- Centre for Crop Health, Institute for Life Sciences and the Environment, University of Southern Queensland, Toowoomba, QLD, Australia
Root-lesion nematodes (Pratylenchus spp.) and arbuscular mycorrhizal fungi (AMF) occupy the same ecological niche in the phytobiome of many agriculturally important crops. Arbuscular mycorrhizal fungi can enhance the resistance or tolerance of a plant to Pratylenchus and previous studies have been undertaken to investigate the relationship between these organisms. A restructuring of the AMF phylum Glomeromycota has reallocated the species into genera according to molecular analysis. A systematic review of the literature was synthesized to assess the interaction between Pratylenchus spp. and AMF using the revised classification. Plants inoculated with AMF generally exhibited greater tolerance as demonstrated by increased biomass under Pratylenchus pressure. Species of AMF from the order Diversisporales tended to increase Pratylenchus population densities compared to those from the order Glomerales. Species from the genera Funneliformis and Glomus had a reductive effect on Pratylenchus population densities. The interaction between AMF and Pratylenchus spp. showed variation in responses as a result of cultivar, crop species, and AMF species. Putative mechanisms involved in these interactions are discussed.
Introduction
Pratylenchus spp. or root-lesion nematodes, are migratory endoparasites (Singh et al., 2013). They feed and move through the root cortex, penetrating parenchyma cells with their stylet, excreting cell degrading enzymes, ingesting the cellular contents, and destroying cortical tissue. This results in necrotic lesions, loss of root function and consequently, reductions in plant vigor, and yield of economic products (Jones et al., 2013).
Root-lesion nematodes are polyphagous and have the broadest host range of all plant-parasitic nematodes. They are responsible for substantial yield losses of many important crop species including cereals, legumes, sugarcane, coffee, banana, potato, vegetables and fruit trees (Castillo and Vovlas, 2007). There are over 68 recognized species of Pratylenchus associated with the phytobiome and they are distributed in diverse habitats worldwide (Castillo and Vovlas, 2007). Historically, Pratylenchus spp. were distinguished on the basis of their morphometric characteristics. With the advent of molecular techniques, differences in the sequences of ribosomal DNA can distinguish between species despite high levels of intraspecific variation in some Pratylenchus spp. High levels of intraspecific variability occur within some Pratylenchus spp. such as P. coffeae and P. penetrans while other species exhibit less intraspecific internal transcribed spacer (ITS) variation, for example, P. goodeyi and P. vulnus (de Luca et al., 2011; Jones et al., 2013).
Arbuscular mycorrhizal fungi (AMF), from the phylum Glomeromycota are a ubiquitous group of soil microorganisms associated with the phytobiome. Arbuscular mycorrhizal fungi form a complex symbiosis with land plants which originated in the Ordovician period 400 million years ago (Parniske, 2008). They have remained morphologically unchanged since then, forming an intrinsic part of ecosystem functionality (Powell and Rillig, 2018). These obligate biotrophs form beneficial mutualistic associations with the roots of an estimated 80% of land plants including many agriculturally important crop species with the notable exception of most species in the families Brassicaceae and Chenopodiaceae (Lambers and Teste, 2013). Their characteristic arbuscules (microscopic tree-like structures) within the root cortical cells of compatible plants enable the photosynthetically derived organic compounds supplied by the plant to be exchanged for inorganic nutrients and water supplied by the fungus from the soil. The fungus also aids in the stabilization of soil aggregates through hyphal binding and exudation of glomalin (Smith and Read, 2008; Leifheit et al., 2014). It is estimated that up to 20% of the photosynthetic carbon of plants is allocated to maintaining the fungal association (Smith and Read, 2007). This carbon cost to the plant is outweighed by the many benefits conferred by the fungi, foremost of which are improved acquisition by the fungal hyphae of immobile nutrients from the soil such as phosphorus (P) and zinc (Zn) (Parniske, 2008).
Arbuscular mycorrhizal fungi have been promoted as a natural tool to maintain and promote sustainable agriculture due to their role as natural biofertilizers; increasing the levels of nitrogen (N), P and Zn in the crop (Thompson, 1993; Parniske, 2008; Smith et al., 2011; Baum et al., 2015; Berruti et al., 2016). They also play a role in drought tolerance (Zhao et al., 2015) and as bio-protectants against fungal, bacterial, and nematode pathogens (Whipps, 2004; Pozo and Azcón-Aguilar, 2007; Veresoglou and Rillig, 2012; Yang et al., 2014).
Early classifications defined species within the order Glomerales of the phylum Glomeromycota on the basis of spore morphology (Morton and Benny, 1990). Schüßler and Walker (2010) restructured the phylum Glomeromycota according to molecular phylogenies based on the small subunit (SSU) rRNA gene, the large subunit (LSU) rRNA gene, β-tubulin sequence data and the ITS region. Consequently, the current classification of the order Glomerales consists of two families — the Glomeraceae and the Claroidoglomeraceae. A number of Glomus species have been transferred to the genera Funneliformis and Rhizophagus. Table 1 shows the phylum Glomeromycota and the subdivisions into the orders Glomerales, Diversisporales, Archaeosporales, and Paraglomerales (Redecker et al., 2013).
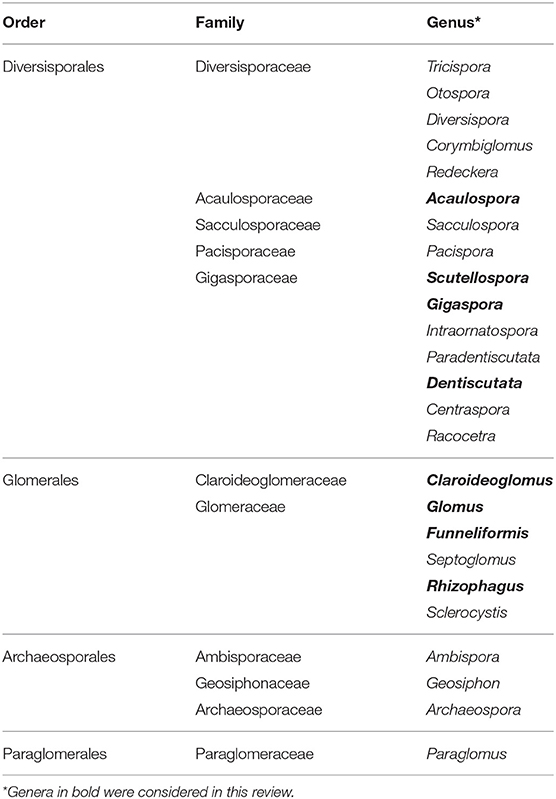
Table 1. Classification of the phylum Glomeromycota according to Redecker et al. (2013).
Plant-parasitic nematodes are classified according to their feeding strategies. These include (i) ecto-parasitic nematodes which feed externally on root cells and remain in the rhizosphere such as Tylenchorhynchus spp., (ii) migratory endo-parasitic nematodes which enter the plant root, feed, and move through the root tissues destroying cells as they migrate such as Pratylenchus spp., and, (iii) sedentary endo-parasitic nematodes which convert vascular cells into specialized feeding cells where they remain, such as the root-knot nematodes (Meloidogyne spp.) and the cyst nematodes (Heterodera and Globodera spp.) (Decraemer and Hunt, 2013).
The coexistence of AMF and nematodes in the phytobiome has prompted a number of investigations into their interactive effects on plants (reviews: Pinochet et al., 1996; meta-analyses: Borowicz, 2001; Hol and Cook, 2005; Veresoglou and Rillig, 2012; Yang et al., 2014). Published meta-analyses describe the generally suppressive effect that AMF have on nematodes (Veresoglou and Rillig, 2012; Yang et al., 2014). These analyses included nematodes belonging to different genera and they grouped plant-parasitic nematodes into their feeding modes (sedentary or migratory). AMF reduced the numbers of the sedentary endo-parasitic nematodes (Meloidogyne, Heterodera, and Globodera spp.) and the ectoparasitic nematodes (Tylenchorhynchus spp.). However, some analyses showed an increase in migratory endo-parasitic nematode numbers on inoculation with AMF (Borowicz, 2001; Hol and Cook, 2005). Grouping the nematodes into their broad feeding modes has the effect of obscuring the data on interactions of AMF with Pratylenchus spp. and those with other migratory endo-parasites including Radopholus spp. and Hirschmanniella spp.
Due to the ubiquitous distribution and the great economic importance of Pratylenchus spp. to agricultural crops worldwide, this systematic review examines the relationship exclusively between Pratylenchus spp. and AMF taking into account the current classification of AMF genera. All life stages of Pratylenchus spp., adults, juveniles, and eggs occupy the same root cortex tissue as the AMF structures of hyphae, arbuscules, and vesicles (Pinochet et al., 1996) and co-occur with AMF extraradical hyphae and spores in the rhizosphere soil.
The aims of this review are to determine (a) the responses in Pratylenchus population densities to AMF, (b) the effects of AMF on the growth of plants infested with Pratylenchus and, (c) the effects of degree of AMF colonization on Pratylenchus population density. The outcomes of the systematic review are discussed in relation to putative mechanisms involved in the interaction between Pratylenchus spp. and AMF. These mechanisms may include: (a) enhanced plant tolerance to Pratylenchus as a result of increased nutrient uptake and altered root morphology, (b) direct competition between Pratylenchus and AMF for resources and space, (c) effects on Pratylenchus through plant defense mechanisms such as induced systemic resistance in the plant from AMF colonization, and (d) altered rhizosphere interactions (Pozo and Azcón-Aguilar, 2007; Schouteden et al., 2015).
Methods
Selection of Studies
A systematic review of the literature was performed according to PRISMA systematic review guidelines (Moher et al., 2009). Studies investigating interactions between Pratylenchus spp. and AMF were obtained from the databases,—Web of Science (www.webofknowledge.com), SCOPUS (https://www.scopus.com) and Google Scholar (https://scholar.google.com/).
The search parameters included the following terms, “Pratylenchus,” “arbuscular mycorrhizal fungi” AND “root-lesion nematode.” The papers were further screened to select original research with quantitatively measured data of the following response variables: (a) effects of AMF on Pratylenchus population densities, (b) effects of Pratylenchus spp. on degree of AMF colonization in the roots (mycorrhization), and (c) effects of both organisms on plant biomass. Other pre-requisites for eligibility for inclusion in the review were (a) studies with one or more AMF species, but not mixed treatments with other beneficial organisms, (b) studies with Pratylenchus species alone not mixed with other plant-parasitic nematodes, and (c) studies with a non-inoculated control. Reviews, meta-analyses and book chapters were excluded from the analyses, but the original research papers cited within were cross referenced and assessed for suitability for inclusion.
Analyses of Response Variables
The “nematode response” was calculated using the following formula:
where “Pratylenchus” is the final population density of Pratylenchus in nematode only treatments and “Pratylenchus plus AMF” is the population density of Pratylenchus in co-inoculated AMF and nematode treatments.
The “biomass response” was calculated using the following formula:
Where “Pratylenchus biomass” is the plant biomass in nematode only inoculated treatments and “Pratylenchus plus AMF biomass” is the plant biomass in co-inoculated AMF and nematode treatments. Biomass data were expressed as shoot, root and total biomass where available.
The “AMF response” was calculated using the following formula:
where “AMF % colonization” is the percentage of mycorrhization of plants with AMF alone and “AMF % colonization plus Pratylenchus” is the percentage of mycorrhization of plants co-inoculated with AMF and nematodes.
The effect of inoculation with AMF on the Pratylenchus population density was categorized as decrease, no effect, or increase based on statistical significance (P<0.05) of studies in the original publications. A chi-squared test for independence was performed to assess the relationship between order of AMF (Glomerales and Diversisporales) and effect on Pratylenchus population densities. Chi-squared values were calculated from two-way contingency tables (Steel and Torrie, 1960) of AMF order by Pratylenchus density effect for the 56 studies using the following function:
The percentage AMF colonization of the roots of the plants in these three categories of AMF effects on Pratylenchus population densities for the studies with relevant data was subjected to one-way analysis of variance (ANOVA) using GenStat (VSN International, 2014).
The data were examined under other independent groupings such as (a) restructured AMF genera according to the current classification by Schüßuler and Walker (Schüßler and Walker, 2010) and (b) host plant functional group (grasses, trees, herbs, shrubs).
Results
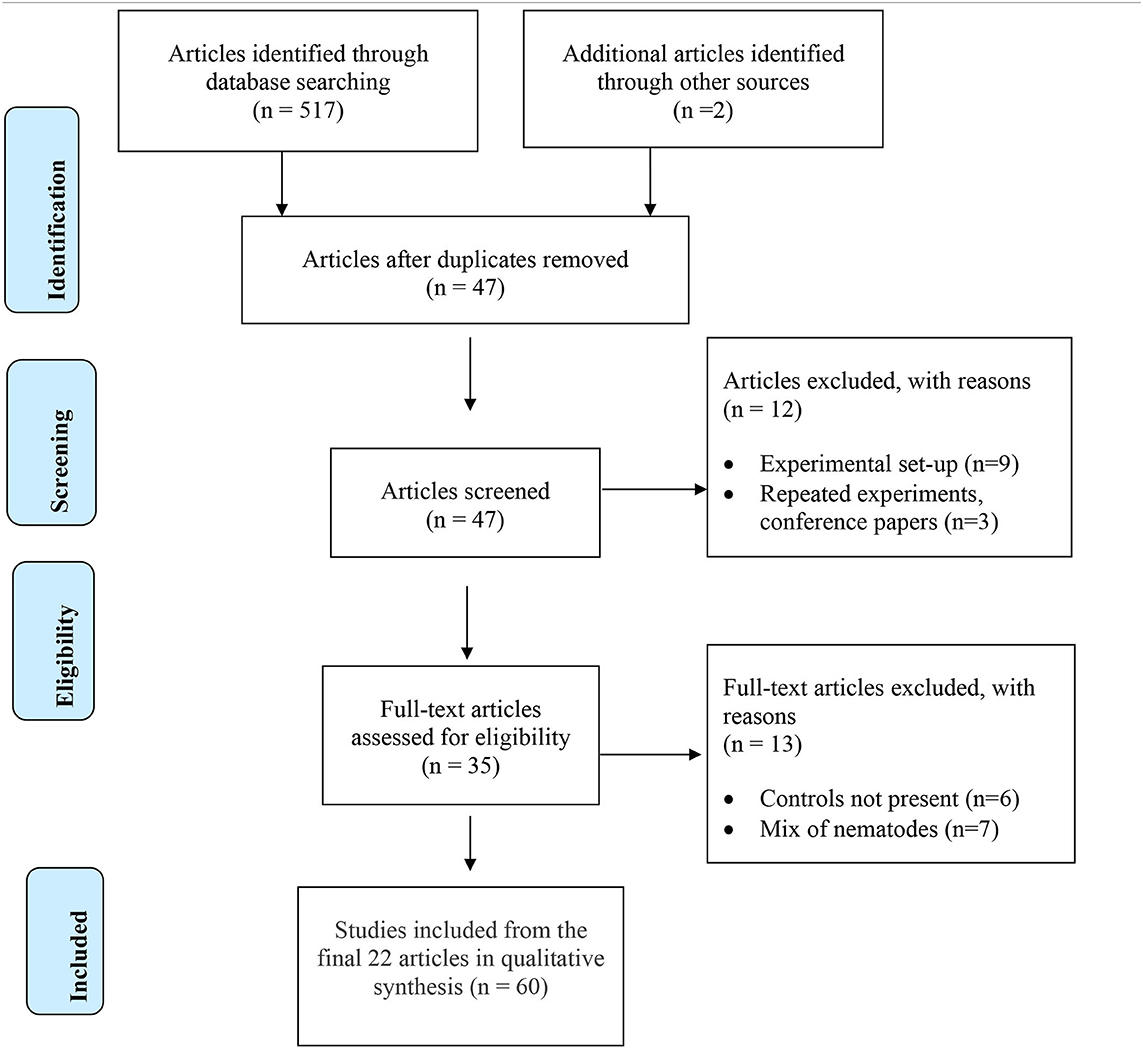
The initial search conducted on all available literature in the three databases provided 519 potential papers for inclusion. Further screening by removing duplicates and ineligible papers resulted in 22 full text articles selected for the systematic review (Table 2). Experiments within papers were treated as separate studies when; (a) two or more AMF species were studied independently, (b) more than one plant cultivar was included, and (c) more than one time of inoculation was used. If there were various times of assessment for plant biomass over multiple years, the most recent data set was used. In total, 60 studies were analyzed (Supplementary Table 1).
Table 3 shows the response of Pratylenchus sp., arbuscular mycorrhizal fungi (AMF) and plants to co-inoculation of AMF and Pratylenchus sp. compared to Pratylenchus sp. alone in glasshouse and microplot experiments. The data is statistically significant as stated in the original papers. The majority of the crops assessed were agriculturally or horticulturally important with the exception of dune grass (Ammophilia arenaria). In general, the experiments were undertaken in glasshouses with some transplanting of pre-inoculated AMF colonized plants to field microplots. There were 14 individual species of AMF used in 43 studies, one undetermined species in ten studies, and a mix of AMF species in seven studies. These species came from both the order Glomerales which included the genera Rhizophagus, Glomus, Funneliformis, Claroideoglomus, and the order Diversisporales, which included the genera Acaulospora, Dentiscutata, Gigaspora, and Scutellospora.
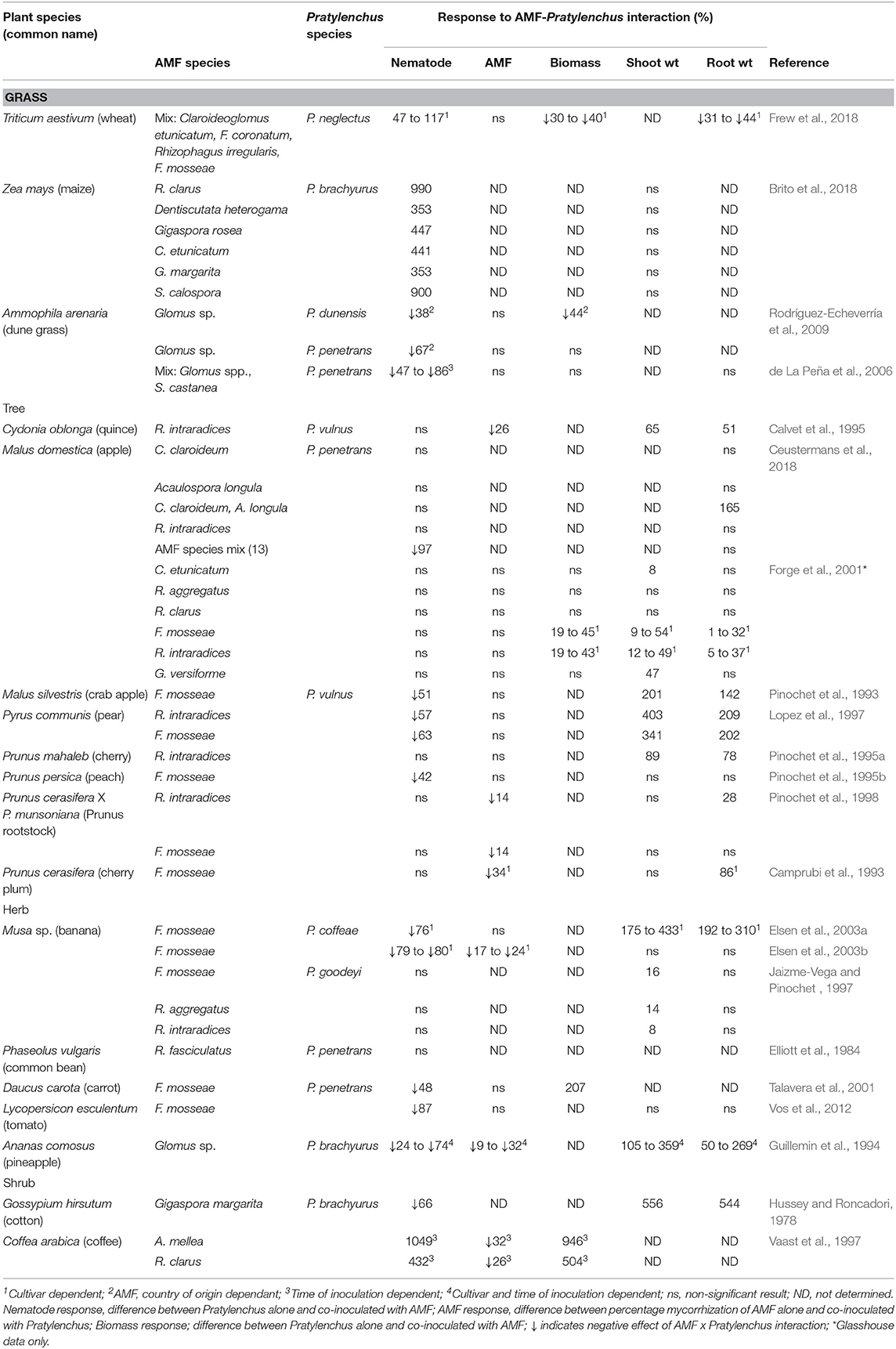
Table 3. Response of Pratylenchus spp., arbuscular mycorrhizal fungi (AMF) and plants to co-inoculation of AMF and Pratylenchus spp. compared to Pratylenchus sp. alone in glasshouse and microplot experiments.
The studies involved seven Pratylenchus spp. namely P. penetrans, P. vulnus, P. neglectus, P. coffeae, P. goodeyi, P. brachyurus and P. dunensis. These species reviewed are many of the species of Pratylenchus causing the most economic damage worldwide (Jones and Fosu-Nyarko, 2014).
Responses in Pratylenchus Population Densities to AMF
The effects of AMF inoculation on Pratylenchus population densities varied from a decrease in population densities (n = 22), no effect on Pratylenchus population densities (n = 28), to an increase in Pratylenchus population densities (n = 10).
The taxonomic order of AMF species used had an effect on Pratylenchus densities, whereby inoculation with species from the order Glomerales tended to decrease Pratylenchus population densities compared with species from the order Diversisporales which tended to increase Pratylenchus population densities (Table 4). Although there were fewer studies with comparisons for Diversisporales than for Glomerales, the differences in response between these groupings were highly significant (Table 4). Within the Glomerales, inoculation with the genera Glomus and Funnelifomis had a neutral to reductive effect on Pratylenchus population densities.
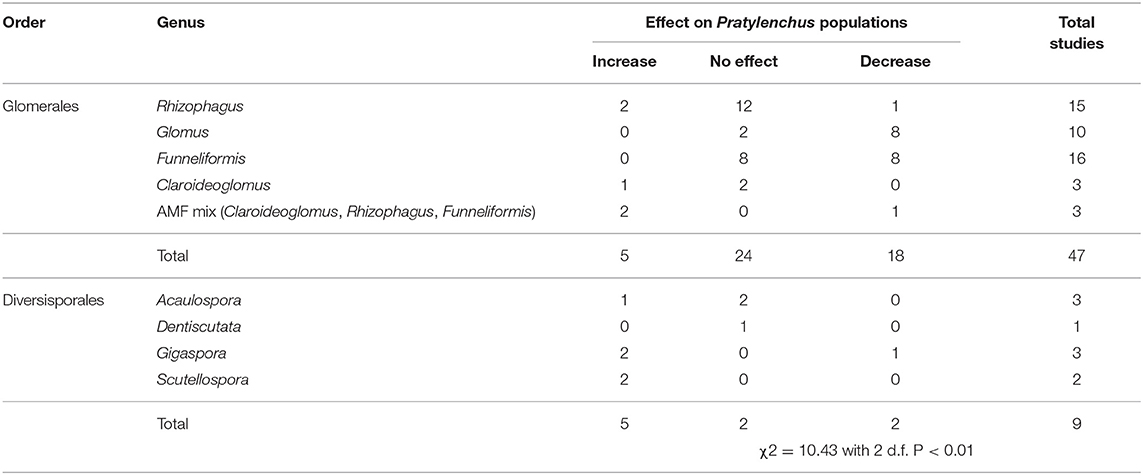
Table 4. Number of studies investigating AMF-Pratylenchus interaction included in the systematic review and the effect of AMF order on Pratylenchus populations.
Increases in Pratylenchus population densities due to AMF inoculation in studies subdivided in relation to the host plant functional group were predominantly found in the grasses (increases in 8 out of 15 studies). No increase in Pratylenchus population densities were found in trees (0 increases in 24 studies), or herbs (0 increases in 16 studies).
Effects of AMF on the Growth of Plants Infested With Pratylenchus
Plant shoot biomass increased when AMF were co-inoculated with Pratylenchus compared with infection with Pratylenchus alone. From the 34 studies with data providing comparisons on shoot biomass, 24 showed an increase in shoot biomass while 10 had no effect. No studies showed a reduction in shoot biomass. Most studies calculated shoot biomass (n = 35) and root biomass independently (n = 41), with fewer reporting results on total biomass (n = 28). From these 28 studies, eight showed an increase in total plant biomass, and three studies a decrease in total plant biomass with 17 having no significant effect.
The change in root biomass between plants inoculated with Pratylenchus and the plants co-inoculated with AMF and Pratylenchus is shown in Table 3. The majority of the studies showed an increase in root biomass when inoculated with AMF (n = 22) in the presence of Pratylenchus with the exception of two studies by Frew et al. (2018).
Effects of Degree of AMF Colonization on Pratylenchus Population Density
There were 58 studies with data on the degree of AMF colonization of the roots. In most studies there was a decrease (n = 21) or no effect (n = 27) on Pratylenchus population densities, which were associated with relatively high percentage AMF colonization of the roots (43.9 and 42.2% respectively), compared to an increase in Pratylenchus population densities (n = 10), which were associated with a significantly lower percentage AMF colonization (20.1%) (Table 5).
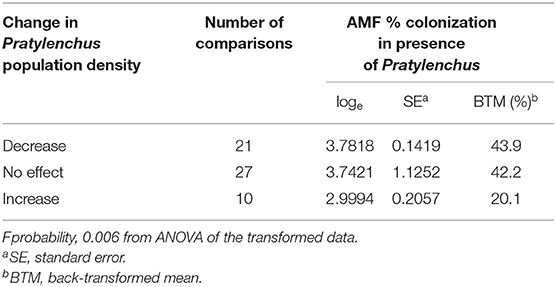
Table 5. Effects of AMF inoculation on change in Pratylenchus population densities in relation to degree of AMF colonization in the roots.
Discussion
This review is the first to examine the effects of specific genera and order of AMF acting on Pratylenchus population densities and demonstrates that the taxonomic order of AMF has a significant influence on Pratylenchus population densities. Previous reviews and meta-analyses showed a varied response of AMF on migratory endo-parasites ranging from a suppressive (Veresoglou and Rillig, 2012; Yang et al., 2014) to a stimulatory effect (Borowicz, 2001; Hol and Cook, 2005).
Variation in functionalities between AMF families has been reported (Smith et al., 2004). Members of the Glomeraceae are typically fast colonizers, concentrating their hyphae within the plant roots and can increase P uptake and promote plant growth under pathogen attack and drought stress (Klironomos, 2000; Hart and Reader, 2002; Maherali and Klironomos, 2007; Yang et al., 2015; Seymour et al., 2019). Members of the Diversisporales are typically slower to colonize roots, concentrating hyphae externally to the plant root in the soil and are effective at enhancing plant phosphorus uptake (Klironomos, 2000; Hart and Reader, 2002; Maherali and Klironomos, 2007). However, from the studies in this review, there was lack of data on the percentage of AMF colonization of the controls in the order Diversisporales (n = 2) therefore it remains unclear if Diversisporales are slower to colonize from these studies.
From our review, species from the genera Glomus or Funneliformis, in the order Glomerales decreased or had no significant effect on the Pratylenchus population densities compared with Rhizophagus and Claroideoglomus. The difference in effects that AMF genera have on Pratylenchus population densities could be due to differences in the secondary metabolites produced under the symbiotic relationship. For example, in tomato, although the metabolic pathways altered by the AMF symbiosis were similar, different metabolites were produced, depending on inoculation with F. mosseae or R. irregularis (Pozo et al., 2002). An increase in the accumulation of bioactive forms of jasmonic acid was found in roots colonized by F. mosseae (Rivero et al., 2015). Jasmonic acid and its derivative methyl jasmonate play a role in plant defense against herbivores and they can reduce susceptibility of plants to infestation by Pratylenchus (Soriano et al., 2004). Root metabolites may influence populations of plant parasitic nematodes by acting as attractants, repellents or affecting hatch rates of nematodes (Sidker and Vestergård, 2019). Mycorrhizal colonization can increase phenolics such as ferulic acid and gallic acid in the host plants (López-Ráez et al., 2010; Li et al., 2015). Ferulic acid inhibits mobility and is toxic to the burrowing nematode R. similis but is ineffective against Pratylenchus penetrans (Wuyts et al., 2006). Gallic acid acts as a nematicide to the root-knot nematode M. incognita (Seo et al., 2013). High constitutive total phenol contents were found in synthetic hexaploid wheat genotypes resistant to P. thornei combined with high levels of induced phenol oxidases (Rahaman et al., 2020). These studies indicate that the biochemical responses of host plants to both inoculation with AMF and infestation by plant-parasitic nematodes are highly complex.
Even within populations of a single species of AMF, there is a high genetic variability which may affect the host/fungal relationship (Koch et al., 2006, 2017). Variations in the effects that a single species of AMF have on Pratylenchus population densities were observed in the studies by Elsen et al. (2003b) and Jaizme-Vega and Pinochet (1997). Both studies used the same cultivar of banana and the same species of AMF, but obtained different results depending on the Pratylenchus sp. tested. Elsen et al. (2003b) stated that it was difficult to explain the contrary results, however, the AMF strain and the environmental conditions differed between experiments. As a different isolate of F. mosseae was used as inoculum, it is important to emphasize the traceability of isolates that are used in experiments. A similar observation was made in dune grass whereby Pratylenchus sp. were only reduced in the interaction with a community of AMF isolated from Wales and not from an AMF community isolated from Belgium (Rodríguez-Echeverría et al., 2009). This highlights the need to study interactions between specific crops, cultivars and AMF species or communities.
Plant functional group influenced Pratylenchus population densities in grasses but not in herbs and trees. Interestingly, response to AMF can be attributed to plant functional groups in which non-nitrogen fixing forbs and woody plants, and C4 grasses benefit more in plant growth by the fungal association, compared to nitrogen fixing plants and C3 grasses (Hoeksema et al., 2010). However, Yang et al. (2016) concluded that nitrogen fixing plants had a greater mycorrhizal growth response only when the host plant was a forb and not woody. A practical application to improve tolerance, or plant growth, when Pratylenchus is present may therefore be to pre-inoculate tree species with AMF prior to transplanting into orchards, taking into account the interaction between cultivars, their mycorrhizal dependency and AMF species used as inoculum sources (Pinochet et al., 1996). The potential of AMF inoculum conferring benefits to crop production in high economic value vegetable crops has been reviewed by Baum et al. (2015). These include advantages such as increases in yield, increases in commercial quality of the crop, protection against nematodes and other pathogens, tolerance to drought and other abiotic stressors and nutrient uptake. As the interaction between host, AMF inoculum and environment can be very specific, future research is needed to optimize the inoculation protocols to target specific crop production limitations.
The outcomes of the present systematic review, in relation to putative mechanisms involved in the interaction between Pratylenchus spp. and AMF, are discussed below.
Enhanced Plant Tolerance
Plant shoot biomass increased when AMF were co-inoculated with Pratylenchus compared with infection with Pratylenchus alone. A number of studies investigated tolerance to Pratylenchus spp. as a reflection of increasing vegetative plant nutrition. AMF can increase the uptake of P and other nutrients such as Zn from the soil (Parniske, 2008; Seymour et al., 2019). This increase in nutrition can lead to a greater plant biomass response conferring a compensatory effect against the damage done by nematodes. Previous studies have shown that AMF confers tolerance to Pratylenchus spp. by compensating for root damage caused by Pratylenchus spp. through increasing the uptake of P and other micronutrients, such as Fe, Mn, Zn, and Cu (Calvet et al., 1995; Pinochet et al., 1998). However, improvement in the nutritional status of the plant is not believed to be wholly responsible for the biocontrol effect of AMF (Bødker et al., 1998; Jung et al., 2012).
Tolerance conferred by AMF to a crop under Pratylenchus pressure has been described in the majority of the reviewed papers (n = 41) with the exception of the following; peach, Musa sp., maize, tomato, dune grass and wheat (Pinochet et al., 1995b; Elsen et al., 2003a; Rodríguez-Echeverría et al., 2009; Vos et al., 2012; Brito et al., 2018; Frew et al., 2018). This may be a reflection of the mycorrhizal dependency of the cultivars assessed as some tomato and wheat cultivars have a low mycorrhizal dependency (Smith et al., 2009) while cultivars of maize, Musa sp. and peach generally have higher mycorrhizal dependency (Pinochet et al., 1995b; Kaeppler et al., 2000; Elsen et al., 2003a). A study by Martín-Robles et al. (2018) found that domesticated crops benefit more from the symbiosis with AMF under P limiting conditions. It is worthwhile to note that most of the studies analyzed in this review were undertaken in low P experimental conditions where AMF function most efficiently (Supplementary Table 1).
The studies assembled in Table 3 demonstrate the predominantly beneficial effects AMF have on crop species, alleviating the damage to the root and shoot biomass caused by Pratylenchus. There were only three studies where AMF decreased total biomass and root weight when co-inoculated with Pratylenchus. These studies were on wheat and dune grass, both C3 crops (Rodríguez-Echeverría et al., 2009; Frew et al., 2018). Variations in root morphology between C3 and C4 grasses determine their dependency on the mycorrhizal symbiosis (Hetrick et al., 1991), which may help explain the reduction in biomass. Wheat has a low to intermediate dependency on mycorrhiza depending on genotype (Lehnert et al., 2017) and modern plant breeding may contribute to a reduction in dependency on the mycorrhizal symbiosis by screening and selecting new varieties in high phosphate or highly fertile soils (Hetrick et al., 1993). However, a modern wheat cultivar Batavia was found to have high dependency on AMF colonization under drought conditions on a field site infested with P. thornei (Owen et al., 2010). Dune grass forms an association with AMF promoting plant growth (Tadych and Blaszkowski, 1999). de La Peña et al. (2006) suggested that evidence of biomass reduction in dune grass was related to a species-specific interaction between a geographically unique community of AMF from Wales and the species of Pratylenchus (P. dunensis) studied. Biomass reduction was not significant in another study of the interaction between AMF and P. penetrans on dune grass (de La Peña et al., 2006).
Previous reviews have also demonstrated this positive effect that AMF have on increasing plant growth under attack by migratory nematodes (Hol and Cook, 2005; Yang et al., 2014). This is contrary to the study by Borowicz (2001) that concluded AMF increased the negative effects of nematodes on plant biomass, indicating a reduced nematode tolerance.
The majority of studies showed an increase in root biomass in the presence of Pratylenchus when inoculated with AMF. Pratylenchus infestation negatively impacts root biomass, resulting in a reduction in the quantity and length of root branches (Fosu-Nyarko and Jones, 2016). Colonization by AMF can also result in alterations to root morphology, causing either an increase or decrease in root branching (Hooker et al., 1992; Sikes, 2010). A study on morphological changes within the root system in Musa sp. under Pratylenchus pressure showed that AMF increased root branching counteracting the negative consequences of Pratylenchus infection (Elsen et al., 2003a). Berta et al. (1995) also demonstrated in cherry plum (Prunus cerasifera) that AMF increased the branching of all root orders. However, there were variable effects on root diameter depending on which genera of AMF were used.
Baylis (1975) hypothesized that plants with extensive fine root systems with long dense root hairs were less reliant on the mycorrhizal symbiosis in comparison to coarsely rooted plants. However, recent evidence suggests that coarse roots are not necessarily a good predictor of crop dependency on the AMF symbiosis (Maherali, 2014). A meta-analysis by Yang et al. (2016) found that although plants with fibrous roots responded less to mycorrhizal colonization than tap rooted plant species, this was only evident for C3 and not C4 grass species. Notwithstanding this, plants that have a highly branched root system may still benefit from the AMF association via other ecosystem functions such as pathogen protection (Newsham et al., 1995).
Competition for Space Between Pratylenchus and AMF
Degree of AMF colonization had an effect on the population densities of Pratylenchus. Inoculation with AMF that resulted in low levels of AMF colonization was associated with increases in Pratylenchus population densities compared with other cases with high levels of AMF colonization that were associated with decreases or no effects on Pratylenchus population densities. The nematode population density could also affect the rate of colonization by AMF indicating a competition between species. Both AMF and Pratylenchus occupy the same ecological niche within the root cortical cells as described in various crop species, for example, quince, cherry, peach, pear, banana, plum, and coffee (Calvet et al., 1995; Pinochet et al., 1995a,b, 1998; Lopez et al., 1997; Vaast et al., 1997; Elsen et al., 2003b). Pratylenchus sp. and AMF were considered to have competed for space within the cortical cells in quince, coffee, banana and dune grass (Calvet et al., 1995; Vaast et al., 1997; Elsen et al., 2003b; de La Peña et al., 2006).
Arbuscules are the metabolically active sites of exchange between the plant and the fungus and a mature mycorrhizal colonization of the plant, as evidenced by the production of arbuscules, has been thought to be the prerequisite for a biocontrol effect (Khaosaad et al., 2007). It has been hypothesized that a greater colonization of AMF in plant roots would lead to a greater biocontrol effect on nematodes.
Pratylenchus can affect the quantity and morphology of AMF within the root cortical cells. For example, in quince, AMF increased the production of arbuscules reflecting a metabolically active state under Pratylenchus infestation, compared to an increase in the production of vesicles in the absence of infestation (Calvet et al., 1995). In banana, nematodes reduced the frequency of colonization but not the intensity (Elsen et al., 2003b). In pineapple, although nematodes reduced the frequency of arbuscules when applied at a later time point during transplanting, they did not affect the efficiency of the symbiosis (Guillemin et al., 1994).
The time of inoculation was not a factor in how the nematode population densities responded to AMF inoculation. AMF was applied to the plants prior to nematode inoculation in the majority of studies (n = 42), which gave the symbiosis a chance to establish before being challenged with Pratylenchus. However, this established symbiosis was not reflected in a decrease in nematode population density, but may have aided the plant in tolerance to nematode infestation through increased vegetative growth as previously discussed.
Plant Defense and Induced Systemic Resistance
Mycorrhiza-induced resistance that can operate systemically can be effective against plant-parasitic nematodes and may contribute toward the biocontrol effect of AMF (Jung et al., 2012). Induced systemic resistance has no association with pathogenesis related proteins or salicylic acid but is regulated by jasmonic acids and ethylene (Pieterse et al., 1998).
There is little available research on induced systemic resistance by AMF against Pratylenchus as compared to other plant pathogens. However, using split root experiments, the systemic biocontrol effects of the AMF species F. mosseae and R. irregularis on Pratylenchus were demonstrated in banana and tomato. Rhizophagus irregularis induced a systemic suppression of P. coffeae and R. similis in banana, though the pathways involved in this suppression were not determined (Elsen et al., 2008). In tomato, inoculation with F. mosseae reduced the number of females of P. penetrans through a localized mechanism and the number of juveniles through a systemic mechanism (Vos et al., 2012). Contrary to this, only a localized suppression of Pratylenchus population densities was observed in dune grass (de La Peña et al., 2006).
Investigations into the metabolomics of AMF showed that AMF colonization increased the production of AMF plant signaling compounds and anti-herbivory defenses (Hill et al., 2018). There is still very little research available on the interactions between Pratylenchus and AMF on effects on the metabolome. Frew et al. (2018) reported that AMF reduced plant defense metabolites, specifically benzoxazinoids, which accounted for an increase in P. neglectus population densities in wheat. Studies involving root organ cultures of carrot showed significant suppressive effects of AMF on P. coffeae female population densities believed to be a result of biochemical changes in the mycorrhized root (Elsen et al., 2003c). Exudates from AMF can reduce the motility and penetration of sedentary nematodes (Vos et al., 2012) but little research has been done on their effects on migratory endo-parasites. An in-vitro chemotaxic assay on the migratory endo-parasite R. similis demonstrated that the exudation of a water-soluble compound, produced by mycorrhizal roots, reduced attraction at a pre-infection stage (Vos et al., 2012), but there is little information on how exudates affect Pratylenchus spp. Further research is needed to assess the mechanisms of AMF in influencing Pratylenchus population densities.
Alterations in the Rhizosphere
Alterations in chemical compounds in the rhizosphere as a result of interactions between plant-parasitic nematodes and AMF have been reviewed (Schouteden et al., 2015). These involve changes in exudation of sugars, organic acids, amino acids, phenolic compounds, flavonoids and strigolactones in AMF colonized plants as compared to non-AMF plants. AMF exudations into the rhizosphere promote beneficial microorganisms such as plant-growth promoting rhizobacteria (PGPR) (Jung et al., 2012; Javaid, 2017) and resultant changes can be induced systemically, influencing the bacterial community structure (Marschner and Baumann, 2003). This enhanced microbial activity around plant roots has been termed the mycorrhizosphere effect (Linderman, 1988). Plant growth promoting rhizobacteria have been implicated in nitrogen fixation, phosphate solubilization, modulating phytohormone levels and the production of antibiotics and lytic enzymes (Glick, 2012). Cameron et al. (2013) proposed that AMF and PGPR act together to increase plant defenses against biotic stressors in mycorrhiza-induced resistance. Studies on multipartite interactions between Pratylenchus, AMF, PGPR and crop hosts are lacking in the literature.
Species of PGPR in the genera Pseudomonas, Bacillus, Streptomyces and Lysobacter have been implicated in reducing Pratylenchus population densities (Walker et al., 1966; Stirling, 2014; Castillo et al., 2017), and some research has been conducted on the interaction between AMF and these PGPR. In strawberry, Pseudomonas chlororaphis suppressed populations of P. penetrans (Hackenberg et al., 2000) while extracts from the AMF species R. irregularis stimulated the growth of Pseudomonas chlororaphis in vitro (Filion et al., 1999). Streptomyces spp. can reduce Pratylenchus population densities (Meyer and Linderman, 1986; Samac and Kinkel, 2001) and they can also stimulate spore germination in F. mosseae and Gigaspora margarita (Tylka et al., 1991). This indicates a link between the three types of phytobiome organisms, though further research is needed to assess AMF and PGPR combined effects on Pratylenchus population densities.
Limitations of the Review and Future Research
The crops assessed in this review were agriculturally or horticulturally important with the exception of dune grass (Ammophilia arenaria). Most studies looked at a single species of AMF alone and not in combination with species from different orders and genera of AMF, or other beneficial microbes such as PGPR. The taxonomic orders of AMF used in the studies reviewed were limited to the Glomerales and Diversisporales. Other orders such as the Archaeosporales and the Paraglomerales are also present in soils, though they are under-represented in experimental work. A study by Gosling et al. (2014), found a wide distribution of the Paraglomerales in agricultural soils in the UK. AMF species such as F. mosseae and R. irregularis have a tendency to be over represented in this type of experimental work due to their ease of multiplication in trap cultures. The studies in this review were undertaken in low P soils, predominantly in glasshouses, with some transplantations to microplots. Arbuscular mycorrhizal fungi function most efficiently under low to moderately high P conditions, and therefore the benefit of AMF in improving plant nutrition and plant biomass under Pratylenchus pressure could be overstated for agricultural systems receiving continued high rates of P fertilizers. Better matching of P fertilizer inputs to crop removal is required in some agricultural systems to avoid excessive levels of available P in soils for better harnessing of AMF functions, stewardship of global P supplies and environmental quality (Gianinazzi et al., 2010).
The number of studies in this highly specific review of the interaction between Pratylenchus spp. and AMF was limited to only 60 studies suitable for inclusion. Further research needs to be undertaken in the area, using a broad range of crop cultivars and AMF species from diverse orders to further increase our understanding of the relationship between these organisms in the rhizosphere.
Further research needs to be done in assessing the mechanisms involved in the effect of AMF on Pratylenchus population densities through investigations into induced systemic resistance and changes in the metabolome. As research is lacking on the effects of AMF, Pratylenchus and beneficial bacteria in the rhizosphere, more studies need to be undertaken on multipartite interactions between these organisms in crop hosts.
Conclusion
The interactions between Pratylenchus and AMF reveal some unique effects as influenced by crop species, crop cultivar, AMF order and AMF genus. Our review showed increased Pratylenchus densities in plants inoculated with species from the order Diversisporales. Inoculation with the AMF genera Glomus and Funneliformis from the order Glomerales, reduced or had no effect on Pratylenchus densities in host roots. AMF aids the tolerance of plants to Pratylenchus through increased vegetative growth. The biocontrol effect of AMF is likely to be a combination of increasing host tolerance, competition between organisms, and systemic resistance, though further research is needed to identify the mechanisms involved. Further studies will need to take into account the specific interactions between crop, cultivar and AMF species in both glasshouse and field trials.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
JT and EG conceptualized the paper. EG performed the database search, collated the data, and drafted the manuscript. JT and EG conducted the statistical analyses. EG, KO, and RZ integrated information on tables. All authors contributed to revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
EG acknowledges support from the University of Southern Queensland Research Training Program Scholarship and Grains Research and Development Corporation (GRDC) Research Scholarship Project USQ1912-003RSX. RZ acknowledges support from USQ. KO acknowledges co-funding by the GRDC through Project DJP1907-002RMX. KO and JT acknowledge support from the Queensland Department of Agriculture and Fisheries (QDAF) through the Broadacre Cropping Initiative with USQ.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2020.00923/full#supplementary-material
References
Baum, C., El-Tohamy, W., and Gruda, N. (2015). Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: a review. Sci. Hortic. 187, 131–141. doi: 10.1016/j.scienta.2015.03.002
Baylis, G. T. S. (1975). The magnolioid mycorrhiza and mycotrophy in root systems derived from it. in: Endomycorrhizas, eds F.E. Sanders, B. Mosse, and P.B. Tinker (London, UK: Academic Press), 373–389.
Berruti, A., Lumini, E., Balestrini, R., and Bianciotto, V. (2016). Arbuscular mycorrhizal fungi as natural biofertilizers: let's benefit from past successes. Front. Microbiol. 6:1559. doi: 10.3389/fmicb.2015.01559
Berta, G., Trotta, A., Fusconi, A., Hooker, J. E., Munro, M., Atkinson, D., et al. (1995). Arbuscular mycorrhizal induced changes to plant growth and root system morphology in Prunus cerasifera. Tree Physiol. 15, 281–293. doi: 10.1093/treephys/15.5.281
Bødker, L., Kjøller, R., and Rosendahl, S. (1998). Effect of phosphate and the arbuscular mycorrhizal fungus Glomus intraradices on disease severity of root rot of peas (Pisum sativum) caused by Aphanomyces euteiches. Mycorrhiza 8, 169–174. doi: 10.1007/s005720050230
Borowicz, V. A. (2001). Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82, 3057–3068. doi: 10.1890/0012-9658(2001)082[3057:DAMFAP]2.0.CO;2
Brito, O. D. C., Hernandes, I., Ferreira, J. C. A., Cardoso, M. R., Alberton, O., and Dias-Arieira, C. R. (2018). Association between arbuscular mycorrhizal fungi and Pratylenchus brachyurus in maize crop. Chilean J. Agric. Res.78, 521–527. doi: 10.4067/S0718-58392018000400521
Calvet, C., Pinochet, J., Camprub,í, A., and Fernández, C. (1995). Increased tolerance to the root-lesion nematode Pratylenchus vulnus in mycorrhizal micropropagated BA-29 quince rootstock. Mycorrhiza 5, 253–258. doi: 10.1007/BF00204958
Cameron, D. D., Neal, A. L., van Wees, S. C. M., and Ton, J. (2013). Mycorrhiza-induced resistance: more than the sum of its parts? Trends Plant Sci. 18, 539–545. doi: 10.1016/j.tplants.2013.06.004
Camprubi, A., Pinochet, J., Calvet, C., and Estaun, V. (1993). Effects of the root-lesion nematode Pratylenchus vulnus and the vesicular-arbuscular mycorrhizal fungus Glomus mosseae on the growth of three plum rootstocks. Plant Soil 153, 223–229. doi: 10.1007/BF00012995
Castillo, J. D., Vivanco, J. M., and Manter, D. K. (2017). Bacterial microbiome and nematode occurrence in different potato agricultural soils. Microb. Ecol. 74, 888–900. doi: 10.1007/s00248-017-0990-2
Castillo, P., and Vovlas, N. (2007). “Pratylenchus (Nematoda: Pratylenchidae): diagnosis, biology, pathogenicity and management,” in Nematology Monographs and Perspectives, Vol. 6, eds D. J Hunt and R. N Perry (Brill: Leiden), 1–7. doi: 10.1163/ej.9789004155640.i-523
Ceustermans, A., van Hemelrijck, W., Van Campenhout, J., and Bylemans, D. (2018). Effect of arbuscular mycorrhizal fungi on Pratylenchus penetrans infestation in apple seedlings under greenhouse conditions. Pathogens 7:76. doi: 10.3390/pathogens7040076
de La Peña, E., Echeverría, S. R., van der Putten, W. H., Freitas, H., and Moens, M. (2006). Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. N. Phytol. 169, 829–840. doi: 10.1111/j.1469-8137.2005.01602.x
de Luca, F., Reyes, A., Troccoli, A., and Castillo, P. (2011). Molecular variability and phylogenetic relationships among different species and populations of Pratylenchus (Nematoda: Pratylenchidae) as inferred from the analysis of the ITS rDNA. Eur. J. Plant Pathol. 130, 415–426. doi: 10.1007/s10658-011-9763-9
Decraemer, W., and Hunt, D. J. (2013). “Structure and classification,” in: Plant Nematology, 2nd Edn, eds R. N. Perry, and D. J. Hunt (Wallingford: CAB International), 3–39. doi: 10.1079/9781780641515.0003
Elliott, A., Bird, G., and Safir, G. (1984). Joint influence of Pratylenchus penetrans (Nematoda) and Glomus fasciculatum (Phycomyceta) on the ontogeny of Phaseolus vulgaris. Nematropica 14, 111–119.
Elsen, A., Baimey, H., Swennen, R., and de Waele, D. (2003b). Relative mycorrhizal dependency and mycorrhiza-nematode interaction in banana cultivars (Musa spp.) differing in nematode susceptibility. Plant Soil 256, 303–313. doi: 10.1023/A:1026150917522
Elsen, A., Beeterens, R., Swennen, R., and de Waele, D. (2003a). Effects of an arbuscular mycorrhizal fungus and two plant-parasitic nematodes on Musa genotypes differing in root morphology. Biol. Fertil. Soils 38, 367–376. doi: 10.1007/s00374-003-0669-3
Elsen, A., Declerck, S., and Waele, D. (2003c). Use of root organ cultures to investigate the interaction between Glomus intraradices and Pratylenchus coffeae. Appl. Environ. Microbiol. 69, 4308–4311. doi: 10.1128/AEM.69.7.4308-4311.2003
Elsen, A., Gervacio, D., Swennen, R., and de Waele, D. (2008). AMF-induced biocontrol against plant parasitic nematodes in Musa sp.: a systemic effect. Mycorrhiza 18, 251–256. doi: 10.1007/s00572-008-0173-6
Filion, M., St-Arnaud, M., and Fortin, J. (1999). Direct interaction between the arbuscular mycorrhizal fungus Glomus intraradices and different rhizosphere microorganisms. N. Phytol. 141, 525–533. doi: 10.1046/j.1469-8137.1999.00366.x
Forge, T., Muehlchen, A., Hackenberg, C., Neilsen, G., and Vrain, T. (2001). Effects of preplant inoculation of apple (Malus domestica Borkh.) with arbuscular mycorrhizal fungi on population growth of the root-lesion nematode, Pratylenchus penetrans. Plant Soil 236, 185–196. doi: 10.1023/A:1012743028974
Fosu-Nyarko, J., and Jones, M. G. (2016). Advances in understanding the molecular mechanisms of root lesion nematode host interactions. Annu. Rev. Phytopathol. 54, 253–278. doi: 10.1146/annurev-phyto-080615-100257
Frew, A., Powell, J. R., Glauser, G., Bennett, A. E., and Johnson, S. N. (2018). Mycorrhizal fungi enhance nutrient uptake but disarm defences in plant roots, promoting plant-parasitic nematode populations. Soil Biol. Biochem. 126, 123–132. doi: 10.1016/j.soilbio.2018.08.019
Gianinazzi, S., Gollote, A., Binet, M. N., van Tuinen, D., Redecker, D., and Wipf, D. (2010). Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20, 519–530. doi: 10.1007/s00572-010-0333-3
Glick, B. R. (2012). Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:963401. doi: 10.6064/2012/963401
Gosling, P., Proctor, M., Jones, J., and Bending, G. D. (2014). Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 24, 1–11. doi: 10.1007/s00572-013-0505-z
Guillemin, J.-P., Gianinazzi, S., Gianinazzi-Pearson, V., and Marchal, J. (1994). Control by arbuscular endomycorrhizae of Pratylenchus brachyurus in pineapple microplants. Agric. Food Sci. 3, 253–262. doi: 10.23986/afsci.72703
Hackenberg, C., Muehlkchen, A., Forge, T., and Vrain, T. (2000). Pseudomonas chlororaphis strain Sm3, bacterial antagonist of Pratylenchus penetrans. J. Nematol. 32, 183–189.
Hart, M. M., and Reader, R. J. (2002). Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. N. Phytol. 153, 335–344. doi: 10.1046/j.0028-646X.2001.00312.x
Hetrick, B. A. D., Wilson, G. W. T., and Cox, T. S. (1993). Mycorrhizal dependence of modern wheat cultivars and ancestors: a synthesis. Can. J. Bot. 71, 512–518. doi: 10.1139/b93-056
Hetrick, B. A. D., Wilson, G. W. T., and Leslie, J. F. (1991). Root architecture of warm-and cool-season grasses: relationship to mycorrhizal dependence. Can. J. Bot. 69, 112–118. doi: 10.1139/b91-016
Hill, E. M., Robinson, L. A., Abdul-Sada, A., Vanbergen, A. J., Hodge, A., and Hartley, S. E. (2018). Arbuscular mycorrhizal fungi and plant chemical defence: effects of colonisation on aboveground and belowground metabolomes. J. Chem. Ecol. 44, 198–208. doi: 10.1007/s10886-017-0921-1
Hoeksema, J. D., Chaudhary, V. B., Gehring, C. A., Johnson, N. C., Karst, J., Koide, R. T., et al. (2010). A meta-analysis of context-dependency in plant response to inoculation with mycorrhizal fungi. Ecol. Lett. 13, 394–407. doi: 10.1111/j.1461-0248.2009.01430.x
Hol, W. H. G., and Cook, R. (2005). An overview of arbuscular mycorrhizal fungi–nematode interactions. Basic Appl. Ecol. 6, 489–503. doi: 10.1016/j.baae.2005.04.001
Hooker, J., Munro, M., and Atkinson, D. (1992). Vesicular-arbuscular mycorrhizal fungi induced alteration in poplar root system morphology. Plant Soil 145, 207–214. doi: 10.1007/BF00010349
Hussey, R., and Roncadori, R. (1978). Interaction of Pratylenchus brachyurus and Gigaspora margarita on cotton. J. Nematol. 10, 16–20.
Jaizme-Vega, M., and Pinochet, J. (1997). Growth response of banana to three mycorrhizal fungi in Pratylenchus goodeyi infested soil. Nematropica 27, 69–76. doi: 10.1023/A:1004236310644
Javaid, A. (2017). “Role of AMF in nitrogen fixation in legumes,” in Microbes for Legume Improvement, eds A. Zaidi, M. S. Khan, and J. Musarrat (Cham: Springer International Publishing), 409–426.
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., Jones, M. G. K., et al. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 14, 946–961. doi: 10.1111/mpp.12057
Jones, M. G. K., and Fosu-Nyarko, J. (2014). Molecular biology of root lesion nematodes (Pratylenchus spp.) and their interaction with host plants: molecular biology of root lesion nematodes. Ann. Appl. Biol. 164, 163–181. doi: 10.1111/aab.12105
Jung, S. C., Martinez-Medina, A., Lopez-Raez, J. A., and Pozo, M. J. (2012). Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 38, 651–664. doi: 10.1007/s10886-012-0134-6
Kaeppler, S. M., Parke, J. L., Mueller, S. M., Senior, L., Stuber, C., and Tracy, W. F. (2000). Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 40, 358–364. doi: 10.2135/cropsci2000.402358x
Khaosaad, T., Garcia-Garrido, J., Steinkellner, S., and Vierheilig, H. (2007). Take-all disease is systemically reduced in roots of mycorrhizal barley plants. Soil Biol. Biochem. 39, 727–734. doi: 10.1016/j.soilbio.2006.09.014
Klironomos, J. N. (2000). “Host-specificity and functional diversity among arbuscular mycorrhizal fungi,” in Microbial Biosystems: New Frontiers. Proceedings of the Eighth International Symposium on Microbial Ecology, eds C. R. Bell, M. Brylinsky, P. Johnson-Green (Halifax: Atlantic Canada Society for Microbial Ecology), 845–851.
Koch, A. M., Antunes, P. M., Maherali, H., Hart, M. M., and Klironomos, J. N. (2017). Evolutionary asymmetry in the arbuscular mycorrhizal symbiosis: conservatism in fungal morphology does not predict host plant growth. N. Phytol. 214, 1330–1337. doi: 10.1111/nph.14465
Koch, A. M., Croll, D., and Sanders, I. R. (2006). Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecol. Lett. 9, 103–110. doi: 10.1111/j.1461-0248.2005.00853.x
Lambers, H., and Teste, F. P. (2013). Interactions between arbuscular mycorrhizal and non-mycorrhizal plants: do non-mycorrhizal species at both extremes of nutrient availability play the same game? Plant Cell Environ. 36, 1911–1915. doi: 10.1111/pce.12117
Lehnert, H., Serfling, A., Enders, M., Friedt, W., and Ordon, F. (2017). Genetics of mycorrhizal symbiosis in winter wheat (Triticum aestivum). N. Phytol. 215, 779–791. doi: 10.1111/nph.14595
Leifheit, E. F., Veresoglou, S. D., Lehmann, A., Morris, E. K., and Rillig, M. C. (2014). Multiple factors influence the role of arbuscular mycorrhizal fungi in soil aggregation—a meta-analysis. Plant Soil 374, 523–537. doi: 10.1007/s11104-013-1899-2
Li, J. F., He, X. H., Li, H., Zheng, W. J., Liu, J. F., and Wang, M. Y. (2015). Arbuscular mycorrhizal fungi increase growth and phenolics synthesis in Poncirus trifoliata under iron deficiency. Sci. Hortic. 183, 87–92. doi: 10.1016/j.scienta.2014.12.015
Linderman, R. (1988). Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizosphere effect. Phytopathology 78, 366–371.
Lopez, A., Pinochet, J., Fernandez, C., Calvet, C., and Camprubi, A. (1997). Growth response of OHF-333 pear rootstock to arbuscular mycorrhizal fungi, phosphorus nutrition and Pratylenchus vulnus infection. Fundam. Appl. Nematol. 20, 87–93.
López-Ráez, J. A., Flors, V., García, J. M., and Pozo, M. J. (2010). AM symbiosis alters phenolic acid content in tomato roots. Plant Signal. Behav. 5, 1138–1140. doi: 10.4161/psb.5.9.12659
Maherali, H. (2014). Is there an association between root architecture and mycorrhizal growth response? New Phytol. 204, 192–200. doi: 10.1111/nph.12927
Maherali, H., and Klironomos, J. N. (2007). Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316, 1746–1748. doi: 10.1126/science.1143082
Marschner, P., and Baumann, K. (2003). Changes in bacterial community structure induced by mycorrhizal colonisation in split-root maize. Plant Soil 251, 279–289. doi: 10.1023/A:1023034825871
Martín-Robles, N., Lehmann, A., Seco, E., Aroca, R., Rillig, M. C., and Milla, R. (2018). Impacts of domestication on the arbuscular mycorrhizal symbiosis of 27 crop species. N. Phytol. 218, 322–334. doi: 10.1111/nph.14962
Meyer, J. R., and Linderman, R. (1986). Selective influence on populations of rhizosphere or rhizoplane bacteria and actinomycetes by mycorrhizas formed by Glomus fasciculatum. Soil Biol. Biochem. 18, 191–196. doi: 10.1016/0038-0717(86)90026-X
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Morton, J. B., and Benny, G. L. (1990). Revised classification of arbuscular mycorrhizal fungi (Zygomycetes): a new order Glomales, two new suborders, Glomineae and Gigasporineae and two new families, Acaulosporaceae and Gigasporaceae, with an emendation to Glomaceae. Mycotaxon 37, 471–491.
Newsham, K. K., Fitter, A. H., and Watkinson, A. R. (1995). Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends Ecol. Evol. 10, 407–411. doi: 10.1016/S0169-5347(00)89157-0
Owen, K. J., Clewett, T. G., and Thompson, J. P. (2010). Pre-cropping with canola decreased Pratylenchus thornei populations, arbuscular mycorrhizal fungi, and yield of wheat. Crop Pasture Sci. 61, 399–410. doi: 10.1071/CP09345
Parniske, M. (2008). Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6, 763–775. doi: 10.1038/nrmicro1987
Pieterse, C. M., van Wees, S. C., van Pelt, J. A., Knoester, M., Laan, R., Gerrits, H., et al. (1998). A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10, 1571–1580. doi: 10.1105/tpc.10.9.1571
Pinochet, J., Calvet, C., Camprubi, A., and Fernandez, C. (1995a). Growth and nutritional response of Nemared peach rootstock infected with Pratylenchus vulnus and the mycorrhizal fungus Glomus mosseae. Fundam. Appl. Nematol. 18, 205–210.
Pinochet, J., Calvet, C., Camprubi, A., and Fernández, C. (1995b). Interaction between the root-lesion nematode Pratylenchus vulnus and the mycorrhizal association of Glomus intraradices and Santa Lucia 64 cherry rootstock. Plant Soil 170, 323–329. doi: 10.1007/BF00010485
Pinochet, J., Calvet, C., Camprubí, A., and Fernández, C. (1996). Interactions between migratory endoparasitic nematodes and arbuscular mycorrhizal fungi in perennial crops: a review. Plant Soil 185, 183–190. doi: 10.1007/BF02257523
Pinochet, J., Camprubi, A., and Calvet, C. (1993). Effects of the root-lesion nematode Pratylenchus vulnus and the mycorrhizal fungus Glomus mosseae on the growth of EMLA-26 apple rootstock. Mycorrhiza 4, 79–83. doi: 10.1007/BF00204062
Pinochet, J., Camprubi, A., Calvet, C., Fernandez, C., and Kabana, R. R. (1998). Inducing tolerance to the root-lesion nematode Pratylenchus vulnus by early mycorrhizal inoculation of micropropagated Myrobalan 29 C plum rootstock. J. Am. Soc. Horti. Sci. 123:342. doi: 10.21273/JASHS.123.3.342
Powell, J. R., and Rillig, M. C. (2018). Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. N. Phytol. 220, 1059–1075. doi: 10.1111/nph.15119
Pozo, M. J., and Azcón-Aguilar, C. (2007). Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 10, 393–398. doi: 10.1016/j.pbi.2007.05.004
Pozo, M. J., Cordier, C., Dumas-Gaudot, E., Gianinazzi, S., Barea, J. M., and Azcón-Aguilar, C. (2002). Localized versus systemic effect of arbuscular mycorrhizal fungi on defence responses to Phytophthora infection in tomato plants. J. Exp. Bot. 53, 525–534. doi: 10.1093/jexbot/53.368.525
Rahaman, M. M., Zwart, R. S., and Thompson, J. P. (2020). Constitutive and induced expression of total phenol and phenol oxidases in wheat genotypes ranging in resistance/susceptibility to the root-lesion nematode Pratylenchus thornei. Plants 9:485. doi: 10.3390/plants9040485
Redecker, D., Schüßler, A., Stockinger, H., Stürmer, S. L., Morton, J. B., and Walker, C. (2013). An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23, 515–531. doi: 10.1007/s00572-013-0486-y
Rivero, J., Gamir, J., Aroca, R., Pozo, M. J., and Flors, V. (2015). Metabolic transition in mycorrhizal tomato roots. Front. Microbiol. 6:598. doi: 10.3389/fmicb.2015.00598
Rodríguez-Echeverría, S., de La Peña, E., Moens, M., Freitas, H., and Van Der Putten, W. H. (2009). Can root-feeders alter the composition of AMF communities? Experimental evidence from the dune grass Ammophila arenaria. Basic Appl. Ecol. 10, 131–140. doi: 10.1016/j.baae.2008.01.004
Samac, D. A., and Kinkel, L. L. (2001). Suppression of the root-lesion nematode (Pratylenchus penetrans) in alfalfa (Medicago sativa) by Streptomyces spp. Plant Soil 235, 35–44. doi: 10.1023/A:1011820002779
Schouteden, N., de Waele, D., Panis, B., and Vos, C. M. (2015). Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: a review of the mechanisms involved. Front. Microbiol. 6:1280. doi: 10.3389/fmicb.2015.01280
Schüßler, A., and Walker, C. (2010). The Glomeromycota: A Species List With New Families and New Genera. The Royal Botanic Garden Kew, Botanische Staatssammlung Munich, and Oregon State University.
Seo, D. J., Nguyen, V. N., Kim, K. Y., Park, R. D., and Jung, W. J. (2013). Nematicidal activity of gallic acid purified from Terminalia nigrovenulosa bark against the root-knot nematode Meloidogyne incognita. Nematology 15, 507–518. doi: 10.1163/15685411-00002696
Seymour, N. P., Edwards, D. G., and Thompson, J. P. (2019). A dual rescaled Mitscherlich model of the simultaneous savings in phosphorus and zinc fertiliser from arbuscular mycorrhizal fungal colonisation of linseed (Linum usitatissimum L). Plant Soil 440, 97–118. doi: 10.1007/s11104-019-04065-2
Sidker, M. M., and Vestergård, M. (2019). Impacts of root metabolites on soil nematodes. Front. Plant Sci. 10:1792. doi: 10.3389/fpls.2019.01792
Sikes, B. A. (2010). When do arbuscular mycorrhizal fungi protect plant roots from pathogens? Plant Signal. Behav. 5, 763–765. doi: 10.4161/psb.5.6.11776
Singh, S. K., Hodda, M., Ash, G. J., and Banks, N. C. (2013). Plant-parasitic nematodes as invasive species: characteristics, uncertainty and biosecurity implications. Ann. Appl. Biol. 163, 323–350. doi: 10.1111/aab.12065
Smith, F. A., Grace, E. J., and Smith, S. E. (2009). More than a carbon economy: nutrient trade and ecological sustainability in facultative arbuscular mycorrhizal symbioses. N. Phytol. 182, 347–358. doi: 10.1111/j.1469-8137.2008.02753.x
Smith, S. E., Jakobsen, I., Grønlund, M., and Smith, A. (2011). Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiol. 156, 1050–1057. doi: 10.1104/pp.111.174581
Smith, S. E., Smith, F. A., and Jakobsen, I. (2004). Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 162, 511–524. doi: 10.1111/j.1469-8137.2004.01039.x
Soriano, I., Asenstorfer, R., Schmidt, O., and Riley, I. (2004). Inducible flavone in oats (Avena sativa) is a novel defense against plant-parasitic nematodes. Phytopathology 94, 1207–1214. doi: 10.1094/PHYTO.2004.94.11.1207
Steel, G. D., and Torrie, J. H. (1960). Principles and Procedures of Statistics. New York, NY: McGraw Hill 19, 366–387.
Stirling, G. R. (2014). Biological Control of Plant-Parasitic Nematodes: Soil Ecosystem Management in Sustainable Agriculture. Wallingford: CABI.
Tadych, M., and Blaszkowski, J. (1999). Growth responses of maritime sand dune plant species to arbuscular mycorrhizal fungi. Acta Mycol. 34, 115–124. doi: 10.5586/am.1999.010
Talavera, M., Itou, K., and Mizukubo, T. (2001). Reduction of nematode damage by root colonization with arbuscular mycorrhiza (Glomus spp.) in tomato-Meloidogyne incognita (Tylenchida: Meloidogynidae) and carrot-Pratylenchus penetrans (Tylenchida: Pratylenchidae) pathosystems. Appl. Entomol. Zool. 36, 387–392. doi: 10.1303/aez.2001.387
Thompson, J. P. (1993). “What is the potential for management of mycorrhizas in agriculture?,” in: Management of Mycorrhizas in Agriculture, Horticulture and Forestry, Proceedings of an International Symposium on Management of Mycorrhizas in Agriculture, Horticulture and Forestry (Perth, WA, Australia), eds A. D. Robson, L. K. Abbott, and N. Malajczuk (Dordecht: Kluwer Academic Publishers), 191–200.
Tylka, G., Hussey, R., and Roncadori, R. (1991). Axenic germination of vesicular-arbuscular mycorrhizal fungi: effects of selected Streptomyces species. Phytopathology 81, 754–759. doi: 10.1094/Phyto-81-754
Vaast, P., Caswell-Chen, E. P., and Zasoski, R. J. (1997). Influences of a root-lesion nematode, Pratylenchus coffeae, and two arbuscular mycorrhizal fungi, Acaulospora mellea and Glomus clarum on coffee (Coffea arabica L.). Biol. Fertil. Soils 26, 130–135. doi: 10.1007/s003740050355
Veresoglou, S. D., and Rillig, M. C. (2012). Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol. Lett. 8, 214–217. doi: 10.1098/rsbl.2011.0874
Vos, C. M., Tesfahun, A. N., Panis, B., de Waele, D., and Elsen, A. (2012). Arbuscular mycorrhizal fungi induce systemic resistance in tomato against the sedentary nematode Meloidogyne incognita and the migratory nematode Pratylenchus penetrans. Appl. Soil Ecol. 61, 1–6. doi: 10.1016/j.apsoil.2012.04.007
Walker, J., Specht, C., and Bekker, J. (1966). Nematocidal activity to Pratylenchus penetrans by culture fluids from actinomycetes and bacteria. Can. J. Microbiol. 12, 347–351. doi: 10.1139/m66-047
Whipps, J. M. (2004). Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 82, 1198–1227. doi: 10.1139/b04-082
Wuyts, N., Swennen, R., and de Waele, D. (2006). Effects of plant phenylpropanoid pathway products and selected terpenoids and alkaloids on the behaviour of the plant-parasitic nematodes Radopholus similis, Pratylenchus penetrans and Meloidogyne incognita. Nematology 8, 89–101. doi: 10.1163/156854106776179953
Yang, H., Dai, Y., Wang, X., Zhang, Q., Zhu, L., and Bian, X. (2014). Meta-analysis of interactions between arbuscular mycorrhizal fungi and biotic stressors of plants. Sci. World J. 2014:746506. doi: 10.1155/2014/746506
Yang, H., Xu, J., Guo, Y., Koide, R. T., Dai, Y., Xu, M., et al. (2016). Predicting plant response to arbuscular mycorrhizas: the role of host functional traits. Fungal Ecol. 20, 79–83. doi: 10.1016/j.funeco.2015.12.001
Yang, H., Zhang, Q., Dai, Y., Liu, Q., Tang, J., Bian, X., et al. (2015). Effects of arbuscular mycorrhizal fungi on plant growth depend on root system: a meta-analysis. Plant Soil 389, 361–374. doi: 10.1007/s11104-014-2370-8
Keywords: arbuscular mycorrhizal fungi, Pratylenchus, root-lesion nematodes, phytobiome interactions, Glomeromycota, systematic review
Citation: Gough EC, Owen KJ, Zwart RS and Thompson JP (2020) A Systematic Review of the Effects of Arbuscular Mycorrhizal Fungi on Root-Lesion Nematodes, Pratylenchus spp. Front. Plant Sci. 11:923. doi: 10.3389/fpls.2020.00923
Received: 27 February 2020; Accepted: 05 June 2020;
Published: 14 July 2020.
Edited by:
Johannes Hallmann, Julius Kühn-Institut - Braunschweig, GermanyReviewed by:
Martina Janouskova, Institute of Botany (ASCR), CzechiaRaffaella Balestrini, Italian National Research Council, Italy
Copyright © 2020 Gough, Owen, Zwart and Thompson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elaine C. Gough, RWxhaW5lLkdvdWdoQHVzcS5lZHUuYXU=
 Elaine C. Gough
Elaine C. Gough Kirsty J. Owen
Kirsty J. Owen Rebecca S. Zwart
Rebecca S. Zwart John P. Thompson
John P. Thompson