- 1Department of Psychiatry, Faculty of Medicine, University of British Columbia, Vancouver, BC, Canada
- 2British Columbia Children's Hospital Research Institute, Vancouver, BC, Canada
- 3BC Mental Health and Substance Use Services Research Institute, Vancouver, BC, Canada
A commentary on
Neurobiology and Therapeutic Potential of Cyclooxygenase-2 (COX-2) Inhibitors for Inflammation in Neuropsychiatric Disorders
by Sethi R, et al. (2019). Front. Psychiatry. 10:605. doi: 10.3389/fpsyt.2019.00605
Introduction
Enzymes of the cyclooxygenase (COX) family catalyze the metabolism of arachidonic acids to prostanoids. In the central nervous system (CNS), COX-1 is constitutively expressed by neurons, astrocytes, and microglia; COX-2 is expressed by glutamatergic neurons in the cerebral cortex, hippocampus, and amygdala and is inducible in other cell types (1, 2). COX-2 and its products play important physiological role in synaptic plasticity and long-term potentiation but may also contribute to neuropathology by enhancing glutamate excitotoxicity, promoting neuronal cell death, and oxidizing endogenous cannabinoids (3, 4). Some studies suggest upregulation of COX-2 expression in inflammatory and neurodegenerative diseases as well as schizophrenia and bipolar disorder (1). Non-steroidal anti-inflammatory drugs (NSAIDs) inhibit COX enzymes either selectively or non-selectively. In rat models, selective COX-2 inhibitors such as celecoxib inhibit microglial activation (5) and glutamate release (6) and enhance serotonergic and noradrenergic output in the prefrontal cortex (7, 8). Meta-analyses suggest possible benefit of adjunctive COX-2 inhibitors in the treatment of major depressive disorder (MDD) (9) and first-episode psychosis (10, 11); the general role of immunomodulation in these disorders has been recently reviewed (12).
Sethi and colleagues provide an important and timely review of pre-clinical and clinical studies investigating the use of COX-2 inhibitors across multiple psychiatric disorders including major depressive disorder, schizophrenia, bipolar affective disorder, autism spectrum disorder (ASD), and obsessive compulsive disorder (OCD). Other than a clinical trial protocol for celecoxib as an adjunct to vortioxetine in depression published in 2018 (13), their review of randomized controlled trials (RCT's) through November 2017 remains up to date 2 years later. In this commentary, we highlight three factors arising from their results that are essential to advancing this research agenda. First, there is a critical need to move beyond schema that use individual markers to characterize peripheral “pro-” and “anti-” inflammatory states, as well as M1/M2 microglial polarization. This model has the potential to vastly oversimplify the role of innate immunity in brain homeostasis and to limit biomarker discovery efforts. Second, COX inhibitors have direct and indirect effects on the functions of both neurons and glia. COX selectivity as well as COX-independent mechanisms of action vary among NSAIDs. More work is needed to determine which drugs of this class are the best candidates for adjunctive therapy targeting specific neuropsychiatric symptoms. Finally, childhood-onset neuropsychiatric symptoms represent important targets for early intervention with low-risk therapies, but there is little evidence to inform their use. Figure 1 outlines the potential role of COX signalling in psychiatric disorders and related research priorities.
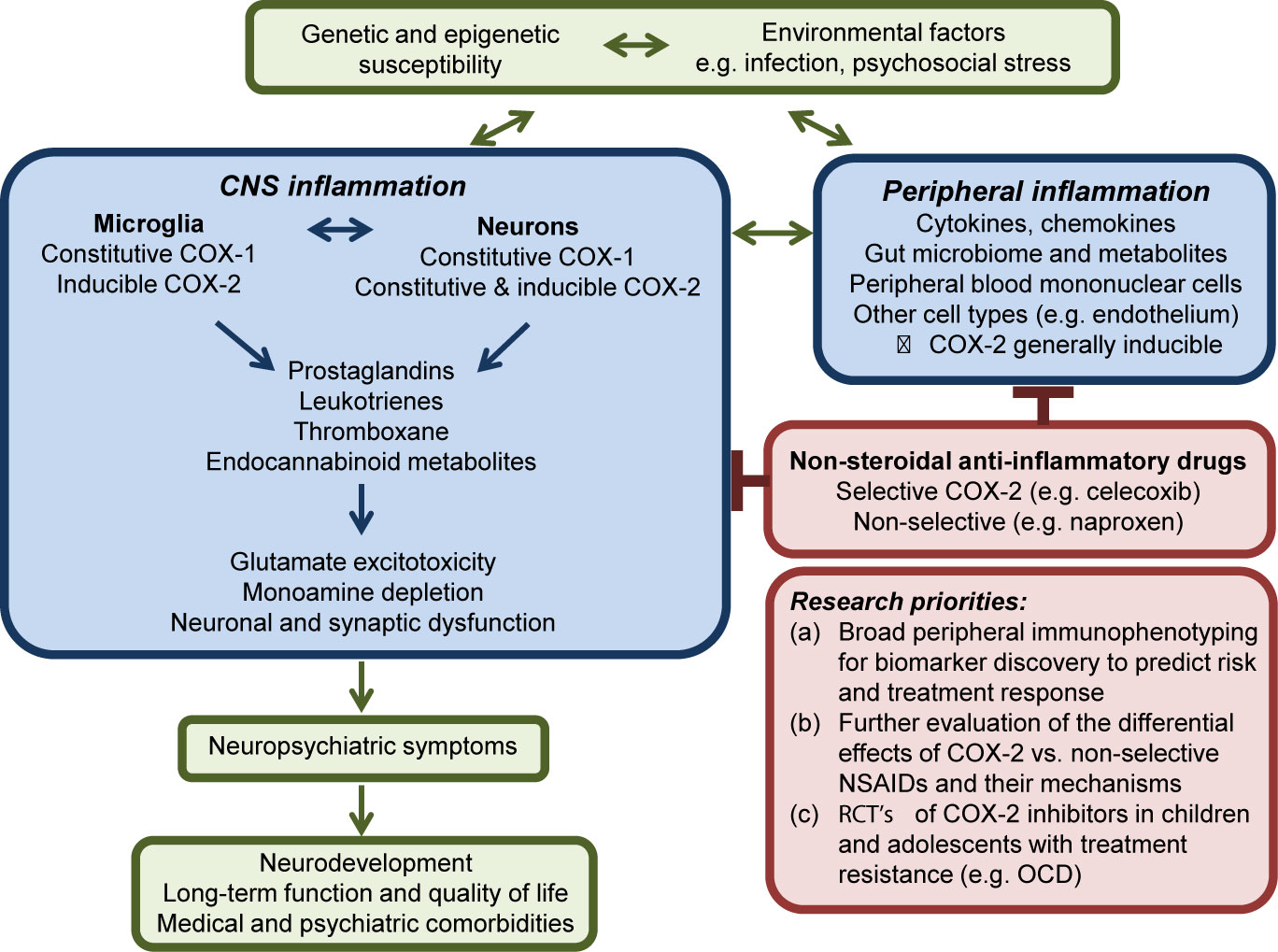
Figure 1 Simplified schematic overview of COX activity in the central nervous system in psychiatric disorders. The inflammatory response may represent one mechanism by which environmental factors including psychosocial stress contribute to the development or perpetuation of neuropsychiatric symptoms in individuals with underlying genetic susceptibility. While typically inducible in other cell types, COX-2 is expressed constitutively at post-synaptic membranes by groups of neurons in the prefrontal cortex, hippocampus, and amygdala. Release of prostanoids that act on pre-synaptic receptors may contribute to dysfunctional synaptic remodeling, altered calcium homeostasis, glutamate excitotoxicity, and neuronal cell death. Research priorities are further described in the text.
Measuring Inflammation in the Brain and Periphery: Beyond Polarization
The identification of immune-related bio-signatures will ideally assist in predicting risk of disease, prognosis, and response to therapy (14). Sethi et al. describe elevated pro-inflammatory markers as “consistently associated with neuropsychiatric symptoms.” While promising studies have begun to identify subpopulations of patients with MDD likely to respond to anti-inflammatory therapy (15), results of immune phenotyping in other disorders have been variable. A recent systematic review found that meta-analyses for MDD, ASD, bipolar disorder, and schizophrenia have consistently reported changes in only 16, 7, 8, and 7 individual inflammation-related factors in peripheral blood, respectively (16). The single meta-analysis of immune phenotyping studies in OCD was filtered out because of insufficient statistical power (16). Longitudinal data were lacking and state versus trait markers difficult to distinguish, markers were restricted to a few per study based on a biased candidate gene/cytokine approach, and the contribution of confounding factors—including childhood adversity, diet, and smoking—was potentially significant (16). Ultimately, longitudinal clinical characterization combined with a broad approach to immune phenotyping—as employed in a recent analysis of microarray data in MDD (17)—is likely a higher-yield approach both for biomarker discovery and for improving our understanding of how peripheral inflammation reflects or perpetuates psychiatric symptoms.
Sethi et al. also provide a common yet limiting perspective on microglial activation states. The disadvantages of the M1/M2 conceptualization of macrophage (18) and microglial (19) polarity in vivo have been previously discussed. As an in vitro construct that relies on stimulating cultured cells with a defined set of factors (20), its application to in vivo conditions is generally limited (21). Moreover, so-called M1 and M2 gene signatures often coexist in complex mixed phenotypes; the dichotomous paradigm is not supported by transcriptional profiling of human macrophages or monocytes activated by diverse ligands (22). Emerging evidence suggests that microglial subtype categorization should consider both their environment-dependent plasticity and subtypes with inherent functional specificity (23). Technologies that can assist in these efforts include two-photon imaging, whole-genome transcriptomic and epigenomic analyses at the cellular level, mass cytometry, and high-content experimental models (19).
Evidence for activation of microglia in patients with psychiatric disorders does not appear to be specific for any particular diagnostic category. Post-mortem characterization of microglia together with evaluation of translocator protein positron emission tomography imaging in patients with MDD suggest that the severity of illness—marked by limited response to traditional medications and increased suicidality—rather than the presence of the disorder itself is associated with altered microglial phenotypes (24, 25). This has led to the hypothesis that inflammation in the CNS primarily reflects psychological stress (26). COX inhibition may therefore be most effective for individuals with severe pathology of diverse etiologies, and when employed at the earliest clinical stage possible may alter the path toward chronic aberrant innate immune activity.
Diverse Non-Steroidal Anti-Inflammatory Drug Mechanisms of Action: The Specific Drug Matters
While all NSAIDS have anti-inflammatory, antipyretic, and analgesic properties attributable to prostaglandin inhibition, they vary with respect to COX selectivity (27) and likely with respect to COX-independent mechanisms (28–30). In the CNS, modulation of glutamate, serotonin, norepinephrine, and endocannabinoid signaling has been demonstrated for COX-2 inhibitors, while the role of non-selective NSAIDs in neurotransmitter function is less clear (3, 4, 6–8). NSAID use has also been associated with distinct gut microbial populations (31), an additional mechanism by which this class of drugs could affect neural development, cognition, and behaviour (32).
Few RCT's have evaluated non-selective NSAIDs in primary psychiatric disorders, although the literature includes a negative RCT of naproxen in geriatric depression (33) and a study of adjuvant aspirin in schizophrenia suggesting some benefit (34). Clinical practice guidelines for the treatment of children with pediatric acute-onset neuropsychiatric syndrome (PANS) and pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection (PANDAS) recommend the use of naproxen before celecoxib because of its “greater potency” (35), despite clinical studies showing benefit of adjunctive celecoxib in OCD (36, 37) and pre-clinical data demonstrating celecoxib-mediated enhancement of the serotonergic effects of fluoxetine in a rat model of anxiety (38). Moreover, observational studies have focused on NSAIDs as a class in children with PANS/PANDAS (39, 40). Given the significance of different COX isoforms and their unknown relative “potencies” in the CNS, careful attention must be given to selection and evaluation of specific NSAIDs.
Need for Pediatric Studies and Early Intervention
A recent Danish population-based study suggested that environmental factors related to infection and inflammation are associated with the development of multiple mental disorders in children (41), adding to growing support for the link between immune activity and psychiatric symptoms early in life. However, there is currently little evidence to inform the use of adjunctive anti-inflammatory agents in children and adolescents with psychiatric disorders. Studies of peripheral inflammatory markers in this population have been equivocal, largely limited by similar methodological factors as adult studies (42, 43).
Early-life stress is more clearly associated with overt inflammation prior to the development of neuropsychiatric symptoms. For example, childhood trauma is associated with significantly elevated peripheral levels of C-reactive protein, interleukin (IL)-6, tumor necrosis factor-a, and soluble urokinase plasminogen activator receptor (44, 45). Elevated IL-6 in childhood is in turn associated with increased risk of future depressive and psychotic symptoms in adolescence (46, 47). Stress-related epigenetic dysregulation in immune networks represents one mechanism by which childhood experiences may become biologically embedded (48), and a potential target for early intervention. Epigenetic modifications facilitate the phenotypic plasticity of macrophages, are critical to their role in maintenance of tissue homeostasis, and contribute to a form of innate immune “memory” that persists across the lifespan (49, 50).
Randomized controlled trials of COX-2 inhibitors as adjunctive therapies in children with treatment-resistant psychiatric disorders with a potential immune-mediated component may be warranted, beyond the single study of celecoxib in ASD noted by Sethi et al. Reassuring safety data exist for both celecoxib and non-selective NSAIDs, derived from studies of children with juvenile idiopathic arthritis (51) and familial adenomatous polyposis (52). This approach may be particularly relevant in OCD given that the majority of affected individuals experience disease onset in childhood or adolescence, with a persistence rate of approximately 40% (53). Clinical practice guidelines suggesting the use of celecoxib as a third-line agent in adults with OCD (54) and naproxen or celecoxib in children with PANS/PANDAS (35) provide a further clinical imperative for these studies.
Conclusion
Multiple lines of evidence suggest that aberrant inflammatory processes contribute to the pathogenesis of psychiatric disorders. Altered immune homeostasis may represent the consequence of exposure to environmental factors including psychosocial stress together with cumulative genetic and epigenetic risk. Changes in neuroendocrine regulation, metabolism, gut microbiota, and health behaviours in turn affect peripheral and central immune cell phenotypes. For individuals with the most severe symptoms refractory to traditional treatments, modulation of the innate immune system with COX-2 inhibitors appears to be an attractive—though understudied—therapeutic approach.
In characterizing state and trait markers of disease and identifying appropriate patients for anti-inflammatory treatments, broad immunophenotyping is likely to be essential. Moreover, preclinical studies suggesting effects of COX-2 inhibition on neurotransmitter function would suggest that traditional markers of inflammation in the periphery may not be required for therapeutic effect. The implications of differences in COX selectivity as well as COX-independent effects of individual NSAIDs in the CNS require further study. Finally, stressful events in childhood drive peripheral inflammation and affect neurodevelopment. Given our increasing understanding of innate immune memory and its potential role in neurodevelopment and neurodegeneration, the likely bidirectional relationship between inflammation and psychiatric symptoms, and the known benefits of early intervention, treatment trials of COX-2 inhibitors in carefully-selected pediatric populations are warranted.
Author Contributions
CW-R conceived of and drafted the article. SES provided critical feedback and reviewed the final version to be submitted.
Funding
Work by SES is supported by the Canadian Institutes of Health Research and Michael Smith Foundation for Health Research. CW-R is the recipient of a 2019 International OCD Foundation Young Investigator Award.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Yagami T, Koma H, Yamamoto Y. Pathophysiological Roles of Cyclooxygenases and Prostaglandins in the Central Nervous System. Mol Neurobiol (2016) 53(7):4754–71. doi: 10.1007/s12035-015-9355-3
2. Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A (1996) 93(6):2317–21. doi: 10.1073/pnas.93.6.2317
3. Yang H, Chen C. Cyclooxygenase-2 in synaptic signaling. Curr Pharm Des (2008) 14(14):1443–51. doi: 10.2174/138161208784480144
4. Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol (2002) 87(6):2851–7. doi: 10.1152/jn.2002.87.6.2851
5. Kaizaki A, Tien LT, Pang Y, Cai Z, Tanaka S, Numazawa S, et al. Celecoxib reduces brain dopaminergic neuronaldysfunction, and improves sensorimotor behavioral performance in neonatal rats exposed to systemic lipopolysaccharide. J Neuroinflammation (2013) 10:45. doi: 10.1186/1742-2094-10-45
6. Lin T-Y, Lu C-W, Wang C-C, Huang SK, Wang S-J. Cyclooxygenase 2 inhibitor celecoxib inhibits glutamate release by attenuating the PGE2/EP2 pathway in rat cerebral cortex endings. J Pharmacol Exp Ther (2014) 351(1):134–45. doi: 10.1124/jpet.114.217372
7. Sandrini M, Vitale G, Pini LA. Effect of rofecoxib on nociception and the serotonin system in the rat brain. Inflammation Res (2002) 51(3):154–9. doi: 10.1007/PL00000287
8. Johansson D, Falk A, Marcus MM, Svensson TH. Celecoxib enhances the effect of reboxetine and fluoxetine on cortical noradrenaline and serotonin output in the rat. Prog Neuropsychopharmacol Biol Psychiatry (2012) 39(1):143–8. doi: 10.1016/j.pnpbp.2012.06.003
9. Faridhosseini F, Sadeghi R, Farid L, Pourgholami M. Celecoxib: a new augmentation strategy for depressive mood episodes. A systematic review and meta-analysis of randomized placebo-controlled trials. Hum Psychopharmacol (2014) 29(3):216–23. doi: 10.1002/hup.2401
10. Marini S, De Berardis D, Vellante F, Santacroce R, Orsolini L, Valchera A, et al. Celecoxib Adjunctive Treatment to Antipsychotics in Schizophrenia: A Review of Randomized Clinical Add-On Trials. Mediators Inflammation (2016) 2016(1):3476240–8. doi: 10.1155/2016/3476240
11. Zheng W, Cai D-B, Yang X-H, Ungvari GS, Ng CH, Müller N, et al. Adjunctive celecoxib for schizophrenia: A meta-analysis of randomized, double-blind, placebo-controlled trials. J Psychiatr Res (2017) 92:139–46. doi: 10.1016/j.jpsychires.2017.04.004
12. Muller N. COX-2 Inhibitors, Aspirin, and Other Potential Anti-Inflammatory Treatments for Psychiatric Disorders. Front Psychiatry (2019) 10:375. doi: 10.3389/fpsyt.2019.00375
13. Fourrier C, Sampson E, Mills NT, Baune BT. Anti-inflammatory treatment of depression: study protocol for a randomised controlled trial of vortioxetine augmented with celecoxib or placebo. Trials (2018) 19(1):447. doi: 10.1186/s13063-018-2829-7
14. Leboyer M, Berk M, Yolken RH, Tamouza R, Kupfer D, Groc L. Immuno-psychiatry: an agenda for clinical practice and innovative research. BMC Med (2016) 14(1):173. doi: 10.1186/s12916-016-0712-5
15. Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, et al. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry (2019) 214(1):11–9. doi: 10.1192/bjp.2018.66
16. Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry (2019) 9(1):233. doi: 10.1038/s41398-019-0570-y
17. Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S, et al. Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol Psychiatry (2018) 83(1):70–80. doi: 10.1016/j.biopsych.2017.01.021
18. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep (2014) 6:13. doi: 10.12703/P6-13
19. Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci (2016) 19(8):987–91. doi: 10.1038/nn.4338
20. Mills CD, Kincaid K, Alt JM, Heilman MJ. Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol (2000) 164(12):6166–73. doi: 10.4049/jimmunol.164.12.6166
21. Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol (2019) 10:1084. doi: 10.3389/fimmu.2019.01084
22. Nahrendorf M, Swirski FK. Abandoning M1/M2 for a Network Model of Macrophage Function. Circ Res (2016) 119(3):414–7. doi: 10.1161/CIRCRESAHA.116.309194
23. Stratoulias V, Venero JL, Tremblay ME, Joseph B. Microglial subtypes: diversity within the microglial community. EMBO J (2019) 38(17):e101997. doi: 10.15252/embj.2019101997
24. Holmes SE, Hinz R, Conen S, Gregory CJ, Matthews JC, Anton-Rodriguez JM, et al. Elevated Translocator Protein in Anterior Cingulate in Major Depression and a Role for Inflammation in Suicidal Thinking: A Positron Emission Tomography Study. Biol Psychiatry (2018) 83(1):61–9. doi: 10.1016/j.biopsych.2017.08.005
25. Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry (2015) 72(3):268–75. doi: 10.1001/jamapsychiatry.2014.2427
26. Mondelli V, Vernon AC, Turkheimer F, Dazzan P, Pariante CM. Brain microglia in psychiatric disorders. Lancet Psychiatry (2017) 4(7):563–72. doi: 10.1016/S2215-0366(17)30101-3
27. Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin (2010) 26(7):1715–31. doi: 10.1185/03007995.2010.486301
28. Ajmone-Cat MA, Bernardo A, Greco A, Minghetti L. Non-Steroidal Anti-Inflammatory Drugs and Brain Inflammation: Effects on Microglial Functions. Pharmaceuticals (Basel) (2010) 3(6):1949–65. doi: 10.3390/ph3061949
29. Diaz-Gonzalez F, Sanchez-Madrid F. NSAIDs: learning new tricks from old drugs. Eur J Immunol (2015) 45(3):679–86. doi: 10.1002/eji.201445222
30. Calvo-Rodriguez M, Nunez L, Villalobos C. Non-steroidal anti-inflammatory drugs (NSAIDs) and neuroprotection in the elderly: a view from the mitochondria. Neural Regener Res (2015) 10(9):1371–2. doi: 10.4103/1673-5374.165219
31. Rogers MAM, Aronoff DM. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect (2016) 22(2):178 e1–e9. doi: 10.1016/j.cmi.2015.10.003
32. Diaz Heijtz R. Fetal, neonatal, and infant microbiome: Perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med (2016) 21(6):410–7. doi: 10.1016/j.siny.2016.04.012
33. Fields C, Drye L, Vaidya V, Lyketsos C, Group AR. Celecoxib or naproxen treatment does not benefit depressive symptoms in persons age 70 and older: findings from a randomized controlled trial. Am J Geriatr Psychiatry (2012) 20(6):505–13. doi: 10.1097/JGP.0b013e318227f4da
34. Laan W, Grobbee DE, Selten JP, Heijnen CJ, Kahn RS, Burger H. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry (2010) 71(5):520–7. doi: 10.4088/JCP.09m05117yel
35. Frankovich J, Swedo S, Murphy T, Dale RC, Agaliu D, Williams K, et al. Clinical Management of Pediatric Acute-Onset Neuropsychiatric Syndrome: Part II—Use of Immunomodulatory Therapies. J Child Adolesc Psychopharmacol (2017) 27(7):574–93. doi: 10.1089/cap.2016.0148
36. Shalbafan M, Mohammadinejad P, Shariat SV, Alavi K, Zeinoddini A, Salehi M, et al. Celecoxib as an Adjuvant to Fluvoxamine in Moderate to Severe Obsessive-compulsive Disorder: A Double-blind, Placebo-controlled, Randomized Trial. Pharmacopsychiatry (2015) 48(4-5):136–40. doi: 10.1055/s-0035-1549929
37. Sayyah M, Boostani H, Pakseresht S, Malayeri A. A preliminary randomized double-blind clinical trial on the efficacy of celecoxib as an adjunct in the treatment of obsessive-compulsive disorder. Psychiatry Res (2011) 189(3):403–6. doi: 10.1016/j.psychres.2011.01.019
38. Garabadu D, Kumar V. Celecoxib potentiates the antianxiety and anticompulsive-like activity of fluoxetine against chronic unpredictable mild stress in experimental animals. Behav Pharmacol (2019) 30(2 and 3-Spec Issue):251–9. doi: 10.1097/FBP.0000000000000468
39. Brown KD, Farmer C, Freeman GM, Spartz EJ, Farhadian B, Thienemann M, et al. Effect of Early and Prophylactic Nonsteroidal Anti-Inflammatory Drugs on Flare Duration in Pediatric Acute-Onset Neuropsychiatric Syndrome: An Observational Study of Patients Followed by an Academic Community-Based Pediatric Acute-Onset Neuropsychiatric Syndrome Clinic. J Child Adolesc Psychopharmacol (2017) 27(7):619–28. doi: 10.1089/cap.2016.0193
40. Spartz EJ, Freeman GM, Brown K, Farhadian B, Thienemann M, Frankovich J. Course of Neuropsychiatric Symptoms After Introduction and Removal of Nonsteroidal Anti-Inflammatory Drugs: A Pediatric Observational Study. J Child Adolesc Psychopharmacol (2017) 27(7):652–9. doi: 10.1089/cap.2016.0179
41. Kohler-Forsberg O, Petersen L, Gasse C, Mortensen PB, Dalsgaard S, Yolken RH, et al. A Nationwide Study in Denmark of the Association Between Treated Infections and the Subsequent Risk of Treated Mental Disorders in Children and Adolescents. JAMA Psychiatry (2019) 76(3):271–9. doi: 10.1001/jamapsychiatry.2018.3428
42. Milkowska P, Popko K, Demkow U, Wolanczyk T. Pro-Inflammatory Cytokines in Psychiatric Disorders in Children and Adolescents: A Review. Adv Exp Med Biol (2017) 1021:73–80. doi: 10.1007/5584_2017_24
43. Mitchell RH, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. J Am Acad Child Adolesc Psychiatry (2014) 53(3):274–96. doi: 10.1016/j.jaac.2013.11.013
44. Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry (2016) 21(5):642–9. doi: 10.1038/mp.2015.67
45. Rasmussen LJH, Moffitt TE, Arseneault L, Danese A, Eugen-Olsen J, Fisher HL, et al. Association of Adverse Experiences and Exposure to Violence in Childhood and Adolescence With Inflammatory Burden in Young People. JAMA Pediatr (2020) 174(1):38–47. doi: 10.1001/jamapediatrics.2019.3875
46. Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry (2014) 71(10):1121–8. doi: 10.1001/jamapsychiatry.2014.1332
47. Flouri E, Francesconi M, Midouhas E, Lewis G. Prenatal and childhood adverse life events, inflammation and depressive symptoms across adolescence. J Affect Disord (2020) 260:577–82. doi: 10.1016/j.jad.2019.09.024
48. Pfeiffer JR, Mutesa L, Uddin M. Traumatic Stress Epigenetics. Curr Behav Neurosci Rep (2018) 5(1):81–93. doi: 10.1007/s40473-018-0143-z
49. Chen S, Yang J, Wei Y, Wei X. Epigenetic regulation of macrophages: from homeostasis maintenance to host defense. Cell Mol Immunol (2019) 17(1):36–49. doi: 10.1038/s41423-019-0315-0
50. Salani F, Sterbini V, Sacchinelli E, Garramone M, Bossu P. Is Innate Memory a Double-Edge Sword in Alzheimer's Disease? A Reappraisal of New Concepts and Old Data. Front Immunol (2019) 10:1768. doi: 10.3389/fimmu.2019.01768
51. Sobel RE, Lovell DJ, Brunner HI, Weiss JE, Morris PW, Gottlieb BS, et al. Safety of celecoxib and nonselective nonsteroidal anti-inflammatory drugs in juvenile idiopathic arthritis: results of the Phase 4 registry. Pediatr Rheumatol Online J (2014) 12:29. doi: 10.1186/1546-0096-12-29
52. Lynch PM, Ayers GD, Hawk E, Richmond E, Eagle C, Woloj M, et al. The safety and efficacy of celecoxib in children with familial adenomatous polyposis. Am J Gastroenterol (2010) 105(6):1437–43. doi: 10.1038/ajg.2009.758
53. Stewart SE, Geller DA, Jenike M, Pauls D, Shaw D, Mullin B, et al. Long-term outcome of pediatric obsessive-compulsive disorder: a meta-analysis and qualitative review of the literature. Acta Psychiatr Scand (2004) 110(1):4–13. doi: 10.1111/j.1600-0447.2004.00302.x
Keywords: innate immunity, inflammation, obsessive compulsive disorder, child and adolescent psychiatric disorders, COX-2 inhibitor, immunopsychiatry, prostanoid, non-steroidal anti-inflammatory drug
Citation: Westwell-Roper C and Stewart SE (2020) Commentary: Neurobiology and Therapeutic Potential of Cyclooxygenase-2 (COX-2) Inhibitors for Inflammation in Neuropsychiatric Disorders. Front. Psychiatry 11:264. doi: 10.3389/fpsyt.2020.00264
Received: 28 November 2019; Accepted: 18 March 2020;
Published: 22 April 2020.
Edited by:
Ming D. Li, Zhejiang University, ChinaReviewed by:
Akira Monji, Saga University, JapanCopyright © 2020 Westwell-Roper and Stewart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clara Westwell-Roper, Y3dlc3R3ZWxscm9wZXJAYmNjaHIudWJjLmNh
 Clara Westwell-Roper
Clara Westwell-Roper S. Evelyn Stewart1,2,3
S. Evelyn Stewart1,2,3