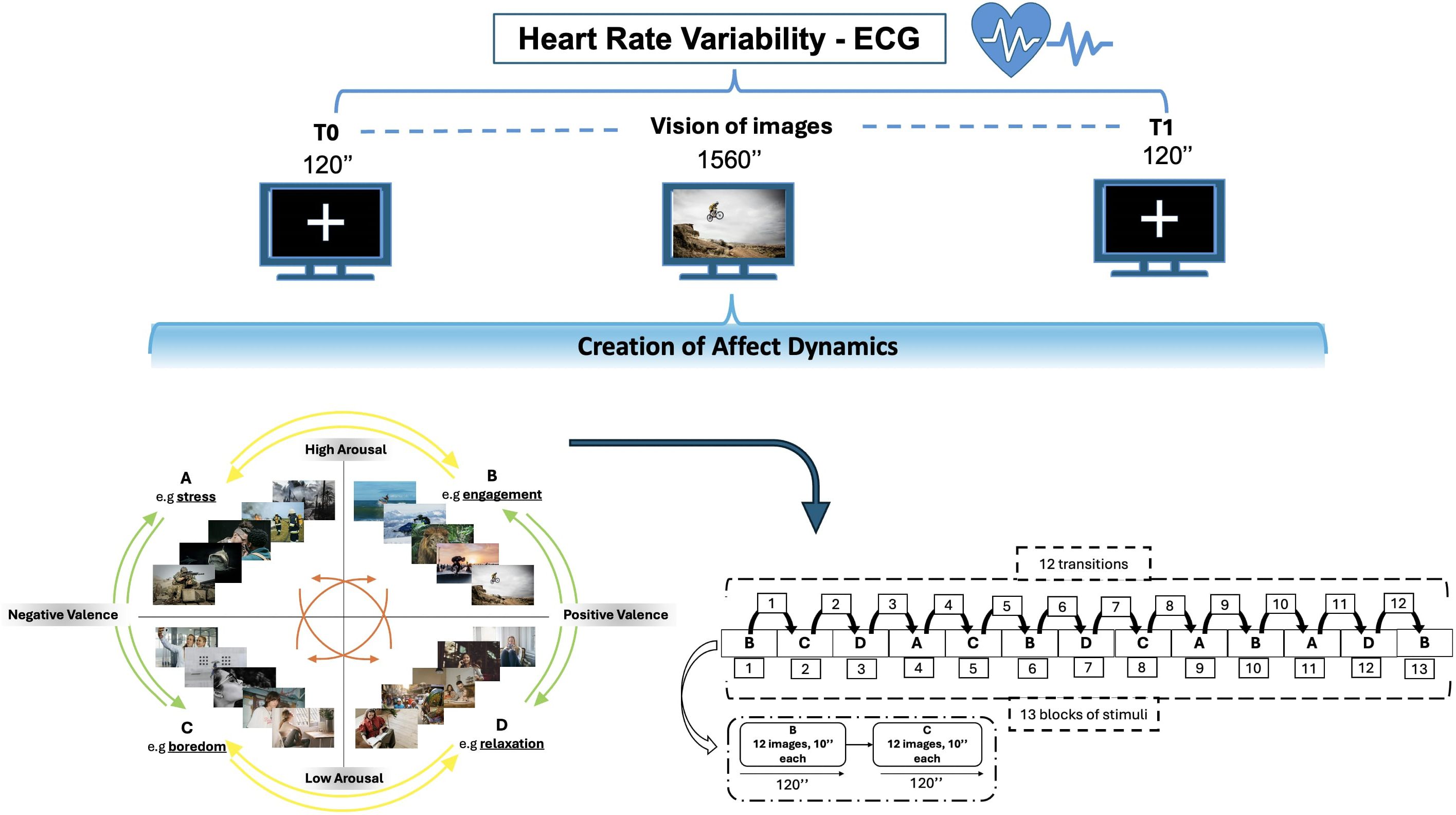
Figure 1. Experimental Design: Cardiac activity is recorded using ECG while observing IAPS images. There are 12 possible transitions between affective states, leading to affect dynamics. The transitions consist of horizontal movements (AB-CD-BA-DC) denoted by yellow arrows, vertical movements (AC-BD-CA-DB) shown by green arrows and diagonal movements (AD-BC-DA-CB) denoted by orange arrows.
During the entire experimental session, heart rate variability was measured, with electrocardiogram (ECG). A baseline phase was run before and after each experimental session, observing a fixed crossing for 2 minutes (120 seconds), taking account intra-variability measure of the participant.
The data on the autonomic nervous systems were collected by measuring physiological responses, i.e., Electrocardiogram. Embletta MPR acquired these responses. The responses were then processed with custom software developed using MATLAB 9.13.0 (R2023a) (The Mathworks, Inc.; Natick, MA, USA). Every channel was acquired synchronously at 1000 Hz. Visual inspection is used to the artefact correction option, instead artefacts due to ectopic beats, missed beat detections, etc. are corrected adapting an appropriate window correction level (threshold for detecting artefact beats) which removes the artefacts but does not distort normal RR intervals.
For ECG analysis, cardiovascular analysis is focused on the RR interval, representing the time between successive heartbeats, was used to derive heart rate (HR) and assess autonomic function. This conversion provided an HR mean (in beats per minute) and an equivalent RR mean (calculated as 60,000/HR), offering insights into heart rate variability and cardiovascular health.
RMSSD is a key time-domain measure used in heart rate variability (HRV) analysis to quantify the short-term variability of heart rate, reflecting the beat-to-beat variance in heart rate. It is calculated by taking the square root of the average of the squared differences between successive normal heartbeats over a specified period. This measure is primarily used to estimate vagally mediated changes reflected in HRV, making it a valuable index for assessing autonomic nervous system function, especially parasympathetic activity. RMSSD is considered a reliable indicator of the autonomic nervous system’s resilience and adaptability to stress. While typically a 5-minute recording period is conventional for HRV analysis, recent research supports the validity of using ultra-short-term periods, such as 10 seconds, for RMSSD calculation, providing a practical approach for both clinical and research settings. This adaptability in measurement duration facilitates the efficient assessment of autonomic function in various contexts, emphasizing RMSSD’s utility in capturing the dynamic nature of autonomic nervous system activity related to emotional and physiological states (53–55).
To better understand the complex and ever-changing nature of emotional transitions, we used Discrete Time and Discrete Space Markov chains, a mathematical framework for modeling sequential processes with intrinsic transitions (38, 56, 57). The changes are not entirely deterministic, but instead are regulated by probability distributions. A Discrete-time and Discrete space Markov chain is characterized by a set of states and a transition matrix P. We call a discrete-states stochastic processes a sequence of random variables X0, X1, … Xn where each Xn is a discrete random variable taking values in a set S, called the state space. The set S is finite or at most countably infinite. Without losing generality, we assume that S is a subset of the relative integers .
The transition matrix P for a Markov chain with Xn states is an n×n matrix where each element P represents the probability of transitioning from state i to state j in one discrete step (58). Inside the transition matrix, the most important Markovian property is conditional probability that the system transitions to state xn+1 at time n+1, given that it was in state xn at time n. The term Pij denotes how the system evolves over time based on its current state (59).
Essentially, the future of the system is determined by its current state rather than the specific route it took to get that condition. Note that we define this probability as a function of just i and j, but of course, it could depend on n as well. The time homogeneity restriction mentioned in the previous footnote is just the assumption that this probability does not depend on the time n, but rather remains constant over time.
The use of Markov chains is inspired by their ability to express probabilistic transitions between discrete states - in this case stress (A), engagement (B), boredom (C), and relaxation (D), where the probability of each event depends only on the state attained in the previous event.
In the context of affect dynamics, Markov chains can be employed to analyze and predict emotional state transitions over time, utilizing physiological measures like RMSSD to understand the probabilistic nature of emotional fluctuations (40). RMSSD is one of the most suitable indexes for heart rate variability in short term: it aligns perfectly with the properties of Markovian chains, a stochastic model of transition, which considers pairs of transitions one at a time, disregarding the path preceding that transition (60, 61).
The Markov chain uses a transition matrix as its primary tool for describing the probability of moving between those affective states: It is quadratic matrix, in this case a 4*4 matrix, considering the 4 affective states. Hence, 12 elements represent probability of transition, and 4 self-transition indexes represent the inside variability of four quadrants (e.g., AA-BB-CC-DD). The 12 transition values could be divided in vertical transition (e.g., AC-CA-BD-DB), horizontal one (e.g., AB-BA-CD-DC), oblique one (AD-DA-BC-CB) (Figure 2).
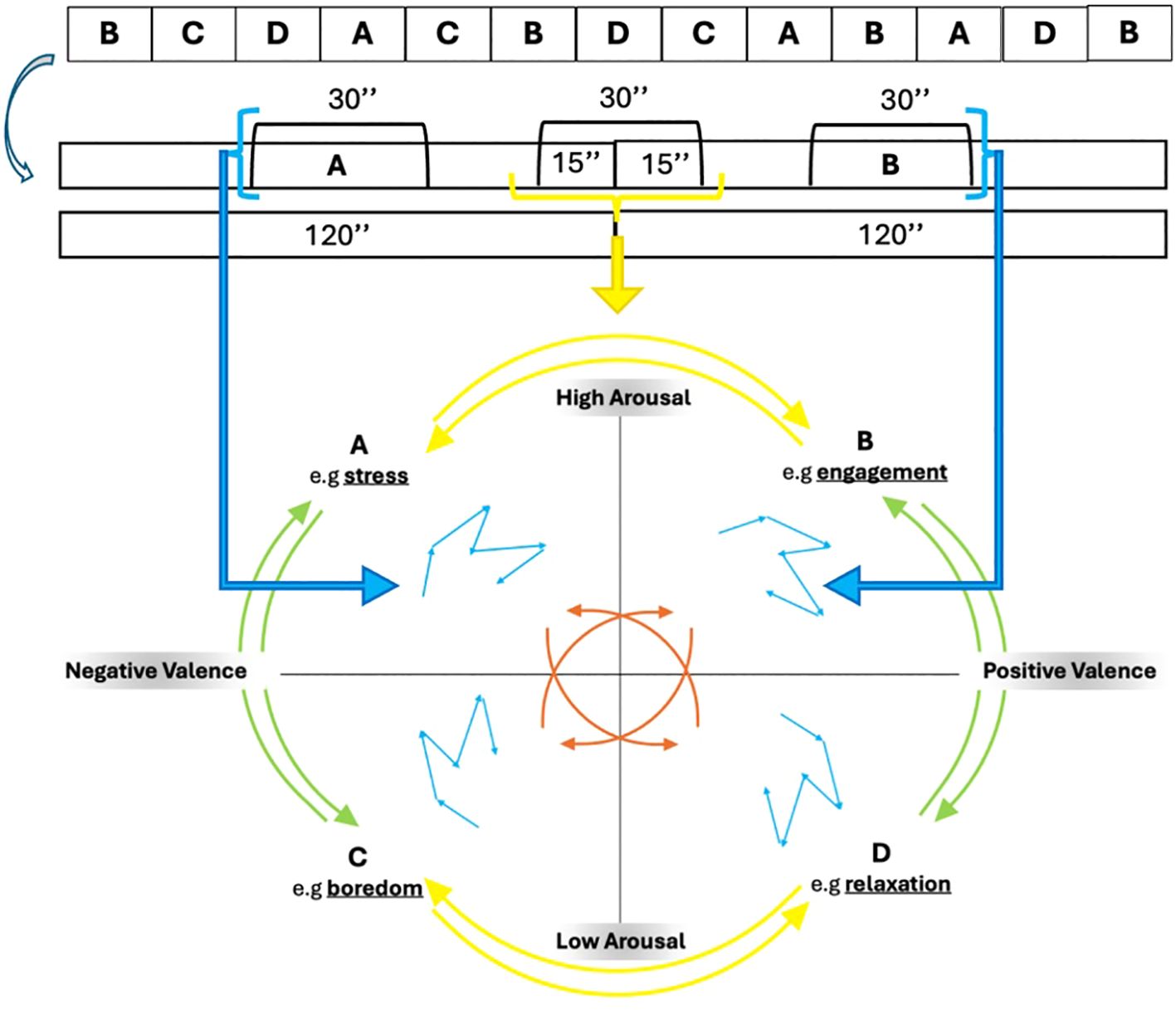
Figure 2. Example of transition between affective state (A, B) and the inside variability of each quadrant.
Hence, we are able to describe all possible affective transitions of the participant, considering the outward and return of each, and also the variability inside each affective state (Figure 2). To express the variability between and within states, we considered the 30 seconds between two transitions (120 15) and the 30 seconds between the first blocks presented by each state (60 15).
The Markov chain, due to its stochastic property, necessitates that the sum of each row in the transition matrix is equal to 1:
For this reason, each RMSSD for 12 transitions is relativized and included into the Markov chain (Δ indexes). These transition probabilities within each row denote the likelihoods of transitioning from a given state to all feasible states within the system (Figure 3).
Furthermore, the Markov chain is also a predictive property, calculating the steady states vector values. The steady state distribution provides insights into the long-term behavior of the system, offering insights into the equilibrium distribution of states after a large number of transitions.
The steady states iterative calculation is not arbitrary but are significantly influenced by two factors: the initial state vector (0) and the participant-specific transition matrix (P). The initial state vector (0) posits an a priori probability of the participant’s presence in one of the four quadrants, serving as the starting point for the Markov chain: i.e. the participant before being subjected to stimuli is equally likely to be in one of the four affective states.
For each state I ∈ S, we denote by π0(i) probability P{X0=i} that the Markov chain starts out in state i. Formally, π0 is a function taking S into the interval [0,1] such that:
Equivalently, instead of thinking of π0 as a function from S to [0,1], we could think of π0 as the vector whose i-th entry is (i)=P{X0=i}. This is the probability distribution of the Markov chain at time 0.
Our initial vector considered equiprobability between initial affective states (PA=0.25, PB=0.25, PC=0.25, PD=0.25). Through iterative multiplication (10 steps) of the initial states vector ( and the transition matrix (P), we found the steady states. To see this numerically, we compute for increasing n to check for convergence to steady states (π).
Thus, the steady states serve as an updated version of the original matrix, modifying the initial equilibrium based on the empirical affective transitions experienced by the participant: They describe the likelihood of discovering one of the four states after attempting ten different hypothetical transitions. The iterative process between the initial states vector of probability and the transition matrix, culminating in the steady states, underscores the dynamic interplay between predisposition and experience in shaping the affective journey of individuals. This calculation of steady states is not the only method available (e.g., eigenvalue method, also used in “process data” in the Supplementary Materials’ section), but we believe it is the most accessible and readily applicable. All methods used have the same results. Finally, In the Supplementary Material, we provide a detailed explanation of all algorithms and the methodology used to calculate Discrete Time and Discrete Space Markov Chains.
A comprehensive Word file is available, guiding users on how to implement the experimental design and compute transitions for physiological data using a Discrete Time and Discrete Space Markov Chain approach.
The results are based on the Markovization process, which translates the variability measured by RMSSD into probabilistic terms. The RMSSD is calculated in milliseconds, for each 30’’ transition and for each singular 30’’ self-transition. This approach assesses the participant’s likelihood of variation during transitions between different affective states and the variability within the self-transition (e.g., AA-BB-CC-DD). The Figure 4 presented the value of RMSSD of each transition, before and after the Markovization process.
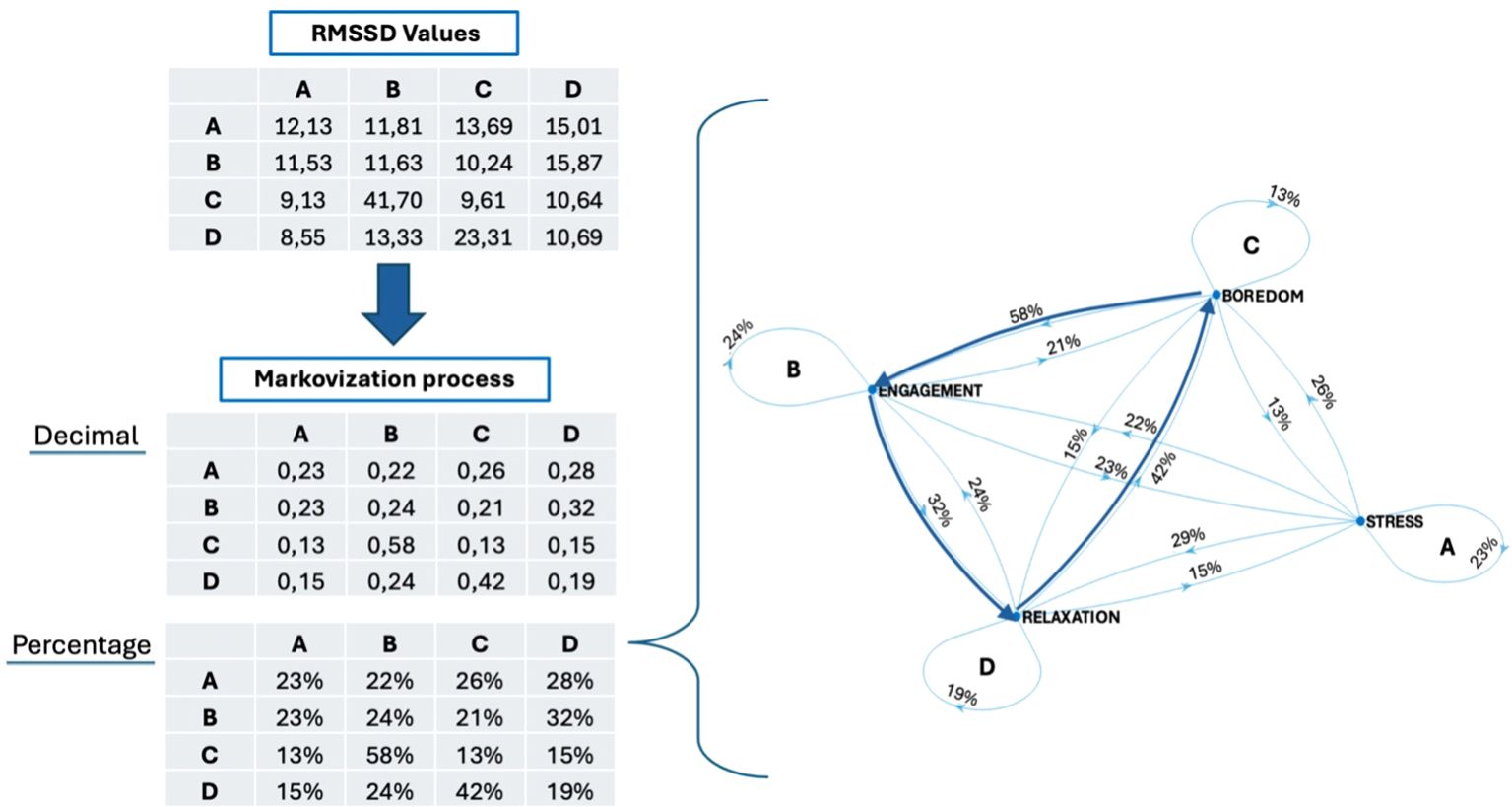
Figure 4. RMSSD values and Markovization processes (expressed in both decimal form and as a percentage) of the AN patient, with also its mathematical grapho representation.
After a Markovization process, we computed twelve RMSSD transitional indexes (vertical, horizontal, oblique), four static self-transition indexes, and four predictive indexes of steady state. Vertical probability indexes (AC-CA-BD-DB) represent the percentage of transitions between affective states with the same valence levels but opposing arousal levels (high-to-low and vice versa). The horizontal probability indexes (AB-BA-CD-DC) show changes between emotional states that have the same level of arousal but differ in valence (from positive to negative and vice versa). The oblique indexes (AD-DA-BC-CB) indicate the likelihood of transitions between emotional states that involve changes in both arousal and valence. The self-transition indexes (AA-BB-CC-DD) suggest that the participant’s affective state is consistent and unchanging. Steady states represent the likelihood of a person being in a specific emotional state after n step, hypothetically moving between the four quadrants ten steps.
Upon analyzing Figure 5, discrepancies in the probability of moving from one emotional state to another were noticeable.
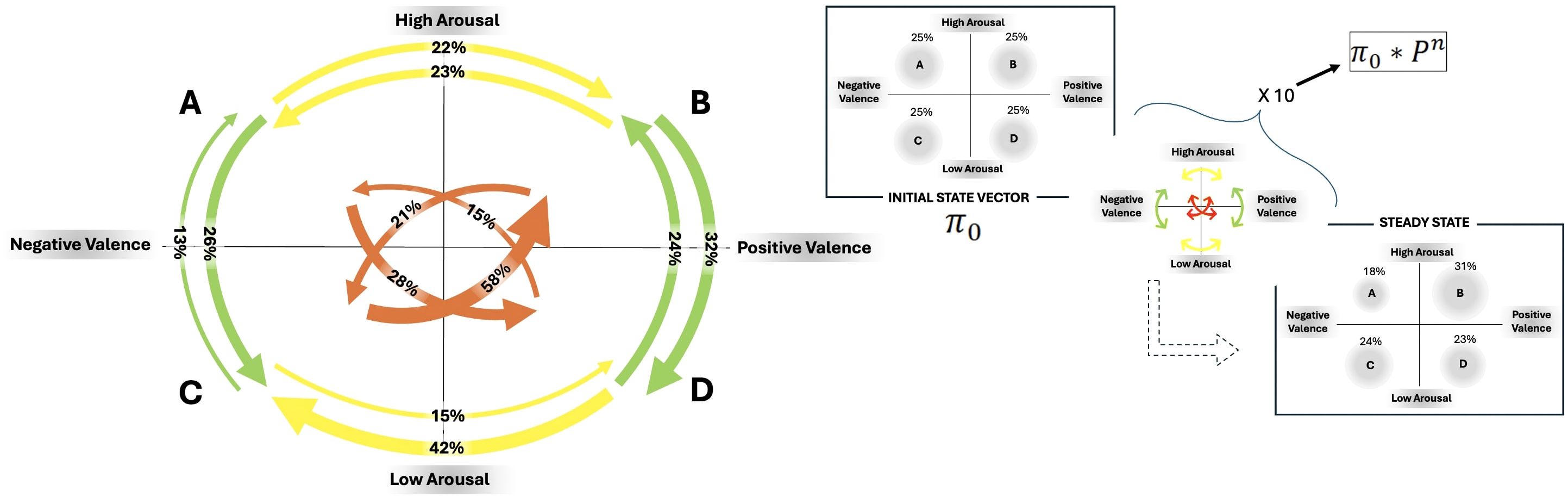
Figure 5. On the left side: Descriptive Markov chain containing transition values: Green arrows represent vertical transitions, yellow arrows depict horizontal transitions, and orange arrows signify oblique transitions. The percentage probability of RMSSD transitioning between states is indicated on each arrow. On the right side: Predictive Markov chain with initial state vector (π0) and steady state after 10 steps.
Self-transition indexes indicate that the individual with anorexia is more likely to maintain an activated (both positive and negative) emotional state compared to other states ( AA = 0.23; BB = 0.24; CC = 0.13; DD = 0.19).
Vertical transitions from high to low activation exhibited a higher probability (for both degrees of valence) than the opposite ones ( AC = 0.26; CA = 0.13; BD = 0.32; DB = 0.24).
When transitioning horizontally, there was a clear difference in the probability of moving from positive to negative states compared to the reverse. Particularly, during periods of low arousal, shifts from positive emotions to negative states were more probable ( AB = 0.22; BA = 0.23; CD = 0.15; DC = 0.42).
The oblique shifts showed more imbalances in transitioning between the four quadrants, with a greater likelihood of moving from highly activated negative states to low-activated positive states and from low-activated negative states to highly activated positive states ( AD = 0.28; DA = 0.15; BC = 0.21; CB = 0.58).
Observing Figure 5, it is also possible to state that the participant is predicted to experience a significant activation state with positive valence following repeated shifts between emotional states, as indicated by the steady state prediction vector ( A = 0.18; B = 0.31; C = 0.24; D = 0.23).
Although the impact of affective dynamics in well-being and health has been studied (62), addressing the physiological layer of this process, also involving an AN patient, poses technical and methodological issues. Our experimental and methodological aim was to develop a patient-centered assessment of dynamism of affective states. First, we assessed the physiological correlate of affect dynamics during the viewing of emotional pictures from the IAPS database, using a laboratory experimental design. Then, using RMSSD indexes into a Discrete-Time and Discrete Space Markov Chain, we were able to compute the probability of transitioning between various emotional states or remaining in a specific state.
Utilizing the Markov chain grapho, a quantitative idiographic approach, we noticed that the patient with anorexia nervosa exhibited particular patterns of behavior. When experiencing lower levels of arousal, the patient was 41% more likely to switch from positive emotional states (such as relaxation) to negative ones (such as boredom), showing strong physiological reactivity. This finding corresponds to other research that emphasizes the difficulties that persons with AN have in regulating their emotions and often encountering heightened negative affect (NA) when exposed to stimuli (18, 63). In conditions of heightened arousal, more balance was observed. This could be attributed to the body’s efforts to maintain homeostasis during stress or engagement, resulting in more regulated emotional responses. Functional MRI studies show that during high arousal, there is increased regulation in brain regions responsible for emotional control, supporting the observation of more balanced emotional states in these conditions (64).
Vertical axis showed increased variability during the shift from high to low activation in conditions with positive valence. Some transitions showed extremely reduced probabilities, particularly when transitioning from low to high activation levels.
As oblique transitions were considered, there was a strong likelihood that the patient would shift from negative to positive emotional states. Observations differed from the outcomes in horizontal transitions, which showed a higher likelihood of moving from positive to negative affective states. To transition from a negative to a positive affective state, the patient needed to regulate their level of activation by switching between low and high arousal. This trend aligns with prior research, which demonstrates the maladaptive techniques for regulating emotions in individuals with AN, such as suppression and avoidance. The need for extreme changes in emotion regulation may suggest the presence of maladaptive methods (18).
The self-transition indexes revealed a greater probability of the patient staying in quadrant B, which represented highly engaged positive emotional states. The likelihood of staying in the other quadrants diminished, resulting in lower variability and reduced sensitivity to these conditions. This observation is consistent with research findings that suggest individuals with anorexia nervosa demonstrate increased functional connectivity in certain brain regions linked to emotional regulation, such as the dorsolateral prefrontal cortex (DLPFC), while simultaneously displaying decreased connectivity in other regions. This disparity adds to their emotional inflexibility and the inclination to persist in certain emotional states for extended durations, thereby reducing the range and responsiveness to shifting emotional circumstances (64, 65).
The steady state matrix displayed a reduced balance among the four quadrants. After ten repetitions of emotional transitions, the patient with AN was more likely to migrate to a highly activated pleasant emotional state, followed by the possibility of experiencing low activating negative emotional states. Once more, our observations align with the findings of Wayda-Zalewska et al. (18), indicating that individuals with AN struggle to maintain psychological balance.
The AN patient exhibited a higher likelihood to engage in oblique transitions, involving changes in arousal and valence. This suggested the requirement of more opposing emotional inputs to facilitate a shift from one emotional state to another. The variability indexes of the patient with anorexia nervosa were diversified: her indexes exhibited substantial variation in some cases and were nearly absent in others, revealing various physiological response patterns based on the emotional transition’s characteristics.
Emotional difficulties in AN include inappropriate emotion regulation (ER) and expression (66), increased negative affect (NA) in response to unpleasant stimuli (63), and alexithymia (67). Among various emotional experience patterns, NA is critical for the development and maintenance of AN. Studies show that higher daily levels of NA increase the likelihood of food restrictions. NA can also increase following specific AN behaviors such as loss of control (LOC) eating, purging, a combination of LOC and purging, or weight checks. Conversely, NA levels tend to fall after drinking fluids or engaging in physical exercise. Research has focused on the states of NA and their specific manifestations (e.g., anxiety or tension) in AN, along with behaviors such as dietary restriction, LOC, or purging, as well as frequent weighing (18).
To contribute to the advancement of affect dynamics’ assessment research, we introduced a novel experimental and methodological approach that examines cardiac variability, specifically RMSSD, in a clinical and laboratory setting. (68, 69). Using standardized IAPS emotional imagery, researchers can effectively investigate affective transitions in a controlled manner, allowing the detection of cardiac variability parameters.
Using an index like RMSSD, one of the most important indicators for cardiac variability, we could evaluate the short-term variability of the various affective transitions. Furthermore, employing Discrete Time and Discrete Space Markov chains enables us to statistically translate the variability associated with transitions into indexes that show the likelihood of variation between states. The Markov matrix measures descriptive indexes—those calculated on the transition matrix, such as verticals, obliques, horizontals, and trait states—as well as predictive indexes—such as steady states—resulting from the matrix product of the transition matrix and the initial state vector (39). Descriptive indexes provide a snapshot of the patient’s current affective state, while predictive indexes forecast the likelihood of being in one of four affective states after multiple transitions. Empirically, we’re curious about how the participant reacts to an emotional storm caused by the encountered emotional shifts. Will the patient feel stressed, relaxed, or engaged?
The combination of an experimental design with analytical techniques like Markov matrixes creates a patient-centric approach, providing detailed insights into a patient’s affectivity, complementing for a holistic understanding of a patient’s emotional health. Our findings suggest that understanding affect dynamics through a combination of Markov Chains and HRV might improve current therapeutic approaches for treating AN. This would enable the implementation of an emotional assessment that is experimentally simple to measure and clinically simple to interpret for both the clinician and the patient. Indeed, the graph of transitions and steady states are quantitative idiographic methods that provide an instant comprehension of the patient’s emotional dynamism.
The current study has limitations by its nature as a case study, which restricts the generalizability of the observed results. Further studies should employ larger and more varied samples to improve generalizability. Extending this methodology to people with differing severities of AN, comorbidities, or other disorders—such as mood or anxiety disorders—necessitates consideration of condition-specific variations in autonomic and emotional control. Additionally, the absence of assessments on conscious emotional processing, such as explicit questions, hinders our understanding of the specific emotions acknowledged by patients. It would be intriguing to use this component in future research to detect any alignment or disparity between the implicit and explicit regulation aspects. Furthermore, self-report surveys assessing the individual’s emotional regulation styles could be implemented.
Future directions also involve incorporating virtual reality (VR) (70–72) to create dynamic stimuli that can better replicate the transitions and emotional dynamics experienced by individuals in the real world. Future research will also explore the idea of creating tools for the generation and examination of Markov matrixes.
The dataset used and/or analyzed during the current study is available from the corresponding author on reasonable request.
The studies involving humans were approved by Ethics Committee of IRCCS Istituto Auxologico Italiano (Prot. 2022_10_25_05). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
FB: Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. GS: Writing – original draft, Writing – review & editing. RC: Writing – review & editing. LB: Writing – review & editing. LM: Writing – review & editing. SiC: Writing – review & editing. FB: Writing – review & editing. StC: Writing – review & editing. EP: Writing – review & editing. AC: Supervision, Writing – review & editing. AM: Supervision, Writing – review & editing. PC: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The publication fee has been supported by Ricerca Corrente from Italian Ministry of Health. The project has been supported by the Grants PRIN 2022 PNRR P2022PXAZW funded by European Union NextGenerationEU. The project has been partially supported by the Department of Excellence Grant of the Italian Ministry of University and Research to the ‘Rita Levi Montalcini’ Department of Neuroscience.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2025.1502217/full#supplementary-material
1. Hsueh B, Chen R, Jo YJ, Tang D, Raffiee M, Kim YS, et al. Cardiogenic control of affective behavioural state. Nature. (2023) 615:7951. doi: 10.1038/s41586-023-05748-8
2. Waugh CE, Kuppens P. Affect dynamics. (2021). (Switzerland AG: Springer Nature), pp. 1–332. doi: 10.1007/978-3-030-82965-0.
3. Wirth M, Voss A, Wirth S, Rothermund K. Affect dynamics and well-being: explanatory power of the model of intraindividual variability in affect (MIVA). Cogn Emotion. (2021) 36:188–210. doi: 10.1080/02699931.2021.1993148
4. Borghesi F, Chirico A, Cipresso P. Outlining a novel psychometric model of mental flexibility and affect dynamics. Front Psychol. (2023) 14:1183316. doi: 10.3389/fpsyg.2023.1183316
5. Kuppens P, Verduyn P. Emotion dynamics. Curr Opin Psychol. (2017) 17:22–6. doi: 10.1016/J.COPSYC.2017.06.004
6. Trull TJ, Lane SP, Koval P, Ebner-Priemer UW. Affective dynamics in psychopathology. Emotion Review. (2015) 7:355–61. doi: 10.1177/1754073915590617
7. Wichers M, Wigman JTW, Myin-Germeys I. Micro-level affect dynamics in psychopathology viewed from complex dynamical system theory. Emotion Rev. (2015) 7:362–7. doi: 10.1177/1754073915590623
8. Borghesi F, Simoncini G, Chirico A, Cipresso P. In medio stat virtus: moderate cognitive flexibility as a key to affective flexibility responses in long-term HRV. Sensors (Basel Switzerland). (2024) 24:8047. doi: 10.3390/S24248047
9. Bonanno GA, Papa A, Lalande K, Westphal M, Coifman K. The importance of being flexible: the ability to both enhance and suppress emotional expression predicts long-term adjustment. psychol Sci. (2004) 15:482–7. doi: 10.1111/J.0956-7976.2004.00705.X
10. Borghesi F, Mancuso V, Bruni F, Cremascoli R, Bianchi L, Mendolicchio L, et al. Mental flexibility assessment: A research protocol for patients with Parkinson’s Disease and Anorexia Nervosa. PloS One. (2023) 18. doi: 10.1371/journal.pone.0293921
11. Koval P, Ogrinz B, Kuppens P, Van Den Bergh O, Tuerlinckx F, Sütterlin S. Affective instability in daily life is predicted by resting heart rate variability. PloS One. (2013) 8:e81536. doi: 10.1371/JOURNAL.PONE.0081536
12. Agorastos A, Mansueto AC, Hager T, Pappi E, Gardikioti A, Stiedl O. Heart rate variability as a translational dynamic biomarker of altered autonomic function in health and psychiatric disease. Biomedicines. (2023) 11:1591. doi: 10.3390/BIOMEDICINES11061591
13. Bosley HG, Soyster PD, Fisher AJ. Affect dynamics as predictors of symptom severity and treatment response in mood and anxiety disorders: evidence for specificity. J Person-Orient Res. (2019) 5:101. doi: 10.17505/JPOR.2019.09
14. So HWS, Kai A, Chau C, Kin-Hei-Chung L, Leung C-M, Chong GHC, et al. Moment-to-moment affective dynamics in schizophrenia and bipolar disorder. Eur Psychiatry. (2023) 66:e67. doi: 10.1192/J.EURPSY.2023.2438
15. Mukherjee D, Lee SA, Almeida D. Daily affective dynamics in major depressive disorder: the role of daily stressors and positive events. Affect Sci. (2023) 4:1–13. doi: 10.1007/s42761-023-00209-x
16. Wang J, Fang S, Yang C, Tang X, Zhu L, Nie Y. The relationship between psychological flexibility and depression, anxiety and stress: A latent profile analysis. Psychol Res Behav Manage. (2023) 16:997–1007. doi: 10.2147/PRBM.S400757
17. Vansteelandt K, Probst M, Pieters G. Assessing affective variability in eating disorders: affect spins less in anorexia nervosa of the restrictive type. Eating Behav. (2013) 14:263–8. doi: 10.1016/J.EATBEH.2013.03.004
18. Wayda-Zalewska M, Grzegorzewski P, Kot E, Skimina E, Santangelo PS, Kucharska K. Emotion dynamics and emotion regulation in anorexia nervosa: A systematic review of ecological momentary assessment studies. Int J Environ Res Public Health 2022. (2022) 19:13659. doi: 10.3390/IJERPH192013659
19. Friederich HC, Herzog W. Cognitive-behavioral flexibility in anorexia nervosa. Curr Topics Behav Neurosci. (2011) 6:111–23. doi: 10.1007/7854_2010_83
20. Santos BM, Haynos AF. Difficulties with positive emotion regulation in anorexia nervosa. Eur Eating Disord Rev. (2023) 31:520–8. doi: 10.1002/erv.2973
21. Koval P, Burnett PT, Zheng Y. Emotional inertia: On the conservation of emotional momentum. Affect Dynamics. (2021) 63–94. doi: 10.1007/978-3-030-82965-0_4/
22. Pirla S, Taquet M, Quoidbach J. Measuring affect dynamics: An empirical framework. Behav Res Methods. (2023) 55:285–300. doi: 10.3758/S13428-022-01829-0
23. Williams-Kerver GA, Wonderlich SA, Crosby RD, Cao L, Smith KE, Engel SG, et al. Differences in affective dynamics among eating-disorder diagnostic groups. Clinical Psychological Sci. (2020) 8:857–71. doi: 10.1177/2167702620917196
24. Russell JA. A circumplex model of affect. J Pers Soc Psychol. (1980) 39:1161–78. doi: 10.1037/H0077714
25. Kwasnicka D, Kale D, Schneider V, Keller J, Yeboah-Asiamah-Asare B, Powell D, et al. Systematic review of ecological momentary assessment (EMA) studies of five public health-related behaviours: review protocol. BMJ Open. (2021) 11:e046435. doi: 10.1136/BMJOPEN-2020-046435
26. Boemo T, Nieto I, Vazquez C, Sanchez-Lopez A. Relations between emotion regulation strategies and affect in daily life: A systematic review and meta-analysis of studies using ecological momentary assessments. Neurosci Biobehav Rev. (2022) 139:104747. doi: 10.1016/J.NEUBIOREV.2022.104747
27. Cipresso P, Serino S, Borghesi F, Tartarisco G, Riva G, Pioggia G, et al. Continuous measurement of stress levels in naturalistic settings using heart rate variability: An experience-sampling study driving a machine learning approach. Acta IMEKO. (2021) 10:239–48. doi: 10.21014/ACTA_IMEKO.V10I4.1183
28. Smith A, Page KA, Smith KE. Associations between affect dynamics and eating regulation in daily life: a preliminary ecological momentary assessment study. Cogn Emotion. (2024) 1–7. doi: 10.1080/02699931.2024.2323478
29. Schoevers RA, Van Borkulo CD, Lamers F, Servaas MN, Bastiaansen JA, Beekman ATF, et al. Affect fluctuations examined with ecological momentary assessment in patients with current or remitted depression and anxiety disorders. psychol Med. (2021) 51:1906–15. doi: 10.1017/S0033291720000689
30. Colombo D, Fernández-Álvarez J, Patané A, Semonella M, Kwiatkowska M, García-Palacios A, et al. Current state and future directions of technology-based ecological momentary assessment and intervention for major depressive disorder: A systematic review. J Clin Med. (2019) 8:465. doi: 10.3390/jcm8040465
31. Dejonckheere E, Mestdagh M, Houben M, Rutten I, Sels L, Kuppens P, et al. Complex affect dynamics add limited information to the prediction of psychological well-being. Nat Hum Behav. (2019) 3:5. doi: 10.1038/s41562-019-0555-0
32. Scott LN, Victor SE, Kaufman EA, Beeney JE, Byrd AL, Vine V, et al. Affective dynamics across internalizing and externalizing dimensions of psychopathology. Clin psychol Sci. (2020) 8:412–27. doi: 10.1177/2167702619898802
33. Delbroek T, Vermeylen W, Spildooren J. The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: a randomized controlled trial. J Phys Ther Sci. (2017) 29:1137–43. doi: 10.1589/JPTS.29.1137
34. Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. (2012) 36:747–56. doi: 10.1016/J.NEUBIOREV.2011.11.009
35. Schmalbach I, Herhaus B, Pässler S, Runst S, Berth H, Wolff S, et al. Autonomic nervous system response to psychosocial stress in anorexia nervosa: A cross-sectional and controlled study. Front Psychol. (2021) 12:649848. doi: 10.3389/FPSYG.2021.649848
36. Appelhans BM, Luecken LJ. Heart rate variability as an index of regulated emotional responding. Rev Gen Psychol. (2006) 10:229–40. doi: 10.1037/1089-2680.10.3.229
37. Lang PJ, Bradley MM, Cuthbert, Greenwald M, Dhman A, Vaid D, et al. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. NIMH Center for the Study of Emotion and Attention: International Affective Picture System (IAPS) (1997).
38. Meini B. Markov Chains. Handbook of Linear Algebra. (2006). (Boca Raton, Florida: Chapman and Hall/CRC), pp. 54–1. doi: 10.1201/9781420010572-54.
39. Borghesi F, Cipresso P. “From states to transitions: Discrete time markov chains for affect dynamics,” in Proceedings of the 22nd international conference on cognitive modelling. Ed. Sibert C. (University Park, PA: Applied Cognitive Science Lab, Penn State) (2024), 16–21.
40. Cipresso P, Borghesi F, Chirico A. Affects affect affects: A Markov Chain. Front Psychol. (2023) 14:1162655. doi: 10.3389/fpsyg.2023.1162655
41. Simoncini G, Borghesi F, Cipresso P. Linking affect dynamics and well-being: A novel methodological approach for mental health. Healthcare. (2024) 12:1690. doi: 10.3390/HEALTHCARE12171690/S1
42. Costa A, Bagoj E, Monaco M, Zabberoni S, De Rosa S, Papantonio AM, et al. Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol Sci. (2014) 35:365–72. doi: 10.1007/S10072-013-1520-8
43. Giovagnoli AR, Del Pesce M, Mascheroni S, Simoncelli M, Laiacona M, Capitani E. Trail Making Test: Normative values from287 normal adult controls. Ital J Neurol Sci. (1996) 17:305–9. doi: 10.1007/BF01997792
44. Bruni F, Toraldo A, Scarpina F. Italian normative data for the original version of the tower of London test: A bivariate analysis on speed and accuracy scores. Assessment. (2022) 29:209–24. doi: 10.1177/1073191120961834
45. Grigoletto F, Zappalà G, Anderson DW, Lebowitz BD. Norms for the mini-mental state examination in a healthy population. Neurology. (1999) 53:315–20. doi: 10.1212/WNL.53.2.315
46. Monaco M, Costa A, Caltagirone C, Carlesimo GA. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol Sci. (2013) 34:749–54. doi: 10.1007/S10072-012-1130-X
47. Carlesimo GA, Caltagirone C, Gainotti G, Facida L, Gallassi R, Lorusso S, et al. The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur Neurol. (1996) 36:378–84. doi: 10.1159/000117297
48. Caffarra P, Gardini S, Zonato F, Concari L, Dieci F, Copelli S, et al. Italian norms for the Freedman version of the Clock Drawing Test. J Clin Exp Neuropsychol. (2011) 33:982–8. doi: 10.1080/13803395.2011.589373
49. Appollonio I, Leone M, Isella V, Piamarta F, Consoli T, Villa ML, et al. The frontal assessment battery (FAB): Normative values in an Italian population sample. Neurol Sci. (2005) 26:108–16. doi: 10.1007/S10072-005-0443-4
50. Basso A, Capitani E, Laiacona M. Raven’s coloured progressive matrices: normative values on 305 adult normal controls. Funct Neurol. (1987) 2:189–94. https://europepmc.org/article/med/3666548 (Accessed February 27, 2024).
51. Russell JA. Core affect and the psychological construction of emotion. psychol Rev. (2003) 110:145–72. doi: 10.1037/0033-295X.110.1.145
52. Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. (1994) 25:49–59. doi: 10.1016/0005-7916(94)90063-9
53. Acharya UR, Joseph KP, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. (2006) 44:1031–51. doi: 10.1007/S11517-006-0119-0
54. Baevsky RМ, Chernikova AG. Heart rate variability analysis: physiological foundations and main methods. Cardiometry. (2017) 10:66–76. doi: 10.12710/CARDIOMETRY.2017.10.6676
55. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
56. Miller GA. Finite markov processes in psychology. Psychometrika. (1952) 17:149–67. doi: 10.1007/BF02288779
57. Suhov Y, Kelbert M. Probability and statistics by example: II Markov chains: A primer in random processes and their applications. Probability and Statistics by Example: II Markov Chains: A Primer in Random Processes and Their Applications. Cambridge University Press. (2008) 2:1–487. doi: 10.1017/CBO9780511813641
58. Crayen C, Eid M, Lischetzke T, Courvoisier DS, Vermunt JK. Exploring dynamics in mood regulation-mixture latent markov modeling of ambulatory assessment data. Psychosom Med. (2012) 74:366–76. doi: 10.1097/PSY.0B013E31825474CB
59. Kaplan D. An overview of Markov chain methods for the study of stage-sequential developmental processes. Dev Psychol. (2008) 44:457–67. doi: 10.1037/0012-1649.44.2.457
60. Craig BA, Sendi PP. Estimation of the transition matrix of a discrete-time Markov chain. Health Econ. (2002) 11:33–42. doi: 10.1002/HEC.654
61. Pham T, Lau ZJ, Chen SHA, Makowski D. Heart rate variability in psychology: A review of HRV indices and an analysis tutorial. Sensors (Basel Switzerland). (2021) 21:3998. doi: 10.3390/S21123998
62. Ullah N, Treur J, Koole SL. Flexibility and adaptivity of emotion regulation: from contextual dynamics to adaptation and control. Affect Dynamics. (2021), 261–92. doi: 10.1007/978-3-030-82965-0_11
63. Culicetto L, Ferraioli F, Lucifora C, Falzone A, Martino G, Craparo G, et al. Disgust as a transdiagnostic index of mental illness: A narrative review of clinical populations. Bulletin Menninger Clinic. (2023) 87:53–91. doi: 10.1521/BUMC.2023.87.SUPPA.53
64. Göller S, Nickel K, Horster I, Endres D, Zeeck A, Domschke K, et al. State or trait: the neurobiology of anorexia nervosa — contributions of a functional magnetic resonance imaging study. J Eating Disord. (2022) 10:1–12. doi: 10.1186/S40337-022-00598-7
65. Sudo Y, Ota J, Takamura T, Kamashita R, Hamatani S, Numata N, et al. Comprehensive elucidation of resting-state functional connectivity in anorexia nervosa by a multicenter cross-sectional study. psychol Med. (2024) 1–14. doi: 10.1017/S0033291724000485
66. Stapp EK, Zipunnikov V, Leroux A, Cui L, Husky MM, Dey D, et al. Specificity of affective dynamics of bipolar and major depressive disorder. Brain Behav. (2023) 13:e3134. doi: 10.1002/brb3.3134
67. Sfärlea A, Dehning S, Keller LK, Schulte-Körne G. Alexithymia predicts maladaptive but not adaptive emotion regulation strategies in adolescent girls with anorexia nervosa or depression. J Eating Disord. (2019) 7:1–9. doi: 10.1186/S40337-019-0271-1
68. Borghesi F, Chirico A, Pedroli E, Cipriani GE, Canessa N, Amanzio M, et al. Exploring biomarkers of mental flexibility in healthy aging: A computational psychometric study. Sensors. (2023) 23:6983. doi: 10.3390/s23156983
69. De Longis E, Alessandri G, Ottaviani C. Inertia of emotions and inertia of the heart: Physiological processes underlying inertia of negative emotions at work. Int J Psychophysiol. (2020) 155:210–8. doi: 10.1016/J.IJPSYCHO.2020.06.007
70. Bermudez S, Quintero LV, Cameirão MS, Chirico A, Triberti S, Cipresso P, et al. Toward emotionally adaptive virtual reality for mental health applications. IEEE J Biomed Health Inf. (2019) 23:1877–87. doi: 10.1109/JBHI.2018.2878846
71. Borghesi F, Murtas V, Chirico A, Pizzolante M, Cipresso P. Affect dynamics through virtual reality. Annu Rev Cyberther Telemed. (2023) 11:258–76. doi: 10.4018/978-1-6684-4854-0
Keywords: affect dynamics, heart rate variability, Markov chain, psychometrics, stochastic model, neuroscience, Anorexia Nervosa
Citation: Borghesi F, Simoncini G, Cremascoli R, Bianchi L, Mendolicchio L, Cappelli S, Brusa F, Cattaldo S, Prina E, Chirico A, Mauro A and Cipresso P (2025) A stochastic model for affect dynamics: methodological insights from heart rate variability in an illustrative case of Anorexia Nervosa. Front. Psychiatry 16:1502217. doi: 10.3389/fpsyt.2025.1502217
Received: 26 September 2024; Accepted: 28 January 2025;
Published: 25 February 2025.
Edited by:
Carmelo Mario Vicario, University of Messina, ItalyReviewed by:
Michela Ponticorvo, University of Naples Federico II, ItalyCopyright © 2025 Borghesi, Simoncini, Cremascoli, Bianchi, Mendolicchio, Cappelli, Brusa, Cattaldo, Prina, Chirico, Mauro and Cipresso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Borghesi, Zi5ib3JnaGVzaUBhdXhvbG9naWNvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.