- 1College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang, China
- 2Wageningen Seed Science Centre, Laboratory of Plant Physiology, Wageningen University, Wageningen, Netherlands
- 3Henan International Joint Laboratory of Stress Resistance Regulation and Safe Production of Protected Vegetables, Luoyang, China
- 4Henan Engineering Technology Research Center for Horticultural Crop safety and Disease Control, Luoyang, China
Fusarium wilt, caused by Fusarium oxysporum f. sp. cucumerinum (Fo), is a severe soil-borne disease affecting cucumber production worldwide, particularly under monocropping in greenhouses. Silicon (Si) plays an important role in improving the resistance of crops to Fusarium wilt, but the underlying mechanism is largely unclear. Here, an in vitro study showed that 3 mmol·l-1 Si had the best inhibitory effect on the mycelial growth of F. oxysporum in potato dextrose agar (PDA) culture for 7 days. Subsequently, the occurrence of cucumber wilt disease and its mechanisms were investigated upon treatments with exogenous silicon under soil culture. The plant height, stem diameter, root length, and root activity under Si+Fo treatment increased significantly by 39.53%, 94.87%, 74.32%, and 95.11% compared with Fo only. Importantly, the control efficiency of Si+Fo was 69.31% compared with that of Fo treatment. Compared with Fo, the activities of peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) significantly increased by 148.92%, 26.47%, and 58.54%, while the contents of H2O2, O·−2O⋅−2, and malondialdehyde (MDA) notably decreased by 21.67%, 59.67%, and 38.701%, respectively, in roots of cucumber plants treated with Si + Fo. Compared with Fo treatment, the net photosynthesis rate (Pn), stomatal conductance (Gs), transpiration rate (Tr), maximum RuBisCO carboxylation rates (Vcmax), maximum RuBP regeneration rates (Jmax), and activities of ribulose-1,5-bisphosphate carboxylase (RuBisCO), fructose-1,6-bisphosphatase (FBPase), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the expression of FBPA, TPI, SBPase, and FBPase in Si+Fo treatment increased significantly. Furthermore, Si alleviated stomatal closure and enhanced endogenous silicon content compared with only Fo inoculation. The study results suggest that exogenous silicon application improves cucumber resistance to Fusarium wilt by stimulating the antioxidant system, photosynthetic capacity, and stomatal movement in cucumber leaves. This study brings new insights into the potential of Si application in boosting cucumber resistance against Fusarium wilt with a bright prospect for Si use in cucumber production under greenhouse conditions.
Introduction
Cucumber (Cucumis sativus L.) is currently one of the vastly cultivated and economically beneficial vegetables of the Cucurbitaceae family in the world. However, its production is increasingly being affected by fungal diseases, especially Fusarium wilt, one of the three most serious fungal diseases of cucumber plants (Raza et al., 2017). Fusarium wilt, caused by F. oxysporum, is a destructive soil-borne fungal disease (Zhai et al., 2021). The cucumber-specific type of F. oxysporum can colonize the vascular bundles of cucumber rhizomes, preventing the transportation of nutrients and water (Zhou et al., 2017), resulting in the wilting of aboveground parts of cucumber plants (Sun et al., 2021). Fusarium wilt significantly reduces the photosynthesis capacity and photosynthetic enzyme activity and can also cause a 15%–50% reduction in cucumber yield (Ahammed et al., 2020). Since pesticides are still used to prevent and control cucumber Fusarium wilt, the pesticide residue has brought a great threat to human health and environmental sustainability (Jain et al., 2022). Therefore, it is a hot topic in recent years to find a pollution-free and effective method to prevent cucumber wilt.
Silicon (Si) is a tetravalent chemical element, which is mainly taken by plants in the form of monosilicic acid (H4SiO4) from the soil (Shivaraj et al., 2022). Si is widely recognized as an element beneficial to plant growth and biomass production. Studies have demonstrated that exogenous Si treatment can significantly improve the yield of rice, tomato, and other crops (Hoffmann et al., 2020; Chaiwong and Prom-u-thai, 2022). Si can thicken the main stems of plants, improve photosynthesis, and enhance the development of vascular bundles involved in the uptake and transport of nutrients (Pavlovic et al., 2021). Moreover, Si can form a “keratin-silicon double-layer” physical barrier on the plant leaf surface to resist insects and fungi (Yoshid, 1965). Si is also known to effectively mitigate various abiotic stresses such as heavy metal toxicities, salinity, drought, chilling, and freezing stresses (Savvas and Ntatsi, 2015). The application of Si increased the salt tolerance of tomato plants by enhancing substomatal CO2, net photosynthetic rate, photosynthetic water use efficiency, and mesophyll conductance (Haghighi and Pessarakli, 2013). Si could alleviate adverse effects of salt stress by reducing the Na+ concentration and boosting antioxidant enzyme activities in Glycyrrhiza uralensis, and these alleviating effects were dependent on Si concentrations and the time of Si treatment (Li et al., 2016).
It is reported that Si addition to soil can significantly enhance the resistance of plants against disease and increase plant immune response. Supplying Si to tomato seedlings can reduce the disease severity of Fusarium crown and root rot in tomato; the increase in the Si content of roots was significantly correlated with the reduction of disease severity of root, crown, and stem (Huang et al., 2011). Furthermore, Si alleviated soil-borne disease stress by adjusting soil microbial composition and diversity, in which Si-added soil harbored a lower proportion of Pseudomonas, Fusarium, and Faecalibacterium (Lin et al., 2020). It was reported that exogenous Si and silicate salts significantly stimulated systemic defense enzymes in onion and garlic plants and decreased the incidence of white rot disease (Elshahawy et al., 2021). Si treatment could increase the late blight resistance of potato plants by increasing ethylene and jasmonic acid metabolism in both detached leaves and intact plants (Xue et al., 2021).
Application of Si is a preventive strategy against many soil-borne fungal diseases, such as Pythium damping off in cucumber (Al Sadi et al., 2010), Fusarium wilt in banana plants (Fortunato et al., 2014), F. oxysporum on cotton (Whan et al., 2016), and black pepper plants (D'Addazio et al., 2020). Silicon can induce a higher lignin concentration in moderately resistant cultivars than that in less resistant cultivars. However, the contribution of antioxidant potential and the photosynthetic capacity of cucumber to Si-mediated Fusarium wilt stress alleviation remain unclear.
In the current study, we examined the effects of sodium silicate (Na2SiO3·9H2O) treatment on the occurrence of cucumber wilt disease and its mechanisms. Our results revealed that Si-induced enhanced resistance to Fusarium wilt was closely related to the stimulation of photosynthetic capacity and antioxidant system of cucumber leaves. The study suggested the great potential of Si as a means of biological control of F. oxysporum in cucumber plants that could not only reduce losses caused by Fusarium wilt but also minimize the use of chemical fungicides.
Materials and methods
Plant materials, fungal strains, and treatments
In the present study, cucumber (Cucumis sativus L. cv. Jinyan No. 4) which is susceptible to Fusarium wilt was used as plant material (Liu et al., 2010). F. oxysporum f. sp. cucumerinum isolates were cultured on Komada’s Fusarium-selective medium (Isack et al., 2014) and confirmed on the basis of microscopic studies of the shape and size of macro- and micro-conidia (Booth, 1971). Analytically pure sodium silicate (Na2SiO3·9H2O) was purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The tested soil was imported peat soil from Germany K brand, with soil organic matter content of 25.25 g/kg, alkali hydrolyzable nitrogen content of 66.8 mg/kg, available phosphorus content of 12.37 mg/kg, available potassium content of 96.53 mg/kg, and pH of 6.50. Seeds of cucumber were surface sterilized with 0.1% potassium permanganate solution and germinated in peat: vermiculite = 2:1 (v:v).
Screening of silicon concentrations
The in vitro plate culture method was used to evenly smear the sterilized Na2SiO3·9H2O solution with concentrations of 1, 2, 3, 4, and 5 mmol L-1 Si on the surface of potato dextrose agar (PDA) medium. F. oxysporum f. sp. cucumerinum was inoculated on the culture medium with a 5-mm-diameter punch. At the same time, the plate coated with distilled water and inoculated with Fusarium was cultured as the reference control. Each treatment was repeated three times. The mycelial growth diameter was observed and recorded for 5–7 days. The inhibition rate was calculated, and the differences were analyzed to select the most suitable concentration of Si.
Experimental design
The experiment was performed in a greenhouse at Henan University of Science and Technology, Luoyang, China (longitude 112°26′E and latitude 34°40′N), with the following growth conditions: 28°C/20°C (day/night) temperatures, 85% relative humidity, and a 12h photoperiod with an average photosynthetic flux of 600 μmol m-2 s-1. The cucumber seedlings at the four-leaf stage were irrigated with 100 ml (per pot) of 3 mmol·l-1 Na2SiO3·9H2O, and the treatment was repeated every 7 days (in total four times). The control was irrigated with 100 ml of sterilized water. Then, one-half of Si-treated seedlings and one-half of water-inoculated seedlings were inoculated with F. oxysporum by adding a conidial suspension of F. oxysporum into the nutrient solutions to achieve a final concentration of 106 conidia per milliliter. The details of the preparation of F. oxysporum suspension and treatment have been described in our previous study (Ahammed et al., 2020). To determine the changes in plant height, stem diameter and root length were measured at 0 day (the day when inoculation was performed) and at 9 days after inoculation with F. oxysporum.
Gas exchange and chlorophyll fluorescence measurements
Gas exchange analysis including the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular carbon dioxide concentration (Ci), and transpiration rate (Tr) of leaves was conducted using an infrared gas analyzer-based portable photosynthesis system (LI-6400; LI-COR, Lincoln, NE, USA) on the fourth leaf of each plant. Asat was measured at an ambient CO2 concentration of 360 µmol mol-1 and saturating PPFD (1,000 µmol m-2 s-1) with a leaf temperature of 25 ± 1.5°C and air relative humidity of 80%–90%. Assimilation versus intercellular CO2 concentration (A/Ci) curves were measured based on the method of von Caemmerer and Farquhar (1981). The maximum RuBisCO carboxylation rates (Vcmax) and maximum RuBP regeneration rates (Jmax) were estimated from the A/Ci curves according to Ethier and Livingston (2004).
Determination of enzyme activity involved in the Calvin cycle
Ribulose-1,5-bis-phosphate (RuBP) carboxylase/oxygenase (RuBisCO) activity was measured spectrophotometrically by coupling 3-phosphoglyceric acid formation with NADH oxidation at 25°C, following the method described by Lilley and Walker (1974). FBPase activity was determined by monitoring the increase in A340 using an extinction coefficient of 6.2 mM-1 cm-1 (Scheibe et al., 1986; Zhou et al., 2007). The initial activity was assayed immediately after homogenization. Total activity was assayed on aliquots of enzyme extract incubated for 20 min with 100 mM dithiothreitol, 2 mM Fru-1,6-bisP, 10 mM MgCl2, and 0.1 M HEPES-NaOH (pH 8.0). The measurement of GAPDH activity was according to the method of Piattoni et al. (2013).
Estimation of antioxidant enzyme activity
The 0.5g sample was ground to a homogenate with 3 ml precooled potassium phosphate buffer (50 mol l-1, pH = 7.0) in an ice bath followed by centrifugation for 20 min at 10,000 g at 4°C. Extracting solution was used to determine the superoxide dismutase (SOD), guaiacol peroxidase (G-POD), catalase (CAT), and ascorbate peroxidase (APX) activity. SOD activity was examined by measuring the ability of SOD to inhibit the photochemical reduction of nitro blue tetrazolium at 560 nm (Aebi, 1984). CAT activity was determined via the reduction at 240 nm for H2O2 extinction (Giannakoula et al., 2010). APX activity was measured based on the rate of AsA oxidation. A 0.1-ml supernatant was mixed with 1 ml extracting solution (50 mol l-1 potassium phosphate with pH = 7.0, 750 µmol l-1 AsA, and 100 mol l-1 H2O2). The absorbance at 290 nm was measured at every 15-s interval for 3 min (Durner and Klessig, 1995). The enzyme extracts of guaiacol peroxidase (G-POD) were isolated following the method of Lamikanra and Watson (2001). G-POD activity (EC 1.11.1.7) was determined by the enhancement in absorbance at 470 nm (ϵ, 26.6 mM-1 cm-1) which resulted from the oxidation of guaiacol (Cakmak and Marschner, 1992).
Biochemical quantification of reactive oxygen species and malondialdehyde levels
H2O2 content in roots was determined spectrophotometrically by a peroxidase assay according to Willekens et al. (1997). The O·−2O⋅−2 production rate was measured according to the method of Elstner and Heupel (Elstner and Heupel, 1976). The level of lipid peroxidation in roots was determined by quantifying the malondialdehyde (MDA) equivalents using 2-thiobarbituric acid (TBA) as described by Hodges et al. (1999).
RNA extraction and qRT-PCR for gene expression analysis
Total RNA was extracted from root tissues using TRIzol reagent (Sangon, China) at 1 dpi with FO according to the manufacturer’s instructions. Genomic DNA was removed by DNase treatment (BBI, Canada). One milligram of total RNA was reverse-transcribed using ReverTra Ace qPCR RT Kit (Toyobo, Japan) according to the manufacturer’s instruction. The iCycler iQ™ real-time PCR detection system (Bio-Rad, Hercules, CA, USA) was used for quantitative real-time PCR. Twenty-five-microliter reaction mixtures consisted of 12.5 µl SYBR Green PCR Master Mix (Takara, Japan), 1 µl of diluted cDNA, and 0.2 µM of forward and reverse primers. The conditions for PCR cycling were as follows: 95°C for 3 min and 40 cycles of 95°C for 10 s 58°C for 45 s. The gene-specific primers used for the amplification were determined on the basis of gene or EST sequences and are listed in Supplementary Table S1. mRNA levels were quantified according to the method of Livak and Schmittgen (2001). To obtain a ΔCt value, the threshold cycle (Ct) value of actin was subtracted from that value of the gene of interest.
Statistical analysis
All data presented are mean values of three repetitions of each treatment. Data were statistically analyzed using analysis of variance (AVONA) and expressed as mean ± standard deviation (SD). We used Tukey’s least significant difference test at 5% for multiple pairwise comparisons.
Results
Screening of optimal silicon concentrations
Five concentration gradients of Na2SiO3·9H2O were used in the in vitro test. After the culture of F. oxysporum f. sp. cucumerinum on the PDA plate for 7 days, the mycelial growth of Fusarium on the control plate reached close to the full plate, while the mycelial growth in the plate coated with Na2SiO3·9H2O was significantly inhibited.
The five concentrations of Si tested all had certain antifungal effects on Fusarium growth in vitro. After 7 days of PDA culture, 3 mmol·l-1 Na2SiO3·9H2O showed the best inhibitory effect on Fusarium growth, and the inhibition rate reached 23.4% (Figure 1). Five millimoles per liter of Na2SiO3·9H2O had the least inhibitory effect on Fusarium growth. Therefore, 3 mmol·l-1 Na2SiO3·9H2O was selected as the optimal Si concentration for the following test.
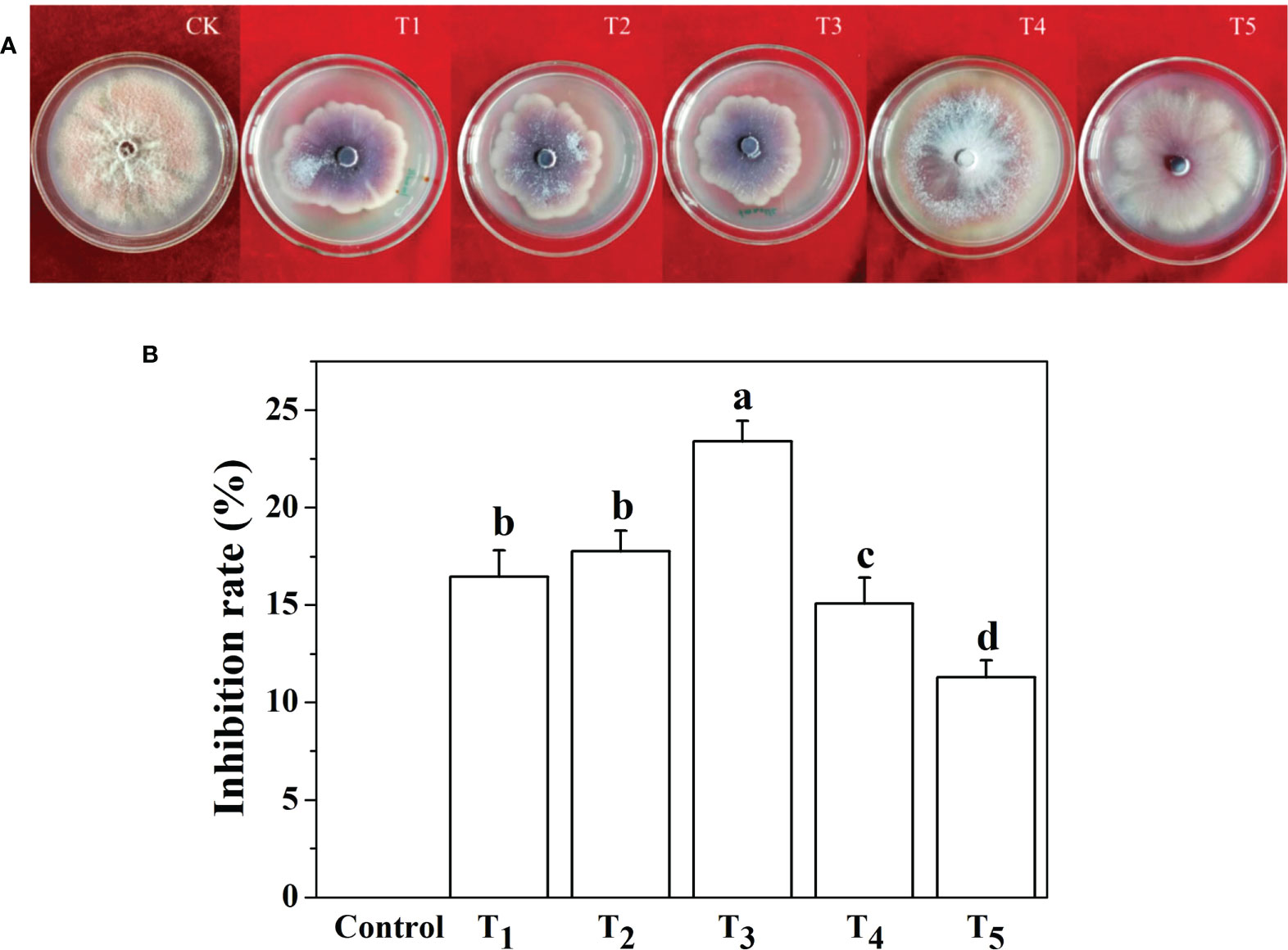
Figure 1 Screening of optimal silicon (Si) concentrations against Fusarium oxysporum (f) sp. cucumerinum fungi. (A) Photographs of in vitro culture of Fusarium fungi in five different silicon concentrations on PDA plates for 7 days. (B) Inhibition rate of five silicon concentrations on mycelial growth of Fusarium when cultured for 7 days. CK or control, 0 mmol·l-1 Si; T1, 1 mmol·l-1 Si; T2, 2 mmol·l-1 Si; T3, 3 mmol·l-1 Si; T4, 4 mmol·l-1 Si; and T5, 5 mmol·l-1 Si. Means denoted by the different lowercase letters indicate a significant difference according to Tukey’s test (P ≤ 0.05).
Silicon enhances cucumber growth and resistance to F. oxysporum
The plants treated with exogenous Si were more vigorous than other treatments, and the plants have more roots. However, the leaves of only Fo-treated plants showed leaf withering, and the roots were gradually rotted. However, with Si application to the roots (i.e., Si + Fo), the withering symptom of cucumber leaves and the rotted symptom of cucumber roots were significantly alleviated (Figure 2).

Figure 2 Phenotypes of cucumber plants under different treatments. Upper panels: whole plant phenotype, middle panels: root phenotype, and lower panels: images of scanned roots under different treatments. CK or control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium.
Compared with the control, the plant height, stem diameter, root length, and root activity of Fo treatment decreased by 39.86%, 54.47%, 49.32%, and 60.53%, respectively. However, the plant height, stem diameter, root length, and root activity of Si+Fo treatment increased significantly by 39.53%, 94.87%, 74.32%, and 95.11%, respectively, compared with Fo only (Table 1). Additionally, exogenous Si application remarkably improved various root traits (Table 2) Importantly, exogenous Si application significantly decreased disease index and the control efficiency of Si+Fo was 69.31% compared with that of Fo treatment (Table 3).
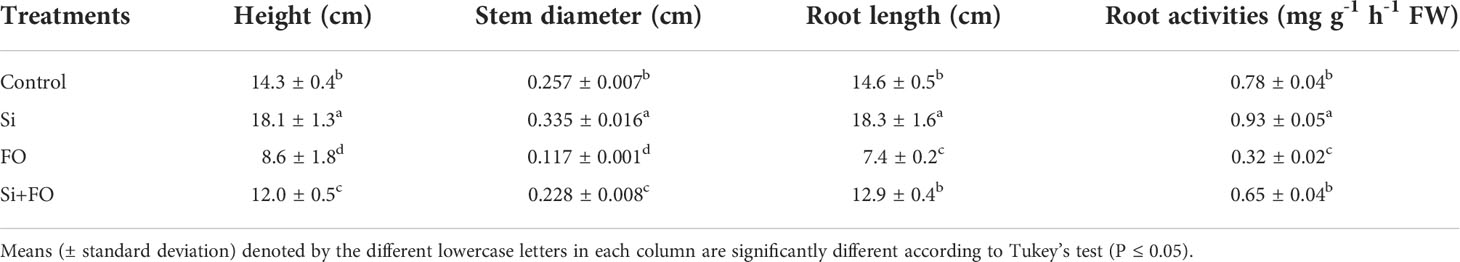
Table 1 Effects of exogenous silicon on root length, plant height, and stem diameter of cucumber as influenced by Fusarium wilt.
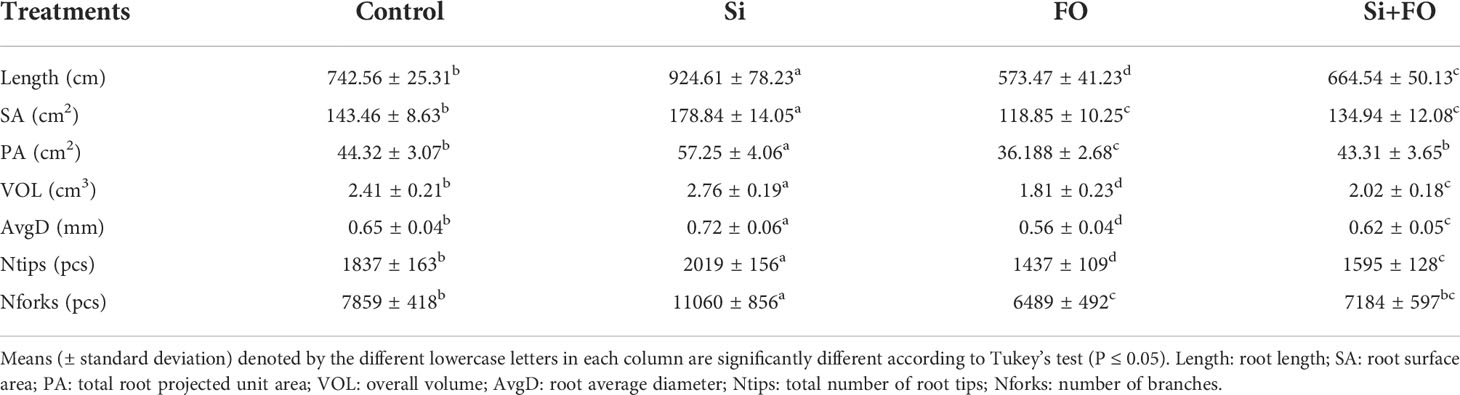
Table 2 Effects of exogenous silicon application on root traits of cucumber plants as influenced by Fusarium wilt.
Silicon alleviates Fusarium wilt-induced oxidative stress
To assess the effect of Fusarium inoculation on oxidative stress markers, the contents of hydrogen peroxide (H2O2), superoxide anion (O·−2O⋅−2), and malondialdehyde (MDA) in the roots and leaves were determined. Compared with the control, the H2O2, O·−2O⋅−2, and MDA contents in roots under Fo treatment increased by 0.56, 11.06, and 0.39 times, respectively, and the H2O2, O·−2O⋅−2, and MDA contents in leaves increased by 0.82, 10.85, and 3.21 times, respectively (Figure 3). However, Si application significantly decreased Fusarium wilt-induced reactive oxygen species (ROS) accumulation in cucumber roots and leaves. Compared with Fo treatment, the contents of H2O2, O·−2O⋅−2, and MDA in roots of Si+Fo treatment decreased by 21.67%, 59.67%, and 38.70%, while those in leaves decreased by 35.98%, 66.02%, and 47.51%, respectively.
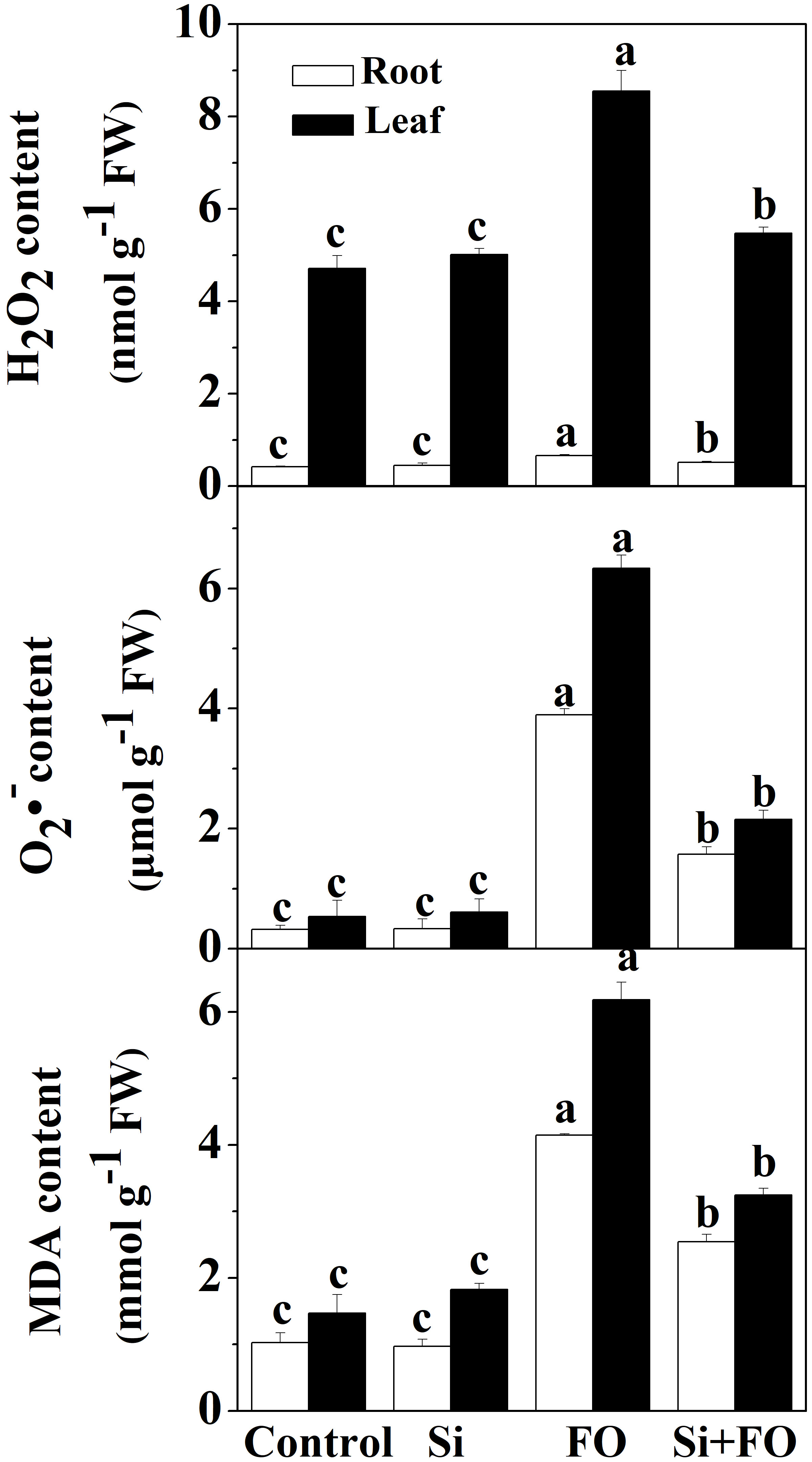
Figure 3 Effects of exogenous silicon application on the contents of H2O2, O·−2O⋅−2, and MDA in cucumber roots. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05); the mean represents the average of three replicates ± standard deviation (SD). Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium.
Exogenous silicon activates antioxidant enzymes
As shown in Figure 4, the activities of antioxidant enzymes, such as POD, CAT, SOD, and APX, in roots of cucumber treated with Si significantly increased by 95.82%, 108.33%, 9.16%, and 546.67%, and in the meantime, those in leaves increased by 72.04%, 29.56%, 10.77%, and 278.82% compared with the control, respectively. However, Fusarium decreased POD and SOD activities in roots significantly by 36.41% and 12.98%, respectively, compared with the control. Compared with Fo, the activities of POD and APX in cucumber plants treated with Si + Fo significantly increased by 148.92% and 115.26% in roots and 58.54% and 71.55% in leaves, respectively.
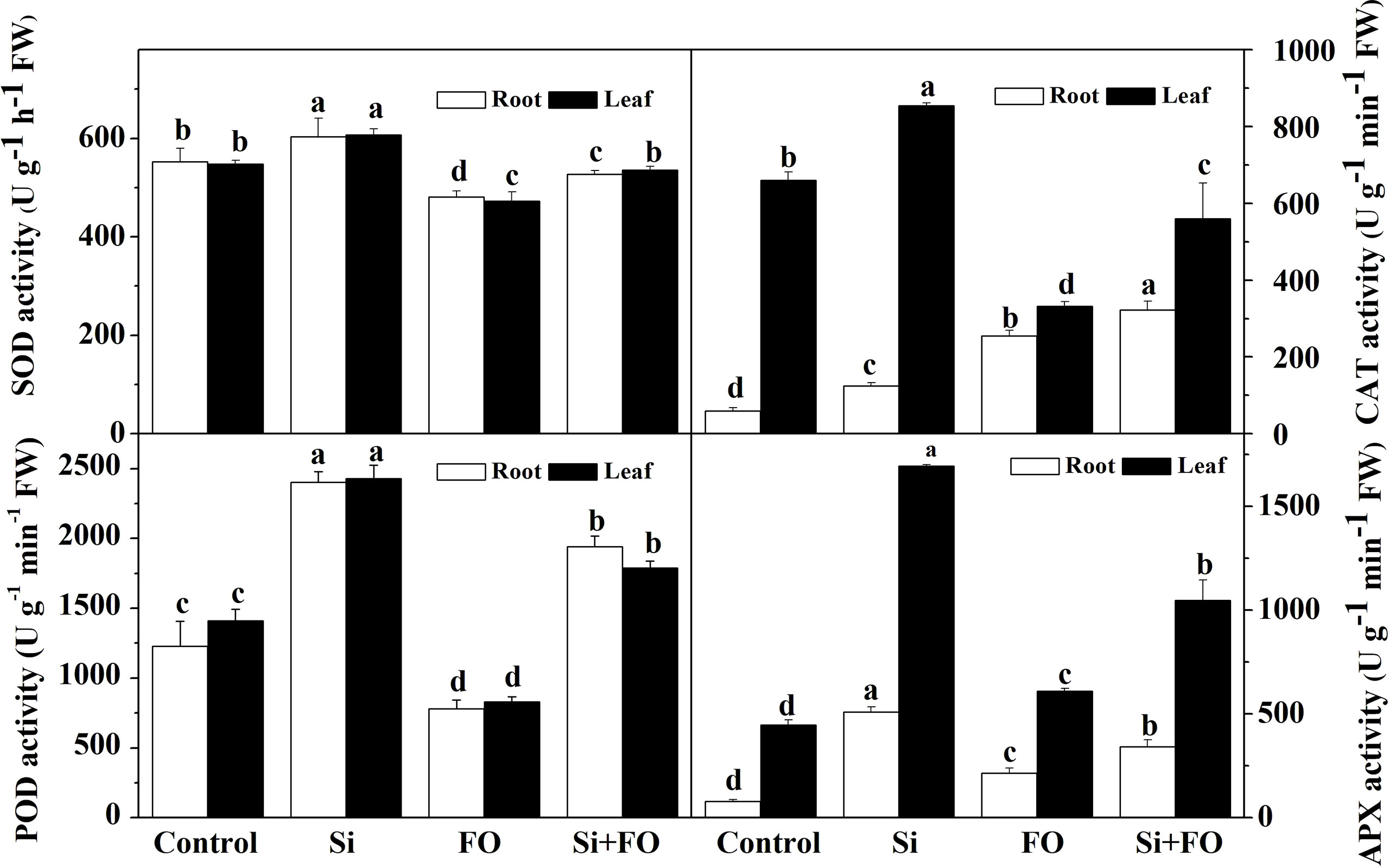
Figure 4 Effects of exogenous silicon application on the activities of POD, CAT, SOD, and APX in cucumber roots. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05); the mean represents the average of three replicates ± standard deviation (SD). Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium..
Silicon enhances photosynthesis rate, Jmax, and Vcmax
Compared with the control, the net photosynthetic rate (Pn), stomatal conductance (Gs), and transpiration rate (Tr) of cucumber leaves in the Si treatment group were significantly increased by 16.05%, 38.15%, and 11.49%, respectively, while the intercellular carbon dioxide concentration (Ci) was significantly reduced by 6.62%. Inoculation with Fo decreased Pn, Gs, and Tr compared with control. Compared with Fo treatment, Pn, Gs, and Tr increased by 40.02%, 28.70%, and 23.84%, while Ci decreased by 6.92% in Si+Fo treatment. The results showed that exogenous Si application could enhance the photosynthetic capacity of cucumber leaves (Figure 5).
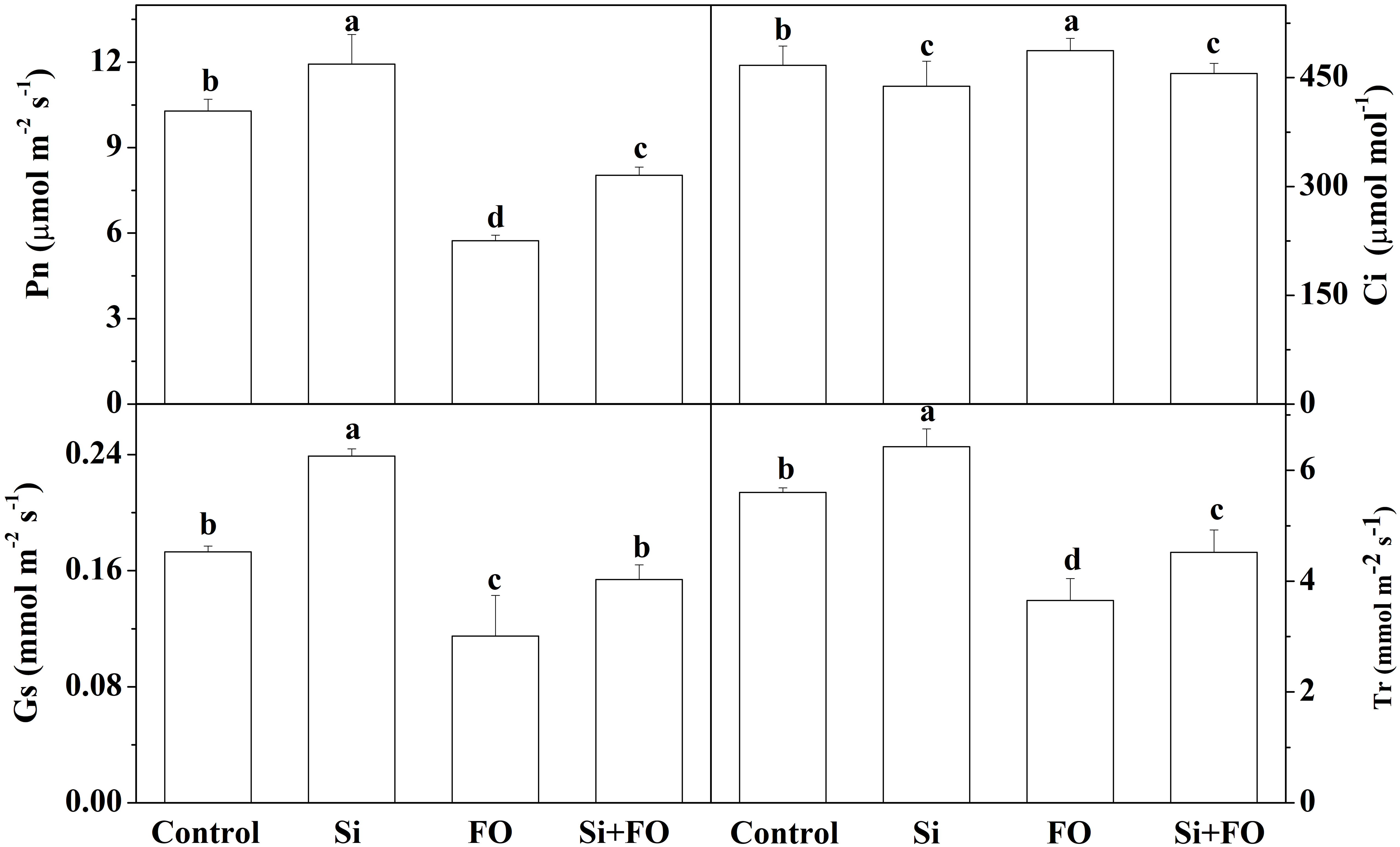
Figure 5 Effects of exogenous silicon application on Pn, Ci, Gs, and Tr in cucumber leaves. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05); the mean represents the average of three replicates ± standard deviation (SD). Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium..
Compared with the control, Vcmax and Jmax in Si treatment increased by 16.82% and 20.56%, respectively. However, Vcmax and Jmax in cucumber leaves decreased significantly after inoculation with Fusarium for 14 days. Meanwhile, Vcmax and Jmax in plants pretreated with Si and then inoculated with Fusarium wilt were significantly increased by 40.97% and 27.07% compared with the cucumber plants inoculated with Fusarium only (Figure 6).
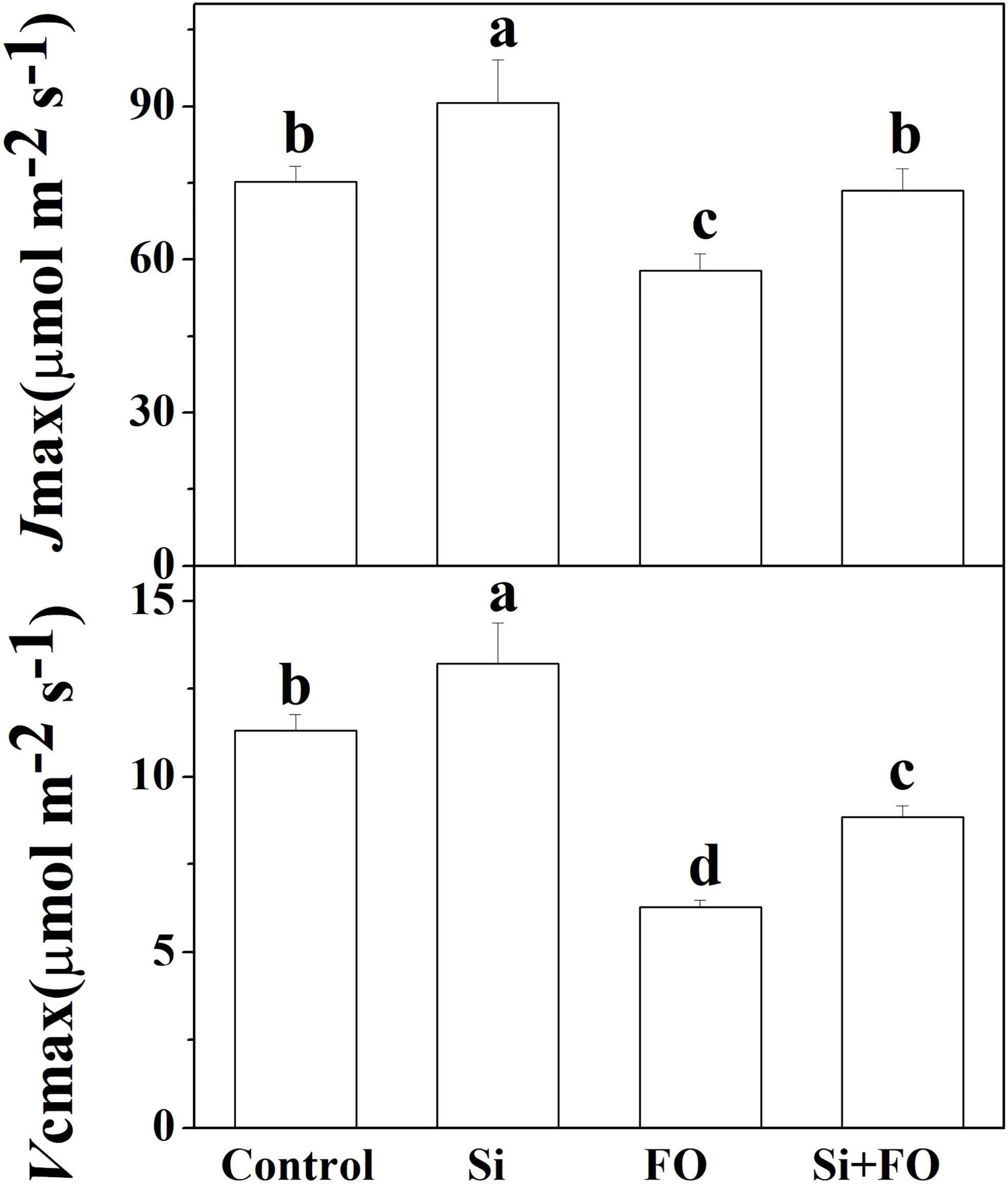
Figure 6 Effects of exogenous silicon application on Jmax and Vcmax of cucumber leaves. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05); the mean represents the average of three replicates ± standard deviation (SD). Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium..
Effects of exogenous silicon application on activities of RuBisCO, FBPase, and GAPDH in cucumber leaves
Exogenous Si treatment induced the activities of three photosynthetic enzymes, namely, RuBisCO, FBPase, and GAPDH. However, inoculation with FO decreased the activities of RuBisCO, FBPase, and GAPDH. Furthermore, the activities of RuBisCO, FBPase, and GAPDH of the plants pretreated with Si and then inoculated with Fusarium significantly increased by 71.51%, 53.96%, and 32.55%, respectively, compared with the plants inoculated with Fusarium only (Figure 7).
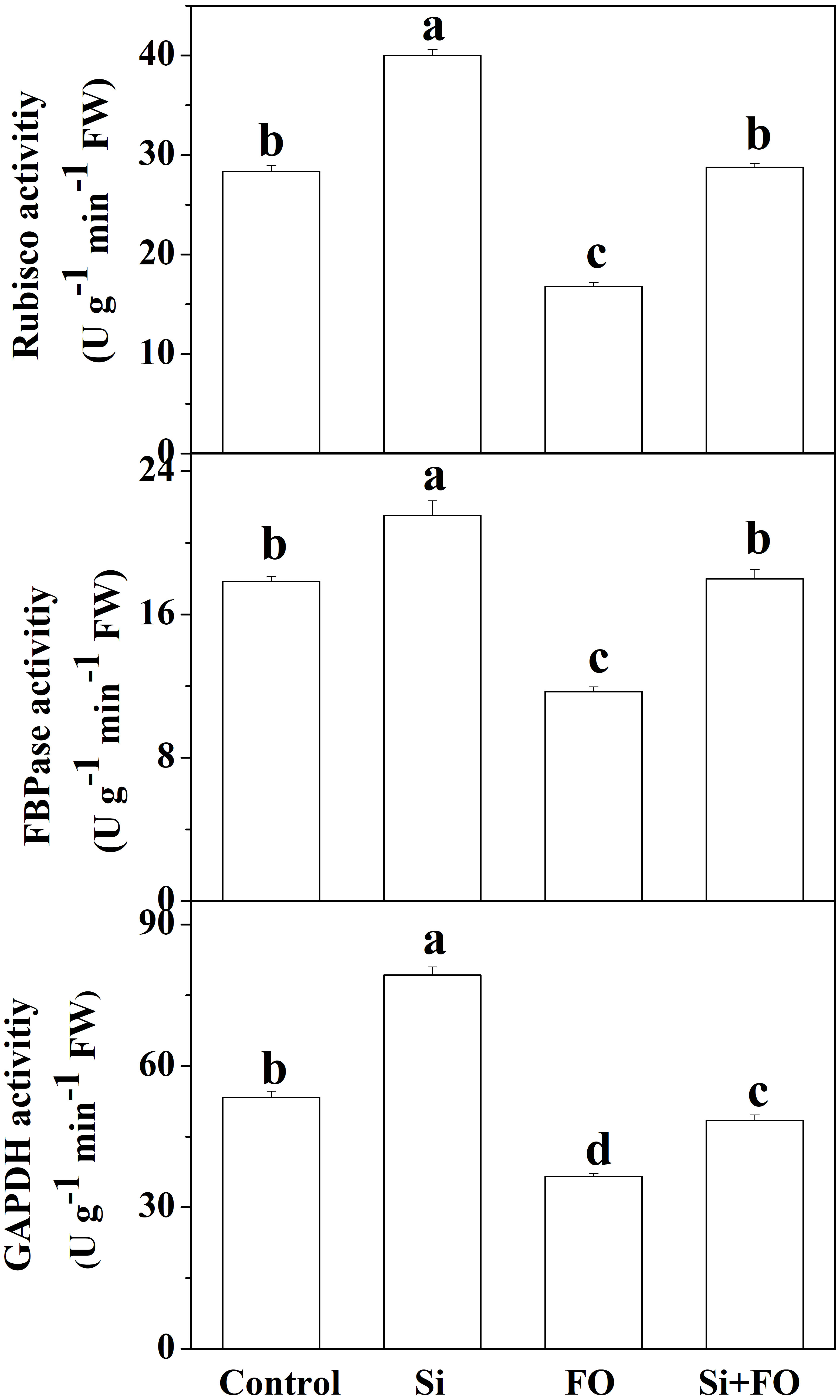
Figure 7 Effects of exogenous silicon application on activities of Rubisco, FBPase, and GAPDH in cucumber leaves. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05); the mean represents the average of three replicates ± standard deviation (SD). Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium.
The effect of exogenous silicon on the expression of Calvin cycle-related genes in cucumber leaves
To further study the role of exogenous silicon in photosynthesis, the expression of four genes involved in RuBP regeneration was determined. As shown in Figure 8, the expression of FBPA, TPI, SBPase, and FBPase of the plants pretreated with Si increased significantly compared with that of the control. On the contrary, the expression of FBPA, TPI, SBPase, and FBPase of the treatments inoculated with Fo decreased by 66.78%, 57.83%, 68.16%, and 49.59%, respectively, compared with that of the control. Meanwhile, the expression of FBPA, TPI, SBPase, and FBPase of the plants pretreated with Si and then inoculated with Fusarium significantly increased 10.61, 6.60, 12.77, and 8.84 times, respectively, compared with the plants inoculated with Fusarium only (Figure 8).
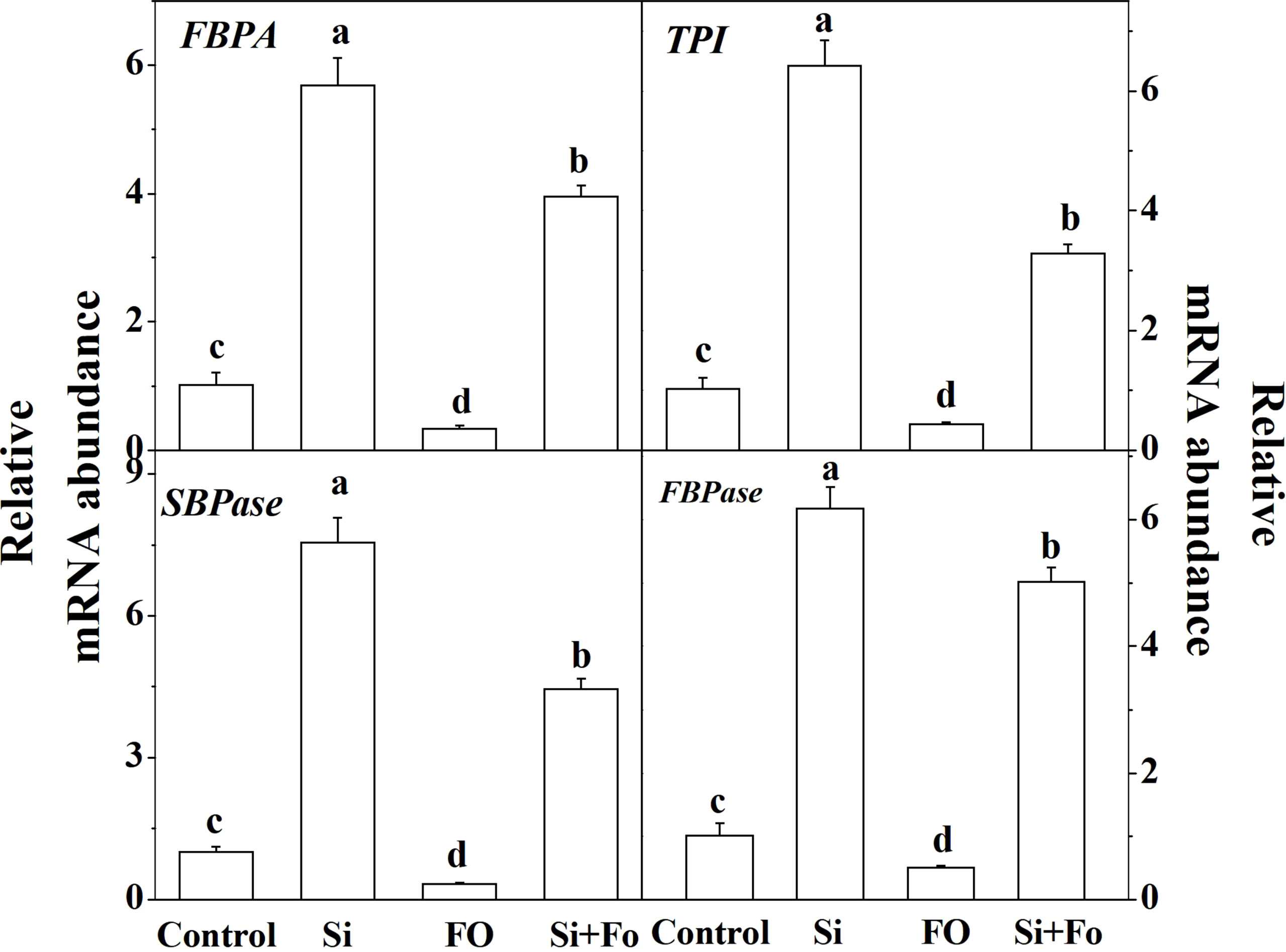
Figure 8 Effect of exogenous silicon on the expression of genes involved in the Calvin cycle in tomato leaves. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05); the mean represents the average of three replicates ± standard deviation (SD). Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium..
Exogenous silicon application alleviates the stomatal closure of cucumber caused by F. oxysporum
The stomatal opening of cucumber plants pretreated with Si was significantly higher than in other treatments. The stomata of the abaxial epidermis of cucumber leaves are almost completely closed at 14 days after inoculation with F. oxysporum. However, Si application alleviated the stomatal closure caused by F. oxysporum, and the stomatal opening was 42.39% higher than that of the plants inoculated with F. oxysporum only (Figure 9).

Figure 9 Effects of exogenous silicon application on stomatal movement in the lower epidermis of cucumber leaves. (A) Microscopic photographs of stomata in the lower epidermis, and (B) stomatal aperture as influenced by different treatments. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05). CK or Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium.
Effects of exogenous silicon application on endogenous silicon content in cucumber
The contents of endogenous Si in roots and leaves of cucumber plants pretreated with Si were 4.41 times and 3.21 times higher than those of the control, respectively, which indicated that cucumber roots can absorb Si from soil and transport it to leaves. The content of endogenous Si in roots and leaves in Fo treatment was 46.06% and 39.46%, respectively, higher than those in the control. Compared with Fo treatment, the content of endogenous Si in roots and leaves of Si+Fo treatment increased by 3.756 times and 3.63 times, respectively (Figure 10).
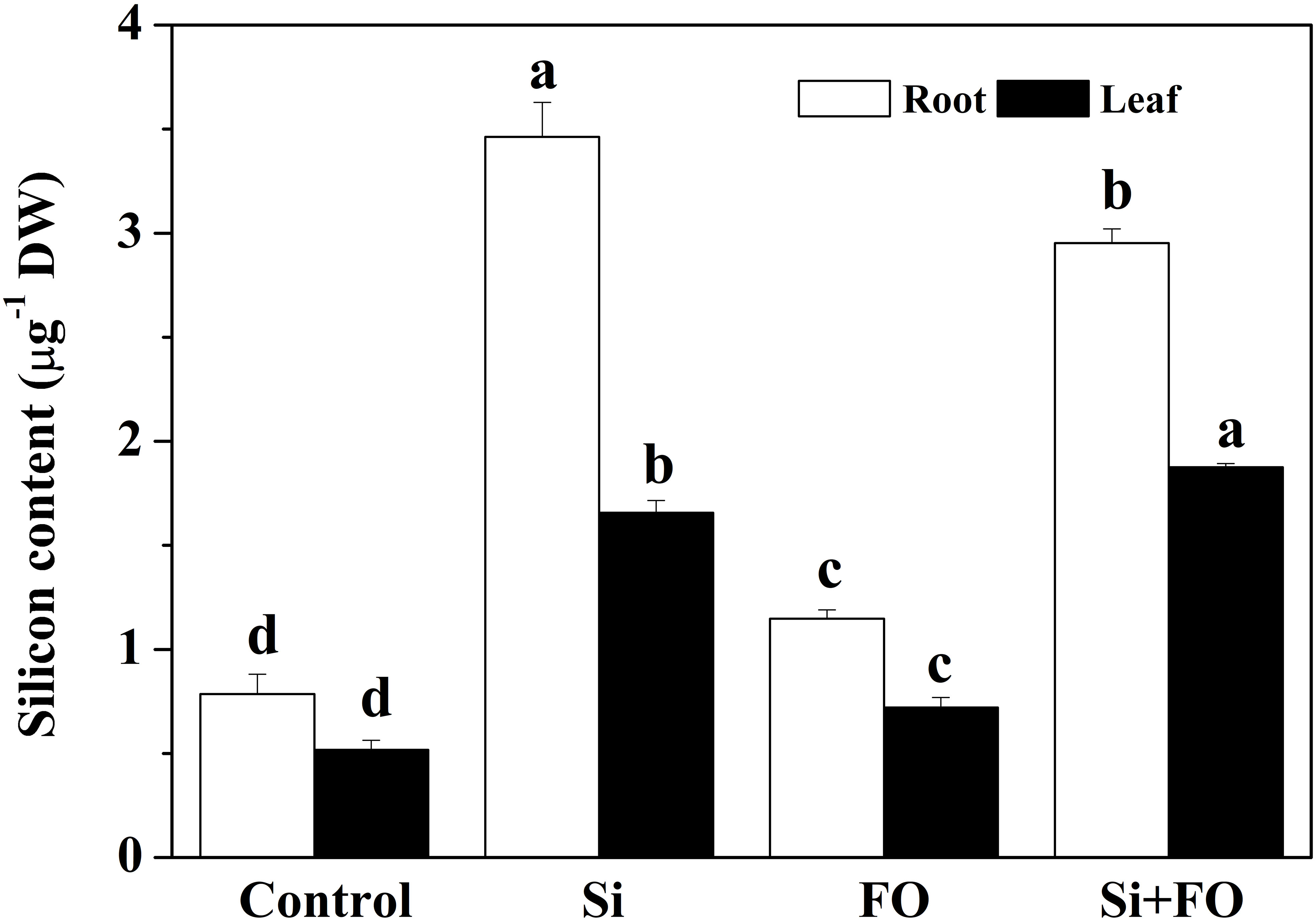
Figure 10 Effects of exogenous silicon application on endogenous silicon content in cucumber plants. Means denoted by the different lowercase letters are significantly different according to Tukey’s test (P ≤ 0.05); the mean represents the average of three replicates ± standard deviation (SD). Control: plants were irrigated with sterilized water; Si: plants were irrigated with exogenous silicon (3 mmol·l-1 Si); FO: plants were only inoculated with Fusarium; Si+FO: plants were irrigated with exogenous silicon followed by inoculation with Fusarium..
Discussion
The exogenous application of Si has demonstrated an efficient ability in reducing both soil and airborne fungal diseases in a wide variety of crops (Kaushik and Saini, 2019; Ahammed and Yang, 2021). Silicon induces disease resistance via two key mechanisms. Firstly, the deposition of Si around the cell wall fosters mechanical protection which prevents pathogen penetration. In addition, Si forms complexes with organic compounds in the epidermal cell wall which also strengthen the cell wall mechanically (Mvondo-She et al., 2021). Secondly, Si induces systemic resistance by improving the upregulation of genes involved in metabolism, signal transduction, defense, and stress response against different plant pathogens (Kurabachew et al., 2013). Moreover, silicon may interact with key plant stress signal systems and eventually induce resistance to pathogens. These mechanisms have been revealed in multiple plant species including pepper and melon (Pozo et al., 2015), cucumber (Silva et al., 2020), onion and garlic (Elshahawy et al., 2021), tomato (Madany et al., 2020), and carrot (Ahamad and Siddiqui, 2021). In the present study, we found that Si alleviated oxidative stress and the inhibition of photosynthesis in cucumber caused by the Fusarium wilt by activating the antioxidant system, thereby improving the photosynthetic capacity of cucumber leaves.
Fusarium wilt of cucumber is one of the three major soil-borne diseases, and its incidence rate is as high as 70%, which reduces the yield of cucumber by 15% to 50%, thus severely restricting the production of greenhouse cucumber (Raza et al., 2017). Silicon application can inhibit fungal diseases by increasing Si accumulation in silicified cells of the leaves of gramineous plants, forming a “keratin-silicon double layer”, or inducing plants to produce phytoalexins and antitoxins (Camargo et al., 2021). In this study, the optimum Si concentration (3 mmol/l) was selected from five different concentrations of Si based on their inhibitory effects on Fusarium growth (Figure 1). Subsequent experiments show that exogenous silicon can significantly increase the growth of cucumber plants infected by F. oxysporum, which provides a theoretical basis for the application of Si in the control of Fusarium wilt in cucumber.
Photosynthesis-related factors play a crucial role in plant metabolism and are involved in defense against pathogens (Letousey et al., 2010). Fungal diseases can inhibit photosynthetic capacity by changing the chloroplast structure and reducing the chlorophyll content and photosynthesis-related enzyme activity (Fernandez-Martinez et al., 2013). In the early stage of infection, Fusarium wilt induces decreases in the light-saturated rate of CO2 assimilation, which are accompanied by decreases in the maximum carboxylation rate and the capacity for RuBP regeneration as well as increases in stomatal limitation, in the absence of any significant photodamage to photosystem II (Nogues et al., 2002). F. oxysporum infection also induces a decrease in net photosynthetic rate in the early stage in banana plants, which is mainly resulted from stomatal limitation, and the damage to chloroplasts contributes to the reduction in the photosynthetic capacity in the later stages of infection (Dong et al., 2016). The supply of Si to rice plants played a central role in decreasing leaf scald symptoms and enhanced the maximum electron transport rate and RuBisCO activity (Pereira et al., 2020).
The expression of four Calvin cycle-related genes encoding proteins such as FBPA, TPI, SBPase, and FBPase was repressed in leaves of Fusarium-inoculated cucumber plants (Figure 8). The repression of the gene expression was in agreement with the reduction in the activity of Rubisco, FBPas, and GAPDH. Notably, SBP and Rubisco are known to have an essential role in the control of photosynthetic carbon fixation (Letousey et al., 2010), suggesting that a metabolic alteration in photosynthetic reaction occurred in leaves due to Fusarium inoculation in cucumber plants. However, Si application significantly increased the Pn, Gs, Tr, Vcmax, and Jmax activities of RuBisCO, FBPase, and GAPDH and the expression of related genes such as FBPA, TPI, SBPase, and FBPase in cucumber leaves and reduced Ci. It indicated that the decline in photosynthetic capacity caused by F. oxysporum was not only related to the decrease in stomatal conductance and the obstruction of CO2 supply but also related to the non-stomatal factors that led to the decrease in photosynthetic rate caused by the decrease in photosynthetic activity of mesophyll cells (Ahammed et al., 2020). Moreover, non-stomatal limitations of photosynthesis often involve disruptions in metabolic pathways of photosynthesis and are common in response to biotic stress (Letousey et al., 2010). In view of the close relationship between the disease resistance effect of Si and the photosynthetic physiology of plant leaves, the photosynthetic rate of leaves can be used as a reference index for screening the Si effect.
Silicon application has been shown to have a dose-dependent effect on enhancing plant resistance to diverse pathogens (Kaushik and Saini, 2019; Ahammed and Yang, 2021). The shoot Si content was negatively correlated with the severity of red crown rot caused by Calonectria ilicicola in soybean (Win et al., 2021). Moreover, aboveground biomass and seed yield at harvest increased with increasing Si concentration in soil (0.0–3.0 g Na2SiO3 kg-1 soil). However, a certain high dose of Si (6.0 g Na2SiO3 kg-1 soil) could reduce seed yield (Win et al., 2021). There were also some opposite reports. Silicon application significantly reduced the incidence of white rot and improved the growth of onion and garlic plants (Elshahawy et al., 2021). However, there were no significant differences between some treatments at 0.1%, 0.2%, and 0.3% of silicon and silicate salts. In the present study, the silicon concentration of 3 mmol/l had the best effect on the improvement of photosynthesis, but when the silicon concentration increased to 5 mmol/l, the effect was largely decreased. This might be correlated with the alterations of the homeostatic network of mineral elements (Etesami and Jeong, 2018).
More evidence showed that Si may participate in the metabolic process to enhance plant disease resistance through a series of physiological and biochemical reactions and signal transduction (Ahammed and Yang, 2021). Enhanced resistance is achieved by activating host defense genes and inducing the production of a series of low molecular weight metabolites. Firstly, Si can enhance the activities of protective enzymes to improve disease resistance (Etesami and Jeong, 2018). Silicon was associated with increased activity of superoxide dismutase in shoots and roots and increased tissue concentrations of phenolics, proline, and antioxidants, but reduced levels of H2O2 (Moradtalab et al., 2018). Secondly, Si can improve host resistance to diseases by inducing the production of some secondary metabolites or antibacterial substances such as phytoalexin, lignin, phenols, and pathogen-related proteins (Ahammed and Yang, 2021). Notably, the induction of antioxidant defense is often associated with ROS generation under stress conditions, particularly in response to pathogen attack. In the present study, Si application (3 mmol/l) significantly increased the antioxidant enzyme activities and decreased H2O2, O·−2O⋅−2, and MDA contents in leaves of cucumber under the stress caused by F. oxysporum inoculation, thus mitigating oxidative damage.
In conclusion, in the current study, we screened out effective concentrations of Si that could significantly inhibit the mycelial growth of Fo and revealed the plant defense mechanisms triggered by exogenous silicon in response to Fo inoculation in cucumber plants. Exogenous Si (3 mmol l-1) application combined with Fo inoculation substantially boosted plant growth, root activity, and antioxidant enzyme activity but reduced ROS accumulation and lipid peroxidation compared to Fo alone (Figure 11). Silicon treatment also increased photosynthetic capacity by alleviating both stomatal and non-stomatal limitations, particularly by attenuating stomatal closure and increasing activities of CO2 assimilation-related enzymes such as RuBisCO. Taken together, our study suggests that exogenous silicon can increase cucumber resistance to Fusarium wilt by increasing antioxidant defense, photosynthetic capacity, and stomatal opening. However, further studies are still required to better understand how silicon regulates plant signal transduction to enhance plant resistance against pathogenic fungi.
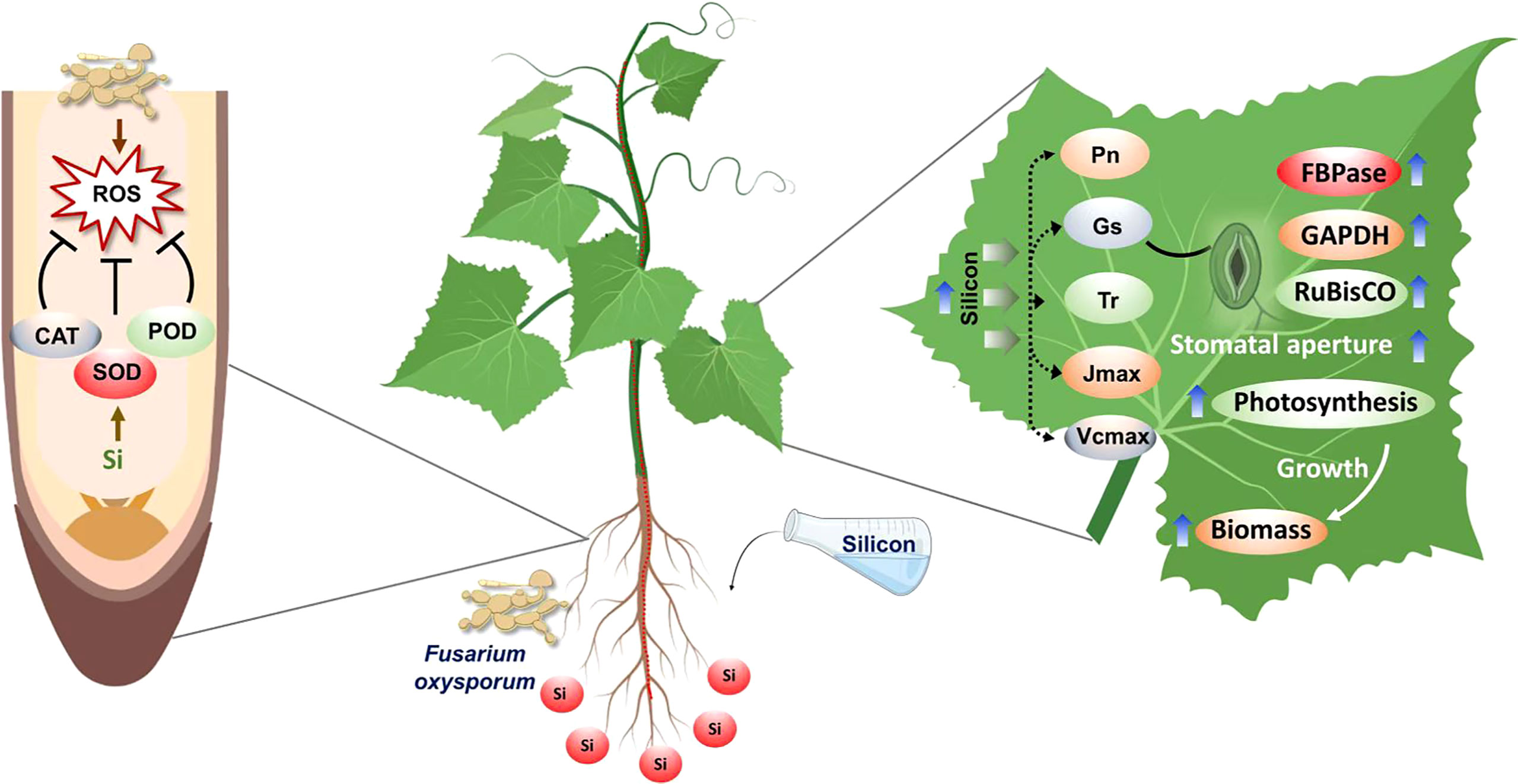
Figure 11 A working model depicting the mechanism of silicon-mediated alleviation of Fusarium wilt stress as revealed in the present study.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
SS: methodology, formal analysis, writing—review and editing. ZY: methodology, formal analysis, investigation, writing—original draft. ZS: formal analysis, investigation. NW: formal analysis, investigation. NG: formal analysis, investigation. JN: formal analysis, investigation. AL: conceptualization, supervision, funding acquisition. BB: supervision. GJA: conceptualization, writing—review and editing, supervision, funding acquisition. SC: conceptualization, writing—original draft, writing—review and editing, supervision, funding acquisition, project administration. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Key Research and Development Program of China (2018YFD1000800), National Natural Science Foundation of China (31872092, 31872157, 31950410555), Natural Science Foundation of Henan (202300410152), Innovative Research Team (in Science and Technology) in University of Henan Province (23IRTSTHN024), Scientific and Technological Research Project of Henan (222102110078), Luoyang Rural Revitalization Project (2101101A), and Ministry of Science and Technology of the People’s Republic of China (DL2022026004L, QNJ2021026001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2022.1011859/full#supplementary-material
References
Aebi, H. (1984). Catalase in vitro. Method. Enzymol. 105, 121–30. doi: 10.1016/S0076-6879(84)05016-3
Ahamad, L., Siddiqui, Z.A. (2021). Effects of silicon dioxide, zinc oxide and titanium dioxide nanoparticles on Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani disease complex of carrot. Exp. Parasitol. 230, 108176. doi: 10.1016/j.exppara.2021.108176
Ahammed, G. J., Mao, Q., Yan, Y. R., Wu, M. J., Wang, Y., Ren, J. J., et al. (2020). Role of melatonin in arbuscular mycorrhizal fungi-induced resistance to Fusarium wilt in cucumber. Phytopathology 110, 999–1009. doi: 10.1094/PHYTO-11-19-0435-R
Ahammed, G. J., Yang, Y. X. (2021). Mechanisms of silicon-induced fungal disease resistance in plants. Plant Physiol. Bioch. 165, 200–206. doi: 10.1016/j.plaphy.2021.05.031
Al Sadi, A. M., Al Said, F. A., Deadman, M. L., Drenth, A., Aitken, E. A. B. (2010). Can silicon improve defense responses of cucumber seedlings to Pythium-induced damping off disease? Acta Hortic. 871, 441–444. doi: 10.17660/ActaHortic.2010.871.61
Cakmak, I., Marschner, H. (1992). Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 98, 1222–1227. doi: 10.1104/pp.98.4.1222
Camargo, M. S., Honaine, M. F., Osterrieth, M., Bozza, N. G., Silva, V. D., Benvenuto, M. L., et al. (2021). Silicon fertilization increases gas-exchange and biomass by silicophytolith deposition in the leaves of contrasting drought-tolerant sugarcane cultivars under well-watered conditions. Plant Soil 466, 581–595. doi: 10.1007/s11104-021-05063-z
Chaiwong, N., Prom-u-thai, C. (2022). Significant roles of silicon for improving crop productivity and factors affecting silicon uptake and accumulation in rice: a review. J. Soil Sci. Plant Nutt. 22, 1970–1982. doi: 10.1007/s42729-022-00787-y
D'Addazio, V., Silva, J. V. G., Jardim, A. S., Longue, L. L., Santos, R. A. A., Fernandes, A. A., et al. (2020). Silicon improves the photosynthetic performance of black pepper plants inoculated with Fusarium solani f. sp. piperis. Photosynthetica 58, 692–701. doi: 10.32615/ps.2019.182
Dong, X., Wang, M., Ling, N., Shen, Q. R., Guo, S. W. (2016). Potential role of photosynthesis-related factors in banana metabolism and defense against Fusarium oxysporum f. sp cubense. Environ. Exp. Bot. 129, 4–12. doi: 10.1016/j.envexpbot.2016.01.005
Durner, J., Klessig, D. F. (1995). Inhibition of ascorbate peroxidase by salicylic acid and 2,6-dichloroisonicotinic acid, two inducers of plant defense responses. P. Natl. Acad. Sci. U.S.A. 92, 11312–6. doi: 10.1073/pnas.92.24.11312
Elshahawy, I. E., Osman, S. A., Abd-El-Kareem, F. (2021). Protective effects of silicon and silicate salts against white rot disease of onion and garlic, caused by Stromatinia cepivora. J. Plant Pathol. 103, 27–43. doi: 10.1007/s42161-020-00685-1
Elstner, E. F., Heupel, A. (1976). Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 70, 616–620. doi: 10.1016/0003-2697(76)90488-7
Etesami, H., Jeong, B. R. (2018). Silicon (Si): Review and future prospects on the action mechanisms in alleviating biotic and abiotic stresses in plants. Ecotox. Environ. Safe 147, 881–896. doi: 10.1016/j.ecoenv.2017.09.063
Ethier, G. J., Livingston, N. J. (2004). On the need to incorporate sensitivity to CO2 transfer conductance into the farquhar-von caemmerer berry leaf photosynthesis model. Plant Cell Environ. 27, 137–153. doi: 10.1111/j.1365-3040.2004.01140.x
Fernandez-Martinez, J., Zacchini, M., Elena, G., Fernandez-Marin, B., Fleck, I. (2013). Effect of environmental stress factors on ecophysiological traits and susceptibility to pathogens of five Populus clones throughout the growing season. Tree Physiol. 33, 618–627. doi: 10.1093/treephys/tpt039
Fortunato, A. A., da Silva, W. L., Rodrigues, F. A. (2014). Phenylpropanoid pathway is potentiated by silicon in the roots of banana plants during the infection process of Fusarium oxysporumf. sp. cubense. Phytopathology 104, 597–603. doi: 10.1094/PHYTO-07-13-0203-R
Giannakoula, A., Moustakas, M., Syros, T., Yupsanis, T. (2010). Aluminum stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ. Exp. Bot. 67, 487–94. doi: 10.1016/j.envexpbot.2009.07.010
Haghighi, M., Pessarakli, M. (2013). Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum l.) at early growth stage. Sci. Hortic. 161, 111–117. doi: 10.1016/j.scienta.2013.06.034
Hodges, D. M., DeLong, J. M., Forney, C. F., Prange, R. K. (1999). Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207, 604–611. doi: 10.1007/s004250050524
Hoffmann, J., Berni, R., Hausman, J. F., Guerriero, G. (2020). A review on the beneficial role of silicon against salinity in non-accumulator crops: tomato as a model. Biomolecules 10, 1284. doi: 10.3390/biom10091284
Huang, C. H., Roberts, P. D., Datnoff, L. E. (2011). Silicon suppresses Fusarium crown and root rot of tomato. J. Phytopathol. 159, 546–554. doi: 10.1111/j.1439-0434.2011.01803.x
Isack, Y., Benichis, M., Gillet, D., Gamliel, A. (2014). A selective agar medium for isolation, enumeration and morphological identification of Fusarium proliferatum. Phytoparasitica 42, 541–547. doi: 10.1007/s12600-014-0392-7
Jain, U., Saxena, K., Hooda, V., Balayan, S., Singh, A. P., Tikadar, M., et al. (2022). Emerging vistas on pesticides detection based on electrochemical biosensors - an update. Food Chem. 371, 131126. doi: 10.1016/j.foodchem.2021.131126
Kaushik, P., Saini, D. K. (2019). Silicon as a vegetable crops modulator–a review. Plants 8, 148. doi: 10.3390/plants8060148
Kurabachew, H., Stahl, F., Wydra, K. (2013). Global gene expression of rhizobacteria-silicon mediated induced systemic resistance in tomato (Solanum lycopersicum) against Ralstonia solanacearum. Physiol. Mol. Plant P. 84, 44–52. doi: 10.1016/j.pmpp.2013.06.004
Lamikanra, O., Watson, M. A. (2001). Effects of ascorbic acid on peroxidase and polyphenoloxidase activities in fresh-cut cantaloupe melon. J. Food Sci. 66, 1283–1286. doi: 10.1111/j.1365-2621.2001.tb15202.x
Letousey, P., Baillieul, F., Perrot, G., Rabenoelina, F., Boulay, M., Vaillant-Gaveau, N., et al. (2010). Early events prior to visual symptoms in the apoplectic form of grapevine esca disease. Phytopathology® 100 (5), 424–431. doi: 10.1094/phyto-100-5-0424
Lilley, R. M., Walker, D. A. (1974). An improved spectrophotometric assay for ribulose-bisphosphate carboxylase. Biochim. Biophys. Acta 358, 226–229. doi: 10.1016/0005-2744(74)90274-5
Lin, W. P., Jiang, N. H., Peng, L., Fan, X. Y., Gao, Y., Wang, G. P., et al. (2020). Silicon impacts on soil microflora under Ralstonia solanacearum inoculation. J. Integr. Agr. 19, 251–264. doi: 10.1016/S2095-3119(18)62122-7
Liu, A. R., Chen, S. C., Chen, K., Lin, X. M., Wang, F. H. (2010). Antagonism effect of Trichoderma harzianum against Fusarium oxysporum on cucumber and related gene expression analysis. Acta Phytophylacica Sin. 37, 249–254.
Livak, K. J., Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Li, Y. T., Zhang, W. J., Cui, J. J., Lang, D. Y., Li, M., Zhao, Q. P., et al. (2016). Silicon nutrition alleviates the lipid peroxidation and ion imbalance of Glycyrrhiza uralensis seedlings under salt stress. Acta Physiol. Plant 38, 96. doi: 10.1007/s11738-016-2108-8
Madany, M. M. Y., Saleh, A. M., Habeeb, T. H., Hozzien, W. N., AbdElgawad, H. (2020). Silicon dioxide nanoparticles alleviate the threats of broomrape infection in tomato by inducing cell wall fortification and modulating ROS homeostasis. Environ. Sci.-Nano 7, 1415–1430. doi: 10.1039/C9EN01255A
Moradtalab, N., Weinmann, M., Walker, F., Hoglinger, B., Ludewig, U., Neumann, G. (2018). Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 9, 420. doi: 10.3389/fpls.2018.00420
Mvondo-She, M. A., Gatabazi, A., Laing, M. D., Ndhlala, A. R. (2021). A review on the role of silicon treatment in biotic stress mitigation and citrus production. Agronomy-Basel 11, 2198. doi: 10.3390/agronomy11112198
Nogues, S., Cotxarrera, L., Alegre, L., Trillas, M. I. (2002). Limitations to photosynthesis in tomato leaves induced by Fusarium wilt. New Phytol. 154, 461–470. doi: 10.1046/j.1469-8137.2002.00379.x
Pavlovic, J., Kostic, L., Bosnic, P., Kirkby, E. A., Nikolic, M. (2021). Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci. 12, 697592. doi: 10.3389/fpls.2021.697592
Pereira, L. F., Martins, S. C. V., Aucique-Perez, C. E., Silva, E. T., Avila, R. T., DaMatta, F. M., et al. (2020). Silicon alleviates mesophyll limitations of photosynthesis on rice leaves infected by Monographella albescens. Theor. Exp. Plant Phys. 32, 163–174. doi: 10.1007/s40626-020-00178-7
Piattoni, C., Guerrero, S., Iglesias, A. (2013). A differential redox regulation of the pathways metabolizing glyceraldehyde-3-phosphate tunes the production of reducing power in the cytosol of plant cells. Int. J. Mol. Sci. 14, 8073–8092. doi: 10.3390/ijms14048073
Pozo, J., Urrestarazul, M., Morales, I., Sanchez, J., Santos, M., Dianez, F., et al. (2015). Effects of silicon in the nutrient solution for three horticultural plant families on the vegetative growth, cuticle, and protection against Botrytis cinerea. Hortscience 50, 1447–1452. doi: 10.21273/HORTSCI.50.10.1447
Raza, W., Ling, N., Zhang, R. F., Huang, Q. W., Xu, Y. C., Shen, Q. R. (2017). Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 37, 202–212. doi: 10.3109/07388551.2015.1130683
Savvas, D., Ntatsi, G. (2015). Biostimulant activity of silicon in horticulture. Sci. Hortic. 196, 66–81. doi: 10.1016/j.scienta.2015.09.010
Scheibe, R., Fickenscher, K., Ashton, A. R. (1986). Studies on the mechanisms of the reductive activation of NADP-malate dehydrogenase by thioredoxin m and low molecular weight thiols. Biochim. Biophys. Acta 870, 191–197. doi: 10.1016/0167-4838(86)90221-9
Shivaraj, S. M., Mandlik, R., Bhat, J. A., Raturi, G., Elbaum, R., Alexander, L., et al. (2022). Outstanding questions on the beneficial role of silicon in crop plants. Plant Cell Physiol. 63, 4–18. doi: 10.1093/pcp/pcab145
Silva, P. R. A., Araujo, L., Resende, R. S., Maffia, L. A., Einhardt, A. M., Oliveira, H. R., et al. (2020). Silicon, Clonostachys rosea, and their interaction for gray mold management in cucumber. J. Plant Pathol. 102, 1257–1262. doi: 10.1007/s42161-020-00643-x
Sun, R. L., Jing, Y. L., de Boer, W., Guo, R. J., Li, S. D. (2021). Dominant hyphae-associated bacteria of Fusarium oxysporum f. sp. cucumerinum in different cropping systems and insight into their functions. Appl. Soil Ecol. 165, 103977. doi: 10.1016/j.apsoil.2021.103977
von Caemmerer, S., Farquhar, G. D. (1981). Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387. doi: 10.1007/BF00384257
Whan, J. A., Dann, E. K., Aitken, E. A. B. (2016). Effects of silicon treatment and inoculation with Fusarium oxysporum f. sp vasinfectum on cellular defences in root tissues of two cotton cultivars. Ann. Bot. 118, 219–226. doi: 10.1093/aob/mcw095
Willekens, H., Chamnongpol, S., Davey, M., Schraudner, M., Langebartels, C., Van Montagu, M., et al. (1997). Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 16, 4806–4816. doi: 10.1093/emboj/16.16.4806
Win, K. T., Maeda, S., Kobayashi, M., Jiang, C.-J. (2021). Silicon enhances resistance to red crown rot caused by Calonectria ilicicola in soybean. Agronomy 11, 899. doi: 10.3390/agronomy11050899
Xue, X. J., Geng, T. T., Liu, H. F., Yang, W., Zhong, W. R., Zhang, Z. L., et al. (2021). Foliar application of silicon enhances resistance against Phytophthora infestans through the ET/JA- and NPR1-dependent signaling pathways in potato. Front. Plant Sci. 12, 609870. doi: 10.3389/fpls.2021.609870
Yoshid, A. S. (1965). Chemical aspects of the role of silicon in physiology rice plant. Bull. Natl. Institute Agric. Sci. Ser. B 15, 1–58.
Zhai, Y., Zhu, J. X., Tan, T. M., Xu, J. P., Shen, A. R., Yang, X. B., et al. (2021). Isolation and characterization of antagonistic Paenibacillus polymyxa HX-140 and its biocontrol potential against Fusarium wilt of cucumber seedlings. BMC Microbiol. 21, 75. doi: 10.1186/s12866-021-02131-3
Zhou, Y. H., Lam, H. M., Zhang, J. H. (2007). Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J. Exp. Bot. 58, 1207–1217. doi: 10.1093/jxb/erl291
Keywords: silicon, cucumber, Fusarium oxysporum, photosynthesis, antioxidant system
Citation: Sun S, Yang Z, Song Z, Wang N, Guo N, Niu J, Liu A, Bai B, Ahammed GJ and Chen S (2022) Silicon enhances plant resistance to Fusarium wilt by promoting antioxidant potential and photosynthetic capacity in cucumber (Cucumis sativus L.). Front. Plant Sci. 13:1011859. doi: 10.3389/fpls.2022.1011859
Received: 04 August 2022; Accepted: 16 September 2022;
Published: 13 October 2022.
Edited by:
Sek-Man Wong, National University of Singapore, SingaporeReviewed by:
Arafat Abdel Hamed Abdel Latef, South Valley University, EgyptKrishan K. Verma, Guangxi Academy of Agricultural Sciences, China
Copyright © 2022 Sun, Yang, Song, Wang, Guo, Niu, Liu, Bai, Ahammed and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Golam Jalal Ahammed, ahammed@haust.edu.cn; Shuangchen Chen, chen_shuangchen@126.com; Airong Liu, evallyn@163.com
 Shuangsheng Sun1
Shuangsheng Sun1