- Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, La Jolla, CA, United States
3,4-Methylenedioxymethamphetamine (MDMA) is being investigated in controlled clinical trials for use as an adjunct medication treatment for post-traumatic stress disorder. MDMA is metabolized by N-demethylation, primarily by CYP2D6, to its main inactive metabolite, 4-hydroxy-3-methoxymethamphetamine. It is also metabolized to a lesser extent by CYP1A2, CYP2B6, and CYP3A4 to its active metabolite, 3,4-methylenedioxyamphetamine. Considering the extensive hepatic metabolism and excretion, MDMA use in psychiatry raises concerns over drug-induced liver injury (DILI), a rare but dangerous event. Majority of the drugs withdrawn from the market for liver injury caused death or transplantation at frequencies under 0.01%. Unfortunately, markers for liver injury were not measured in most published clinical trials. At the same time, no visible DILI-related symptoms and adverse events were observed. Idiosyncratic DILI cases are rarely registered during clinical trials due to their rare nature. In this study, we surveyed a larger, over 1,500, and a more diverse set of reports from the FDA Adverse Event Reporting System and found 23 cases of hepatic injury and hepatic failure, in which MDMA was reported to be taken in addition to one or more substances. Interestingly, 22 out of 23 cases had one or more listed drugs with a known DILI concern based on the FDA’s DILIrank dataset. Furthermore, only one report had MDMA listed as the primary suspect. Considering the nearly 20 million doses of MDMA used annually, this single report is insufficient for establishing a significant association with DILI.
Introduction
MDMA or 3,4-methylenedioxymethamphetamine is currently a controlled Class A substance in the United Kingdom and Schedule I substance in the United States and the European Union. Based on efficacy and safety findings in multiple clinical trials (1–5), there is an ever-increasing interest in MDMA use in psychiatry.
MDMA is metabolized through N-demethylation primarily by cytochrome P450 2D6 (CYP2D6). This pathway produces its main inactive metabolite 4-hydroxy-3-methoxymethamphetamine. MDMA is also metabolized to a lesser extent by CYP1A2, CYP2B6, and CYP3A4 to its active metabolite, 3,4-methylenedioxyamphetamine. Most of the drugs associated with drug-induced liver injury (DILI) are metabolized through the hepatic pathway (6). However, there is no correlation with any specific CYP450 metabolic pathway and liver injury or failure. Most DILI cases are of idiosyncratic nature (7, 8) with a few exceptions such as in the case with acetaminophen (paracetamol) (9). Although DILI has not been observed in controlled clinical trials using MDMA (1, 2, 10, 11), considering the rare nature of this adverse event [13.9–24.0 per 100,000, and severe cases under 10 per 100,000 (12, 13)], it is rarely captured in clinical trials and cannot be completely ruled out. There have been published case reports of liver injury and liver failure with detectable MDMA levels, suggesting a possible association of its use with DILI (14–20). However, in every single case, the confounding factors, such as known DILI-concern concomitant drugs and infectious or other hepatitis, had not been addressed to justify causality assessment of MDMA as a culprit.
The lack of any concrete evidence of MDMA association with DILI warranted a further evaluation of MDMA user reports associated with liver injury and liver failure from the United States Food and Drug Administration Adverse Event Reporting System (FDA AERS or FAERS) reported to the FDA through MedWatch (21). In this study, FAERS reports were evaluated for the presence of MDMA as the sole reported drug and for the presence of any additional drugs with a known association with DILI based on the DILIrank database (13, 22).
Methods
FDA Adverse Event Reporting System
The FDA Adverse Event Reporting System (FAERS) is dataset repository, which hosts adverse events (AEs) submitted to the FDA through MedWatch AE forms 3,500 and 3500A by manufacturers, consumers, lawyers, and healthcare professionals (21).
Although initially intended for postmarketing safety surveillance of approved drugs and biologics, FAERS is a useful and significant source of safety information on drugs still under investigation, drugs such as Schedule I/Class A substances not yet approved by regulatory authorities, but available to the public through illicit means.
Case selection
FAERS/AERS quarterly datasets, each including separate tables for demographics, country, drug, indication, outcome, reaction, and report source, were downloaded individually from the FDA public repository in dollar sign-separated text format (23–25). Unix shell scripts were used for data restructuring and filtering (26).
At the time of the analysis, FAERS/AERS contained 19,190,582 reports from January 2004 to March 2023. A total of 1,575 reports involving reported MDMA/ecstasy were selected for further analysis. Liver failure and liver injury FDA medical queries (FMQs) were used to filter out the cases of interest (see Table 1 for a comprehensive list of the search terms). Cases with any of the FMQs reported to be associated with viral or ischemic hepatitis were excluded. Each individual remaining case was reviewed for exclusion of any duplicates by multiple reporters.
Results
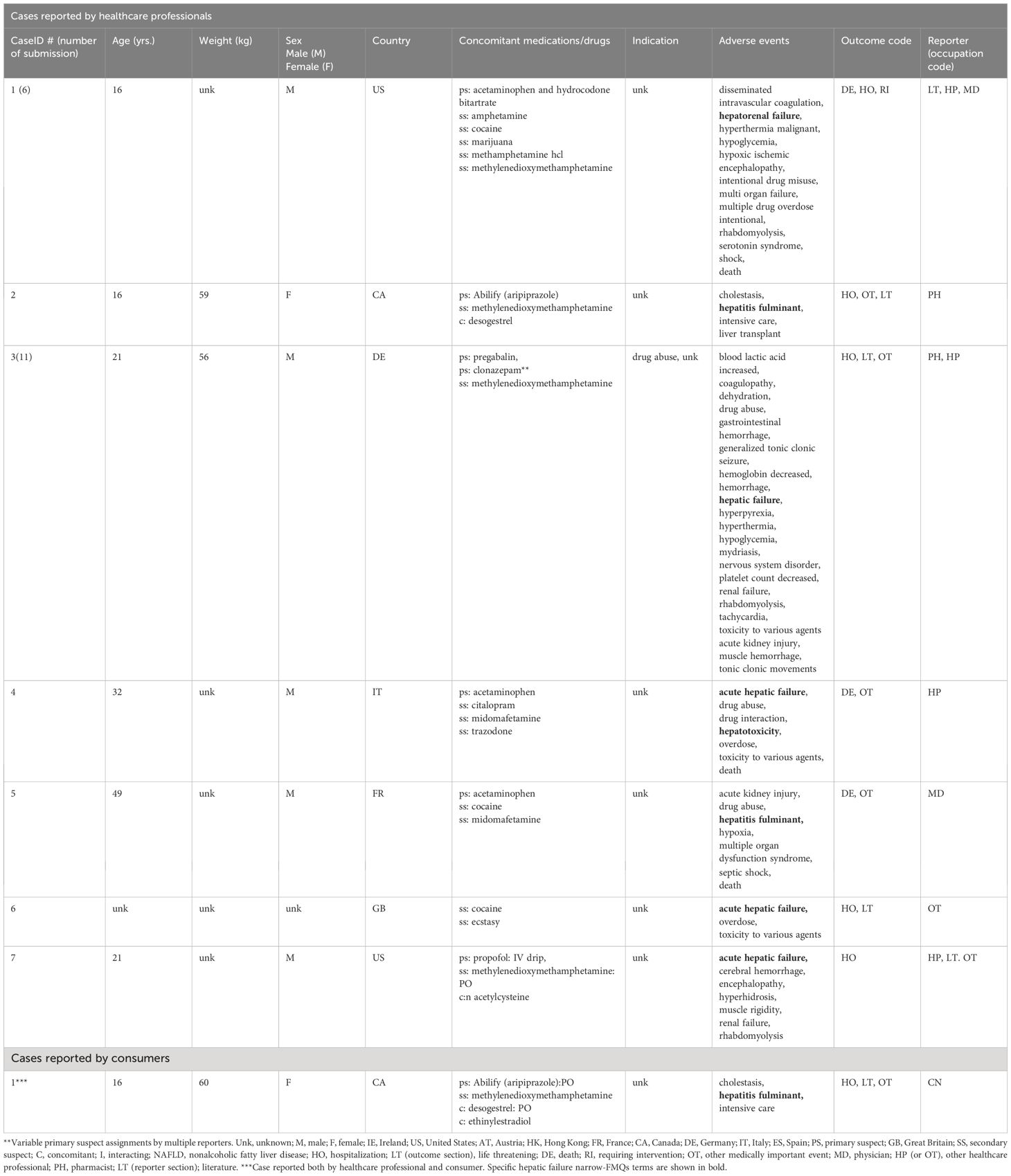
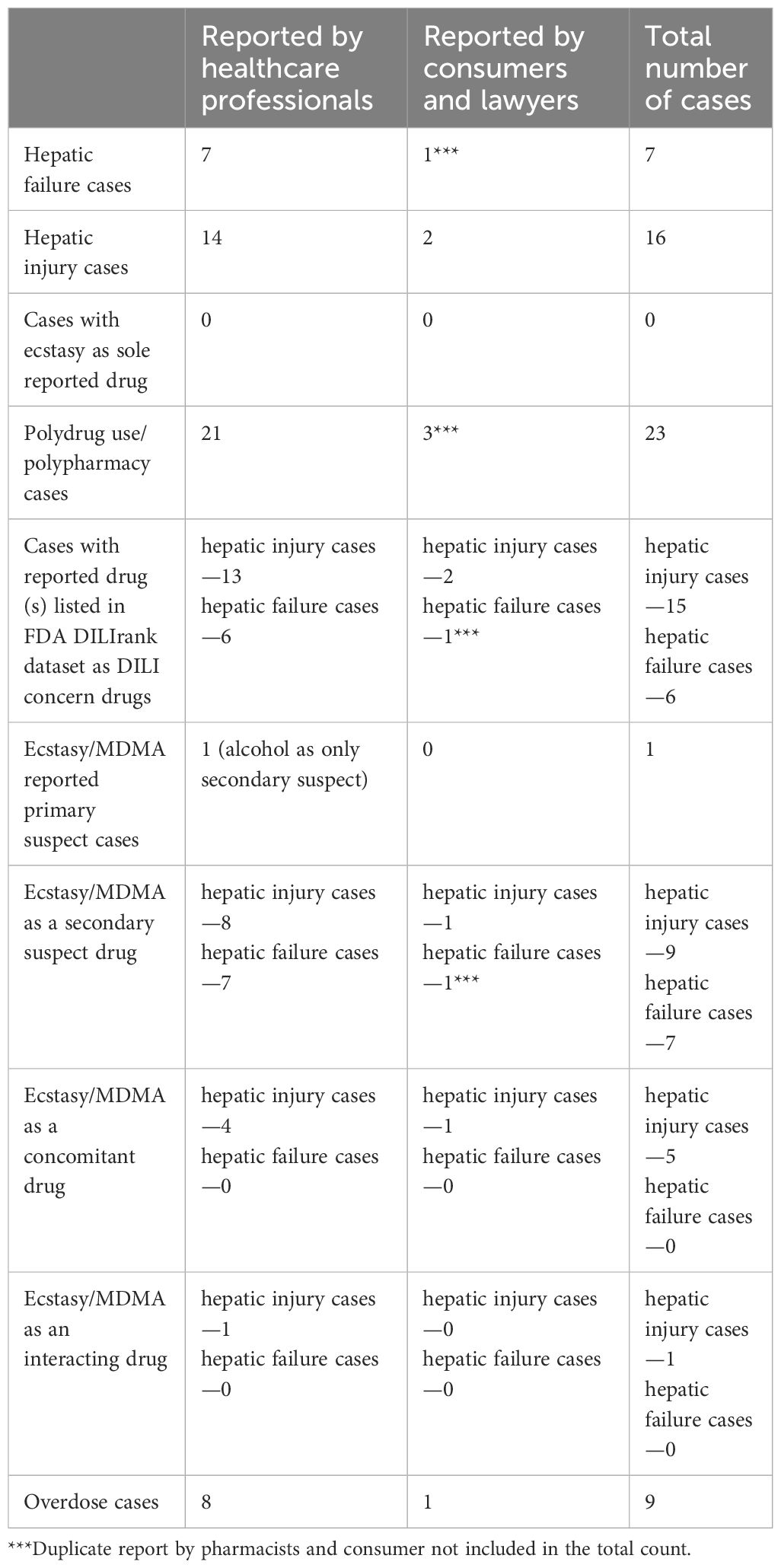
There was a total of seven unique liver failure narrow-FMQ AE cases in the FEARS database. All of these cases were reported by healthcare professionals, and one of the seven was also reported by a consumer. Four out of seven cases were overdose cases, and six of these reported one or more drugs known to be associated with liver injury according to the DILIrank dataset. No cases reported MDMA as a “primary suspect” of the AEs. One overdose case had cocaine, and MDMA both reported as “secondary suspects” with no “primary suspect” listed (Table 2).
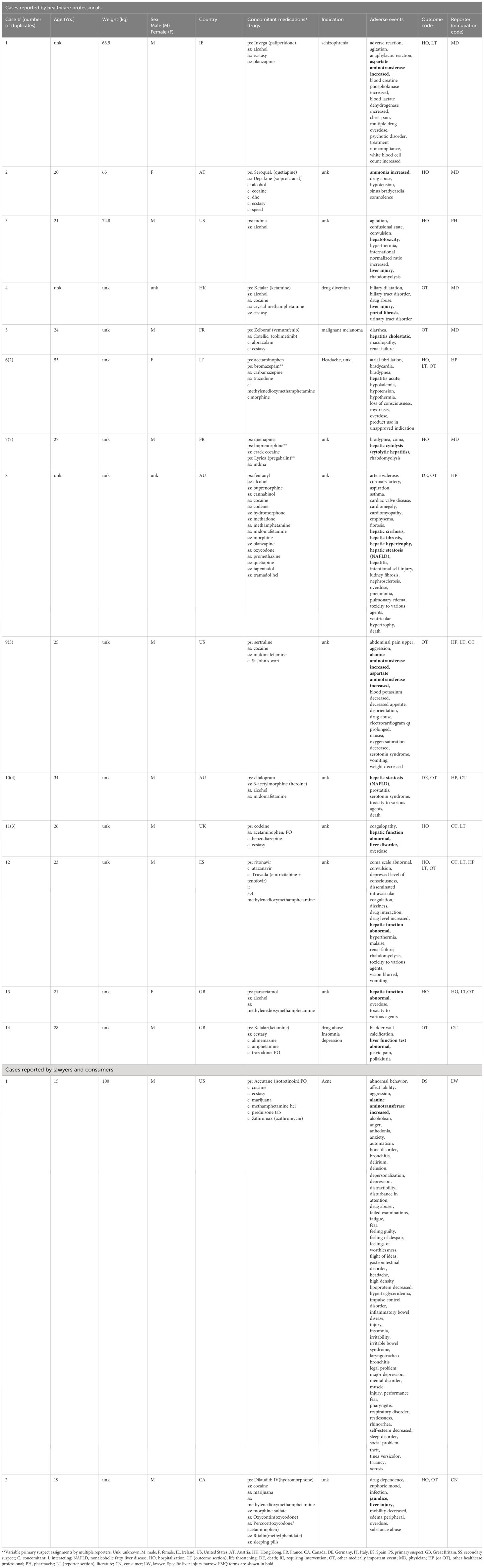
There were 16 unique liver injury narrow-FMQ AE cases in the FEARS database, including 14 cases reported by healthcare professionals, one by a lawyer, and one by a consumer. Five out of 16 cases were overdose cases, and as many as 15 out of the 16 cases had reported one or more drugs known to be associated with liver injury according to the DILIrank. One case was reported with MDMA as a “primary suspect” with alcohol listed as a “secondary suspect” (Table 3). The cases for both hepatic failure and injury are summarized in Table 4.
Discussion
In this study, we evaluated liver failure and liver injury cases reported as associated with a list of drugs including MDMA, from the United States Food and Drug Administration FAERS. We found no cases where MDMA was the sole reported compound. This was in line with the absence of liver injury or liver failure AEs in the clinical trials. Additionally, we observed a limited number of 23 (seven hepatic failure and 16 hepatic injury cases) originating from a list of drugs including MDMA in the last ~18 years. Of these 23 cases, 21 listed one or more co-reported drugs associated with liver injury or liver failure according to DILIrank (13) (Table 5). MDMA was listed as a primary suspect only in a single case, which contained alcohol use, a known substance to cause liver damage, concurrent with MDMA. There is still the possibility that MDMA may have played a role in the other drugs’ hepatotoxic effects due to a potential CYP2D6-mediated drug–drug interaction (27, 28). However, considering the worldwide widespread MDMA use (~20 million annually) (29), the single report with liver injury/failure reported to the FAERS database was insufficient to establish a meaningful association signal.
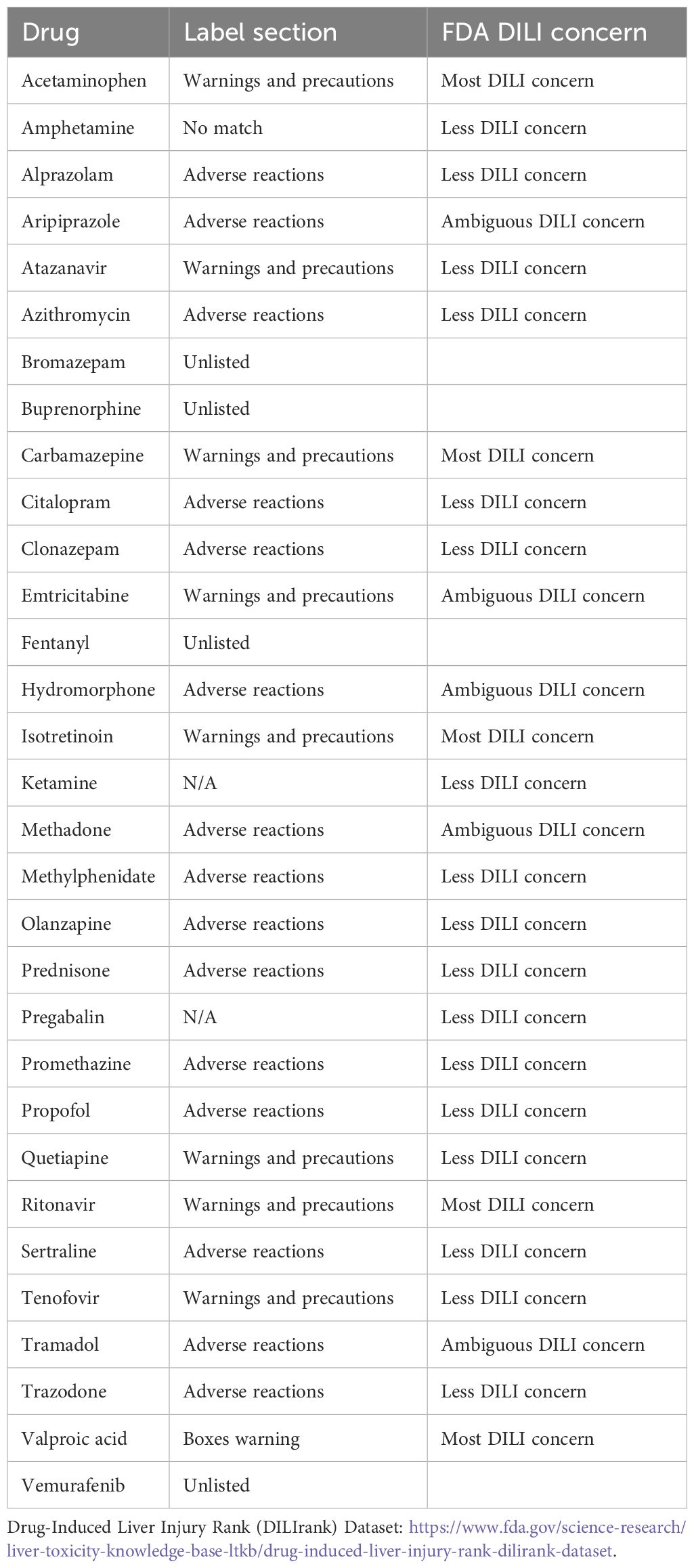
Table 5 Drugs of concern according to DILIrank list in the FAERS liver injury and liver failure FMQ reports.
Study limitations
Reporting to FAERS is mostly voluntary, apart from spontaneous reports forwarded from the manufacturers/authorization holders. Thus, the dataset represents only a subset of actual cases and should not be confused with absolute population frequencies. Most of the cases are not clinically assessed for causality. There was no consistent means for reporters to provide information on drug identification or detection. Since manufacture and distribution of MDMA is not regulated, it is uncertain whether the chemical compound listed in the cases could be confirmed as MDMA or MDMA laced with another compound.
Conclusion
In summary, reported use of MDMA as the only administered drug produced a single report of liver injury or liver failure in the FAERS system; it was far more common for hepatotoxicity-related AEs to arise when MDMA was reportedly combined with an additional substance with well-documented DILI-concern (22). The current findings in the FAERS system are in line with the failure of clinical trials to report DILI.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
TM: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. RA: Writing – review & editing, Writing – original draft, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded in part by NIH R35GM131881.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wolfson PE, Andries J, Feduccia AA, Jerome L, Wang JB, Williams E, et al. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study. Sci Rep. (2020) 10:20442. doi: 10.1038/s41598-020-75706-1
2. Mithoefer MC, Feduccia AA, Jerome L, Mithoefer A, Wagner M, Walsh Z, et al. MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacol (Berl). (2019) 236:2735–45. doi: 10.1007/s00213-019-05249-5
3. Jerome L, Feduccia AA, Wang JB, Hamilton S, Yazar-Klosinski B, Emerson A, et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacol (Berl). (2020) 237:2485–97. doi: 10.1007/s00213-020-05548-2
4. Mitchell JM, Bogenschutz M, Lilienstein A, Harrison C, Kleiman S, Parker-Guilbert K, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. (2021) 27:1025–33. doi: 10.1038/s41591-021-01336-3
5. Yazar-Klosinski BB, Mithoefer MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. (2017) 101:194–6. doi: 10.1002/cpt.565
6. Corsini A, Bortolini M. Drug-induced liver injury: the role of drug metabolism and transport. J Clin Pharmacol. (2013) 53:463–74. doi: 10.1002/jcph.23
7. Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an overview. Expert Opin Drug Saf. (2007) 6:673–84. doi: 10.1517/14740338.6.6.673
8. Hussaini SH, Farrington EA. Idiosyncratic drug-induced liver injury: an update on the 2007 overview. Expert Opin Drug Saf. (2014) 13:67–81. doi: 10.1517/14740338.2013.828032
9. Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol. (2016) 4:131–42. doi: 10.14218/JCTH
10. Vizeli P, Liechti ME. Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol. (2017) 31:576–88. doi: 10.1177/0269881117691569
11. Vizeli P, Straumann I, Duthaler U, Varghese N, Eckert A, Paulus MP, et al. Effects of 3,4-methylenedioxymethamphetamine on conditioned fear extinction and retention in a crossover study in healthy subjects. Front Pharmacol. (2022) 13:906639. doi: 10.3389/fphar.2022.906639
12. Suk KT, Kim DJ. Drug-induced liver injury: present and future. Clin Mol Hepatol. (2012) 18:249–57. doi: 10.3350/cmh.2012.18.3.249
13. Chen M, Suzuki A, Thakkar S, Yu K, Hu C, Tong W. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discovery Today. (2016) 21:648–53. doi: 10.1016/j.drudis.2016.02.015
14. Ellis AJ, Wendon JA, Portmann B, Williams R. Acute liver damage and ecstasy ingestion. Gut. (1996) 38:454–8. doi: 10.1136/gut.38.3.454
15. Greene SL, Dargan PI, O'connor N, Jones AL, Kerins M. Multiple toxicity from 3,4-methylenedioxymethamphetamine ("ecstasy"). Am J Emerg Med. (2003) 21:121–4. doi: 10.1053/ajem.2003.50028
16. Milroy CM, Clark JC, Forrest AR. Pathology of deaths associated with "ecstasy" and "eve" misuse. J Clin Pathol. (1996) 49:149–53. doi: 10.1136/jcp.49.2.149
17. Lang CN, Sommer MJ, Neukamm MA, Staudacher DL, Supady A, Bode C, et al. Use of the CytoSorb adsorption device in MDMA intoxication: a first-in-man application and in vitro study. Intensive Care Med Exp. (2020) 8:21. doi: 10.1186/s40635-020-00313-3
18. Kunitz O, Ince A, Kuhlen R, Rossaint R. [Hyperpyrexia and rhabdomyolysis after ecstasy (MDMA) intoxication]. Anaesthesist. (2003) 52:511–5. doi: 10.1007/s00101-003-0474-2
19. Fahal IH, Sallomi DF, Yaqoob M, Bell GM. Acute renal failure after ecstasy. BMJ. (1992) 305:29. doi: 10.1136/bmj.305.6844.29
20. Brown C, Osterloh J. Multiple severe complications from recreational ingestion of MDMA ('Ecstasy'). JAMA. (1987) 258:780–1. doi: 10.1001/jama.258.6.780
21. Craigle V. MedWatch: the FDA safety information and adverse event reporting program. J Med Libr Assoc. (2007) 95:224–5. doi: 10.3163/1536-5050.95.2.224
22. FDA. Drug Induced Liver Injury Rank (DILIrank) Dataset (2023). Available online at: https://www.fda.gov/science-research/liver-toxicity-knowledge-base-ltkb/drug-induced-liver-injury-rank-dilirank-dataset: US Food and Drug Administration.
23. Joulfayan H, Makunts T, Abagyan R. Anti-TNF-α therapy induced psoriasis in rheumatoid arthritis patients according to FDA postmarketing surveillance data. Sci Rep. (2023) 13:10448. doi: 10.1038/s41598-023-37010-6
24. Makunts T, Dahill D, Jerome L, de Boer A, Abagyan R. Concomitant medications associated with ischemic, hypertensive, and arrhythmic events in MDMA users in FDA adverse event reporting system. Front Psychiatry. (2023) 14:1149766. doi: 10.3389/fpsyt.2023.1149766
25. Makunts T, Jerome L, Abagyan R, de Boer A. Reported cases of serotonin syndrome in MDMA users in FAERS database. Front Psychiatry. (2021) 12:824288. doi: 10.3389/fpsyt.2021.824288
26. Stein LD. Unix survival guide. Curr Protoc Bioinf. (2015) 51:A1.C–A.C.27. doi: 10.1002/0471250953.bia01cs51
27. Rodgers JT, Davydova NY, Paragas EM, Jones JP, Davydov DR. Kinetic mechanism of time-dependent inhibition of CYP2D6 by 3,4-methylenedioxymethamphetamine (MDMA): Functional heterogeneity of the enzyme and the reversibility of its inactivation. Biochem Pharmacol. (2018) 156:86–98. doi: 10.1016/j.bcp.2018.08.010
28. de la Torre R, Yubero-Lahoz S, Pardo-Lozano R, Farré M. MDMA, methamphetamine, and CYP2D6 pharmacogenetics: what is clinically relevant? Front Genet. (2012) 3:235. doi: 10.3389/fgene.2012.00235
29. UN. World Drug Report 2021. (Wiley) (2021). Available at: https://currentprotocols.onlinelibrary.wiley.com/doi/10.1002/0471250953.bia01cs51
Keywords: DILI (drug-induced liver injury), MDMA, DDI (drug-drug interaction), FAERS, adverse events, MDMA (3,4-methylenedioxymethamphetamine)
Citation: Makunts T and Abagyan R (2024) Hepatic injury and hepatic failure adverse events in 3,4-methylenedioxymethamphetamine users reported to the FDA Adverse Event Reporting System. Front. Psychiatry 15:1414622. doi: 10.3389/fpsyt.2024.1414622
Received: 09 April 2024; Accepted: 29 May 2024;
Published: 18 June 2024.
Edited by:
Patricia Di Ciano, University of Toronto, CanadaReviewed by:
Anastasio Tini, Marche Polytechnic University, ItalySusie H. Park, Riverside University Health System, United States
Copyright © 2024 Makunts and Abagyan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruben Abagyan, cmFiYWd5YW5AaGVhbHRoLnVjc2QuZWR1
 Tigran Makunts
Tigran Makunts Ruben Abagyan*
Ruben Abagyan*