- 1Bionet Group, Department of Electrical Engineering, Columbia University, New York, NY, United States
- 2Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, United States
- 3Department of Computer and Information Science, Fordham University, New York, NY, United States
Associative memory in the Mushroom Body of the fruit fly brain depends on the encoding and processing of odorants in the first three stages of the Early Olfactory System: the Antenna, the Antennal Lobe and the Mushroom Body Calyx. The Kenyon Cells (KCs) of the Calyx provide the Mushroom Body compartments the identity of pure and odorant mixtures encoded as a train of spikes. Characterizing the code underlying the KC spike trains is a major challenge in neuroscience. To address this challenge we start by explicitly modeling the space of odorants using constructs of both semantic and syntactic information. Odorant semantics concerns the identity of odorants while odorant syntactics pertains to their concentration amplitude. These odorant attributes are multiplicatively coupled in the process of olfactory transduction. A key question that early olfactory systems must address is how to disentangle the odorant semantic information from the odorant syntactic information. To address the untanglement we devised an Odorant Encoding Machine (OEM) modeling the first three stages of early olfactory processing in the fruit fly brain. Each processing stage is modeled by Divisive Normalization Processors (DNPs). DNPs are spatio-temporal models of canonical computation of brain circuits. The end-to-end OEM is constructed as cascaded DNPs. By extensively modeling and characterizing the processing of pure and odorant mixtures in the Calyx, we seek to answer the question of its functional significance. We demonstrate that the DNP circuits in the OEM combinedly reduce the variability of the Calyx response to odorant concentration, thereby separating odorant semantic information from syntactic information. We then advance a code, called first spike sequence code, that the KCs make available at the output of the Calyx. We show that the semantics of odorants can be represented by this code in the spike domain and is ready for easy memory access in the Mushroom Body compartments.
1 Introduction
Odor signal processing in the olfactory system of diverse organisms is the result of millennia of convergent evolution (Ache and Young, 2005). Unlike other sensory systems such as the visual system, the odor processing pathways are not embedded within topographically organized circuits (such as retinotopy in visual systems (Sanes and Zipursky, 2010)). Instead, olfactory circuits are organized non-topographically (Mombaerts, 1999; Buck, 2005; Shepherd, 2004; Cleland and Sethupathy, 2006), and their affinities to given odorant molecules directly encode the identities of the said stimuli (Buck and Axel, 1991; Firestein, 2001). This unique sensory characterization of the olfactory stimulus space also led to a highly efficient odor signal processing neural circuit.
In Drosophila Melanogaster, more than 40% of the total neural real estate is dedicated to processing visual signals (Barish and Volkan, 2015), while about 5% is dedicated to processing olfactory inputs (Scheffer et al., 2020; Masse et al., 2009; Zheng et al., 2017). Neverthless, Drosophila have remarkable olfactory-based foraging, mating, and predator avoidance (Vosshall and Stocker, 2007) capabilities. Given the rich olfactory-related behavior repertoire of Drosophila (Benton, 2022), its well-mapped olfactory neural circuit and powerful genetic tools, its olfactory system serves as the ideal platform for unraveling the mysteries of olfactory processing.
In the fruit fly, natural odorant scenes (see Figure 1 first column) are first sensed in the Antenna and Maxillary Palps by the dendrites of thousands of Olfactory Sensory Neurons (OSNs), each expressing a single olfactory receptor (OR) type (Vosshall, 2000) (see Figure 1 second column). The second layer of olfactory sensory processing is the Antennal Lobe (AL, see Figure 1 third column). OSNs expressing the same OR type typically project their axons into a single glomerulus, a dense connectivity region in the AL. The dendritic trees of Projection Neurons (PNs) typically also innervate a single glomerulus. A large number of Local Neurons (LNs) shape the I/O of the AL circuit (Lazar et al., 2022). PNs project their axons to the Mushroom Body Calyx and/or the Lateral Horn (see Figure 1 third column top and fourth column). In the Calyx, some 50 types of PNs synapse onto 2,000 Kenyon Cells (KCs), a rapid expansion of the number of neurons (Modi et al., 2020). A key circuit element in the Calyx is the giant Anterior Paired Lateral (APL) feedback neuron receiving input from all KCs. The third and fourth columns in Figure 1 can also be viewed online as interactive 3D visualizations provided by the Fruit Fly Brain Observatory (Ukani et al., 2019; 2024). The URLs can be found in NeuroNLP (2024a) and NeuroNLP (2024b).
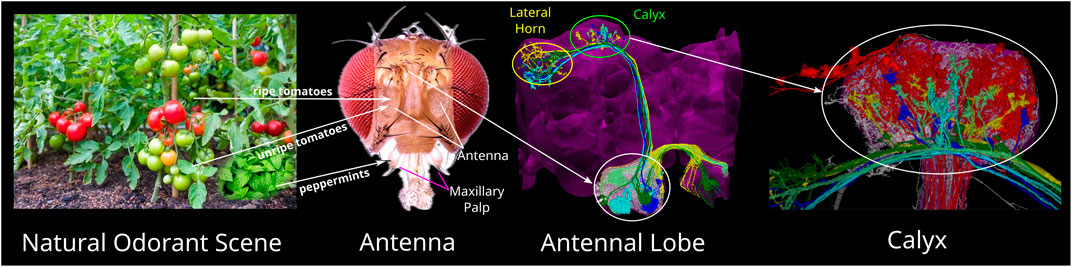
Figure 1. Odorant mixture processing pathways of the Early Olfactory System of the fruit fly. A natural odorant scene (left) may consist of many odorant mixtures, including ripe and unripe tomatoes, and peppermints that repel flies. Odorant mixtures are sensed by the Olfaction Sensory Neurons (OSNs) whose dendrites are located on the Antenna and Maxillary Pulps (second to left, adapted from Fabian and Sachse (2023), under Creative Commons Attribution License (CC-BY)). OSNs project their axons into the Antennal Lobe (AL) (white ellipse in third to left). AL is innervated by a large number of Local Neurons (LNs, white transparent). Projection Neurons (PNs) (colored neurons), the outputs of the AL, send their axons to the Calyx (green ellipse in third to left) and the Lateral Horn (yellow ellipse in third to left). In Calyx (right most), PNs (colors other than red and white) provide inputs into Kenyon Cells (KCs) (red). The Calyx is also innervated by the APL neuron (white transparent) that interacts with the KCs. For interactive 3D visualization of the connectome of the AL and Calyx, see NeuroNLP (2024a) and NeuroNLP (2024b).
There has been an extensive amount of work in discerning the odorant identity and concentration in the olfactory system of the fruit flies, other insects and vertebrates. It has been shown that odorants typically retain their perceptual identities over a range of concentrations (Blazing and Franks, 2020). In Drosophila, the same odorant may recall the memory associated with the odorant over more than an order of magnitude of concentration amplitude values (Masek and Heisenberg, 2008).
Concentration-invariant representation of odorant identity has been proposed at almost every stage of the olfactory circuit, in the Antenna (Egea-Weiss et al., 2018), in the Antennal Lobe (or Olfactory Bulb in vertebrates) (Stopfer et al., 2003; Wilson et al., 2017; Chong et al., 2020; Lazar et al., 2023) and at the KC level of the Mushroom Body (or Piriform Cortex in mammals) (Stopfer et al., 2003; Bolding and Franks, 2018). These studies assumed, however, that the odorant identity is known. In other words, odorant identity has been viewed akin to labels used in supervised learning. A major goal has been to record from the neural activity arising at different stages of the Early Olfactory System and to examine when the recorded signal can be used to increase the accuracy of identifying or classifying odorants (Egea-Weiss et al., 2018; Jeanne and Wilson, 2015; Stopfer et al., 2003). These approaches do not reveal, however, the functional logic of the underlying neural circuits.
In previous work, we proposed computational models for mono-molecular odorant encoding and processing in both the Antenna (Lazar and Yeh, 2020) and the Antennal Lobe (Lazar et al., 2023). We advanced a model of olfactory objects of the odorant space that explicitly describes both their identity (odorant semantics) and their concentration amplitude (odorant syntax). Our model of the Antenna then encodes a multiplicatively-coupled representation of the semantic and syntactic information streams, resulting in a confounding representation that is disentangled by the inhibitory and excitatory Local Neurons of the Antennal Lobe. Both models of the Antenna and the Antennal Lobe reproduce with a very high precision the experimentally obtained physiological responses of the Olfactory Sensory Neurons (output neurons of the Antenna) and Projection Neurons (output neurons of the Antennal Lobe) (Kim et al., 2011; 2015). Importantly, by developing a model of the Antennal Lobe that recovers the odorant identity information from the confounding representation of the Antenna, we showed that the functional significance of the Antennal Lobe (in particular its highly diverse inhibitory Local Neurons) is to separate the odorant semantics from syntax, thereby undoing the multiplicatively coupled odorant encoding in the Antenna (see also Figure 2).
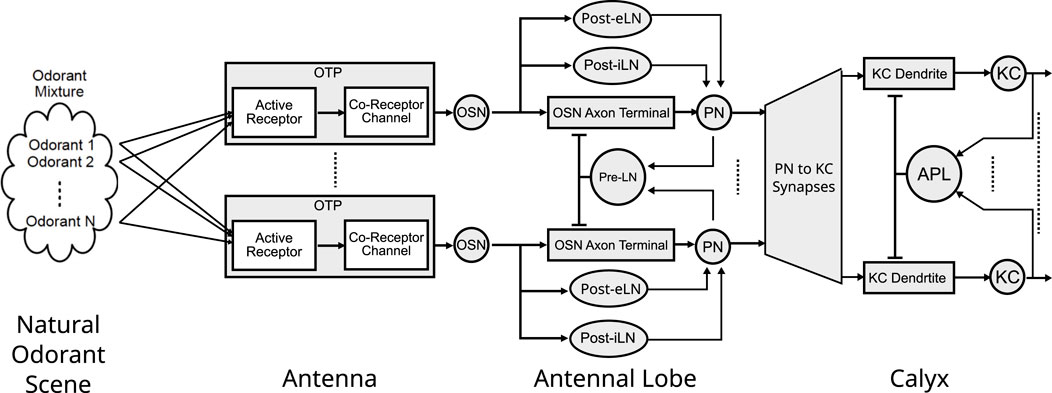
Figure 2. The architecture of the Odorant Encoding Machine (OEM) modeling the early olfactory system of the fruit fly. The OEM consists of a cascade of spatio-temporal Divisive Normalization Processors (DNPs) each modeling the Antenna, Antennal Lobe and Calyx. All odorants in a mixture are sensed and encoded by a molecular Odorant Transduction Process (OTP) of each OSN type. Each OSN type then provides input to an AL channel (glomerulus) with a Projection Neuron (PN) as channel output. Three types of local neurons, Presynaptic Local Neurons (Pre-LNs), Postsynaptic excitatory LNs (Post-eLNs) and Postsynaptic inhibitory LNs (Post-iLNs) are modeled as 3 types of differential DNPs. The Calyx features an expansion of the PN to KC connectivity, as well as a DNPs circuit consisting of the KC dendrites, KC biological spike generators and the APL spatio-temporal feedback neuron.
To emphasize, the novelty of our approach rests on explicitly modeling the space of odorants using constructs of both semantic and syntactic information, a subtle but profound distinction from the existing literature that solely invokes methods of traditional syntactic information processing. These prior works focused on methods of processing odorant concentration, with odorant identity mentioned in passing and/or lacking computational or theoretical rigor. However, when it comes to understanding the functional logic of olfactory circuits, processing odorant concentration alone turns out to be, as we argue here and elsewhere, a major limitation. Our present work extends the I/O modeling and characterization of semantic/syntactic information processing that we obtained for the Antenna and Antennal Lobe to the MB Calyx circuit. We show how the Calyx extracts and represents semantic information in the spike domain.
The first three layers of the Early Olfactory System depicted in Figure 1 are modeled as the Odorant Encoding Machine (OEM) shown in Figure 2 (Lazar et al., 2020a). The architecture of the OEM consists of three cascaded Divisive Normalization Processors (DNPs), a spatio-temporal extension of the static divisive normalization model previously analyzed by (Olsen and Wilson, 2008; Carandini and Heeger, 2012; Lazar et al., 2020b; Lazar and Zhou, 2023). Note that in vision, it has been recently shown that the motion detection pathway of the early visual system of the fruit fly can also be modeled as a cascade of DNPs (Lazar et al., 2020b; Lazar and Zhou, 2023), suggesting that DNPs as building blocks of computation can be combined to realize more complex processing in the fruit fly brain.
In the current work, we seek to answer the question regarding the functional significance of the Mushroom Body Calyx, the last building block of the OEM cascade shown in Figure 2. Note that in the MB Calyx most of biological real estate is devoted for re-representing odorant identities - with, on average, 40 Kenyon Cells in the Calyx for each 1 Projection Neuron type in the Antennal Lobe. While previous studies have explored the Calyx’s role in associative learning (e.g., Heisenberg, 2003), our focus shall be on modeling the pre-associative representation of odorant identity and exploring how semantics of pure and odorant mixtures are coded for memory access by the Mushroom Body. By (i) abstracting the structural connectome datasets into executable circuit diagrams, and by (ii) focusing on the exploration of the functional logic of the underlying circuits, we follow here the workflow established in Lazar et al. (2021).
This paper is organized as follows. In Section 2, we present the architecture of the OEM. We review the model of the space of odorants and the input/output (I/O) model of the Antenna and Antennal Lobe. The model of the Calyx is detailed next. In Section 3, we extensively characterize and evaluate the processing of pure and odorant mixtures in the Calyx. The KC generated spike train at the output of the Calyx, called the first spike sequence code, represents the odorant identity made available to the Mushroom Body memory circuit. In Section 4 we conclude with a brief discussion.
2 Odorant Encoding Machine
A schematic diagram of the Odorant Encoding Machine (OEM) is shown in Figure 2. In what follows we describe the four cascaded building blocks of the OEM, respectively, modeling the space of odorants (see section 2.1), the molecular encoding of mono-molecular odorants and odorant mixtures in the antenna (see section 2.2), the I/O modeling of the antennal lobe (see section 2.3) and the I/O modeling of mushroom body calyx (see section 2.4).
2.1 Modeling the space of odorants
The space of mono-molecular odorants (see also Figure 2, left) was first formally modeled and biologically validated in Lazar and Yeh (2020). In this model, the Odorant Transduction Process (OTP) taking place in the cilia of the Olfactory Sensory Neurons (OSNs) (see also Section 2.2) encodes odorants as objects defined by the tensor of binding rates, dissociation rates and concentration amplitude
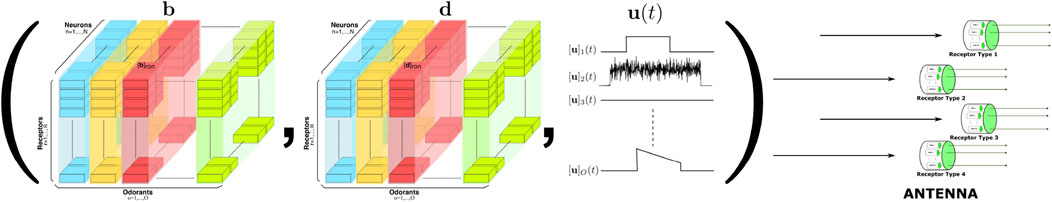
Figure 3. Modeling the space of mono-molecular odorants. Elements of the odorant space are defined by the tensor of odorant-receptor binding rate, dissociation rate and concentration amplitude
With this odorant object model, the semantics of the space of mono-molecular odorants (Lazar et al., 2023) is defined by the 2-tuple of binding/dissociation rate tensors
2.2 Modeling odorant encoding in the antenna
In order to study pure and odorant mixture processing in the Mushroom Body, we first extended our model of the Antenna to account for competitive binding of a mixture of odorant molecules (Nagel and Wilson, 2011; Olsen et al., 2010).
Odorant molecules are first sensed in either the second-segment of the Antenna or Maxillary-Palp (see Figure 1 2nd column) that are both covered with sensory hairs, called sensilla. Cilia (dendrites) of a few OSNs are housed in each sensillum. Odorants that enter sensilla through the pores on its surface are subsequently transported to the Odorant Receptors (ORs) located on the OSN sensory cilia (Larter et al., 2016). Odorant molecules then bind to the ORs and induce the OSN to generate action potentials. This process is modeled here as the Olfactory Transduction Process (OTP) (see also Figure 4).
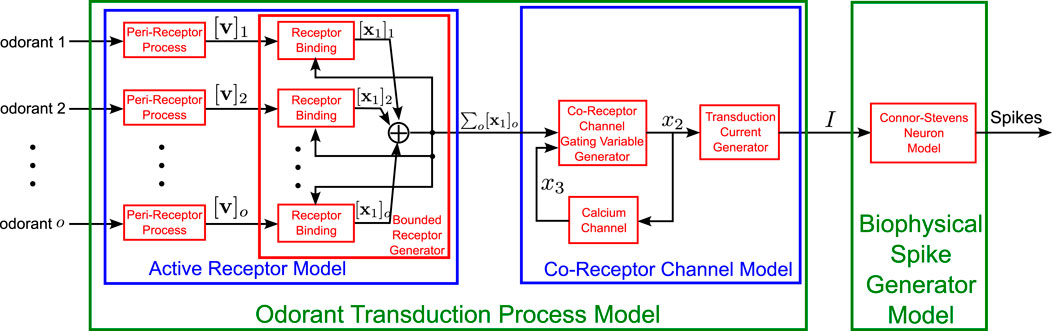
Figure 4. Schematic diagram of the Olfactory Transduction Process for a set
We start by briefly reviewing the OTP for a single mono-molecular odorant. The output of the peri-receptor process, that models the overall effect of odorant molecules entering the sensilla before binding to ORs (see also Figure 4), is given by Equation 1 (Lazar and Yeh, 2020):
where
The bound-receptor model describes the dynamics of binding of odorant molecules to the ORs (see also Figure 4) and is given by
where
where
To study odorant mixture representation and processing, we now extended the OTP model to odorant mixtures. In the odorant mixture model, we denote the set of mixture components as
It is easy to see that by summing up the Equation 4 over all the odorants present in the mixture, the ratio of the total bound receptors in steady-state amounts to
and the steady-state solution to the set of Equations 4 is
If we consider the odorant mixture at a particular component ratio as a new “pure” odorant, then we can define, up to a scaling factor, its effective affinity as
The co-receptor channel that models the dynamics of the activation of ligand-gated channels in the mixture model (see also Figure 4) can then be compactly described by
where
Taken together, the OTP process of an odorant mixture
In the last Equation 9,
Revisiting Equation 9, we note that, similar to the mono-molecular odorant, the encoding of odorant mixtures exhibits multiplicative coupling in a confounding representation of odorant identities and concentration waveforms.
Finally, we note that the spike train generated by the Biophysical Spike Generator (BSG, see also Figure 4) of the OSN expressing receptor
where
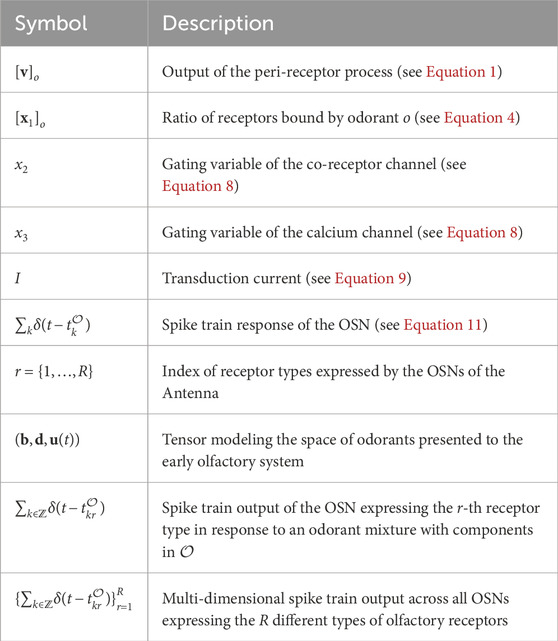
In conclusion, the notation of the key parameters and input/output variables of the Antenna circuit (see Figures 3, 4) are shown in detail in Table 1.
2.3 I/O modeling of the antennal lobe
The Antennal Lobe (AL) can be anatomically divided into some 52 regions called glomeruli, where all the OSNs expressing the same olfactory receptor project their axons into (Buck and Axel, 1991; Firestein, 2001). The dendrites of a uniglomerular projection neurons (uPNs) exclusively innervate a single glomerulus (Scheffer et al., 2020) (see also Figure 1 third column, each color marks the PNs innervating a single glomerulus). Therefore each glomerulus can be considered a separate coding channel in which the odorants sensed by a single olfactory receptor type all converge onto the same uPNs. In addition to uPNs, multiglomerular PNs innervate multiple glomeruli and most of them project to the Lateral Horn (LH) while skipping the Mushroom Body (MB). Following (Lazar et al., 2023), multiglomerular PNs are ignored in our AL model described below as physiological recordings are only available for uPNs (Kim et al., 2015). An extensive group of Local Neurons (LNs) exclusively innervates the AL (Scheffer et al., 2020; Lazar et al., 2022). LNs are known to mediate presynaptic inhibition on the OSN axon terminals (Olsen and Wilson, 2008).
The I/O modeling of the Antennal Lobe is extensively covered in the Supplementary Material, Section 1. Here, we briefly describe the I/O of the Antennal Lobe circuit with spatio-temporal feedback. The schematic diagram of this circuit is shown in Figure 5. This circuit consists of
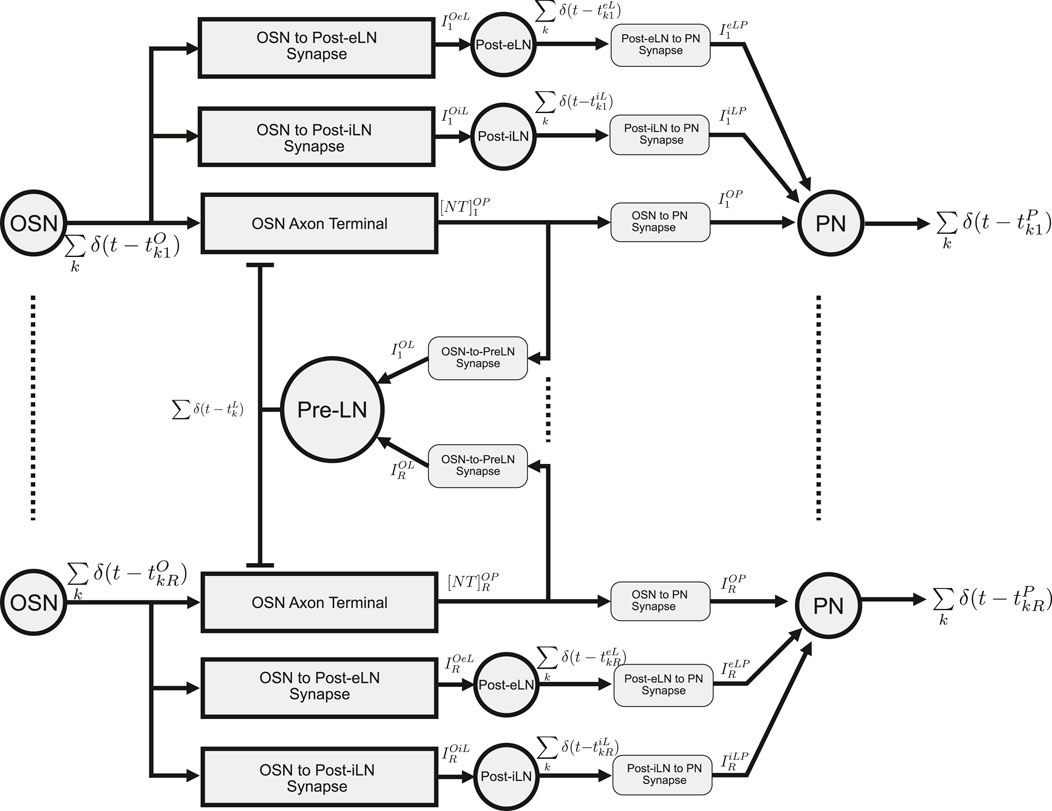
Figure 5. Schematic diagram of the multi-channel AL circuit with spatio-temporal Pre-LN feedback. “OSN” represent a group of OSNs that express the same OR. Their axon terminals provide inputs to uPNs (“PN”) in the same channel (glomerulus). OSN spikes are fed into both Post-eLN and Post-iLN in the channel. These two neurons also drive PNs. In addition to providing inputs to their corresponding PNs, neurotransmitter release at all OSN axon terminals also drive the Pre-LN, which then feedback into the OSN axon terminals. Channels 1 and
In what follows, in response to the spike train generated by an OSN, we will evaluate the currents injected by each of the three DNPs of a single channel
The
where
Pre-LN BSG is modeled as a Noisy Connor-Stevens point neuron model (Lazar and Yeh, 2020), similar to the OSN BSGs. The generated spike train is given by
where
The
where
where
In conclusion, the key parameters and input/output variables of the Antennal Lobe circuit with spatio-temporal feedback (see Figure 5) are shown in detail in Table 2.
2.4 I/O modeling of the mushroom body calyx
The primary circuit architecture of the Mushroom Body Calyx (MB Calyx) exhibits 3 types of neurons. The first neuron type, the uPNs of the Antennal Lobe, projects into the MB Calyx and provides inputs to the second neuron type, the Kenyon Cells (KCs). In the fruit fly, there are about 2,000 KCs on each hemisphere (Li et al., 2020). The connectivity between PNs and KCs is considered random and differs among individual flies (Caron et al., 2013; Masuda-Nakagawa et al., 2005), although a more recent connectome study suggested the existence of more discernible structures (Zheng et al., 2022). Nevertheless, the connectivity is stereotypic with each KC receiving inputs, on average, from 6 to 7 PNs. The third type is an Anterior Paired Lateral (APL) neuron. It covers the entire MB, including the Calyx, and has reciprocal interactions with all the KCs throughout. It has been recently shown that the APL neuron normalizes the magnitude of the overall responses of all the KCs in the MB Calyx (Prisco et al., 2021).
Here, we refine the MB Calyx circuit with two primary structures. First, the PN to KC connectivity is modeled as a bipartite graph, as PNs and KCs can be considered two disjoint sets of vertices in the graph and all edges connect a PN to a KC. Second, we model the interactions between KCs and the APL as a spatio-temporal feedback DNP circuit, similar to the Pre-LN feedback circuit in the Antennal Lobe.
The schematic diagram of the MB Calyx circuit with spatio-temporal Anterior Paired Lateral (APL) feedback is shown in Figure 6. The
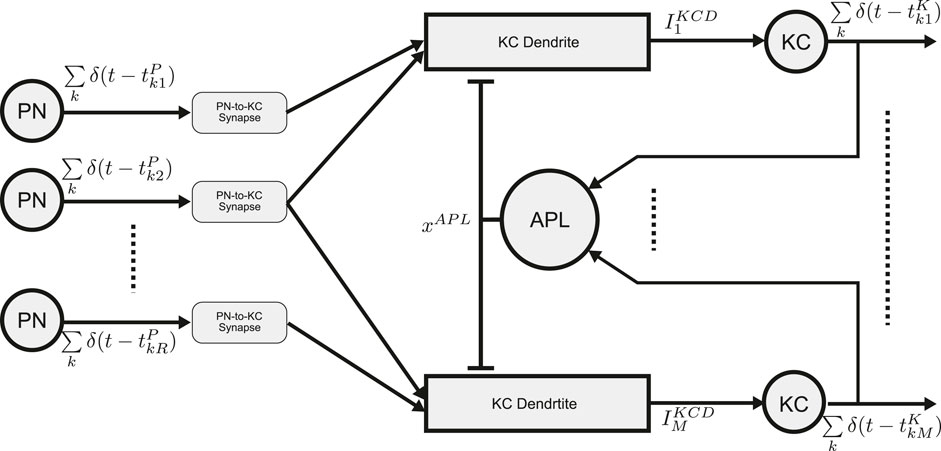
Figure 6. Schematic diagram of the Calyx circuit with spatio-temporal APL feedback. Spiking outputs of the PNs provide inputs to KC dendrites. Each KC receives inputs from
The output current
where
For simplicity, the APL feedback signal
where
The KC BSG is modeled by the NoisyConnorStevens point neuron, with noise variance
where
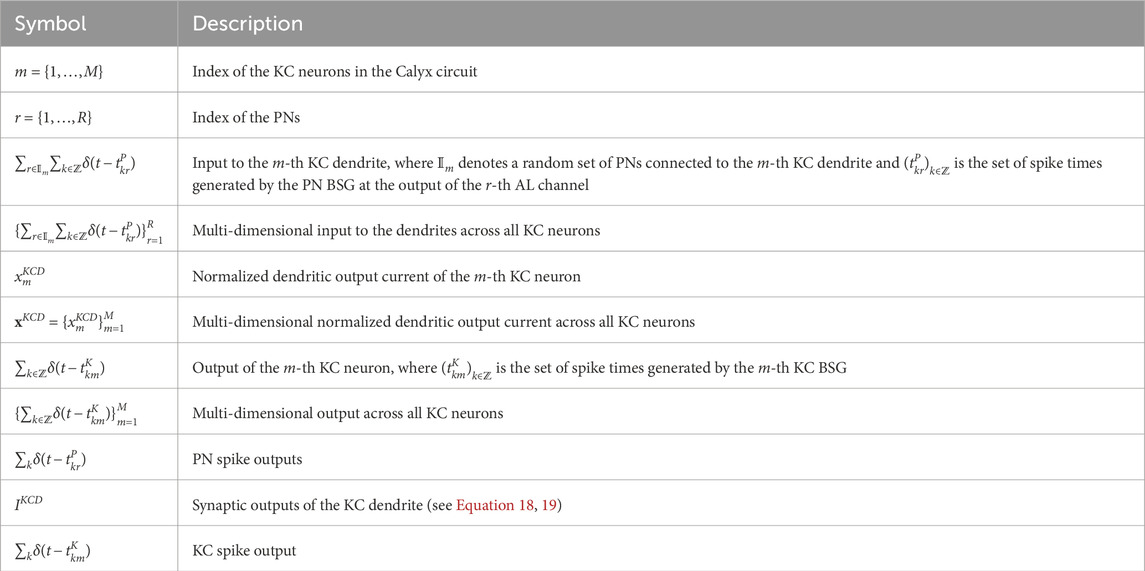
In conclusion, the key parameters and input/output variables of the Calyx circuit (see Figure 6) are shown in detail in Table 3.
3 I/O characterization of odor signal processing in the MB calyx
In what follows, our goal is to characterize the I/O of the MB Calyx, the last building block of the OEM cascade depicted in Figure 2. Given the prior modeling of the space of odorants in Section (2.1), the odorant encoding process in the Antenna described in Section (2.2) and, the odor signal processing taking place in the Antennal Lobe and detailed in Section (2.3), the input to the Mushroom Body Calyx can be readily evaluated as the PN response at the output of the Antennal Lobe for pure and odorant mixtures.
Recall that, we evaluated the odorant encoding process described in Section (2.2) with 110/23 odorant/receptor pairs stored in the DoOR dataset (Münch and Galizia, 2016). Each of the 110 odorants was associated with a 23-dimensional affinity vector whose entries were estimated using the algorithm advanced in (Lazar and Yeh, 2020). Given the PN output provided by the Antennal Lobe model (Lazar et al., 2023), we shall investigate whether the Mushroom Body Calyx extracts semantic information, i.e., the identity of pure odorants and odorant mixtures, faithfully and distortion free.
This section is organized as follows. In section 3.1 we evaluate the effect of the PN-KC connectivity on the KC dendritic input for both pure (mono-molecular) and odorant mixtures. In section 3.2 we evaluate the effect of the KC-APL feedback on the KC dendritic output for both pure and odorant mixtures. Finally, in section 3.3 we show how the Calyx extracts and represents semantic information in the spike domain.
3.1 The effect of the PN-KC connectivity on the KC dendritic input for pure and odorant mixtures
A key descriptor of the Calyx circuit is the connectivity between PNs and KCs, i.e., the adjacency matrix of the PN-KC bipartite graph. The topology of the bipartite graph is determined by two factors. First, each KC receives inputs from a number of
We first evaluate the dependency of the KC dendritic inputs on
A recent experimental study has examined the effect of the number of KC claws on fly’s ability to discriminate odorants (Ahmed et al., 2023). Genetic manipulation allowed the authors to obtain flies that have an increased or decreased number of dendritic claws. Here we evaluate the effect of computationally changing the value of
3.1.1 The effect of the PN-KC connectivity on the dendritic KC input for pure odorants
In this section we evaluate the dependence of the KC dendritic inputs on
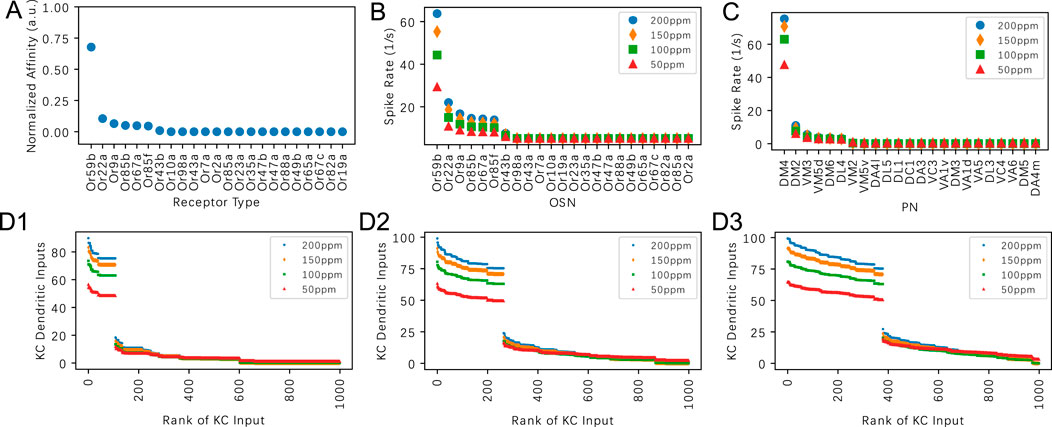
Figure 7. Evaluating the effect of the PN-KC connectivity parameter
Visualizing the KC responses in Figures 7D1–D3, we observe that the number of KCs activated by a given odorant is strongly influenced by the
We note that the ranking of the KC dendritic inputs is largely determined by the number of top responding PNs. For example, if the
In Figure 8, we characterize responses to the odorant Nerol in the same way as in Figure 7 for the odorant Acetone. We note that the affinity values of 3 receptors are relatively higher. This creates a different signature in the ordered ranking of the KC inputs. The general trend is similar to the case when Acetone is presented. With a smaller
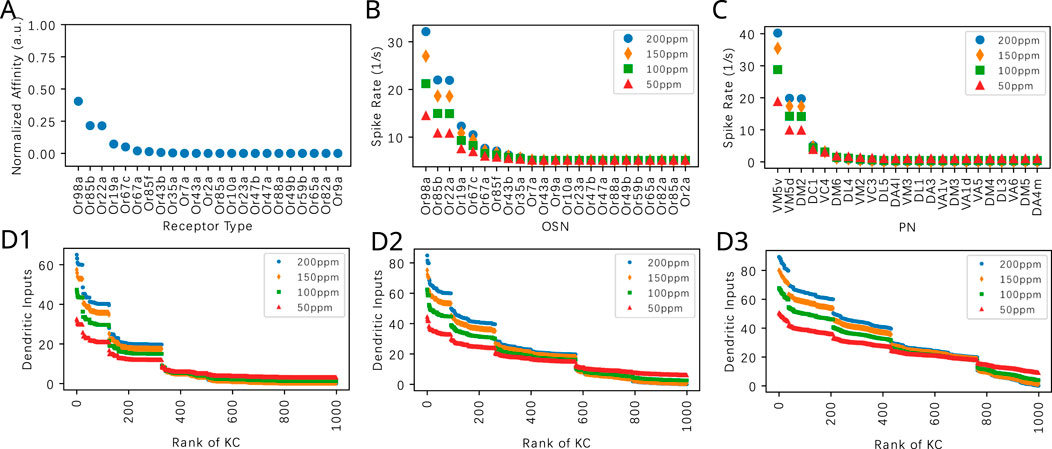
Figure 8. Evaluating the effect of the PN-KC connectivity parameter
With an increasing number of PNs responding to a pure odorant, the dendritic input-ranking curve becomes smoother. See, for example, the results for Diethyl Succinate and Ethyl Butyrate shown, respectively, in Supplementary Figures S3, S4. Ethyl Butyrate elicits responses in a wide range of PNs, and the dendritic input-rank curves are smoother without noticeable gaps between KC dendritic inputs.
Similar dendritic input-rank plots can be obtained for randomly instantiated PN-KC bipartite graphs (see Supplementary Figure S5). Note, however, that for random connectivity, the exact ranking order of each KC might differ. Since the connectivity between PNs and KCs has been shown to be random and may differ from fly to fly (Caron et al., 2013), the preservation of the input-ranking for different odorants across concentration amplitudes applies across individual flies.
3.1.2 The effect of the PN-KC connectivity on the KC dendritic input for odorant mixtures
In Figure 9, we evaluate the dependence of the KC dendritic inputs on the connectivity parameter
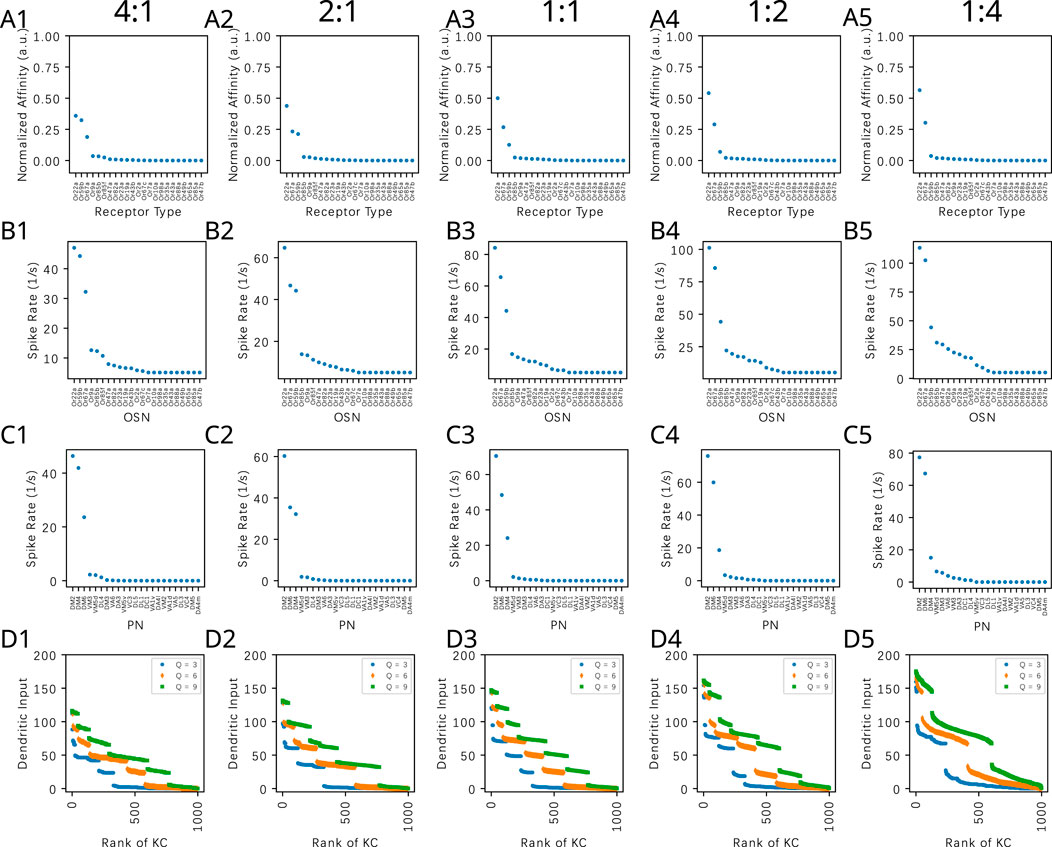
Figure 9. Evaluating the effect of the PN-KC connectivity parameter
We observe that across all
Concluding, the connectivity between the PNs and KCs modeled by a bipartite graph with parameter
3.2 The effect of the KC-APL feedback on the KC dendritic output for pure and odorant mixtures
In this section we analyze the dependence of the Mushroom Body Calyx circuit on the APL feedback. We focus on the effect of APL feedback on the KC dendritic outputs that drive the KC spike generation. For simplicity, we set the connectivity parameter of the PN-KC bipartite graph to
3.2.1 The effect of the KC-APL feedback on the KC dendritic output for pure odorants
We first note that the differential DNP described by Equations 18, 20 is, in steady-state, approximately characterized by a monotonically increasing sigmoid function of KC dendritic inputs. Therefore, we expect that the ranking of the magnitude of KC dendritic inputs is preserved by the KC dendritic outputs.
In Figure 10, we depict the transformation of KC dendritic inputs (left column) into dendritic outputs (middle column) in the presence of APL feedback. Each row of Figure 10 shows the transformation for one of the four odorants that we tested (Acetone, Diethyl Succinate, Nerol and Ethyl Butyrate) each with four different constant amplitude concentration values. The dendritic output amounts to
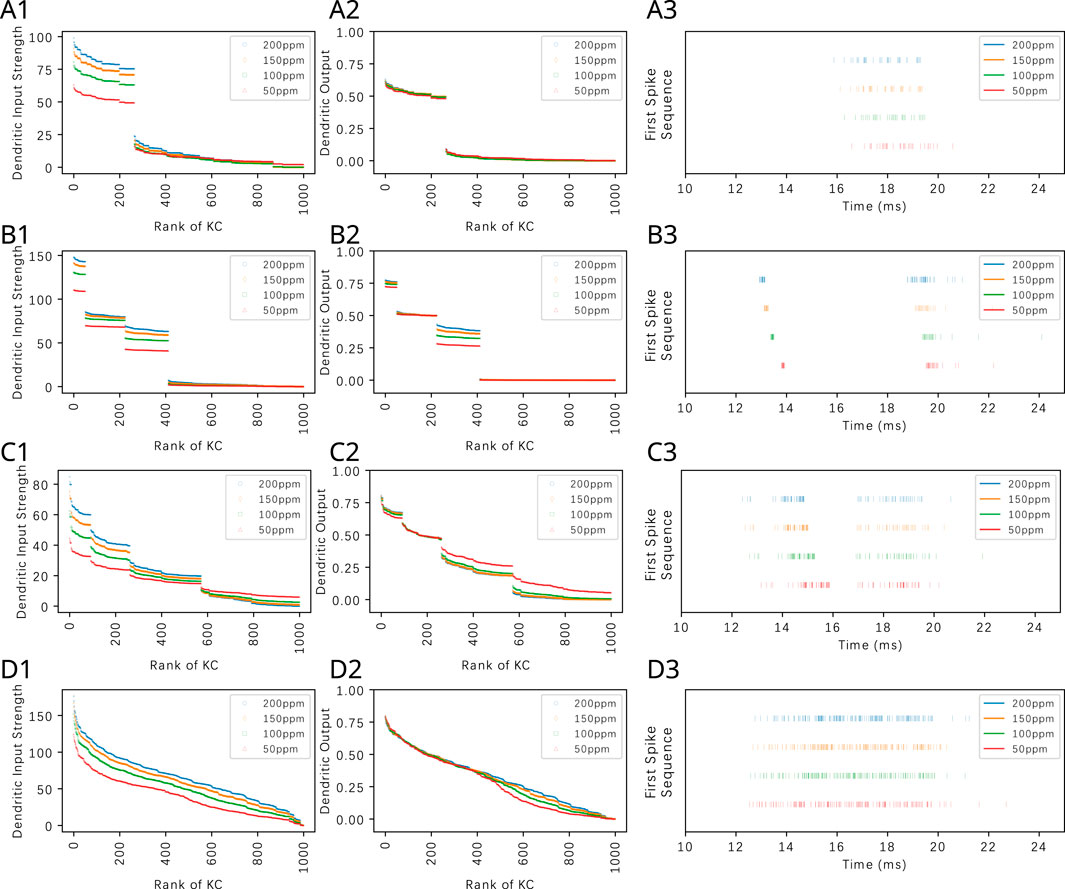
Figure 10. APL feedback facilitates the extraction of odorant semantic information by normalizing KC responses and by reducing odorant concentration dependence of the KC dendritic output. The connectivity parameter of the PN-KC bipartite graph is
As shown in Figure 10, the presence of APL feedback largely removes the concentration dependence of the KC dendritic output if the latter is above threshold. This demonstrates that the proposed divisive normalization circuit is capable of further reducing the variability of KC responses to odorants of different concentration levels (Prisco et al., 2021) beyond the normalization effect induced by the Local Neurons of the Antennal Lobe (Lazar et al., 2023), thereby further separating odorant semantic information from syntactic information (Lazar et al., 2023). The aggregation of the KC responses in Figure 10 (right column) will be discussed in Section 3.3.
3.2.2 The effect of the KC-APL feedback on the KC dendritic output for odorant mixtures
APL feedback is equally effective for extracting the semantic information of odorant mixtures. In Figure 11, we consider a binary mixture consisting of Acetone and diethyl succinate at different component constant amplitude concentration ratios. For each component ratio, we also varied the total concentration while keeping the ratio fixed. The OSN responses to the mixtures are shown in Figure 11A. PN responses, as shown in Figure 11B, exhibited reduced variability to constant concentration ratios. The KC dendritic inputs and and the dendritic outputs are, respectively, shown in Figures 11C. While the magnitude of dendritic inputs varies across component ratios and total concentration, the dendritic outputs display a markedly reduced variability across concentration amplitudes. Among the different component ratios tested, the overall range of responses at the KC dendritic outputs are also similar.
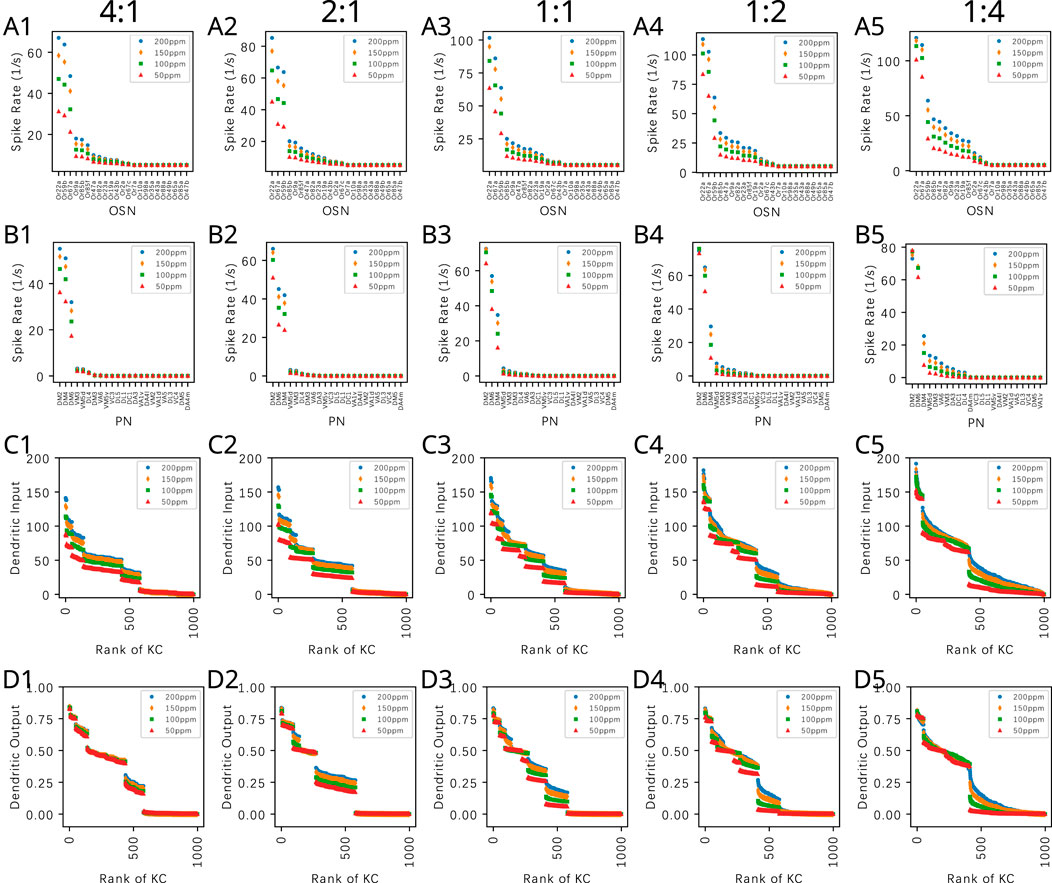
Figure 11. By normalizing KC responses and by reducing odorant concentration dependence of the KC dendritic output, APL feedback reduces the variability of KC responses to component concentration ratios of odorant mixtures. (A) OSN responses to mixture of Acetone and Diethyl Succinate at five different component ratios and different total concentration levels. Legend shows the concentration of Acetone. Concentration of Diethyl Succinate can be derived from the component ratio. (B) PN responses. (C) KC dendritic inputs. (D) KC dendritic outputs. Ratios of acetone to Diethyl Succinate are (column 1) 4:1, (column 2) 2:1, (column 3) 1:1, (column 4) 1:2, (column 5) 1:4. The horizontal axes in rows (C) and (D) list the KCs in ranking order. The connectivity parameter of the PN-KC bipartite graph is
Normalized KC dendritic outputs naturally maintain the number of active KCs with a single spiking threshold. From Figures 10, 11, we can see that about 20% of the KCs are above the spiking threshold. With a different threshold or
3.3 The Calyx extracts and represents odorant semantic information in the spike domain
Ranking the dendritic input and output KCs in Figures 10, 11 provides insights into the structure of the affinity vector of pure and mixture odorants under consideration. The ranking operation, however, exhibits a combinatorial complexity. This forbidding complexity can be computationally readily avoided by mapping, for each KC, the dendritic output into the spike domain. The proposed code takes the first spike of each active KC and joins them all together at generation time into a single first spike sequence. Figure 10 (right column) shows the first spike sequences for four different odorants, each at four different concentration levels. We note that these spike sequences are not generated by a single neuron. Rather, each sequence consists of a train of spikes received by, e.g., a Mushroom Body Output Neuron (MBON) (or APL neuron) innervating its presynaptic KCs in a MB compartment. Therefore, the order of the KC dendritic output that is invariant to odorant concentration can be naturally read out by an MBON (or APL) based on the timing of the proposed first spike sequence.
Since the KC dendritic output is largely concentration invariant for the KCs with dendritic output above the threshold, the variability of the sequence of spikes across a range of concentration amplitude values is small. The first spike sequences in Figure 10 (right column) are clearly different when due to two different odorants but rather similar when due to two different concentration waveforms of the same odorant. In the Supplementary Figure S6, we display the ranked KC dendritic inputs, the ranked KC dendritic outputs, the first spike sequence and the cumulative interspike intervals for all 110 odorants whose OSN responses have been characterized for 23 ORs at a single concentration level in the DoOR dataset (Münch and Galizia, 2016). Note that the cumulative interspike distance plots are largely concentration invariant. This is amply displayed in the last column of the Supplementary Figure S6 for 110 mono-molecular odorants evaluated at four different concentration amplitude values. Thus, we hypothesize that the sequence of first spikes generated by each individual KC represents the odorant semantic information in the time domain largely unaltered by the syntactic information of the odorant concentration waveform.
The key advantage of the first spike sequence code across the active KCs in the spike domain is that the readout of the sequence of spikes arriving at the MBONs does not require the knowledge of the KCs that the spike originated from. The entire sequence becomes a single code. Therefore, the code remains the same for different flies with different instantiations of the PN-KC bipartite graph.
The first spike sequence code can also be used to distinguish odorant mixtures with different mixture ratios. Figure 12 (right column) shows the first spike sequence code for mixtures of Methanol and Benzyl Alcohol at five different ratios. For each fixed ratio, the concentration of the mixture components are presented at four different Methanol concentration levels. Again, the first spike sequence code shows different patterns for each ratio but similar patterns for different concentration ampitudes of the same ratio. The corresponding cumulative interspike intervals are shown in Supplementary Figure S7. The response of the OEM to two other binary mixtures are shown in Supplementary Figures S8, S9.
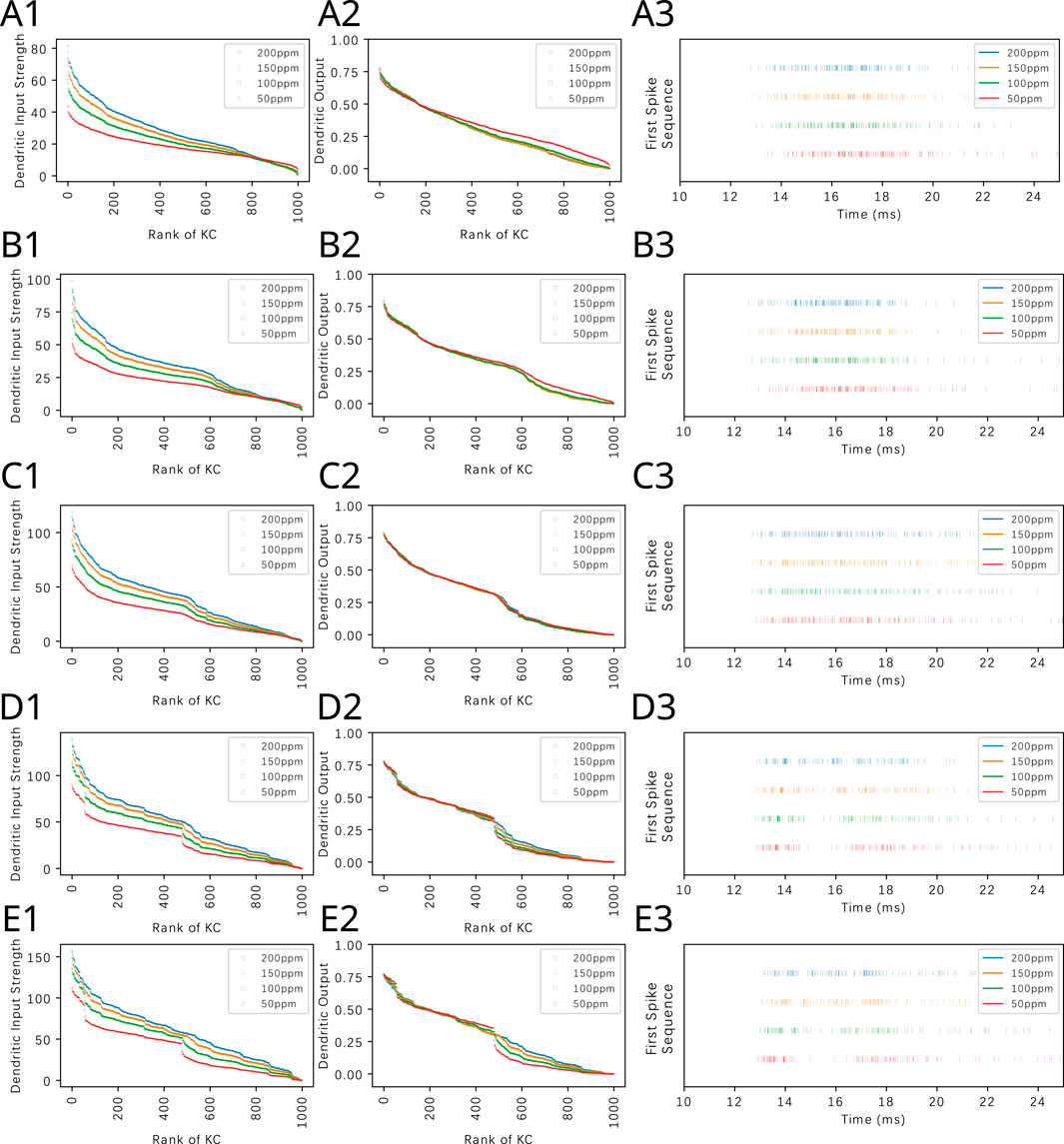
Figure 12. Odorant semantics information of a mixture of Methanol and Benzyl Alcohol with different constant concentration amplitude ratios encoded in the time domain across the population of KCs. The mixtures are presented at a fixed ratio in row (A) 4:1, (B) 2:1, (C) 1:1, (D) 1:2 and (E) 1:4. For each fixed ratio, 4 Methanol concentration levels are used, (red) 50ppm, (green) 100ppm, (orange) 150ppm and (blue) 200ppm. (left column) Ranking of KC dendritic inputs. (middle column) Ranking of KC dendritic outputs. (right column) Odorant semantics encoded in the time domain across the population of KCs. The first spikes of each of the active KCs in response to each odorant are collected onto a single row for each of the odorant concentration amplitude values.
4 Discussion
The early olfactory sensory circuits evolved to encode and identify odorants in various ecological niches, thereby raising the structure and features of the odorant space to be key determinants of the encoding mechanisms adapted in the olfactory pathways. Despite its importance, however, an explicit modeling of the odorant space has often been neglected when discussing odor signal processing in the early olfactory circuits (Endo and Kazama, 2022).
In the present work, we explicitly modeled odorant stimuli in terms of their semantic and syntactic information content, and explored how the early olfactory system of the fruit fly separates semantic and syntactic information. Recall that, Shannon (Shannon, 1948) made a clear distinction between meaning (semantic) and syntactic information. He felt, rightly so, that syntactic information can be formalized and that led to, among others, key concepts in information theory such as channel capacity, coding theorems, etc. One of his main arguments was that “a bit is a bit” and there is no meaning associated with “bits”. He did not address the challenges of formalizing the concept of semantic information.
In accordance to Shannon’s distinction between syntax and semantics, our key prior research results (Lazar and Yeh, 2020; Lazar et al., 2023) have pointed out that the traditional application of methods of information theory, signal processing and control theory to odor signal processing is lacking the notion of “meaning” or semantics. An example might help clarify our point of view. When a neuroscientist applies a mono-molecular odorant such as Acetone, to the Antennae of the fruit fly, and only provides single OSN recordings to a second neuroscientist without mentioning the odorant identity, the recordings alone provide no clues that the odorant in question is Acetone. This is because different odorant identity and concentration pairs can lead to the same OSN spike train response (Lazar and Slutskiy, 2012). Most of the experiments in the olfactory literature, assume that the odorant identity is known. As such, prior arts (Blazing and Franks, 2020; Endo and Kazama, 2022; Bandyopadhyay and Sachse, 2023) have primarily focused on the representation of odorant syntactic information (i.e., concentration amplitude) and cannot, therefore, serve as baseline methods without a formal computational/theoretic model of odorant identity. In contrast we argue that odor signal processing in the Early Olfactory System (EOS) of the fruit fly, is mostly focussed on extracting semantic information. Consequently, we argue that olfactory research needs to shift from solely focusing on processing syntactic (or Shannon) information to processing semantic, i.e., odorant identity information.
To that end, by extending our previous work on the functional logic of odor signal processing in the Antennal Lobe (Lazar et al., 2023), we have established that the Antennal Lobe and Calyx jointly remove the concentration dependency of the odorant information from the confounding representation of the Antenna (Lazar and Yeh, 2020). We demonstrated that these circuits separate the odorant semantics from syntax, thereby undoing the multiplicative coupling of these two information streams in the Antenna.
We showed that in the Calyx the sought after semantic information underlies the ranking of the KC dendritic output after the KC dendritic input undergoes the PN-KC random connectivity and the spatio-temporal feedback provided by the APL neuron. Consequently, expansion recoding in the Calyx characterizes the structure of vector PN responses by computing fixed mean random additive combinations, and use their ranking as a simple yet powerful way of extracting the semantic information of the odorant. More importantly, we addressed the combinatorial complexity of ranking by mapping, for each KC, the concentration-invariant dendritic output into the spike domain. The proposed time code takes the first spike of each active KC and joins them all together at generation time into a single first spike sequence. Clearly, the order of the first spikes across the population of KCs reflects the ranking order at negligible complexity. The existence of such concentration-invariant spike code is supported by increasing evidence in the Antenna and Antennal Lobe (Haddad et al., 2013; Wilson et al., 2017; Zwicker, 2019; Chong et al., 2020; Egea-Weiss et al., 2018), the piriform cortex (Bolding and Franks, 2018), the visual system (Rullen and Thorpe, 2001; Gollisch and Meister, 2008), and at the neuron level in general (Branco et al., 2010).
Time is an intrinsic variable of the concentration waveform, but not of the odorant object identity. Interestingly, the key result of the modeling and characterization of the early olfactory system we advanced here asserts that the semantic information is mapped into the time domain by the Calyx circuit, in the form of the first spike sequence code. This allows a low complexity single readout of the semantic information at the downstream MBONs regardless of the exact connectivity between PNs and KCs in individual flies. The code itself is temporally bounded, making it possible for timely memory access in the MB compartments.
Overall, our work argues that the main information pattern processed by the early olfactory system is supplied by the odorant semantics and not the syntax. The odorant semantics is mapped by the Calyx circuit into a first spike sequence in the time domain. This is, clearly, central to understanding the functional logic of the neural circuits involved in odor signal processing in the EOS of the fruit fly brain. Our approach, backed up by the analysis of the fist spike sequence code and the robustness of the cumulative interspike intervals of 110 odorants in the DoOR dataset (Münch and Galizia, 2016), represents a radical departure in understanding the logic of odor signal processing in the EOS. Among others, it calls for recordings of the KCs in the MB with the ultimate goal of addressing the existence of the first spike sequence code that we advanced here.
Furthermore, we extended the model of mono-molecular odor signal processing in the Antenna, Antennal Lobe and Calyx to odorant mixtures. Our model covers the syntopic interactions (Rospars et al., 2008) among odorants competing for the unbound receptors in the OSN cilia while abstracting additional resources (e.g., the number of permeable pores on the surface of sensilla binding proteins (Larter et al., 2016)) into the peri-receptor processes. No further interactions between odorants and the same receptor type have been modeled that may result in binding/dissociation facilitation or suppression (Singh et al., 2019). We note that alternative extensions to the OTP model may be developed for describing other phenomena of odorant mixture encoding, such as masking (Reddy et al., 2018), and ephaptic coupling (Su et al., 2012; Wu et al., 2022; Pannunzi and Nowotny, 2021). However, despite recent insight into the structure of Machili hrabei olfactory receptor (del Mármol et al., 2021), additional recording datasets are required to determine which model most accurately describes odorant mixture binding to Drosophila olfactory receptors.
Algorithmically, our model of the first three stages of the EOS anchors on Divisive Normalization Processors (DNPs). DNPs are models of biological neural circuits (Lazar et al., 2020b; Lazar and Zhou, 2023) with spatio-temporal feedforward and/or feedback control. The power of DNPs in modeling key computational building blocks in the early olfactory system suggests their applicability in many other sensory processing systems in and beyond those of the fruit fly brain.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AL: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing–original draft, Writing–review and editing. TL: Conceptualization, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft, Writing–review and editing. C-HY: Conceptualization, Investigation, Methodology, Software, Validation, Visualization, Writing–original draft. YZ: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The research reported here was supported by AFOSR under grant #FA9550-16-1-0410, DARPA under contract #HR0011-19-9-0035 and NSF under grant #2024607. YZ was also supported by a Fordham Research Fellowship from the Office of Research at Fordham University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2024.1410946/full#supplementary-material
References
Ache B. W., Young J. M. (2005). Olfaction: diverse species, conserved principles. Neuron 48, 417–430. doi:10.1016/j.neuron.2005.10.022
Ahmed M., Rajagopalan A. E., Pan Y., Li Y., Williams D. L., Pedersen E. A., et al. (2023). Input density tunes kenyon cell sensory responses in the drosophila mushroom body. Curr. Biol. 33, 2742–2760.e12. doi:10.1016/j.cub.2023.05.064
Bandyopadhyay P., Sachse S. (2023). Mixing things up! — how odor blends are processed in drosophila. Curr. Opin. Insect Sci. 59, 101099. doi:10.1016/j.cois.2023.101099
Barish S., Volkan P. C. (2015). Mechanisms of olfactory receptor neuron specification in drosophila. WIREs Dev. Biol. 4, 609–621. doi:10.1002/wdev.197
Benton R. (2022). Drosophila olfaction: past, present and future. Proc. R. Soc. B 289, 20222054. doi:10.1098/rspb.2022.2054
Blazing R. M., Franks K. M. (2020). Odor coding in piriform cortex: mechanistic insights into distributed coding. Curr. Opin. Neurobiol. 64, 96–102. doi:10.1016/j.conb.2020.03.001
Bolding K. A., Franks K. M. (2018). Recurrent cortical circuits implement concentration-invariant odor coding. Science 361, eaat6904. doi:10.1126/science.aat6904
Branco T., Clark B. A., Häusser M. (2010). Dendritic discrimination of temporal input sequences in cortical neurons. Science 329, 1671–1675. doi:10.1126/science.1189664
Buck L., Axel R. (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187. doi:10.1016/0092-8674(91)90418-x
Buck L. B. (2005). Unraveling the sense of smell (Nobel Lecture). Angewandte Chemie International Edition, 44, 6128–6140. doi:10.1002/anie.200501120
Bushdid C., Magnasco M. O., Vosshall L. B., Keller A. (2014). Humans can discriminate more than 1 trillion olfactory stimuli. Science 343, 1370–1372. doi:10.1126/science.1249168
Carandini M., Heeger D. J. (2012). Normalization as a canonical neural computation. Nat. Rev. Neurosci. 13, 51–62. doi:10.1038/nrn3136
Caron S. J. C., Ruta V., Abbott L. F., Axel R. (2013). Random convergence of olfactory inputs in the Drosophila mushroom body. Nature 497, 113–117. doi:10.1038/nature12063
Chong E., Moroni M., Wilson C., Shoham S., Panzeri S., Rinberg D. (2020). Manipulating synthetic optogenetic odors reveals the coding logic of olfactory perception. Science 368, eaba2357. doi:10.1126/science.aba2357
Cleland T. A., Sethupathy P. (2006). Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci. 7, 7. doi:10.1186/1471-2202-7-7
Connor J. A., Stevens C. F. (1971). Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J. Physiology 213, 31–53. doi:10.1113/jphysiol.1971.sp009366
Dasgupta S., Stevens C. F., Navlakha S. (2017). A neural algorithm for a fundamental computing problem. Science 358, 793–796. doi:10.1126/science.aam9868
del Mármol J., Yedlin M. A., Ruta V. (2021). The structural basis of odorant recognition in insect olfactory receptors. Nature 597, 126–131. doi:10.1038/s41586-021-03794-8
Egea-Weiss A., Renner A., Kleineidam C. J., Szyszka P. (2018). High precision of spike timing across olfactory receptor neurons allows rapid odor coding in drosophila. iScience 4, 76–83. doi:10.1016/j.isci.2018.05.009
Endo K., Kazama H. (2022). Central organization of a high-dimensional odor space. Curr. Opin. Neurobiol. 73, 102528. doi:10.1016/j.conb.2022.102528
Fabian B., Sachse S. (2023). Experience-dependent plasticity in the olfactory system of drosophila melanogaster and other insects. Front. Cell. Neurosci. 17, 1130091. doi:10.3389/fncel.2023.1130091
Firestein S. (2001). How the olfactory system makes sense of scents. Nature 413, 211–218. doi:10.1038/35093026
Gkanias E., McCurdy L. Y., Nitabach M. N., Webb B. (2022). An incentive circuit for memory dynamics in the mushroom body of Drosophila melanogaster. eLife 11, e75611. doi:10.7554/eLife.75611
Gollisch T., Meister M. (2008). Rapid neural coding in the retina with relative spike latencies. Science 319, 1108–1111. doi:10.1126/science.1149639
Haddad R., Lanjuin A., Madisen L., Zeng H., Murthy V. N., Uchida N. (2013). Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat. Neurosci. 16, 949–957. doi:10.1038/nn.3407
Heisenberg M. (2003). Mushroom body memoir: from maps to models. Nat. Rev. Neurosci. 4, 266–275. doi:10.1038/nrn1074
Jeanne J. M., Wilson R. I. (2015). Convergence, divergence, and reconvergence in a feedforward network improves neural speed and accuracy. Neuron 88, 1014–1026. doi:10.1016/j.neuron.2015.10.018
Kim A. J., Lazar A. A., Slutskiy Y. B. (2011). System identification of drosophila olfactory sensory neurons. J. Comput. Neurosci. 30, 143–161. doi:10.1007/s10827-010-0265-0
Kim A. J., Lazar A. A., Slutskiy Y. B. (2015). Projection neurons in drosophila antennal lobes signal the acceleration of odor concentrations. eLife 4, e06651. doi:10.7554/eLife.06651
Larter N. K., Sun J. S., Carlson J. R. (2016). Organization and function of Drosophila odorant binding proteins. eLife 5, e20242. doi:10.7554/eLife.20242
Lazar A. A., Liu T., Turkcan M. K., Zhou Y. (2021). Accelerating with flybrainlab the discovery of the functional logic of the Drosophila brain in the connectomic and synaptomic era. eLife 10, e62362. doi:10.7554/eLife.62362
Lazar A. A., Liu T., Yeh C.-H. (2020a). “An odorant encoding machine for sampling, reconstruction and robust representation of odorant identity,” in IEEE international Conference on acoustics, Speech and signal processing (barcelona, Spain), 1743–1747.
Lazar A. A., Liu T., Yeh C. H. (2023). The functional logic of odor information processing in the drosophila antennal lobe. PLOS Comput. Biol. 19, e1011043. doi:10.1371/journal.pcbi.1011043
Lazar A. A., Slutskiy Y. B. (2012). “Identifying dendritic processing in drosophila osns,” in Computational and systems neuroscience meeting (COSYNE 2012).
Lazar A. A., Turkcan M. K., Zhou Y. (2022). A programmable ontology encompassing the functional logic of the drosophila brain. Front. Neuroinformatics 16, 853098. doi:10.3389/fninf.2022.853098
Lazar A. A., Ukani N. H., Zhou Y. (2020b). Sparse identification of contrast gain control in the fruit fly photoreceptor and amacrine cell layer. J. Math. Neurosci. 10, 3.doi:10.1186/s13408-020-0080-5
Lazar A. A., Yeh C.-H. (2020). A molecular odorant transduction model and the complexity of spatio-temporal encoding in the Drosophila antenna. PLOS Comput. Biol. 16, e1007751. doi:10.1371/journal.pcbi.1007751
Lazar A. A., Zhou Y. (2023). Divisive normalization processors in the early visual system of the drosophila brain. Biol. Cybern. 117, 411–431. doi:10.1007/s00422-023-00972-x
Li F., Lindsey J. W., Marin E. C., Otto N., Dreher M., Dempsey G., et al. (2020). The connectome of the adult Drosophila mushroom body provides insights into function. eLife 9, e62576. doi:10.7554/eLife.62576
Masek P., Heisenberg M. (2008). Distinct memories of odor intensity and quality in Drosophila. Proc. Natl. Acad. Sci. 105, 15985–15990. doi:10.1073/pnas.0804086105
Masse N. Y., Turner G. C., Jefferis G. S. (2009). Olfactory information processing in drosophila. Curr. Biol. 19, R700–R713. doi:10.1016/j.cub.2009.06.026
Masuda-Nakagawa L. M., Tanaka N. K., O’Kane C. J. (2005). Stereotypic and random patterns of connectivity in the larval mushroom body calyx of Drosophila. Proc. Natl. Acad. Sci. 102, 19027–19032. doi:10.1073/pnas.0509643102
Modi M. N., Shuai Y., Turner G. C. (2020). The Drosophila mushroom body: from architecture to algorithm in a learning circuit. Annu. Rev. Neurosci. 43, 465–484. doi:10.1146/annurev-neuro-080317-0621333
Mombaerts P. (1999). Molecular biology of odorant receptors in vertebrates. Annu. Rev. Neurosci. 22, 487–509. doi:10.1146/annurev.neuro.22.1.487
Münch D., Galizia C. G. (2016). DoOR 2.0 - comprehensive mapping of drosophila melanogaster odorant responses. Sci. Rep. 6, 21841. doi:10.1038/srep21841
Nagel K. I., Wilson R. I. (2011). Biophysical mechanisms underlying olfactory receptor neuron dynamics. Nat. Neurosci. 14, 208–216. doi:10.1038/nn.2725
NeuroNLP (2024a). Url for figure 1 3rd column. Available at: https://hemibrain.neuronlp.fruitflybrain.org?tag=lazar_2024_fig1c (Accessed August 24, 2024).
NeuroNLP (2024b). Url for figure 1 4th column. Available at: https://hemibrain.neuronlp.fruitflybrain.org?tag=lazar_2024_fig1d (Accessed August 24, 2024).
Olsen S. R., Bhandawat V., Wilson R. I. (2010). Divisive normalization in olfactory population codes. Neuron 66, 287–299. doi:10.1016/j.neuron.2010.04.009
Olsen S. R., Wilson R. I. (2008). Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960. doi:10.1038/nature06864
Pannunzi M., Nowotny T. (2021). Non-synaptic interactions between olfactory receptor neurons, a possible key feature of odor processing in flies. PLOS Comput. Biol. 17, e1009583. doi:10.1371/journal.pcbi.1009583
Prisco L., Deimel S. H., Yeliseyeva H., Fiala A., Tavosanis G. (2021). The anterior paired lateral neuron normalizes odour-evoked activity in the Drosophila mushroom body calyx. eLife 10, e74172. doi:10.7554/eLife.74172
Reddy G., Zak J. D., Vergassola M., Murthy V. N. (2018). Antagonism in olfactory receptor neurons and its implications for the perception of odor mixtures. eLife 7, e34958. doi:10.7554/eLife.34958
Rospars J.-P., Lansky P., Chaput M., Duchamp-Viret P. (2008). Competitive and noncompetitive odorant interactions in the early neural coding of odorant mixtures. J. Neurosci. 28, 2659–2666. doi:10.1523/JNEUROSCI.4670-07.2008
Rullen R. V., Thorpe S. J. (2001). Rate coding versus temporal order coding: what the retinal ganglion cells tell the visual cortex. Neural Comput. 13, 1255–1283. doi:10.1162/08997660152002852
Sanes J. R., Zipursky S. L. (2010). Design principles of insect and vertebrate visual systems. Neuron 66, 15–36. doi:10.1016/j.neuron.2010.01.018
Saumweber T., Rohwedder A., Schleyer M., Eichler K., Chen Y.-c., Aso Y., et al. (2018). Functional architecture of reward learning in mushroom body extrinsic neurons of larval drosophila. Nat. Commun. 9, 1104. doi:10.1038/s41467-018-03130-1
Scheffer L. K., Xu C. S., Januszewski M., Lu Z., Takemura S.-y., Hayworth K. J., et al. (2020). A connectome and analysis of the adult Drosophila central brain. eLife 9, e57443. doi:10.7554/eLife.57443
Schürmann F.-W. (1974). Bemerkungen zur funktion der corpora pedunculata im gehirn der insekten aus morphologischer sicht. Exp. Brain Res. 19, 406–432. doi:10.1007/BF00234464
Shannon C. E. (1948). A mathematical theory of communication. Bell Syst. Tech. J. 27, 623–656. doi:10.1002/j.1538-7305.1948.tb00917.x
Shepherd G. M. (2004). The human sense of smell: are we better than we think? PLoS Biol. 2, e146. doi:10.1371/journal.pbio.0020146
Singh V., Murphy N. R., Balasubramanian V., Mainland J. D. (2019). Competitive binding predicts nonlinear responses of olfactory receptors to complex mixtures. Proc. Natl. Acad. Sci. U. S. A. 116, 9598–9603. doi:10.1073/pnas.1813230116
Stopfer M., Jayaraman V., Laurent G. (2003). Intensity versus identity coding in an olfactory system. Neuron 39, 991–1004. doi:10.1016/j.neuron.2003.08.011
Su C.-Y., Menuz K., Reisert J., Carlson J. R. (2012). Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature 492, 66–71. doi:10.1038/nature11712
Ukani N. H., Yeh C.-H., Tomkins A., Zhou Y., Florescu D., Ortiz C. L., et al. (2019). The fruit fly brain observatory: from structure to function. bioRxiv. doi:10.1101/580290
Ukani N. H., Yeh C.-H., Tomkins A., Zhou Y., Turkcan M. K., Liu T., et al. (2024). Fruit fly brain observatory. Available at: https://fruitflybrain.org (Accessed August 24, 2024).
Vosshall L. B. (2000). Olfaction in drosophila. Curr. Opin. Neurobiol. 10, 498–503. doi:10.1016/S0959-4388(00)00111-2
Vosshall L. B., Stocker R. F. (2007). Molecular architecture of smell and taste in drosophila. Annu. Rev. Neurosci. 30, 505–533. doi:10.1146/annurev.neuro.30.051606.094306
Wilson C. D., Serrano G. O., Koulakov A. A., Rinberg D. (2017). A primacy code for odor identity. Nat. Commun. 8, 1477. doi:10.1038/s41467-017-01432-4
Wu S.-T., Chen J.-Y., Martin V., Ng R., Zhang Y., Grover D., et al. (2022). Valence opponency in peripheral olfactory processing. Proc. Natl. Acad. Sci. 119, e2120134119. doi:10.1073/pnas.2120134119
Yusuyama K., Meinertzhagen I. A., Schürmann F. W. (2002). Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J. Comp. Neurology 445, 211–226. doi:10.1002/cne.10155
Zheng Z., Lauritzen J. S., Perlman E., Robinson C. G., Nichols M., Milkie D., et al. (2017). A complete electron microscopy volume of the brain of adult drosophila melanogaster. cell 174, 730–743.e22. doi:10.1016/j.cell.2018.06.019
Zheng Z., Li F., Fisher C., Ali I. J., Sharifi N., Calle-Schuler S., et al. (2022). Structured sampling of olfactory input by the fly mushroom body. Curr. Biol. 32, 3334–3349.e6. doi:10.1016/j.cub.2022.06.031
Keywords: Drosophila, olfaction, divisive normalization, calyx, APL, kenyon cell, odorant mixtures, pure odorants
Citation: Lazar AA, Liu T, Yeh C-H and Zhou Y (2024) Modeling and characterization of pure and odorant mixture processing in the Drosophila mushroom body calyx. Front. Physiol. 15:1410946. doi: 10.3389/fphys.2024.1410946
Received: 02 April 2024; Accepted: 05 September 2024;
Published: 16 October 2024.
Edited by:
André Fiala, University of Göttingen, GermanyReviewed by:
Bart R. H. Geurten, University of Otago, New ZealandAndreas Stephan Thum, Leipzig University, Germany
Copyright © 2024 Lazar, Liu, Yeh and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aurel A. Lazar, YXVyZWxAZWUuY29sdW1iaWEuZWR1
†The authors’ names are listed in alphabetical order
 Aurel A. Lazar
Aurel A. Lazar Tingkai Liu
Tingkai Liu Chung-Heng Yeh
Chung-Heng Yeh Yiyin Zhou
Yiyin Zhou