- 1Fujian Key Laboratory on Conservation and Sustainable Utilization of Marine Biodiversity, Fuzhou Institute of Oceanography, Minjiang University, Fuzhou, China
- 2College of Resource and Environmental Science, Fujian Agriculture and Forestry University, Fuzhou, China
- 3Technology Innovation Center for Monitoringand Restoration Engineering of Ecological Fragile Zonein Southeast China, Ministry of Natural Resources, Fuzhou, China
- 4College of Environment and Safety Engineering, Fuzhou University, Fuzhou, China
As an important mangrove species, Kandelia obovata plays an irreplaceable role in the coastal ecosystem. However, due to a lack of genetic technology, there is limited research on its functional genes. As such, establishing an efficient and rapid functional verification system is particularly important. In this study,tobacco rattle virus (TRV) and the phytoene desaturase gene KoPDS were used as the vector and target gene, respectively, to establish a virus-induced gene silencing system (VIGS) in K. obovata. Besides, the system was also used to verify the role of a Chlorophyll a/b binding protein (Cab) gene KoCAB in leaf carbon sequestration of K. obovata.
RNA-Seq and qRT-PCR showed that the highest gene-silencing efficiency could reach 90% after 10 days of inoculation and maintain above 80% after 15 days, which was achieved with resuspension buffer at pH 5.8 and Agrobacterium culture at OD600 of 0.4-0.6. Taken together, the TRV-mediated VIGS system established herein is the first genetic analysis tool for mangroves, which may greatly impel functional genomics studies in mangrove plants.
Introduction
Kandelia obovata is the dominant mangrove species along the southeast coast of China and is tolerant to a variety of complex environments such as high organic matter, low oxygen, and tidal flooding in the sea-land ecotone (Senger et al., 2021; Jiang et al., 2022). In addition, it is an integral part of blue carbon ecosystems, which can absorb large quantities of carbon dioxide from the atmosphere and alleviate the negative impact of global climate change. Li LF. et al. (2015) showed that the daily net carbon sequestration of K.obovata was 13.83 g·m-2·d-1 in the Zhanjiang mangrove forest area, which was significantly more than that of Avicennia marina, Aegiceras corniculatum, Bruguiera gymnorrhiza, and Rhizophora stylosa. Traditional studies have shown that a wide range of factors affect the photosynthetic efficiency of K.obovata; however, the primary regulatory mechanism remains unclear.
The chlorophyll a/b binding proteins (Cab) are a class of membrane proteins that bind to pigment molecules in the plant photosystem. They are encoded by nuclear genes and are widely involved in light energy capture, energy transfer, and respond to various stresses. Previous studies revealed that K.obovata chlorophyll a/b binding protein KoCab (OR479794) is significantly differentially expressed during photosynthesis, but its exact function is still unknown. Analysis of the functions of KoCab underlying photosynthetic carbon sequestration is critical for enhancing our understanding of the special physiological and ecological characteristics of mangrove plants. It can also provide guidance for further improving the carbon sequestration efficiency of mangrove plants during mangrove restoration.
Although it is currently possible to characterize the function of mangrove genes, to some extent, using the model plants such as Arabidopsis thaliana (Hong et al., 2018) and rice (Prashanth et al., 2008), results from heterologous transformations may not faithfully reveal the biological function of the target gene in mangrove plants. Moreover, K. obovata is rich in secondary metabolites such as tannin, which can cause leaf hypersensitivity when transformation systems of common model plants are used. As such, researches on mangrove plants at the molecular level lag far behind those of other model plants.
Virus-induced gene silencing (VIGS) is a powerful reverse genetics technology that can be used for rapid analysis of gene function. To verify the function of target genes, VIGS uses virus vectors to carry cDNA fragments from target genes to induce gene silence and related phenotypic mutation in plant tissues (Li et al., 2021). Virus vectors currently used for VIGS mainly include DNA virus vectors, RNA virus vectors, and satellite virus vectors. Tobacco rattle virus (TRV) is a soil-borne RNA virus and belongs to the genus Tobravirus (Senthil-Kumar and Mysore, 2014). Due to the wide host range, high silencing efficiency, and mild virus symptoms associated with rapid propagation in plant meristems, TRV has become the preferred vector for most VIGS systems (Sui et al., 2018). At present, many studies have reported the use of TRV-VIGS technology in gene function research for many non-model plants, such as the carotenoid cleavage dioxygenase gene of peach (Bai et al., 2015), the phytoene desaturase (PDS) gene of strawberry (Tian et al., 2015), the chalcone isomerase gene of apple (Wang, 2014), the P450 monooxygenase gene of cherry (Li Q. et al., 2015), the flavonoid-3-O-glucosyltransferase gene of Litchi chinensis (Li X. et al., 2015), the acyltransferase 3 gene AT3 of Capsicum annuum (Arce-Rodríguez et al., 2015), the actin depolymerizing factor gene TaADF7 of wheat (Chen et al., 2022), the neomycin phosphotransferase gene in cotton (Wu et al., 2021), the methionine sulfoxide reductase gene of eggplant (Zhao et al., 2015), and PDS in the beet genome (Gong et al., 2015). However, whether TRV-VIGS technology is suitable for mangrove plants is unclear, as is how a TRV-VIGS silencing system for mangrove plants should be constructed.
The phytoene desaturase gene is a rate-limiting enzyme involved in carotenoid synthesis in plants, and PDS silencing can generate obvious chlorosis of green plant tissues which is easy for detection (Zeng et al., 2019). Therefore, in this study, the TRV virus and the K. obovata phytoene desaturase gene KoPDS were used respectively as the virus vector and the marker gene to establish a VIGS system in K. obovata. We also used the established system, together with a comparative transcriptomics analysis, to verify the function of the Cab gene in K. obovata. The establishment of the VIGS system in K. obovata can promote functional verification of genes that are related to important traits of mangroves and provide technical support for later high-throughput functional genomics analysis.
Materials and methods
Plant materials
Mature K. obovata propagules were collected from the Zhangjiang estuary in Fujian Province, China (23°53′45″-23°56′00″N, 117°24′07″-117°30′00″E). The propagules had been cultivated in pots containing sand and irrigated with Hoagland’s nutrient solution for one year. The plants were then placed in a greenhouse under controlled conditions [1000 µmol photons m-2s-1, 28/25°C (day/night)] for cultivation and experiments. Uniform size robust seedlings (1-year cultivation) were selected and the 3rd–5th mature leaves from the top were used for studies.
Vector construction
The extraction of total RNA from the samples was carried out based on the manufacturer’s instructions for the Easy Spin plus complex plant RNA kit. cDNA synthesis was performed using a PrimeScript RT reagent kit with gDNA Eraser (Takara, Dalian, China). The gene fragments (KoPDS and KoCab) were amplified using specific primers (KoPDS-OF, KoPDS-OR, KoCab-OF, and KoCab-OR) and inserted into pTRV2 to generate pTRV2-KoPDS and pTRV2-KoCab, respectively. The primer sequences used for vector construction are listed in Supplementary Table 1.
RNAi transient assay using a virus-induced gene silencing system
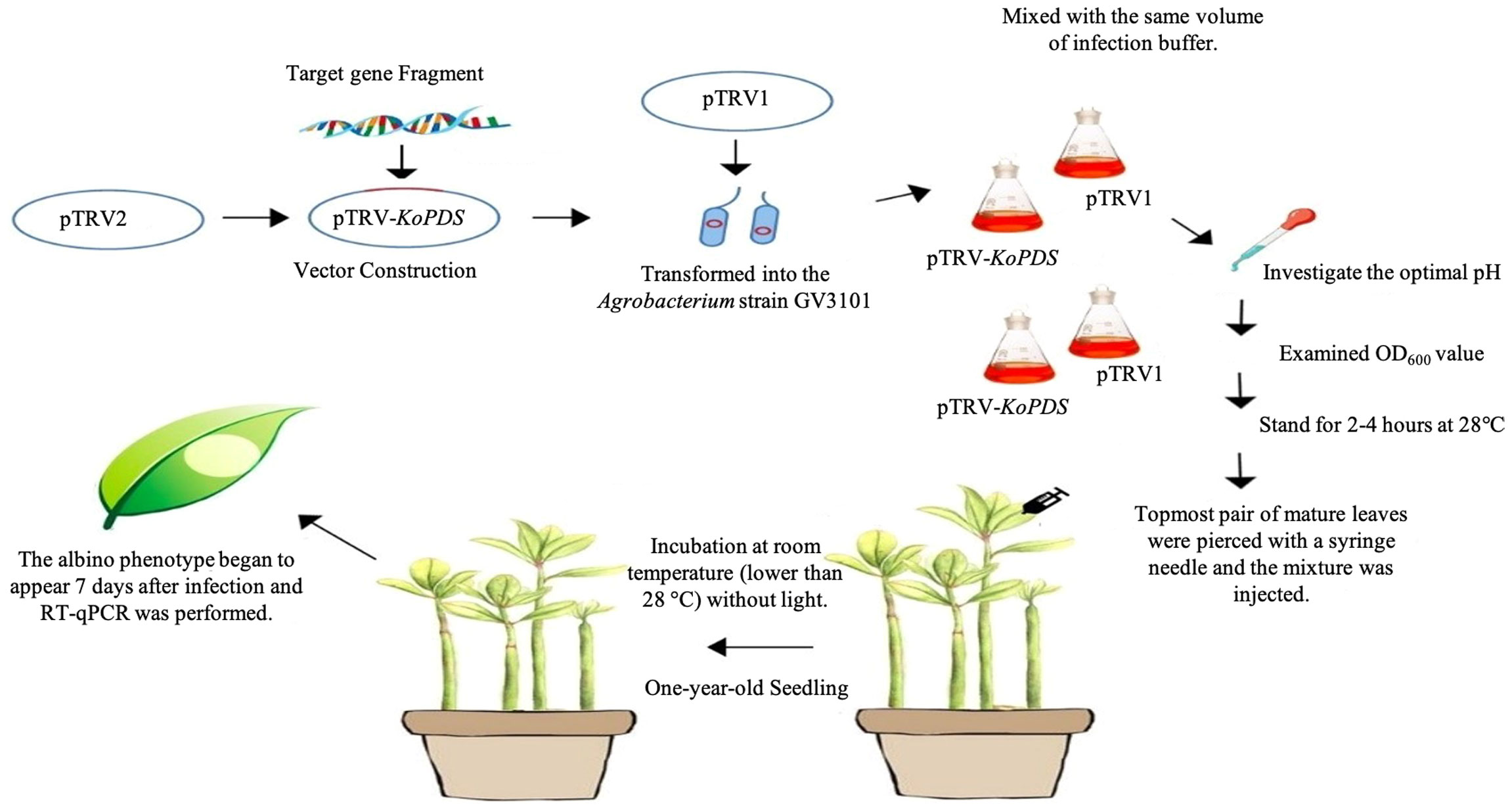
After the vector sequence was verified by sequencing, pTRV1, pTRV2, and pTRV2-KoPDS were individually transformed into the Agrobacterium strain GV3101. Seeding solutions were prepared by overnight incubation of the transformed Agrobacterium in LB medium containing kanamycin (Kan) and rifampicin (Rif) at 28°C with shaking at 250 rpm. The solutions were then diluted in fresh LB (Kan + Rif) medium at a ratio of 1:50 (vol:vol) and were then placed on an incubator shaker and grown to the required concentration as measured by OD600 and then centrifuged at 4,000 rpm for 10 min. Then, pTRV1 and pTRV2-KoPDS (pTRV1 and pTRV2 as a control) were mixed with the same volume of infection buffer, and the infection mixture was allowed to stand for 2-4 hours at 28°C before injection into plants. Infection buffer solution (0.1 mM MES, 0.1 mM MgCl2·6H2O, with 200 µM acetosyringone in 100 mL dd H2O) was adjusted to the indicated pH value (pH 4.5-6.5) with 1M NaOH. For infection, the topmost pair of mature leaves were pierced with a syringe needle and the indicated mixture (pTRV1/pTRV2-KoPDS; pTRV1/pTRV2 as a control) was injected into 1-2 blades of leaves under the meristem via the wound, so that the mixture could completely infect the entire leaf. The leaves used for the three replicates were selected from different mangrove plants, such that no replicates for one experimental condition were taken from the same plant. Phenotype silencing in the plants appeared within 7-15 days after incubation at room temperature (lower than 28°C) with protection from light.
Evaluation of infection effect
To evaluation the infection effect of the TRV, we used the total chlorophyll content of the leaves and effective infection rate as the reference indicator. Chlorophyll was quantitatively determined with the Meter Model SPAD-502 (SPAD). The effective infection rate is the ratio of the leaves with chlorosis symptoms near the infection point to the total number of leaves receiving the same treatment. Based on the results of the previous experiments, the total chlorophyll content and effective infection rate were determined starting at 7 days after infection.
RT-qPCR analysis of KoPDS expression levels
K. obovata leaves infected with TRV for 7, 10, and 15 days were sampled and pooled into three replicates that were immediately frozen in liquid nitrogen and kept at -80°C for use in further experiments. RNA extraction and reverse transcription were performed as described above. qRT-PCR was performed using the Eppendorf Realplex 4 real-time PCR system (Hamburg, Germany) as described previously (Fang et al., 2016). KoPDS-F/R was designed to isolate and clone the KoPDS coding region of the phytoene desaturase gene from K. obovata. The Koactin gene was used as a reference. Expression levels were calculated as described previously (Fang et al., 2016).
Comparative transcriptome to verify the function of the Cab gene in K. obovata
K. obovata leaves infected with the pTRV1+pTRV2 vector or pTRV1+pTRV-KoCab (described above) for 15 days were sampled and were used for transcriptome analysis. Extraction of total RNA, library construction, and RNA-Seq were performed by staff at Beijing BioMarker Technologies (Beijing, China). Sequencing of the cDNA library was carried out on the Illumina HiSeqTM X Ten sequencing platform. RNA-seq was performed in three replicates (Fang et al., 2021). Photosynthetic parameters were measured in an incubation chamber at 25°C with a light intensity of 400 μmol·m-2·s-1. KoCab expression levels were analyzed by RT-qPCR as described above.
Results
Construction of the TRV-VIGS system
An overview of the protocol for Agrobacterium-mediated delivery of TRV1 and TRV2 constructs into K. obovata by syringe inoculation was shown in Figure 1. By using TRV-VIGS, KoPDS can be silenced in 7 days after TRV inoculation and used for subsequent analysis. In this study, the pTRV2 vector was digested with EcoRI and KpnI, and the KoPDS fragment (312 bp) was used to construct the recombinant pTRV2-KoPDS vector (Supplementary Data Figure S1).
Chlorosis symptoms appeared on the leaves about 7 days after infection, followed by a gradual whitening of the spots that did not further intensify after about 15 days. As shown in Figure 2A, the infection rate of K. obovata leaves was significantly and positively correlated with pH at 5.0-6.0, and the infection efficiency was maximized at pH 6.0. The total chlorophyll content was negatively correlated with pH values. As the pH increased from 5.0 to 6.0, the chlorophyll content decreased significantly and reached its lowest value at pH 6.0. When the pH value exceeded 6.0 to 6.5, the infection efficiency declined, and the leaves wilted to different degrees (Figure 2B). Therefore, the data show that the optimum re-suspension pH of the VIGS system is 6.0, which is where the optimum VIGS infection efficiency was achieved.
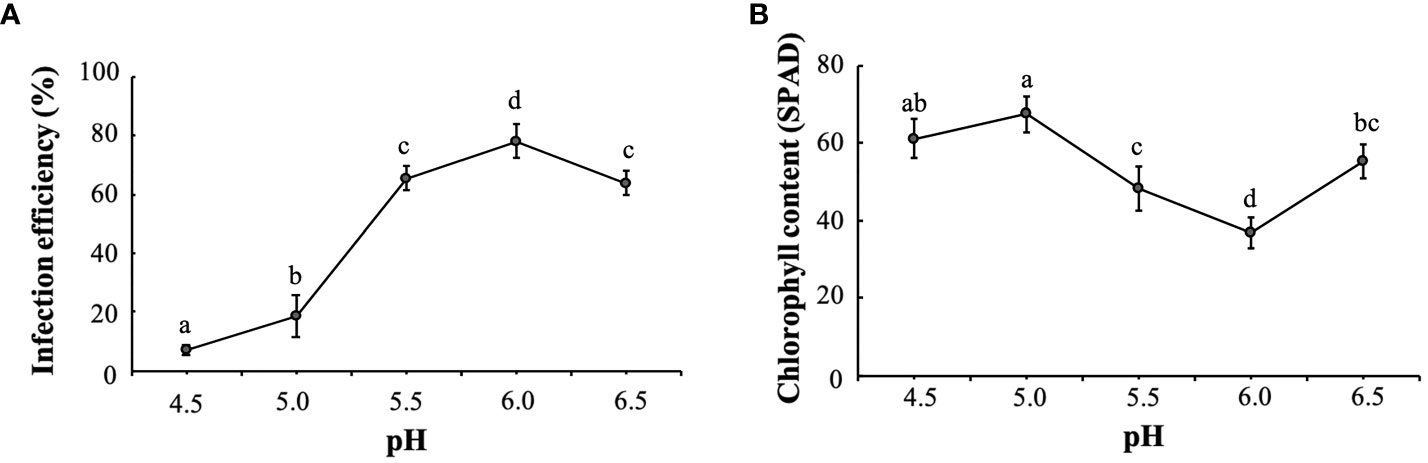
Figure 2 Optimal infection pH for the K. obovata VIGS system. (A) Effect of resuspension buffers having different pH on infection efficiency. (B) Effect of resuspension buffers having different pH on leaf chlorophyll content. Statistical analyses were performed using SPSS 17.0, and significance was assessed with Tukey’s Test at P < 0.05. All experiments included three biological replicates. Five leaves derived from five individual plants were collected for each biological replicate in each experiment. Different letters indicate that the data are directly significantly different (P < 0.05).
Based on the results, equal volumes of pTRV1 and pTRV2-KoPDS were injected into a resuspension solution at pH 6.0 and then into the abaxial surface of leaves. Chlorosis symptoms began to appear approximately 7 days after incubation. The results of the infection efficiency showed that the increase in the agrobacterium density (OD600) was significantly and positively correlated with the viral infection efficiency. When the OD600 was 0.6, the infection efficiency was significantly increased. However, a significant decrease in infection efficiency occurred when the OD600 exceeded 0.6 (Figure 3A). On the other hand, as OD600 increased from 0.3 to 0.6, chlorophyll content decreased significantly (Figure 3B). Although the data showed a significant decrease in chlorophyll content when the OD600 was greater than 0.6 (Figure 3B), the ulceration phenotype of some of the leaves was observed. Therefore, we inoculated the leaves using three different OD600 levels to determine the agrobacterium density that caused necrosis of leaf tissues (Figure 3C).
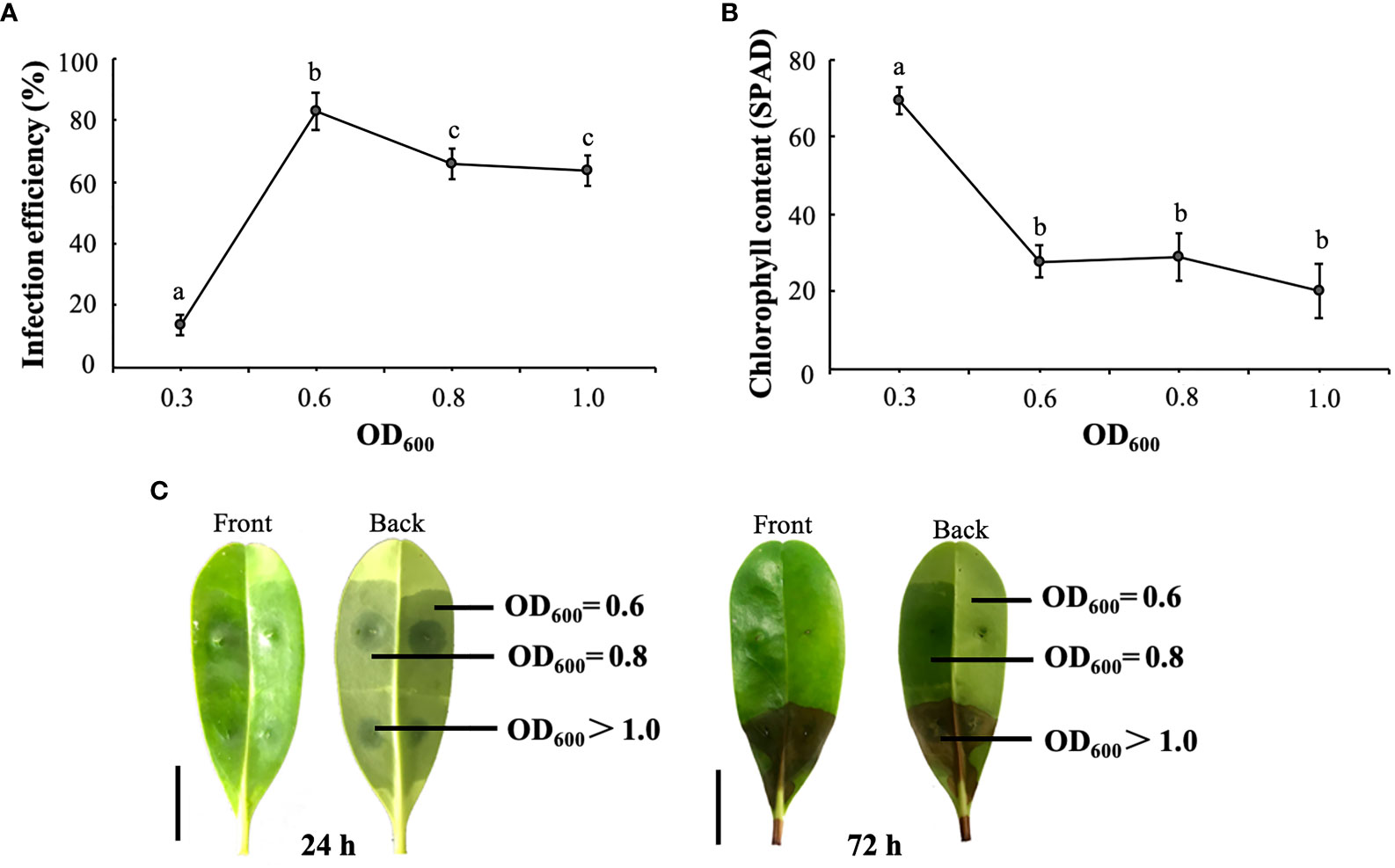
Figure 3 Optimal OD600 of the K. obovata VIGS system. (A) Effect of different OD600 values on infection efficiency. (B) Effect of different OD600 on leaf chlorophyll content. (C) Albino phenotype of leaves 24 and 72 hours after infection. The front and back of the leaf were showed, respectively. At 72 hours after infection, necrotic spots began to appear at OD600 >1. The scale bar on the left corresponds to 1 cm. Statistical analyses were performed using SPSS 17.0, and significance was assessed with Tukey’s Test at P < 0.05. All experiments included three biological replicates. Five leaves derived from five individual plants were collected for each biological replicate in each experiment. Different letters indicate that the data are directly significantly different (P < 0.05).
For resuspension solutions with OD600 > 0.8, the leaves showed necrotic spots and rapidly wilted and died (Figure 3C). Therefore, although the infection rate increased with increasing OD600 values, considering the activity of the practical factors, we used an OD600 of 0.6 as the optimum concentration for the K. obovata VIGS infection system.
RNAi transient assay of KoPDS gene using the established VIGS system
Silencing of the PDS gene, a key gene in carotenoid synthesis, can promote chlorosis symptoms in plants. In this study, chlorosis spots on the leaves of K. obovata mangrove plants appeared approximately 7 days after infection (Figure 4A). We selected infected leaves for RT-qPCR quantification at 7, 15, and 30 days after infection. The gene silencing was effective and lasting, as evidenced by significant reductions in PDS gene expression of 92.5%, 95.6% and 85.6% on day 7, 15 and 30 after infection, respectively (Figure 4B). Together, these results show that the VIGS system established in this study can achieve more than 90% silencing efficiency at 7 days, with peak of silencing efficiency occurring around 15 days and maintenance of 80% effective gene silencing by 30 days post infection.
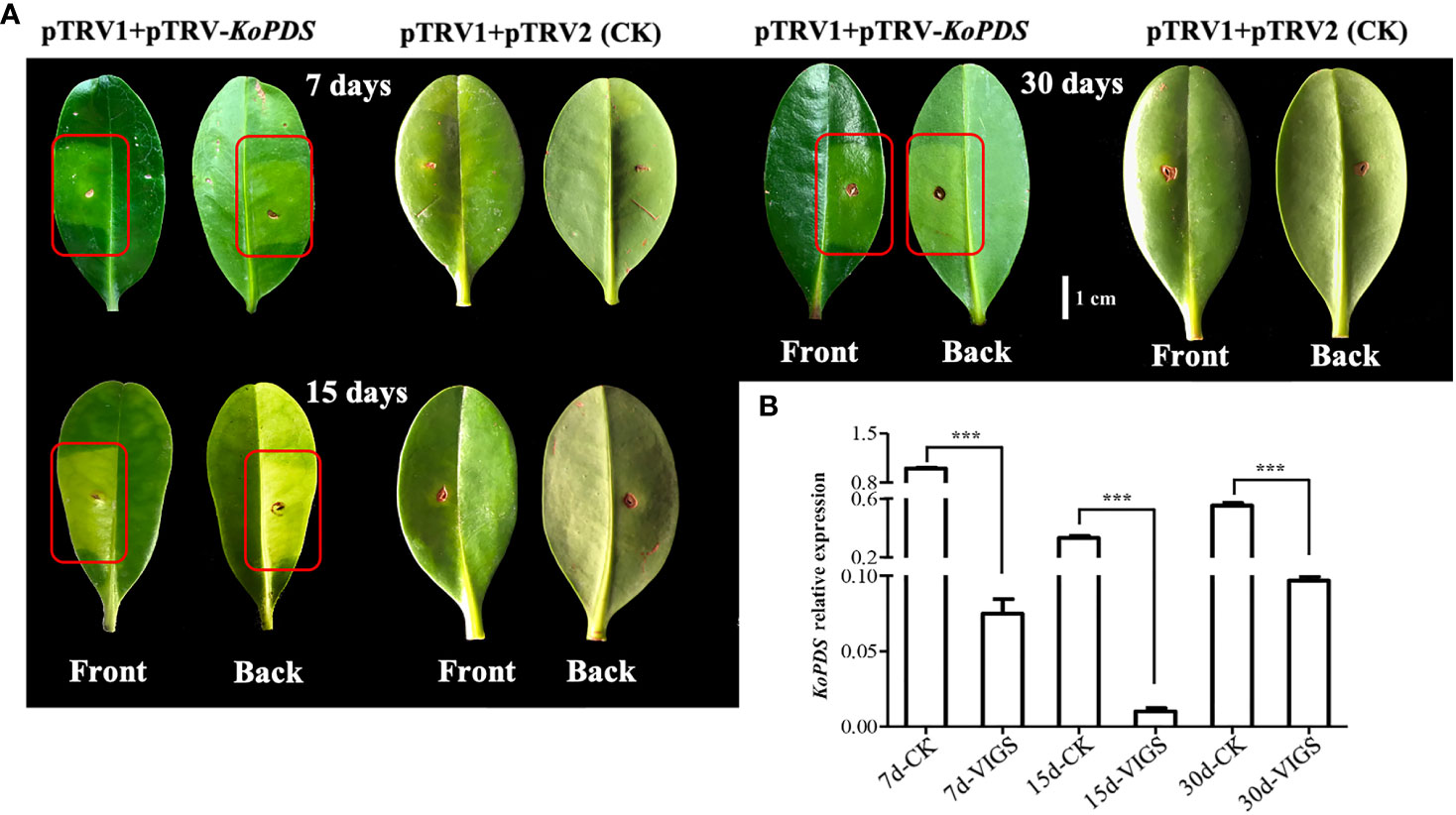
Figure 4 KoPDS silencing in Kandelia obovata and KoPDS relative expression after infection. (A) Silencing of KoPDS in K. obovata leaves by pTRV2-KoPDS (VIGS). The albino phenotype began to appear 7 days after infection, with the most severe effects seen at 15 days and some recovery apparent at 30 days. pTRV2-pTRV1 was used as control (CK). The red boxes indicate areas of infected leaves that appear to be fading green. (B) Comparison of relative KoPDS gene expression in control and VIGS plants at 7, 15, and 30 days after infection. Koactin was used to as an internal control. The 2-△△Ct method was used to calculated expression data, which were replicated three times. Statistical analyses were performed using SPSS 17.0, and significance was assessed with Tukey’s Test at P < 0.05. All experiments included three biological replicates. Five leaves derived from five individual plants were collected for each biological replicate in each experiment. *** indicate that the data are directly significantly different (P < 0.01).
RNA-seq analysis and identification of KoCab functions on K. obovata leaves
To investigate the silencing effect of the VIGS system on the target genes and whether it has additional effects on the leaves that interfere with the experimental results, we analyzed the effects of the pTRV1+pTRV2 system on normal leaves using comparative transcriptome analysis. RNA-seq analysis was performed on normal leaves, leaves infected with the pTRV1+pTRV2 vector, or leaves infected with pTRV1+pTRV-KoCab. After discarding low-quality reads and adaptor sequences, a total of 55.70 Gb clean data were obtained from these samples. The Clean Reads of each sample were sequenced against the designated reference genome separately, and the mapping ratio ranged between 96.38% and 97.13%. Correlation analysis showed that the biological duplicates of leaves from different treatments were highly correlated. A total of 2,323 putative new genes not present in the genome were identified, of which 1,421 genes were annotated by databases, including the NCBI Non-Redundant Protein Database (NR), Swiss-Prot Protein Database (Swiss-Prot), Gene Ontology (GO), Orthologous Groups Database (COG), EuKaryotic Ortholog Groups of proteins database (KOG), Protein Family Database (Pfam), and the Kyoto Encyclopedia of Genes and Genomes (KEGG). A total of 8,395 genes were identified to be differentially expressed by pairwise comparison. Among them, 3,008 and 3,890 genes were differentially expressed on leaves infected with the pTRV1+pTRV2 vector and pTRV1+pTRV-KoCab, respectively.
According to the results of KEGG enrichment analysis and GO annotation, infection of the pTRV1+pTRV2 system resulted in a total of 411 differentially expressed genes that were assigned mainly to plant-pathogen interactions, the MAPK signaling pathway, phytohormone signaling, and the phenylalanine biosynthesis pathway (Figure 5A). Notably, on leaves infected with pTRV1+pTRV- KoCab, we not only saw a chlorophyll fading phenotype, but also found significant down-regulation of eight photosynthetic-antenna protein genes, together with a down-regulation of the flavonoid biosynthesis and flavone and flavonol biosynthesis pathways (Figure 5B). Net photosynthetic rate measurements showed that the difference between the infection with pTRV1+pTRV2 and the normal leaves was not significant, while the photosynthetic rate of the leaves infected with pTRV1+pTRV-KoCab was barely detectable after the appearance of the albino phenotype (data not shown).
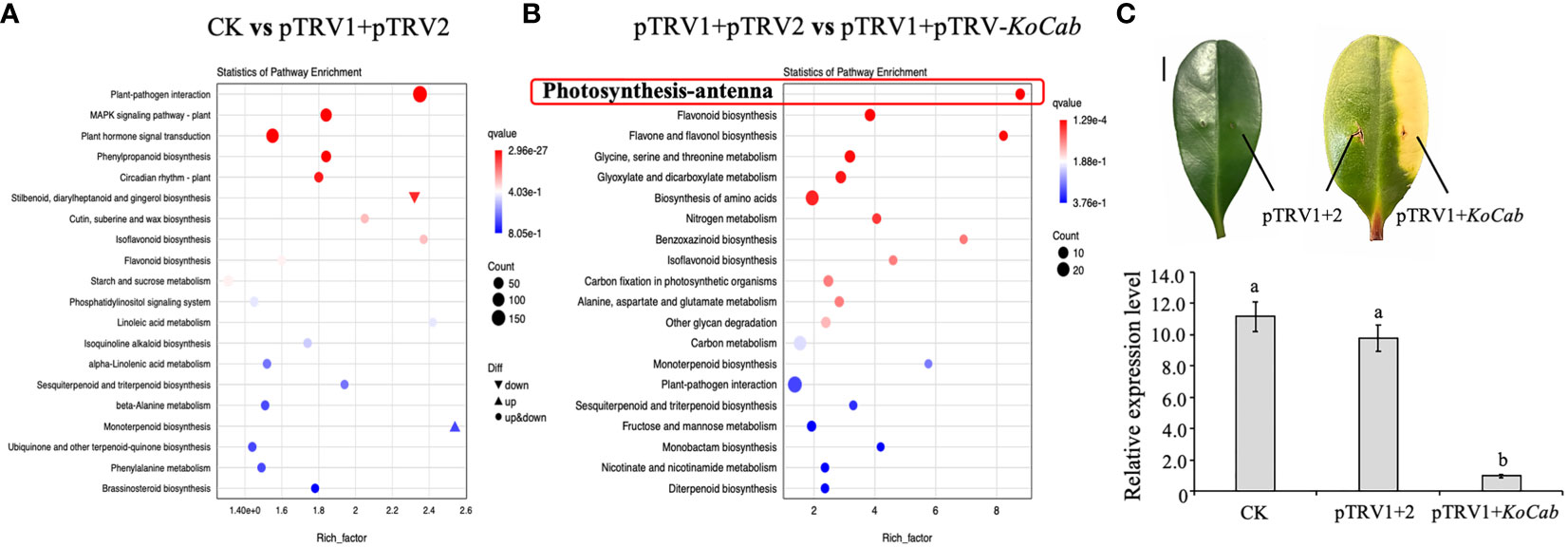
Figure 5 Transcriptome analysis of the effect of the VIGS silencing system on K. obovata leaves. (A) K. obovata leaves infected with pTRV1+pTRV2 for 15 days were used for transcriptome analysis, and normal growing leaves were used as controls (CK). (B) After the appearance of greening in the leaves (about 15 days of infection), the regions infected by pTRV1+pTRV2 and pTRV1+pTRV-KoCab were assessed separately for comparative transcriptome analysis. (C) Leaves was infected with different treatments and the phenotypic differences can be detected by 15 days after infection. Normal growing leaves were used as controls (CK). The regions infected with pTRV1+pTRV2 or pTRV1+pTRV-KoCab were labeled pTRV1 + 2 or pTRV1+KoCab, respectively. The scale indicates 1 cm. The relative expression of KoCab under different infection treatments is shown. Koactin was used to as an internal control. The 2-△△Ct method was used to calculated expression data, which were replicated three times. Different letters indicate that the data are directly significantly different (P < 0.05).
On day 15 post-infection, a distinct greenish phenotype was observed and KoCab gene expression was significantly reduced by more than 95% (Figure 5C). These results again demonstrate that gene silencing is effective.
Discussion
As one of the most carbon-rich ecosystems worldwide (Senger et al., 2021), mangroves are mostly found in tropical and subtropical coastal regions. Carbon sequestration by mangroves also plays a vital role in mitigating global warming and reducing greenhouse gas emissions (Gu et al., 2022). However, mangrove forests worldwide are declining at a rate of 1-2% per year due to continuous human disturbance (Goldberg et al., 2020). Therefore, conservation of mangroves is a matter of great urgency.
Photosynthesis is an indispensable part of mangrove survival and the carbon sink in special coastal habitats. PSII is the site where water is oxidized to oxygen during photosynthesis (Pagliano et al., 2013). The chlorophyll a-b binding protein is an important part of PSII, which is a class of proteins encoded by nuclear genes. In addition to light energy absorption and transfer, they play important roles in maintaining the structure of the cystoid membrane, regulating the distribution of excitation energy between the PSI and PSII photosystems, photoprotection, and adaptation to various environments. Therefore, studying the function of chlorophyll a-b binding protein in mangrove plants may be the key to resolving the regulatory mechanism of their photosynthetic carbon fixation. However, the identification of functional genes in mangrove plants has always been difficult, since few genetic transformation systems for mangroves have been developed. Establishment of the VIGS system for Kandelia obovata mangrove is therefore particularly important for accelerating research on functional mangrove genes.
Virus-induced gene silencing (VIGS) involves virus vectors to induce gene silencing in host plants. In recent years, VIGS has been used extensively in non-model plant species and, because of its simplicity, VIGS allows rapid phenotyping of the silenced genes while being virtually harmless to the plant from a genetic perspective. The success of the VIGS system is largely determined by the efficiency of infection and of silencing. Previous studies showed that infection efficiency is largely related to environmental conditions and infection method, whereas silencing efficiency is mostly related to the suitability of the virus vector used for the host plant (Deng et al., 2021). The TRV vector used in this study is able to infect most plants with few symptoms related to infection.
Susceptibility to TRV infection can vary from plant to plant or from plant to plant of the same species at different stages of growth (Deng et al., 2012; Naing et al., 2019). In this study, K.obovasta leaves exhibited a typical photobleaching phenotype, but leaves at different levels of development varied in their susceptibility to TRV. The 3rd-5th leaves from the top were selected as the best for this experiment because they had the highest silencing rate. In addition, we attempted to optimize the infection conditions by screening for pH and Agrobacterium density for more efficient VIGS use. The expression of virulence genes that are key for Agrobacterium infection was previously shown to be significantly increased with use of acidic virus resuspension buffer, and this increased expression translated to increased Agrobacterium infection rates (Baron et al., 2001). In the present study, we obtained similar results with the highest infection efficiency seen for pH 5.5-6.0, suggesting that Agrobacterium infection of mangrove plants is similar to that of other plants in some respects, but pH induces a degree of hypersensitivity in mangrove plants, such as delayed leaf greening, for reasons that require further investigation.
Although the silencing rate increased with the increase of Agrobacterium density, an Agrobacterium density that was too high (OD600>0.8) tended to cause ulceration of leaf tissues in K. obovata, while the intensity of photobleaching was highest at OD600 0.6. Therefore, OD600 0.6 was selected as the optimum density of Agrobacterium. In addition, the temperature of infection and cultivation also significantly affects Agrobacterium infection rates. Although higher temperatures favor spread of the pathogen after infection, the tumor-inducing activity of Agrobacterium rhizogenes is significantly inhibited above 29°C and may lead to a reduction in symptoms and duration of disease (Riker, 1926). In addition, the ability of Agrobacterium to form extracellular hairs (extracellular pilus, T-pilus) after infection of dicotyledonous plants is a major influence on infection rates. Temperatures >28°C significantly inhibit formation of T-pili and reduce the temperature stability of VIR proteins that further reduces the efficiency of Agrobacterium infection (Baron et al., 2001). Fullner and Nester (1996) showed that the optimal temperature for Agrobacterium-mediated transformation during infection and cultivation is 25°C, or as far below 28°C as possible. To activate the infection activity of Agrobacterium, the bacteria are resuspended in buffer before injection. Acetosyringone (AS) appears to be an essential element of this buffer, and mainly serves as a source of phenolic molecules that further promote expression of vir genes that promote Agrobacterium infection (Zou, 2011). Notably, although mangrove plant tissues contain high levels of phenolics and tannins, the concentration of AS in Agrobacterium-infected K. obovata was not different from that of other plants.
When the deletion of a gene shows significant morphological or color changes on the surface of the plant body, the use of this gene can quickly identify whether the VIGS system of the plant is established or not. In this study, based on the successful silencing of the phytoene desaturase gene, we also silenced the Cab gene and verified the function of this gene at the same time. The chlorophyll a-b binding protein has important effects on plant growth and development as well as physiological status. Knockdown of three genes, Lhcb4, Lhcb5, and Lhcb6, in Arabidopsis thaliana resulted in smaller, thinner leaves and fewer seeds (Chen et al., 2016; Chen et al., 2018). The results of the comparative transcriptome analysis showed that the Cab gene was successfully silenced under the VIGS system, but also triggered a reduction in the expression of some columns of Cab genes, together with the appearance of localized leaf bleaching. However, whether the leaf bleaching phenotype is caused by silencing of the target genes or is associated with down-regulation of multiple Cab genes will require further investigation. But it is certain that we confirmed that the target gene was silenced by qRT-PCR.
In this study, a VIGS system for Kandelia obovata mangrove plants was established for the first time that provides a good experimental system for study of functional genes in mangrove plants and provides new ideas and methods to further improve the silencing efficiency of the Agrobacterium-mediated VIGS system, which has high practical value.
Conclusion
In this study, we constructed the first pTRV-PDS silencing vector for Kandelia obovata mangrove using tobacco rattle virus (TRV) and optimized infection conditions. The system was effective with a solution for infection that had OD600 0.6 and pH 6.0. Under these conditions, the gene silencing efficiency could reach 95%.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: CNCB Genome Sequence Archive, CRA011646.
Author contributions
CP and JC conceived and designed the project. All authors collected samples and performed the experiment. MZ, YS, and CP analyzed the data. YS and YR wrote the manuscript. MZ and YR revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (32001198) and the Fujian Provincial Natural Resources Science and Technology Innovation Project (Grants KY-090000-04-2021-010).
Acknowledgments
We would like to thank Bioscience Editing Solutions for English language editing of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1245555/full#supplementary-material
References
Arce-Rodríguez, M. L., Ochoa-Alejo, N. (2015). Silencing AT3 gene reduces the expression of pAmt, BCAT, kas, and acl genes involved in capsaicinoid biosynthesis in chili pepper fruits. Biol. Plant 59, 477–484. doi: 10.1007/s10535-015-0525-y
Bai, S., Tuan, P., Tatsuki, M., Yaegaki, H., Ohmiya, A., Yamamizo, C., et al. (2015). Knockdown of carotenoid cleavage dioxygenase 4 (CCD4) via virus-induced gene silencing confers yellow coloration in peach fruit: evaluation of gene function related to fruit traits. Plant Mol. Biol. Reporter. 34 (1), 257–264. doi: 10.1007/s11105-015-0920-8
Baron, C., Domke, N., Beinhofer, M., Hapfelmeier, S. (2001). Elevated temperature differentially affects virulence, VirB protein accumulation, and T-pilus formation in different Agrobacterium tumefaciens and Agrobacterium Vitis strains. J. Bacteriol 183, 6852–6861. doi: 10.1128/JB.183.23.6852-6861.2001
Chen, H., Jia, X., Ran, H., Niu, J., Du, M. (2022). Application of VIGS system to explore the function of common wheat TaADF7. BIOCELL 46 (2), 559–565. doi: 10.32604/BIOCELL.2022.01743
Chen, Y. E., Liu, W. J., Su, Y. Q., Cui, J. M., Zhang, Z. W., Yuan, M., et al. (2016). Different response of photosystem II to short and long-term drought stress in Arabidopsis thaliana. Physilolgia Plantarum. 158, 2:225–2:235. doi: 10.1111/ppl.12438
Chen, Y. E., Ma, J., Wu, N., Su, Y. Q., Zhang, Z. W., Yuan, M., et al. (2018). The roles of Arabidopsis proteins of Lhcb4, Lhcb5 and Lhcb6 in oxidative under natural light conditions. Plant Physiol. Biochem. 130, 267–276. doi: 10.1016/j.plaphy.2018.07.014
Deng, X., Elomaa, P., Nguyen, C. X., Hytönen, T., Valkonen, J. R. P. T., Teeri, T. H., et al. (2012). Principles and practice of virus induced gene silencing for Asteraceae-A reverse genetics approach for functional genomics in Gerbera hybrida. Plant Biotechnol. J. 10, 970–978. doi: 10.1007/S11262-022-01941-5
Deng, C., Fan, Z., Wang, J., Li, Y., Huang, H., Dai, S. (2021). Tobacco Rattle Virus-induced Phytoene Deasturase (PDS) silencing in Centaurea cyanus. Hortic. Plant J. 7 (2), 159–166. doi: 10.1016/j.hpj.2020.08.002
Fang, Z. Z., Zhou, D. R., Ye, X. F., Jiang, C. C., Pan, S. L. (2016). Identification of candidate anthocyanin-related genes by transcriptomic analysis of ‘Furongli’ plum (Prunus salicina lindl.) during fruit ripening using RNA-seq. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01338
Fang, Z., Lin-Wang, K., Zhou, D., Lin, Y., Jiang, C., Pan, S., et al. (2021). Activation of PsMYB10.2 transcription causes anthocyanin accumulation in flesh of the red-fleshed mutant of ‘Sanyueli’ (Prunus salicina Lindl.). Front. Plant Sci. 12. doi: 10.3389/FPLS.2021.680469
Fullner, K. J., Nester, E. W. (1996). Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J. Bacteriol. 178, 1498–1504. doi: 10.1128/jb.178.6.1498-1504
Goldberg, L., Lagomasino, D., Thomas, N., Fatoyinbo, T. (2020). Global declines in human-driven mangrove loss. Global Change Biol. 26 (10), 5844–5855. doi: 10.1111/gcb.15275
Gong, P., Cui, J., Li, J., Luo, C., Yang, H. (2015). Application of virus-induced gene silencing in beta vulgaris. Chin. J. Bioinform., 18–22. doi: 10.3969/j.issn.1672-5565.2015.01.04
Gu, X., Zhao, H., Peng, C., Guo, X., Lin, Q., Yang, Q., et al. (2022). The mangrove blue carbon sink potential: Evidence from three net primary production assessment methods. For. Ecol. Manag. 504, 119848. doi: 10.1016/J.FORECO.2021.119848
Hong, L., Su, W., Zhang, Y., Ye, C., Shen, Y., Li, Q. (2018). Transcriptome profiling during mangrove viviparity in response to abscisic acid. Sci. Rep. 8, 770. doi: 10.1038/s41598-018-19236-x
Jiang, B. C., Li, R. L., Shen, S. X., Zhang, Z., Zhang, Y. Q. (2022). A meta-analysis of salt-flood tolerance in mangrove plants and its applied countermeasures. J. Peking University: Natural Sci. Edition 58 (04), 687–699. doi: 10.13209/j.0479-8023.2022.062. (058-004).
Li, Q., Chen, P., Dai, S., Sun, Y., Yuan, B., Kai, W., et al. (2015). PacCYP707A2 negatively regulates cherry fruit ripening while PacCYP707A1 mediates drought tolerance. J. Exp. Botany. 66 (13), 3765–3774. doi: 10.1093/jxb/erv169
Li, L. F., Wu, X. F., Liu, S. Q. (2015). Characteristics of photosynthesis and photosynthetic carbon fixation capacity of five mangrove tree species in zhangjiang city. Guihaia 35 (6), 825–832. doi: 10.11931/guihaia.gxzw201412028
Li, J., Luo, J. H., Wan, Z. L., Wan, Z., Yang, P. (2021). Advances in the application of VIGS technology in the functional study of pepper genes. Henan Agric. Sci. 50 (6), 7. doi: 10.15933/j.cnki.1004-3268.2021.06.002
Li, X., Zhang, J., Wu, Z., Lai, B., Huang, X., Qin, Y., et al. (2015). Functional characterization of a glucosyltransferase gene, LcUFGT1, involved in the formation of cyanidin glucoside in the pericarp of Litchi chinensis. Plant Cell Tiss Organ Culture. 120 (3), 1131–1138. doi: 10.1111/ppl.12391
Naing, A. H., Song, H. Y., Lee, J. M., Lim, K. B., Kim, C. K. (2019). Development of an efficient virus-induced gene silencing method in petunia using the pepper phytoene desaturase (PDS) gene. Plant Cell Tiss Org 138, 507–515. doi: 10.1007/s11240-019-01646-y
Pagliano, C., Saracco, G., Barber, J. (2013). Structural, functional and auxiliary proteins of photosystem II. Photosynth Res. 116, 167–188. doi: 10.1007/s11120-013-9803-8
Prashanth, S. R., Sadhasivam, V., Parida, A. (2008). Overexpression of cytosolic copper/zinc superoxide dismutase from a mangrove plant Avicennia marina in India Rice var Pusa Basmati-1 confers abiotic stress tolerance. Transgenic Res. 17, 281–291. doi: 10.1007/s11248-007-9099-6
Riker, A. J. (1926). Studies on the influence of some environmental factors on the development of crown gall. J. Agric. Res. 32, 83–96.
Senger, D. F., Saavedra Hortua, D. A., Engel, S., Schnurawa, M., Moosdorf, N., Gillis, L.G. (2021). Impacts of wetland dieback on carbon dynamics: A comparison between intact and degraded mangroves. Sci. Total Environ. 753, 141817. doi: 10.1016/j.scitotenv.2020.141817
Senthil-Kumar, M., Mysore, K. S. (2014). Tobacco rattle virus-based virus-induced gene silencing in. Nicotiana benthamiana. Nat. Protoc. 9 (7), 1549–1562. doi: 10.1038/nprot.2014.092
Sui, X., Yan, S., Han, X., Zhao, M., Zhao, L., Xu, Z. (2018). Establishment of virus-induced gene silencing (VIGS) system in perennial rosa plants under field conditions. Natural Sci. 10, 319–328. doi: 10.4236/ns.2018.109032
Tian, J., Cheng, L., Han, Z., Yao, Y. (2015). Tobacco rattle virus mediated gene silencing in strawberry plants. Plant Cell Tiss Organ Culture. 120 (3), 1131–1138. doi: 10.1007/s11240-014-0669-z
Wang, L. H. (2014). Regulation of apple pericarp anthocyanin metabolism and related genes. Ph.D. thesis (China Agricultural University).
Wu, J., Liu, S. H., Ma, J. J., Yang, Z., Qiao, Y., Zhao, P., et al. (2021). Study on the silencing efficiency of different VIGS systems in cotton. Southwest J. Agric. 34 (5), 6. doi: 10.16213/j.cnki.scjas.2021.5.007
Zeng, H., Xie, Y., Liu, G., Wei, Y., Hu, W., Shi, H. (2019). Agrobacterium-mediated gene transient overexpression and Tobacco Rattle Virus (TRV)-based gene silencing in cassava. Int. J. Mol. Sci. 20, 3976. doi: 10.3390/ijms20163976
Zhao, Z., Liu, F. Z., Zhang, Y., Qi, D. X., Chen, Y. H., Lian, Y. (2015). Construction and expression analysis of VIGS expression vector for. SmMsrA Gene aubergine. J. Horticulture 42 (8), 1495–1504. doi: 10.16420/j.issn.0513-353x.2015-0177
Keywords: Kandelia obovata, VIGS system, phytoene desaturase, chlorophyll a/b binding protein, carbon sequestration
Citation: Zhang M, Rao Y, Chen X, Shi Y, Wei C, Wang X, Wang L, Xie C, Pan C and Chen J (2023) Function verification of a chlorophyll a/b binding protein gene through a newly established tobacco rattle virus-induced gene silencing system in Kandelia obovata. Front. Plant Sci. 14:1245555. doi: 10.3389/fpls.2023.1245555
Received: 23 June 2023; Accepted: 06 September 2023;
Published: 03 October 2023.
Edited by:
Youxiong Que, Fujian Agriculture and Forestry University, ChinaReviewed by:
Ben-Qiang Gong, Sun Yat-sen University, ChinaWang Huasen, Zhejiang Agriculture and Forestry University, China
Copyright © 2023 Zhang, Rao, Chen, Shi, Wei, Wang, Wang, Xie, Pan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenglang Pan, cGFuX2NoYXJsZXlAMTYzLmNvbQ==; Jianming Chen, Y2hlbmptQG1qdS5lZHUuY24=
†These authors share first authorship
 Mingxiong Zhang1,2†
Mingxiong Zhang1,2† Yunrui Shi
Yunrui Shi Chenglang Pan
Chenglang Pan