- 1State Key Laboratory of Severe Weather, Chinese Academy of Meteorological Sciences, Beijing, China
- 2College of Applied Meteorology, Nanjing University of Information Science & Technology, Nanjing, China
- 3Gucheng Agrometeorological Field Scientific Experiment Base, China Meteorological Administration, Baoding, China
- 4Collaborative Innovation Center on Forecast Meteorological Disaster, Warning and Assessment, Nanjing University of Information Science & Technology, Nanjing, China
The maximizing of water use efficiency (WUE) and radiation use efficiency (RUE) is vital to improving crop production in dryland farming systems. However, the fundamental question as to the association of WUE with RUE and its underlying mechanism under limited-water availability remains contentious. Here, a two-year field trial for maize designed with five progressive soil drying regimes applied at two different growth stages (three-leaf stage and seven-leaf stage) was conducted during the 2013–2014 growing seasons. Both environmental variables and maize growth traits at the leaf and canopy levels were measured during the soil drying process. The results showed that leaf WUE increased with irrigation reduction at the early stage, while it decreased with irrigation reduction at the later stage. Leaf RUE thoroughly decreased with irrigation reduction during the progressive soil drying process. Aboveground biomass (AGB), leaf area index (LAI), a fraction of absorbed photosynthetically active radiation (fAPAR), and light extinction coefficient (k) of the maize canopy were significantly decreased by water deficits regardless of the growth stages when soil drying applied. The interrelationships between WUE and RUE were linear across the leaf and canopy scales under different soil drying patterns. Specifically, a positive linear relationship between WUE and RUE are unexpectedly found when soil drying was applied at the three-leaf stage, while it turned out to be negative when soil drying was applied at the seven-leaf stage. Moreover, the interaction between canopy WUE and RUE was more regulated by fAPAR than LAI under soil drying. Our findings suggest that more attention must be paid to fAPAR in evaluating the effect of drought on crops and may bring new insights into the interrelationships of water and radiation use processes in dryland agricultural ecosystems.
Introduction
Maize (Zea mays L.), one of the world’s top three cultivated kinds of cereal along with wheat and rice (FAO, 2020), is typically cultivated in semi-arid and semi-humid areas in China. Even though maize is a C4 plant with higher temperature adaptation and lower water consumption than wheat and rice (Sanchez et al., 2014), its growth and productivity are more vulnerable to climate change than other staple crops (Tao et al., 2008). Therefore, it is not surprising that a worldwide significant decrease in maize yield trend can be attributed to the climate anomalies and extremes, especially seasonal drought and heat stress (Tao and Zhang, 2010; Lobell et al., 2011; Zampieri et al., 2019). North China Plain (NCP) is a drought-prone region, and also one of the foremost dryland agricultural production areas in China (Fang et al., 2021). Past and present climate trends and variability in NCP were characterized as increased air temperature and decreased precipitation (Ming et al., 2015). To make matters worse, the situation is expected to be further intensified under projected future climate, accompanied by a decrease in solar radiation (Liu et al., 2013; Huang et al., 2017). Adapting maize growth to the changing climate environments (decreasing solar radiation, decreasing precipitation, increasing temperature, etc.) will be an important innovation to increase total biomass and then grain yield (Yang et al., 2019; Kan et al., 2020). Therefore, it is particularly urgent and desirable to evaluate the relative potential of resource use efficiency and then enhance them for the sustainability of agricultural development in this drought-prone region.
Water use efficiency (WUE), defined as the ratio of carbon gain to water loss, is an important physiological indicator in assessing the interactions between the carbon and water cycles (Farquhar and Richards, 1984; Beer et al., 2009). Previous studies have enhanced the understanding that moderate drought has a stimulatory effect on crop WUE [e.g., durum wheat (Bhattarai et al., 2020), maize (Kang et al., 1998), and sweet sorghum (Dercas and Liakatas, 2006)]. However, the degree and duration of drought also play an important role in affecting WUE (Ma et al., 2019), as well as crop species and developmental stages. The underlying mechanism of progressive soil drying on WUE and its connection with other resource use efficiency remains unclear. Like soil water, solar radiation is the ultimate energy source for crop development and production (Monteith et al., 1977; Wild et al., 2005), but it also can cause stress to plants and modulate response to water deficit (Roeber et al., 2020). Radiation use efficiency (RUE), an important determinant of carbon sequestration by terrestrial ecosystems, indicates the efficiency of a plant to convert absorbed photosynthetically active radiation (PAR) of 400–700 nm wavelength into biomass (Monteith, 1972). In dryland agriculture, crop productivity is directly related to the efficiency to convert resources into biological materials, especially water and solar radiation. Therefore, improving our understanding of how to quantify the interaction of WUE and RUE and how they affect crop productivity is necessary for optimizing dryland agricultural management practice (Ding et al., 2021).
A large body of literature has emphasized the effects of water deficit only on WUE or RUE either at the leaf or canopy level (Steduto and Albrizio, 2005; Yi et al., 2010; VanLoocke et al., 2012; Marwein et al., 2016; Song et al., 2018; Hatfield and Dold, 2019; Cai et al., 2020; Yu et al., 2020). High WUE was proved to be related to low RUE under probable drought (Dercas and Liakatas, 2006). In contrast, WUE and RUE are directly proportional under optimal growth conditions (Ullah et al., 2019; Kukal and Irmak, 2020). However, little is known about the interrelationships of radiation and water use processes at both leaf and canopy levels in agricultural ecosystems. In addition, the connection between WUE and RUE response to progressive soil drying at different growth stages remains unclear. WUE and RUE are inversely related to canopy conductance (gc) (Sadras et al., 1991; Hernández et al., 2021), which may be suitable to distinguish and communicate the WUE and RUE relations (Kukal and Irmak, 2020). In addition, changes in canopy structure [e.g., leaf angle, plant height, leaf area index (LAI)] reflect both in light transmittance, and reflectance under water stress conditions (Holmes and Keiller, 2002; Onoda et al., 2014). The variation of crop evapotranspiration is greatly affected by environmental and vegetation factors, which are directly or indirectly mediated by changes in biotic factors, such as LAI (Zhou et al., 2019). LAI is a primary descriptor of vegetation function and structure, and is functionally related to the exchange of water and energy between vegetation and the atmosphere (Running, 1984; Liu et al., 2016). What’s more, the fraction of absorbed photosynthetically active radiation (fAPAR) by the canopy is a key variable not only in assessing the production of the vegetation, but also in the efficiency of light usage (Gitelson et al., 2014). However, it’s still not very clear about how the interrelationship between WUE and RUE are mediated by LAI and fAPAR and their relative influence pathway.
Here, we used an experiment-based approach to explore the link between WUE and RUE and its underlying mechanism under soil drying at different developmental stages. Specifically, the objectives were to address the following questions: (i) How does WUE and RUE respond to soil drying? (ii) How does WUE commutate with RUE at both leaf and canopy scales? (iii) Does the association between WUE and RUE change with different progressive soil drying patterns and growth stages? (iv) What are the direct and indirect pathways by which LAI and fAPAR mediate the association of WUE with RUE under limited-water availability?
Materials and Methods
Experiment Site
The field experiment was conducted in two growing seasons (from June to October 2013 and 2014) at the Gucheng Agro-meteorological Field Scientific Experiment Base (39°08′N, 115°40′E and 15.2 m a.s.l.) in Baoding, Hebei Province, China. The site is a typical maize production region located on NCP, with a warm temperate continental monsoon climate. The mean annual temperature (1981–2010), annual precipitation, and average sunshine duration are 12.2°C, 515.5 mm, and 2264 h yr–1, respectively. The variations in the daily mean air temperature (T), PAR, and precipitation (P) for the growing seasons in 2013 and 2014 are shown in Figure 1. The soil type is classified as sandy loam, with total potassium, total phosphorus, and total nitrogen contents of 17.26 g kg–1, 1.02 g kg–1, and 0.98 g kg–1, respectively (Fang et al., 2013). The mean pH and soil bulk density within a depth of 50 cm are 8.19 and 1.37 g cm–3 (Wang Q. et al., 2018). The average field capacity is 22.7% (g g–1) and the wilting point is 5.0% (g g–1). Double cropping with two crop harvestings (wheat–maize rotation) in a year is conventional farming practice in this region. The principal crops are winter wheat (Triticum aestivum), maize (Zea mays), potato (Solanum tuberosum), peanut (Arachis hypogaea), and soybean (Glycine max).
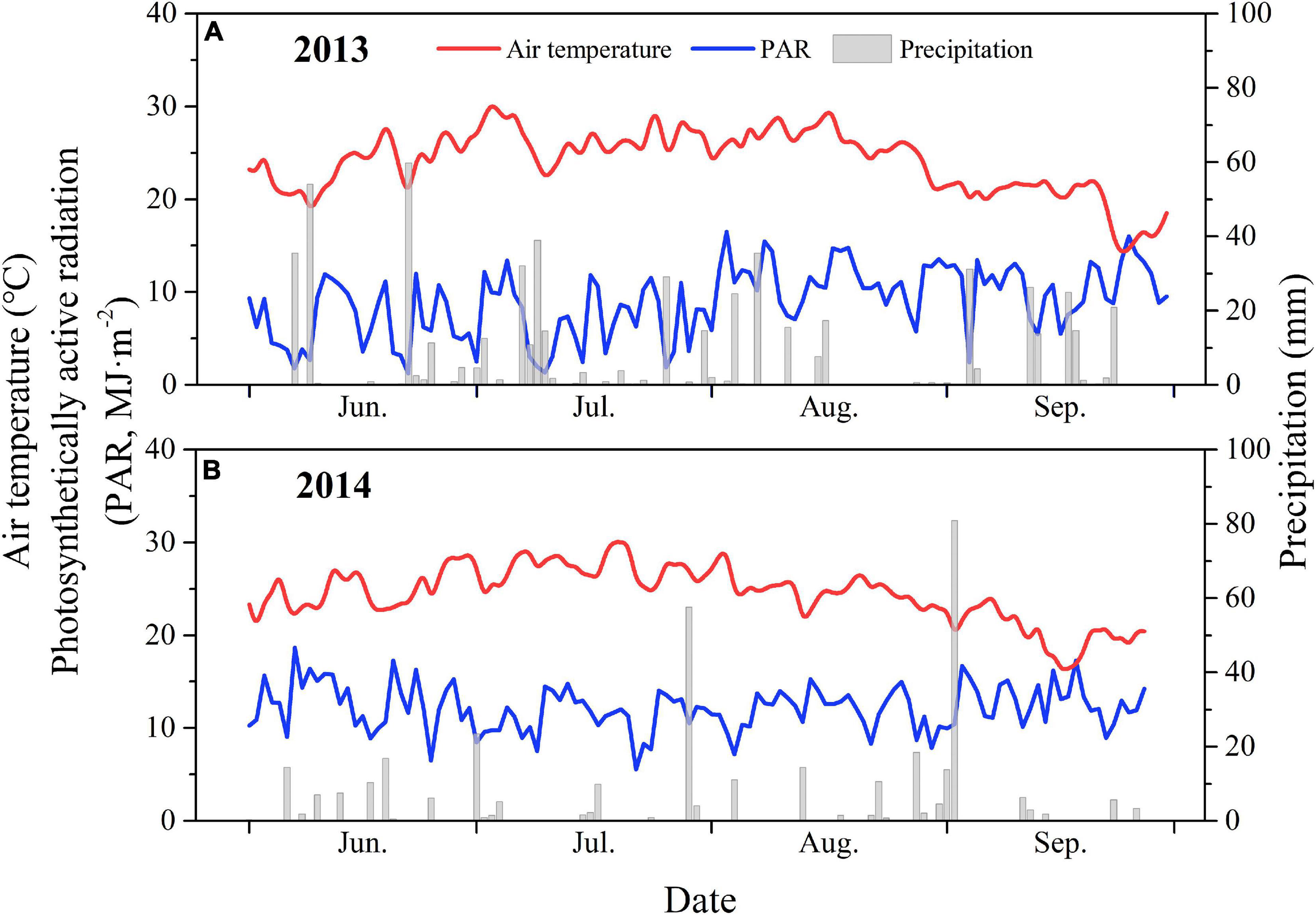
Figure 1. Daily mean air temperature (T, °C), precipitation (P, mm), photosynthetically active radiation (PAR, MJ m–2) at the study site in 2013 (A) and 2014 (B) growing seasons.
Experimental Design and Farm Management
The study was carried out in the field with a randomized complete block design. The area of each plot was 8 m2 (4 m long, 2 m wide). To block out the natural precipitation, a large electric-powered waterproof shelter was used to cover the plots when it was rainy, otherwise, it was moved away to keep the experimental plots fully exposed to the ambient conditions. In addition, cement walls with a depth of 3.0 m were installed between each plot to avoid horizontal water exchange across plots. The selected cultivar was zhengdan-958, which is a drought-tolerant variety that has been widely planted on the NCP over the last two decades. Approximately 1 month before seeding, the soil moisture of each plot from 0 to 100 cm at 10 cm interval was measured and calculated, and then each plot was irrigated to reach the same water condition. A controlled-release fertilizer called diammonium phosphate (DAP), with N and P2O5 accounting for 16 and 45% of the total mass, was conventionally applied before sowing at a rate of 320 kg⋅hm–2. Maize seeds were sown on June 27, 2013, and June 24, 2014, during the two growing seasons. The plant density was set to 52 plants per plot (65000 plants hm–2). Prior to the imposed prolonged drought, all plots were well irrigated to encourage seedling emergence. The irrigation amounts were designed based on the mean precipitation (150 mm) in July from 1981 to 2010. In 2013, five irrigation amounts were conducted at the beginning of the seven-leaf stage (July 24, 27 days after sowing (DAS)), 80 mm (T1, equal to 53.3% of 150 mm precipitation in July), 60 mm (T2, 40%), 40 mm (T3, 26.7%), 25 mm (T4, 16.7%), and 15 mm (T5, 10%). In 2014, another five irrigation treatments were carried out at the beginning of the three-leaf stage (July 2, 8 DAS), 150 mm (W1, 100%), 120 mm (W2, 80%), 90 mm (W3, 60%), 60 mm (W4, 40%), and 30 mm (W5, 20%) (Zhou et al., 2021). Each treatment was designed with three replicate plots. All irrigations were recorded by a water meter. After the irrigation, no more irrigation water or precipitation was applied during the remaining growing seasons. The maize crops were harvested on October 8, 2013, and October 9, 2014, respectively.
Measurements of Environmental Variables and Maize Growth Traits
Available Soil Water Content and Soil Water Storage
During the two growing seasons, the soil water content was measured using the oven-drying method with an interval of 7–14 days. As more than 95% root biomass of maize was expected to grow within the 0–30 cm soil layers (Wang S. et al., 2018), and it was laborious to take soil samples under drought conditions, the sampling depth was set up to 50 cm. Soil samples were collected at a 10-cm interval, and soil cores were randomly chosen from the middle area of two rows of maize plants in the center of each plot. The gravimetric water content of each soil layer was measured by taking weights before and after drying the soil at 105°C to a constant weight. The average soil water content was determined by the mean value of three different sampling positions for each treatment. There were 8 measurements performed throughout the 2013 and 2014 growing seasons. The available soil water content (ASWC, %) of each layer and soil water storage (SWS, mm) was calculated according to the following equations (Haghverdi et al., 2015; Cosentino et al., 2016):
where SWC (g g–1) is the soil water content on a mass basis; SFM (g) and SDM (g) are the soil fresh and dry mass, respectively. FC (g g–1) is the field capacity, and WP (g g–1) is the wilting point. SWCi is the soil water content of the ith soil layer; hi (cm) is the thickness of the ith soil layer; ρi (g cm–3) is the soil bulk density of the ith soil layer, and n is the number of soil layers measured.
Evapotranspiration (ET, mm) which includes soil water evaporation and crop transpiration for different growth periods of maize was calculated based on the water balance equation as follows:
where I (mm) is the irrigation amount, P (mm) is precipitation, which is blocked out by the shelter since sowing (here P = 0), K (mm) is the groundwater influx into the root zone, D (mm) is the drainage, and R (mm) is surface runoff. In this experiment, K, D, and R were negligible. Therefore, the soil–water balance equation is reduced as (Guan et al., 2015):
where SWSstart (mm) and SWSend (mm) are SWS at the start and end of the growth period at interest, respectively.
Measurement of Leaf Gas Exchange Parameters
Leaf gas exchange parameters were measured by using a portable photosynthesis system (Li-6400XT; Li-Cor, Lincoln, NE, United States) with a fluorescence leaf chamber (Li-6400-40). Three to five healthy and representative maize plants (at least 0.5 m from the plot edge) were selected in each treatment with the same sampling interval as the soil water content measurement. In detail, the measurements were conducted on 33 DAS and 16 DAS in 2013 and 2014 growing seasons, respectively. Therefore, there were 6 and 7 measurements during experimental periods in 2013 and 2014, respectively. Measurements were conducted in the middle area of the newly fully expanded leaves in the morning (9:30–11:30) on clear days. The environmental conditions in the leaf chamber were controlled with a reference CO2 concentration of 380–410 μmol⋅mol–1 and an airflow rate of 500 μmol⋅s–1. The air temperature, relative air humidity, and photosynthetic photon flux density were maintained under ambient conditions. The net photosynthetic rate (Pn), transpiration rate (Tr), and leaf photosynthetically active radiation (PARleaf) were recorded. Water use efficiency (WUEleaf) and radiation use efficiency (RUEleaf) at the leaf level was determined by the following equations (Farquhar and Richards, 1984; Yang et al., 2018):
Leaf Area Index and Aboveground Biomass
Three healthy maize plants were randomly selected from each treatment and destructively harvested with the same sampling interval as leaf gas exchange measurement. The aboveground plant organs were clearly separated and quickly weighed to avoid excessive water loss. Aboveground biomass (AGB) included all the leaves, leaf sheath, shoot, tassel, and ear. The plant leaf area was determined by the Montgomery method (Stewart and Dwyer, 1999), with the maximum length (L, cm) and width (W, cm) of each leaf measured by a ruler. All fresh plant organs were oven dried at a temperature of 105°C for 1 h and were kept at 80°C until a constant dry mass was obtained (Sun et al., 2019). The leaf area (LA, cm2) of each maize plant, LAI (m2 m–2), and AGB (g m–2) at the canopy level were calculated as follows:
where n is the total number of leaves per maize plant, m is the number of repetitions, d is the plant density (plants m–2), and AGBj is the total aboveground biomass of the selected plant (g plant–1).
Measurement of Photosynthetically Active Radiation, Fraction of Absorbed Photosynthetically Active Radiation, and the Cumulative Amount PAR Intercepted by the Canopy
Photosynthetically active radiation was measured by a line quantum sensor with 64 photodiodes (SunScan, Delta T Devices Ltd., Cambridge, United Kingdom) at 11:30–14:00 on clear days. The measured solar radiation included the incoming PAR above the canopy (PARin), PAR reflected by the canopy and soil (PARout), PAR transmitted through the canopy (PARtran), and PAR reflected by the soil (PARsoil). PARin and PARout were measured using the quantum sensor placed horizontally 1.0 m above the maize canopy surface pointing toward the sky and ground, respectively; PARtran was measured with line quantum sensors placed at about 5 cm above the ground, pointing upward; and PARsoil was measured with line quantum sensors placed about 12 cm above the ground, pointing downward (Gitelson et al., 2014). Each kind of PAR was obtained with the mean value of three different measurement directions. The fAPAR was determined by the following formula, which excluded the noise of the bare soil background (Gallo and Daughtry, 1984):
Then, daily LAI values were calculated by a cubic spline interpolation between measured points of LAI, assuming a linear relationship between subsequent sampling dates (Cosentino et al., 2016). Daily intercepted PAR was calculated using Beer’s law, with a simple assumption of constant k estimated by Eq. 13 throughout the growing season (Ceotto and Castelli, 2002). As the remaining radiation flux after passing through a leaf area declines exponentially, which can be expressed by the Lambert-Beer model (Monsi and Saeki, 2005; Cosentino et al., 2016).
where k is the extinction coefficient.
The cumulative amount of PAR intercepted by the maize canopy (IPAR) during the growth period was calculated as follows (Yang et al., 2004):
where 0.5 is the fraction of PAR relative to total incident solar radiation (Rn), and fAPARi is the daily fAPAR of the ith day.
Measurement of Meteorological Variables
During the 2013–2014 growing seasons, the main meteorological parameters, such as the mean daily air temperature (T, °C), daily PAR, and daily precipitation (P, mm) were measured by a weather station located nearly 20 m from the experimental field.
Statistics
Canopy water use efficiency (WUEAGB, g m–2 mm–1) and radiation use efficiency (RUEAGB, g MJ–1) were calculated as the slope of the linear regression between AGB and cumulative ET and IPAR, respectively, during the experimental period. Canopy conductance (gc) is defined as the ratio of RUEAGB to WUEAGB (Sadras et al., 1991). The effects of the progressive soil drying treatments on WUEleaf, RUEleaf, fAPAR, LAI, and AGB were evaluated by repeated measures analysis of variance (RM-ANOVA). The least significant difference (LSD) test was applied to examine differences between treatments according to Duncan’s test. The relationship between WUE and RUE was evaluated by simple linear regression at both leaf and canopy levels. The standardized major axis (SMA) was employed to determine the WUE and RUE relationships among different treatments by using the “smatr” package in R 4.0.2 (R Core Team). The structural equation model (SEM) was employed using AMOS 21.0 (Amos Development Co., Greene, ME, United States) to analyze the direct and indirect pathway by that LAI and fAPAR influence AGB then WUE and RUE under progressive soil drying. In all cases, differences were deemed to be significant if p < 0.05. All the figures were plotted in Origin 9.1 (Origin Lab Corporation, Northampton, MA, United States).
Results
Soil Water Storage Dynamic Variations Under Various Progressive Soil Drying Treatments
Soil water storage in different soil layers decreased significantly and exhibited a similar trend with DAS in two growing seasons (Figure 2). In 2013, prior to the treatment period (16–27 DAS), the SWS values of five soil layers showed no significant difference among treatments (T1–T5) based on SM-ANOVA (Figures 2A–E). The SWS values in each soil layer showed significant differences among the T1–T5 treatments after treatments began until 42 DAS. Since then, no obvious differences were observed between the T1 and T5 treatments at different soil layers, except for 0–10 cm in 59 DAS and 20–30 cm in 85 DAS. Moreover, SWS in the upper soil layers (0–10, 10–20, and 20–30 cm) showed even more significant differences than those in the lower layers (30–40, 40–50 cm). In 2014, since irrigation treatments were applied (8 DAS), the average duration of significant differences in the SWSs of the W1–W5 treatments for different soil layers were longer (approximately 1 month) than those of the T1–T5 treatments in 2013 (Figures 2F–J).
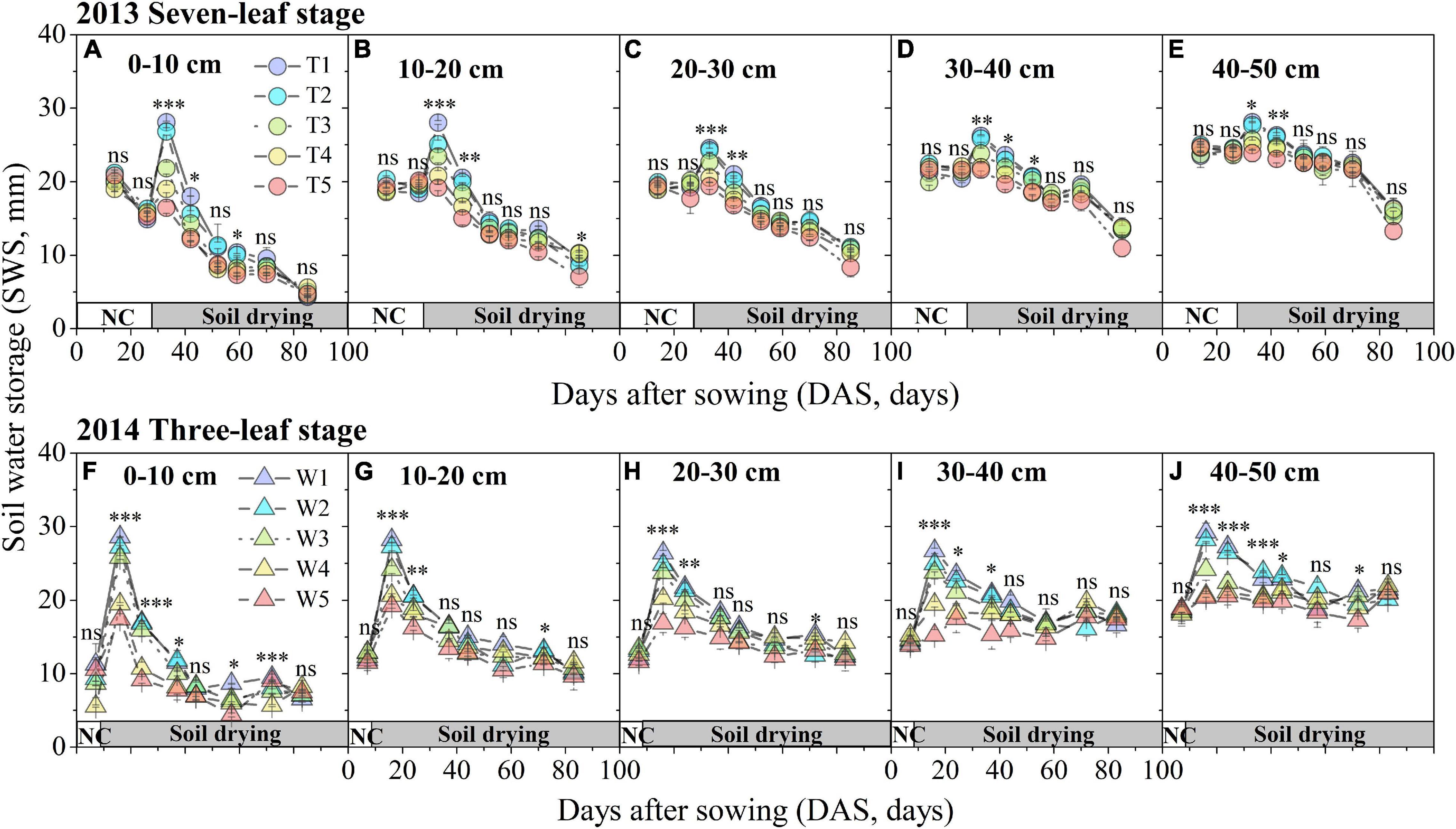
Figure 2. Time series of SWS in the different soil layers under different progressive soil drying treatments during 2013 (A–E) and 2014 (F–J) growing seasons. The growth period prior to treatment is indicated by the horizontal white bar named “NC,” while the period during treatment is represented by the horizontal gray bar named “progressive soil drying”. “***”, “**”, and “*” denote significant differences between treatments at the 0.001, 0.01, and 0.05 levels, respectively, while “ns” indicates no significant differences. The error bars indicate the standard errors of the replications.
Water Use Efficiency and Radiation Use Efficiency Response to Progressive Soil Drying at the Leaf Level
Leaf water use efficiency and RUEleaf under different progressive soil drying treatments displayed a similar trend of first increasing, then gradually decreasing, and finally increasing again (Figures 3A,B,D), which was not obvious for RUEleaf in the T1–T5 treatments (Figure 3C). At the early stage, progressive soil drying stress increased WUEleaf, but depressed it at the later stage (Figures 3A,B). However, progressive soil drying stress had a negative effect on RUEleaf throughout the experimental period (Figures 3C,D). Even though progressive soil drying treatments began at different growth stages, the sensitivity of the WUEleaf response to ASWC in 2013 (slope = –0.037) was not significantly different for that in 2014 (slope = –0.035), while the sensitivity of RUEleaf to ASWC showed an obvious difference between 2013 (slope = 0.00017) and 2014 (slope = 0.00040) growing seasons (Figure 4).
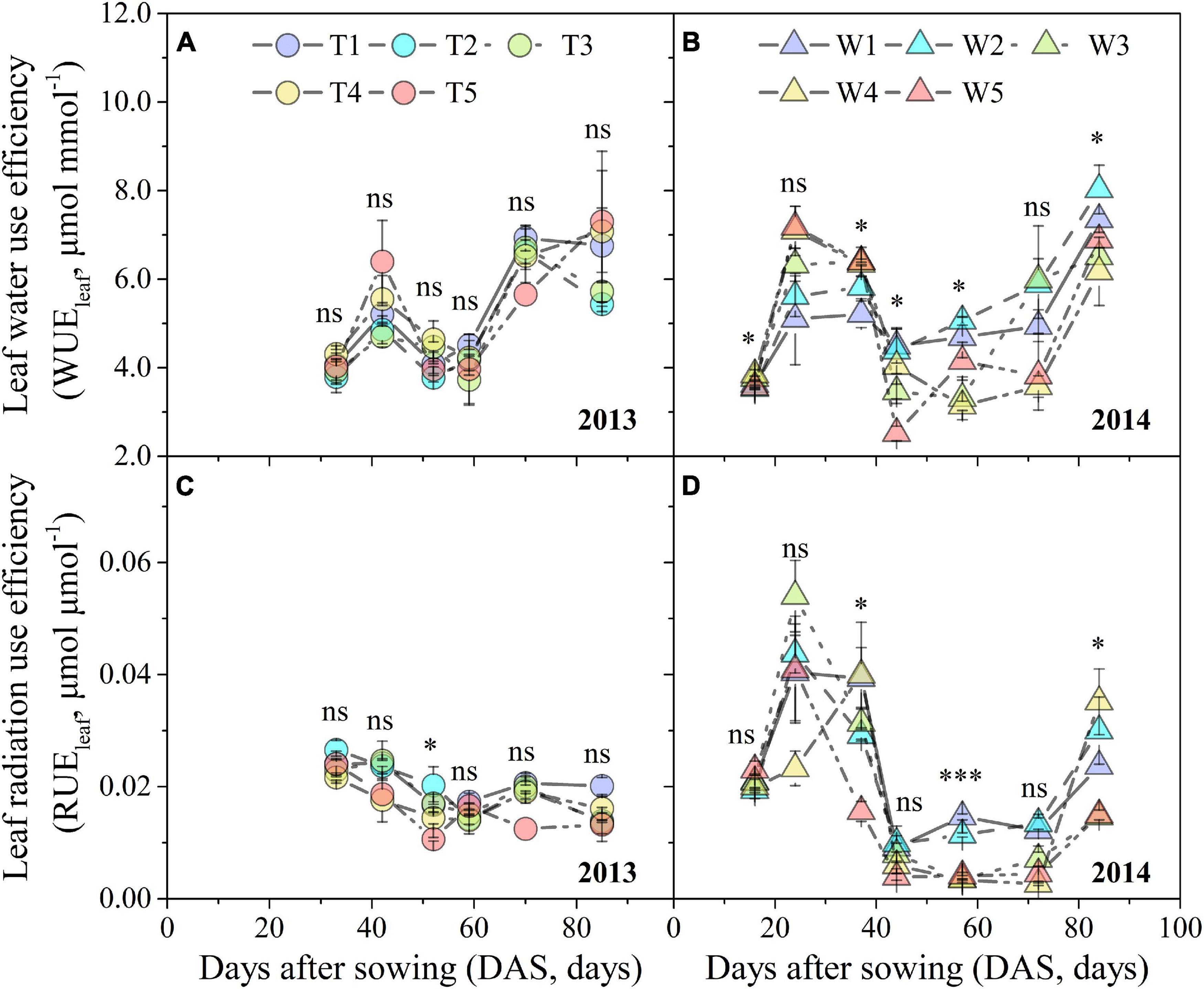
Figure 3. Changes in WUEleaf and RUEleaf under different progressive soil drying treatments during the 2013 (A,C) and 2014 (B,D) growing seasons. “***”, “**”, and “*” denote significant differences between treatments at the 0.001, 0.01, and 0.05 levels, respectively, while “ns” indicates no significant differences. Error bars denote the standard errors of the replications.
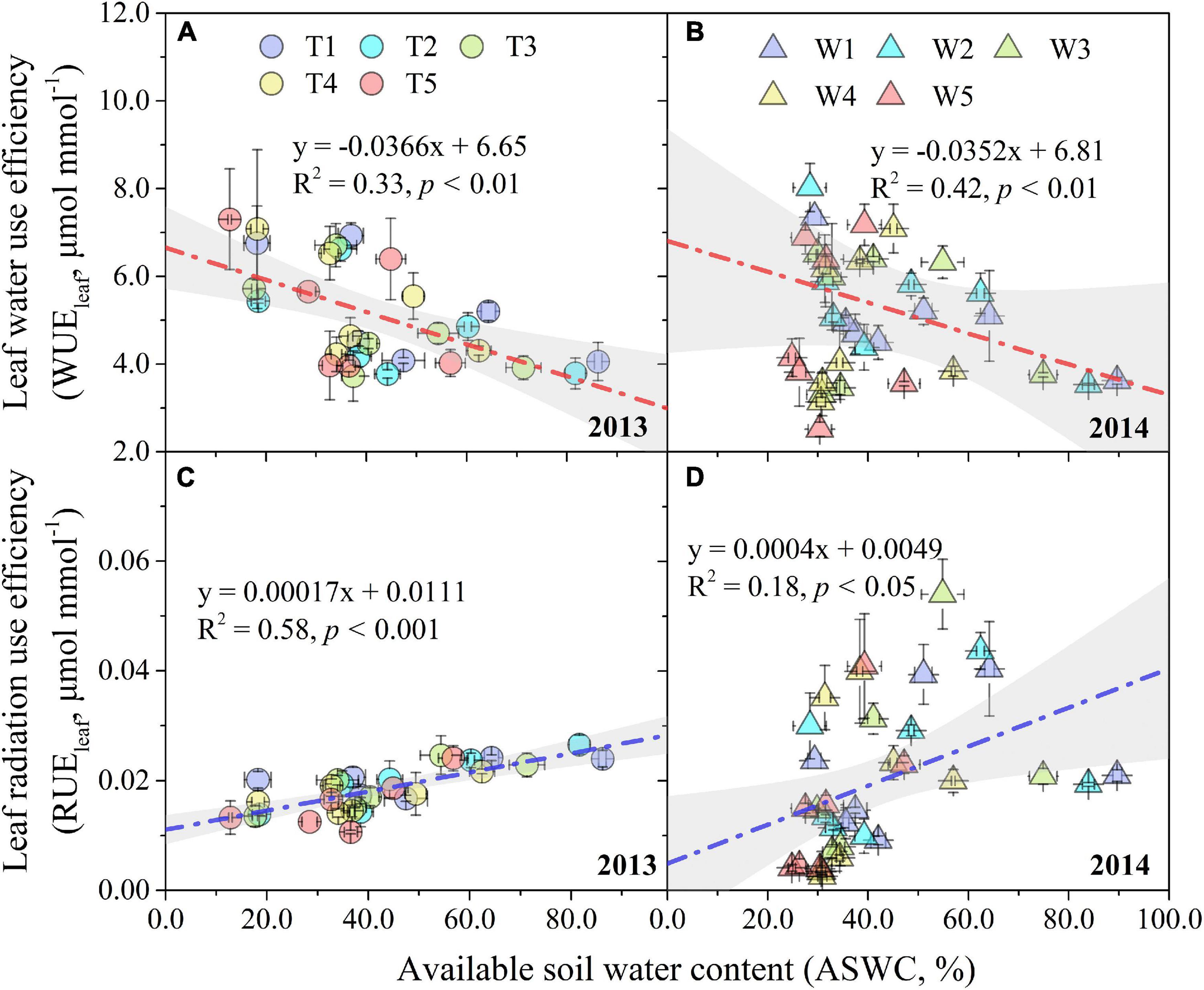
Figure 4. Response of WUEleaf and RUEleaf to ASWC under different progressive soil drying treatments in 2013 (A,C) and 2014 (B,D). The gray areas are the 95% confidence bands of the linear fitting. Error bars denote the standard errors of the replications.
Aboveground Biomass, Leaf Area Index, and Fraction of Absorbed Photosynthetically Active Radiation Response to Progressive Soil Drying
Progressive soil drying significantly decreased AGB, LAI, and fAPAR based on RM-ANOVA (Figure 5). In 2013, water deficit had a significant negative effect on biomass accumulation (Figure 5A). The dynamic characteristics of LAI and fAPAR shared a similar trend (Figures 5C,E). LAI values of T2–T5 treatments reached maximum values approximately 18 days later than fAPAR, except for T1. In 2014, the rates of increment in AGB among the W1–W5 treatments were much slower than those of the T1–T5 treatments, especially after 59 DAS (Figure 5B). There were obvious maximum values for LAI and fAPAR at 59 DAS, followed by a subsequent declining trend with progressive soil drying (Figures 5D,F).
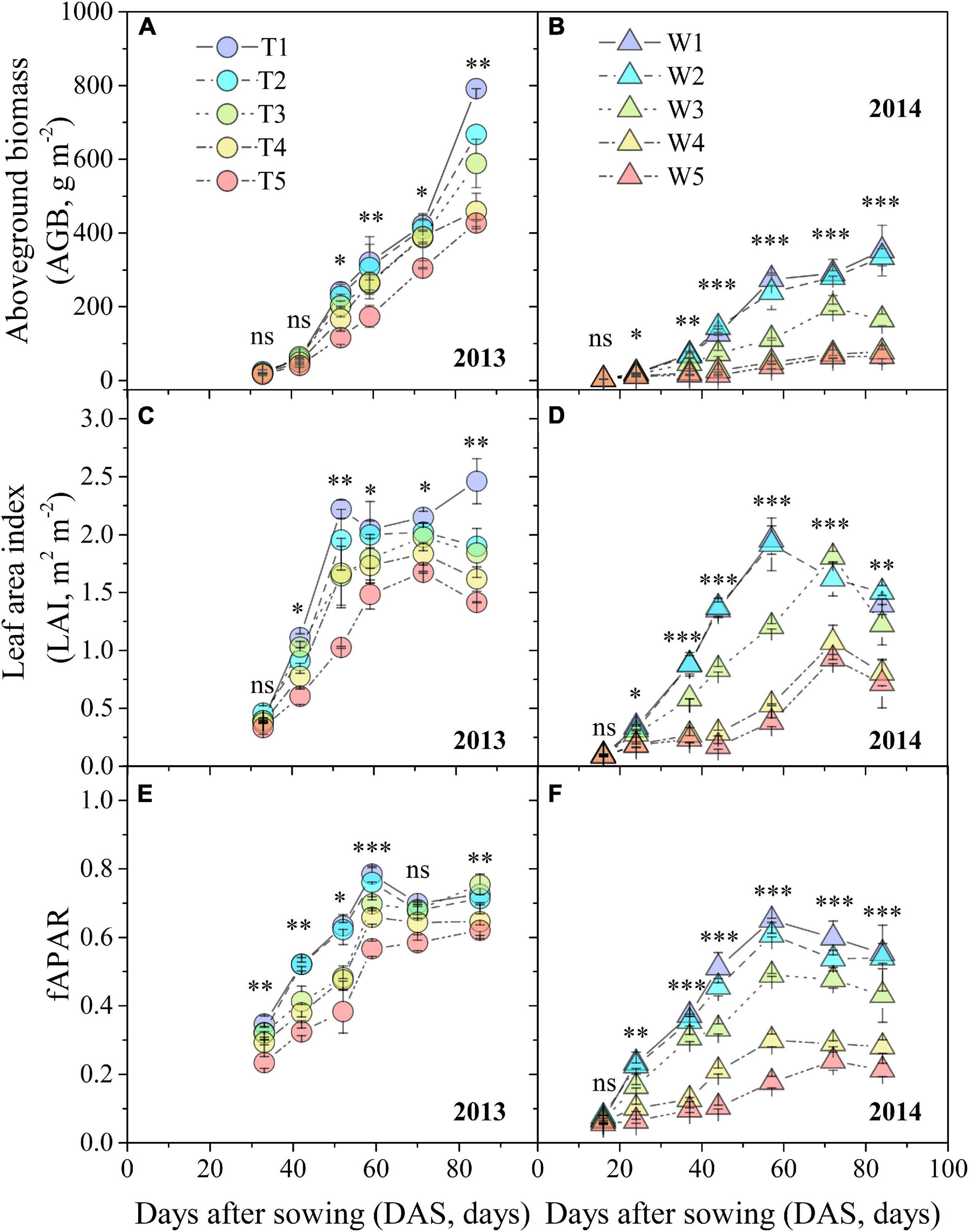
Figure 5. Changes in AGB, fAPAR, and LAI under different progressive soil drying treatments during the 2013 (A,C,E) and 2014 (B,D,F) growing seasons. “***”, “**”, and “*” denote significant differences between treatments at the 0.001, 0.01, and 0.05 levels, respectively, while “ns” indicates no significant differences. The error bars indicate the standard errors of the replications.
The relationships between fAPAR and LAI under different irrigation treatments were well represented by a non-linear Eq. 13 (Figure 6). Progressive soil drying had significant adverse effects on k derived from the asymptotic equation, especially in 2014 (Table 1). Moreover, the k values under different progressive soil drying treatments generally decreased with the irrigation reductions.
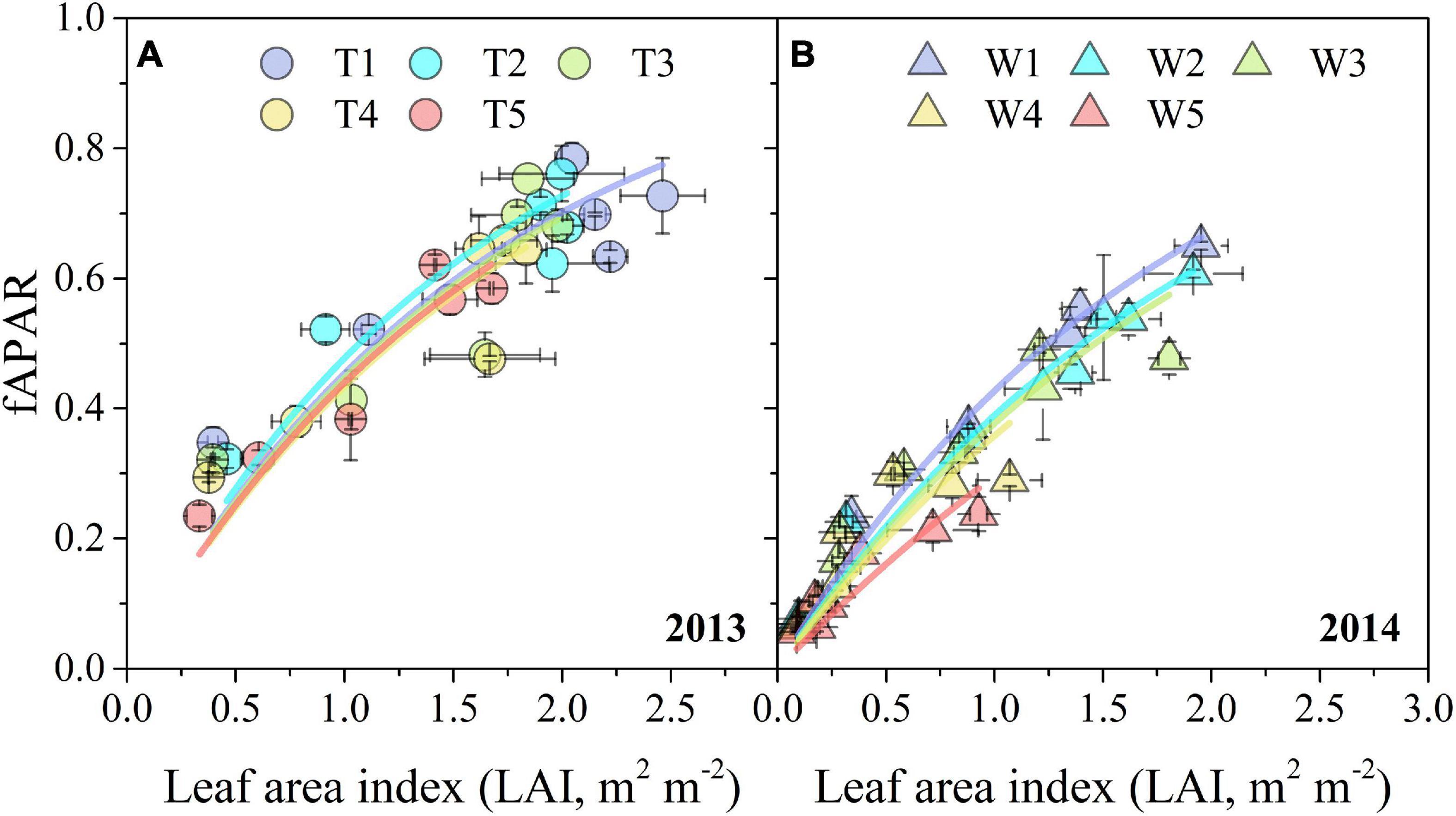
Figure 6. Relationships between periodic measurements of LAI and fAPAR under different progressive soil drying treatments during the 2013 (A) and 2014 (B) growing seasons. The colorful fitting line was formed as Eq. 13. The error bars indicate the standard errors of the replications.
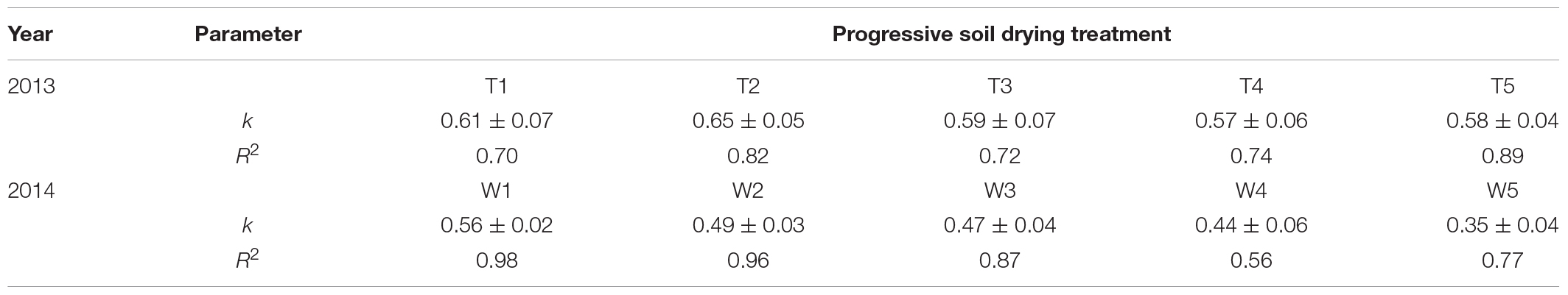
Table 1. Estimated k under different progressive soil drying treatments during the 2013 (T1–T5 treatments) and 2014 (W1–W5 treatments) growing seasons.
As indicated by SMA analysis, the estimated SMA slopes of the linear relationship between AGB and cumulative ET remained stable in both 2013 and 2014, while the SMA elevations were significantly affected by progressive soil drying (Figures 7A,B). In contrast, progressive soil drying significantly affected the estimated SMA slope of the linear relationship between AGB and cumulative IPAR in both 2 years, and significantly affected the elevation only in 2014 (Figures 7C,D).
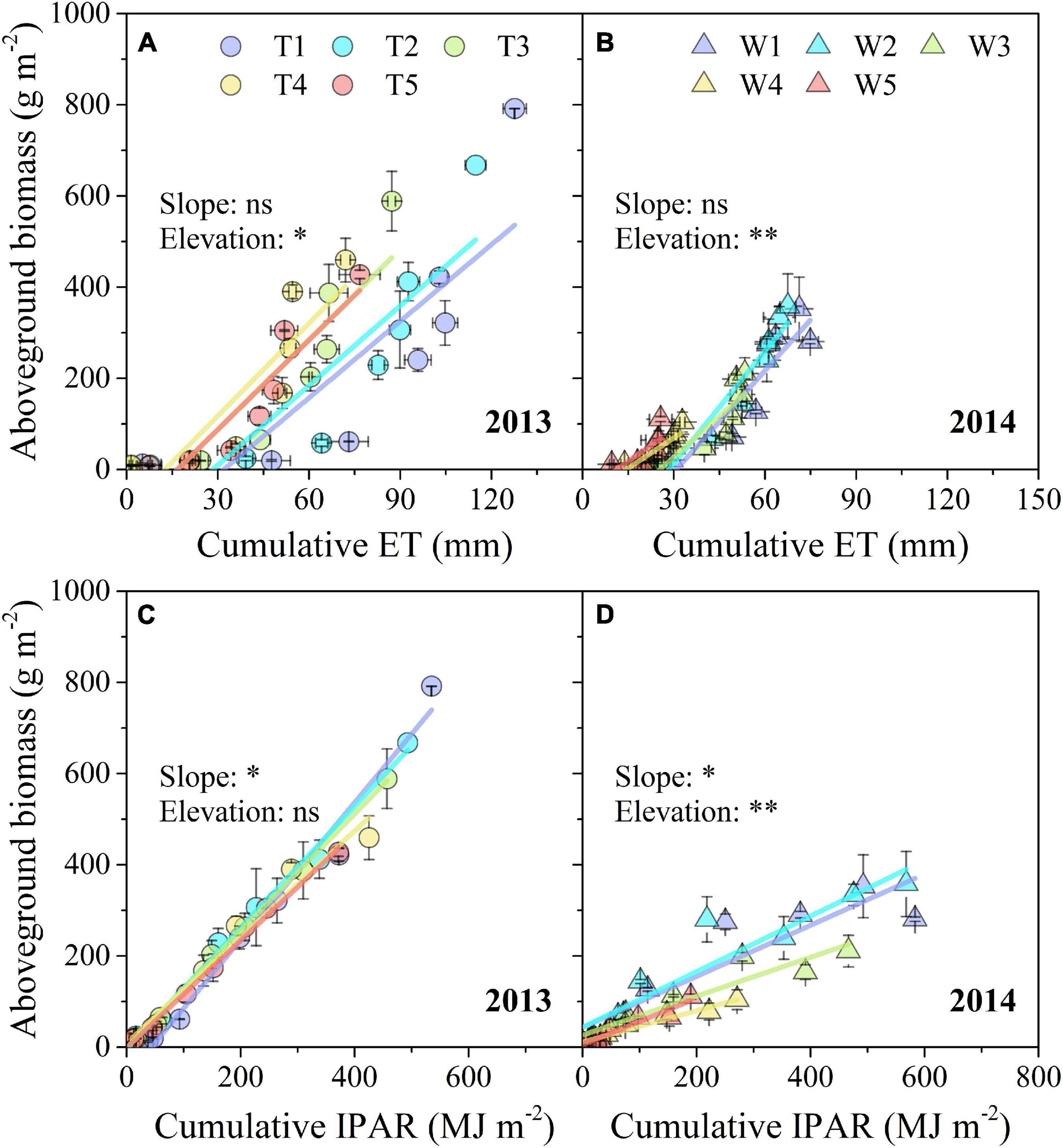
Figure 7. Response of AGB to cumulative ET and IPAR under different progressive soil drying treatments during the 2013 (A,C) and 2014 (B,D) growing seasons. “**” and “*” denote significant differences between treatments at the 0.01 and 0.05 levels, respectively, while “ns” indicates no significant differences. The error bars indicate the standard errors of the replications.
The Association of Water Use Efficiency With Radiation Use Efficiency Across the Leaf and Canopy Levels
The linear relationships between WUE and RUE were opposite in 2013 compared with those in 2014, even if not statistically significant in 2013 (p > 0.05) (Figure 8). In detail, the slope (RUEleaf vs. WUEleaf) was negative for the pooled data of the T1–T5 treatments in 2013, while it was positive for the W1–W5 treatments in 2014 (Figures 8A,B). Moreover, the relationship between WUEAGB and RUEAGB was significantly negative (R2 = 0.81, p < 0.01) in 2013, while it was significantly positive (R2 = 0.65, p < 0.01) in 2014 (Figures 8C,D). In addition, the absolute value of the linear slope (RUEAGB vs. WUEAGB) in 2013 was nearly 3.8 times larger than that in 2014.

Figure 8. Relationships between WUE and RUE at leaf and canopy levels in 2013 (A,C) and 2014 (B,D) growing seasons. The gray areas are the 95% confidence bands of the linear fitting. The error bars denote the standard errors of the replications.
Effects of Leaf Area Index and Fraction of Absorbed Photosynthetically Active Radiation on the Association Between Water Use Efficiency and Radiation Use Efficiency
Water use efficiency of AGB shared a quadratic relationship with maximum fAPAR and LAI (Figures 9A,B), while it was a linear relationship for RUEAGB (Figures 9C,D). Moreover, WUEAGB and RUEAGB were more linked with fAPAR than LAI as the R2 explained. Maize seasonal gc ranged between 0.07 and 0.27 mm MJ–1. A quadratic relationship was also fitted between gc and maximum fAPAR (R2 = 0.80, p < 0.001) and LAI (R2 = 0.45, p < 0.01) across irrigation treatments, respectively; where gc were more strongly linked with maximum fAPAR relative to maximum LAI (Figures 9E,F).
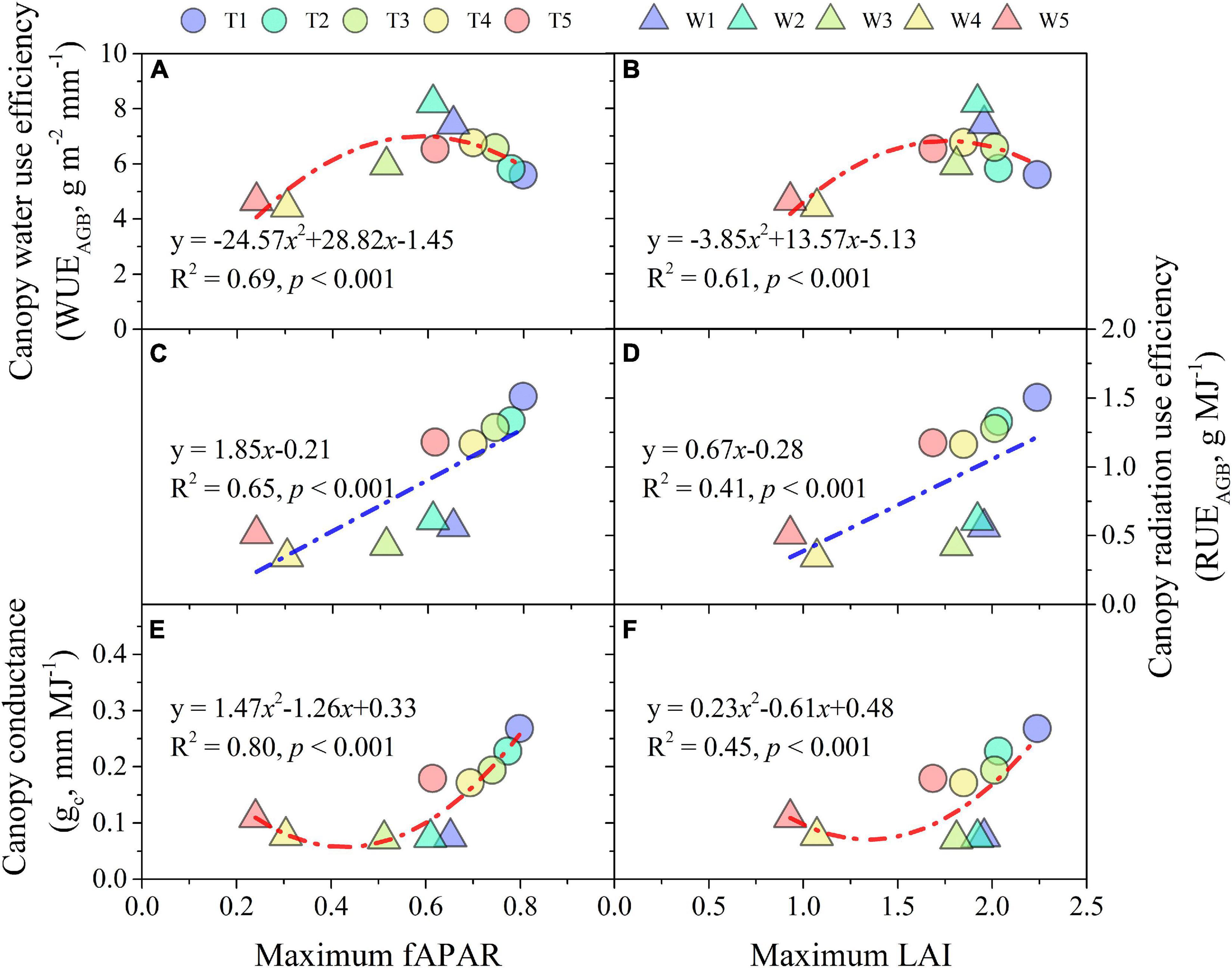
Figure 9. Water use efficency (A,B), radiation use efficiency (C,D) of AGB, and canopy conductance (E,F) response to maximum LAI and fAPAR during 2013 and 2014 growing seasons.
Moreover, SEM analysis showed that the ET, fAPAR, IPAR, and LAI explained 98% and 92% of the variation of AGB in 2013 and 2014, respectively (Figure 10). In 2013, the primary latent variable that affected AGB was IPAR (total standardized path coefficient: 0.95), whereas it was ET (0.37) and IPAR (0.52) in 2014. Although fAPAR did not directly affect AGB, LAI only had a weak direct effect on AGB in both growing seasons. ET affected AGB via fAPAR with a path coefficient of 0.40 and −0.18 in 2013 and 2014, respectively, while they were 0.04 and 0.06 through LAI. Besides, the effect of LAI on AGB through fAPAR was 0.33, which was larger than that the direct effect of LAI on AGB (0.05) in 2013 (Figure 10A). Overall, the models showed that the influences of ET and IPAR on AGB were more mediated by fAPAR than LAI under progressive soil drying.

Figure 10. Structural equation models for the direct and indirect pathway of ET, fAPAR, LAI, and IPAR on AGB in 2013 (A) and 2014 (B). Numbers on arrows are standardized path coefficients, blue arrows are positive and red are negative, solid arrows indicate significant standardized path coefficients (p < 0.05) (significance levels are *p < 0.05, **p < 0.01, and ***p < 0.001), dashed arrows indicate non-significant standardized path coefficients (p > 0.05). R2 values close to the rectangles indicate the variance explained by the model. ET, actual evapotranspiration; fAPAR, the fraction of absorbed photosynthetically active radiation; IPAR, intercepted photosynthetically active radiation; LAI, leaf area index; AGB, aboveground biomass.
Discussion
Effects of Progressive Soil Drying on the Dynamic Variations of Water Use Efficiency and Radiation Use Efficiency
Understanding the dynamic variations of WUE and RUE and their underlying mechanisms can improve our ability to predict the effects of climate change on carbon and water cycles. In fact, the temporal variations in WUE and RUE are affected by various climatic factors (Yu et al., 2008) and genetic variables (Furbank et al., 2019), including common environmental stresses (e.g., drought, heat, salt, and solar dimming) and physiological, developmental, and phenological variations in plants (Turner et al., 2003; Leakey et al., 2019). Furthermore, previous studies revealed that the seasonal variation in WUEleaf was closely coupled with leaf stoichiometry (Du et al., 2020), and also driven by air temperature, vapor pressure deficit (VPD), and solar radiation (Jiang et al., 2020). Our results indicated that progressive water deficit slightly changed the seasonal variations of WUEleaf, which were characterized by obvious fluctuations (Figures 3A,B). This finding was similar to a study for grape (Medrano et al., 2015). At the canopy level, diurnal WUE peaked in the early morning, while RUE topped at sunset, seasonal WUE and RUE reached a maximum value in summer (Gao et al., 2018). However, our results suggested that the dynamic variations of RUEleaf showed different changing patterns in the 2013 and 2014 growing seasons (Figures 3C,D). In 2013, RUEleaf decreased with DAS, whereas the RUEleaf had significant fluctuations in 2014, which might be contributed to the differences in the degree and duration of soil drying. The values of RUE for maize in the later growth stage were often lower than those of the early growth stage (Otegui et al., 1995), which were also found in the 2013 growing season (Figure 3C), as the progressive loss of leaf photosynthetic capacity with soil drying and increasing leaf age (Earl and Tollenaar, 1999).
Effects of Progressive Soil Drying on Aboveground Biomass, Leaf Area Index, and Fraction of Absorbed Photosynthetically Active Radiation
Drought stress significantly reduces crop production through its adverse effects on root water absorption, nutrient uptake, leaf net assimilation, and subsequently on dry matter accumulation (like AGB) and distribution (Ullah et al., 2019). The light interception capacity of a crop is mainly determined by the LAI and fAPAR (Hikosaka et al., 2016). Smaller leaf area and biomass lead to a smaller LAI and fAPAR, which is not beneficial for light interception (Baret et al., 2013; Mantilla-Perez and Salas Fernandez, 2017). In addition, leaf rolling is a mechanism developed by plants to mitigate the impact of environmental stresses, especially under severe stress conditions (Baret et al., 2018). Our study indicated that progressive soil drying significantly decreased AGB, LAI, and fAPAR (Figure 5), and accelerated leaf senescence (Figures 5E,F). Moreover, the adverse effects of progressive soil drying applied at the three-leaf stage on k were more significant than those at the seven-leaf stage (Figure 6 and Table 1). In general, WUEAGB increased with irrigation reduction (T1–T5 treatments) in 2013 when soil drying began at the seven-leaf stage, whereas WUEAGB decreased with irrigation reduction (W1–W5 treatments) in 2014 when soil drying began at the three-leaf stage (Figures 7A,B). The results suggest that moderate water stress increased WUEAGB, while severe water stress decreased WUEAGB. The main reason is that drought affected leaf development and expansion as soil drying. Consequently, the RUE values of different irrigation treatments were significantly reduced by water deficits (Figures 7C,D). The theoretical RUE of maize under optimal conditions is about 3.84 ± 0.08 g MJ–1 (Lindquist et al., 2005), which is much higher than our results which were severely decreased by soil drying. Recent breeding efforts have no evident effect on crop water use but have significant improvements on crop biomass production and partitioning (Curin et al., 2020). The maize ideotypes planted under the future climate of the NCP should have a longer reproductive growing period, faster potential grain filling rate, higher maximum grain numbers, and higher RUE (Xiao et al., 2020). Therefore, there is still a great potential to breed new maize varieties with both high WUE and RUE and improve field management for future climate.
Influencing Factors on the Interaction Between Water Use Efficiency and Radiation Use Efficiency
The relationship between WUE and RUE may exhibit various patterns at different spatial-temporal scales and environmental backgrounds (Liu et al., 2019). At the leaf level, low transpiration induced by stomatal closure leads to increased WUE as water loss exceeds photosynthesis (Kang et al., 1998). Low photosynthesis tends to decrease RUE, causing WUE to be negatively correlated with RUE (Tarvainen et al., 2015). Our results also confirmed that leaf WUE and RUE were negatively correlated when progressive soil drying was applied at the seven-leaf stage (Figure 8A). However, when photosynthetic downregulation was caused by non-stomatal limitation during serious water stress (Song et al., 2020), leaf WUE dropped as photosynthesis decreased more than transpiration. As a result, the relationship between leaf WUE and RUE was positively correlated (Figure 8B). However, the critical value of the soil or plant water content where arouses the relationship between WUE and RUE to shift remains unknown and needs to be quantified in future study. The similar relationships between WUE and RUE were also found at canopy level, and even stronger than those at leaf level (Figures 8C,D). At the canopy level, high WUE of sweet sorghum tends to be related to low RUE under probable drought in sweet sorghum (a C4 plant similar to maize) (Dercas and Liakatas, 2006), which was also confirmed in our study (Figure 8C). A trade-off may occur between the use efficiency of two resources when there are differences in the relative costs of the resources, with an increase in the use efficiency of an “expensive” resource and a decrease in a “cheaper” resource (Bloom et al., 1985). Soil water is an “expensive” resource, while radiation is a “cheaper” resource under water-deficit conditions. The trade-off was confirmed in the interrelationship between canopy WUE and RUE when progressive soil drying treatments were applied at the seven-leaf stage (Figures 8A,C). However, the relationship between WUE and RUE turned out to be synergistic when progressive soil drying treatments were applied at the three-leaf stage (Figures 8B,D).
Leaf area index plays an important mediating role in the relationship among climate, soil variables, and evapotranspiration (Zhou et al., 2019). Meanwhile, fAPAR is a key variable not only in assessing vegetation productivity but also in light use efficiency (Gitelson et al., 2014). Accurately quantifying LAI and fAPAR is important for characterizing the dynamics of carbon and energy exchanges between vegetation and the atmosphere (Serbin et al., 2013). Our results indicated that fAPAR was more related to canopy conductance (gc, representing transpired water per unit of IPAR) than LAI (Figure 9). Even though LAI and fAPAR are deemed to be closely related (Zhou et al., 2002; Monsi and Saeki, 2005), their direct and indirect effects on AGB were significantly different according to an SEM analysis (Figure 10). fAPAR played a stronger role in mediating the influence of soil drying on AGB than LAI. Therefore, our results suggest that the association between WUE and RUE was more regulated by fAPAR than LAI, which would be important for WUE- and RUE-based models of crop production, especially under water-limited conditions.
Source of Limitation
The two-year field experiment provided us with an opportunity to investigate the interrelationships between WUE and RUE and their underlying mechanisms across leaf and canopy scales under soil drying. Nevertheless, some limitations would still exist in the implication and generalization of the results, even though data was collected from standardized experimental measurements and after strict data quality control and analysis. Neglecting the differences in meteorological conditions between 2013 and 2014 growing seasons may be a source of uncertainties. As the three-leaf and seven-leaf stage of maize was only evaluated in a single growing season, which might not capture the effects of the year and its interaction with a development stage. Furthermore, only one maize genotype was adopted in this study, the evaluation of the cultivar variations was not conducted. Therefore, future studies can address the effects of meteorological conditions and genotypes on the interaction between WUE and RUE and their underlying mechanisms.
Conclusion
To understand the interrelationship between WUE and RUE and their underlying mechanisms across the leaf and canopy levels under the soil drying process, a field experiment with different irrigation regimes on maize was designed and conducted during two growing seasons (2013–2014) on North China Plain. The results indicated that leaf WUE increased with irrigation reduction at an early stage, while decreased with irrigation reduction at a later stage. Leaf RUE decreased with irrigation reduction during the progressive soil drying process. Maize canopy traits (e.g., AGB, LAI, fAPAR, and k) were significantly decreased by water deficit regardless of the growth stage when soil drying was applied. The relationships between WUE and RUE were linear across the leaf and canopy scales under different soil drying patterns. However, the interrelationship between canopy WUE and RUE unexpectedly appeared to be positive when soil drying was applied at the three-leaf stage, while negative when soil drying was applied at the seven-leaf stage. Canopy conductance (gc) was more related to fAPAR than LAI. The effects of ET and IPAR on AGB were achieved via fAPAR more than LAI. Our finding demonstrated that the association between canopy WUE and RUE was more regulated by fAPAR than LAI under soil drying.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
GZ, HZ, LZ, and MZ made a substantial contribution to conception and experimental design, and critically revised the manuscript. HZ, XL, and YJ performed the experiment. HZ and GZ analyzed the data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was jointly supported by the National Key Research and Development Program of China (No. 2018YFA0606103) and the Basic Research Fund of the Chinese Academy of Meteorological Sciences (2020Z004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors appreciate Xueyan Ma, Yaohui Shi, Feng Zhang, Qiuling Wang, Minzheng Wang, Xiaoyu Feng, and Fan Wang for their work assistant both in the field and laboratory analysis. Sincere thanks also go to the editor and reviewers for their thoughtful comments that improved this manuscript.
Abbreviations
ASWC, available soil water content; AGB, aboveground biomass; DAS, days after sowing; ET, evapotranspiration; fAPAR, fraction of absorbed photosynthetically active radiation; IPAR, the cumulative amount PAR intercepted by the canopy; LAI, leaf area index; NCP, North China Plain; PAR, photosynthetically active radiation; RUE, radiation use efficiency; RUEAGB, radiation use efficiency of AGB; RUEleaf, leaf radiation use efficiency; SFM, soil fresh mass; SDM, soil dry mass; WP, wilting point; FC, field capacity; SWC, soil water content; SWS, soil water storage; VPD, vapor pressure deficit; WUE, water use efficiency; WUEAGB, water use efficiency of AGB; WUEleaf, leaf water use efficiency.
References
Baret, F., Madec, S., Irfan, K., Lopez, J., Comar, A., Hemmerle, M., et al. (2018). Leaf-rolling in maize crops: from leaf scoring to canopy-level measurements for phenotyping. J. Exp. Bot. 69, 2705–2716. doi: 10.1093/jxb/ery071
Baret, F., Weiss, M., Lacaze, R., Camacho, F., Makhmara, H., Pacholcyzk, P., et al. (2013). GEOV1: LAI and FAPAR essential climate variables and FCOVER global time series capitalizing over existing products. Part1: principles of development and production. Remote Sens. Environ. 137, 299–309. doi: 10.1016/j.rse.2012.12.027
Beer, C., Ciais, P., Reichstein, M., Baldocchi, D., Law, B. E., Papale, D., et al. (2009). Temporal and among-site variability of inherent water use efficiency at the ecosystem level. Global Biogeochem. Cycles 23:GB2018. doi: 10.1029/2008GB003233
Bhattarai, B., Singh, S., West, C. P., Ritchie, G. L., and Trostle, C. L. (2020). Water depletion pattern and water use efficiency of forage sorghum, pearl millet, and corn under water limiting condition. Agric. Water Manage. 238:106206. doi: 10.1016/j.agwat.2020.106206
Bloom, A. J., Chapin, F. S., and Mooney, H. A. (1985). Resource limitation in plants-an economic analogy. Annu. Rev. Ecol. Syst 16, 363–392. doi: 10.1146/annurev.es.16.110185.002051
Cai, F., Zhang, Y. S., Mi, N., Ming, H. Q., Zhang, S. J., Zhang, H., et al. (2020). Maize (Zea mays L.) physiological responses to drought and rewatering, and the associations with water stress degree. Agric. Water Manage. 241, 106379. doi: 10.1016/j.agwat.2020.106379
Ceotto, E., and Castelli, F. (2002). Radiation-use efficiency in flue-cured tobacco (Nicotiana tabacum L.): response to nitrogen supply, climatic variability and sink limitations. Field Crops Res. 74, 117–130. doi: 10.1016/S0378-4290(01)00201-5
Cosentino, S. L., Patanè, C., Sanzone, E., Testa, G., and Scordia, D. (2016). Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 72, 56–69. doi: 10.1016/j.eja.2015.09.011
Curin, F., Severini, A. D., González, F. G., and Otegui, M. E. (2020). Water and radiation use efficiencies in maize: breeding effects on single-cross Argentine hybrids released between 1980 and 2012. Field Crops Res. 246:107683. doi: 10.1016/j.fcr.2019.107683
Dercas, N., and Liakatas, A. (2006). Water and radiation effect on sweet sorghum productivity. Water Resour. Manage. 21, 1585–1600. doi: 10.1007/s11269-006-9115-2
Ding, D., Wang, N., Zhang, X., Zou, Y., Zhao, Y., Xu, Z., et al. (2021). Quantifying the interaction of water and radiation use efficiency under plastic film mulch in winter wheat. Sci. Total Environ. 794:148704. doi: 10.1016/j.scitotenv.2021.148704
Du, B., Zheng, J., Ji, H., Zhu, Y., Yuan, J., Wen, J., et al. (2020). Stable carbon isotope used to estimate water use efficiency can effectively indicate seasonal variation in leaf stoichiometry. Ecol. Indic. 121:107250. doi: 10.1016/j.ecolind.2020.107250
Earl, H. J., and Tollenaar, M. (1999). Using chlorophyll fluorometry to compare photosynthetic performance of commercial maize (Zea mays L.) hybrids in the field. Field Crops Res. 61, 201–210. doi: 10.1016/S0378-4290(98)00162-2
Fang, H., Li, Y., Gu, X., Yu, M., Du, Y., Chen, P., et al. (2021). Evapotranspiration partitioning, water use efficiency, and maize yield under different film mulching and nitrogen application in northwest China. Field Crops Res. 264:108103. doi: 10.1016/j.fcr.2021.108103
Fang, S., Su, H., Liu, W., Tan, K., and Ren, S. (2013). Infrared warming reduced winter wheat yields and some physiological parameters, which were mitigated by irrigation and worsened by delayed sowing. PLoS One 8:e67518. doi: 10.1371/journal.pone.0067518
FAO (2020). World Food And Agriculture – Statistical Yearbook 2020. Rome: Agriculture Organization of the United Nations.
Farquhar, G. D., and Richards, R. A. (1984). Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Funct. Plant Biol. 11, 539–552. doi: 10.1071/PP9840539
Furbank, R. T., Jimenez-Berni, J. A., George-Jaeggli, B., Potgieter, A. B., and Deery, D. M. (2019). Field crop phenomics: enabling breeding for radiation use efficiency and biomass in cereal crops. New Phytol. 223, 1714–1727. doi: 10.1111/nph.15817
Gallo, K. P., and Daughtry, C. S. T. (1984). Techniques for measuring intercepted and absorbed photosynthetically active radiation in corn canopies. Agron. J. 78, 752–756. doi: 10.2134/agronj1986.00021962007800040039x
Gao, X., Gu, F., Mei, X., Hao, W., Li, H., Gong, D., et al. (2018). Light and water use efficiency as influenced by clouds and/or aerosols in a rainfed spring maize cropland on the Loess Plateau. Crop Sci. 58, 853–862. doi: 10.2135/cropsci2017.06.0341
Gitelson, A. A., Peng, Y., and Huemmrich, K. F. (2014). Relationship between fraction of radiation absorbed by photosynthesizing maize and soybean canopies and NDVI from remotely sensed data taken at close range and from MODIS 250m resolution data. Remote Sens. Environ. 147, 108–120. doi: 10.1016/j.rse.2014.02.014
Guan, D., Zhang, Y., Al-Kaisi, M. M., Wang, Q., Zhang, M., and Li, Z. (2015). Tillage practices effect on root distribution and water use efficiency of winter wheat under rain-fed condition in the North China Plain. Soil Tillage Res. 146, 286–295. doi: 10.1016/j.still.2014.09.016
Haghverdi, A., Leib, B. G., Washington-Allen, R. A., Ayers, P. D., and Buschermohle, M. J. (2015). High-resolution prediction of soil available water content within the crop root zone. J. Hydrol. 530, 167–179. doi: 10.1016/j.jhydrol.2015.09.061
Hatfield, J. L., and Dold, C. (2019). Water-use efficiency: advances and challenges in a changing climate. Front. Plant Sci. 10:103. doi: 10.3389/fpls.2019.00103
Hernández, M. D., Alfonso, C., Echarte, M. M., Cerrudo, A., and Echarte, L. (2021). Maize transpiration efficiency increases with N supply or higher plant densities. Agric. Water Manage. 250:106816. doi: 10.1016/j.agwat.2021.106816
Hikosaka, K., Anten, N. P. R., Borjigidai, A., Kamiyama, C., Sakai, H., Hasegawa, T., et al. (2016). A meta-analysis of leaf nitrogen distribution within plant canopies. Ann. Bot. 118, 239–247. doi: 10.1093/aob/mcw099
Holmes, M. G., and Keiller, D. R. (2002). Effects of pubescence and waxes on the reflectance of leaves in the ultraviolet and photosynthetic wavebands: a comparison of a range of species. Plant Cell Environ. 25, 85–93. doi: 10.1046/j.1365-3040.2002.00779.x
Huang, J., Zhai, J., Jiang, T., Wang, Y., Li, X., Wang, R., et al. (2017). Analysis of future drought characteristics in China using the regional climate model CCLM. Clim. Dyn. 50, 507–525. doi: 10.1007/s00382-017-3623-z
Jiang, S., Liang, C., Cui, N., Zhao, L., Liu, C., Feng, Y., et al. (2020). Water use efficiency and its drivers in four typical agroecosystems based on flux tower measurements. Agric. For. Meteorol. 295:108200. doi: 10.1016/j.agrformet.2020.108200
Kan, Z.-R., Liu, Q.-Y., He, C., Jing, Z.-H., Virk, A. L., Qi, J.-Y., et al. (2020). Responses of grain yield and water use efficiency of winter wheat to tillage in the North China Plain. Field Crops Res. 249:107760. doi: 10.1016/j.fcr.2020.107760
Kang, S., Liang, Z., Hu, W., and Zhang, J. (1998). Water use efficiency of controlled alternate irrigation on root-divided maize plants. Agric. Water Manage. 38, 69–76. doi: 10.1016/S0378-3774(98)00048-1
Kukal, M. S., and Irmak, S. (2020). Interrelationships between water use efficiency and light use efficiency in four row crop canopies. Agrosyst. Geosci. Environ. 3:e20110. doi: 10.1002/agg2.20110
Leakey, A. D. B., Ferguson, J. N., Pignon, C. P., Wu, A., Jin, Z., Hammer, G. L., et al. (2019). Water use efficiency as a constraint and target for improving the resilience and productivity of C3 and C4 Crops. Annu. Rev. Plant Biol. 70, 781–808. doi: 10.1146/annurev-arplant-042817-040305
Lindquist, J. L., Arkebauer, T. J., Walters, D. T., Cassman, K. G., and Dobermann, A. (2005). Maize radiation use efficiency under optimal growth conditions. Agron. J. 97, 72–78. doi: 10.2134/agronj2005.0072
Liu, P., Black, T. A., Jassal, R. S., Zha, T., Nesic, Z., Barr, A. G., et al. (2019). Divergent long-term trends and interannual variation in ecosystem resource use efficiencies of a southern boreal old black spruce forest 1999–2017. Glob. Chang. Biol. 25, 3056–3069. doi: 10.1111/gcb.14674
Liu, W., Fu, G., Liu, C., Song, X., and Ouyang, R. (2013). Projection of future rainfall for the North China Plain using two statistical downscaling models and its hydrological implications. Stoch. Env. Res. Risk A 27, 1783–1797. doi: 10.1007/s00477-013-0714-1
Liu, Y., Xiao, J., Ju, W., Xu, K., Zhou, Y., and Zhao, Y. (2016). Recent trends in vegetation greenness in China significantly altered annual evapotranspiration and water yield. Environ. Res. Lette. 11:094010. doi: 10.1088/1748-9326/11/9/094010
Lobell, D. B., Bänziger, M., Magorokosho, C., and Vivek, B. (2011). Nonlinear heat effects on African maize as evidenced by historical yield trials. Nat. Clim. Chang. 1, 42–45. doi: 10.1038/nclimate1043
Ma, J., Jia, X., Zha, T., Bourque, C. P. A., Tian, Y., Bai, Y., et al. (2019). Ecosystem water use efficiency in a young plantation in Northern China and its relationship to drought. Agric. For. Meteorol. 275, 1–10. doi: 10.1016/j.agrformet.2019.05.004
Mantilla-Perez, M. B., and Salas Fernandez, M. G. (2017). Differential manipulation of leaf angle throughout the canopy: current status and prospects. J. Exp. Bot. 68, 5699–5717. doi: 10.1093/jxb/erx378
Marwein, M., Choudhury, B., Chakraborty, D., Kumar, M., Das, A., and Rajkhowa, D. (2016). Response of water deficit regime and soil amelioration on evapotranspiration loss and water use efficiency of maize (Zea mays L.) in subtropical northeastern Himalayas. Int. J. Biometeorol. 61, 845–855. doi: 10.1007/s00484-016-1262-4
Medrano, H., Tomás, M., Martorell, S., Flexas, J., Hernández, E., Rosselló, J., et al. (2015). From leaf to whole-plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J. 3, 220–228. doi: 10.1016/j.cj.2015.04.002
Ming, B., Guo, Y.-Q., Tao, H.-B., Liu, G.-Z., Li, S.-K., and Wang, P. (2015). SPEIPM-based research on drought impact on maize yield in North China Plain. J. Integr. Agric. 14, 660–669. doi: 10.1016/s2095-3119(14)60778-4
Monsi, M., and Saeki, T. (2005). On the factor light in plant communities and its importance for matter production. Ann. Bot. 95, 549–567. doi: 10.1093/aob/mci052
Monteith, J. L. (1972). Solar radiation and productivity in tropical ecosystems. J. Appl. Ecol. 9, 747–766. doi: 10.2307/2401901
Monteith, J. L., Moss, C. J., Cooke, G. W., Pirie, N. W., and Bell, G. D. H. (1977). Climate and the efficiency of crop production in Britain. Philos. Trans. R. Soc. Lond. Ser. B 281, 277–294. doi: 10.1098/rstb.1977.0140
Onoda, Y., Saluñga, J. B., Akutsu, K., Aiba, S.-I., Yahara, T., Anten, N. P. R., et al. (2014). Trade-off between light interception efficiency and light use efficiency: implications for species coexistence in one-sided light competition. J. Ecol. 102, 167–175. doi: 10.1111/1365-2745.12184
Otegui, M. E., Nicolini, M. G., Ruiz, R. A., and Dodds, P. A. (1995). Sowing date effects on grain yield components for different maize genotypes. Agron. J. 87, 29–33. doi: 10.2134/agronj1995.00021962008700010006x
Roeber, V. M., Bajaj, I., Rohde, M., Schmulling, T., and Cortleven, A. (2020). Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 44, 645–664. doi: 10.1111/pce.13948
Running, S. W. (1984). Microclimate control of forest productivity: analysis by computer simulation of annual photosynthesis/transpiration balance in different environments. Agric. For. Meteorol. 32, 267–288. doi: 10.1016/0168-1923(84)90054-6
Sadras, V. O., Whitfield, D. M., and Connor, D. J. (1991). Transpiration efficiency in crops of semi-dwarf and standard-height sunflower. Irrig. Sci. 12, 87–91. doi: 10.1007/BF00190015
Sanchez, B., Rasmussen, A., and Porter, J. R. (2014). Temperatures and the growth and development of maize and rice: a review. Glob. Chang. Biol. 20, 408–417. doi: 10.1111/gcb.12389
Serbin, S. P., Ahl, D. E., and Gower, S. T. (2013). Spatial and temporal validation of the MODIS LAI and FPAR products across a boreal forest wildfire chronosequence. Remote Sens. Environ. 133, 71–84. doi: 10.1016/j.rse.2013.01.022
Song, H., Li, Y., Zhou, L., Xu, Z., and Zhou, G. (2018). Maize leaf functional responses to drought episode and rewatering. Agric. For. Meteorol. 249, 57–70. doi: 10.1016/j.agrformet.2017.11.023
Song, X., Zhou, G., He, Q., and Zhou, H. (2020). Stomatal limitations to photosynthesis and their critical Water conditions in different growth stages of maize under water stress. Agric. Water Manage. 241:106330. doi: 10.1016/j.agwat.2020.106330
Steduto, P., and Albrizio, R. (2005). Resource use efficiency of field-grown sunflower, sorghum, wheat and chickpea II. Water use efficiency and comparison with radiation use efficiency. Agric. For. Meteorol. 130, 269–281. doi: 10.1016/j.agrformet.2005.04.003
Stewart, D. W., and Dwyer, L. M. (1999). Mathematical characterization of leaf shape and area of maize hybrids. Crop Sci. 39, 422–427.
Sun, H., Feng, M., Xiao, L., Yang, W., Wang, C., Jia, X., et al. (2019). Assessment of plant water status in winter wheat (Triticum aestivum L.) based on canopy spectral indices. PLoS One 14:e0216890. doi: 10.1371/journal.pone.0216890
Tao, F., and Zhang, Z. (2010). Adaptation of maize production to climate change in North China Plain: quantify the relative contributions of adaptation options. Eur. J. Agron. 33, 103–116. doi: 10.1016/j.eja.2010.04.002
Tao, F., Yokozawa, M., Liu, J., and Zhang, Z. (2008). Climate–crop yield relationships at provincial scales in China and the impacts of recent climate trends. Clim. Res. 38, 83–94. doi: 10.3354/cr00771
Tarvainen, L., Rantfors, M., and Wallin, G. (2015). Seasonal and within-canopy variation in shoot-scale resource-use efficiency trade-offs in a Norway spruce stand. Plant Cell Environ. 38, 2487–2496. doi: 10.1111/pce.12565
Turner, D. P., Urbanski, S., Bremer, D., Wofsy, S. C., Meyers, T., Gower, S. T., et al. (2003). A cross-biome comparison of daily light use efficiency for gross primary production. Glob. Chang. Biol. 9, 383–395. doi: 10.1046/j.1365-2486.2003.00573.x
Ullah, H., Santiago-Arenas, R., Ferdous, Z., Attia, A., and Datta, A. (2019). “Chapter two – improving water use efficiency, nitrogen use efficiency, and radiation use efficiency in field crops under drought stress: a review,” in Advances in Agronomy, ed. D. L. Sparks (Cambridge, MA: Academic Press), 109–157.
VanLoocke, A., Twine, T. E., Zeri, M., and Bernacchi, C. J. (2012). A regional comparison of water use efficiency for miscanthus, switchgrass and maize. Agric. For. Meteorol. 164, 82–95. doi: 10.1016/j.agrformet.2012.05.016
Wang, Q., He, Q., and Zhou, G. (2018). Applicability of common stomatal conductance models in maize under varying soil moisture conditions. Sci. Total Environ. 628-629, 141–149. doi: 10.1016/j.scitotenv.2018.01.291
Wang, S., Huang, Y., Sun, W., and Yu, L. (2018). Mapping the vertical distribution of maize roots in China in relation to climate and soil texture. J. Plant Ecol. 11, 899–908. doi: 10.1093/jpe/rty015
Wild, M., Gilgen, H., Roesch, A., Ohmura, A., Long, C. N., Dutton, E. G., et al. (2005). From dimming to brightening: decadal changes in solar radiation at Earth’s surface. Science 308, 847–850. doi: 10.1126/science.1103215
Xiao, D., Liu, D. L., Wang, B., Feng, P., and Waters, C. (2020). Designing high-yielding maize ideotypes to adapt changing climate in the North China Plain. Agric. Syst. 181, 102805. doi: 10.1016/j.agsy.2020.102805
Yang, H. S., Dobermann, A., Lindquist, J. L., Walters, D. T., Arkebauer, T. J., and Cassman, K. G. (2004). Hybrid-maize-a maize simulation model that combines two crop modeling approaches. Field Crops Res. 87, 131–154. doi: 10.1016/j.fcr.2003.10.003
Yang, S., Wang, L., Shi, C., and Lu, Y. (2018). Evaluating the relationship between the photochemical reflectance index and light use efficiency in a mangrove forest with Spartina alterniflora invasion. Int. J. Appl. Earth Obs. 73, 778–785. doi: 10.1016/j.jag.2018.08.014
Yang, Y., Xu, W., Hou, P., Liu, G., Liu, W., Wang, Y., et al. (2019). Improving maize grain yield by matching maize growth and solar radiation. Sci. Rep. 9:3635. doi: 10.1038/s41598-019-40081-z
Yi, L., Yang, S. J., Li, S. Q., Chen, X. P., and Chen, F. (2010). Growth and development of maize (Zea mays L.) in response to different field water management practices: resource capture and use efficiency. Agric. For. Meteorol. 150, 606–613. doi: 10.1016/j.agrformet.2010.02.003
Yu, G., Song, X., Wang, Q., Liu, Y., Guan, D., Yan, J., et al. (2008). Water-use efficiency of forest ecosystems in eastern China and its relations to climatic variables. New Phytol. 177, 927–937. doi: 10.1111/j.1469-8137.2007.02316.x
Yu, L., Zhao, X., Gao, X., and Siddique, K. H. M. (2020). Improving/maintaining water-use efficiency and yield of wheat by deficit irrigation: a global meta-analysis. Agric. Water Manage. 228:105906. doi: 10.1016/j.agwat.2019.105906
Zampieri, M., Ceglar, A., Dentener, F., Dosio, A., Naumann, G., Berg, M., et al. (2019). When will current climate extremes affecting maize production become the norm? Earths Future 7, 113–122. doi: 10.1029/2018ef000995
Zhou, H., Zhou, G., He, Q., Zhou, L., Ji, Y., and Lv, X. (2021). Capability of leaf water content and its threshold values in reflection of soil–plant water status in maize during prolonged drought. Ecol. Indic. 124:107395. doi: 10.1016/j.ecolind.2021.107395
Zhou, L., Wang, Y., Jia, Q., Li, R., Zhou, M., and Zhou, G. (2019). Evapotranspiration over a rainfed maize field in northeast China: how are relationships between the environment and terrestrial evapotranspiration mediated by leaf area? Agric. Water Manage. 221, 538–546. doi: 10.1016/j.agwat.2019.05.026
Keywords: fAPAR, leaf area index (LAI), maize, progressive soil drying, RUE, WUE
Citation: Zhou H, Zhou G, Zhou L, Lv X, Ji Y and Zhou M (2021) The Interrelationship Between Water Use Efficiency and Radiation Use Efficiency Under Progressive Soil Drying in Maize. Front. Plant Sci. 12:794409. doi: 10.3389/fpls.2021.794409
Received: 13 October 2021; Accepted: 08 November 2021;
Published: 10 December 2021.
Edited by:
Dejan Dodig, Maize Research Institute Zemun Polje, SerbiaReviewed by:
Weverton Pereira Rodrigues, Universidade Estadual da Região Tocantina do Maranhão (UEMASUL), BrazilSofija Bozinovic, Maize Research Institute Zemun Polje, Serbia
Domagoj Simic, Agricultural Institute Osijek, Croatia
Copyright © 2021 Zhou, Zhou, Zhou, Lv, Ji and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangsheng Zhou, emhvdWdzQGNtYS5nb3YuY24=
 Huailin Zhou
Huailin Zhou Guangsheng Zhou
Guangsheng Zhou Li Zhou1,3
Li Zhou1,3