- Department of Psychiatry, Medical University of Gdansk, Gdansk, Poland
There is a growing evidence for the rapid and robust antidepressive effect of ketamine in unipolar and bipolar treatment resistant depression although evidence for the risk of affective switch is still limited. This case presents bipolar I disorder patient with treatment resistant depressive episode experiencing a switch to manic episode with mixed features shortly after receiving eight subanaesthetic doses of oral ketamine as an add-on treatment preceded by 2-day period of manic symptoms.
Background
Bipolar disorder (BPD) is one of the most severe and lethal of all psychiatric disorders (1, 2). There is an unmet need for more effective treatment strategies in bipolar depression (3). Mounting evidence supports effectiveness of single low dose ketamine in treatment resistant cases of bipolar depression (4–6). Data on the effectiveness and risk of polarity switch in bipolar patients treated with ketamine is still insufficient. This case report presents a 28-year-old BP I patient with severe depressive episode experiencing a switch to mania with mixed features after eight doses of oral ketamine. To our knowledge this is the first report of affective switch associated with oral ketamine.
Case Report
A 28-year-old Caucasian female with bipolar I disorder diagnosed 4 years before, was hospitalized in an inpatient psychiatry clinic due to treatment-resistant depressive episode with extensive suicidal thoughts. Mental status has been deteriorating for 4 months before admission. During her illness, the patient was hospitalized five times due to depressive episodes and had two manic episodes without hospitalization. The patient had been treated with ketamine twice, first during previous hospitalization (IV) and second time during described stay (oral).
Previous Hospitalization
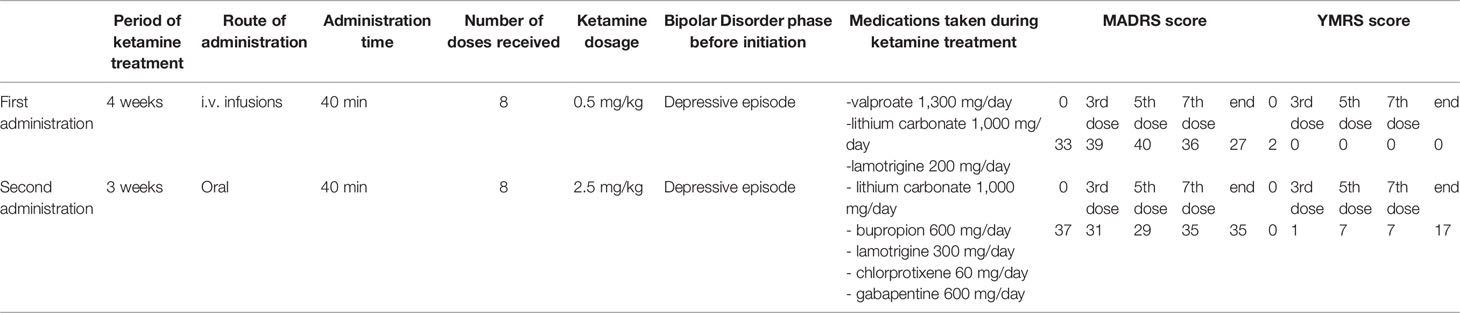
One year before she was hospitalized in the same facility due to severe treatment resistant depressive episode and despite treatment modifications she did not achieve remission, thus she was offered ketamine treatment. A dosage of 0.5mg/kg ketamine hydrochloride intravenous infusion over a period of 40 min was given two times per week for a period of 4 weeks (eight times in total) as add-on treatment to standard of care. After the ketamine administration period, an intermittent mood improvement lasting 1 week has been observed (six points reduction in MADRS score)—no manic symptoms appeared. Further pharmacological modifications were made—after 6-month hospitalization patient achieved partial remission and was discharged on lamotrigine 400 mg/day, lithium carbonate 750 mg/day, clozapine 100 mg/day, and topiramate 400 mg/day.
Present Hospitalization
On admission the patient presented decreased mood, decreased energy, suicidal thoughts, feeling of constant inner tension, difficulties concentrating, withdrawal from social interactions, sleeping difficulties. Somatic causes were excluded after physical examination, neurological examination, and laboratory tests (blood morphology, electrolytes, kidney and liver profile, TSH, FT4, CRP, B12, folate levels, urine test, toxicology) which turned out normal.
During the time since her previous hospitalization, the treatment has been modified in the outpatient care—clozapine has been discontinued, the dose of topiramate has been reduced to 100 mg/day, bupropion 300 mg/day, and chlorprothixene 60 mg/day have been added. Lithium serum level was 0,87 mmol/L on dose of 1,000 mg/day. In the EEG record slow basic activity and slow waves in the front-temporo-parietal region on the left side were described; the MRI scan did not reveal any pathological changes. The pharmacological changes listed above did not cause any improvement; for this reason the patient was qualified for readministration of ketamine, this time in oral form.
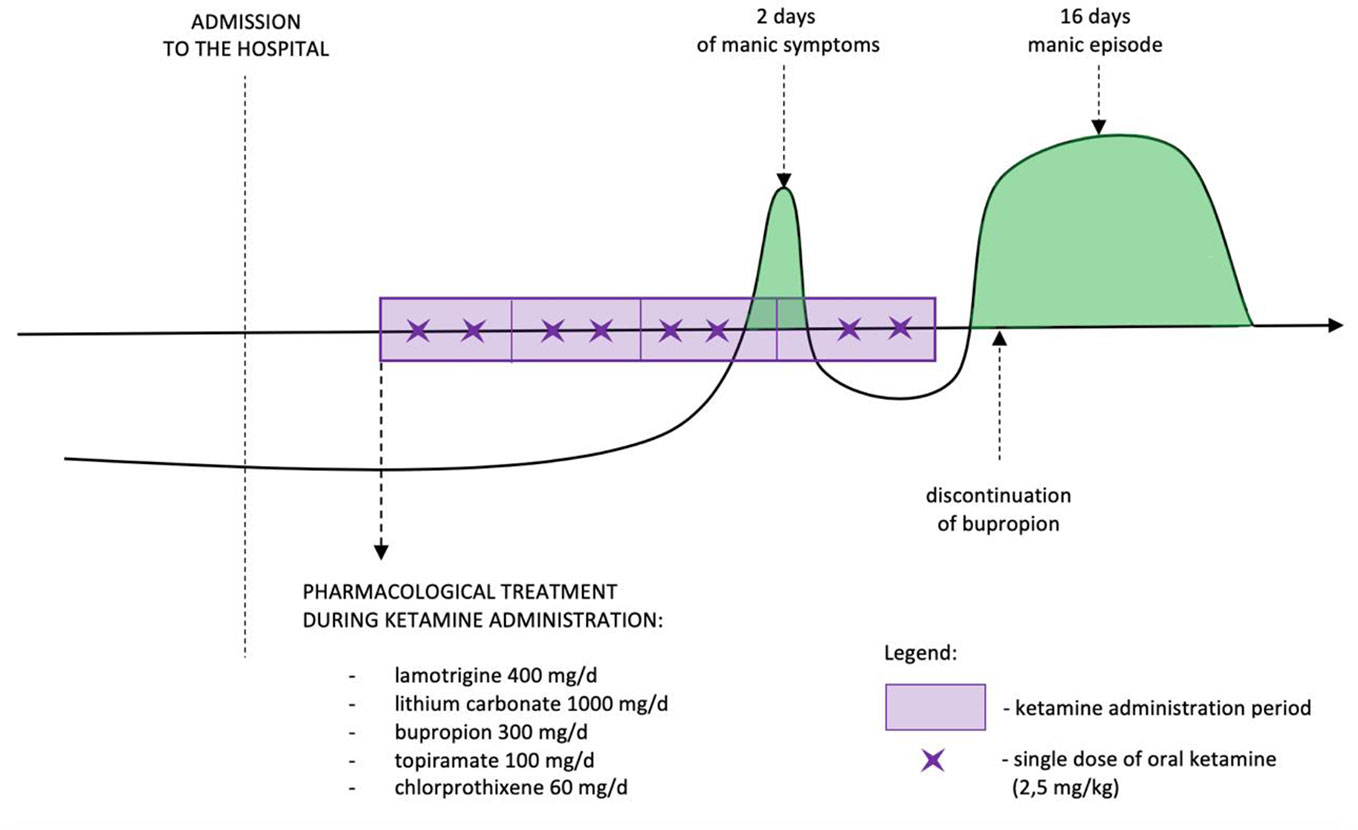
The dose of ketamine administered orally was 2.5 mg/kg. During the first 10 min half of the dosage was administered and in the next 30 min the remaining part was sipped. Ketamine was administered twice weekly during 4 weeks. After 2nd week of ketamine administration the daily dose of lithium was reduced due to its increased serum level (1.42 mmol/L) to 750mg/day which resulted in 0.74 mmol/L serum concentration. During the third week of oral ketamine administration period, manic symptoms were observed. The patient presented increased sex drive, elevated psychomotor activity, racing thoughts, irritability, problems falling asleep and total sleep reduction. She spent a significant amount of money on online shopping, cleaned her locker several times, and became talkative. The symptoms were present for 2 days; after that time depressive symptoms returned with additional appetite increase. Thus decision for continuation of ketamine treatment was made. One day after the last dose of ketamine, the patient presented manic symptoms again. She had intermittent periods of elevated mood, her energy was constantly increased, she presented maladaptive affect regulation, decreased need for sleep, increased sex drive, she became talkative, dressed inappropriately, had increased appetite. The patient demonstrated psychomotor agitation with accompanying suicidal thoughts, lasting for 1 week. Depressive symptoms were still present, but manic symptoms clearly dominated. No psychotic symptoms were present. Drug screen was negative. One week after finishing ketamine administration lithium dose was increased again up to 1,000 mg/day reaching serum level 1.11 mmol/L.
The symptoms resolved after a period of 16 days. During first three doses of ketamine administration patient presented sedation, dizziness, and some dissociative symptoms. The symptoms were of moderate intense and resolved during next 40 min after the last sip of oral ketamine. No other significant adverse events were observed. Treatment timeline is presented in Figure 1.
Safety and Psychometric Scales
Both protocols, intravenous and oral, included recording of basic life parameters such as heart rate, arterial pressure, oxygen saturation level, respiratory rate and body temperature measured every 15 min. In both protocols psychometric assessment included: Montgomery Åsberg Depression Rating Scale structured interview (MADRS-Sigma) and Young Mania Rating Scale (YMRS) completed before IV and oral administrations and after 3rd, 5th, and 7th dose as well as 1 week after finishing the treatment.
Oral Ketamine
The oral route of administration is probably the least costly and most acceptable way to administer ketamine in patients with depression. The existing literature on oral ketamine suggests that oral doses should range from 2–3 mg/kg. It is approximately the dose of 0.5 mg/kg IV multiplied by the oral bioavailability correction factor which is 4–5. This factor is a result of high first-pass metabolism (7). It is also the dose most commonly used in the oral ketamine study by Hartberg (8). Nevertheless it is worth noticing that off-label use of oral ketamine is based on very limited research and no dose-ranging study has been published so far. Additionally antidepressive effect and possible adverse effects of norketamine—the main metabolite of ketamine administered orally are presently unknown although no serious adverse events have been reported so far (7).
The case report is a part of the naturalistic ketamine trial, approved by the bioethical regulatory of the institution, NKBBN/172/2017; 172-674/2019, NCT04226963. The patient was informed about potential risks and limitations as well as reasonable expectations of ketamine treatment for depression and consent for treatment and gave an informed consent for the participation in the trial. The previously mentioned cases are presented according to guidelines for disguising case material.
The MADRS and YMRS scores and concomitant medications are summarized in Table 1.
Discussion
Presented case reports polarity switch in bipolar patient treated with eight doses of oral ketamine. Described symptoms reached the International Society for Bipolar Disorders Task Force criteria of a “likely” treatment-emergent affect switch (i.e., two manic symptoms lasting more than 50% of the day for 2 days, and a Young Mania Rating Scale (YMRS) greater than 12) (9).
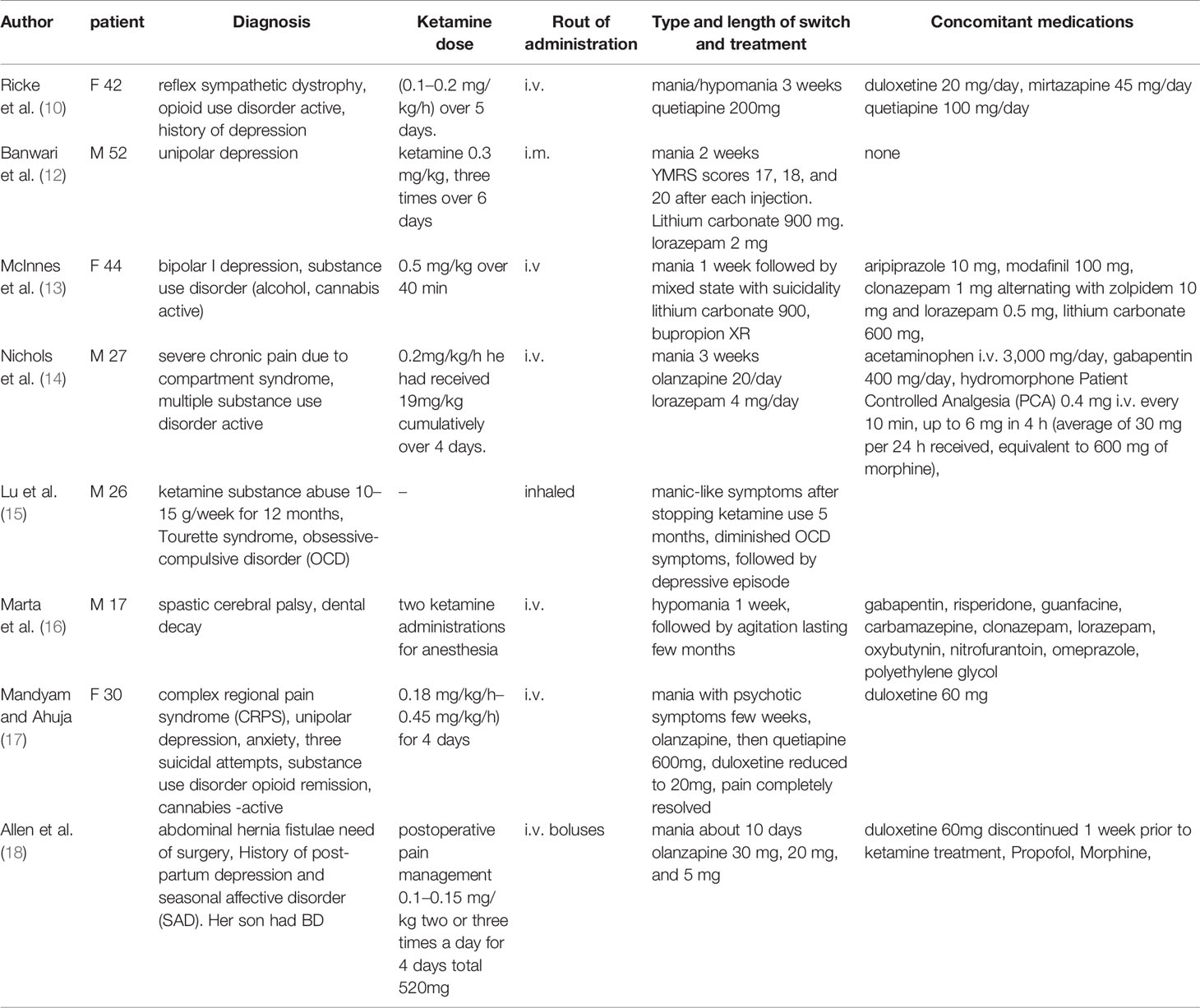
To our knowledge, eight cases of affective switch associated to ketamine have been described so far. None of them however concerned oral administration. The first case report was criticized for the weak evidence of connection to ketamine and the possibility of misdiagnosis (10, 11). Following two cases reported manic symptoms after ketamine infusions for depression. The first case reported a patient with unipolar depression who was administered ketamine 0.3 mg/kg intramuscularly, three times over 6 days, and YMRS scores were 17, 18, and 20 after each injection. In this case, mania emerged in the absence of concomitant medications (12). The second BP I patient after single infusion of 0.5 mg/kg ketamine, developed elevated mood, hypersexuality, and excessive spending despite a therapeutic lithium level (13). The next case describes a patient with active substance use disorder who underwent surgical intervention (14). To reduce postoperative opioid use, ketamine was infused at 0.2 mg/kg/h over 4 days, and expansive affect, sleep-deprived energy enhancement, grandiosity, hypersexuality, and disinhibition occurred. The fifth case of reported ketamine-induced mania described a patient with Tourette syndrome and OCD who abused ketamine by inhaling (15). One case report describes a 17-year-old man with cerebral palsy experiencing hypomanic-like symptoms possibly connected to two ketamine administrations for sedation during dental procedures. Naranjo Adverse Drug Reaction Probability Scale, suggested a probable causal relationship in the described case (16). The next case presents a prolonged manic episode with psychotic symptoms in a young woman with unipolar depression, substance use disorder and CRPS (complex regional pain syndrome) treated with ketamine infusion 10, 15, and 20 mg/h, (her weight was 56 kg). Manic symptoms lasted for about 3 weeks, the pain completely resolved. For months prior to hospitalization she received ketamine treatment for 2 days in similar doses. No change in her mood was observed then (17). The most recent case presents a patient with possible genetic predisposition to BD who developed mania after receiving ketamine as a part of pain management and sedation (18). The above case reports are described in Table 2.
It appears that some specific features predispose to manic switch associated with ketamine treatment. Mentioned cases report mainly subjects with substance use disorder, moreover in half of reported cases monoaminergic antidepressants were used. The patient described in this report was treated with mood stabilizers, but also received bupropion. According to one metanalysis bupropion, previously associated with low risk of polarity switch in bipolar disorder causes it as often as other antidepressants (19). Our observation and previous case reports suggest increased risk of switch associated to ketamine administered together with antidepressants. Although the neurobiological processes causing affective switch are still poorly understood there is evidence suggesting that genes regulating monoaminergic transmission, circadian rhythms and neurotrophic factors like BDNF may be responsible for increased risk of treatment-emergent switch (20). BDNF role in switch process is particularly interesting considering the evidence from animal studies showing increase after ketamine administration (21). Bipolar patients experiencing switches associated to treatment have more severe course of the disorder including cycle acceleration and increased suicide risk (22, 23). They also more often develop substance use disorder (24). It is possible that substance use disorders in bipolar patients predispose to switch, but this question is still to be answered.
Data from three studies of unipolar and bipolar patients with treatment resistant depression, randomized to subanaesthetic ketamine or placebo revealed transient mood elevation in 3 of 44 (7%) patients receiving placebo and 5 of 52 (10%) patients given ketamine, but none met the International Society for Bipolar Disorders Task Force criteria The authors concluded that the data did not support a persistent substance-induced syndrome, since the patients’ mood returned to baseline by the following day and without additional intervention (11).
Taken together it seems that affective switch is a possible complication of ketamine treatment and the risk might be increased in bipolar patients and patients with substance use disorder. Ketamine administered together with antidepressants likely predisposes to affective switch. It also seems that opioid treatment which is particularly important in case of patients with chronic pain can also increase the risk. Available, although limited data suggest that manic symptoms associated with ketamine can be effectively treated with olanzapine, quetiapine and lithium. We hypothesize that treating treatment resistant bipolar depression with ketamine should be accompanied by mood stabilizers and antidepressant should be avoided during this time. It also seems reasonable to conduct the drug screen before starting ketamine treatment. It is still unknown if the route of ketamine administration the level of ketamine metabolite—norketamine can be involved in the risk of affective switch. More studies are needed in order to discover neurobiological processes causing this phenomenon.
Limitations
The case report does not reflect the wider population nor a causative relationship, and thus, replication in a proof of concept study is warranted in a larger population. Longer follow-up might be warranted.
Conclusion
This case report suggests that polarity switch should be considered as a potential side effect while using ketamine for treatment-resistant bipolar depression. There is a significant need for further studies of safety and efficacy of oral and IV ketamine as add-on treatment in bipolar patients.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by Independent Ethics Committee of the Medical University of Gdansk NKBBN/172/2017; 172-674/2019,NCT04226963. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
AW contributed to the manuscript draft and research on the topic. ŁS, MC, and AW contributed to the patient management and elaborated on patients’ medical records JS administered ketamine and evaluated the patient. MG-W directed the naturalistic case registry and elaborated on patients’ records. MW and WC conceptualized the study and corrected the manuscript.
Funding
This paper was granted by the Medical University of Gdańsk, Poland (Grant No. ST-02-0039/07/221).
Conflict of Interest
AW has received research support from Angelini, Biogen, Eli Lilly and Company, Janssen-Cilag, Lundbeck, Polpharma, Sanofi and Valeant. ŁS has received support from Angelini and +Pharma. JS has received research support from Actavis, Celon, Eli Lilly, Minerva Neurosciences, and Sunovion Pharmaceuticals; Speakers bureau: Promed, Teva, Valeant. MC has received support from Angelini, Janssen-Cilag and Adamed. MG-W has received research support from Alkermes, Biogen, Celon, Janssen, KCR, Minerva Neurosciences, Lilly, and Servier. MW has received research support from Alkermes, Auspex Pharmaceuticals, Biogen, Cephalon, Celon, Cortexyme, Eli Lilly, Ferrier, Forest Laboratories, GedeonRichter, GWPharmaceuticals, Janssen, Lundbeck, Orion, Otsuka, and Servier; Speakers bureau: Lundbeck, Servier. WC has received research support from Acadia, Actavis, Alkermes, Allergan, Apodemus, Auspex, Biogen, Bristol-Myers Squibb, Cephalon, Celon, Cortexyme, Eli Lilly, Ferrier, Forest Laboratories, Gedeon Richter, GW Pharmaceuticals, Janssen, KCR, Lundbeck, NIH, NeuroCog, Orion, Otsuka, Sanofi, and Servier; he has served on speakers bureaus for Adamed, Angelini, AstraZeneca, Bristol-Myers Squibb, Celon, GlaxoSmithKline, Janssen, Krka, Lekam, Lundbeck, Novartis, Orion, Pfizer, Polfa Tarchomin, Sanofi, Servier, and Zentiva; and he has served as a consultant for GW Pharmaceuticals, Janssen, KCR, Quintiles, and Roche.
References
1. Nock MK, Hwang I, Sampson N, Kessler RC, Angermeyer M, Beautrais A, et al. Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PloS Med (2009) 6. doi: 10.1371/journal.pmed.1000123
2. Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Arch Gen Psychiatry (2011) 68:1058–64. doi: 10.1001/archgenpsychiatry.2011.113
3. Baldessarini RJ, Vázquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord (2020) 68(1):1. doi: 10.1186/s40345-019-0160-1
4. Zarate CA Jr., Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: A randomized controlled add-on trial. Biol Psychiatry (2012) 71:939–46. doi: 10.1016/j.biopsych.2011.12.010
5. DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry (2010) 71:1605–11. doi: 10.4088/JCP.09m05327blu
6. Grunebaum MF, Ellis SP, Keip JG, Moitra VK, Cooper TB, Marver JE, et al. Ketamine vs. midazolam in bipolar depression with suicidal thoughts. Bipolar Disord (2017) 19(3):176–83. doi: 10.1038/s41386-019-0317-8
7. Andrade C. Oral ketamine for depression, 1: pharmacological considerations and clinical evidence. J Clin Psychiatry (2019) 80(2):19f12820. doi: 10.4088/JCP.19f12820.
8. Hartberg J, Garrett-Walcott S, De Gioannis A. Impact of oral ketamine augmentation on hospital admissions in treatment-resistant depression and PTSD: a retrospective study. Psychopharmacol (Berl) (2018) 235(2):393–8. doi: 10.1007/s00213-017-4786-3
9. Tohen M, Frank E, Bowden CL, Colom F, Ghaemi SN, Yatham LN, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord (2009) 11:453–73. doi: 10.1111/j.1399-5618.2009.00726.x
10. Ricke AK, Snook RJ, Anand A. Induction of prolonged mania during ketamine therapy for reflex sympathetic dystrophy. Biol Psychiatry (2011) 70(4):e13–4. doi: 10.1016/j.biopsych.2011.02.030
11. Niciu MJ, Luckenbaugh DA, Ionescu DF, Mathews DC, Richards EM, Zarate CA Jr. Subanesthetic Dose Ketamine Does Not Induce an Affective Switch in Three Independent Samples of Treatment-Resistant Major Depression. Biol Psychiatry (2013) 15(74(10)):e23–4. doi: 10.1016/j.biopsych.2013.01.038
12. Banwari G, Desai P, Patidar P. Ketamine-induced affective switch in a patient with treatment-resistant depression. Indian J Pharmacol (2015) 47(4):454–5. doi: 10.4103/0253-7613.161277
13. McInnes LA, James-Myers MB, Turner MS. Possible Affective Switch Associated With Intravenous Ketamine Treatment in a Patient With Bipolar I Disorder. Biol Psychiatry (2016) 79(9):e71–2. doi: 10.1016/j.biopsych.2015.07.003
14. Nichols SD, Bulman M, Tisher A, Campbell J. A Case of Possible Iatrogenic Ketamine-Induced Mania in a Patient Being Treated for Postoperative Pain. Psychosomatics (2016) 57(5):543–6. doi: 10.1016/j.psym.2016.06.003
15. Lu YY, Lin CH, Lane HY. Mania following ketamine abuse. Neuropsychiatr Dis Treat (2016) 12:237–8. doi: 10.2147/NDT.S97696
16. Marta CJ, Yudofsky LM, Enenbach MJ. Probable Ketamine-Induced Hypomanic-Like Episode in a Child With Cerebral Palsy. J Neuropsychiat Clin Neurosci (2016) Winter;28(1):e6–7. doi: 10.1176/appi.neuropsych.15040091
17. Mandyam MC, Ahuja NK. Ketamine-Induced Mania During Treatment for Complex Regional Pain Syndrome. Pain Med (2017) 18(10):2040–1. doi: 10.1093/pm/pnx061
18. Allen ND, Rodysill BR, Bostwick JM. A report of affective switching associated with ketamine: The case of ketamine-induced mania is not closed. Bipolar Disord (2019) 21(2):176–8. doi: 10.1111/bdi.12728
19. Li DJ, Tseng PT, Chen YW, Wu CK, Lin PY. Significant Treatment Effect of Bupropion in Patients With Bipolar Disorder but Similar Phase-Shifting Rate as Other Antidepressants: A Meta-Analysis Following the PRISMA Guidelines. Med (Baltimore) (2016) 95(13):e3165. doi: 10.1097/MD.0000000000003165
20. Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA Jr. The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry (2010) 71(11):1488–501. doi: 10.4088/JCP.09r05259gre
21. Réus GZ, Stringari RB, Ribeiro KF, Ferraro AK, Vitto MF, Cesconetto P, et al. Ketamine plus imipramine treatment induces antidepressant- like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav Brain Res (2011) 221(1):166–71. doi: 10.1016/j.bbr.2011.02.024
22. MacKinnon DF, Potash JB, McMahon FJ, Simpson SG, Depaulo JR Jr, Zandi PP. Rapid mood switching and suicidality in familial bipolar disorder. Bipolar Disord (2005) 7(5):441–8. doi: 10.1111/j.1399-5618.2005.00236.x
23. Altshuler LL, Post RM, Leverich GS, Mikalauskas K, Rosoff A, Ackerman L. Antidepressant induced mania and cycle acceleration: a controversy revisited. Am J Psychiatry (1995) 152(8):1130–8. doi: 10.1176/ajp.152.8.1130
Keywords: ketamine, oral and subanaesthetic, affective switch, bipolar I depression, treatment resistance
Citation: Wilkowska A, Szałach Ł, Słupski J, Wielewicka A, Czarnota M, Gałuszko-Węgielnik M, Wiglusz MS and Cubała WJ (2020) Affective Switch Associated With Oral, Low Dose Ketamine Treatment in a Patient With Treatment Resistant Bipolar I Depression. Case Report and Literature Review. Front. Psychiatry 11:516. doi: 10.3389/fpsyt.2020.00516
Received: 23 February 2020; Accepted: 19 May 2020;
Published: 03 June 2020.
Edited by:
Paul Stokes, King’s College London, United KingdomReviewed by:
Mario F. Juruena, King’s College London, United KingdomCasimiro Cabrera Abreu, Queens University, Canada
Copyright © 2020 Wilkowska, Szałach, Słupski, Wielewicka, Czarnota, Gałuszko-Węgielnik, Wiglusz and Cubała. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alina Wilkowska, YWxpLndpbGtvd3NrYUBnbWFpbC5jb20=
 Alina Wilkowska
Alina Wilkowska Łukasz Szałach
Łukasz Szałach Jakub Słupski
Jakub Słupski Małgorzata Czarnota
Małgorzata Czarnota Mariusz S. Wiglusz
Mariusz S. Wiglusz Wiesław J. Cubała
Wiesław J. Cubała